Submitted:
08 January 2025
Posted:
11 January 2025
You are already at the latest version
Abstract
Background/Objectives: Endometriosis has a marked impact on fertility, although the mechanisms behind this relationship remain poorly understood, particularly in cases without significant anatomical distortions or in the context of ovarian endometriomas. This study aimed to investigate the effect of peritoneal endometriosis on ovarian function by assessing ovarian reserve and apoptosis. Methods: Peritoneal endometriosis was surgically induced in Sprague Dawley rats through autotransplantation of uterine fragments onto the bowel mesothelium. One month post-surgery, ovarian structures were counted, follicle and corpora lutea apoptosis were evaluated by TUNEL and apoptotic-related protein expression in ovaries was assessed by western blot. Additionally, a co-culture system using 12Z endometriotic and KGN granulosa cell lines was utilized to evaluate gene expression by RT-qPCR. Results: Rats with peritoneal endometriosis exhibited a significant reduction in ovarian structures characterized by a low number of total follicles, particularly primordial, primary, preantral, and late antral follicles. Consistently, AMH protein expression was decreased in ovaries in the presence of endometriosis. Besides, this disease led to a significant increase in late antral follicles that were TUNEL-positive and in the number of apoptotic cells in corpora lutea, indicating higher apoptosis in endometriosis