Submitted:
28 November 2025
Posted:
01 December 2025
You are already at the latest version
Abstract
With the increase of watermelon cultivation area and continuous cropping, the harm of watermelon sclerotiniosis is becoming more and more serious. It has now risen to an important disease in watermelon production, which seriously affects the quality and yield of watermelon. The pathogen of watermelon Sclerotinia sclerotiorum is [Sclerotinia sclerotiorum(Lib.) De Bary], which is widely distributed in the world, causes plant sclerotia worldwide. The host range of Sclerotinia sclerotiorum is very wide, which not only harms watermelon, but also infects 75 families, 278 genera and 450 species of Cucurbitaceae, Leguminosae, Solanaceae and Cruciferae. To provide a new control method for the biological control of sclerotinia sclerotiorum in watermelon, the biocontrol bacteria in soil were isolated, screened and identified, and the bacteriostasis was studied. (1) The isolation, identification and biological characteristics of the pathogenic bacteria were determined. The fungus strain LY 24 was obtained from watermelon stem and vine infected with Sclerotinia sclerotiorum. The fungus strain LY 24 had good pathogenicity to watermelon plants by pathogenicity. The strain LY 24 was identified by morphology and molecular biology. The results showed that the best carbon source of strain LY 24 was mannitol, the best nitrogen source was tryptone, the best inorganic salt was NaCl, the best pH value was 9.The best growth temperature was 25℃, and the best light condition was whole darkness. (2) The isolation and identification of biocontrol bacteria in soil were carried out in the watermelon planting base around Changchun, such as the watermelon planting base in Jiutai District. Forty soil samples around the rhizosphere of watermelon plants were collected, and 300 strains of bacteria were isolated from the samples. The bacterial strains with significant antagonistic effect were obtained by plate confrontation method, and the strain XJ-04 with good antibacterial effect was selected as the research object. The inhibitory band width of strain XJ-04 was about 5.21 mm. The inhibitory rate of strain XJ-04 against Sclerotinia sclerotiorum was 70.12%. After morphological