Submitted:
20 November 2025
Posted:
20 November 2025
You are already at the latest version
Abstract
Plants synthesize a wide range of secondary metabolites, including phenolic compounds and terpenoids, which play key ecological roles and have relevant agro-industrial applications. The genus Baccharis, belonging to the family Asteraceae, is highly abundant in South America, particularly in Brazil, and has long been used in traditional medicine, supporting its neotropical origin. Given the growing interest in the species that compose this genus and, in their metabolites, the present study aimed to compile a structured database to support the identification of volatile compounds occurring in Baccharis species. A total of 158 volatile compounds were identified across 15 species, most of which belong to the subgenus Baccharis. Eleven compounds were observed that may serve as chemotaxonomic markers for the genus. The species most extensively studied over the past decade were B. dracunculifolia and B. trimera. Altogether, these findings highlight the metabolic potential of the Baccharis genus and point to new prospects for pharmaceutical and agro-industrial applications.
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Search and Selection
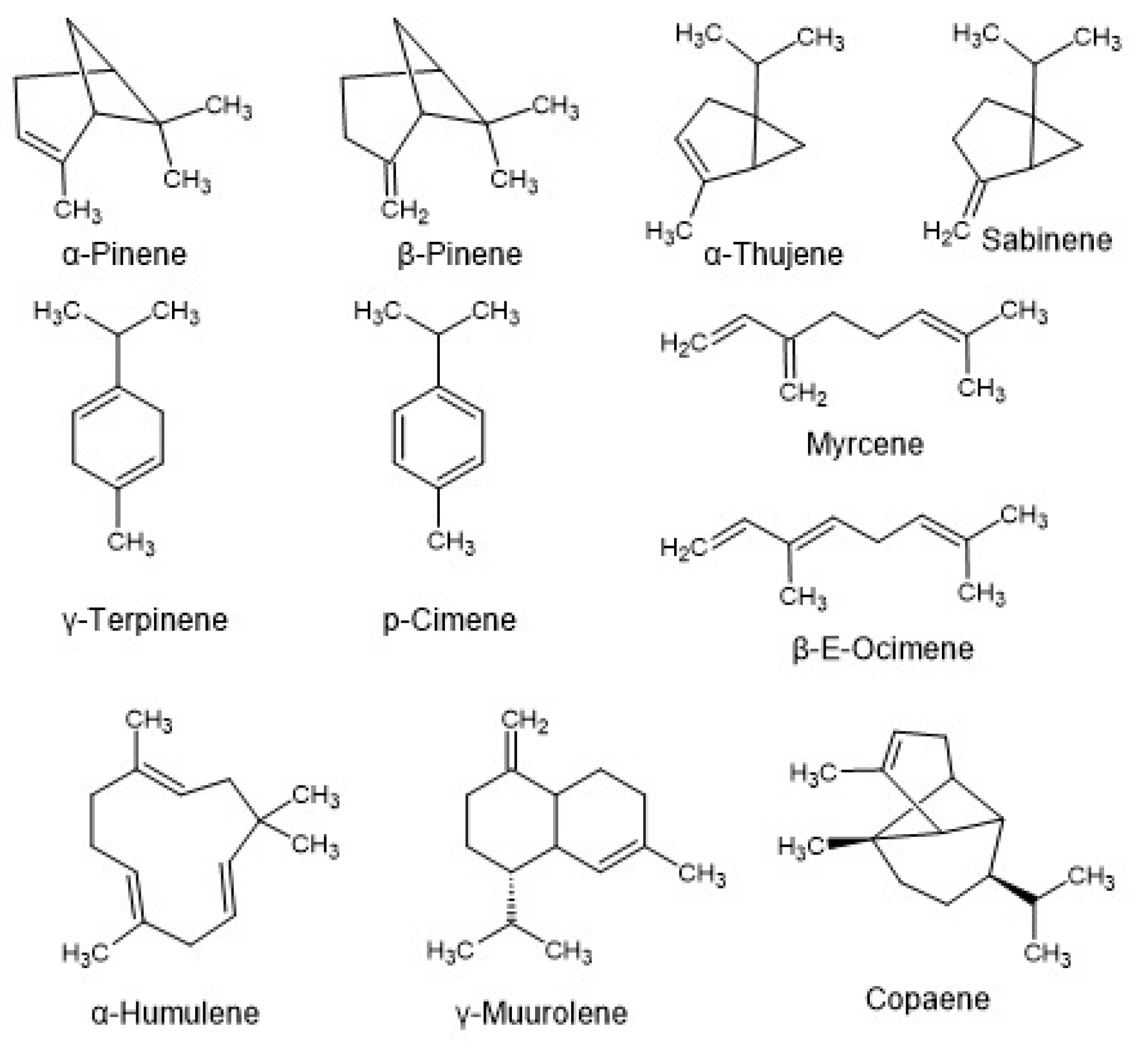
3.2. Volatile Compounds
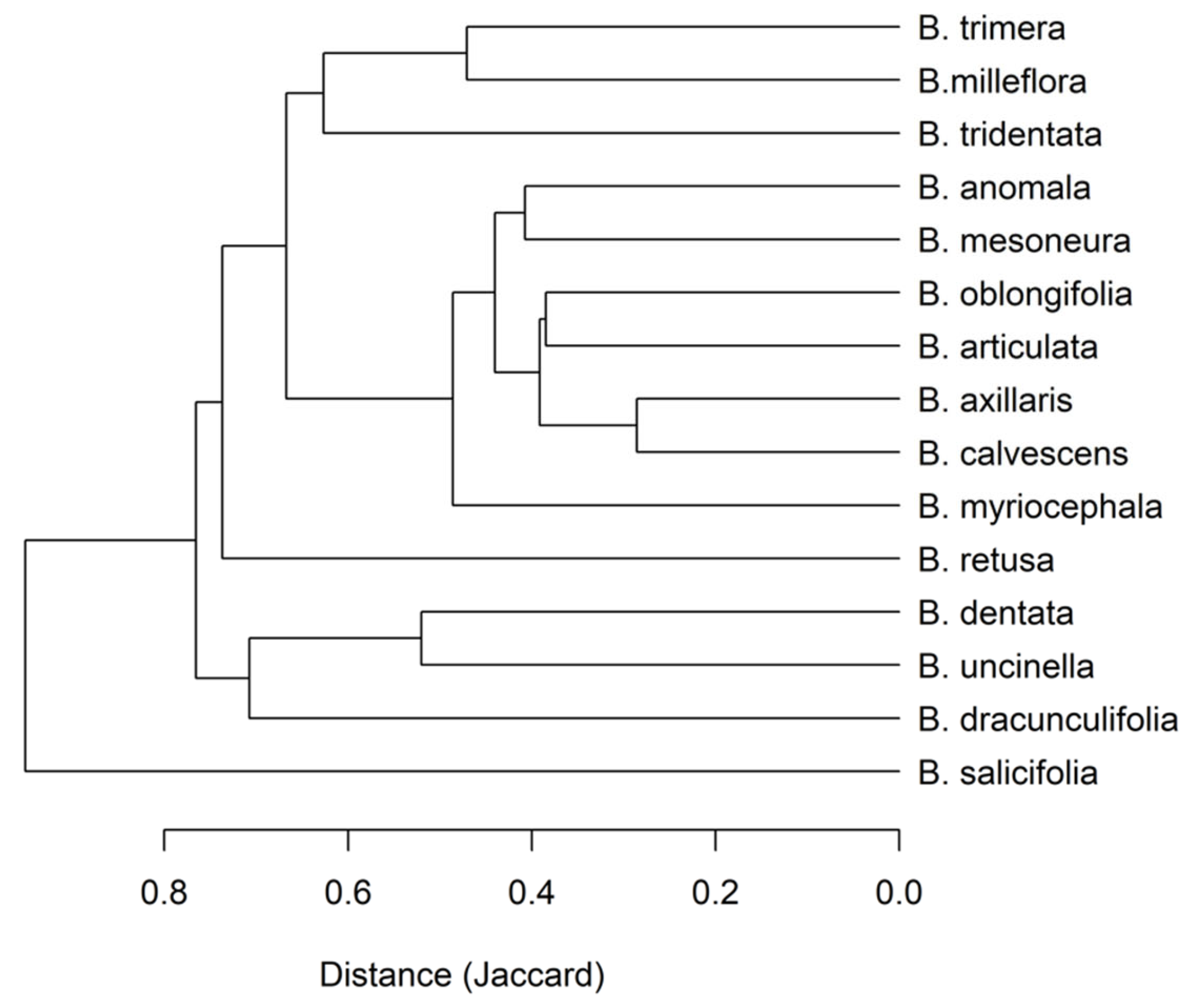
