Submitted:
11 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
The molecular mechanisms through which endometriosis-related ovarian neoplasms (ERONs) develop from benign endometrioma remain unclear. It is especially a long-standing mystery why ovarian endometrioma has the potential to develop into two representative histological subtypes: endometrioid ovarian carcinoma or clear cell ovarian carcinoma. The present study aimed to investigate the molecular carcinogenesis of ERONs using newly developed in vitro and in vivo carcinogenesis models. Epithelial cells were isolated and purified from surgically removed benign endometrioma samples, followed by immortalization by overexpressing cyclinD1/CDK4 in combination with the human TERT gene. Immortalized cells were subjected to various molecular manipulations by combining knockout or overexpression of several candidate drivers, including ARID1A, KRAS, PIK3CA, AKT, and MYC, based on previous comprehensive genome-wide studies of ERONs. These cells were then inoculated into immunocompromised mice and evaluated for malignant transformation. Inoculated cells harboring a combination of three genetic alterations successfully de-veloped tumors with malignant features in mice, whereas those with two genetic mutations failed to do so. Especially, ARID1A gene knockout combined with overexpressing the KRAS oncogenic mutant allele (or overexpressing AKT) and c-Myc overexpression led to efficient tumor formation. Of note, these three combinations of genetic alterations produced tumors that histologically represented typical clear cell carcinoma in SCID mice, while the same combination led to tumors with endometrioid histology in nude mice. A combination of ARID1A mutation, KRAS mutation or AKT activation, and c-Myc overexpression were confirmed to be the main candidate drivers for the development of ERONs, as suggested by comprehensive genetic analyses of ERONs. A tumor immune microenvironment involving B cell signaling may contribute to the diverse histological phenotypes. The present model may help to clarify the molecular mechanisms of ERON carcinogenesis and understand their histological diversity and novel molecular targets.
