1. Introduction
Endometriosis is an inflammatory estrogen-dependent disorder that course with chronic pelvic pain, dyspareunia, dysmenorrhea, and infertility, affecting 5-10% of age-reproductive women worldwide [
1,
2]. On the basis of this clinical entity is the invasion of different organs by endometrial-like tissue. This tissue proliferate and growth in the proper endometrium or outside the uterus (ovary, peritoneum, urinary bladder, intestine), or more distant (lung) originating eutopic or ectopic endometriosis, respectively [
3,
4]. The endometrial infiltrates cause inflammation, angiogenesis, fibrosis, formation of scar tissue, and functional impairments of the hosted organs [
5,
6,
7]. Nevertheless, despite its high prevalence and impact on quality of life, the pathogenesis of endometriosis is poorly understood [
8,
9]. Consistently, the available treatment options are limited and mainly focused on symptom management rather than targeting the underlying mechanisms of the disease [
10,
11,
12].
Two emergent ion channels denominated Piezo1 and Piezo2 which primary work as mechanosensors and mechanotransducers [
13,
14,
15,
16] have demonstrated to also play a role in cellular migration [
17], inflammation [
18,
19,
20], angiogenesis [
21], fibrosis [
22,
23,
24,
25], and pain [
26,
27,
28] that are the primary clinical markers of endometriosis. As far as we know, there is no association of Piezo channels with dysmenorrhea. Thus, Piezo channels are surely more than mechanotransducers or at least they are involved in the linking between forces and different physiological and pathological conditions [
29,
30,
31,
32].
Piezo channels are broadly distributed in tissues and cells, predominating Piezo2 in nervous tissues and Piezo 1 in non-nervous ones. However, little information is only available about the occurrence of Piezo channels in the uterus [
33,
34]. Therefore, we decided to conduct the present research to detect Piezo1 and Piezo2 in healthy endometrium and to investigate whether they change in eutopic and ectopic endometriosis. We used immunohistochemistry and immunofluorescence coupled with laser confocal microscopy, and image analysis.
2. Materials and Methods
2.1. Subjects
This prospective study enrolled 35 of which 30 diagnosed with endometriosis and 5 controls. The age of patients was (mean age ± Standard Deviation (SD) 48 ± 9,16 in the control group and 35,10 ± 8,3 in the endometriosis group.
Medical records were reviewed to collect relevant clinical information. All patients enrolled in the study were confirmed to be premenopausal with regular menstrual cycles. Endometriosis was surgically and histologically diagnosed as stage I, II, III and IV according to the revised American Fertility Society (r-AFS) classification schema. The level of pain was determined using visual analog scale (VAS), from scale 0 (no pain), to 10 (worst pain). The VAS scores were grouped into three levels: mild (ranging from 0 to 3), moderate (ranging from 4 to 6), and severe (ranging from 7 to 10)
The largest proportion in endometriosis group. In this study symptoms of dysmenorrhea (100%), followed by infertility (55,17%). Intense pain with a visual analog scale value (VAS) >7 was found in nearly 60% of women. The findings on laparoscopic examination and anatomical pathology demonstrated ovarian endometriosis in 37,93% of cases peritoneal and digestive endometriosis was detected in 34,48% while the rate of stage III and IV was reported to be 27,58% and 68,96%, respectively. All the signs and symptoms of patients in the endometriosis group are listed in
Table 1.
All patients underwent laparoscopy due to symptomatic endometriosis in which the endometriotic tissue was removed. Moreover, endometrium samples from 5 women without endometriosis who had undergone laparoscopy from benign gynecological diseases were used as controls. Subjects with endometrial cyst, pain, infertility and endometriosis detected through laparoscopic surgery were included in the endometriosis group. Endometriosis was confirmed histologically by the presence of both endometrial glands and stroma in an ectopic location.
2.2. Ethical
Tissue samples were obtained at the Service of Pathology of the Hospital Alvarez Buylla, Mieres, Principality of Asturias, Spain. All materials used in the present study were obtained in compliance with Spanish legislation (RD 1301/2006; Law 14/2007; RD 1716/2011; Order ECC/1404/2013) and in accordance with the guidelines of the Declaration of Helsinki II. Informed consent was obtained from patients and the study was approved by the Research Ethics Committee of the Principality of Asturias (Cod CEIm P Ast: Project 266/18).
2.3. Treatment of Tissues Samples
The excised tissues from both patients with endometriosis and controls were fixed on 10% formalin (in 1 M PBS, pH 7.4) for 12h and routinely embedded in paraffin. Serial sections 7µm and 10 µm thick were cut and mounted onto silane-coated slides. Thereafter sections were deparaffinized and processed for immunohistochemistry or immunofluorescence.
2.4. Structural and Immunohistochemical Diagnosis of Endometriosis
For the certainty diagnosis of endometriosis, representative sections of each specimen were processed as follows: after removing paraffin and permeabilization with 1M PBS at pH 7.6 with 0.5% Tween-20, the endogenous peroxidase activity was blocked with 10% H
2O
2 for 30 minutes, and non-specific binding was then prevented with 25% fetal bovine serum. Sections were incubated overnight at 4ᵒ C in a humid chamber with the primary antibodies against CD10, progesterone receptor and estrogen receptor (
Table 2). Subsequently, the sections were incubated with anti-rabbit or anti-mouse EnVision system-labelled polymer (DakoCytomation) for 30 min. Finally, they were washed with buffer solution, and the immunoreaction was visualized with diaminobenzidine as a chromogen. Finally the slides were washed, dehydrated, and mounted with Entellan (Merck, Dramstadt, Germany). To ascertain structural details, the sections were counterstained with Mayer’s hematoxylin. Images of the immunohistochemical results were taken with a Nikon Eclipse
® 80i optical microscope coupled to a Nokia
® DS-5M camera.