3.3. Cluster Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | Molecular Formula |
| MM | Molar Mass |
| BM | Baccharis milleflora |
| BME | Baccharis mesoneura |
| BR | Baccharis retusa |
| BAR | Baccharis articulata |
| BAX | Baccharis axillaris |
| BMY | Baccharis myriophoella |
| BT | Baccharis trimera |
| BA | Baccharis anomala |
| BS | Baccharis salicifolia |
| BD | Baccharis dentata |
| BO | Baccharis oblongifolia |
| BC | Baccharis calvescens |
| BDR | Baccharis dracunculifolia |
| BU | Baccharis uncinella |
| BTR | Baccharis tridentata |
References
- Pinto-zevallos, D. M.; Martins, C. B. C. Pellegtino, A. C.; & Zarbin, P. H. G. (2013). Compostos orgânicos voláteis na defesa induzida das plantas contra insetos herbívoros. Química Nova, 36 (9), 1395–1405. [CrossRef]
- Picazo-Aragonés, J., Terrab, A., & Balao, F. (2020). Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. International Journal of Molecular Sciences, 21(23), 8956. [CrossRef]
- Budel, J. M., Duarte, M. R., Santos, C. A. M., Farago, P. V., & Matzenbacher, N. I. (2005). O progresso da pesquisa sobre o gênero Baccharis, Asteraceae: I - Estudos botânicos. Revista Brasileira de Farmacognosia, 15(3), 268–271. [CrossRef]
- Müller, J. (2006). Systematics of Baccharis (Compositae-Astereae) in Bolivia, including an overview of the genus. Systematic Botany Monographs, 76, 1–341.
