Submitted:
24 November 2025
Posted:
26 November 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. MECP2: Molecular Biology, Isoforms, and Genetics
2.1. Gene Structure, Isoforms, and Transcriptional Regulation
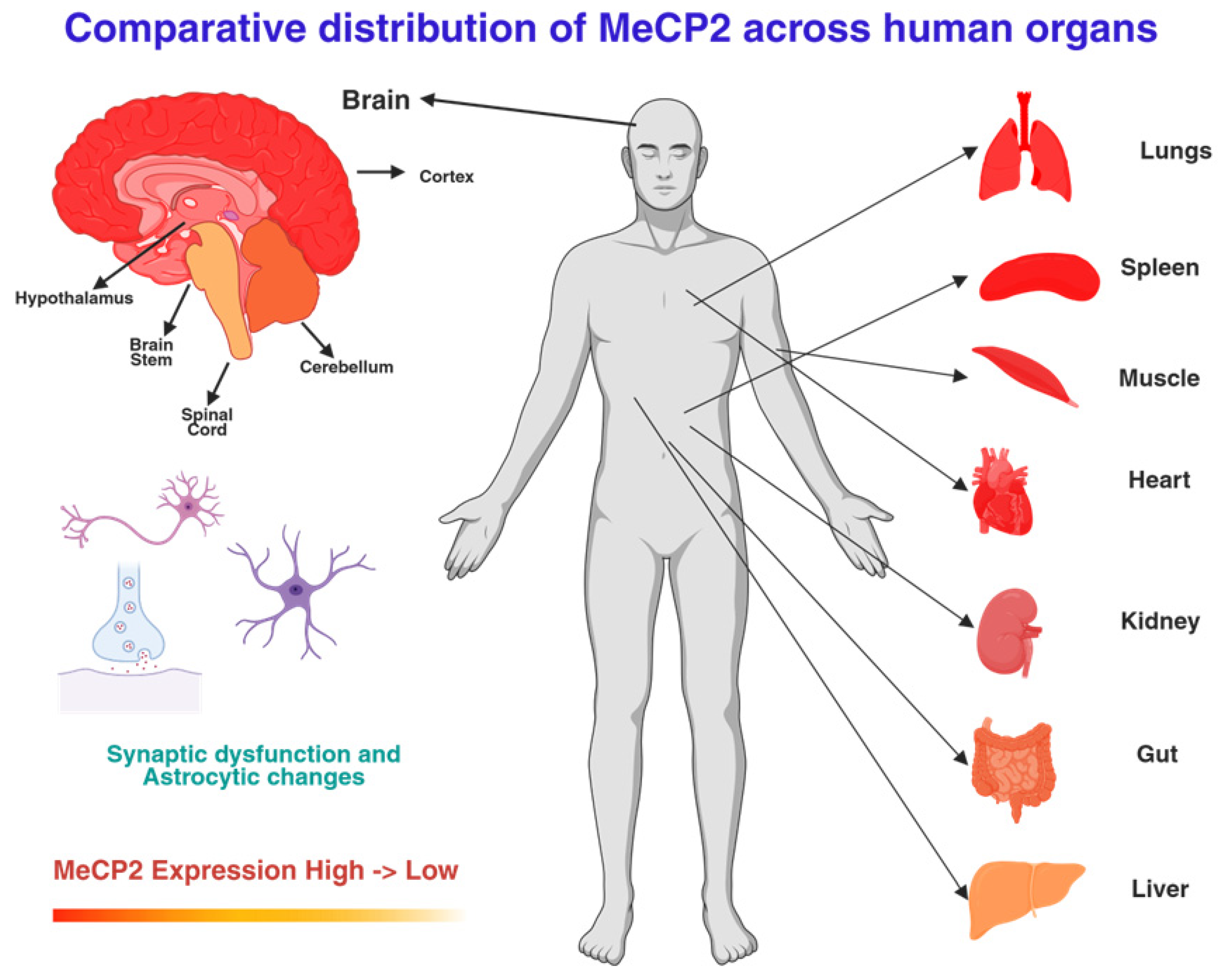
2.2. Expression Patterns and Systemic Role
2.3. Functional Role and Dosage Sensitivity
2.4. MECP2 Mutations and Genotype–Phenotype Correlations
2.5. Preclinical Insights and Therapeutic Implications
3. Central Nervous System Phenotype
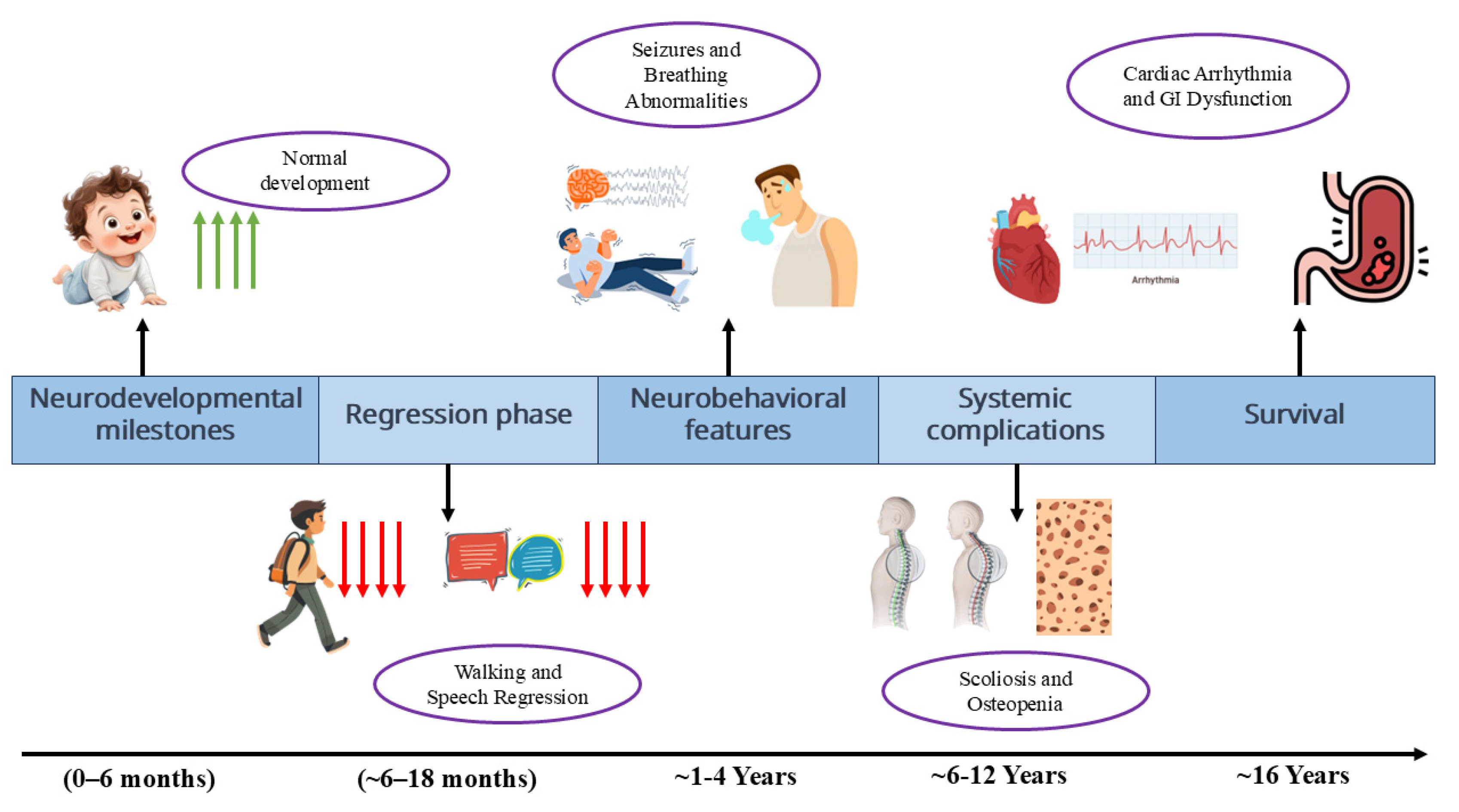
3.1. Developmental Stages and Regression
3.2. Stage I: Early Onset/Developmental Arrest (6–18 Months)
3.3. Stage II: Rapid Progressive/Regression (1–4 Years)
3.4. Stage III: Plateau/Pseudo-Stationary (2–10 Years, Extending into Preadolescence)
3.5. Stage IV: Late Motor Deterioration (Post-10 Years)
3.6. Seizures and Electroencephalography
3.7. Neuroimaging and Brain Structure
3.8. Behavioral Features and Communication
3.9. Natural History Data and Clinical Insights
3.10. Summary of Clinical and CNS Phenotype
4. Beyond the Brain—System-by-System Pathophysiology & Clinical Manifestations
4.1. Respiratory Control & Breathing Irregularities
4.2. Cardiovascular System (Autonomic Dysfunction and Arrhythmia)
4.3. Gastrointestinal System & Nutrition
4.4. Skeletal & Musculoskeletal System
4.5. Metabolic & Mitochondrial Dysfunction
4.6. Immune System & Glial/Peripheral Immune Interactions
4.7. Endocrine & Growth/Reproductive Health
4.8. Sleep, Sensory Systems & Pain
4.9. Oral/Dental & Dental Health
4.10. Other Organ Systems (Renal, Dermatologic, Ophthalmologic)
5. Biomarkers, Outcome Measures, and Trial Endpoints
6. Models and Mechanistic Tools (Preclinical & Translational Platforms)
6.1. Rodent Models of RTT
6.2. Human Cell-Based Models
6.3. Molecular and Multi-Omic Insights