CD10 is routinely used in the diagnostic of endometriosis [
35,
36] and estrogen or progesterone receptors since these hormones promote endometriosis [
37,
38].
2.5. Simple Immunohistochemistry for PIEZOs
Deparaffinized and rehydrated sections were processed as described above and incubated overnight at 4ᵒ C in a humid chamber with the primary antibodies against specific epitopes of PIEZO1 and PIEZO (
Table 2). Images of the immunohistochemical results were taken with a Nikon Eclipse
® 80i optical microscope coupled to a Nokia
® DS-5M camera.
2.6. Evaluation of Intensity of Immunostaining for PIEZOs
The intensity of immunostaining for Piezo1 and Piezo2 developed in the different tissues was evaluated semi quantitatively as follows: whole section sections were scanned at medium (×50) and high magnification (×200) using a SCN400F scanner (Leica Biosystems™); the images were processed with the SlidePath Gateway LAN program (Leica Biosystems™) at the Histopathology Laboratory, University Institute of Oncology of the Principality of Asturias. The results were evaluated thrice by two independent observers (OGS and YGM) blinded to the group of the specimen, i.e., control vs. endometriosis, and the antigen investigated. Results were divided into four groups: -: negative, no staining; +: faint; ++: moderate; +++: strong.
2.6. Evaluation of Intensity of Immunostaining for PIEZOs
The intensity of immunostaining for Piezo1 and Piezo2 developed in the different tissues was evaluated semi quantitatively as follows: whole section sections were scanned at medium (×50) and high magnification (×200) using a SCN400F scanner (Leica Biosystems™); the images were processed with the SlidePath Gateway LAN program (Leica Biosystems™) at the Histopathology Laboratory, University Institute of Oncology of the Principality of Asturias. The results were evaluated thrice by two independent observers (OG-S and YG-M) blinded to the group of the specimens, i.e., control vs. endometriosis, and the investigated antigen. Results were divided into four groups: no staining, 0; weak staining, light yellow, 1; moderate staining, yellowish brown, 2; and strong staining, brown, 3.
2.7. Double Immunofluorescence for Identification of PIEZO Positive Cells
Deparaffinized and rehydrated sections were washed in PBS-T for 20 minutes. Then, sections were incubated overnight at 4 °C in a humid chamber with a 1:1 mixture of two antibodies for the simultaneous detection of Piezo1/CK7 and Piezo2/CK7. Subsequently, sections were washed with PBS-T for 30 minutes and then incubated with the secondary antibodies for 90 minutes: first, Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:100; Serotec™, Oxford, UK), then washed in PBS-T, and followed by Cy3-conjugated donkey anti-mouse IgG (1:200; Jackson-ImmunoResearch™, Baltimore, MD, USA); both incubations were carried out in a humid chamber, in darkness and at room temperature. Finally, the sections were stained with DAPI (4’,6-diamino-2-phenylindole; 10 ng/ml) to label the nuclei (blue color) and mounted with diluted Fluoromount-G mounting medium (Southern-Biotech, Alabama, USA). Triple staining was detected using a Leica DMR-XA automatic fluorescence microscope coupled with a Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg GmbH, Germany), and the images captured were processed using the software Image J version 1.43 g Master Biophotonics Facility, Mac Master University Ontario (
www.macbiophotonics.ca) from the Image Processing Service of the University of Oviedo.
Specific reaction controls were performed in the same way as for simple immunohistochemistry.
For control purposes selected sections were processed identically as described above omitting the primary and/or secondary antibody in incubation or incubating the sections with non-immune serum from rabbits or mice. Under these conditions, no specific immunoreactivity was found. Additional controls were carried out to confirm the absence of autofluorescence that may interfere with immunofluorescence results.