- Hattori, E. K. O., & Nakajima, J. N. (2008). A família Asteraceae na estação de pesquisa e desenvolvimento ambiental galheiro, Perdizes, Minas Gerais, Brasil. Rodriguésia, 59(4), 687–749.
- Heiden, G., Baumgratz, J. F. A., & Esteves, R. L. (2012). Baccharis subgen. Molina (Asteraceae) no estado do Rio de Janeiro, Brasil. Rodriguésia, 63(3), 649–687. [CrossRef]
- Xavier, V. B., Minteguiaga, M., Umpiérrez, N., Vargas, R. M. F., Dellacassa, E., & Cassel, E. (2016). Olfactometry evaluation and antimicrobial analysis of essential oils from Baccharis dentata (Vell.) G.M. Barroso and Baccharis uncinella DC. Journal of Essential Oil Research, 29(2), 137–144. [CrossRef]
- Aires, C. P., Sassaki, G. L., Santana-Filho, A. P., Spadaro, A. C. C., & Cury, J. A. (2016). Baccharis dracunculifolia-based mouthrinse alters the exopolysaccharide structure in cariogenic biofilms. International Journal of Biological Macromolecules, 84, 301–307. [CrossRef]
- De Araújo-Neto, J. B., Da Silva, M. M. C., Lucas Dos Santos, A. T., De Souza, A. B., Oliveira-Tintino, C. D. D. M., Da Silva, L. E., ... & Tintino, S. R. (2023). Terpenic profile of the essential oil of Symphyopappus cuneatus (DC.) Sch.Bip. Ex Baker and its effects on antibiotic resistance in vitro. South African Journal of Botany, 157, 355–359. [CrossRef]
- Hocayen, P. D. A., Grassiolli, S., Leite, N. C., Pochapski, M. T., Pereira, R. A., Da Silva, L. A., & Malfatti, C. R. (2016). Baccharis dracunculifolia methanol extract enhances glucose-stimulated insulin secretion in pancreatic islets of monosodium glutamate induced-obesity model rats. Pharmaceutical Biology, 54(7), 1263–1271.
- Lucas, A. M., Bento, A. F. M. L., Vargas, R. M. F., Scheffel, T. B., Rockenbach, L., Diz, F. M., ... & Cassel, E. (2021). Use of supercritical CO₂ to obtain Baccharis uncinella extracts with antioxidant and antitumor activity. Journal of CO₂ Utilization, 49, 101563. [CrossRef]
- Brandenburg, M. M., Rocha, F. G., Pawloski, P. L., Soley, B. D. S., Rockenbach, A., Scharf, D. R., ... & Otuki, M. F. (2020). Baccharis dracunculifolia (Asteraceae) essential oil displays anti-inflammatory activity in models of skin inflammation. Journal of Ethnopharmacology, 259, 112840. [CrossRef]
- Nogueira, N. P. A., Reis, P. A., Laranja, G. A. T., Pinto, A. C., Aiub, C. A. F., Felzenszwalb, I., ... & Coelho, M. G. P. (2011). In vitro and in vivo toxicological evaluation of extract and fractions from Baccharis trimera with anti-inflammatory activity. Journal of Ethnopharmacology, 138(2), 513–522.
- Ferreira, P. de A. (2012). Desenvolvimento de forma farmacêutica sólida à base de Baccharis trimera (Less.) DC. para o tratamento da artrite reumatóide.
- Casagrande, M., Zanela, J., Júnior, A. W., Busso, C., Wouk, J., Iurckevicz, G., & Malfatti, C. R. M. (2018). Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Industrial Crops and Products, 125, 207–219.
- Silva, M. C. D., Silva, L. A. D., Malfatti, C. R. M., & Soares, K. C. N. (2022). Propriedades antidiabéticas de cerveja enriquecida com Baccharis dracunculifolia. Research, Society and Development, 11(11), e262111133720. [CrossRef]
- Ming, L. C. (2022). Baccharis: De filho de Júpiter a vassoura e carqueja: uma visão botânica e etnobotânica histórica e os problemas de produtos comercializados. Scientia Naturalis, 4(1), 264–286. [CrossRef]
- R Core Team (2021). R: A Language and Environment for Statistical Computing.