6.4. Comparative Strengths and Limitations of Models
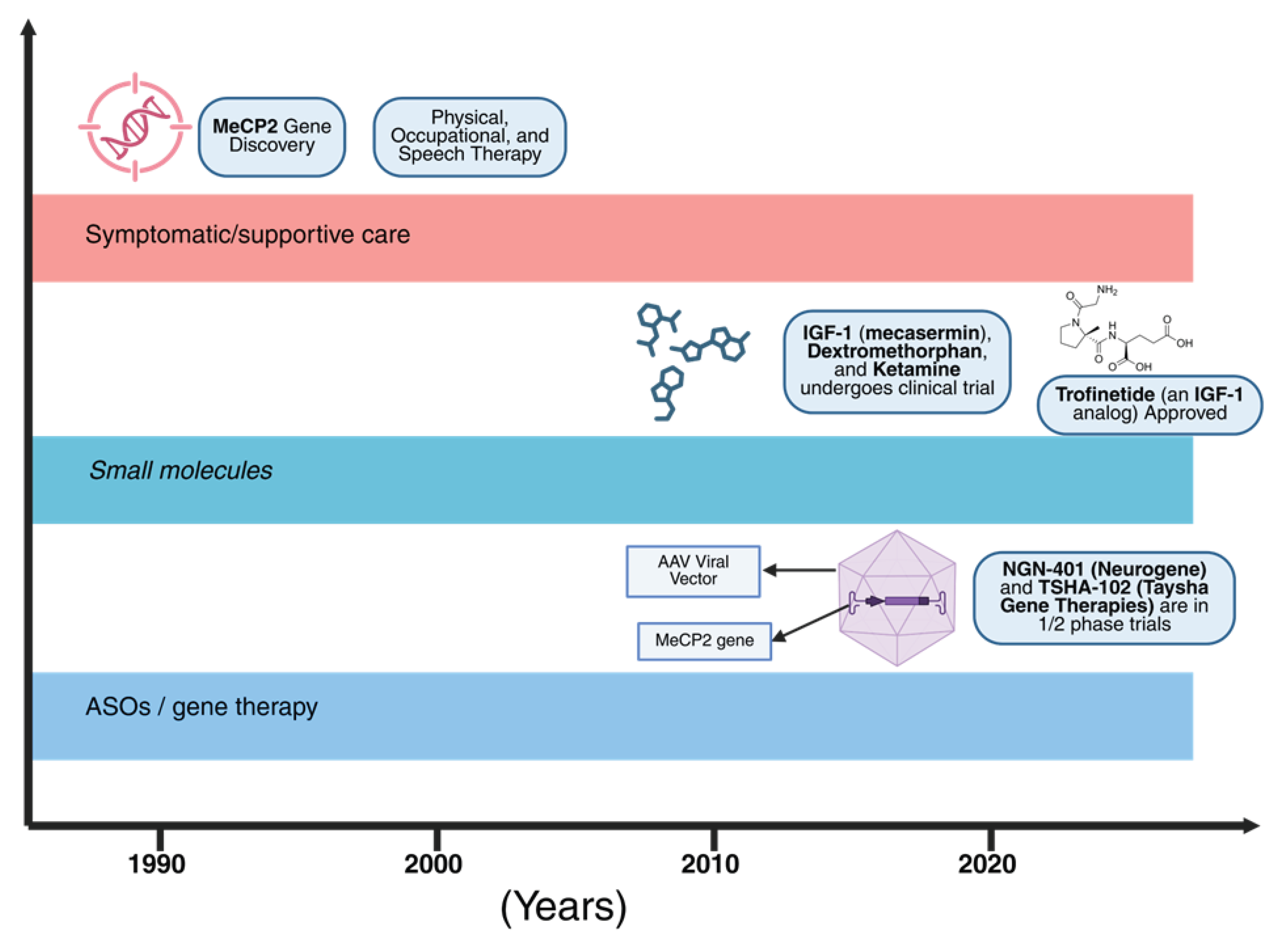
7. Therapeutic Landscape: Symptom Control to Disease Modification
7.1. Standard Supportive Care
7.2. Approved Pharmacotherapy: Trofinetide (DAYBUE™)
7.3. Gene Replacement/Gene Therapy
7.4. Antisense Oligonucleotides and Dosage Normalization
7.5. Small Molecules, Neurotrophic Strategies, and Repurposing
7.6. Cellular and Other Novel Strategies
7.7. Clinical Trial Design and Operational Considerations
8. Safety & Regulatory Considerations
9. Quality of Life, Caregiver Burden, and Health-Services Issues
10. Global Health, Equity & Access to Specialized Care
11. Gaps, Controversies & Prioritized Research Agenda
12. Conclusions
Author Contributions
Funding
Use of Artificial Intelligence
Conflicts of Interest
References
- Bricker, K.; Vaughn, B.V. Rett syndrome: a review of clinical manifestations and therapeutic approaches. Front. Sleep 2024, 3, 1373489. [CrossRef]
- Kyle, S.M.; Vashi, N.; Justice, M.J. Rett syndrome: a neurological disorder with metabolic components. Open Biol. 2018, 8. [CrossRef]
- May, D.; Kponee-Shovein, K.; Neul, J.L.; Percy, A.K.; Mahendran, M.; Downes, N.; Chen, G.; Watson, T.; Pichard, D.C.; Kennedy, M.; et al. Characterizing the journey of Rett syndrome among females in the United States: a real-world evidence study using the Rett syndrome natural history study database. J. Neurodev. Disord. 2024, 16, 1–12. [CrossRef]
- Pejhan, S.; Rastegar, M. Role of DNA Methyl-CpG-Binding Protein MeCP2 in Rett Syndrome Pathobiology and Mechanism of Disease. Biomolecules 2021, 11, 75. [CrossRef]
- Gold, W.A.; Percy, A.K.; Neul, J.L.; Cobb, S.R.; Pozzo-Miller, L.; Issar, J.K.; Ben-Zeev, B.; Vignoli, A.; Kaufmann, W.E. Rett syndrome. Nat Rev Dis Primer. 2024, 10, 84.