3. Results
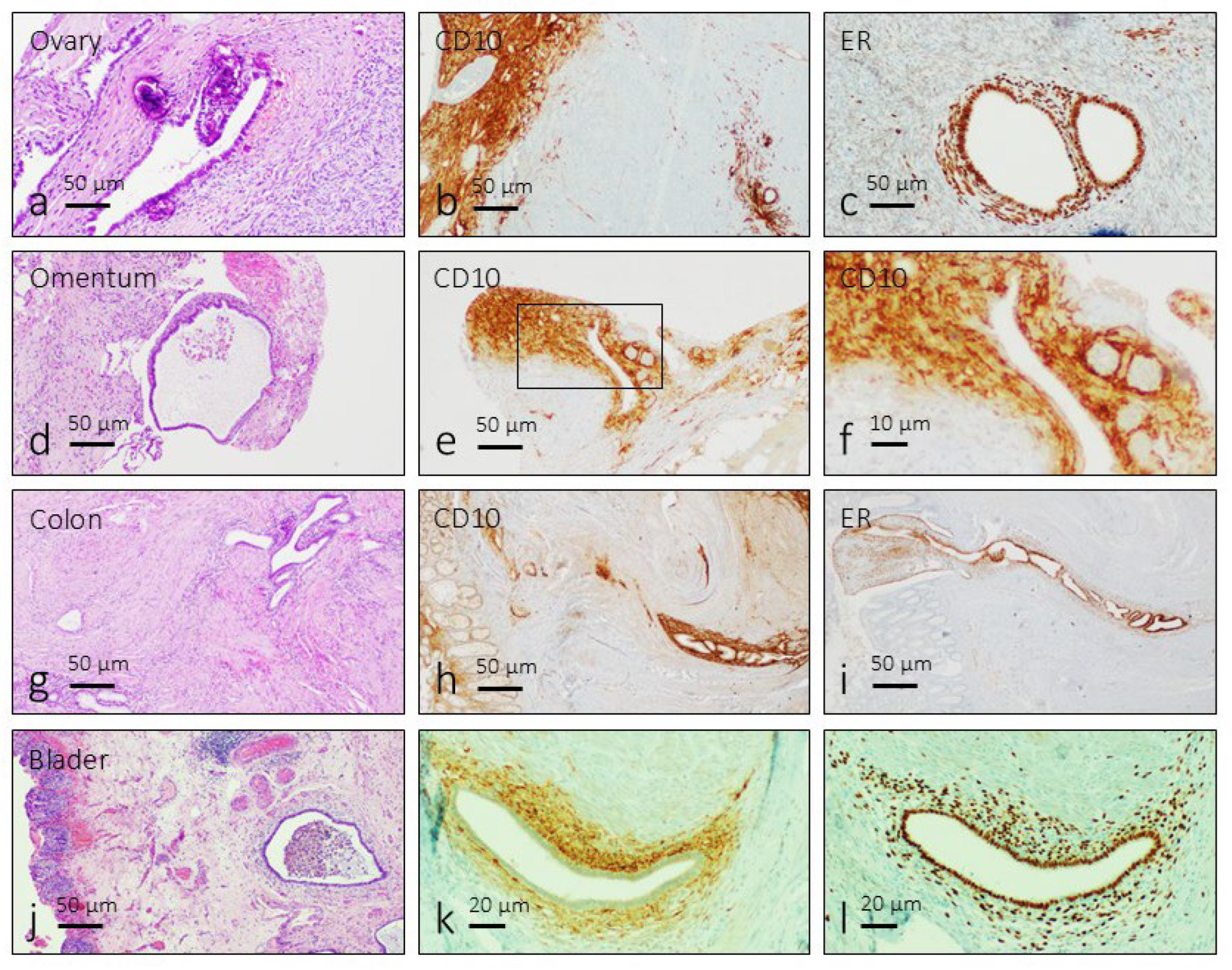
3.1. Identification of the Endometriosis Foci
Endometriosis foci are characterized by the presence of ectopic endometrial glands surrounded by a fibrous endometrial stroma with inflammatory cells. Occasionally they form cysts with heterogeneous content including cell debris, traces of hemorrhages and pigmentation due to hemosiderin deposition (
Figure 1a,d,g and j). Regardless of their anatomical location, the immunoreactivity for CD10 was detected in the stroma of endometriosis lesions (
Figure 1b,e,h and k), and immunoreactivity for estrogen receptors was localized in the nuclei of gland and stromal cells (
Figure 1c,f,i and l).
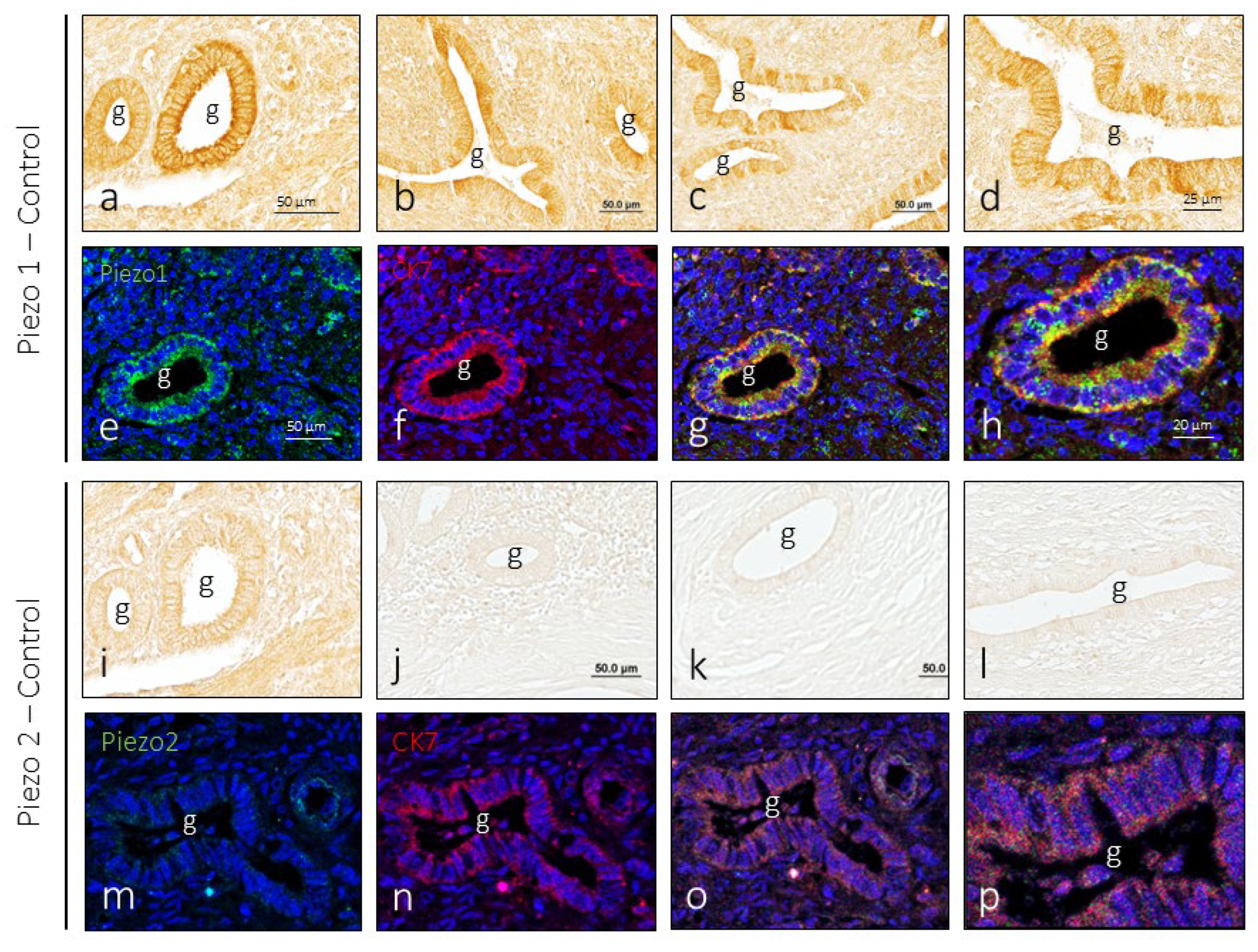
3.2. Piezo1 and Piezo2 Immunoreactivity in the Endometrium
In endometrial samples obtained from healthy women, immunoreaction for PIEZO1 was regularly detected in the glandular epithelium, with small differences in immunolabeling from one gland to another, and no evident differences were noted between the analyzed samples. The immunolabeling pattern was membrane-shaped, being more intense in the luminal pole of the cells (
Figure 2a-d). Doble immunolabeling of the sections to simultaneously detect Piezo1 and cytokeratin 7 (CK7) confirms that Piezo1 immunoreactivity is limited to the cells of the gland epithelium and localized in the cell membrane (
Figure 2e-h).
Regarding Piezo2, its presence in the healthy endometrium was restricted to a small number of glands. As for Piezo1, immunoreactivity presented a membrane pattern of immunolabeling (
Figure 2i-l) and was restricted to epithelial cells (
Figure 2m-p).
When the intensity of immunoreactivity for Piezo and Piezo2 was compared in identically processed serial sections (
Figure 1a and
Figure 1i), the difference between the two in favor of Piezo1 can be clearly seen.
3.2. Localization of PIEZO1 and PIEZO2 in Endometriosis
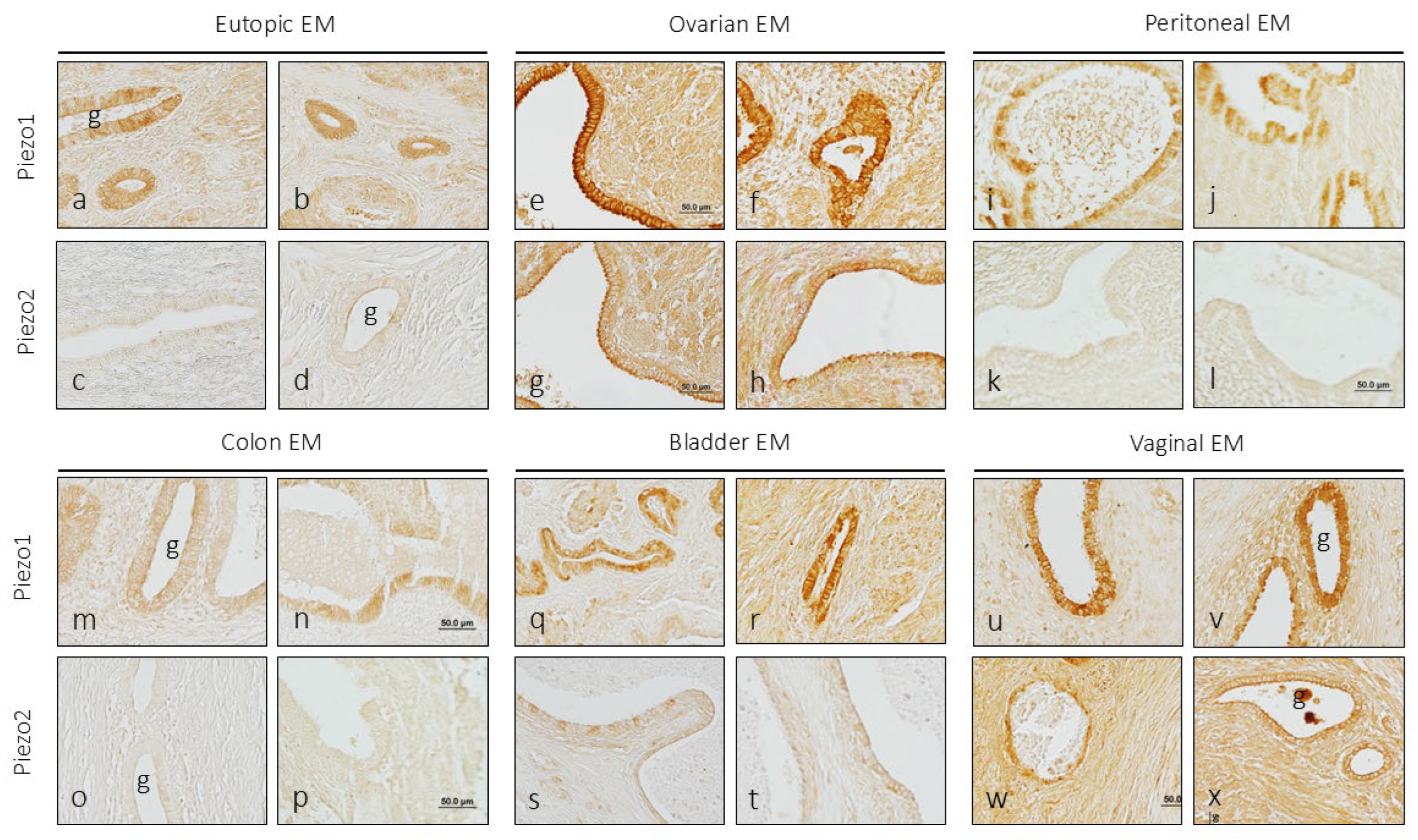
The endometriosis samples analyzed in this study came from endometriosis foci located in the uterus itself (eutopic endometriosis) or in other organs (ectopic endometriosis). It should be emphasized that the variations in the intensity of immunolabeling observed and reported here in endometriosis are always in relation to the epithelium of healthy endometrial tissue.
3.2.1. Eutopic Endometriosis