and molecular biological identification, strain XJ-04 was identified as Bacillus marisflavi. The physiological and biochemical characteristics of biocontrol strain XJ-04 were analyzed by detecting Gram reaction, contact enzyme reaction, methyl red reaction, V-P reaction and hydrogen sulfide reaction. (3) Studies on fermentation optimization of strain and fermentation broth stability. The optimum composition of culture medium was determined by orthogonal test, which was No.8 culture medium (sucrose, fine bran, K2HPO4·3H2O, pH 9). The inhibition rate was about 71.75%. The optimum addition amount of the above three components was determined by response surface methodology. When the addition amounts of sucrose, fine bran and K2HPO4·3H2O were about 21.08 g/L, 9.17 g/L and 9.77 g/L respectively, the highest inhibition rate was about 77. 78% by Design Expert software, which was about 7.66% higher than that before optimization, and 4% higher than that before optimization. The optimal fermentation conditions were 100 ml of liquid, 30℃ of temperature and 3d of fermentation time. The stability study showed that the bacteriostasis rate of fermentation broth treated by different temperature, pH, storage time and light had little change.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test Materials and Methods
2.1.1. Test Material
2.1.1.1. Test Strain
2.1.1.2 Test Medium
2.1.1.3 Main Reagents
2.1.1.4 Main Instruments and Equipment
2.1.2. Test Method
2.1.2.1 Isolation and Purification of Pathogenic Fungi
2.1.2.2 Pathogenicity Test
2.1.2.3 Identification of Pathogens
2.1.2.4 Biological Characteristics
3. Results
3.1. Isolation and Pathogenicity Determination of Pathogenic Fungi
3.1.1.1. Isolation and Purification of Pathogenic Fungi

3.1.1.2. Pathogenicity Test
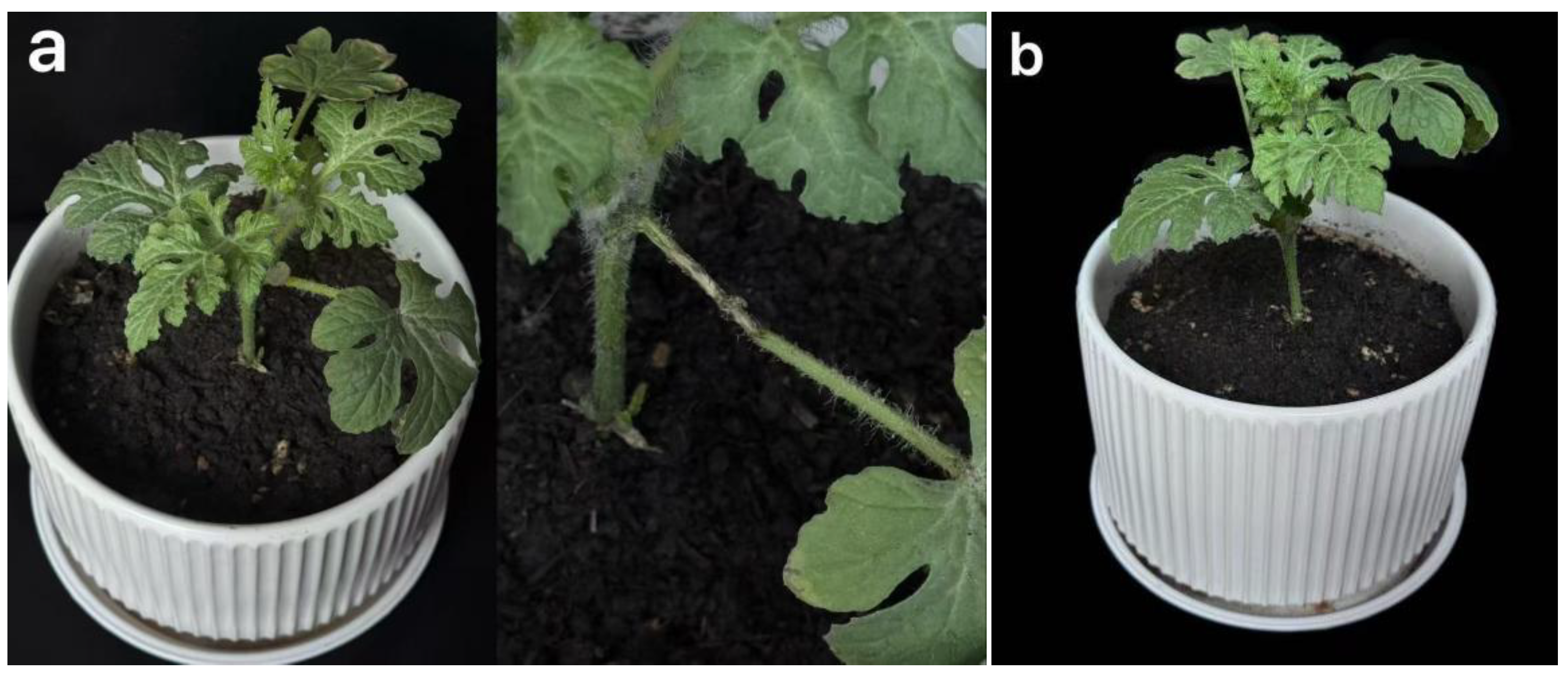
3.1.2. Identification of Pathogens
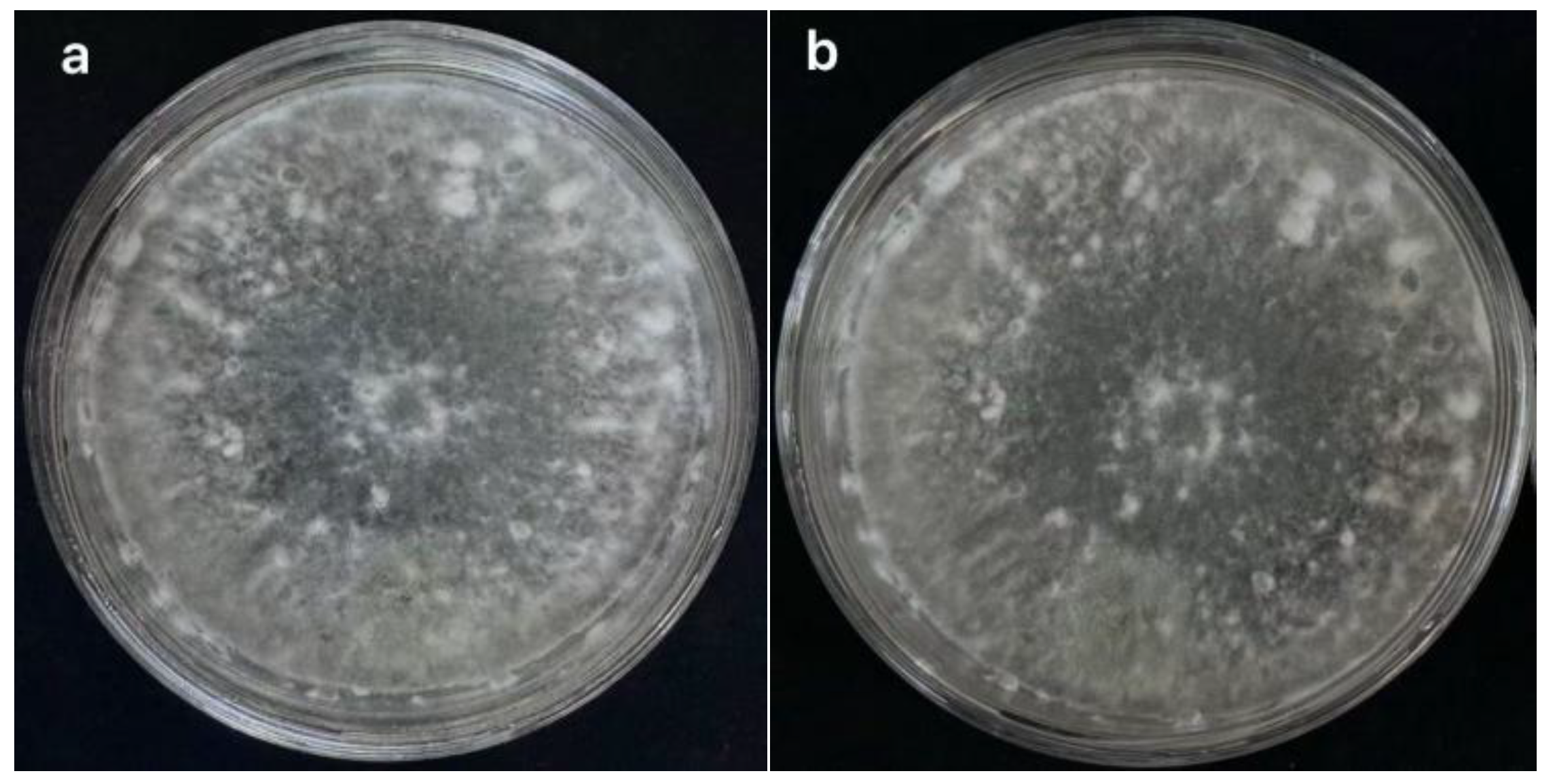
3.1.2.1 Morphological Identification
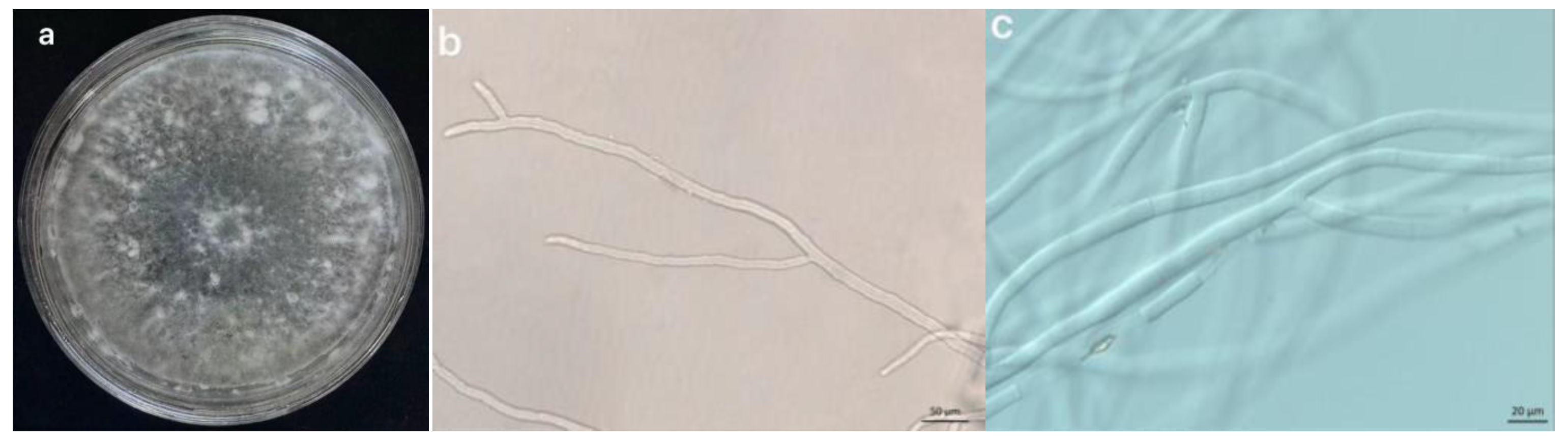
3.1.2.2 Molecular Biological Identification
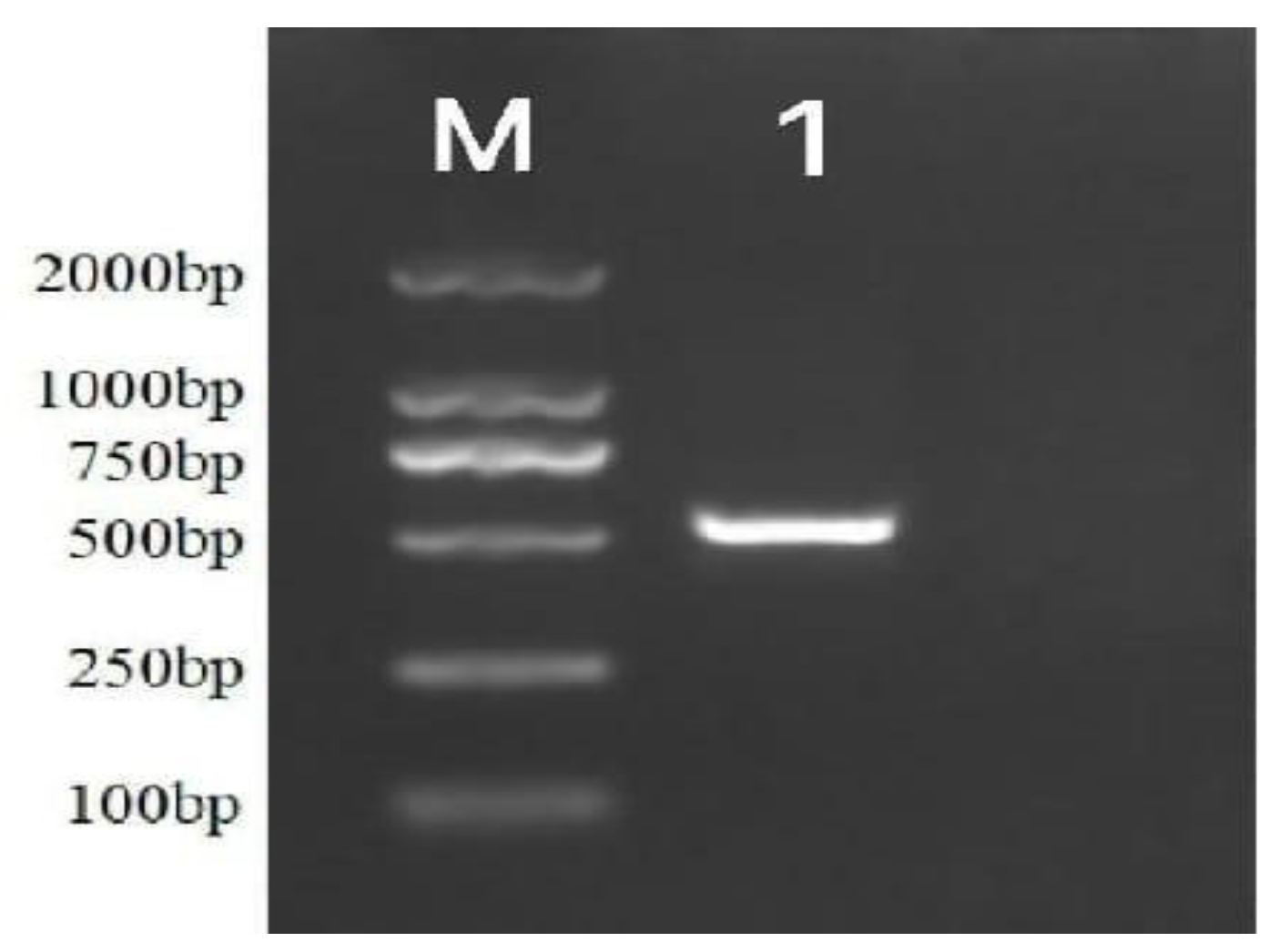
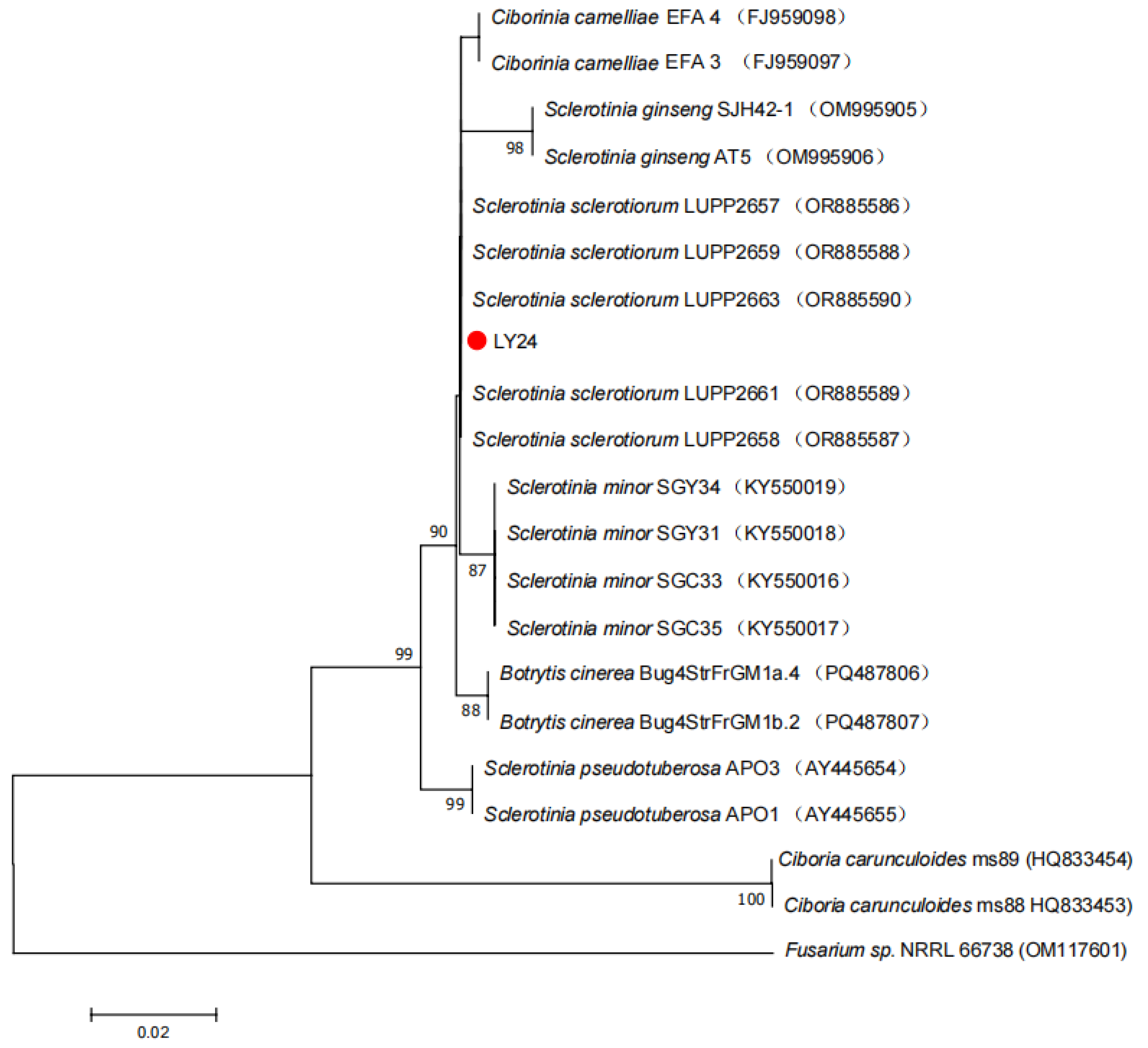
3.1.3. Biological Characteristics
3.1.3.1 Effects of Medium Components on Mycelial Growth of Pathogenic Fungi
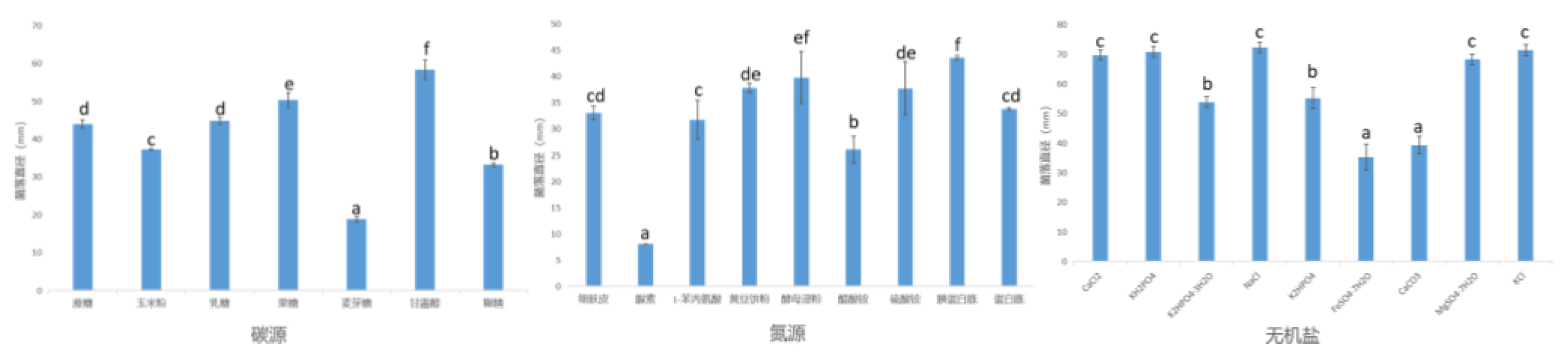