ovaries. Concomitantly, altered expression of apoptosis-related proteins was observed, with increased procaspase 3 and decreased BCL-2 expression. In addition, KGN granulosa cells co-cultured with 12Z endometriotic cells displayed reduced KITLG mRNA expression and increased AMHR2 mRNA expression. Conclusions: Peritoneal endometriosis significantly impairs ovarian health by disrupting folliculogenesis, reducing ovarian reserve, and increasing apoptosis, potentially accelerating ovarian aging and contributing to infertility. These results underscore the need for further research to identify the molecular pathways involved and to develop targeted therapeutic strategies.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Ovarian Morphology
2.3. Apoptosis Detection System
2.4. Western Blot
2.5. Co-Culture
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.7. Data Analysis
3. Results
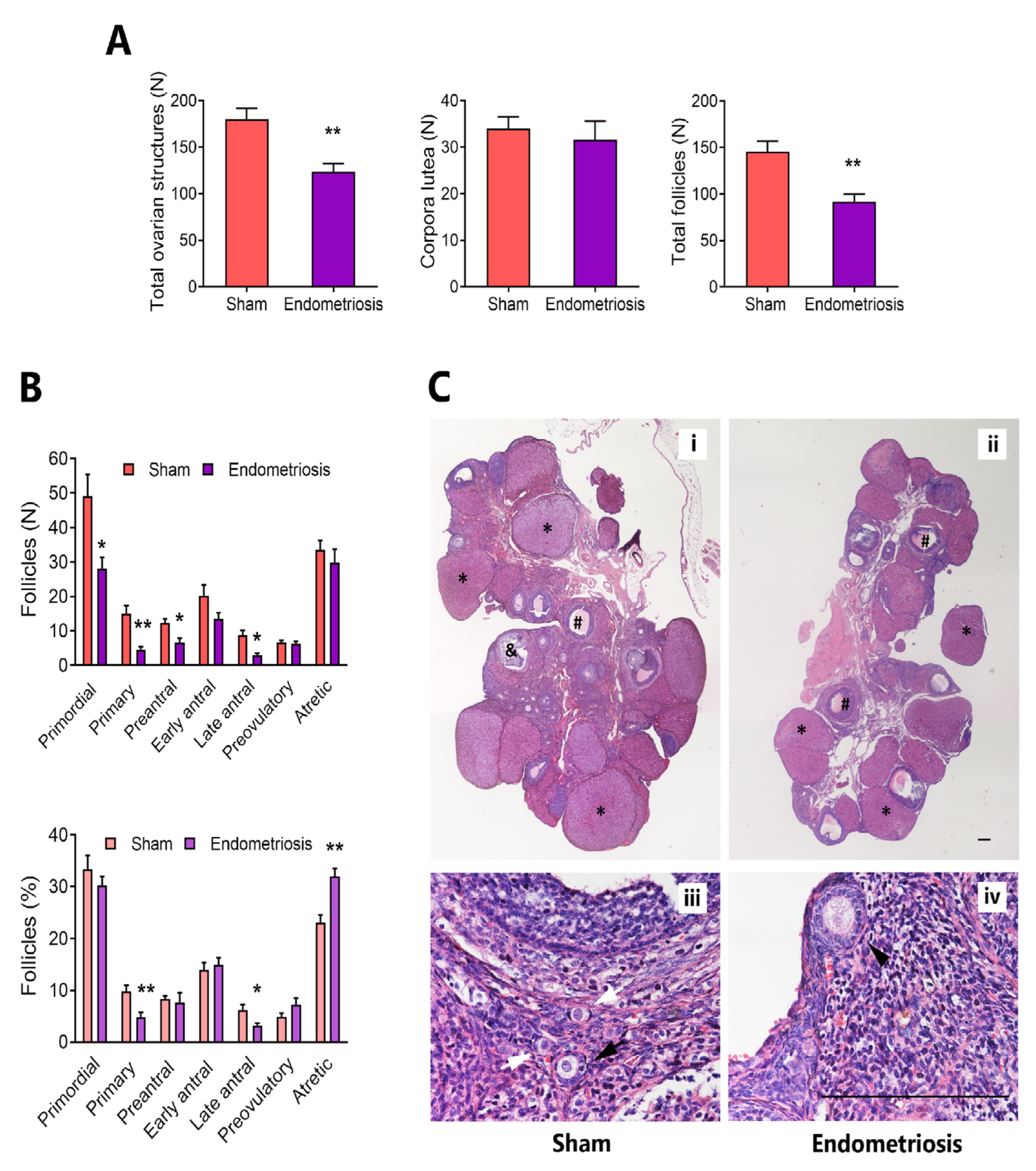
3.1. Endometriosis alters Folliculogenesis In Vivo
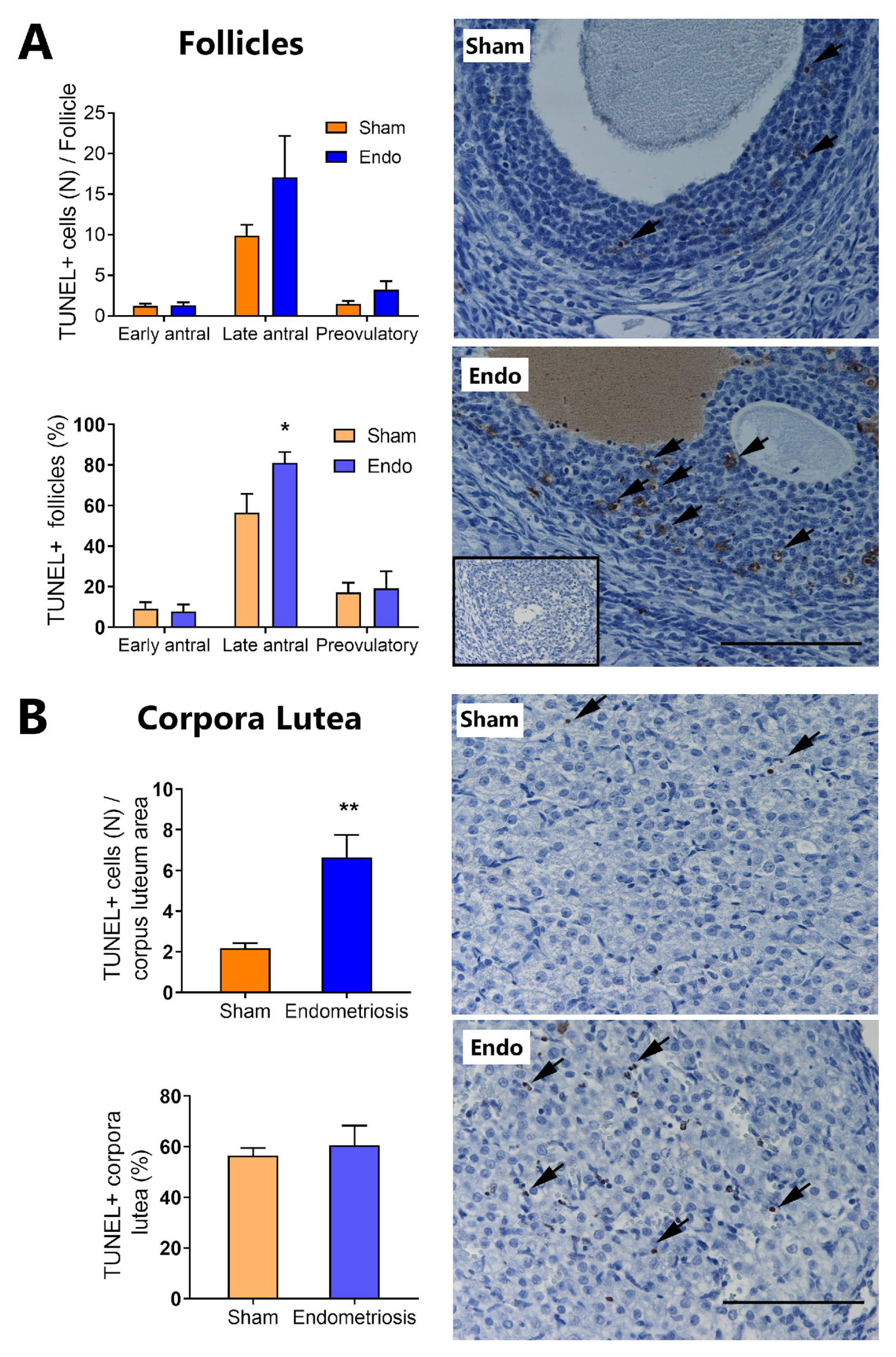
3.2. Endometriosis Increases Ovarian Apoptosis In Vivo
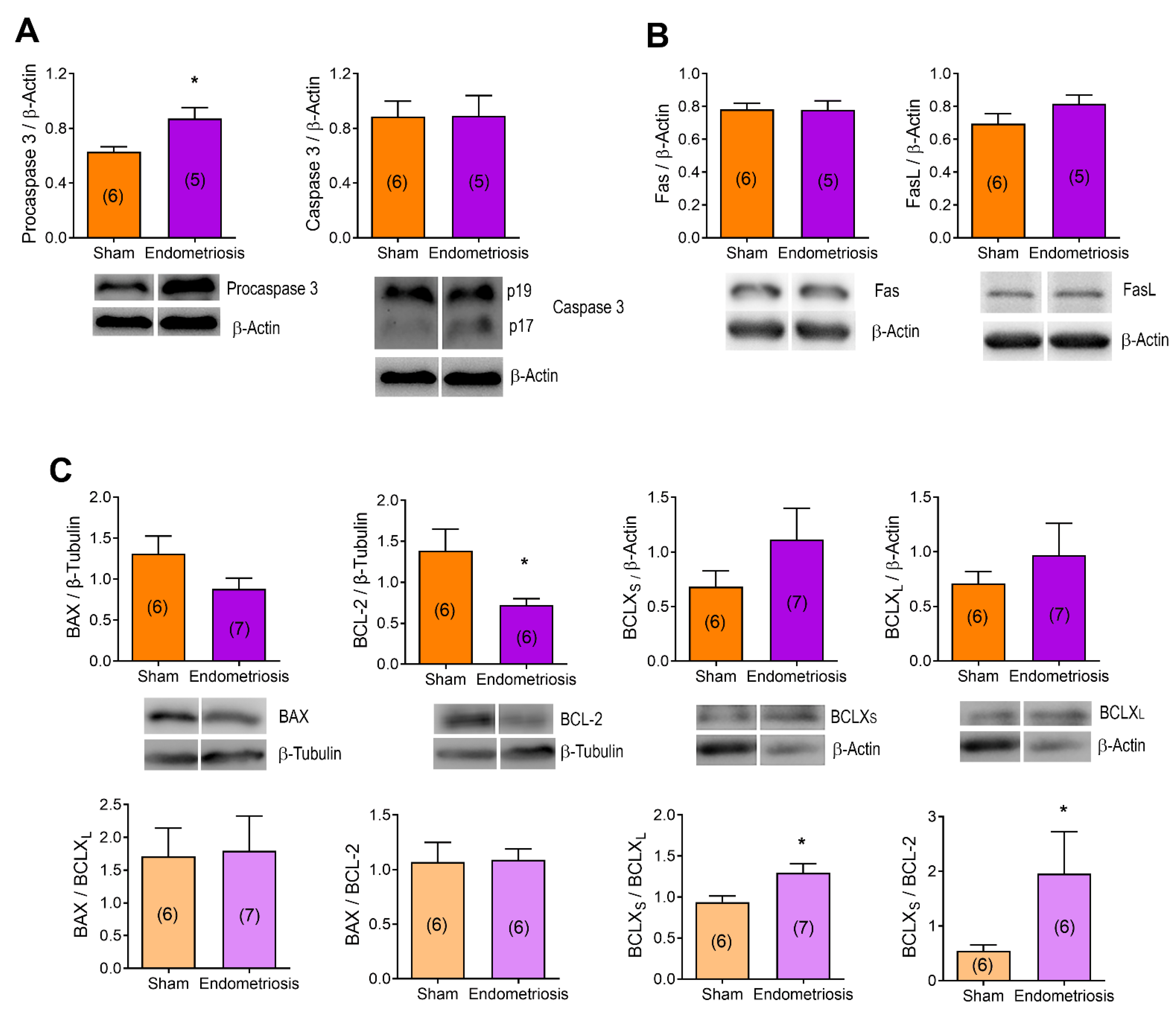
3.3. Endometriosis Alters the Expression of Proteins Related to Apoptosis in the Ovary In Vivo
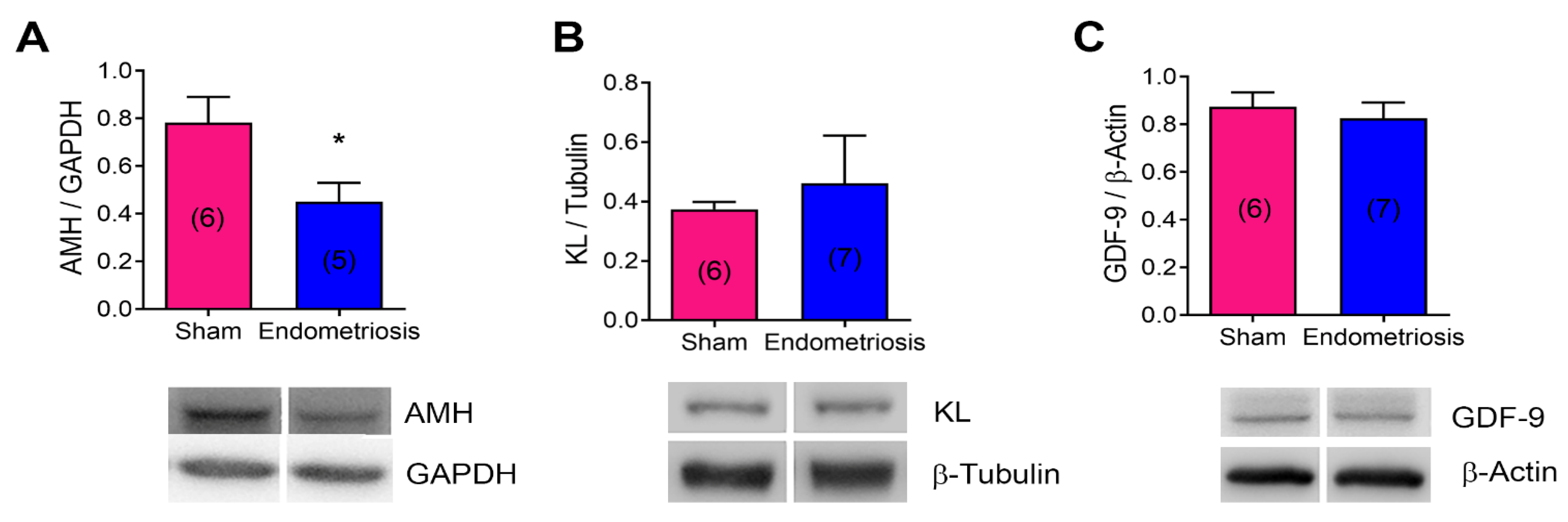
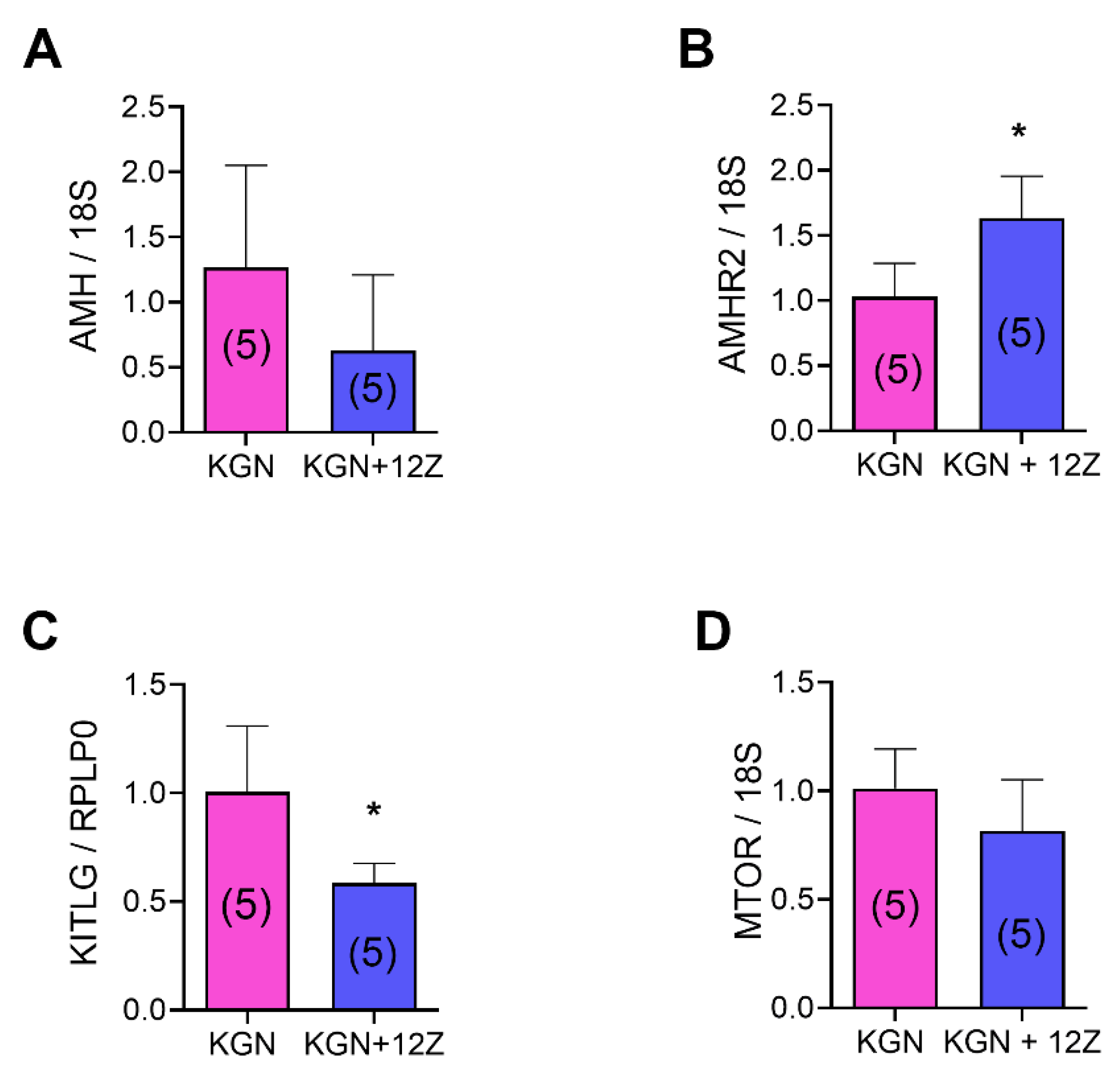