- Bandeira, J. M., Barbosa, F. F., Barbosa, L. M. P., Rodrigues, I. C. S., Bacarin, M. A., Peters, J. A., & Braga, E. J. B. (2011). Composição do óleo essencial de quatro espécies do gênero Plectranthus. Revista Brasileira de Plantas Medicinais, 13(2), 157–164. [CrossRef]
- Do Amaral, W., Deschamps, C., Bizzo, H. R., Pinto, M. A. S., Da Silva, L. E., & Biasi, L. A. (2016). Essential oil yield and composition of native species of the Myrtaceae family from "Campos Gerais" of the Atlantic Forest in Parana State.
- Trombin-Souza, M., Amaral, W., Pascoalino, J. A. L., Oliveira, R. A., Bizzo, H. R., & Deschamps, C. (2017). Chemical composition of the essential oils of Baccharis species from southern Brazil: A comparative study using multivariate statistical analysis. Journal of Essential Oil Research, 29(5), 400–406. [CrossRef]
- Nieves-Silva, E., & Romero-López, A. A. (2019). Chemical profile of the volatiles of Baccharis salicifolia (Asteraceae) and interaction with Macrodactylus nigripes (Coleoptera: Melolonthidae). Acta Agronómica, 68(3), 222–227.
- Minteguiaga, M., Umpiérrez, N., Xavier, V., Lucas, A., Mondin, C., Fariña, L., ... & Dellacassa, E. (2015). Recent findings in the chemistry of odorants from four Baccharis species and their impact as chemical markers. Chemistry & Biodiversity, 12(9), 1339–1348.
- Zimmermann, R. C., de Carvalho Aragao, C. E., de Araújo, P. J. P., Benatto, A., Chaaban, A., Martins, C. E. N., ... & Zawadneak, M. A. (2021). Insecticide activity and toxicity of essential oils against two stored-product insects. Crop Protection, 144, 105575.
- de Assis Lage, T. C., Montanari, R. M., Fernandes, S. A., de Oliveira Monteiro, C. M., Senra, T. D. O. S., Zeringota, V., ... & Daemon, E. (2015). Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae). Experimental Parasitology, 148, 24–29. [CrossRef]
- Cazella, L. N., Glamoclija, J., Soković, M., Gonçalves, J. E., Linde, G. A., Colauto, N. B., & Gazim, Z. C. (2019). Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Frontiers in Plant Science, 10, 27.
- Pedrotti, C., da Silva Ribeiro, R. T., & Schwambach, J. (2019). Control of postharvest fungal rots in grapes through the use of Baccharis trimera and Baccharis dracunculifolia essential oils. Crop Protection, 125, 104912.
- Bonin, E., Carvalho, V. M., Avila, V. D., Aparecida Dos Santos, N. C., Benassi-Zanqueta, É., Contreras Lancheros, C. A., ... & Nunes Do Prado, I. (2020). Baccharis dracunculifolia: Chemical constituents, cytotoxicity and antimicrobial activity. LWT, 120, 108920. [CrossRef]
- Tomazzoli, M. M., Amaral, W. D., Cipriano, R. R., Tomasi, J. D. C., Gomes, E. N., Ferriani, A. P., ... & Deschamps, C. (2021). Chemical composition and antioxidant activity of essential oils from populations of Baccharis dracunculifolia DC. in southern Brazil. Brazilian Archives of Biology and Technology, 64, e21190253. [CrossRef]
- Rigotti, M., Facanali, R., Haber, L. L., Vieira, M. A. R., Isobe, M. T. C., Cavallari, M. M., ... & Marques, M. O. M. (2023). Chemical and genetic diversity of Baccharis dracunculifolia DC. (Asteraceae) from the Cerrado biome. Biochemical Systematics and Ecology, 111, 104735.
- Cruzeiro, R. R. P., Davies, N. W., Obara, M. T., Silveira, D., Gomes-Copeland, K. K. P., De Castro Nizio, D. A., & Fagg, C. W. (2024). Activity of essential oils from Brazilian Cerrado against Aedes aegypti (Diptera: Culicidae) larvae. South African Journal of Botany, 172, 619–626. [CrossRef]
- Von Poser, G. L. (2016). A quimiotaxonomia na sistemática dos seres vivos. In C. M. O. Simões, E. P. Schenkel, J. C. P. Mello, L. A. Mentz, & P. R. Petrovick (Orgs.), Farmacognosia: do produto natural ao medicamento (pp. 23–28). Artmed.