3.1.3.2 Effects of Culture Conditions on Mycelial Growth of Pathogens
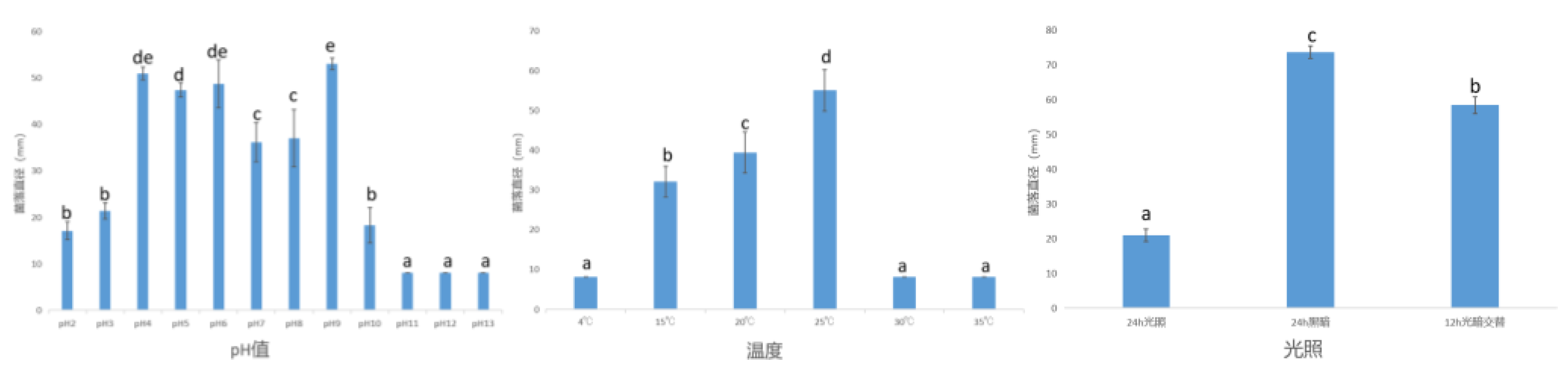
3.1.4. Summary
3.2. Isolation, Screening and Identification of Biocontrol Bacteria
3.2. Test Materials and Methods
3.2.1. Test Material
3.2.1.1 Target Strain
3.2.1.2 Soil Samples and Culture Medium
3.2.1.3 Main Reagents
3.2.1.4 Main Instruments and Equipment
3.2.2. Test Method
3.2.2.1 Collection of Soil Samples and Separation and Purification of Biocontrol Bacteria
3.2.2.2 Screening of Biocontrol Bacteria
3.2.2.3 Identification of Biocontrol Bacteria
3.2.3. Results and Analysis3.2.3. Isolation and Screening of Soil Bacteria
3.2.3.1 Isolation of Bacterial Strains
3.2.3.2 Screening of Antagonistic Bacteria Strains
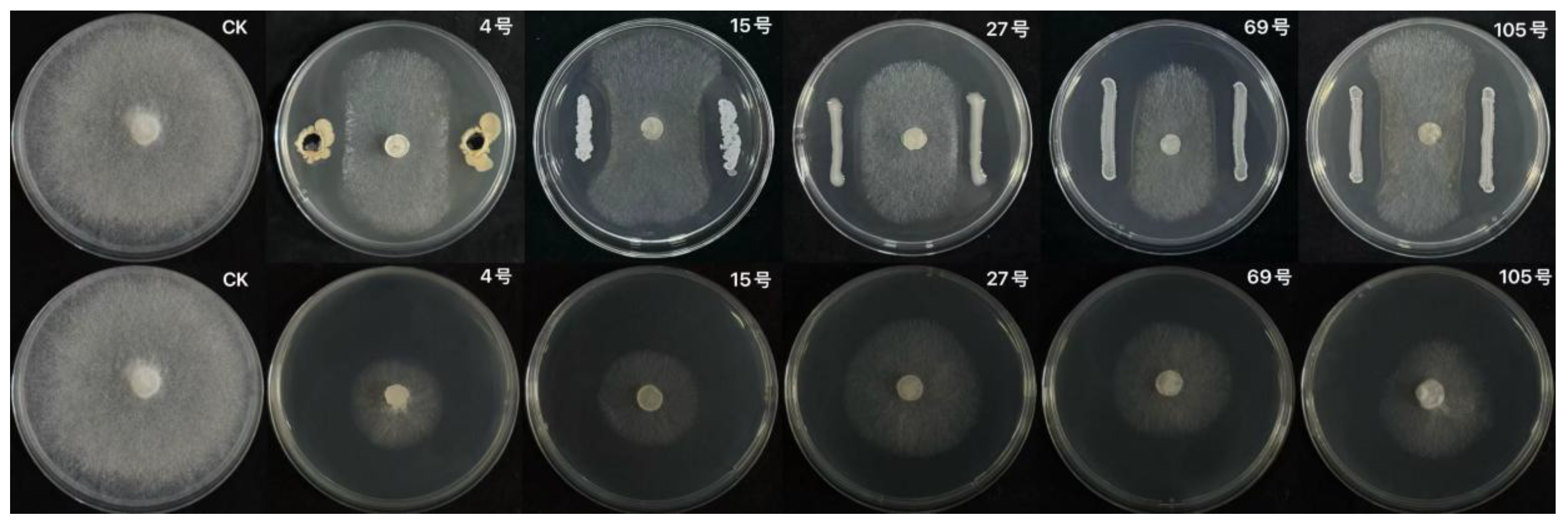
| Treatment | Number of sclerotia germination in 3d | Inhibition rate(%) |
| Fermentation supernatant | 10 | 60 |
| CK | 25 | / |
3.2.2. Identification of Biocontrol Fungi
3.2.2.1 Morphological Identification
3.2.2.2 Physiological and Biochemical Identification

| Item | Result |
| Gram reaction | + |
| Contact enzyme reaction | + |
| Methyl red reaction | + |
| V-P Reaction | - |
| Hydrogen sulfide reaction | - |
3.2.2.3 Molecular Biological Identification
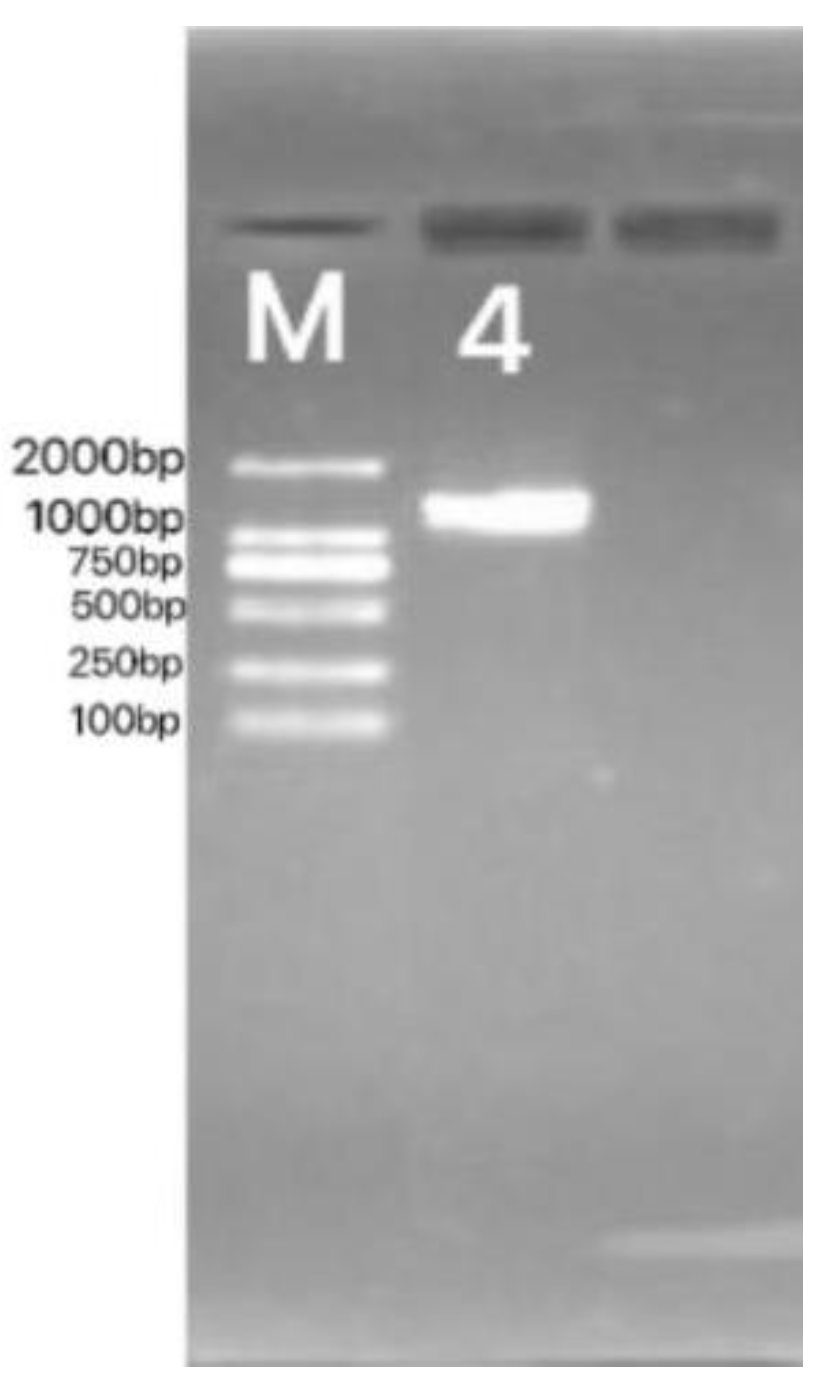
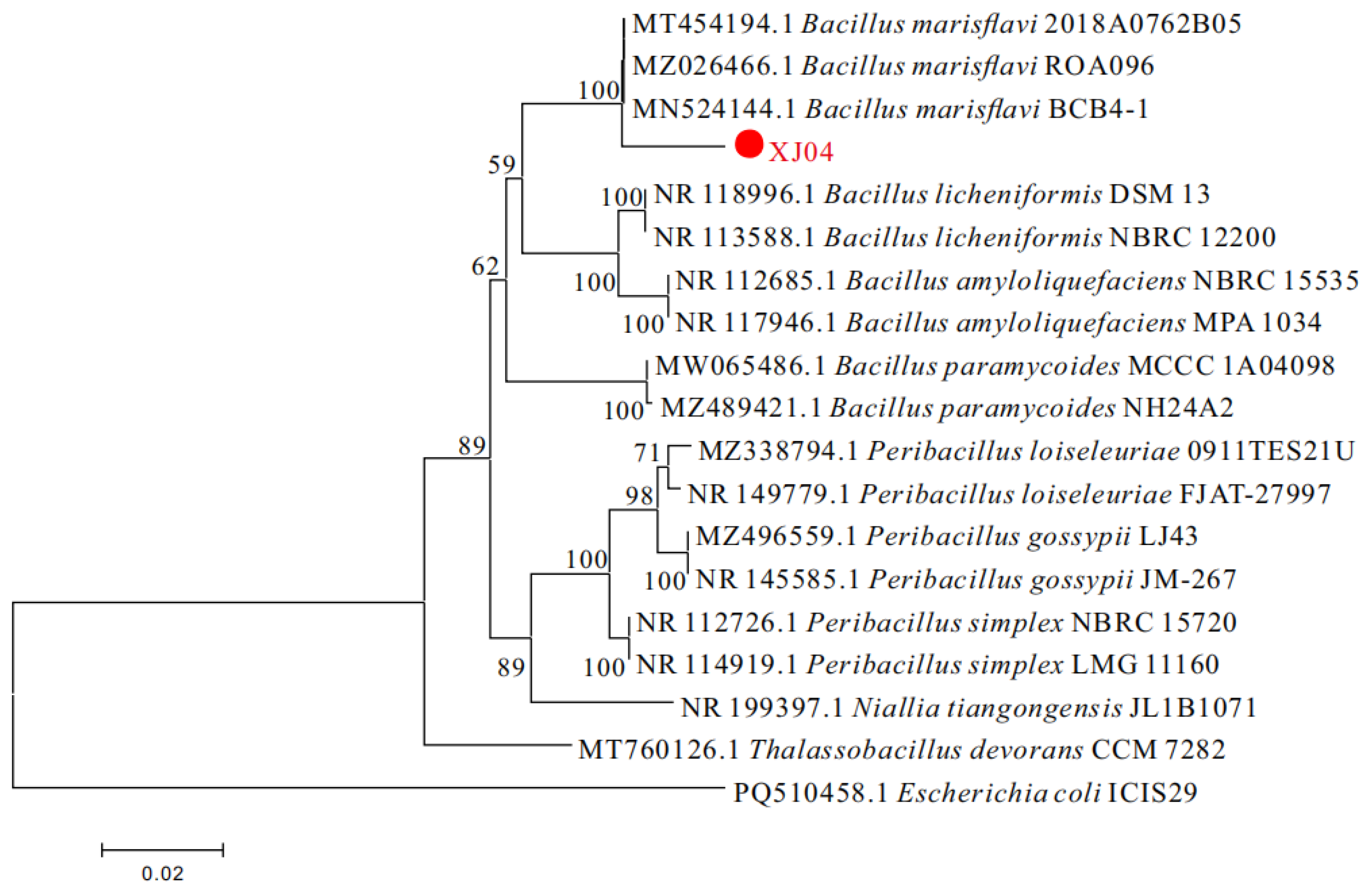
3.2.3. Summary
3.3. Optimization of Fermentation Conditions and Stability of Fermentation Broth of Strain XJ-04
3.3.1. Test Materials and Methods
3.3.1.1 Test Material3.3.1.1.1 Test Strain
3.3.1.1.2 Test reagent and culture medium
3.3.1.2. Test Method3.3.1.2.1 Screening of Optimal Components in Fermentation Culture
3.3.1.2.2. Orthogonal Test
|
Horizontal Level |
Factor Factor | |||
| A Carbon source | B nitrogen source | C Inorganic salt | D pH | |
| 1 | Maltose | Urea | K2HPO4·3H2O | 7 |
| 2 | Mannitol | Fine bran | FeSO4·7H2O | 8 |
| 3 | Sucrose | Diammonium phosphate | CaCO3 | 9 |
3.3.1.2.3. Optimization of the Addition Amount of Fermentation Medium Components
3.3.1.2.4. Optimization of fermentation conditions of medium
3.3.1.2.5. Preliminary Study on the Stability of Fermentation Broth of Strain XJ-04
- (1)
- Heat treatment : The prepared fermentation broth of strain XJ-04 was treated at 20 ℃, 40 ℃, 60 ℃, 80 ℃, 100 ℃ and 120 ℃ for 30 min, 60 min and 90 min, respectively;