3.4. Endometriosis Alters the Expression of mRNA and Proteins Related to Folliculogenesis In Vivo and In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat Rev Dis Primers 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Requadt, E.; Nahlik, A.J.; Jacobsen, A.; Ross, W.T. Patient Experiences of Endometriosis Diagnosis: A Mixed Methods Approach. BJOG 2024, 131, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Bandini, V.; Buggio, L.; Berlanda, N.; Somigliana, E. Association of Endometriosis and Adenomyosis with Pregnancy and Infertility. Fertil Steril 2023, 119, 727–740. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking Mechanisms, Diagnosis and Management of Endometriosis. Nat Rev Endocrinol 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Pankhurst, M.W. Hyperactivation of Dormant Primordial Follicles in Ovarian Endometrioma Patients. Reproduction 2020, 160, R145–R153. [Google Scholar] [CrossRef]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. What Is the “Ovarian Reserve”? Fertil Steril 2015, 103, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The Physiology and Clinical Utility of Anti-Mullerian Hormone in Women. Hum Reprod Update 2014, 20, 370–385. [Google Scholar] [CrossRef]
- Jiang, D.; Nie, X. Effect of Endometrioma and Its Surgical Excision on Fertility (Review). Exp Ther Med 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Casalechi, M.; Di Stefano, G.; Fornelli, G.; Somigliana, E.; Viganò, P. Impact of Endometriosis on the Ovarian Follicles. Best Pract Res Clin Obstet Gynaecol 2024, 92, 102430. [Google Scholar] [CrossRef] [PubMed]
- Rangi, S.; Hur, C.; Richards, E.; Falcone, T. Fertility Preservation in Women with Endometriosis. J Clin Med 2023, 12, 1302–1310. [Google Scholar] [CrossRef]