| Database | Search Strategy | Articles |
|---|---|---|
| Science Direct | "Chemical profile" AND "Volatile compounds" AND "Baccharis" | 76 |
| PubMed | "Chemical profile" AND "Volatile compounds" AND "Baccharis" | 4 |
| CAPES | "Chemical profile" AND "Volatile compounds" AND "Baccharis" | 3 |
| Web of Science | "Chemical profile" AND "Volatile compounds" AND "Baccharis" | 5 |
| Scopus | "Chemical profile" AND "Volatile compounds" AND "Baccharis" | 6 |
| Compound | Formula | Molar Mass (g mol-1) | Baccharis oblongifolia |
|---|---|---|---|
| p-Cymene | C₁₀H₁₄ | 134.22 | X |
| Myrcene | C₁₀H₁₆ | 136.23 | X |
| β-E-Ocimene | C₁₀H₁₆ | 136.23 | X |
| α-Pinene | C₁₀H₁₆ | 136.23 | X |
| β-Pinene | C₁₀H₁₆ | 136.23 | X |
| Sabinene | C₁₀H₁₆ | 136.23 | X |
| γ-Terpinene | C₁₀H₁₆ | 136.23 | X |
| α-Terpineol | C₁₀H₁₈O | 154.25 | X |
| α-Thujene | C₁₀H₁₆ | 136.23 | X |
| Terpinen-4-ol | C₁₀H₁₈O | 154.25 | X |
| δ-Cadinene | C₁₅H₂₄ | 204.35 | X |
| Copaene | C₁₅H₂₄ | 204.35 | X |
| β-Elemene | C₁₅H₂₄ | 204.35 | X |
| Germacrene D | C₁₅H₂₄ | 204.35 | X |
| cis-β-Guaiene | C₁₅H₂₄ | 204.35 | X |
| α-Humulene | C₁₅H₂₄ | 204.35 | X |
| γ-Muurolene | C₁₅H₂₄ | 204.35 | X |
| Compound | Formula | Molar Mass (g mol-1) | B. salicifolia | B. anomala |
|---|---|---|---|---|
| Styrene | C₈H₈ | 104.15 | - | X |
| Phenylmethanol | C₇H₈O | 108.14 | - | X |
| Ethylidenecyclohexane | C₈H₁₄ | 110.2 | - | X |
| 2-Phenylpropene | C₉H₁₀ | 118.18 | - | X |
| 1,2,3-Trimethylbenzene | C₉H₁₂ | 120.19 | - | X |
| 1,2,4-Trimethylbenzene | C₉H₁₂ | 120.19 | - | X |
| 1,3,5-Trimethylbenzene | C₉H₁₂ | 120.19 | - | X |
| Cumene | C₉H₁₂ | 120.19 | - | X |
| p-Cymene | C₁₀H₁₄ | 134.22 | X | - |
| Myrcene | C₁₀H₁₆ | 136.23 | X | - |
| Sabinene | C₁₀H₁₆ | 136.23 | X | - |
| α-Pinene | C₁₀H₁₆ | 136.23 | X | X |
| α-Thujene | C₁₀H₁₆ | 136.23 | X | - |
| β-E-Ocimene | C₁₀H₁₆ | 136.23 | X | - |
| β-Pinene | C₁₀H₁₆ | 136.23 | X | X |
| γ-Terpinene | C₁₀H₁₆ | 136.23 | X | - |
| 1,1,4-Trimethylidane | C₁₀H₁₆ | 160.25 | - | X |
| 1,1,5-Trimethylidane | C₁₂H₁₆ | 160.25 | - | X |
| 1,1,6-Trimethylidane | C₁₂H₁₆ | 160.25 | - | X |
| Cyclohexylbenzene | C₁₂H₁₆ | 160.25 | - | X |
| Trimethylidane | C₁₂H₁₆ | 160.25 | - | X |
| trans-Calamenene | C₁₅H₂₂ | 202.33 | - | X |
| α-Humulene | C₁₅H₂₄ | 204.35 | X | - |
| β-Elemene | C₁₅H₂₄ | 204.35 | X | - |
| Bicyclogermacrene | C₁₅H₂₄ | 204.35 | X | - |
| Copaene | C₁₅H₂₄ | 204.35 | X | - |
| Germacrene D | C₁₅H₂₄ | 204.35 | X | - |
| Modheph-2-ene | C₁₅H₂₄ | 204.35 | X | - |
| β-Cubebene | C₁₅H₂₄ | 204.35 | X | - |
| δ-Cadinene | C₁₅H₂₄ | 204.35 | X | - |
| 1-Epi-cubenol | C₁₅H₂₆O | 222.37 | X | - |
| Viridiflorol | C₁₅H₂₆O | 222.37 | X | - |
| α-Cadinol | C₁₅H₂₆O | 222.37 | X | - |
| α-Muurolol | C₁₅H₂₆O | 222.37 | X | - |
| Compound | MF | MM (g mol-1) |
BM | BT | BMY | BTR | BD | BAX | BAR | BME | BR | BDR | BC | BU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Hexenal | C₆H₁₀O | 98.14 | - | - | - | - | - | - | - | - | X | - | - | - |
| Hexanal | C₆H₁₂O | 100.16 | - | - | - | - | - | - | - | - | X | - | - | - |
| (E)-3-Hexen-1-ol | C₆H₁₂O | 100.16 | - | - | - | - | - | - | - | - | X | - | - | - |
| 1-Hexanol | C₆H₁₄O | 102.17 | - | - | - | - | - | - | - | - | X | - | - | - |
| Acetophenone | C₈H₈O | 120.15 | - | - | - | - | - | - | - | - | - | X | - | - |
| p-Cymenene | C₁₀H₁₂ | 132.2 | - | - | - | - | X | - | - | - | - | - | - | X |
| p-Methylacetophenone | C₉H₁₀O | 134.17 | - | - | - | - | X | - | - | - | - | - | - | X |
| m-Cymene* | C₁₀H₁₄ | 134.22 | - | - | - | - | - | - | - | - | X | - | - | - |
| p-Cymene | C₁₀H₁₄ | 134.22 | X | X | X | - | X | X | X | X | - | X | X | X |
| δ-3-Carene | C₁₀H₁₆ | 136.23 | - | - | - | - | - | - | - | - | X | - | - | - |