No differences with healthy endometrial tissue were found in eutopic endometriosis for the immunoreactivity of PIEZO1 or PIEZO2, nor in the number of glands marked, nor in the pattern or intensity of immunolabeling (
Figure 3a-d).
3.2.2. Ovarian Endometriosis
In the endometriotic foci placed in the ovary, there was an increase in the intensity of the immunostaining for Piezo1, and only at the level of the luminal pole of the epithelial cells for Piezo2 (
Figure 3e-h). In addition, immunoreaction of variable intensity for both mechanoproteins was detected in the stroma surrounding the glands.
3.2.3. Peritoneal Endometriosis
One of the most common locations of ectopic endometriosis is peritoneum. In the samples analyzed, there was no evidence of changes in the expression of the immunoreactivity for Piezo2, that is, it was not detected, and that for Piezo1 was higher than that of the control uterine glandular epithelium (
Figure 3i-l).
3.2.4. Intestinal Endometriosis
The levels of immunoreaction intensity for Piezo1 and Piezo2 in endometriosis foci in the colon were similar to those observed in the control endometrium (
Figure 3m-p).
3.2.5. Vesical Endometriosis
In the foci of endometriosis implanted in the urinary bladder, an increase in the intensity of the immunoreaction for Piezo1 was observed both in the glandular cells and in the stroma, especially in a case in which the foci were implanted in the thickness of the detrusor muscle. Regarding Piezo2, it was observed that some isolated epithelial cells had a moderate immunoreaction intensity the others being unreactive (
Figure 3q-t).
3.2.6. Vaginal Endometriosis
As in the case of the ovary, an increase in the intensity of the immunoreaction for Piezo1 and Piezo2 was observed in both the glandular epithelium and the stroma (
Figure 3u-x).
The results of the semi-quantitative study are shown in
Table 3 and have been carried out by specialists in the analysis of histological samples processed for the detection of antigens in human tissues fixed and embedded in paraffin. Although the pieces were processed identically, technical factors may have influenced the final result, and it is well known that in immunohistochemistry there is no stoichiometric relationship between antigen quantity and immunoreactivity intensity.
4. Discussion