- (2)
- Acid-base treatment : The fermentation broth was adjusted to pH 2.0,4.0,6.0,8.0,10.0 and 12.0 with 0.1 mol / L NaOH and 0.1 mol / L HCL, respectively [27]. The fermentation broth was placed at room temperature for 30 min, 60 min and 90 min, then adjusted to the original pH and filtered with a filter of 0.22 μm in size;
- (3)
- Light treatment : The fermentation broth was irradiated for 1 h, 2 h, 3 h, 4 h, 5 h and 6 h under 30 W ultraviolet lamp and fluorescent lamp at a vertical distance of 30 cm;
- (4)
- Storage treatment : The fermentation broth of the strain was placed at room temperature and 4 °C for 10 d, 20 d and 30 d;
- (5)
- Passage treatment : The initial strain XJ-04 was subcultured, and then the colony was transferred to the plate every 5 days to obtain 1-5 generations of strains;
3.3.2. Results and analysis
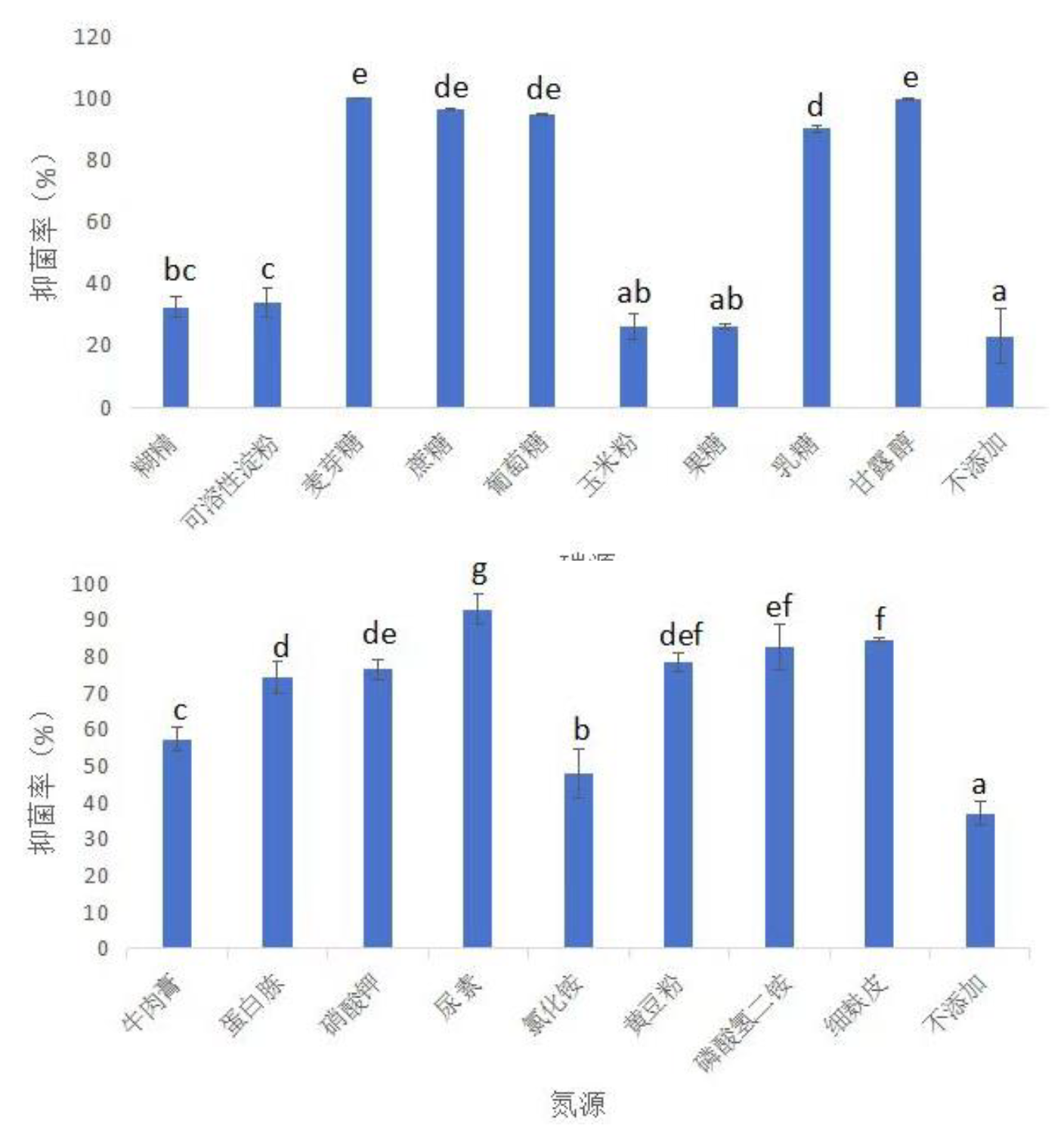
3.3.2.1. Optimization of Fermentation Medium Composition3.3.2.1.1. Single Factor Test
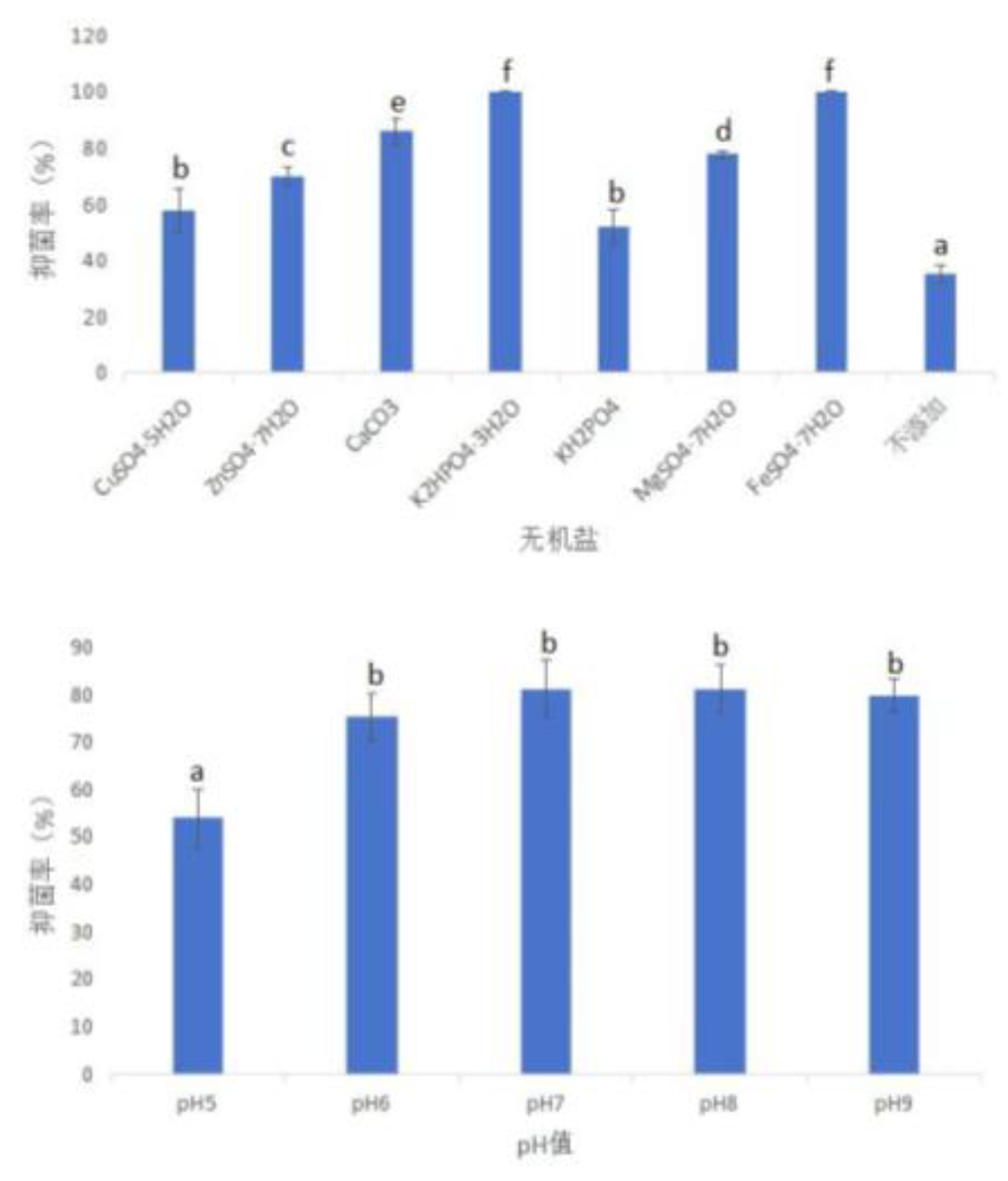
3.3.2.1.2. Intuitive Analysis of Orthogonal Test Results of Inhibition Rate of Fermentation Broth
| Test number | Test factor Factor |
Inhibition rate(%) |
|||
|
A Carbon source |
B nitrogen source |
C Inorganic salt |
D pH | ||
| 1 (A1B1C1D1) | Maltose | Urea | K2HPO4·3H2O | 7 | 98.77±2.1362d |
| 2 (A1B2C3D2) | Maltose | Fine bran | CaCO3 | 8 | 64.42±2.4606a |
| 3 (A1B3C2D3) | Maltose | Diammonium hydrogen phosphate | FeSO4·7H2O | 9 | 100.00±0.0000d |
| 4 (A2B1C3D3) | Mannitol | Urea | CaCO3 | 9 | 99.79±0.1050d |
| 5 (A2B2C2D1) | Mannitol | Fine bran | FeSO4·7H2O | 7 | 47.31±6.3369a |
| 6 (A2B3C1D2) | Mannitol | Diammonium hydrogen phosphate | K2HPO4·3H2O | 8 | 65.59±1.4816b |
| 7 (A3B1C2D2) | Sucrose | Urea | FeSO4·7H2O | 8 | 67.73±2.9556b |
| 8 (A3B2C1D3) | Sucrose | Fine bran | K2HPO4·3H2O | 9 | 71.75±4.5160c |
| 9 (A3B3C3D1) | Sucrose | Diammonium hydrogen phosphate | CaCO3 | 7 | 100.00±0.0000d |
|
Indicators Index |
Test factor Factor | |||
| A Carbon source | B Nitrogen source | C Inorganic salt |
D pH |
|
| K1 | 244.19 | 262.29 | 232.11 | 246.08 |
| K2 | 208.69 | 164.48 | 211.04 | 197.74 |
| K3 | 235.48 | 261.59 | 245.21 | 271.54 |
| k1 | 81.40 | 87.43 | 77.37 | 82.03 |
| k2 | 69.56 | 54.83 | 70.35 | 65.91 |
| k3 | 78.49 | 87.20 | 81.74 | 90.51 |