| α-Ocimene | C₁₀H₁₆ | 136.23 | - | - | - | - | - | - | - | - | X | - | - | - |
| α-Fenchene (if intended; otherwise α-Felandrene kept as submitted) | C₁₀H₁₆ | 136.23 | - | - | - | - | - | - | - | - | - | - | - | X |
| (Z)-β-Ocimene | C₁₀H₁₆ | 136.23 | - | - | - | - | - | - | - | - | - | - | - | X |
| cis-β-Ocimene | C₁₀H₁₆ | 136.23 | - | X | - | - | - | - | - | - | - | X | - | - |
| Terpinolene | C₁₀H₁₆ | 136.23 | - | - | - | - | X | - | - | - | X | X | - | - |
| α-Terpinene | C₁₀H₁₆ | 136.23 | - | - | - | - | X | - | - | - | - | X | - | X |
| β-Felandrene | C₁₀H₁₆ | 136.23 | X | X | - | X | - | - | - | - | - | - | - | X |
| Camphene | C₁₀H₁₆ | 136.23 | - | X | - | X | X | - | - | - | X | - | - | X |
| α-Pinene | C₁₀H₁₆ | 136.23 | X | X | X | X | - | X | X | X | X | X | X | X |
| Myrcene | C₁₀H₁₆ | 136.23 | X | X | X | - | X | X | X | X | X | X | X | X |
| γ-Terpinene | C₁₀H₁₆ | 136.23 | X | X | X | - | X | X | X | X | X | X | X | X |
| β-E-Ocimene | C₁₀H₁₆ | 136.23 | X | X | X | X | X | X | X | X | - | X | X | X |
| β-Pinene | C₁₀H₁₆ | 136.23 | X | X | X | X | X | X | X | X | X | X | X | X |
| α-Thujene | C₁₀H₁₆ | 136.23 | X | X | X | X | X | X | X | X | X | X | X | X |
| Sabinene | C₁₀H₁₆ | 136.23 | X | X | X | X | X | X | X | X | X | X | X | X |
| β-Isophorone | C₉H₁₄O | 138.21 | - | X | - | - | - | - | - | - | - | - | - | - |
| δ-Isopulegol | C₉H₁₄O | 138.21 | - | - | - | - | - | - | - | - | - | X | - | - |
| Nopinone | C₉H₁₄O | 138.21 | - | - | - | - | - | - | - | - | - | - | - | X |
| Cryptone | C₉H₁₄O | 138.21 | - | X | - | - | X | - | - | - | - | - | - | X |
| n-Nonanal | C₉H₁₈O | 142.24 | - | - | - | - | X | - | - | - | - | - | - | - |
| Cuminaldehyde | C₁₀H₁₂O | 148.2 | - | - | - | - | X | - | - | - | - | - | - | X |
| Pinocarvone | C₁₀H₁₄O | 150.22 | - | - | - | - | - | - | - | - | - | - | - | X |
| Safranal | C₁₀H₁₄O | 150.22 | - | - | - | - | - | - | - | - | - | - | - | X |
| Carvone | C₁₀H₁₄O | 150.22 | - | - | - | - | X | - | - | - | - | - | - | - |
| Myrtenal | C₁₀H₁₄O | 150.22 | - | - | - | - | X | - | - | - | - | X | - | - |
| Verbenone | C₁₀H₁₄O | 150.22 | - | - | - | - | X | - | - | - | - | - | - | X |
| α-Campholenal | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | - |
| (E)-Pinocarveol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | - |
| (E)-Sabinol | C₁₀H₁₆O | 152.23 | - | - | - | - | - | - | - | - | - | - | - | X |
| (E)-Verbenol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | - |
| (Z)-Carveol | C₁₀H₁₆O | 152.23 | - | - | - | - | - | - | - | - | - | - | - | X |
| Geranial | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | - |
| (E)-Tagetone | C₁₀H₁₆O | 152.23 | - | - | - | - | - | - | - | - | - | - | - | X |
| Myrtenol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | X | - | - |
| Camphor | C₁₀H₁₆O | 152.23 | - | X | - | - | X | - | - | - | - | - | - | - |
| p-Menthadienol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | X |
| (E)-Carveol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | X |
| Perillyl alcohol | C₁₀H₁₆O | 152.23 | - | - | - | - | X | - | - | - | - | - | - | X |
| Dihydrotagetone | C₁₀H₁₈O | 154.25 | - | - | - | - | - | - | - | - | - | X | - | - |
| Citronellal | C₁₀H₁₈O | 154.25 | - | - | - | - | X | - | - | - | - | - | - | X |
| Borneol | C₁₀H₁₈O | 154.25 | - | - | - | - | X | - | - | - | - | - | - | X |
| (Z)-Piperitol | C₁₀H₁₈O | 154.25 | - | - | - | - | X | - | - | - | - | - | - | X |
| Geraniol | C₁₀H₁₈O | 154.25 | - | - | - | - | X | - | - | - | - | - | - | X |
| Linalool | C₁₀H₁₈O | 154.25 | - | - | - | - | X | - | - | - | X | X | - | - |
| Terpinen-4-ol | C₁₀H₁₈O | 154.25 | X | - | - | - | - | - | X | X | - | X | X | X |