Piezo1 and Piezo2 ion channels were originally described as mediators of mechanotransduction [
13] but is now accepted that they participate in multiple physiological and pathophysiological processes involving forces such as touch, proprioception, pain, angiogenesis, tissue remodeling, fibrosis, and inflammation [
14,
16,
39,
40,
41]. On the other hand, it is well known that dysregulation of Piezo1 function is associated with fibrotic disorders [
42], chronic pain syndromes and inflammatory diseases [
18], and cancer [
43]. Furthermore, mutations in the genes encoding PIEZO1 and PIEZO2 are responsible for multiple hereditary human diseases [
44]. Therefore the Piezo channels are linked to a wide range of diseases but as far as we know there is no data linking them to endometriosis.
In the present research, the expressions of Piezo1 and Piezo2 in normal endometrium and in eutopic and ectopic endometriosis has been studied using immunohistochemistry. Our results show that in normal healthy endometrium Piezo1 and Piezo2 are expressed in the epithelial cells of the glands, although the immunoreactivity for Piezo1 is much more potent than for Piezo2. Numerous studies using different techniques have shown the expression of both ion channels in epithelial cells, for example in the breast, skin, intestine, etc [
13,
45,
46,
47,
48]. Our results extends to the epithelium of the human uterus the expression of these channels, most notably Piezo1. We have also observed immunoreactivity for Piezo1 and Piezo2 in the smooth muscle cells of the blood vessels and in the myometrium, but always at lower levels than in the epithelial tissue. Previous studies already demonstrated the presence of Piezo1 mRNA in primary human endometrial epithelial and stromal cells. Moreover, consistently with previous research which observed low levels of Piezo2 mRNA in human and murine endometrial epithelial and stromal cells [
33] we observed weak or negative staining for Piezo2 in some endometrial glands. Additionally, we detected Piezo1 staining in human endometrial blood vessels, aligning with earlier findings of Piezo1 expression in rat uterine blood vessels [
34]. Mechanical forces influence endometrial cell behavior throughout the menstrual cycle, preparing the endometrium for embryo implantation [
49] acting through various types of mechanosensitive channels [
50].
