1. Introduction
In Asia, OCCC accounts for 20-25% of epithelial ovarian cancer (EOC) and unlike western countries where it comprises only 5-10% of cases, it represents the second or third most common histology of EOC [
1]. Although OCCC usually presents at early stages with better survival compared to early-stage high-grade serous carcinoma, the disease typically shows poor response to platinum-based treatment and other conventional chemotherapy regimens, with response rates below 30% in advanced or recurrent cases [
2].
Loss of PTEN expression related to genetic or epigenetic alterations is particularly common in OCCC, occurring in approximately 40-50% of cases, but its association with clinical characteristics and other immune-related biomarkers remains unclear [
3]. PTEN loss has been linked to increased PI3K/AKT pathway activation, which may contribute to chemotherapy resistance and disease progression. Previous genome-wide sequencing studies have revealed that PTEN mutations frequently co-occur with PIK3CA mutations in OCCC, suggesting a synergistic effect in promoting oncogenesis through enhanced PI3K/AKT signaling [
4].
ARID1A mutations, present in approximately 50-60% of OCCC cases, often result in loss of protein expression and dysregulation of chromatin remodeling [
5]. The co-occurrence of ARID1A and PTEN alterations has been reported in about 30% of OCCC cases, potentially indicating a cooperative role in tumor development [
6]. Mechanistic studies have demonstrated that ARID1A loss leads to impaired DNA damage repair and altered expression of genes involved in cell cycle regulation and apoptosis [
7]. Recent studies have shown that immune checkpoint inhibitors may demonstrate efficacy in recurrent OCCC, with objective response rates of 15-20%, a response pattern not typically observed in other ovarian cancer subtypes [