- de Carvalho, M.R.; Cavalcante, T.T.; Oliveira, P.S.; Naves, P.V.F.; Cunha, P.E.L. Rett syndrome due to mutation in the MECP2 gene and electroencephalographic findings. Arq Neuropsiquiatr. 2024, 82, 1–2. [CrossRef]
- Ribeiro, M.C.; MacDonald, J.L. Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development. Brain Res. 2020, 1729, 146644–146644. [CrossRef]
- Buchanan, C.B.; Stallworth, J.L.; Scott, A.E.; Glaze, D.G.; Lane, J.B.; Skinner, S.A.; Tierney, A.E.; Percy, A.K.; Neul, J.L.; Kaufmann, W.E. Behavioral profiles in Rett syndrome: Data from the natural history study. Brain Dev. 2019, 41, 123–134. [CrossRef]
- Percy, A.K.; Benke, T.A.; Marsh, E.D.; Neul, J.L. Rett syndrome: The Natural History Study journey. Ann. Child Neurol. Soc. 2024, 2, 189–205. [CrossRef]
- Chin, E.W.M.; Goh, E.L.K. MeCP2 Dysfunction in Rett Syndrome and Neuropsychiatric Disorders. Methods Mol Biol 2019, 2011, 573–591.
- Vidal, S.; Xiol, C.; Pascual-Alonso, A.; O’callaghan, M.; Pineda, M.; Armstrong, J. Genetic Landscape of Rett Syndrome Spectrum: Improvements and Challenges. Int. J. Mol. Sci. 2019, 20, 3925. [CrossRef]
- Fang, X.; Baggett, L.M.; Caylor, R.C.; Percy, A.K.; Neul, J.L.; Lane, J.B.; Glaze, D.G.; Benke, T.A.; Marsh, E.D.; Motil, K.J.; et al. Parental age effects and Rett syndrome. Am. J. Med Genet. Part A 2023, 194, 160–173. [CrossRef]
- Kim, J.A.; Kwon, W.K.; Kim, J.W.; Jang, J.H. Variation spectrum of MECP2 in Korean patients with Rett and Rett-like syndrome: a literature review and reevaluation of variants based on the ClinGen guideline. J Hum Genet. 2022, 67, 601–606.
- Ip, J.P.K.; Mellios, N.; Sur, M. Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 2018, 19, 368–382. [CrossRef]
- Liu, Y.; Flamier, A.; Bell, G.W.; Diao, A.J.; Whitfield, T.W.; Wang, H.-C.; Wu, Y.; Schulte, F.; Friesen, M.; Guo, R.; et al. MECP2 directly interacts with RNA polymerase II to modulate transcription in human neurons. Neuron 2024, 112, 1943–1958.e10. [CrossRef]
- Ali, N.E.; Tariq, N.; Naz, G.; Abalkhail, A.; Kausar, T.; Mazhar, I.; Zia, S.; Aqib, A.I.; Khan, N.U. Rett syndrome: advances in Understanding MeCP2 function, potential gene therapies, and public health implications. Mol. Biol. Rep. 2025, 52, 1–19. [CrossRef]
- Golubiani, G.; van Agen, L.; Tsverava, L.; Solomonia, R.; Müller, M. Mitochondrial Proteome Changes in Rett Syndrome. Biology 2023, 12, 956. [CrossRef]
- Pascual-Alonso, A.; Xiol, C.; Smirnov, D.; Kopajtich, R.; Prokisch, H.; Armstrong, J. Identification of molecular signatures and pathways involved in Rett syndrome using a multi-omics approach. Hum. Genom. 2023, 17, 1–15. [CrossRef]
- Qian, J.; Guan, X.; Xie, B.; Xu, C.; Niu, J.; Tang, X.; Li, C.H.; Colecraft, H.M.; Jaenisch, R.; Liu, X.S. Multiplex epigenome editing of MECP2 to rescue Rett syndrome neurons. Sci. Transl. Med. 2023, 15, eadd4666–eadd4666. [CrossRef]
- E Collins, B.; Neul, J.L. Rett Syndrome and MECP2 Duplication Syndrome: Disorders of MeCP2 Dosage. Neuropsychiatr. Dis. Treat. 2022, ume 18, 2813–2835. [CrossRef]
- Zahorakova, D.; Lelkova, P.; Gregor, V.; Magner, M.; Zeman, J.; Martasek, P. MECP2 mutations in Czech patients with Rett syndrome and Rett-like phenotypes: novel mutations, genotype–phenotype correlations and validation of high-resolution melting analysis for mutation scanning. J. Hum. Genet. 2016, 61, 617–625. [CrossRef]
- Zeid, M.A.; Elrosasy, A.; Mohamed, R.G.; Ghazou, A.; Goufa, E.; Hassan, N.; Abuzaid, Y. A meta-analysis of the efficacy and safety of trofinetide in patients with rett syndrome. Neurol. Sci. 2024, 45, 4767–4778. [CrossRef]
- Lou, S.; Tihagam, R.D.; Wasko, U.N.; Equbal, Z.; Venkatesan, S.; Braczyk, K.; Przanowski, P.; Koo, B.I.; Saltani, I.; Singh, A.T.; et al. Targeting microRNA-dependent control of X chromosome inactivation improves the Rett Syndrome phenotype. Nat. Commun. 2025, 16, 1–17. [CrossRef]
- Takahashi, S.; Takeguchi, R.; Kuroda, M.; Tanaka, R. Atypical Rett syndrome in a girl with mosaic triple X and MECP2 variant. Mol. Genet. Genom. Med. 2020, 8, e1122. [CrossRef]
- Collins, B.E.; Merritt, J.K.; Erickson, K.R.; Neul, J.L. Safety and efficacy of genetic MECP2 supplementation in the R294X mouse model of Rett syndrome. Genes, Brain Behav. 2021, 21, e12739. [CrossRef]
- Powers, S.; Likhite, S.; Gadalla, K.K.; Miranda, C.J.; Huffenberger, A.J.; Dennys, C.; Foust, K.D.; Morales, P.; Pierson, C.R.; Rinaldi, F.; et al. Novel MECP2 gene therapy is effective in a multicenter study using two mouse models of Rett syndrome and is safe in non-human primates. Mol. Ther. 2023, 31, 2767–2782. [CrossRef]
- Akaba, Y.; Takahashi, S. MECP2 duplication syndrome: Recent advances in pathophysiology and therapeutic perspectives. Brain Dev. 2025, 47, 104371. [CrossRef]
- Chen, X.; Han, X.; Blanchi, B.; Guan, W.; Ge, W.; Yu, Y.-C.; Sun, Y.E. Graded and pan-neural disease phenotypes of Rett Syndrome linked with dosage of functional MeCP2. Protein Cell 2020, 12, 639–652. [CrossRef]
- Haase, F.; Gloss, B.S.; Tam, P.P.L.; Gold, W.A. WGCNA Identifies Translational and Proteasome-Ubiquitin Dysfunction in Rett Syndrome. Int. J. Mol. Sci. 2021, 22, 9954. [CrossRef]
- Anitha, A.; A Poovathinal, S.; Viswambharan, V.; Thanseem, I.; Iype, M.; Anoop, U.; Sumitha, P.S.; Parakkal, R.; Vasu, M.M. MECP2 Mutations in the Rett Syndrome Patients from South India. Neurol. India 2022, 70, 249–253. [CrossRef]
- Chin, E.W.M.; Goh, E.L.K. Behavioral Characterization of MeCP2 Dysfunction-Associated Rett Syndrome and Neuropsychiatric Disorders. Methods Mol Biol 2019, 2011, 593–605.