In general, endometriosis produced slight to moderate increases in Piezo1 and no changes in Piezo2 expression. However, in the endometriosis foci found in the ovary and vagina the immunoreactivity for both ion channels increased markedly. Thus, the organs where the increase in the expression of Piezo1 and Piezo2 has been most evident were those with well-known hormonal regulation. As far as we know, this fact has never been described in endometriosis. However, if it is known that in other organs under hormonal control such as the breast that Piezo2 expression positively correlated with estrogen receptor (ER) and progesterone receptor [
51].
Endometriosis is a chronic inflammatory condition characterized by the presence of endometrial-like tissue within or outside the uterus, resulting in pelvic pain and infertility [
52]. In all the hallmarks of endometriosis, i.e., pain, fibrosis, inflammation, Piezo channels could participate [
16]. For example, frequently in endometriosis there is fibrotic tissue inside and around the glandular foci, especially in stages III and IV, which are the majority of our cases, and Piezo1 is related to fibrosis [
23]. In addition, endometriosis courses with inflammation and both Piezo1 and Piezo2 promotes pro-inflammatory signaling [
53,
54] and with inflammation-related pain states [
55,
56].
Understanding the roles of Piezo1 and Piezo2 in endometriosis could provide novel insights into the disease’s pathogenesis and identify new molecular targets for therapeutic intervention. Recent advances in Piezo channels pharmacology [
57,
58,
59] make this an especially timely and relevant avenue of research. Such studies could pave the way for more effective treatments that address the mechanobiological underpinnings of this debilitating condition, improving patient outcomes.
Various limitations to this study should be considered, especially the size of the sample, the organs where the foci of endometriosis are located and that only one study technique is used. We consider this work to be the basis for further studies using molecular biology techniques and a larger number of samples and locations to confirm the present data. However, the data still demonstrate a tendency for increased Piezo1 in endometriosis and point to a hormonal regulation of the expression of Piezo1 and Piezo2. Despite these limitations, our findings provide valuable evidence of the relationship between Piezo channels and the hallmarks of endometriosis.
5. Conclusions
As a whole, present results demonstrate for the first time the occurrence of Piezo1, and with less expression, the Piezo2 in healthy human endometrium, as well as an increase in Piezo1 in ectopic endometriosis, regardless of the anatomical location of the endometriosis foci. Piezo2 is only evidently increased in the foci of endometriosis of the ovary and vagina, which suggests a hormonal control of its expression.
Author Contributions
Conceptualization: O.G.-S. and J.A.-V.; methodology, A.S.-R., A.G.-P., P.C., E.V., G.M.-B. and Y.G.-M.; validation, O.G.-S., J.A.-V. and Y.G.-M.; formal analysis, A.G-P, Y.G.-M. and O.G.-S.; investigation, A.S.-R., A.G.-F., P.C., E.V., G.M.-B. and Y.G.-M.; resources, A.S.-R. and A.G.-F.; data curation, Y.G.-M.; writing—original draft preparation, writing—review and editing, O.G.-S. and J.A.-V.; visualization, Y.G.-M.; supervision, O.G.-S. and J.A.-V.; project administration, O.G.-S. and J.A.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. P.C. was supported by a grant, “Severo Ochoa Program,” from the Government of the Principality of Asturias (PA-21-PF-BP20-122).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod, CElm, Past: Proyecto 266/18, 19 November 2018).
Informed Consent Statement
The samples were obtained at the Pathology Department of the Álvarez Buylla Hospital, Mieres (Principality of Asturias, Spain). All materials used in the present study were obtained in accordance with Spanish legislation (RD 1301/2006; Law 14/2007; RD 1716/2011; Order ECC/1404/2013) and in compliance with the guidelines of the Declaration of Helsinki II. All patients provide written informed consent for the use of their pathological specimens for research purposes.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The data are anonymized and there are no ethical or contractual restrictions on its use. They are also not subject to intellectual property.