8]. Recent investigations have begun exploring the relationship between ARID1A expression and immunotherapy response, with preliminary data suggesting that ARID1A deficiency may enhance sensitivity to immune checkpoint blockade [
9].
Given that MMR deficiency and PD-L1 expression serve as important biomarkers for immunotherapy response, with positive predictive values reported in multiple solid tumors, studies have shown that MMR deficiency occurs in approximately 2-3% of ovarian cancers and is associated with increased tumor mutational burden and enhanced response to immunotherapy [
10]. Meanwhile, PD-L1 expression has been reported in 30-40% of OCCC cases and appears to correlate with tumor-infiltrating lymphocytes and improved survival outcomes [
11]. Based on these findings, our study aimed to investigate the associations between these immune-related markers and clinical features in OCCC and explore the correlations between these biomarkers and clinical outcomes as a reference for future treatment of this challenging tumor.
2. Results
2.1. Clinicopathological Characteristics of included patients
Among the 69 ovarian clear cell carcinoma cases analyzed, PTEN expression was negative in 78.3% (54/69) of cases, while ARID1A loss was observed in 48.8% (39/69) of cases. PD-L1 expression was positive in 26.1% (18/69) of cases. dMMR was observed in 10.1% (n=7) of cases, while 89.9% (n=62) maintained pMMR function. PD-L1 expression was positive in 26.1% (n=18) of tumors and negative in 73.9% (n=51) (
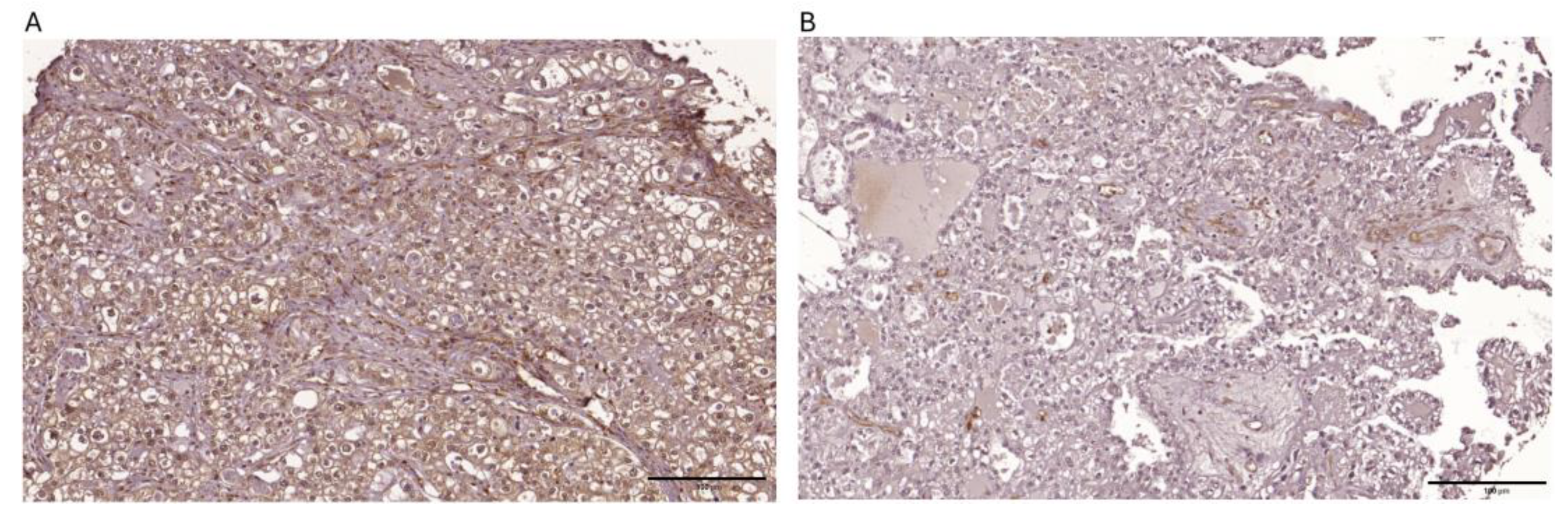
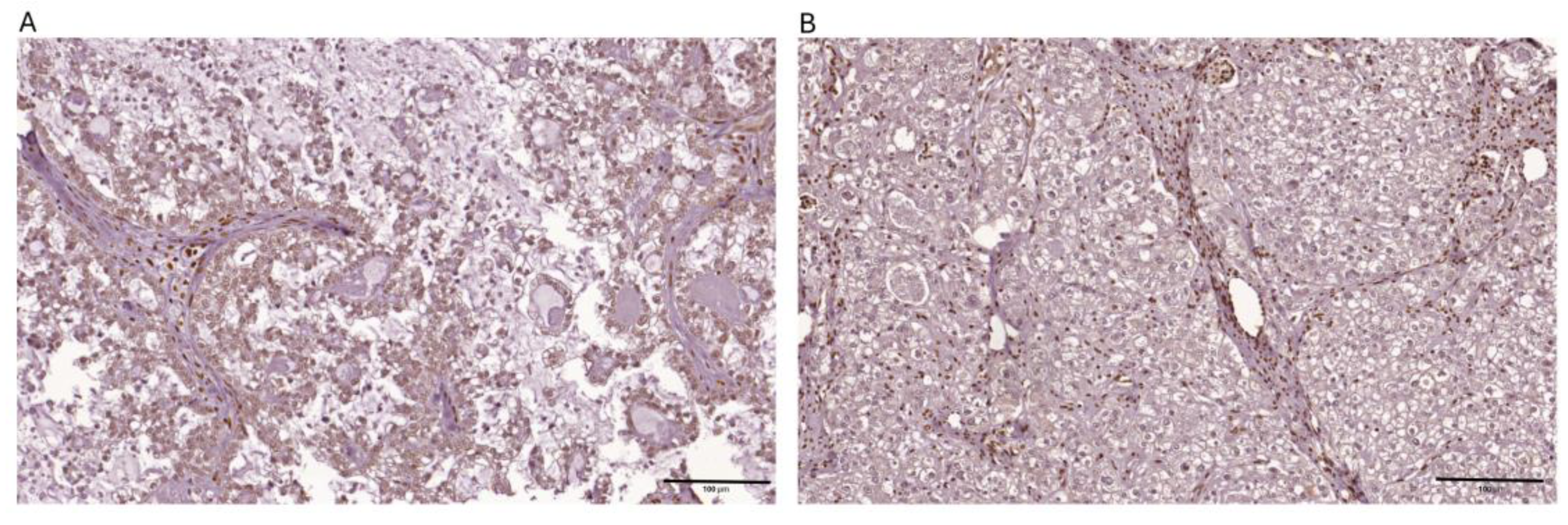
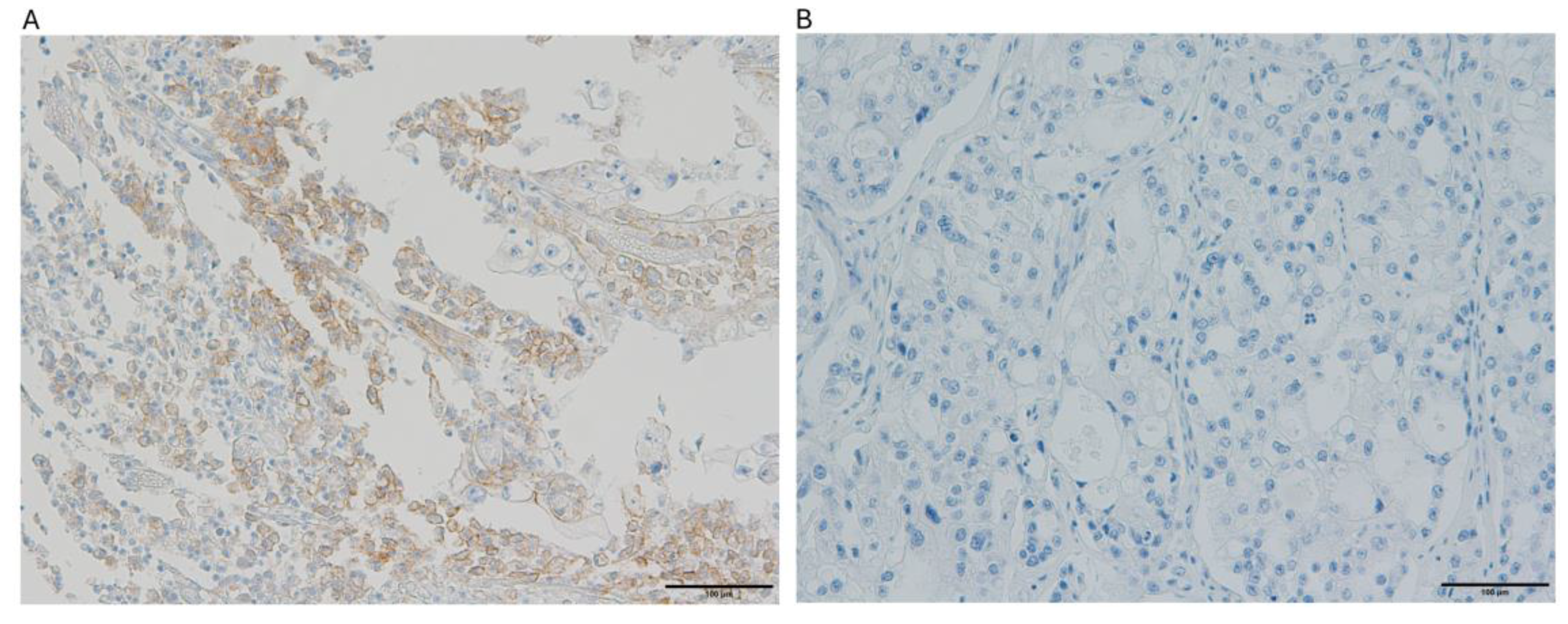
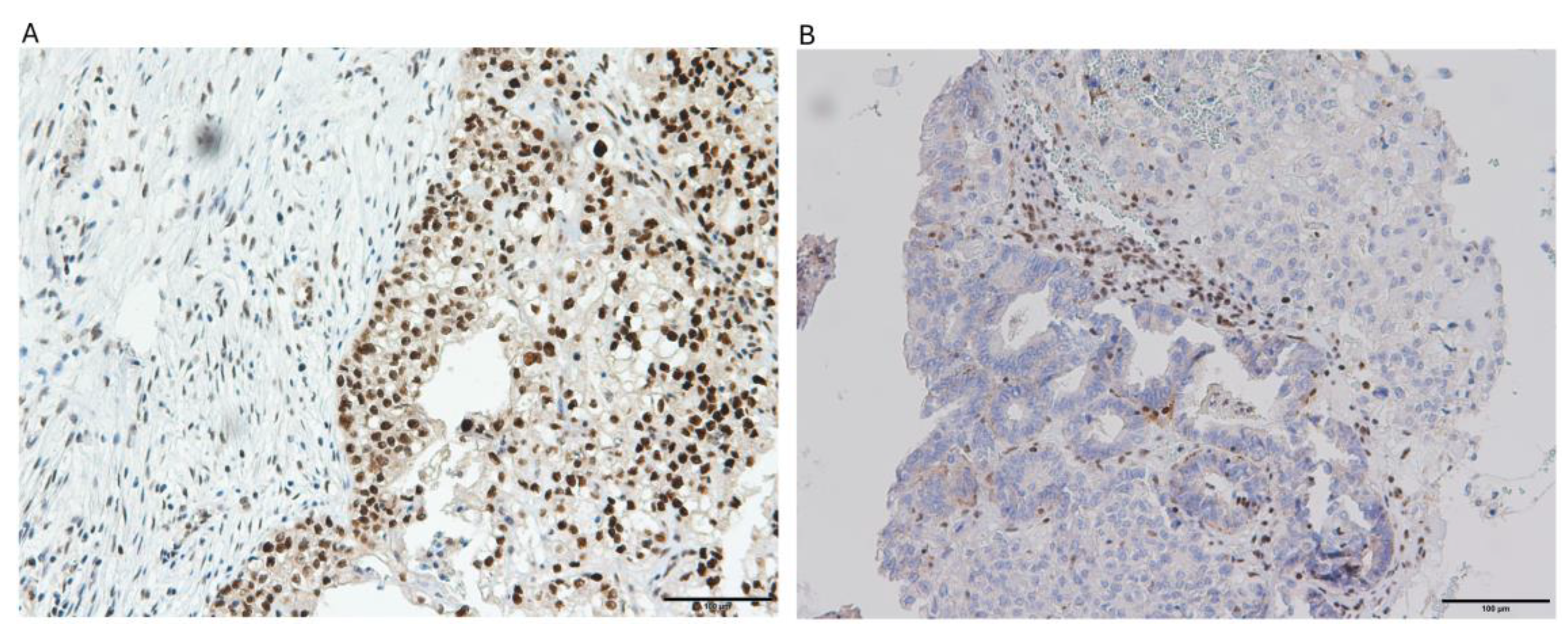
Table 1). The representative images depicting immunohistochemical expression patterns of the 4 targeted biomarkers are presented in
Figure 1,
Figure 2,
Figure 3 and
Figure 4.
2.2. Expression Patterns According to Disease Stage and Molecular Interrelationships Between Biomarkers