Keywords:
1. Introduction
2. Results
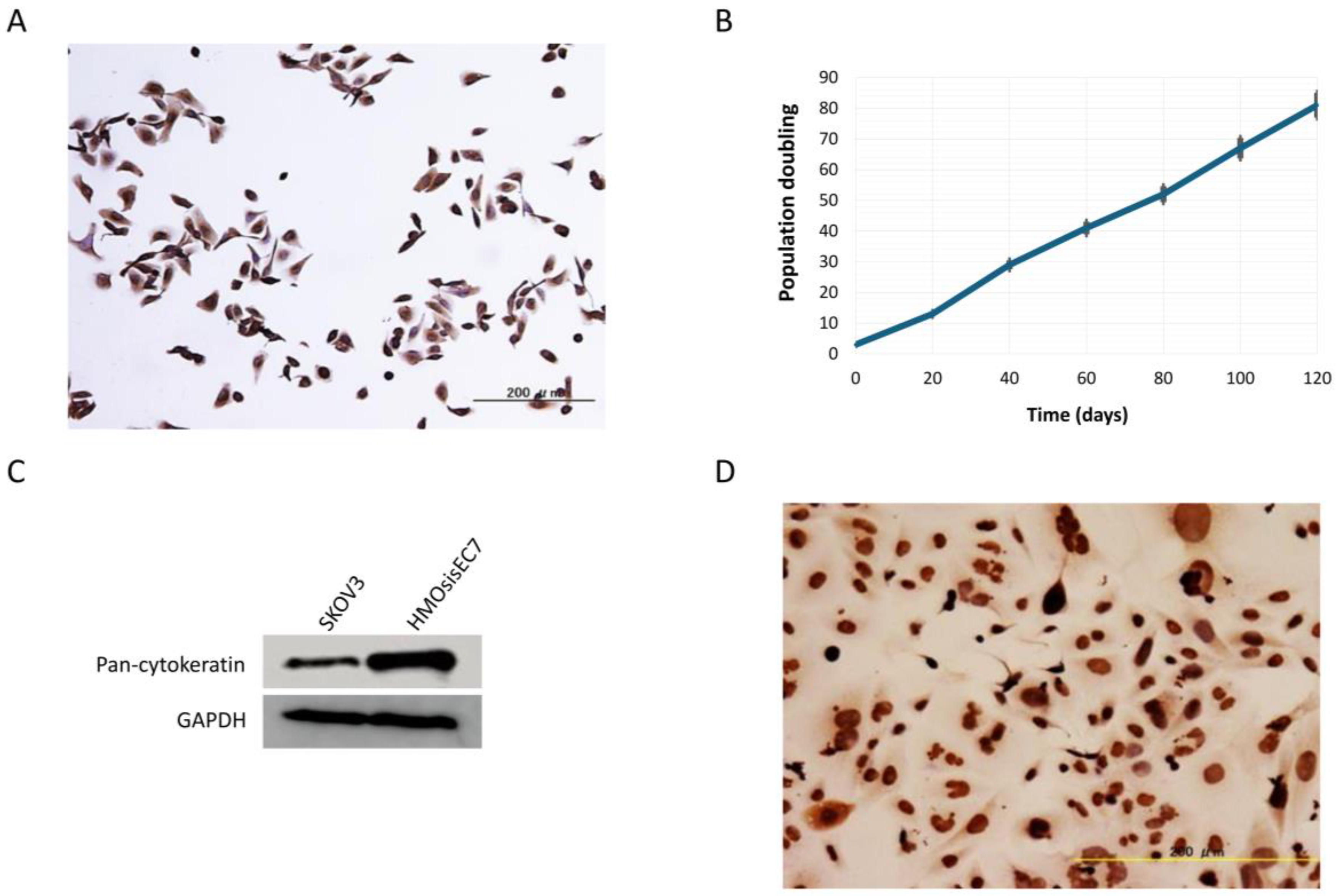
2.1. Immortalization of Primary Endometriotic Epithelial Cells
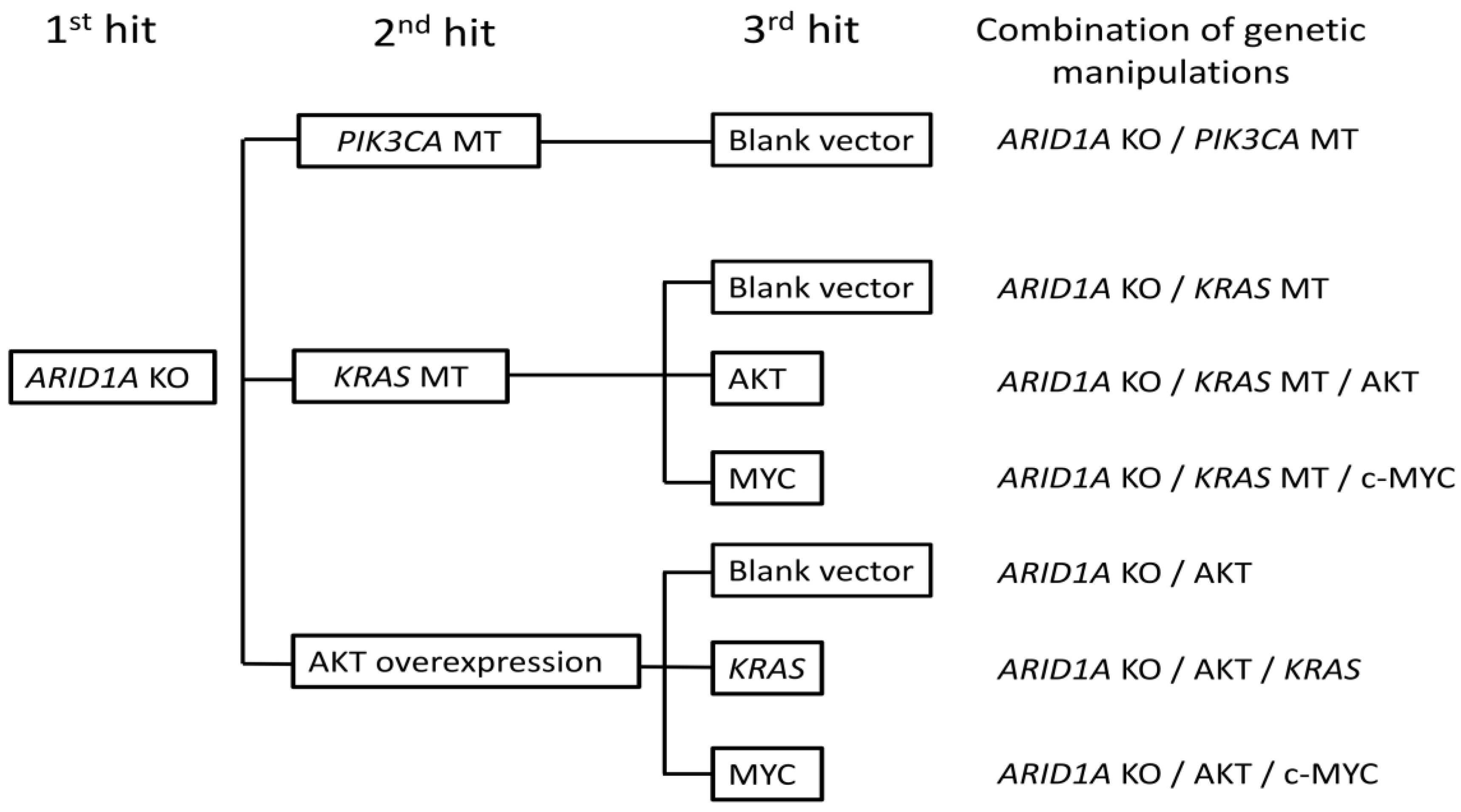
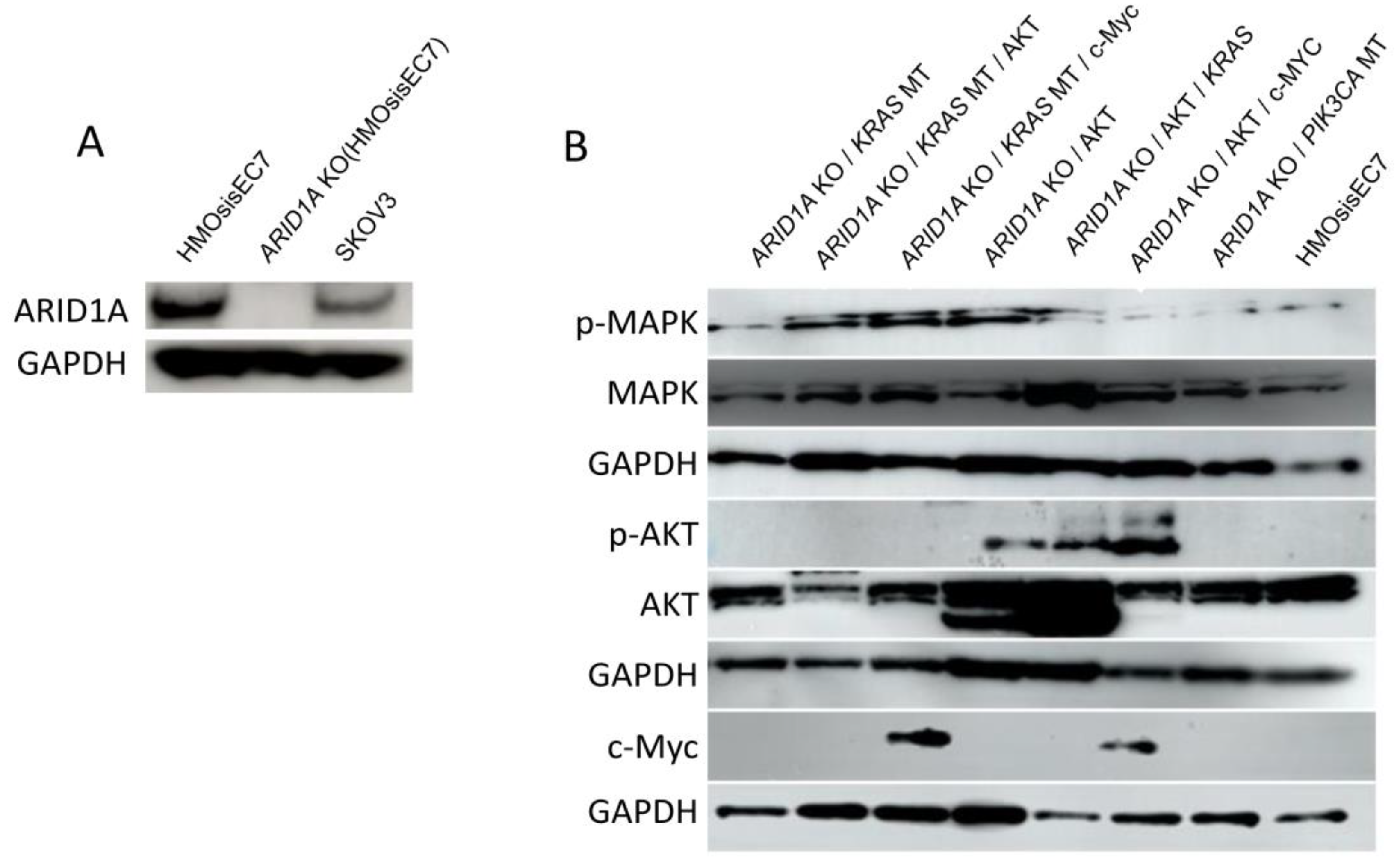
2.2. Genetic Manipulation of Immortalized Endometriotic Epithelial Cells
2.3. The Effects of Genetic Alterations on the Growth Property of Immortalized Endometriotic Epithelial Cells
2.4. The Effects of Genetic Alterations on the Tumor Forming Ability in Mice
3. Discussion
4. Materials and Methods
4.1. Isolation and Primary Culture of Human Endometriotic Epithelial Cells
4.2. Whole-Exome Sequencing of Ovarian Endometriotic Tissues
4.3. Immortalization of Endometriotic Epithelial Cells
4.4. Genetic Manipulations of Immortalized HMOsisEC7 Cells
4.5. Analysis of Population Doubling
4.6. Analysis of Population Doubling
4.7. Western Blot Analysis
4.8. Cell Viability Assay
4.9. The Scratch-Wound Healing Assay
4.10. In Vitro Matrigel Invasion Assay
4.11. The Clonogenic Assay
4.12. Xenograft in Mice
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERON | Endometriosis-related ovarian neoplasm |
| OCCC | Ovarian clear cell carcinoma |
| OEC | Ovarian endometrioid carcinoma |
| KO | Knock out |
| KRAS | Kirsten rat sarcoma virus |
| ARID1A | AT-rich interaction domain 1A |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| c-Myc | Cellular myelocytomatosis oncogene |
| AKT | Ak strain transforming |
| TIME | Tumor immune microenvironment |
| MT | Mutation |
References