- Li, Y.; Anderson, A.G.; Qi, G.; Wu, S.R.; Revelli, J.P.; Liu, Z.; Zoghbi, H.Y. Early transcriptional signatures of MeCP2 positive and negative cells in Rett syndrome. BioRxiv Prepr Serv Biol 2025.
- Müller, M. Disturbed redox homeostasis and oxidative stress: Potential players in the developmental regression in Rett syndrome. Neurosci. Biobehav. Rev. 2019, 98, 154–163. [CrossRef]
- Chin Wong, L.; Hung, P.L.; Jan, T.Y.; Lee, W.T.; Taiwan Rett Syndrome Association. Variations of stereotypies in individuals with Rett syndrome: A nationwide cross-sectional study in Taiwan. Autism Res Off J Int Soc Autism Res. 2017, 10, 1204–1214. [CrossRef]
- Karoum, A.; Fathalla, W.; Lazek, R.A. Genotype–Phenotype Correlation and Therapeutic Amenability in a Cohort of Rett Syndrome Patients: A Single-Center Study. Cureus 2025, 17, e86953. [CrossRef]
- Zhou, J.; Cattoglio, C.; Shao, Y.; Tirumala, H.P.; Vetralla, C.; Bajikar, S.S.; Li, Y.; Chen, H.; Wang, Q.; Wu, Z.; et al. A novel pathogenic mutation of MeCP2 impairs chromatin association independent of protein levels. Genes Dev. 2023, 37, 883–900. [CrossRef]
- Sangani, N.B.; Koetsier, J.; Gomes, A.R.; Diogo, M.M.; Fernandes, T.G.; Bouwman, F.G.; Mariman, E.C.M.; Ghazvini, M.; Gribnau, J.; Curfs, L.M.G.; et al. Involvement of extracellular vesicle microRNA clusters in developing healthy and Rett syndrome brain organoids. Cell. Mol. Life Sci. 2024, 81, 1–16. [CrossRef]
- Lopes, A.G.; Loganathan, S.K.; Caliaperumal, J. Rett Syndrome and the Role of MECP2: Signaling to Clinical Trials. Brain Sci. 2024, 14, 120. [CrossRef]
- Pascual-Alonso, A.; Martínez-Monseny, A.F.; Xiol, C.; Armstrong, J. MECP2-Related Disorders in Males. Int. J. Mol. Sci. 2021, 22, 9610. [CrossRef]
- Shiohama, T.; Tsujimura, K. Quantitative Structural Brain Magnetic Resonance Imaging Analyses: Methodological Overview and Application to Rett Syndrome. Front. Neurosci. 2022, 16, 835964. [CrossRef]
- Kong, Y.; Li, Q.-B.; Yuan, Z.-H.; Jiang, X.-F.; Zhang, G.-Q.; Cheng, N.; Dang, N. Multimodal Neuroimaging in Rett Syndrome With MECP2 Mutation. Front. Neurol. 2022, 13, 838206. [CrossRef]
- Buchanan, C.B.; Stallworth, J.L.; Joy, A.E.; Dixon, R.E.; Scott, A.E.; Beisang, A.A.; Benke, T.A.; Glaze, D.G.; Haas, R.H.; Heydemann, P.T.; et al. Anxiety-like behavior and anxiolytic treatment in the Rett syndrome natural history study. J. Neurodev. Disord. 2022, 14, 31. [CrossRef]
- Stallworth, J.L.; Dy, M.E.; Buchanan, C.B.; Chen, C.F.; Scott, A.E.; Glaze, D.G.; Lane, J.B.; Lieberman, D.N.; Oberman, L.M.; Skinner, S.A.; Tierney, A.E.; Cutter, G.R.; Percy, A.K.; Neul, J.L.; Kaufmann, W.E. Hand stereotypies: Lessons from the Rett Syndrome Natural History Study. Neurology 2019, 92, e2594–e2603.
- Vilvarajan, S.; McDonald, M.; Douglas, L.; Newham, J.; Kirkland, R.; Tzannes, G.; Tay, D.; Christodoulou, J.; Thompson, S.; Ellaway, C. Multidisciplinary Management of Rett Syndrome: Twenty Years’ Experience. Genes 2023, 14, 1607. [CrossRef]
- Andoh-Noda, T.; Inouye, M.O.; Miyake, K.; Kubota, T.; Okano, H.; Akamatsu, W. Modeling Rett Syndrome Using Human Induced Pluripotent Stem Cells. CNS Neurol. Disord.-Drug Targets 2016, 15, 544–550. [CrossRef]
- Parent, H.; Ferranti, A.; Niswender, C. Trofinetide: a pioneering treatment for Rett syndrome. Trends Pharmacol. Sci. 2023, 44, 740–741. [CrossRef]
- Sweatt, J.D.; Tamminga, C.A. An epigenomics approach to individual differences and its translation to neuropsychiatric conditions. Dialog- Clin. Neurosci. 2016, 18, 289–298. [CrossRef]
- Vogel Ciernia, A.; Yasui, D.H.; Pride, M.C.; Durbin-Johnson, B.; Noronha, A.B.; Chang, A.; Knotts, T.A.; Rutkowsky, J.R.; Ramsey, J.J.; Crawley, J.N.; LaSalle, J.M. MeCP2 isoform e1 mutant mice recapitulate motor and metabolic phenotypes of Rett syndrome. Hum Mol Genet. 2018, 27, 4077–93. [CrossRef]
- Marano, D.; Fioriniello, S.; D’esposito, M.; Della Ragione, F. Transcriptomic and Epigenomic Landscape in Rett Syndrome. Biomolecules 2021, 11, 967. [CrossRef]
- Sandweiss, A.J.; Brandt, V.L.; Zoghbi, H.Y. Advances in understanding of Rett syndrome and MECP2 duplication syndrome: prospects for future therapies. Lancet Neurol. 2020, 19, 689–698. [CrossRef]
- Haase, F.D.; Coorey, B.; Riley, L.; Cantrill, L.C.; Tam, P.P.L.; Gold, W.A. Pre-clinical Investigation of Rett Syndrome Using Human Stem Cell-Based Disease Models. Front. Neurosci. 2021, 15. [CrossRef]
- Pramanik, S.; Bala, A.; Pradhan, A. Zebrafish in understanding molecular pathophysiology, disease modeling, and developing effective treatments for Rett syndrome. J. Gene Med. 2024, 26, e3677. [CrossRef]
- MacKay, J.; Leonard, H.; Wong, K.; Wilson, A.; Downs, J. Respiratory morbidity in Rett syndrome: an observational study. Dev. Med. Child Neurol. 2018, 60, 951–957. [CrossRef]
- Rashid, N.; Darer, J.D.; Ruetsch, C.; Yang, X. Aspiration, respiratory complications, and associated healthcare resource utilization among individuals with Rett syndrome. Orphanet J. Rare Dis. 2025, 20, 1–8. [CrossRef]
- Crosson, J.; Srivastava, S.; Bibat, G.M.; Gupta, S.; Kantipuly, A.; Smith-Hicks, C.; Myers, S.M.; Sanyal, A.; Yenokyan, G.; Brenner, J.; et al. Evaluation of QTc in Rett syndrome: Correlation with age, severity, and genotype. Am. J. Med Genet. Part A 2017, 173, 1495–1501. [CrossRef]
- Rodrigues, G.D.; Cordani, R.; Veneruso, M.; Chiarella, L.; Prato, G.; Ferri, R.; Carandina, A.; Tobaldini, E.; Nobili, L.; Montano, N. Predominant cardiac sympathetic modulation during wake and sleep in patients with Rett syndrome. Sleep Med. 2024, 119, 188–191. [CrossRef]
- Meyyazhagan, A.; Balasubramanian, B.; Kathannan, S.; Alagamuthu, K.K.; Easwaran, M.; Shanmugam, S.; Pappusamy, M.; Bhotla, H.K.; Mustaqahamed, S.; Arumugam, V.A.; et al. Scrutinizing the molecular, biochemical, and cytogenetic attributes in subjects with Rett syndrome (RTT) and their mothers. Epilepsy Behav. 2020, 111, 107277. [CrossRef]
- Singh, J.; Lanzarini, E.; Santosh, P. Autonomic dysfunction and sudden death in patients with Rett syndrome: a systematic review. J. Psychiatry Neurosci. 2020, 45, 150–181. [CrossRef]
- Berger, T.D.; Berger, C.F.; Gara, S.; Ben-Zeev, B.; Weiss, B. Nutritional and gastrointestinal manifestations in Rett syndrome: long-term follow-up. Eur. J. Pediatr. 2024, 183, 4085–4091. [CrossRef]
- Caputi, V.; Hill, L.; Figueiredo, M.; Popov, J.; Hartung, E.; Margolis, K.G.; Baskaran, K.; Joharapurkar, P.; Moshkovich, M.; Pai, N. Functional contribution of the intestinal microbiome in autism spectrum disorder, attention deficit hyperactivity disorder, and Rett syndrome: a systematic review of pediatric and adult studies. Front. Neurosci. 2024, 18, 1341656. [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell 2023, 14, 762–775.