We evaluated MMR status and PD-L1 expression alongside other clinicopathological characteristics. The mean age was comparable across both MMR and PD-L1 subgroups (approximately 50 years). Most patients presented with early-stage disease (FIGO stages I/II). Notably, there was a significant association between ARID1A expression and MMR status, with ARID1A loss being significantly more frequent in dMMR tumors (85.7% vs. 43.5% in pMMR tumors, p=0.049). Strikingly, all dMMR tumors (100%) showed PTEN loss, compared to 75.8% of pMMR tumors, although this difference did not reach statistical significance (p=0.333). A striking finding in our study was all PD-L1 positive tumors (100%) demonstrated PTEN loss, which was significantly higher than in PD-L1 negative tumors (70.6%, p=0.007). There was no significant association between PD-L1 expression and MMR status (p=0.667), nor was there any significant correlation between these molecular features and platinum sensitivity or FIGO stage. (
Table 2)
2.3. Co-Expression Analysis of ARID1A and PTEN According to Disease Stage
The concurrent expression patterns of ARID1A and PTEN were analyzed, revealing four distinct groups: ARID1A+/PTEN+ (14.5%), ARID1A+/PTEN- (37.7%), ARID1A-/PTEN+ (7.2%), and ARID1A-/PTEN- (40.6%). In early-stage disease, the distribution shifted notably, with ARID1A-/PTEN- being the predominant pattern (45.4%), followed by ARID1A+/PTEN- (36.4%), ARID1A-/PTEN+ (11.3%), and ARID1A+/PTEN+ (6.8%). In advanced-stage disease, a different pattern emerged with ARID1A+/PTEN- being most common (41.7%), followed by ARID1A-/PTEN- (33.3%) and ARID1A+/PTEN+ (25%), while no cases showed ARID1A-/PTEN+ pattern (p=0.066) (
Table 3). These findings demonstrate the heterogeneous molecular profile of ovarian clear cell carcinoma in our patient population, with a predominance of early-stage disease and high frequency of PTEN loss and ARID1A alteration.