| α-Terpineol | C₁₀H₁₈O | 154.25 | X | - | - | - | X | - | X | X | X | X | X | X |
| Methyl eugenol | C₁₁H₁₄O₂ | 178.23 | - | - | - | - | - | - | - | - | - | X | - | - |
| β-Damascenone | C₁₃H₁₈O | 190.28 | - | - | - | - | - | - | - | - | X | - | - | - |
| Carquejol acetate | C₁₂H₁₅O₂ | 191.25 | - | X | - | - | - | - | - | - | - | - | - | - |
| (E)-β-Ionone | C₁₃H₂₀O | 192.3 | - | - | - | - | - | - | - | - | - | - | - | X |
| trans-Pinocarvyl acetate | C₁₂H₁₈O₂ | 194.27 | - | X | - | - | - | - | - | - | - | - | - | - |
| Bornyl acetate | C₁₂H₁₈O₂ | 196.29 | - | X | - | X | - | - | - | - | - | - | - | - |
| Cadalene | C₁₅H₁₈ | 198.3 | - | - | - | - | - | - | - | - | - | - | - | X |
| α-Calacorene | C₁₅H₂₀ | 200.32 | - | - | - | - | X | - | - | - | - | - | - | - |
| α-Curcumene | C₁₅H₂₂ | 202.33 | - | - | - | - | - | - | - | - | - | X | - | - |
| trans-Calamenene | C₁₅H₂₂ | 202.33 | - | - | X | - | - | - | X | - | - | - | - | - |
| ar-Curcumene | C₁₅H₂₂ | 202.33 | X | - | - | - | - | - | - | - | - | - | - | X |
| β-Eudesmene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | X | - | - | - |
| β-Cadinene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | X | - | - | - |
| α-Elemene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| α-Bourbonene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Ylangene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| δ-Elemene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Dauca-5,8-diene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| (E)-Muurola-4(14),5-diene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | - |
| δ-Amorphene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | - |
| Aromadendrene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | X | X | - | - |
| α-Gurjunene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | - | X | - | X |
| δ-Amorphene (duplicate preserved) | C₁₅H₂₄ | 204.35 | X | - | - | - | - | - | - | - | - | - | - | X |
| α-Longipinene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| 2-Epi-β-Funebrene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| β-Gurjunene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| α-(E)-Bergamotene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| (E)-β-Farnesene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| Germacrene A | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | - | - | X |
| Allo-aromadendrene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | X | X | - | - |
| α-Muurolene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | - | - | - | X | X | - | X |
| β-Copaene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | X | - | X |
| α-Cubebene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | X | - | X |
| trans-α-Bergamotene | C₁₅H₂₄ | 204.35 | X | - | - | X | - | - | - | - | - | - | - | X |
| (E,E)-α-Farnesene | C₁₅H₂₄ | 204.35 | X | - | - | X | - | - | - | - | - | - | - | X |
| β-Cubebene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | X | - | - | - | X |
| (E)-Cadina-1(6)-4-diene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | - | X | - | X |
| β-Guaiene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | X | X | - | X |
| Ledene | C₁₅H₂₄ | 204.35 | - | - | X | - | X | - | - | - | X | - | - | X |
| γ-Cadinene | C₁₅H₂₄ | 204.35 | - | - | - | - | X | - | - | - | X | X | - | X |
| α-Amorphene | C₁₅H₂₄ | 204.35 | X | X | - | X | - | - | - | - | - | - | - | X |
| cis-β-Guaiene | C₁₅H₂₄ | 204.35 | - | - | - | - | - | X | X | - | - | - | X | X |
| β-Elemene | C₁₅H₂₄ | 204.35 | - | X | - | - | X | X | X | - | X | X | X | X |
| Germacrene D | C₁₅H₂₄ | 204.35 | - | - | - | - | X | X | X | X | X | X | X | X |
| Bicyclogermacrene | C₁₅H₂₄ | 204.35 | X | X | - | X | X | - | - | X | X | X | - | X |