Acknowledgments
P.C was supported by a grant, “Severo Ochoa Program,” from the Government of the Principality of Asturias (PA-21-PF-BP20-122). The authors thank Marta Alonso Guervos for her technical assistance with confocal microscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- As-Sanie, S.; Black, R.; Giudice, L.C.; Gray Valbrun, T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef]
- As-Sanie S, Mackenzie SC, Morrison L, Schrepf A, Zondervan KT, Horne AW, Missmer SA. Endometriosis: A Review. JAMA 2025, 334, 64–78. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Bo, C.; Wang, Y. Angiogenesis signaling in endometriosis: Molecules, diagnosis and treatment (Review). Mol. Med. Rep. 2024, 29, 43. [Google Scholar] [CrossRef]
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef]
- Velho, R.V.; Taube, E.; Sehouli, J.; Mechsner, S. Neurogenic Inflammation in the Context of Endometriosis-What Do We Know? Int. J. Mol. Sci. 2021, 22, 13102. [Google Scholar] [CrossRef]
- Mariadas, H.; Chen, J.H.; Chen, K.H. The Molecular and Cellular Mechanisms of Endometriosis: From Basic Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 2458. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Rethinking the pathogenesis of endometriosis: Complex interactions of genomic, epigenetic, and environmental factors. J. Obstet. Gynaecol. Res. 2024, 50, 1771–1784. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Kou, L.; Huang, C.; Xiao, D.; Liao, S.; Li, Y.; Wang, Q. Pharmacologic Interventions for Endometriosis-Related Pain: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2025, 146, e23–e35. [Google Scholar] [CrossRef]
- Mick, I.; Freger, S.M.; van Keizerswaard, J.; Gholiof, M.; Leonardi, M. Comprehensive endometriosis care: a modern multimodal approach for the treatment of pelvic pain and endometriosis. Ther. Adv. Reprod. Health 2024, 18, 26334941241277759. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef]
- Delmas, P.; Parpaite, T.; Coste, B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron 2022, 110, 2713–2727. [Google Scholar] [CrossRef]
- Xiao, B. Mechanisms of mechanotransduction and physiological roles of PIEZO channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 886–903. [Google Scholar] [CrossRef]
- Sforna, L.; Michelucci, A.; Morena, F.; Argentati, C.; Franciolini, F.; Vassalli, M.; Martino, S.; Catacuzzeno, L. Piezo1 controls cell volume and migration by modulating swelling-activated chloride current through Ca2+ influx. J. Cell Physiol. 2022, 237, 1857–1870. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, C.; Zhuang, Y.; Zhong, A.; Wang, M.; Zhang, W.; Zhu, L. Mechanosensitive Piezo1 protein as a novel regulator in macrophages and macrophage-mediated inflammatory diseases. Front. Immunol. 2023, 14, 1149336. [Google Scholar] [CrossRef]
- Du, S.; Liu, K. Mechanosensitive ion channels and inflammation: key links in cellular signal transduction. Inflamm. Res. 2025, 74, 104. [Google Scholar] [CrossRef]
- Pirri, C. PIEZO Channels in Mechano-Inflammation: Gatekeepers of Neuroimmune Crosstalk. Diseases 2025, 13, 263. [Google Scholar] [CrossRef]
- Alibrandi, S.; Rinaldi, C.; Vinci, S.L.; Conti, A.; Donato, L.; Scimone, C.; Sidoti, A.; D’Angelo, R. Mechanotransduction in Development: A Focus on Angiogenesis. Biology (Basel) 2025, 14, 346. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Signal Transduct Target Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Cheng, X.; Hu, B.; Jiang, D.; Wu, L.; Peng, S.; Hu, J. Mechanotransductive receptor Piezo1 as a promising target in the treatment of fibrosis diseases. Front. Mol. Biosci. 2023, 10, 1270979. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Borkar, N.A.; Yao, Y.; Ye, X.; Vogel, E.R.; Pabelick, C.M.; Prakash, Y.S. Mechanosensitive channels in lung disease. Front. Physiol. 2023, 14, 1302631. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, M.; Smólski, J.; Grądalski, Ł.; Niemirka, S.; Młynarska, E.; Rysz, J.; Franczyk, B. Mechanosensitive Cation Channel Piezo1 Is Involved in Renal Fibrosis Induction. Int. J. Mol. Sci. 2024, 25, 1718. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, J.; Li, H. The Role of Mechanosensitive Piezo Channels in Chronic Pain. J. Pain Res. 2024, 17, 4199–4212. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Mei, S.; Hu, J.; Wu, L.; Xu, L.; Bao, L.; Fang, X. The mechanism and potential therapeutic target of piezo channels in pain. Front. Pain Res. (Lausanne) 2024, 5, 1452389. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Hang, L. The research progress into cellular mechanosensitive ion channels mediating cancer pain. Channels (Austin) 2025, 19, 2517109. [Google Scholar] [CrossRef]
- Burridge, K.; Monaghan-Benson, E.; Graham, D.M. Mechanotransduction: from the cell surface to the nucleus via RhoA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180229. [Google Scholar] [CrossRef]
- Martino, S. Mechanobiology in Cells and Tissues. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Lacroix, J.J.; Wijerathne, T.D. PIEZO channels as multimodal mechanotransducers. Biochem. Soc. Trans. 2025, 53, BST20240419. [Google Scholar] [CrossRef]