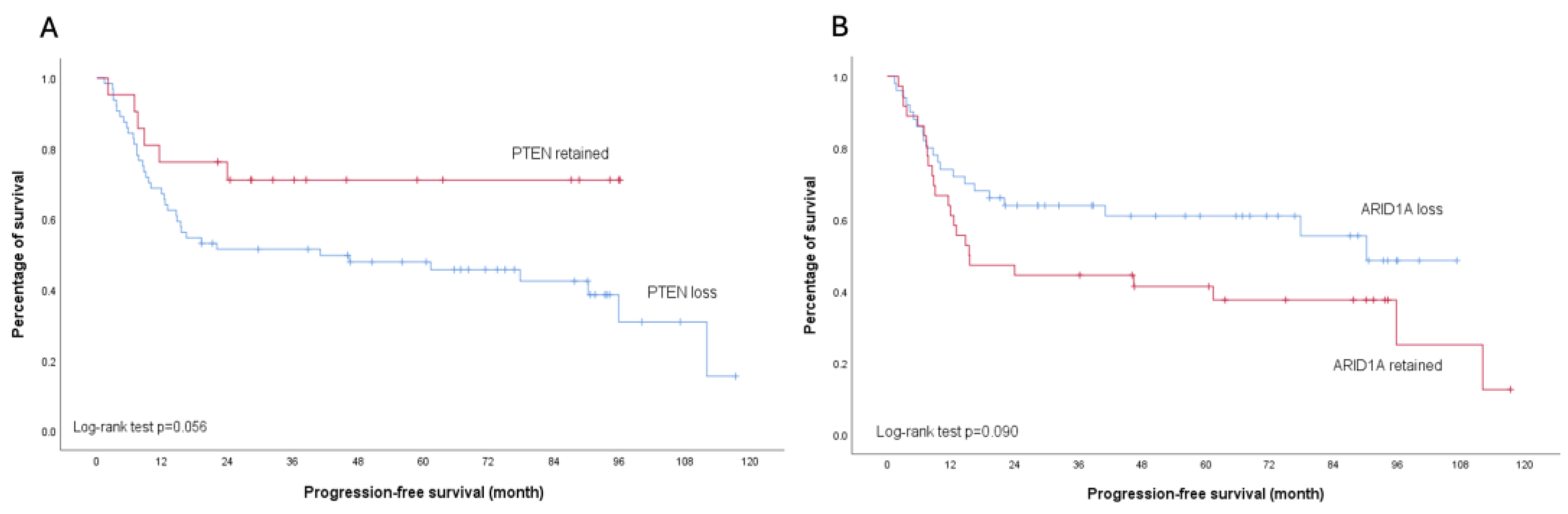
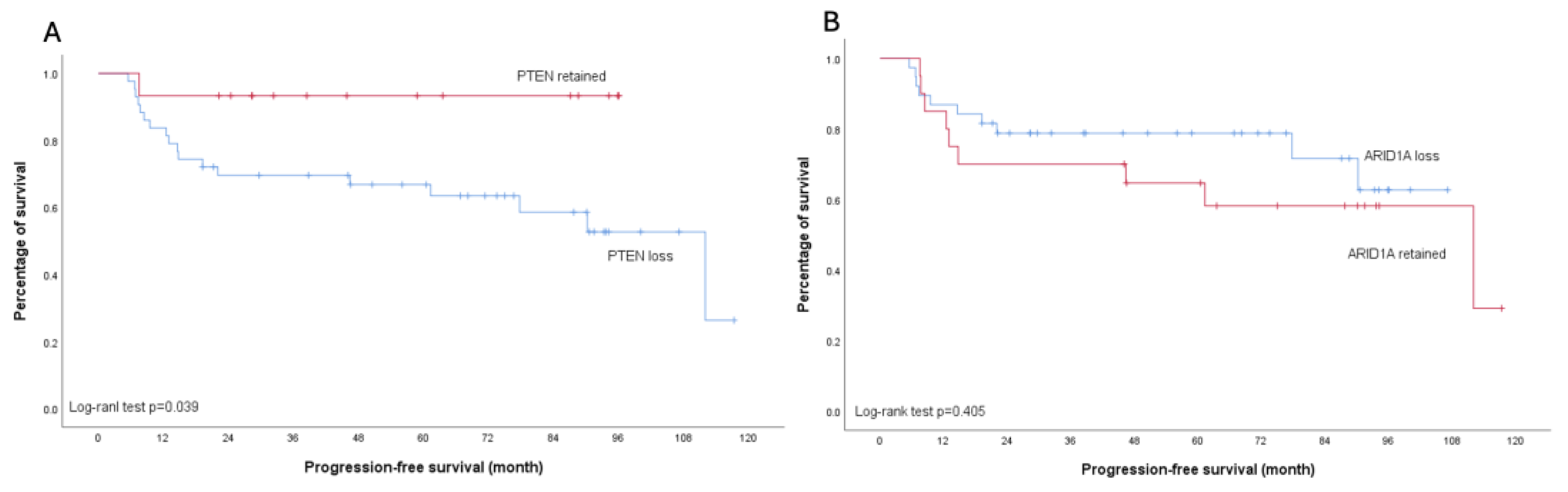
2.4. Treatment Response and Survival Analysis
None of the analyzed molecular markers showed significant association with platinum sensitivity (all p>0.05) (
Table 2). Regarding survival outcomes, PTEN loss was associated with worse PFS, particularly in early-stage disease (p=0.039). This trend was also observed in the overall cohort, though with borderline significance (p=0.056). In contrast, patients with ARID1A loss demonstrated a trend toward improved PFS compared to those with retained ARID1A expression across all stages, although this did not reach statistical significance (p=0.090). Importantly, subgroup analysis of early-stage disease revealed no difference in PFS between ARID1A-loss and ARID1A-retained patients (p=0.405) (
Figure 5 and
Figure 6).