- Eskenazi, B.; Warner, M.L. Epidemiology of Endometriosis. Obstet Gynecol Clin North Am 1997, 24, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Yamaguchi, K.; Matsumura, N.; Baba, T.; Konishi, I. Ovarian Cancer in Endometriosis: Molecular Biology, Pathology, and Clinical Management. Int J Clin Oncol 2009, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between Endometriosis and Risk of Histological Subtypes of Ovarian Cancer: A Pooled Analysis of Case-Control Studies. Lancet Oncol 2012, 13, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.; Shih, I.-M. Pathogenesis and the Role of ARID1A Mutation in Endometriosis-Related Ovarian Neoplasms. Adv Anat Pathol 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Mandai, M.; Suzuki, A.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Hamanishi, J.; Yoshioka, Y.; Kosaka, K.; Konishi, I. Clinical Management of Ovarian Endometriotic Cyst (Chocolate Cyst): Diagnosis, Medical Treatment, and Minimally Invasive Surgery. Curr Obstet Gynecol Rep 2012, 1, 16–24. [Google Scholar] [CrossRef]
- Kuhn, E.; Kurman, R.J.; Shih, I.-M. Ovarian Cancer Is an Imported Disease: Fact or Fiction? Curr Obstet Gynecol Rep 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sumimoto, K.; Kitanaka, T.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; et al. Ovarian Endometrioma--Risks Factors of Ovarian Cancer Development. Eur J Obstet Gynecol Reprod Biol 2008, 138, 187–193. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol 2016, 186, 733–747. [Google Scholar] [CrossRef]
- Shibuya, Y.; Tokunaga, H.; Saito, S.; Shimokawa, K.; Katsuoka, F.; Bin, L.; Kojima, K.; Nagasaki, M.; Yamamoto, M.; Yaegashi, N.; et al. Identification of Somatic Genetic Alterations in Ovarian Clear Cell Carcinoma with next Generation Sequencing. Genes Chromosomes Cancer 2018, 57, 51–60. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Noske, A.; Dedes, K.J.; Fink, D.; Imesch, P. ARID1A Mutations and PI3K/AKT Pathway Alterations in Endometriosis and Endometriosis-Associated Ovarian Carcinomas. Int J Mol Sci 2013, 14, 18824–18849. [Google Scholar] [CrossRef]