| R | 2.91 | 0.23 | 4.37 | 24.6 |
3.3.2.2. Optimization of Fermentation Medium Composition Addition Amount3.3.2.2.1. Single Factor Test
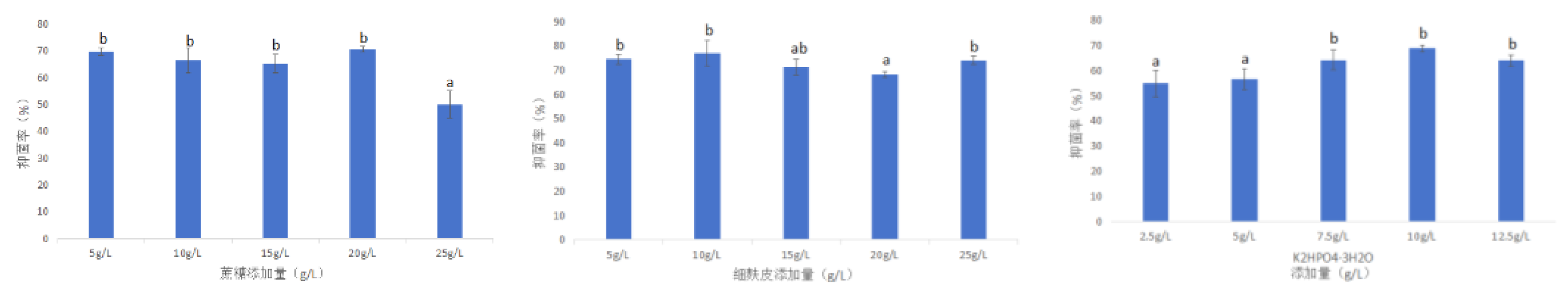
3.3.2.2.2. Response Surface Optimization Experiment
|
Horizontal Level |
Factor | ||
| X1Sucrose addition ( g / L) | X2The amount of fine bran added ( g / L) | X3The addition of K2HPO4·3H2O ( g / L) | |
| -1 | 15 | 5 | 7. 5 |
| 0 | 20 | 10 | 10 |
| 1 | 25 | 15 | 12.5 |
| Code | Factors and levels | YInhibition rate(%) | ||
| X1 (g / L) | X2 (g / L) | X3 (g /L) | ||
| 1 | 15.00 | 5.00 | 10.00 | 74.87 |
| 2 | 25.00 | 5.00 | 10.00 | 76.16 |
| 3 | 15.00 | 15.00 | 10.00 | 73.96 |
| 4 | 25.00 | 15.00 | 10.00 | 74.57 |
| 5 | 15.00 | 10.00 | 7.50 | 75.60 |
| 6 | 25.00 | 10.00 | 7.50 | 76.64 |
| 7 | 15.00 | 10.00 | 12.50 | 75.69 |
| 8 | 25.00 | 10.00 | 12.50 | 76.42 |
| 9 | 20.00 | 5.00 | 7.50 | 76.48 |
| 10 | 20.00 | 15.00 | 7.50 | 74.05 |
| 11 | 20.00 | 5.00 | 12.50 | 75.02 |
| 12 | 20.00 | 15.00 | 12.50 | 75.85 |
| 13 | 20.00 | 10.00 | 10.00 | 77.85 |
| 14 | 20.00 | 10.00 | 10.00 | 77.92 |
| 15 | 20.00 | 10.00 | 10.00 | 78.12 |
| 16 | 20.00 | 10.00 | 10.00 | 77.87 |
| 17 | 20.00 | 10.00 | 10.00 | 77.75 |
| Source | Sum of Squares | Degrees of freedom | Mean Square | F Value | P-value Prob > F | Significance |
| Model | 30.86 | 9 | 3.43 | 116.70 | <0.0001 | ** |
| X1 | 1.68 | 1 | 1.68 | 57.31 | 0.0001 | |
| X2 | 2.10 | 1 | 2.10 | 71.52 | <0.0001 | |
| X3 | 0.0055 | 1 | 0.0055 | 0.19 | 0.6779 | |
| X1X2 | 0.12 | 1 | 0.12 | 3.93 | 0.0877 | |
| X1X3 | 0.024 | 1 | 0.024 | 0.82 | 0.3959 | |
| X2X3 | 2.66 | 1 | 2.66 | 90.43 | <0.0001 | |
| X12 | 5.45 | 1 | 5.45 | 185.36 | <0.0001 | ** |
| X22 | 14.80 | 1 | 14.80 | 503.71 | <0.0001 | ** |
| X32 | 1.93 | 1 | 1.93 | 65.73 | <0.0001 | |
| Residual | 0.21 | 7 | 0.029 | |||
| Lack of Fit | 0.13 | 3 | 0.044 | 2.34 | 0.2149 | |
| Pure Error | 0.075 | 4 | 0.019 | |||
| Cor Total | 31.06 | 16 | ||||
| Note: Using Design Expert software for data analysis, “*”indicated significant impact on the results (0.01<P<0.05); “**”indicated that the impact on the results was extremely significant (P<0.01). | ||||||
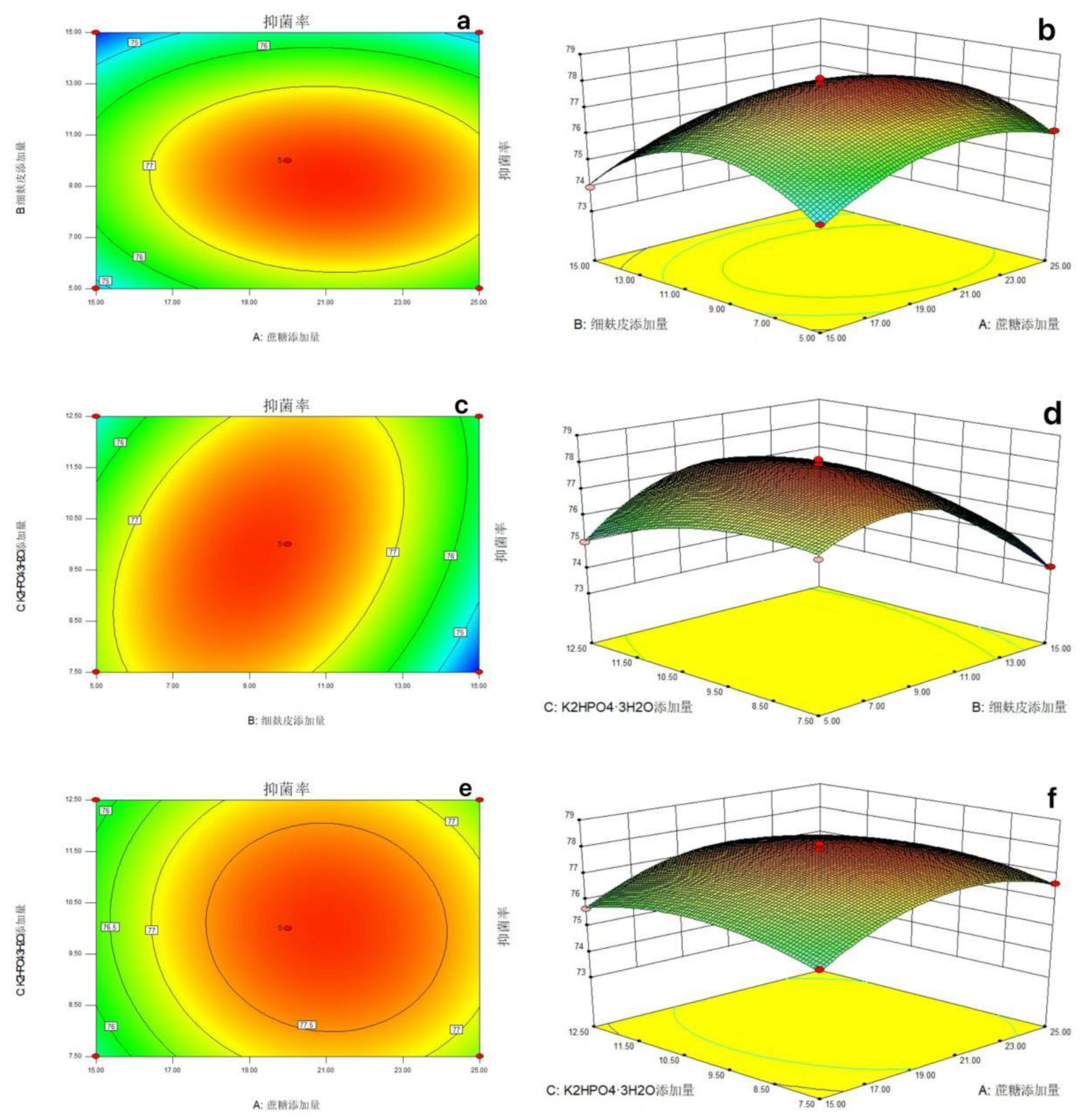
3.3.2.2.3. Response Surface Curve Analysis