- Pedachenko, N.; Anagnostis, P.; Shemelko, T.; Tukhtarian, R.; Alabbas, L. Serum Anti-Mullerian Hormone, Prolactin and Estradiol Concentrations in Infertile Women with Endometriosis. Gynecol Endocrinol 2021, 37, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Uncu, G. Impact of Endometriomas and Their Removal on Ovarian Reserve. Curr Opin Obstet Gynecol 2015, 27, 235–241. [Google Scholar] [CrossRef]
- Mc Cormack, B.A.; Olivares, C.N.; Madanes, D.; Ricci, A.G.; Bilotas, M.A.; Barañao, R.I. Effect of Urolithins A and B on Ectopic Endometrial Growth in a Murine Model of Endometriosis. Food Funct 2021, 12, 9894–9903. [Google Scholar] [CrossRef]
- Woodruff, T.K.; Lyon, R.J.; Hansen, S.E.; Rice, G.C.; Mather, J.P. Inhibin and Activin Locally Regulate Rat Ovarian Folliculogenesis. Endocrinology 1990, 127, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Irusta, G.; Abramovich, D.; Tesone, M.; Parborell, F. Administration of a Gonadotropin-Releasing Hormone Agonist Affects Corpus Luteum Vascular Stability and Development and Induces Luteal Apoptosis in a Rat Model of Ovarian Hyperstimulation Syndrome. Mol Cell Endocrinol 2011, 335, 116–125. [Google Scholar] [CrossRef]
- MM, B. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an Invasive, N-Cadherin-Expressing Epithelial Cell Type in Endometriosis Using a New Cell Culture Model. Am J Pathol 2001, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res 2001, 29, E45. [Google Scholar] [CrossRef]