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to Immunotherapy of Ovarian Clear Cell Carcinoma: Unique Opportunities for Management. Gynecol Oncol 2018, 151, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N Engl J Med 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Mao, T.-L.; Seckin, T.; Wu, C.-H.; Guan, B.; Ogawa, H.; Futagami, M.; Mizukami, H.; Yokoyama, Y.; Kurman, R.J.; et al. Loss of ARID1A Expression Is an Early Molecular Event in Tumor Progression from Ovarian Endometriotic Cyst to Clear Cell and Endometrioid Carcinoma. Int J Gynecol Cancer 2012, 22, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.; Barbosa, C.P.; Trevisan, C.M.; Laganà, A.S.; Montagna, E. Endometrial Cancer: A Genetic Point of View. Transl Cancer Res 2020, 9, 7706–7715. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Ono, R.; Sanuki, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; et al. Affinity-Purified DNA-Based Mutation Profiles of Endometriosis-Related Ovarian Neoplasms in Japanese Patients. Oncotarget 2018, 9, 14754–14763. [Google Scholar] [CrossRef]
- Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [CrossRef]
- Bamias, A.; Psaltopoulou, T.; Sotiropoulou, M.; Haidopoulos, D.; Lianos, E.; Bournakis, E.; Papadimitriou, C.; Rodolakis, A.; Vlahos, G.; Dimopoulos, M.A. Mucinous but Not Clear Cell Histology Is Associated with Inferior Survival in Patients with Advanced Stage Ovarian Carcinoma Treated with Platinum-Paclitaxel Chemotherapy. Cancer 2010, 116, 1462–1468. [Google Scholar] [CrossRef]
- Itamochi, H.; Kigawa, J.; Sultana, H.; Iba, T.; Akeshima, R.; Kamazawa, S.; Kanamori, Y.; Terakawa, N. Sensitivity to Anticancer Agents and Resistance Mechanisms in Clear Cell Carcinoma of the Ovary. Jpn J Cancer Res 2002, 93, 723–728. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical Characteristics of Clear Cell Carcinoma of the Ovary: A Distinct Histologic Type with Poor Prognosis and Resistance to Platinum-Based Chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Winter, W.E.; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P.; et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J Clin Oncol 2007, 25, 3621–3627. [Google Scholar] [CrossRef]