- Borghi, E.; Vignoli, A. Rett Syndrome and Other Neurodevelopmental Disorders Share Common Changes in Gut Microbial Community: A Descriptive Review. Int. J. Mol. Sci. 2019, 20, 4160. [CrossRef]
- Downs, J.; Wong, K.; Leonard, H. Associations between genotype, phenotype and behaviours measured by the Rett syndrome behaviour questionnaire in Rett syndrome. J. Neurodev. Disord. 2024, 16, 1–13. [CrossRef]
- Moore, R.; Poulsen, J.; Reardon, L.; Samples-Morris, C.; Simmons, H.; Ramsey, K.M.; Whatley, M.L.; Lane, J.B. Managing Gastrointestinal Symptoms Resulting from Treatment with Trofinetide for Rett Syndrome: Caregiver and Nurse Perspectives. Adv. Ther. 2024, 41, 1305–1317. [CrossRef]
- Motil, K.J.; Beisang, A.; Smith-Hicks, C.; Lembo, A.; Standridge, S.M.; Liu, E. Recommendations for the management of gastrointestinal comorbidities with or without trofinetide use in Rett syndrome. Expert Rev. Gastroenterol. Hepatol. 2024, 18, 227–237. [CrossRef]
- Borloz, E.; Villard, L.; Roux, J.-C. Rett syndrome: think outside the (skull) box. Fac. Rev. 2021, 10, 59. [CrossRef]
- Singh, J.; Fiori, F.; Law, M.L.; Ahmed, R.; Ameenpur, S.; Basheer, S.; Chishti, S.; Lawrence, R.; Mastroianni, M.; Mosaddegh, A.; et al. Development and Psychometric Properties of the Multi-System Profile of Symptoms Scale in Patients with Rett Syndrome. J. Clin. Med. 2022, 11, 5094. [CrossRef]
- Y L, R G, R J. Rett Syndrome: Thinking Beyond Brain Borders. Adv Exp Med Biol [Internet]. 2025 [cited 2025 Sep 3];1477. [CrossRef]
- Wang, W.; Li, H.; Xiao, M.; Mu, M.; Xu, H.; Wang, B. Clinical Research on Rett Syndrome: Central Hypoxemia and Hypokalemic Metabolic Alkalosis: 2023.
- Zlatic, S.A.; Duong, D.; Gadalla, K.K.; Murage, B.; Ping, L.; Shah, R.; Fink, J.J.; Khwaja, O.; Swanson, L.C.; Sahin, M.; et al. Convergent cerebrospinal fluid proteomes and metabolic ontologies in humans and animal models of Rett syndrome. iScience 2022, 25, 104966. [CrossRef]
- A V, V C, A P, G V. Ox-inflammasome involvement in neuroinflammation. Free Radic Biol Med [Internet]. 2023 Oct [cited 2025 Sep 3];207. [CrossRef]
- Gonçalez, J.L.; Shen, J.; Li, W. Molecular Mechanisms of Rett Syndrome: Emphasizing the Roles of Monoamine, Immunity, and Mitochondrial Dysfunction. Cells 2024, 13, 2077. [CrossRef]
- Das, D.K.; Raha, S.; Sanghavi, D.; Maitra, A.; Udani, V. Spectrum of MECP2 gene mutations in a cohort of Indian patients with Rett syndrome: Report of two novel mutations. Gene 2013, 515, 78–83. [CrossRef]
- Zepeda-Mendoza, C.J.; Bardon, A.; Kammin, T.; Harris, D.J.; Cox, H.; Redin, C.; Ordulu, Z.; Talkowski, M.E.; Morton, C.C. Phenotypic interpretation of complex chromosomal rearrangements informed by nucleotide-level resolution and structural organization of chromatin. Eur. J. Hum. Genet. 2018, 26, 374–381. [CrossRef]
- Huang, C.-H.; Wong, L.-C.; Chu, Y.-J.; Hsu, C.-J.; Wang, H.-P.; Tsai, W.-C.; Lee, W.-T. The sleep problems in individuals with Rett syndrome and their caregivers. Autism 2024, 28, 3118–3130. [CrossRef]
- Kay, C.; Leonard, H.; Smith, J.; Wong, K.; Downs, J. Genotype and sleep independently predict mental health in Rett syndrome: an observational study. J. Med Genet. 2023, 60, 951–959. [CrossRef]
- Tascini, G.; Dell’ISola, G.B.; Mencaroni, E.; Di Cara, G.; Striano, P.; Verrotti, A. Sleep Disorders in Rett Syndrome and Rett-Related Disorders: A Narrative Review. Front. Neurol. 2022, 13, 817195. [CrossRef]
- Lai, Y.Y.L.; Downs, J.; Zafar, S.; Wong, K.; Walsh, L.; Leonard, H. Oral health care and service utilisation in individuals with Rett syndrome: an international cross-sectional study. J. Intellect. Disabil. Res. 2021, 65, 561–576. [CrossRef]
- Neul, J.L.; Percy, A.K.; Benke, T.A.; Berry-Kravis, E.M.; Glaze, D.G.; Marsh, E.D.; Lin, T.; Stankovic, S.; Bishop, K.M.; Youakim, J.M. Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat. Med. 2023, 29, 1468–1475. [CrossRef]
- Hirano, D.; Goto, Y.; Shoji, H.; Taniguchi, T. Relationship between hand stereotypies and purposeful hand use and factors causing skin injuries and joint contractures in individuals with Rett syndrome. Early Hum. Dev. 2023, 183, 105821–105821. [CrossRef]
- Zhang, E.; Zhao, T.; Sikora, T.; Ellaway, C.; Gold, W.A.; Van Bergen, N.J.; Stroud, D.A.; Christodoulou, J.; Kaur, S. CHD8 Variant and Rett Syndrome: Overlapping Phenotypes, Molecular Convergence, and Expanding the Genetic Spectrum. Hum Mutat. 2025, 2025, 5485987.