- Hennes, A.; Held, K.; Boretto, M.; De Clercq, K.; Van den Eynde, C.; Vanhie, A.; Van Ranst, N.; Benoit, M.; Luyten, C.; Peeraer, K.; Tomassetti, C.; Meuleman, C.; Voets, T.; Vankelecom, H.; Vriens, J. Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Sci. Rep. 2019, 9, 1779. [Google Scholar] [CrossRef]
- Arishe, O.O.; Ebeigbe, A.B.; Webb, R.C. Mechanotransduction and Uterine Blood Flow in Preeclampsia: The Role of Mechanosensing Piezo 1 Ion Channels. Am. J. Hypertens. 2020, 33, 1–9. [Google Scholar] [CrossRef]
- Toki, T.; Shimizu, M.; Takagi, Y.; Ashida, T.; Konishi, I. CD10 is a marker for normal and neoplastic endometrial stromal cells. Int. J. Gynecol. Pathol. 2002, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Potlog-Nahari, C.; Feldman, A.L.; Stratton, P.; Koziol, D.E.; Segars, J.; Merino, M.J.; Nieman, L.K. CD10 immunohistochemical staining enhances the histological detection of endometriosis. Fertil. Steril. 2004, 82, 86–92. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; Arnal, J.F.; Lenfant, F. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed]
- Coroleucă, C.A.; Coroleucă, C.B.; Coroleucă, R.; Brătilă, P.C.; Nodiți, A.R.; Roșca, I.; Brîndușe, L.A.; Brătilă, E.; Boț, M. Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. Int. J. Mol. Sci. 2025, 26, 2983. [Google Scholar] [CrossRef]
- Syeda, R. Physiology and Pathophysiology of Mechanically Activated PIEZO Channels. Annu Rev Neurosci. 2021, 44, 383–402. [Google Scholar] [CrossRef]
- Poole, K. The Diverse Physiological Functions of Mechanically Activated Ion Channels in Mammals. Annu. Rev. Physiol. 2022, 84, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Signal Transduct Target Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Xin, Liu.; Weipin, Niu.; Shuqing, Zhao.; Wenjuan, Zhang.; Ying, Zhao.; Jing, Li. Piezo1. the potential new therapeutic target for fibrotic disease. Progress in Biophysics and molecular Biology 2023, 184, 42–49. [Google Scholar] [CrossRef]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive Ion Channels and Their Role in Cancer Cells. Membranes (Basel). 2023, 13, 167. [Google Scholar] [CrossRef]
- Alper, S.L. Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction. Curr. Top. Membr. 2017, 79, 97–134. [Google Scholar] [CrossRef] [PubMed]
- Piddini, E. Epithelial Homeostasis: A Piezo of the Puzzle. Curr. Biol. 2017, 27, R232–R234. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Cuendias, P.; Alonso-Guervós, M.; García-Piqueras, J.; Martín-Biedma, B.; Cobo, T.; García-Suárez, O.; Vega, J.A. Immunohistochemical detection of PIEZO1 and PIEZO2 in human digital Meissner’s corpuscles. Ann. Anat. 2024, 252, 152200. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhou, J.; Xu, X.; Zhou, P.; Zhong, H.; Liu, M. Piezo channels in the intestinal tract. Front. Physiol. 2024, 15, 1356317. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.H.; Yoon, Y.B.; Jeong, G.H.; Park, S.C.; Lee, G.W.; Aryal, Y.P.; Kwak, H.J.; Cho, S.J. Unveiling salivary gland-specific gene expression of Piezo1 and Neuroendocrine in the leech, Helobdella austinensis. Dev. Comp. Immunol. 2025, 168, 105391. [Google Scholar] [CrossRef]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells. 2021, 10, 2008. [Google Scholar] [CrossRef]
- Davoodi, Nik. B.; Hashemi Karoii, D.; Favaedi, R.; Ramazanali, F.; Jahangiri, M.; Movaghar, B.; Shahhoseini, M. Differential expression of ion channel coding genes in the endometrium of women experiencing recurrent implantation failures. Sci. Rep. 2024, 14, 19822. [Google Scholar] [CrossRef]
- Lou, W.; Liu, J.; Ding, B.; Jin, L.; Xu, L.; Li, X.; Chen, J.; Fan, W. Five miRNAs-mediated PIEZO2 downregulation, accompanied with activation of Hedgehog signaling pathway, predicts poor prognosis of breast cancer. Aging (Albany NY). 2019, 11, 2628–2652. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hang, L. Mechanical gated ion channel Piezo1: Function, and role in macrophage inflammatory response. Innate Immun 2024, 30, 32–39. [Google Scholar] [CrossRef]
- Dubin, A.E.; Schmidt, M.; Mathur, J.; Petrus, M.J.; Xiao, B.; Coste, B.; Patapoutian, A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2012, 2, 511–517. [Google Scholar] [CrossRef]
- Szczot, M.; Liljencrantz, J.; Ghitani, N.; Barik, A.; Lam, R.; Thompson, J.H.; Bharucha-Goebel, D.; Saade, D.; Necaise, A.; Donkervoort, S.; Foley, A.R.; Gordon, T.; Case, L.; Bushnell, M.C.; Bönnemannn, C.G.; Chesler, A.T. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 2018, 10, eaat9892. [Google Scholar] [CrossRef]
- Romero, L.O.; Caires, R.; Nickolls, A.R.; Chesler, A.T.; Cordero-Morales, J.F.; Vásquez, V. A dietary fatty acid counteracts neuronal mechanical sensitization. Nat Commun. 2020, 11, 2997, Erratum in: Nat Commun. 2020, 11, 3938. [Google Scholar] [CrossRef]
- De Logu, F.; Geppetti, P. Ion Channel Pharmacology for Pain Modulation. Handb. Exp. Pharmacol. 2019, 260, 161–186. [Google Scholar] [CrossRef]
- Kinsella, J.A.; Debant, M.; Parsonage, G.; Morley, L.C.; Bajarwan, M.; Revill, C.; Foster, R.; Beech, D.J. Pharmacology of PIEZO1 channels. Br. J. Pharmacol. 2024, 181, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Thien, N.D.; Hai-Nam, N.; Anh, D.T.; Baecker, D. Piezo1 and its inhibitors: Overview and perspectives. Eur. J. Med. Chem. 2024, 273, 116502. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).