- Soovares, P.; Pasanen, A.; Bützow, R.; Lassus, H. L1CAM Expression Associates with Poor Outcome in Endometrioid, but Not in Clear Cell Ovarian Carcinoma. Gynecol Oncol 2017, 146, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Nakayama, K.; Shanta, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Sato, S.; Iida, K.; Kanno, K.; et al. Establishment of a Novel In Vitro Model of Endometriosis with Oncogenic KRAS and PIK3CA Mutations for Understanding the Underlying Biology and Molecular Pathogenesis. Cancers (Basel) 2021, 13, 3174. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, K.; Ishikawa, N.; Ishikawa, M.; Sultana, R.; Kiyono, T.; Kyo, S. Reconstitution of High-Grade Serous Ovarian Carcinoma from Primary Fallopian Tube Secretory Epithelial Cells. Oncotarget 2018, 9, 12609–12619. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Kiyono, T.; Okamura, K.; Uezumi, M.; Goto, Y.; Yasumoto, S.; Shimizu, S.; Hashimoto, N. CDK4 and Cyclin D1 Allow Human Myogenic Cells to Recapture Growth Property without Compromising Differentiation Potential. Gene Ther 2011, 18, 857–866. [Google Scholar] [CrossRef]
- Karst, A.M.; Drapkin, R. Primary Culture and Immortalization of Human Fallopian Tube Secretory Epithelial Cells. Nat Protoc 2012, 7, 1755–1764. [Google Scholar] [CrossRef]
- Maeda, T.; Tashiro, H.; Katabuchi, H.; Begum, M.; Ohtake, H.; Kiyono, T.; Okamura, H. Establishment of an Immortalised Human Ovarian Surface Epithelial Cell Line without Chromosomal Instability. Br J Cancer 2005, 93, 116–123. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of ARID1A Protein Expression Occurs as an Early Event in Ovarian Clear-Cell Carcinoma Development and Frequently Coexists with PIK3CA Mutations. Mod Pathol 2012, 25, 615–624. [Google Scholar] [CrossRef]