- Hsueh, A.J.W.; Billig, H.; Tsafriri, A. Ovarian Follicle Atresia: A Hormonally Controlled Apoptotic Process*. Endocr Rev 1994, 15, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, S.L.; Chen, X.; He, Y.X.; Ye, D.S.; Guo, W.; Zheng, H.Y.; Yang, X.H. Ovarian Damage after Laparoscopic Endometrioma Excision Might Be Related to the Size of Cyst. Fertil Steril 2013, 100, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Torng, P.-L. Clinical Implication for Endometriosis Associated with Ovarian Cancer. Gynecol Minim Invasive Ther 2017, 6, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, V.; Rossi, G.; Di Luigi, G.; Palumbo, P.; D’Alfonso, A.; Iorio, R.; Cecconi, S. Increased Levels of Proapoptotic Markers in Normal Ovarian Cortex Surrounding Small Endometriotic Cysts. Reprod Biol 2019, 19, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.-M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced Follicular Recruitment and Atresia in Cortex Derived from Ovaries with Endometriomas. Fertil Steril 2014, 101, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J 2017, 18, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front Pharmacol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The Role of Oxidative Stress in Ovarian Aging: A Review. J Ovarian Res 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Hasegawa, Y.; Tsukui, Y.; Kobayashi, M.; Hiraishi, H.; Nakazato, T.; Kitahara, Y. Anti-Müllerian Hormone beyond an Ovarian Reserve Marker: The Relationship with the Physiology and Pathology in the Life-Long Follicle Development. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Durlinger, A.; Visser, J.; Themmen, A. Regulation of Ovarian Function: The Role of Anti-Mullerian Hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K. Cellular and Molecular Regulation of the Activation of Mammalian Primordial Follicles: Somatic Cells Initiate Follicle Activation in Adulthood. Hum Reprod Update 2015, 21, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Matsumoto, K.; Murakami, N.; Kajimura, I.; Harada, A.; Kitajima, Y.; Masuzaki, H.; Miura, K. AMH Concentrations in Peritoneal Fluids of Women With and Without Endometriosis. Front Surg 2020, 7. [Google Scholar] [CrossRef]
- Pais, A.S.; Flagothier, C.; Tebache, L.; Almeida Santos, T.; Nisolle, M. Impact of Surgical Management of Endometrioma on AMH Levels and Pregnancy Rates: A Review of Recent Literature. J Clin Med 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Shapso, N.; Ben-Sira, Y.; Nelson, S.M.; Izhaki, I. Endometrioma Surgery-a Systematic Review and Meta-Analysis of the Effect on Antral Follicle Count and Anti-Müllerian Hormone. Am J Obstet Gynecol 2021. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.C.; Sabry, R.; Mastromonaco, G.F.; Favetta, L.A. BPA and BPS Affect the Expression of Anti-Mullerian Hormone (AMH) and Its Receptor during Bovine Oocyte Maturation and Early Embryo Development. Reproductive Biology and Endocrinology 2021, 19, 119. [Google Scholar] [CrossRef]