3. Discussion
This study demonstrated high prevalence of alteration of PTEN and ARID1A expression as well as low percentage of deficient MMR expression in OCCC. Notably, 26.1% of all OCCC cases showed positive PD-L1 expression, and all these PD-L1-positive cases demonstrated concurrent PTEN loss. Additionally, we identified a significant association between MMR deficiency and ARID1A loss, with 85.7% of ARID1A-deficient cases showing MMR loss compared to only 14.3% in ARID1A-preserved cases. Most importantly, PTEN loss correlated significantly with disease PFS, particularly in early-stage disease. These findings reveal distinct molecular patterns that warrant further investigation, particularly in the context of Asian OCCC populations.
3.1. Distinct Molecular Characteristics of ARID1A and PTEN Expression Patterns in Asian OCCC Cohort: Early Alterations and Stage-Specific Dynamics
Ovarian clear cell carcinoma (OCCC) demonstrates distinct geographical distribution patterns, representing 5% to 13% of epithelial ovarian cancer in Western populations while accounting for up to 20-25% in East Asian countries [
13]. Our finding of high PTEN loss rate (78.3%) in this Asian cohort substantially exceeds the previously reported 40-60% in Western populations [
14]. This remarkable difference aligns with recent studies highlighting population-specific molecular profiles in OCCC [
15]. Particularly noteworthy in our cohort is the co-occurrence pattern of PTEN and ARID1A loss, with 46.5% of early-stage cases showing concurrent loss while only 7.0% maintained expression of both markers. These findings further corroborate the theory proposed in previous studies [
16] and indicate that these molecular alterations represent fundamental early events in the pathogenesis of OCCC) [
17,
18].
Despite not reaching statistical significance (p=0.066), possibly due to limited advanced-stage cases (n=24), we observed intriguing patterns in ARID1A/PTEN expression across disease stages. The unexpected higher proportion of A+/P+ cases in advanced versus early stage (25.0% vs 6.8%) suggests complex underlying mechanisms. This molecular complexity in advanced OCCC has been well-documented by Bolton, K.L., et al who identified distinct molecular subgroups with different therapeutic vulnerabilities [
19]. These findings reflect tumor heterogeneity, with advanced tumors maintaining protein expression while harboring alternative pathway alterations. This observation aligns with Chao, A et al., who demonstrated that advanced OCCCs often develop resistance mechanisms through activation of alternative pathways, potentially indicating dynamic protein expression changes through disease progression [
20]. These findings challenge traditional views of tumor suppressor roles and suggest possible involvement of tumor microenvironment interactions or post-translational modifications in OCCC progression. Furthermore, Wijaya, S.T. et al reported improved response rates in advanced OCCC patients treated with combination targeted therapies based on comprehensive molecular profiling, supporting the need for larger cohort studies to validate these stage-specific molecular patterns and their therapeutic implications [
21].
3.2. PTEN Loss as an Early Prognostic Indicator in OCCC: Implications for Stage-Specific Therapeutic Strategies
In our cohort, PTEN loss demonstrated significant correlation with PFS, particularly in early-stage OCCC. This finding aligns with previous studies suggesting PTEN's crucial role in tumor progression through the PI3K/AKT/mTOR pathway [
3,
22]. Previous molecular studies have revealed that PTEN loss leads to constitutive activation of the PI3K/AKT pathway, resulting in enhanced cell survival and proliferation [
23]. Additionally, demonstrated that PTEN-deficient OCCC cells had been demonstrated to exhibit increased resistance to platinum-based chemotherapy, potentially explaining the poor treatment outcomes observed in these patients [
24]. Moreover, despite both ARID1A and PTEN being involved in OCCC pathogenesis, they exhibit distinct prognostic value, particularly in early-stage disease where PTEN may be a more relevant prognostic indicator while ARID1A expression status has minimal impact on survival outcomes. The particularly strong correlation in early-stage disease suggests that PTEN loss might be an early event in OCCC progression. This hypothesis is supported by previous work disclosing PTEN alterations in endometriosis-associated OCCC precursor lesions [
25]. Furthermore, integrated genomic analyses also revealed that PTEN loss often precedes other molecular alterations in OCCC development, highlighting its potential value as an early prognostic marker [
20]. These stage-specific patterns of PTEN loss and their differential impact on survival suggest the need for distinct therapeutic approaches in early versus advanced OCCC. Beyond individual marker analysis, understanding the complex interactions between these molecular alterations is crucial for developing effective therapeutic strategies. The unexpected associations between different molecular markers in our study suggest intricate pathway crosstalk that may have significant implications for treatment approaches.
3.3. MMR Deficiency and PI3K-AKT-mTOR Pathway: Molecular Interplay in Immune Evasion
In our cohort, all tumors with dMMR (100%) exhibited concurrent PTEN protein loss, compared to 75.8% of PTEN loss in pMMR tumors. Although this association did not reach statistical significance, likely due to the limited number of dMMR cases, the consistent co-occurrence of dMMR and PTEN loss warrants further attention. This finding aligns with recent research demonstrating potential molecular crosstalk between the PI3K-AKT-mTOR pathway and mismatch repair proteins. Wang et al. found that in dMMR gastric adenocarcinoma, higher mutation burden in the PI3K-AKT-mTOR pathway correlated with reduced immune cell infiltration and inferior response to immune checkpoint inhibitors [
26]. This suggests that alterations in the PI3K-AKT-mTOR pathway might be one of the mechanisms underlying immune evasion and primary resistance to immunotherapy in dMMR tumors.