- Yachida, N.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Ueda, H.; Sugino, K.; Yamaguchi, M.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. ARID1A Protein Expression Is Retained in Ovarian Endometriosis with ARID1A Loss-of-Function Mutations: Implication for the Two-Hit Hypothesis. Sci Rep 2020, 10, 14260. [Google Scholar] [CrossRef]
- Mao, T.-L.; Ardighieri, L.; Ayhan, A.; Kuo, K.-T.; Wu, C.-H.; Wang, T.-L.; Shih, I.-M. Loss of ARID1A Expression Correlates with Stages of Tumor Progression in Uterine Endometrioid Carcinoma. Am J Surg Pathol 2013, 37, 1342–1348. [Google Scholar] [CrossRef]
- Engelman, J.A.; Chen, L.; Tan, X.; Crosby, K.; Guimaraes, A.R.; Upadhyay, R.; Maira, M.; McNamara, K.; Perera, S.A.; Song, Y.; et al. Effective Use of PI3K and MEK Inhibitors to Treat Mutant Kras G12D and PIK3CA H1047R Murine Lung Cancers. Nat Med 2008, 14, 1351–1356. [Google Scholar] [CrossRef]
- Sugino, K.; Tamura, R.; Nakaoka, H.; Yachida, N.; Yamaguchi, M.; Mori, Y.; Yamawaki, K.; Suda, K.; Ishiguro, T.; Adachi, S.; et al. Germline and Somatic Mutations of Homologous Recombination-Associated Genes in Japanese Ovarian Cancer Patients. Sci Rep 2019, 9, 17808. [Google Scholar] [CrossRef] [PubMed]
- Lal, N.; White, B.S.; Goussous, G.; Pickles, O.; Mason, M.J.; Beggs, A.D.; Taniere, P.; Willcox, B.E.; Guinney, J.; Middleton, G.W. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res 2018, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.M.J.; Berg, A.; Wik, E.; Birkeland, E.; Krakstad, C.; Kusonmano, K.; Petersen, K.; Kalland, K.H.; Oyan, A.M.; Akslen, L.A.; et al. ARID1A Loss Is Prevalent in Endometrial Hyperplasia with Atypia and Low-Grade Endometrioid Carcinomas. Mod Pathol 2013, 26, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.-L.; Shih, I.-M.; Mao, T.-L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.; Vogelstein, B.; et al. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef]