| Treatment | Number of sclerotia germination in 3d | Inhibition rate(%) |
| Fermentation supernatant | 9 | 64 |
| CK | 25 | / |
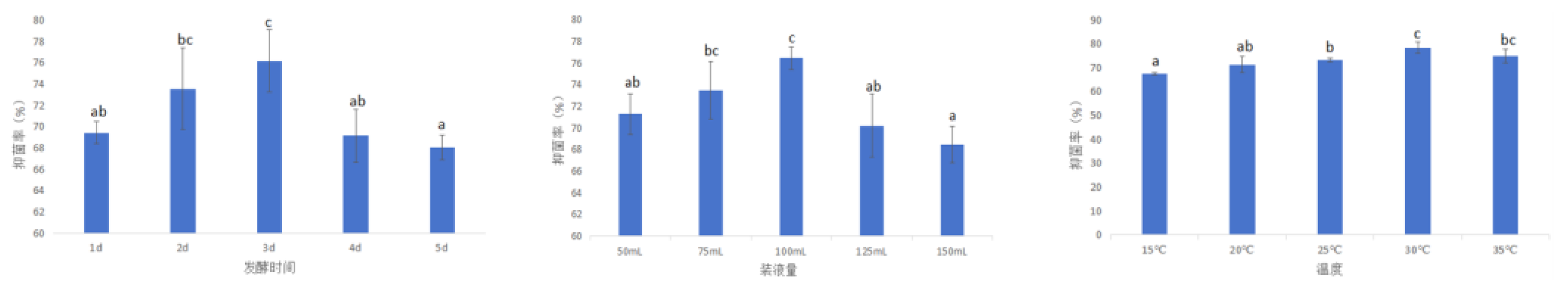
3.3.2.3. Fermentation Condition Optimization
3.3.2.4. Study on the Stability of Fermentation Broth of Strain XJ-04
- (1)
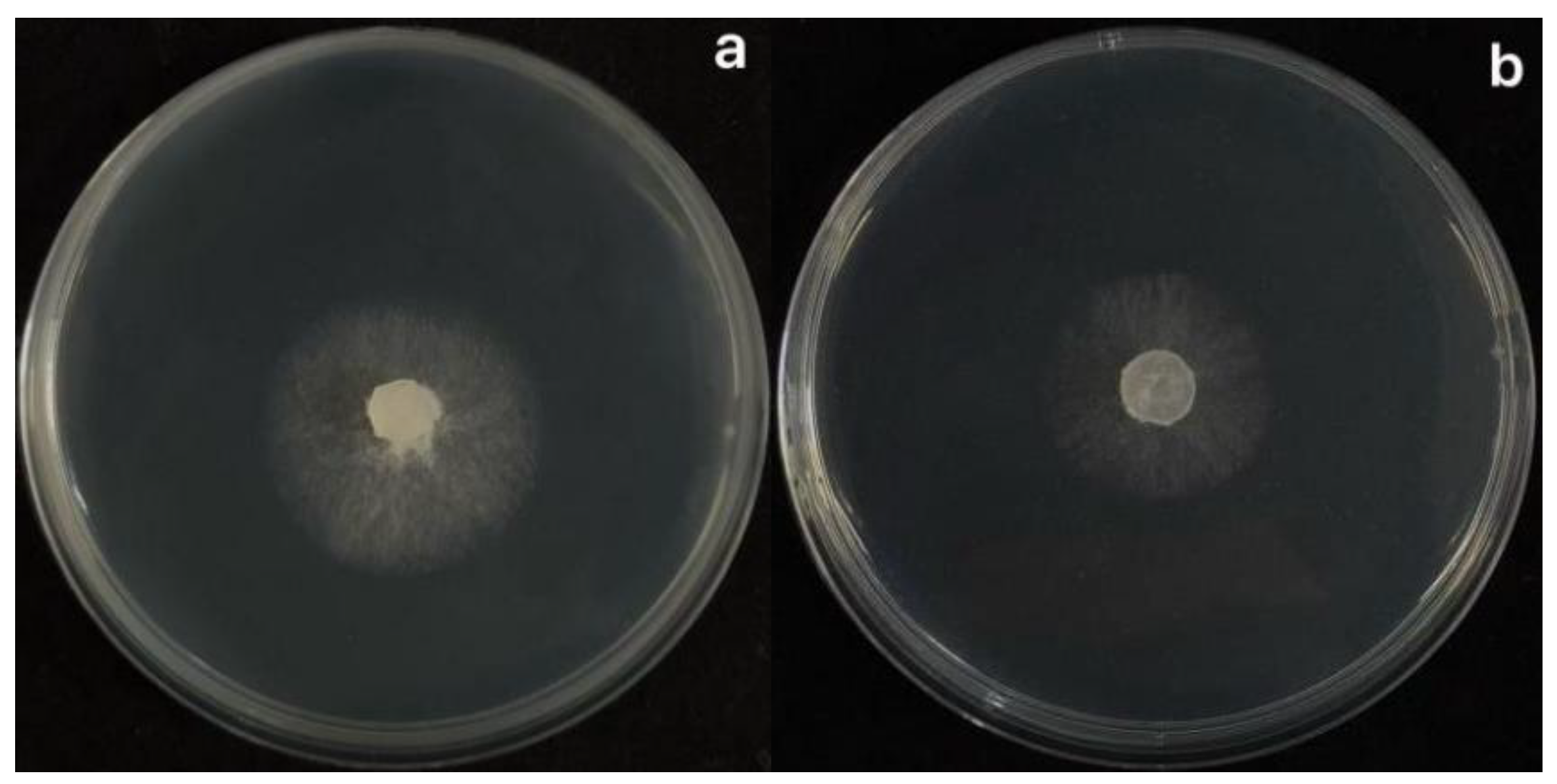
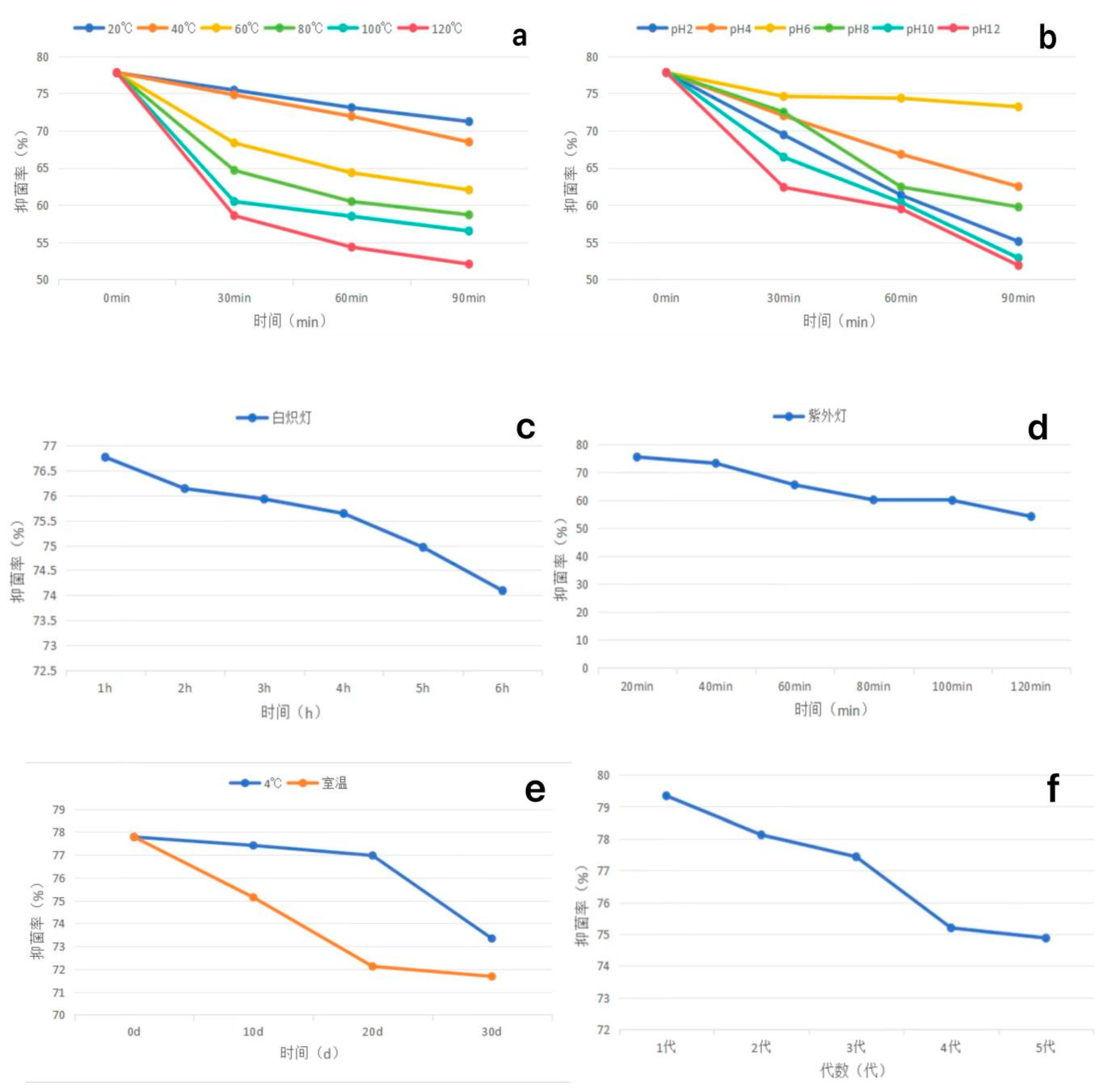
- Thermal stability : The fermentation broth of strain XJ-04 was treated at 20 ℃, 40 ℃, 60 ℃, 80 ℃ water bath for 30 min, 60 min and 90 min, and the bacteriostatic rate decreased by less than 15 %. Treatment at 100 ℃ and 120 ℃ for 60 min and 90 min had a great influence on the inhibition rate, and the inhibition rate decreased by nearly 20 %. Among them, there was no significant difference in the bacteriostatic rate between the 20 ℃ treatment for 30 min and the 40 ℃ treatment for 30 min and the control group ( without water bath heat treatment). It shows that the fermentation broth has good heat resistance within 80 ℃. The results are shown in Figure 5.7-a.
- (2)
- Acid-base stability : After the fermentation broth was treated with acid and alkali, compared with the original environment ( pH 6), the acid or alkali environment had a greater impact on the antibacterial effect of the fermentation broth. When the pH value was in the range of 2~10, the inhibition rate could still reach more than 60 % after treatment for 60 min. However, the inhibition rate was less than 60 % in the environment of pH 12, and the environment of acid and alkali had a great influence on the inhibition rate. The results are shown in Figure 5.7-b.
- (3)
- Light stability : Incandescent lamp irradiation had no effect on the fermentation broth, and the antibacterial rate did not change significantly after 6 h of irradiation. There was no significant difference in the bacteriostatic rate within 40 min of ultraviolet light irradiation. When the irradiation time was more than 60 min, the bacteriostatic rate was significantly different from that of the control group, but the bacteriostatic rate remained above 50 %. The results showed that the fermentation broth of XJ-04 strain had good light stability, and the results were shown in Figure 5.7-c and d.
- (4)