- Migovich, M.; Ullal, A.; Fu, C.; Peters, S.U.; Sarkar, N. Feasibility of wearable devices and machine learning for sleep classification in children with Rett syndrome: A pilot study. Digit. Heal. 2023, 9. [CrossRef]
- Wandin, H.; Lindberg, P.; Sonnander, K. Aided language modelling, responsive communication and eye-gaze technology as communication intervention for adults with Rett syndrome: three experimental single case studies. [CrossRef]
- Monteiro-Fernandes, D.; Charles, I.; Guerreiro, S.; Cunha-Garcia, D.; Pereira-Sousa, J.; Oliveira, S.; Teixeira-Castro, A.; Varney, M.A.; Kleven, M.S.; Newman-Tancredi, A.; et al. Rescue of respiratory and cognitive impairments in Rett Syndrome mice using NLX-101, a selective 5-HT1A receptor biased agonist. Biomed. Pharmacother. 2025, 186, 117989. [CrossRef]
- Cordone, V. Biochemical and molecular determinants of the subclinical inflammatory mechanisms in Rett syndrome. Arch. Biochem. Biophys. 2024, 757, 110046. [CrossRef]
- Kaufmann, W.E.; Oberman, L.M.; Downs, J.; Leonard, H.; Barnes, K.V. Rett Syndrome Behaviour Questionnaire: Variability of Scores and Related Factors. J. Child Adolesc. Psychopharmacol. 2025. [CrossRef]
- Percy, A.K.; Neul, J.L.; Benke, T.A.; Marsh, E.D.; Glaze, D.G. A review of the Rett Syndrome Behaviour Questionnaire and its utilization in the assessment of symptoms associated with Rett syndrome. Front. Pediatr. 2023, 11, 1229553. [CrossRef]
- Neul, J.L.; Percy, A.K.; Benke, T.A.; Berry-Kravis, E.M.; Glaze, D.G.; Peters, S.U.; Jones, N.E.; Youakim, J.M. Design and outcome measures of LAVENDER, a phase 3 study of trofinetide for Rett syndrome. Contemp. Clin. Trials 2022, 114, 106704. [CrossRef]
- Oberman, L.M.; Leonard, H.; Downs, J.; Cianfaglione, R.; Stahlhut, M.; Larsen, J.L.; Madden, K.V.; Kaufmann, W.E. Rett Syndrome Behaviour Questionnaire in Children and Adults With Rett Syndrome: Psychometric Characterization and Revised Factor Structure. Am. J. Intellect. Dev. Disabil. 2023, 128, 237–253. [CrossRef]
- Cohen, S.R.; Helbig, I.; Kaufman, M.C.; Schust Myers, L.; Conway, L.; Helbig, K.L. Caregiver assessment of quality of life in individuals with genetic developmental and epileptic encephalopathies. Dev Med Child Neurol. 2022, 64, 957–964. [CrossRef]
- Killian, J.T.; Lane, J.B.; Lee, H.-S.; Pelham, J.H.; Skinner, S.A.; Kaufmann, W.E.; Glaze, D.G.; Neul, J.L.; Percy, A.K. Caretaker Quality of Life in Rett Syndrome: Disorder Features and Psychological Predictors. Pediatr. Neurol. 2016, 58, 67–74. [CrossRef]
- McGraw, S.A.; Smith-Hicks, C.; Nutter, J.; Henne, J.C.; Abler, V. Meaningful Improvements in Rett Syndrome: A Qualitative Study of Caregivers. J. Child Neurol. 2023, 38, 270–282. [CrossRef]
- Bajikar, S.S.; Zhou, J.; O’hAra, R.; Tirumala, H.P.; Durham, M.A.; Trostle, A.J.; Dias, M.; Shao, Y.; Chen, H.; Wang, W.; et al. Acute MeCP2 loss in adult mice reveals transcriptional and chromatin changes that precede neurological dysfunction and inform pathogenesis. Neuron 2024, 113, 380–395.e8. [CrossRef]
- Ito-Ishida, A.; Ure, K.; Chen, H.; Swann, J.W.; Zoghbi, H.Y. Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron 2015, 88, 651–658. [CrossRef]
- Sadhu, C.; Lyons, C.; Oh, J.; Jagadeeswaran, I.; Gray, S.J.; Sinnett, S.E. The Efficacy of a Human-Ready miniMECP2 Gene Therapy in a Pre-Clinical Model of Rett Syndrome. Genes 2023, 15, 31. [CrossRef]
- Yang, K.; Li, T.; Geng, Y.; Zhang, R.; Xu, Z.; Wu, J.; Yuan, Y.; Zhang, Y.; Qiu, Z.; Li, F. Protocol for the neonatal intracerebroventricular delivery of adeno-associated viral vectors for brain restoration of MECP2 for Rett syndrome. STAR Protoc. 2024, 5, 103344. [CrossRef]
- Gomathi, M.; Balachandar, V. Novel therapeutic approaches: Rett syndrome and human induced pluripotent stem cell technology. Stem Cell Investig. 2017, 4, 20–20. [CrossRef]
- Huber, A.; Sarne, V.; Beribisky, A.V.; Ackerbauer, D.; Derdak, S.; Madritsch, S.; Etzler, J.; Huck, S.; Scholze, P.; Gorgulu, I.; et al. Generation and Characterization of a Human Neuronal In Vitro Model for Rett Syndrome Using a Direct Reprogramming Method. Stem Cells Dev. 2024, 33, 128–142. [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 2021, 24, 1488–1500. [CrossRef]
- Gomes, A.R.; Fernandes, T.G.; Vaz, S.H.; Silva, T.P.; Bekman, E.P.; Xapelli, S.; Duarte, S.; Ghazvini, M.; Gribnau, J.; Muotri, A.R.; et al. Modeling Rett Syndrome With Human Patient-Specific Forebrain Organoids. Front. Cell Dev. Biol. 2020, 8. [CrossRef]
- Mok, R.S.F.; Zhang, W.; Sheikh, T.I.; Pradeepan, K.; Fernandes, I.R.; DeJong, L.C.; Benigno, G.; Hildebrandt, M.R.; Mufteev, M.; Rodrigues, D.C.; et al. Wide spectrum of neuronal and network phenotypes in human stem cell-derived excitatory neurons with Rett syndrome-associated MECP2 mutations. Transl. Psychiatry 2022, 12, 1–16. [CrossRef]
- Pradeepan, K.S.; McCready, F.P.; Wei, W.; Khaki, M.; Zhang, W.; Salter, M.W.; Ellis, J.; Martinez-Trujillo, J. Calcium-Dependent Hyperexcitability in Human Stem Cell–Derived Rett Syndrome Neuronal Networks. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100290. [CrossRef]
- Aldosary, M.; Al-Bakheet, A.; Al-Dhalaan, H.; Almass, R.; Alsagob, M.; Al-Younes, B.; AlQuait, L.; Mustafa, O.M.; Bulbul, M.; Rahbeeni, Z.; et al. Rett Syndrome, a Neurodevelopmental Disorder, Whole-Transcriptome, and Mitochondrial Genome Multiomics Analyses Identify Novel Variations and Disease Pathways. OMICS A J. Integr. Biol. 2020, 24, 160–171. [CrossRef]
- Karaosmanoglu, B.; Imren, G.; Ozisin, M.S.; Reçber, T.; Kiper, P.O.S.; Haliloglu, G.; Alikaşifoğlu, M.; Nemutlu, E.; Taskiran, E.Z.; Utine, G.E. Ex vivo disease modelling of Rett syndrome: the transcriptomic and metabolomic implications of direct neuronal conversion. Mol. Biol. Rep. 2024, 51, 1–15. [CrossRef]
- Pascual-Alonso, A.; Xiol, C.; Smirnov, D.; Kopajtich, R.; Prokisch, H.; Armstrong, J. Multi-omics in MECP2 duplication syndrome patients and carriers. Eur J Neurosci. 2024, 60, 4004–4018.