- Dey, P.; Nakayama, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Kanno, K.; Sato, S.; Kiyono, T.; Kyo, S. Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells. Cancers 2022, 14, 1506. [Google Scholar] [CrossRef]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only Three Driver Gene Mutations Are Required for the Development of Lung and Colorectal Cancers. Proc Natl Acad Sci U S A 2015, 112, 118–123. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The Path to Cancer --Three Strikes and You’re Out. N Engl J Med 2015, 373, 1895–1898. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Tessier-Cloutier, B.; Lawrence, K.M.; Nazeran, T.; Karnezis, A.N.; Salamanca, C.; Cheng, A.S.; McAlpine, J.N.; Hoang, L.N.; Gilks, C.B.; et al. Clear Cell and Endometrioid Carcinomas: Are Their Differences Attributable to Distinct Cells of Origin? J Pathol 2017, 243, 26–36. [Google Scholar] [CrossRef]
- Kolin, D.L.; Dinulescu, D.M.; Crum, C.P. Origin of Clear Cell Carcinoma: Nature or Nurture? J Pathol 2018, 244, 131–134. [Google Scholar] [CrossRef]
- Beddows, I.; Fan, H.; Heinze, K.; Johnson, B.K.; Leonova, A.; Senz, J.; Djirackor, S.; Cho, K.R.; Pearce, C.L.; Huntsman, D.G.; et al. Cell State of Origin Impacts Development of Distinct Endometriosis-Related Ovarian Carcinoma Histotypes. Cancer Res 2024, 84, 26–38. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, L.; Liu, L. Reprogramming the Lipid Metabolism of Dendritic Cells in Tumor Immunomodulation and Immunotherapy. Biomed Pharmacother 2023, 167, 115574. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.-W.; Sun, C.-M.; Roumenina, L.T.; Sautès-Fridman, C. B Cells and Cancer: To B or Not to B? J Exp Med 2021, 218, e20200851. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Kanno, K.; Ishibashi, T.; Ishikawa, M.; Iida, K.; Razia, S.; Kiyono, T.; Kyo, S. Evaluation of ARID1A as a Potential Biomarker for Predicting Response to Immune Checkpoint Inhibitors in Patients with Endometrial Cancer. Cancers 2024, 16, 1999. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK Inhibitor and Feeder Cells Induce the Conditional Reprogramming of Epithelial Cells. Am J Pathol 2012, 180, 599–607. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Fukuda, T.; Yang, L.; Zaha, H.; Akanuma, H.; Zeng, Q.; Yoshinaga, J.; Sone, H. Effects of Bisphenol A Exposure on the Proliferation and Senescence of Normal Human Mammary Epithelial Cells. Cancer Biol Ther 2012, 13, 296–306. [Google Scholar] [CrossRef]
- Nakayama, K.; Miyazaki, K.; Kanzaki, A.; Fukumoto, M.; Takebayashi, Y. Expression and Cisplatin Sensitivity of Copper-Transporting P-Type Adenosine Triphosphatase (ATP7B) in Human Solid Carcinoma Cell Lines. Oncol Rep 2001. [Google Scholar] [CrossRef]
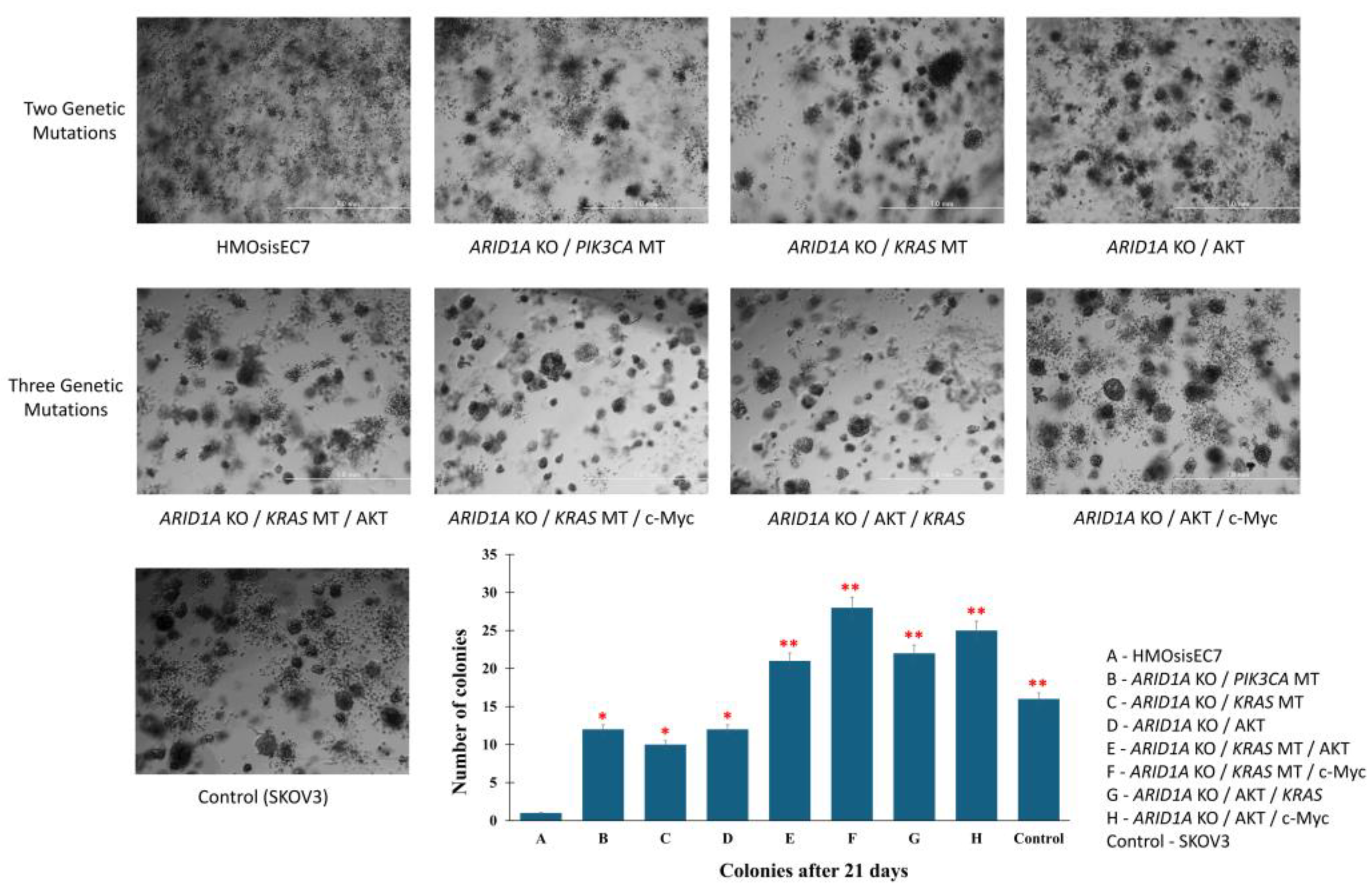
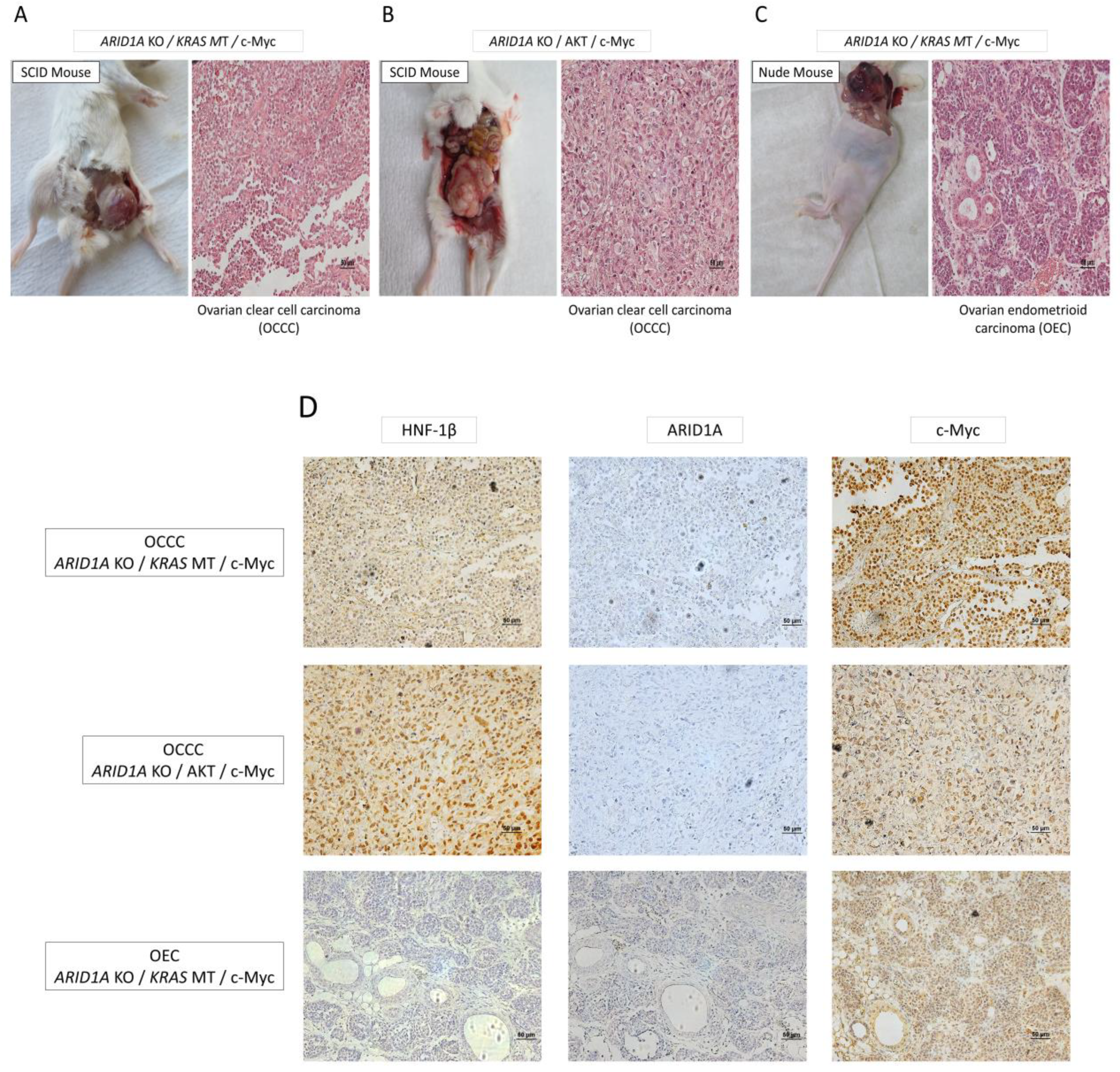
| Mutant Type | Result (Mice with tumor formation/inoculated mice) |
Remarks (Histology) |
|---|---|---|
| Wild type (HMOsisEC7) | 0/5 | |
| ARID1A KO / PIK3CA MT | 0/5 | |
| ARID1A KO / KRAS MT | 0/5 | |
| ARID1A KO / KRAS MT / AKT | 2/5 | No malignancy |
| ARID1A KO / KRAS MT / c-Myc | 4/5 | OCCC |
| ARID1A KO / AKT | 0/5 | |
| ARID1A KO / AKT / KRAS | 2/5 | No malignancy |
| ARID1A KO / AKT / c-Myc | 3/5 | OCCC |
| Mutant Type | Result (Mice with tumor formation/inoculated mice) |
Remarks (Histology) |
|---|---|---|
| Wild type (HOMsisEC7) | 0/5 | |
| ARID1A KO / PIK3CA MT | 0/5 | |
| ARID1A KO / KRAS MT | 0/5 | |
| ARID1A KO / KRAS MT / AKT | 2/5 | No malignancy |
| ARID1A KO / KRAS MT / c-Myc | 2/5 | OEC |
| ARID1A KO / AKT | 0/5 | |
| ARID1A KO / AKT / KRAS | 2/5 | No malignancy |
| ARID1A KO / AKT / c-Myc | 1/5 | No malignancy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