3.4. ARID1A-MMR Relationship: Implications for DNA Repair and Immunotherapy Response
Emerging research has uncovered critical information about OCCC's immunological characteristics and survival outcomes, paving the way for immune-based therapeutic strategies while revealing distinct immune patterns that affect patient prognosis [
27]. Our analysis revealed a significant association between ARID1A loss and MMR deficiency, with 18.6% of ARID1A-loss tumors exhibiting MMR loss compared to only 2.8% in ARID1A-preserved tumors. This finding aligns with previous studies suggesting that ARID1A, a component of the SWI/SNF chromatin remodeling complex, plays a crucial role in DNA damage response and repair mechanism [
28]. While this relationship has been underexplored in OCCC, mechanistic studies in other cancers have demonstrated that ARID1A deficiency impairs MMR protein expression through disruption of MLH1 transcription and compromises DNA mismatch repair through SWI/SNF complex-mediated chromatin remodeling [
29,
30,
31,
32]. These findings suggest a conserved mechanistic link between ARID1A loss and MMR deficiency that may have therapeutic implications, as both ARID1A-deficient tumors and MMR-deficient tumors have shown particular sensitivity to immunotherapy approaches. Although our cohort showed no significant difference in PD-L1 expression between ARID1A-loss and ARID1A-preserved tumors, the MMR status correlation warrants further investigation into immunotherapeutic approaches for ARID1A-deficient cancers.
3.5. PTEN-PD-L1 Axis: Targeting Immune Checkpoint Pathways in OCCC
A striking finding in our study was the perfect correlation between PTEN loss and PD-L1 expression, with all PD-L1-positive cases showing PTEN loss. This clinical observation validates recent preclinical studies demonstrating that PTEN loss directly upregulates PD-L1 expression through PI3K/AKT/mTOR pathway activation and specific transcriptional regulators [
33,
34]. In addition, the influence of PTEN deficiency and PI3K activation on T cell-driven antitumor immunity was examined using a preclinical melanoma model [
35], and recent preclinical studies have also shown synergistic effects when combining PI3K inhibitors with immune checkpoint blockade in PTEN-deficient models [
36]. More and more studies have disclosed changes in PTEN and the interplay with other genes studied in mouse models of prostate cancer have been shown to collectively shape the immune-cell content and expression of immunosuppressive markers in the tumor microenvironment [
37,
38], providing a strong rationale for investigating similar strategies in OCCC patients with this molecular profile.
Collectively, these molecular interactions highlight complex interconnections between chromatin remodeling, DNA repair, and immune regulation pathways in OCCC. While platinum sensitivity did not significantly differ between PD-L1-positive and PD-L1-negative tumors, the complete concordance between PD-L1 positivity and PTEN alteration suggests that PTEN status could serve as a surrogate biomarker for potential responsiveness to PD-1/PD-L1 inhibitors. PTEN, as a critical negative regulator of the PI3K-AKT-mTOR pathway, appears to be a central player in immune evasion through its direct impact on PD-L1 expression. These findings further highlight the importance of assessing both PTEN and PD-L1 status when considering immunotherapeutic strategies in OCCC, as dual targeting of the PI3K/AKT pathway and immune checkpoints may provide synergistic effects in tumors with these molecular features. Larger studies are needed to validate these observations and determine whether these molecular signatures define distinct OCCC subtypes with unique therapeutic vulnerabilities that could optimize treatment selection and improve outcomes in this challenging malignancy.
3.6. Limitation
Despite the significant findings, several limitations of this study warrant consideration. First, the retrospective nature and relatively modest sample size (n=69) may limit the statistical power, particularly for subgroup analyses of advanced-stage disease. Second, our reliance on immunohistochemistry for protein expression assessment, while clinically applicable, does not capture all genetic or epigenetic alterations that might affect these pathways. Specifically, post-translational modifications and functional pathway activation cannot be fully evaluated through IHC analysis alone. Third, our tissue microarray approach, although including three cores per tumor, may not fully account for intratumorally heterogeneity known to exist in OCCC. Further studies with larger cohorts and comprehensive molecular profiling are needed to confirm these associations and their therapeutic implications.
4. Materials and Methods
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (CGMH), Taiwan and patient informed consent was waived (number 102-1076B, 105-0992C). We confirmed that all experiments were performed in accordance with relevant guidelines and regulations.
4.1. Patients and Settings
Cases and their pathology data were retrospectively collected including all stages ovarian CCCs diagnosed at Kaohsiung Chang Memorial Hospital between 2008 and 2013. Diagnosis was based on the conventional histomorphological examination and ancillary immunohistochemical (IHC) study if necessary, and all cases were re-reviewed by 2 surgical pathologists to confirm the diagnosis. Tumor staging was performed according to the 2014 International Federation of Gynecology and Obstetrics (FIGO) classification. All patients were primarily treated with staging surgery or debulking surgery followed by at least 3 cycles of adjuvant platinum-based combination chemotherapy (paclitaxel 175 mg/m2 and carboplatin area under the curve [AUC] 5 or CDDP 75 mg/m2 and cyclophosphamide 750 mg/m2) according to disease stage.
4.2. Assembling of Tissue Array
The paraffin tissue blocks were assembled into tissue arrays using an automated tissue array, TMA Grand Master (3DHISTECH Ltd., Budapest, Hungary). Briefly, the selected tumor areas were marked on the hematoxylin and eosin-stained slides to match the corresponding areas on the paraffin tissue blocks. Considering the tissue heterogeneity, three tissue cores for each tumor were obtained using a tissue cylinder of 1.5 mm in diameter to assemble the tissue array blocks. In addition, leiomyoma tissue was included in each tissue array block for orientation and as external controls.