- Baroncelli, L.; Auel, S.; Rinne, L.; Schuster, A.-K.; Brand, V.; Kempkes, B.; Dietrich, K.; Müller, M. Oral Feeding of an Antioxidant Cocktail as a Therapeutic Strategy in a Mouse Model of Rett Syndrome: Merits and Limitations of Long-Term Treatment. Antioxidants 2022, 11, 1406. [CrossRef]
- Panayotis, N.; Ehinger, Y.; Felix, M.S.; Roux, J.C. State-of-the-art therapies for Rett syndrome. Dev Med Child Neurol. 2023, 65, 162–170.
- Persico, A.M.; Ricciardello, A.; Cucinotta, F. The psychopharmacology of autism spectrum disorder and Rett syndrome. Handb Clin Neurol. 2019, 165, 391–414.
- Kaufmann, W.E.; Stallworth, J.L.; Everman, D.B.; Skinner, S.A. Neurobiologically-based treatments in Rett syndrome: opportunities and challenges. Expert Opin. Orphan Drugs 2016, 4, 1043–1055. [CrossRef]
- Percy, A.K.; Neul, J.L.; Benke, T.A.; Berry-Kravis, E.M.; Glaze, D.G.; Marsh, E.D.; An, D.; Bishop, K.M.; Youakim, J.M. Trofinetide for the treatment of Rett syndrome: Results from the open-label extension LILAC study. Med 2024, 5, 1178–1189.e3. [CrossRef]
- Singh, A.; Balasundaram, M.K.; Gupta, D. Trofinetide in Rett syndrome: A brief review of safety and efficacy. Intractable Rare Dis. Res. 2023, 12, 262–266. [CrossRef]
- Coorey, B.; Haase, F.; Ellaway, C.; Clarke, A.; Lisowski, L.; Gold, W.A. Gene Editing and Rett Syndrome: Does It Make the Cut?. CRISPR J. 2022, 5, 490–499. [CrossRef]
- Fonzo, M.; Sirico, F.; Corrado, B. Evidence-Based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review. Brain Sci. 2020, 10, 410. [CrossRef]
- Saby, J.N.; Peters, S.U.; Roberts, T.P.L.; Nelson, C.A.; Marsh, E.D. Evoked Potentials and EEG Analysis in Rett Syndrome and Related Developmental Encephalopathies: Towards a Biomarker for Translational Research. Front. Integr. Neurosci. 2020, 14, 30. [CrossRef]
- Buckley, N.; Stahlhut, M.; Elefant, C.; Leonard, H.; Lotan, M.; Downs, J. Parent and therapist perspectives on "uptime" activities and participation in Rett syndrome. Disabil. Rehabilitation 2021, 44, 7420–7427. [CrossRef]
- Bajikar, S.S.; Sztainberg, Y.; Trostle, A.J.; Tirumala, H.P.; Wan, Y.-W.; Harrop, C.L.; Bengtsson, J.D.; Carvalho, C.M.B.; Pehlivan, D.; Suter, B.; et al. Modeling antisense oligonucleotide therapy in MECP2 duplication syndrome human iPSC-derived neurons reveals gene expression programs responsive to MeCP2 levels. Hum. Mol. Genet. 2024, 33, 1986–2001. [CrossRef]
- Gogliotti, R.G.; Senter, R.K.; Rook, J.M.; Ghoshal, A.; Zamorano, R.; Malosh, C.; Stauffer, S.R.; Bridges, T.M.; Bartolome, J.M.; Daniels, J.S.; Jones, C.K.; Lindsley, C.W.; Conn, P.J.; Niswender, C.M. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum Mol Genet. 2016, 25, 1990–2004. [CrossRef]
- Merritt, J.K.; Fang, X.; Caylor, R.C.; Skinner, S.A.; Friez, M.J.; Percy, A.K.; Neul, J.L. Normalized Clinical Severity Scores Reveal a Correlation between X Chromosome Inactivation and Disease Severity in Rett Syndrome. Genes 2024, 15, 594. [CrossRef]
- Abbas, A.; Fayoud, A.M.; Moawad, M.H.E.D.; Hamad, A.A.; Hamouda, H.; Fouad, E.A. Safety and efficacy of trofinetide in Rett syndrome: a systematic review and meta-analysis of randomized controlled trials. BMC Pediatr. 2024, 24, 1–12. [CrossRef]
- Cherchi, C.; Chiappini, E.; Amaddeo, A.; Testa, M.B.C.; Banfi, P.; Veneselli, E.; Cutrera, R.; Syndrome, T.P.F.T.P.I.P.W.R. Management of respiratory issues in patients with Rett syndrome: Italian experts’ consensus using a Delphi approach. Pediatr. Pulmonol. 2024, 59, 1970–1978. [CrossRef]
- Furley, K.; Mehra, C.; Goin-Kochel, R.P.; Fahey, M.C.; Hunter, M.F.; Williams, K.; Absoud, M. Developmental regression in children: Current and future directions. Cortex 2023, 169, 5–17. [CrossRef]
- Xiol, C.; Vidal, S.; Pascual-Alonso, A.; Blasco, L.; Brandi, N.; Pacheco, P.; Gerotina, E.; O’callaghan, M.; Pineda, M.; Armstrong, J. X chromosome inactivation does not necessarily determine the severity of the phenotype in Rett syndrome patients. Sci. Rep. 2019, 9, 1–9. [CrossRef]
- Lim, J.; Greenspoon, D.; Hunt, A.; McAdam, L. Rehabilitation interventions in Rett syndrome: a scoping review. Dev. Med. Child Neurol. 2020, 62, 906–916. [CrossRef]
- Downs, J.; Pichard, D.C.; Kaufmann, W.E.; Horrigan, J.P.; Raspa, M.; Townend, G.; Marsh, E.D.; Leonard, H.; Motil, K.; Dietz, A.C.; et al. International workshop: what is needed to ensure outcome measures for Rett syndrome are fit-for-purpose for clinical trials? June 7, 2023, Nashville, USA. Trials 2024, 25, 1–20. [CrossRef]
- Howell, K.B.; White, S.M.; McTague, A.; D’gAma, A.M.; Costain, G.; Poduri, A.; Scheffer, I.E.; Chau, V.; Smith, L.D.; Stephenson, S.E.M.; et al. International Precision Child Health Partnership (IPCHiP): an initiative to accelerate discovery and improve outcomes in rare pediatric disease. npj Genom. Med. 2025, 10, 1–14. [CrossRef]
- Smith, M.; Arthur, B.; Cikowski, J.; Holt, C.; Gonzalez, S.; Fisher, N.M.; Vermudez, S.A.D.; Lindsley, C.W.; Niswender, C.M.; Gogliotti, R.G. Clinical and Preclinical Evidence for M1 Muscarinic Acetylcholine Receptor Potentiation as a Therapeutic Approach for Rett Syndrome. Neurotherapeutics 2022, 19, 1340–1352. [CrossRef]
| Mutation Type | Common Variants | Approximate frequency among classic MECP2 variants in RTT (%)—cohort-dependent | Phenotypic Severity | Associated Features | Recent Data | Inference |
|---|---|---|---|---|---|---|
| Missense | R106W, R133C, T158M | 40–50 | Moderate to Severe | Early regression, epilepsy (60–80%), scoliosis | Disrupt methyl-binding domain; partial function retained. | R133C shows a milder phenotype due to residual binding. RNHS: Better ambulation in R133C (30% vs. 10% in R106W). |
| Nonsense | R168X, R255X, R270X | 30–40 | Severe | Profound intellectual disability, non-ambulatory, respiratory issues | Early truncation leading to a null function. | High sudden death risk (25%). 2025 data: Correlation with mitochondrial dysfunction suggests metabolic crisis. |
| Frameshift/Deletions | C-terminal deletions | 10–15 | Mild to Moderate | Preserved speech variant, later onset | Retain partial domains; 2024 iPSC data show reduced synaptic loss. | Therapeutic window exists for partial restoration. |
| Duplications | MECP2 duplication syndrome | <5 (males predominant) | Severe (males lethal) | Hypotonia, infections, autism-like features | Overexpression toxicity; mouse models demonstrate anxiety phenotypes. | Dosage sensitivity inferred. |
| System | Key Features | Prevalence | Mechanisms (Animal/iPSC Evidence) | Clinical Management | Inference |
|---|---|---|---|---|---|
| Respiratory/Breathing Dysrhythmia | Awake hyperventilation, apnea, breath-holds; brainstem circuit disruption; mitochondrial hypoxia | ~60–80% (RNHS 2024–2025: 70–80%) | Brainstem circuit disruption; mitochondrial hypoxia | NIV, oxygen; training | Central–peripheral interplay; oxidative stress target for therapies. |