- Storage stability : There was no significant difference in the inhibition rate within 10-20 days of storage at 4 °C. The bacteriostatic rate decreased by 4.44 % at 30 d. There was no significant difference in the inhibition rate after 10 days of storage at room temperature. The inhibition rate decreased by 5.66 % after 20 days of storage and decreased by 6.10 % after 30 days of storage. The results are shown in Figure 5.7-e.
- (5)
- Passage stability : The stability test of the fermentation filtrate was carried out. The results showed that the antibacterial activity of the fermentation broth of different generations of XJ-04 strain was small. The antibacterial rate of the fermentation broth of the fifth generation of XJ-04 strain remained above 70 %, and the antibacterial rate was as high as 74.87 %, indicating that the strain had good passage stability. The results are shown in Figure 5.7-f.
3.3.3. Summary
- (1)
- The inhibition rate was used as the main consideration index. The addition components of the fermentation medium of strain XJ-04 were optimized by single factor test and orthogonal test. The No.8 medium ( sucrose, fine bran, K2HPO4·3H2O, pH 9) was the best component combination, and the inhibition rate was about 77.78 %.
- (2)
- The optimal addition parameters of each component of the fermentation medium were determined by the response surface test method and the maximum response value was predicted. It was predicted that when the sucrose addition amount was 21.08 g / L, the fine bran addition amount was 9.17 g / L and the K2HPO4·3H2O addition amount was 9.77 g / L, the inhibition rate was up to about 77.99 %. The bacteriostatic rate was 77.78 %, which was similar to the predicted value, and was about 7.66 % higher than the bacteriostatic rate before optimization. The experiment on the effect of sclerotia germination was carried out, and the inhibition rate of sclerotia germination was 64 %, which was 4 % higher than that before optimization.
- (3)
- The optimum fermentation conditions of strain XJ-04 were determined as follows : fermentation time was 3 d, liquid volume was 100 mL, fermentation temperature was 30 ℃.
- (4)
- The fermentation broth of strain XJ-04 had good thermal stability, acid-base stability, light stability and storage stability. At the same time, strain XJ-04 had good passage stability.