4.3. Immunohistochemistry Study of Targeted Biomarkers
IHC analysis was performed on 3-um sections sliced from the tissue array blocks. Following deparaffinization and rehydration of the tissue’s sections, antigen retrieval was performed at 100°C for 15 min with 1X citrate buffer, pH 6.0 (Thermo, AP-9003-500). Endogenous peroxidase was blocked with 3% H2O2 for 15 min. All the patients included had IHC testing for ARID1A, PTEN, MMR and PD-L1 expression with MMR was evaluated through assessment of 4 proteins including MSH2, MSH6, MLH1, and PMS2. Primary ARID1A antibody (Sigma HPA005456) was applied at the diluted concentration of 1:2000 as well as primary PTEN antibody (Cell signaling #9559) at the diluted concentration of 1:200. The primary antibodies used were those specifically against MLH1 (clone GM011, 1:50, Genemed, South San Francisco), MSH2 (clone G219-1129, 1:100, ZETA), MSH6 (clone GM024, 1:100, Genemed), and PMS2 (clone A16-4, 1:100, BD Biosciences). Programmed death-ligand 1 (PD-L1) was detected using anti-PD-L1 (Agilent Cat# GE00621-2, RRID: AB_2833074) mouse monoclonal primary antibody. All primary antibodies were qualified by the Nordic IHC Quality Control (NordiQC) scientific organization, except for MSH6 antibody (clone GM024). Primary antibody detection was carried out using a polymer system (UltraVision Quanto Detection System HRP Polymer; Thermo TL-125-QHL). Staining development was achieved by incubation with DAB and DAB Enhancer (Thermo TA-125-QHDX).
4.4. Definition for ARID1A and PTEN Expressions
Two pathologists independently examined all the sections of the tumor tissue array. Immunostainings of ARID1A protein expression and PTEN protein in tumor cells were scored following a semiquantitative method. The positive immunostaining was shown in the cytoplasm for PTEN and in the nucleus for ARID1A-encoded protein, BAF250A. The staining intensity in tumor cells was compared to that detected in the adjacent stromal cells which were used as positive controls. The intensity of BAF250A and PTEN immunostainings were scored as 0 (no staining), 1 (weaker staining than that of stromal cells), and 2 (equal to that of stromal cells). Only score 2 of intensity tumor cell was defined as positive. The percentage of positive tumor cells was further scored as 0 (0%), 1+ (≤ 10%), 2+ (11-50%), 3+ (51-80%) and 4+ (> 80 %) using the scoring system proposed by Samartzis et al [
12]. Tumor tissue was classified as having downregulation or loss of ARID1A or PTEN expression when either the percentage of positive tumor cells was scored as 3+ or below (corresponding to ≤80% positive tumor cells) or when the staining intensity was scored as 0 or 1.
4.5. Definition of Positive PD-L1 and Deficient Expression of MMR Proteins
Internal positive controls consisted of non-cancerous ovarian and stroma cells. With appropriate internal positive controls, nuclear staining in tumor cells was referred to be a normal expression (proficiency) of MMR proteins, while a total absence of nuclear staining was seen as a loss of expression (deficiency). Tumor MMR status was assessed and determined by the IHC expression of four MMR proteins (MLH1, MSH2, MSH6 and PMS2); the definition of deficient MMR (dMMR) was the loss of at least one of these four proteins, while proficient MMR (pMMR) was defined as the intact expression of all four MMR proteins. An anti-PD-L1 22C3 IHC assay was used to evaluate the tumor PD-L1 expression. For PD-L1 expression evaluation, we utilized the Combined Positive Score (CPS) method. The CPS was calculated as the number of PD-L1 staining cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100. A CPS ≥1 was defined as PD-L1 positive. All slides were independently assessed by two pathologists blinded to clinical outcomes, with discrepancies resolved by consensus review.
4.6. Statistical Analysis
Progression-free survival (PFS) was calculated from the date of initial diagnosis to the date of first disease progression or death from any cause. Comparisons between categorical variables were conducted using chi-square tests or Fisher's exact tests when appropriate. We estimated survival probabilities using the Kaplan–Meier method and evaluated differences between groups with log-rank tests. All statistical analyses were performed using IBM SPSS Statistics software version 26 (IBM Corporation, Armonk, NY, USA), with p < 0.05 considered statistically significant.
5. Conclusions
Our analysis of PTEN, ARID1A, MMR, and PD-L1 expression in OCCC revealed several key findings with clinical relevance. The frequent co-occurrence of PTEN and ARID1A alterations in early-stage disease suggests these are fundamental early events in OCCC pathogenesis, particularly in Asian populations. PTEN loss shows strong prognostic value in early-stage disease, indicating potential utility for adjuvant therapy decisions. The correlation between PTEN loss and PD-L1 expression, plus the association between ARID1A and MMR deficiency, provides insights that could inform therapeutic strategies. These findings support future investigations of targeted therapies and immunotherapy approaches in OCCC.
Author Contributions
Conceptualization, C.-H.W. and C.-C.H.; methodology, H.L., C.-H.W., Y.-C.O., M.-Y.Y., and C.-C.H.; validation and formal analysis, C.-C.H., H.L., H.-C.F., and C.-H.W.; writing—original draft preparation, C.-H.W., H.L., and H.-C.F.; writing—review and editing, Y.-C.O., , M.-Y.Y., and C.-C.H.; funding acquisition, C.-H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Chang Gung Memorial Hospital in Taiwan (grant number CMRPG8P0271 to Dr. Chen-Hsuan Wu).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval number: 102-1076B, 105-0992C).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the analysis used anonymous clinical data.
Data Availability Statement
All data of this study will be provided on reasonable request from the corresponding author.
Acknowledgments
We thank Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Tissue Bank Core Lab and Biobank (CLRPG8I0032) for their excellent technical support. GenAI (Claude) has been used for superficial text editing including grammar and spelling.
Conflicts of Interest
All authors have no conflicts of interest that could be perceived to bias their work, making known all financial support and any other personal connections.