- Thomas, F.H.; Vanderhyden, B.C. Oocyte-Granulosa Cell Interactions during Mouse Follicular Development: Regulation of Kit Ligand Expression and Its Role in Oocyte Growth. Reproductive Biology and Endocrinology 2006, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K. Cellular and Molecular Regulation of the Activation of Mammalian Primordial Follicles: Somatic Cells Initiate Follicle Activation in Adulthood. Hum Reprod Update 2015, 21, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Yoshino, O.; Wada-hiraike, O.; Fujii, T.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. 2019. [CrossRef]
- Jin, X.; Han, C.; Yu, F.; Wei, P.; Hu, Z.; Liu, Y. Anti-apoptotic Action of Stem Cell Factor on Oocytes in Primordial Follicles and Its Signal Transduction. Mol Reprod Dev 2005, 70, 82–90. [Google Scholar] [CrossRef]
- Panwar, D.; Rawal, L.; Ali, S. The Potential Role of the KFG and KITLG Proteins in Preventing Granulosa Cell Apoptosis in Bubalus Bubalis. Journal of Genetic Engineering and Biotechnology 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; So, W.; Dong, R.; Abazarikia, A.; Kim, S.Y. KIT in Oocytes: A Key Factor for Oocyte Survival and Reproductive Lifespan. EBioMedicine 2024, 106. [Google Scholar] [CrossRef]
- Park, M.J.; Ahn, J.-W.; Kim, K.H.; Bang, J.; Kim, S.C.; Jeong, J.Y.; Choi, Y.E.; Kim, C.-W.; Joo, B.S. Prediction of Ovarian Aging Using Ovarian Expression of BMP15, GDF9, and C-KIT. Exp Biol Med 2020, 245, 711–719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