3.4. Study on the Growth-Promoting Effect of Strain XJ-04 Fermentation Broth on Watermelon and In Vitro Culture of Leaves
3.4.1. Test Materials and Methods
3.4.1.1. Test Material3.4.1.1.1. Test Strains and Plant Materials
3.4.1.1.2. Main Reagents and Equipment
3.4.1.2. Test Method3.4.1.2.1. Determination of Growth-Promoting Effect of Fermentation Broth of Strain XJ-04
3.4.1.2.2. Leaf Culture In Vitro
3.4.2. Results and Analysis
3.4.2.1. Effects of Fermentation Broth of Strain XJ-04 on Seed Germination and Seedling Growth of Watermelon
| Code | Fermentation broth : Water | Germination potential(%) | Germination rate(%) |
| A | 1:5 | 60 | 87 |
| B | 1:10 | 72 | 90 |
| C | 1:25 | 67 | 82 |
| D | 1:50 | 89 | 96 |
| E | 1:75 | 87 | 90 |
| F | 1:100 | 70 | 92 |
| G | CK | 65 | 85 |

| Fermentation broth : Water | Plant height(mm) | Root length(mm) | Stem diameter(mm) | Fresh weight(g) | Dry weight(g) | |
| 1:100 (A) | 135.50±3.88c | 46.72±3.34ab | 3.47±0.19a | 1.28±0.10ab | 0.11±0.02b | |
| 1:75 (B) | 130.19±6.03bc | 49.25±1.19bc | 3.47±0.25a | 1.71±0.10d | 0.16±0.03c | |
| 1:50 (C) | 137.67±2.91c | 53.37±1.43c | 3.46±0.08a | 1.72±0.09d | 0.13±0.03bc | |
| 1:25 (D) | 136.96±8.56c | 50.09±1.51bc | 3.57±0.09a | 1.45±0.07bc | 0.08±0.02ab | |
| 1:10 (E) | 121.33±2.17ab | 47.88±2.49ab | 3.49±0.29a | 1.55±0.15cd | 0.10±0.02ab | |
| 1:5 (F) | 114.80±13.03a | 45.91±3.83ab | 3.42±0.23a | 1.17±0.12a | 0.09±0.04ab | |
| CK (G) | 116.44±8.00a | 43.77±2.57a | 3.39±0.23a | 1.12±0.19a | 0.06±0.02a | |
| Note: Using SPSS for data analysis, different lowercase letters indicate significant differences at the P<0.05 level. | ||||||
3.4.2.2. Leaf In Vitro Culture Experiment

| Code | Fermentation broth : Water | Lesion diameter(mm) |
| A | 0:1 | 54.26±1.5245e |
| B | 1:20 | 49.61±0.5823d |
| C | 1:15 | 44.66±0.8426c |
| D | 1:10 | 33.84±1.6038b |
| E | 1:5 | 20.07±1.0758a |
| Note: Using SPSS for data analysis, Different lowercase letters indicate significant differences at the P<0.05 level. | ||
3.4.2.3. Summary
- (1)
- When the ratio of strain XJ-04 fermentation broth to water was 1:50, the germination rate and germination potential of watermelon seeds and the plant height and root length of watermelon seedlings were significantly improved. When the dilution ratio of the fermentation broth was 1:25, the basal diameter of the seedlings was significantly widened. When the dilution ratio of the fermentation broth was 1:50 and 1:75, the fresh weight of the seedlings was significantly increased.
- (2)
- The in vitro experiment of watermelon leaves showed that the fermentation broth of strain XJ-04 had a good control effect on watermelon sclerotiniosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- BOLTON M D, THOMMA B P, NELSON B D. Sclerotinia sclerotiorum(Lib.) de Bary: biology and molecu- lar traits of a cosmopolitan pathogen[J]. Molecular Plant Pathology, 2006, 7(1): 1. [CrossRef]
- Yang Qingpo, Liu Wancai, Huang Chong, et al.. Statistics and Analysis of Major Diseases and Insect Pests in Rape in Recent Ten Years [J]. Plant Protection, 2018,44: 24. [CrossRef]
- SHARMA P, MEENA P D, VERMA P R, et al.Sclerotinia sclerotiorum (Lib) de Bary causing sclerotinia rot in oilseed Brassicas: a review[J]. Journal of Oilseed Brassica, 2015, 6(S): 1.
- WILLBUR J, MCCAGHEY M, KABBAGE M, et al.An overview of the Sclerotinia sclerotiorum pathosystem in soybean: impact, fungal biology, and current management strategies[J]. Tropical Plant Pathology, 2019, 44(1):3. [CrossRef]
- Derbyshire, M.C.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassicanapus): current practices and future opportunities. Plant Pathology. 2016, 65, 859–887. [Google Scholar] [CrossRef]
- Liu Yun-hui. Existing Problems, Application Status and Development of Biological Control Technology [J]. Journal of Seed Industry, 2019,06: 14-16.
- Fangzhongda. Methods of Plant Disease Research, 3rd Edition [M]. China Agriculture Press, 2007: 46-50.
- Samson, R.A.; Visagie, C.M.; Houbraken, J.J.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus[J]. Studies in Mycology, 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; et al. Identification and nomenclature of the genus Penicillium[J]. Studies in Mycology, 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Houbraken, J.; Visagie, C.M.; Meijer, M.; et al. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides[J]. Studies in Mycology, 2014, 78, 373–451. [Google Scholar] [CrossRef] [PubMed]
- Amein, T.; Omer, Z.; Welch, C. Application and evalution of Pseudomonas strains for biocontrol of wheat seedling blight[J]. Crop Protection, 2008, 27, 532–536. [Google Scholar] [CrossRef]
- Zhou Xiangping, Shu Cuihua, Teng Kai, et al.. Identification of Endophytic Bacillus Starch Xe01 and Optimization of Fermentation Conditions [J]. Chinese Tobacco Science, 2020,: 58-67.
- Zhang Kecheng, Ran Longxian, et al. Isolation and Identification of Antagonistic Actinomycetes from Apple Rotting Bacteria [J]. Hebei Forest Fruit Research. 2008, 23, 5.
- Yasmin Z, Shamsi S. In vitro screening of fungicides and plant extracts against Colletotrichum gloeosporioides(Penz.) Rauwolfia serpentina(L.) Sacc. the causal agent of anthracnose disease of Sacc. the causal agent of anthracnose disease of Benth Ex Kurz[J]. Benth Ex Kurz[J]. Journal of The Asiatic Society of Bengal, 2019, 45(1): 35-43.
- Bacterial Classification Group., Institute of Microbiology, Chinese Academy of Agricultural Sciences Common Identification Methods of General Bacteria [M]. Science Press, 1978.
- R.E. Buchanan, N.E. R.E. Buchanan, N.E. Gibbons et al.. Berger Bacteria Identification Manual (8th Edition) [M]. Science Press, 1984.
- Dongxiuzhu, Cai Miaoying. Manual for Identification of Common Bacterial Systems [M]. Science Press, 2001.
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium[J]. Applied and Environmental Microbiology, 2001, 67, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Pérez, J.S.; Sevilla, J.F.; et al. Fermentation optimization for the production of lovastatin by Aspergillus terreus: use of response surface methodology[J]. Journal of Chemical Technology & Biotechnology Biotechnology, 2010, 79, 1119–1126. [Google Scholar]
- Song Xiao-feng, former Zengyan, Li Peng-xu, et al. Optimization of Extraction and Purification of Total Flavonoids from Hawthorn Leaves by Box-Behnken Design and Study on Their Antibacterial Activity [J]. Henan Science, 2018,36: 6.
- Wei, W.; Yuan, T.; Wang, K.; et al. Statistical optimization of cellulase production by the brown rot fungi, Fomitopsis palustris, and its application in the enzymatic hydrolysis of LHW-pretreated woody biomass[J]. Process Biochemistry, 2012, 47, 2552–2556. [Google Scholar]
- Guowei, S.; Chunju, B.; He, C.; et al. Fermentation optimization of goat milk with Lactobacillus acidophilus and Bifidobacterium bifidum by Box-Behnken design[J]. Acta Scientiarum Polonorum Technologia Alimentaria, 2016, 15, 151–159. [Google Scholar]
- Dertli, E.; Toker, O.S.; Durak, M.Z.; et al. Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: Rheological, molecular, microstructural and sensory characterization[J]. Carbohydrate Polymers, 2016, 136, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chen Xi, Li Guolin, Chen Mengyu, et al.. Optimization of fermentation conditions of blueberry vinegar by response surface methodology [J]. Brewing in China 2018, 37, 67–71.
- Wang Qiang, Huang Meigui, Xie Yuejie, et al.. Optimization of Extraction Process of Hydroxytyrosol from Olive Pomace by Response Surface Methodology [J]. Food Industry Technology, 2018,39: 145-151.
- Morita, K.; Nishijima, Y.; Kana, T. Chemical nature of a desmutagenic factor from burdock (Arctium lappa Linne)[J]. Agricultural and Biological Chemistry, 1985, 49, 925–932. [Google Scholar] [CrossRef]
- Wang Wei. Biological Characteristics of Sclerotinia Sclerotiorum and Screening of Control Agents [D]. Sichuan Agricultural University, 2012.
- Chen Xi, Li Guolin, Chen Mengyu, et al.. Optimization of fermentation conditions of blueberry vinegar by response surface methodology [J]. Brewing in China, 2018,37: 67-71.
- Wang Qiang, Huang Meigui, Xie Yuejie, et al.. Optimization of Extraction Process of Hydroxytyrosol from Olive Pomace by Response Surface Methodology [J]. Food Industry Technology, 2018,39: 145-151.
- Kulandaivelu, V.; Radha, P. Trichoderma–azotobacter Biofilm-based Formulation Enhance Natural Plant Defense Enzyme Activities in Wheat and Cotton Seedlings[J]. National Academy Science Letters, 2023, 47, 61–64. [Google Scholar]
- Emily R, T M M. Data on Plant Defense Enzyme Activity Associated with Three Endophytes Against Cornus Florida Erysiphe Pulchra Powdery Mildew[J]. Data in Brief, 2023, 48: 109220-109220.
|
No. Code |
Inhibitory zone(Mm) Inhibition zone |
Rescreening inhibition rate(%) Inhibition rate of secondary screening |
| XJ-04 | 5.21±0.2450c | 70.12±0.6710d |
| LY-15 | 4.93±0.1652c | 69.32±3.2216d |
| SY-27 | 3.76±0.4328a | 50.32±0.6727a |
| XG-69 | 4.55±0.2658b | 59.63±3.9452b |
| JH-105 | 4.68±0.1747ab | 64.60±0.8278c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