Abbreviations
The following abbreviations are used in this manuscript:
| OCCC |
Ovarian clear cell carcinoma |
| PTEN |
Phosphatase and tensin homolog |
| ARID1A |
AT-rich interactive-domain 1A |
| PD-L1 |
Programmed Cell Death-Ligand 1 |
| MMR |
Mismatch repair |
| IHC |
Immunohistochemistry |
| dMMR |
Deficient mismatch repair |
| pMMR |
Proficient mismatch repair |
| CPS |
Combined Positive Score |
| PFS |
Progression-free survival |
References
- Chan, J.K. , et al., Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T. , et al., Randomized Phase III Trial of Irinotecan Plus Cisplatin Compared With Paclitaxel Plus Carboplatin As First-Line Chemotherapy for Ovarian Clear Cell Carcinoma: JGOG3017/GCIG Trial. J Clin Oncol 2016, 34, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.C. , et al., Clinical and pathological associations of PTEN expression in ovarian cancer: a multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br J Cancer 2020, 123, 793–802. [Google Scholar] [CrossRef]
- Kim, S.I. , et al., Genomic landscape of ovarian clear cell carcinoma via whole exome sequencing. Gynecol Oncol 2018, 148, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C. , et al., ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Khalique, S. , et al., Translational genomics of ovarian clear cell carcinoma. Semin Cancer Biol 2020, 61, 121–131. [Google Scholar] [CrossRef]
- Shen, J. , et al., ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov 2015, 5, 752–767. [Google Scholar] [CrossRef]
- Matulonis, U.A. , et al., Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019, 30, 1080–1087. [Google Scholar] [CrossRef]
- Shen, J. , et al., ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Bennett, J.A. , et al., Mismatch Repair Protein Expression in Clear Cell Carcinoma of the Ovary: Incidence and Morphologic Associations in 109 Cases. Am J Surg Pathol 2016, 40, 656–663. [Google Scholar] [CrossRef]
- Fu, M. , et al., Infiltration of CD8(+) cytotoxic T-cells and expression of PD-1 and PD-L1 in ovarian clear cell carcinoma. Sci Rep 2025, 15, 4716. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P. , et al., Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol 2012, 25, 885–892. [Google Scholar] [CrossRef]
- Takano, M., H. Tsuda, and T. Sugiyama, Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res 2012, 31, 53. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.L. , et al., Molecular Profiling of Clear Cell Ovarian Cancers: Identifying Potential Treatment Targets for Clinical Trials. Int J Gynecol Cancer 2016, 26, 648–654. [Google Scholar] [CrossRef]
- Stružinská, I. , et al., A comprehensive molecular analysis of 113 primary ovarian clear cell carcinomas reveals common therapeutically significant aberrations. Diagn Pathol 2023, 18, 72. [Google Scholar] [CrossRef]
- Zhai, Y. , et al., Arid1a inactivation in an Apc- and Pten-defective mouse ovarian cancer model enhances epithelial differentiation and prolongs survival. J Pathol 2016, 238, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L. , et al., Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun 2015, 6, 6118. [Google Scholar] [CrossRef]
- Tong, A. , et al., Review the progression of ovarian clear cell carcinoma from the perspective of genomics and epigenomics. Front Genet 2023, 14, 952379. [Google Scholar] [CrossRef]
- Bolton, K.L. , et al., Molecular Subclasses of Clear Cell Ovarian Carcinoma and Their Impact on Disease Behavior and Outcomes. Clin Cancer Res 2022, 28, 4947–4956. [Google Scholar] [CrossRef]
- Chao, A. , et al., Molecular profiling reveals novel therapeutic targets and clonal evolution in ovarian clear cell carcinoma. BMC Cancer 2024, 24, 1403. [Google Scholar] [CrossRef]
- Wijaya, S.T. , et al., Comprehensive characterization of genomic features and clinical outcomes following targeted therapy and secondary cytoreductive surgery in OCCC: a single center experience. J Gynecol Oncol 2024, 35, e69. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. , et al., Clinicopathologic and biological analysis of PIK3CA mutation in ovarian clear cell carcinoma. Hum Pathol 2012, 43, 2197–2206. [Google Scholar] [CrossRef]
- Lee, S. , et al., Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol 2005, 97, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Rinne, N. , et al., Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist 2021, 4, 573–595. [Google Scholar] [PubMed]
- Worley, M.J., Jr. , et al., Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur J Cancer 2015, 51, 1831–1842. [Google Scholar] [CrossRef]
- Heong, V. , et al., A multi-ethnic analysis of immune-related gene expression signatures in patients with ovarian clear cell carcinoma. J Pathol 2021, 255, 285–295. [Google Scholar] [CrossRef]
- Wang, Z. , et al., Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med 2022, 20, 133. [Google Scholar] [CrossRef]
- Bakr, A. , et al., ARID1A regulates DNA repair through chromatin organization and its deficiency triggers DNA damage-mediated anti-tumor immune response. Nucleic Acids Res 2024, 52, 5698–5719. [Google Scholar] [CrossRef]
- Allo, G. , et al., ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod Pathol 2014, 27, 255–261. [Google Scholar] [CrossRef]
- Kim, K.J. , et al., Loss of ARID1A Expression in Gastric Cancer: Correlation with Mismatch Repair Deficiency and Clinicopathologic Features. J Gastric Cancer 2015, 15, 201–208. [Google Scholar] [CrossRef]
- Hung, Y.P. , et al., ARID1A mutations and expression loss in non-small cell lung carcinomas: clinicopathologic and molecular analysis. Mod Pathol 2020, 33, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, B.M. , et al., Loss of ARID1A expression is associated with DNA mismatch repair protein deficiency and favorable prognosis in advanced stage surgically resected esophageal adenocarcinoma. Hum Pathol 2019, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cretella, D. , et al., PTEN Alterations as a Potential Mechanism for Tumor Cell Escape from PD-1/PD-L1 Inhibition. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Vidotto, T. , et al., Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Peng, W. , et al., Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Xing, F. , et al., Modulating the tumor microenvironment via oncolytic virus and PI3K inhibition synergistically restores immune checkpoint therapy response in PTEN-deficient glioblastoma. Signal Transduct Target Ther 2021, 6, 275. [Google Scholar] [CrossRef]
- Maxwell, P.J. , et al., Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget 2014, 5, 4895–4908. [Google Scholar] [CrossRef]
- Bezzi, M. , et al., Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat Med 2018, 24, 165–175. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).