| Cardiovascular/Autonomic | QTc prolongation, arrhythmia, HRV ↓ vagal tone; sympathetic overdrive | ~20% QTc↑ (RNHS: 75% instability; 20–30% QTc) | Ion channel dysregulation; sympathetic overdrive; mitochondrial contribution | Annual ECG; beta-blockers | Mitochondrial role inferred; sudden death prevention priority. |
| Gastrointestinal (Constipation, GERD, Dysphagia)* | Severe chronic constipation; reflux disease; swallowing incoordination | Constipation ~80–90% (RNHS: 80%); GERD/Dysphagia ~60–70% (RNHS: 70%) | Enteric neuron hypofunction; microbiome shifts | Laxatives, gastrostomy | Inflammation link; trofinetide GI AEs highlight need for adjuncts. |
| Skeletal (Scoliosis, Low BMD/Fractures)* | Scoliosis often progressive (>40°); osteopenia; frequent fractures | Scoliosis ≥60–85% (RNHS: 85%); Low BMD ≈45–60%; fractures ~30% (RNHS: 20%) | Osteoblast defects; endocrine (low Vitamin D) | Bisphosphonates; surgery | Immobility exacerbates; early PT inferred to mitigate. |
| CNS—Seizures | Onset 2–5 yrs; mix of focal and generalized; treatable | ~60–90% lifetime | Cortical hyperexcitability; neuronal circuit dysfunction | Antiepileptic drugs (AEDs); multidisciplinary seizure management | Seizure control impacts quality of life and neurodevelopment. |
| Growth/Nutrition | Short stature, poor weight gain, microcephaly | ~80–100% | Nutritional/metabolic dysfunction; growth hormone/endocrine contribution | Nutritional support; multidisciplinary monitoring | Growth failure central to prognosis; metabolic basis |
| Sleep | Insomnia, night awakenings, non-restorative sleep | ~60–80% | Circadian rhythm disruption; neurotransmitter imbalance | Behavioral interventions; melatonin | Sleep disturbance linked to cognition and seizures. |
| Anxiety/Mood | Excessive fear, anxious behaviors, mood swings | ~50–70% | Amygdala circuit dysfunction; altered GABA/glutamate signaling | Behavioral therapy; SSRIs (case-based) | Mental health central to QoL; neurochemical imbalance inferred. |
| Dental—Bruxism | Daytime teeth grinding | ~80–100% | Abnormal brainstem reflex pathways; neuromuscular dysregulation | Mouthguards; dental monitoring | Contributes to dental wear; symptomatic care only. |
| Peripheral/Autonomic—Cold Extremities | Vasomotor instability (cyanosis of hands/feet) | ~50% | Autonomic dysregulation; vascular tone impairment | Supportive management; warming measures | Peripheral autonomic dysfunction reinforces systemic involvement |
| Model System | Genetic Alteration/Type | Key Features/Uses | Strengths | Limitations | Inferences |
|---|---|---|---|---|---|
| Mouse Mecp2-null (Bird) males | Mecp2 null (hemizygote) | Rapid-onset RTT-like phenotype; seizures; early death (~10 wks) | High phenotypic fidelity; reproducible phenotype | Small brain size; not representative of female mosaicism | Adult re-expression reverses ~80% of phenotypes; omics show metabolic shifts. |
| Mouse Mecp2-null (heterozygous females) | Mecp2+/– (female heterozygote) | Mosaic neuronal populations; slower progression | Models clinical female RTT; long survival | Variable expressivity due to XCI | Omics reveal compensatory networks in mosaic populations. |
| Mouse Mecp2-duplication | Multiple Mecp2 transgene copies | Models MECP2 duplication syndrome; seizures | Relevant for antisense therapy | Overexpression artifacts; limited survival | Overexpression risks validated in vivo. |
| Conditional knockout mice | Mecp2 deleted in neuron/astrocyte/microglia | Cell-type specific phenotypes | Dissects MECP2 roles | Restricted scope | Highlights glial contributions to disease. |
| Rat Mecp2 knockout | Mecp2 deletion | RTT-like features; larger size | Larger brain; better dosing/autonomic profiling | Fewer strains; less genetic versatility | Enhanced autonomic profiling; better for gene therapy dosing. |
| Human iPSC-derived neurons | Patient fibroblasts reprogrammed | Reduced dendritic arborization, synaptic deficits | Human genotype/phenotype context | No systemic environment | Synaptic rescue demonstrated; 2025: mitochondrial targets identified. |
| Brain organoids (human iPSC) | 3D cortical cultures | Reveal early cortical networks, activity patterns | Human-specific; network-level | Limited maturation | Organoid models validate early neurodevelopmental signatures. |
| Large Animal/NHP Mecp2 models | Transgenic/gene-edited | Translational biodistribution, pharmacology | Closer to human brain | Ethical and costly | AAV safety validated; overexpression risks confirmed. |
| Zebrafish Mecp2-null | MecP2 mutant fish | Transparent larvae; rapid assays | Fast, scalable | Limited behavioral fidelity | Useful for rapid drug screening and in vivo imaging approaches. |
| Therapeutic Class/Strategy | Example(s)/Compound(s) | Mechanism of Action | Status | Key Efficacy/Safety Notes | Inference |
|---|---|---|---|---|---|
| Pharmacotherapy | Trofinetide | IGF-1 analog; neurotrophic/anti-inflammatory | FDA-Approved (2023) | Efficacy on RSBQ, CGI-I, CSBS. GI side effects (diarrhea, vomiting). | Symptom modifier; real-world reports suggest sustained benefits with careful GI management. |
| Neurotrophic/Growth Factor Strategies | IGF-1 (mecasermin) | Neurotrophic support | Phase 2 | Mixed efficacy; not advanced. | Highlights limitations of systemic IGF-1 therapy. |
| Gene Replacement Therapy | NGN-401 (Neurogene) | AAV-based MECP2 delivery | Phase I/II | High-dose halted (fatal inflammatory syndrome). Low-dose: 28–52% RSBQ improvement. | Potential disease modifier; tight dose regulation essential. |
| Dosage Normalization | ASOs (e.g., ISIS-LEGRO) | Silence MECP2 mRNA | Preclinical/early trials | Dosage titration possible; risk of over-suppression. | Conceptually validates dosage control; requires precision for safety. |
| Metabolic/Mitochondrial Modulators | Leriglitazone | PPAR-γ agonist; mitochondrial targeting | Phase 2a | Ongoing; systemic benefit expected. | Mitochondrial/metabolic pathways are relevant therapeutic targets. |
| Cell-based Approaches | Stem cell transplantation, exosome therapy | Replacement/trophic support | Investigational | Safety and targeting limitations. | Currently experimental; requires advanced targeting. |
| Neuromodulation | TNS, VNS | Electrical network modulation | Experimental | Small studies: improvements in arousal/respiration. | Adjunct potential but preliminary. |
| Symptomatic/Supportive Care | PT, OT, speech, nutritional support | Multidisciplinary | Standard of care | Improves quality of life. | Remains essential alongside all experimental therapies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