| δ-Cadinene | C₁₅H₂₄ | 204.35 | X | X | - | X | X | X | - | X | - | X | - | X |
| Copaene | C₁₅H₂₄ | 204.35 | - | - | X | - | X | X | X | X | X | X | X | X |
| α-Humulene | C₁₅H₂₄ | 204.35 | X | X | X | X | X | X | X | X | X | X | X | X |
| γ-Muurolene | C₁₅H₂₄ | 204.35 | X | X | X | X | X | X | X | X | X | X | X | X |
| Tetradecanal | C₁₄H₂₈O | 212.37 | - | X | - | - | - | - | - | - | - | - | - | X |
| Isobicyclogermacrenal | C₁₅H₂₂O | 218.33 | - | - | - | - | - | - | - | - | - | X | - | - |
| (E)-Calamen-10-ol | C₁₅H₂₂O | 218.33 | - | - | - | - | - | - | - | - | - | - | - | X |
| Ledene oxide | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Isospathulenol | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | X | - | - | - |
| Cedrene-13-en-8-ol | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Germacran-δ-4-ol | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Cabreuva oxide B | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| β-Copaen-4α-ol | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Khusimol | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | X | - | - |
| Salviol-4(14)-en-1-one | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | X | - | - | - | - |
| Iso-italicene epoxide | C₁₅H₂₂O | 220.35 | - | - | - | - | - | - | - | - | - | - | - | X |
| Eudesma-4(15),7-dien-1β-ol | C₁₅H₂₂O | 220.35 | X | - | - | - | - | - | - | - | - | - | - | X |
| Aromadendrene oxide | C₁₅H₂₂O | 220.35 | X | X | - | - | - | - | - | - | - | - | - | - |
| Spathulenol | C₁₅H₂₂O | 220.35 | - | X | - | - | - | - | - | - | - | X | - | - |
| Humulene epoxide II | C₁₅H₂₂O | 220.35 | X | - | - | - | - | - | - | X | - | - | X | X |
| Germacra-4(15),5,10(14)-trien-1α-ol | C₁₅H₂₂O | 220.35 | X | X | - | - | - | - | - | - | - | X | - | X |
| Isospathulenol (duplicate preserved) | C₁₅H₂₂O | 220.35 | X | X | X | - | - | - | X | - | - | - | X | X |
| Caryophyllene oxide | C₁₅H₂₂O | 220.35 | X | X | X | X | - | X | - | X | - | X | X | X |
| trans-Nerolidol | C₁₅H₂₆O | 222.37 | - | - | - | - | - | - | - | - | - | X | - | - |
| Junenol | C₁₅H₂₆O | 222.37 | - | - | - | - | - | - | - | - | - | X | - | - |
| α-Muurolol | C₁₅H₂₆O | 222.37 | - | - | - | - | - | - | - | - | - | X | - | - |
| Guaiol | C₁₅H₂₆O | 222.37 | - | - | - | - | - | - | - | - | - | - | X | - |
| α-Acorenol | C₁₅H₂₆O | 222.37 | X | - | - | - | - | - | - | - | - | - | - | - |
| Cubenol | C₁₅H₂₆O | 222.37 | - | - | - | - | - | - | - | - | - | - | - | X |
| Rosifoliol | C₁₅H₂₆O | 222.37 | - | - | X | - | - | - | X | - | - | - | - | X |
| Bulnesol | C₁₅H₂₆O | 222.37 | X | - | - | - | X | - | - | - | - | - | - | X |
| α-Epi-cadinol | C₁₅H₂₆O | 222.37 | - | - | X | - | - | - | X | - | X | X | - | - |
| t-Cadinol | C₁₅H₂₆O | 222.37 | X | X | - | X | - | - | - | - | - | - | - | X |
| Cubeban-11-ol | C₁₅H₂₆O | 222.37 | - | - | X | - | - | - | - | - | - | X | X | X |
| β-Selinene | C₁₅H₂₆O | 222.37 | - | X | - | X | X | X | - | - | - | - | X | - |
| Palustrol | C₁₅H₂₆O | 222.37 | X | X | - | - | - | - | X | - | - | X | - | X |
| Ledol | C₁₅H₂₆O | 222.37 | X | X | - | - | X | - | - | - | - | X | - | X |
| 1-Epi-cubenol | C₁₅H₂₆O | 222.37 | X | - | X | - | - | - | - | - | - | X | X | X |
| β-Eudesmol | C₁₅H₂₆O | 222.37 | X | X | X | - | - | X | - | - | - | - | X | X |
| Globulol | C₁₅H₂₆O | 222.37 | - | X | - | - | X | - | X | X | X | X | - | X |
| Viridiflorol | C₁₅H₂₆O | 222.37 | X | X | X | - | - | X | X | - | X | X | X | X |
| α-Cadinol | C₁₅H₂₆O | 222.37 | X | X | X | - | - | X | X | X | - | X | X | X |
| Epi-α-Muurolol | C₁₅H₂₆O | 222.37 | X | X | X | - | - | X | X | X | - | X | X | X |
| Murolan-3,9(11)-diene-10-peroxide | C₁₅H₂₄O₂ | 236.35 | - | - | - | - | X | - | - | - | - | X | - | X |
| β-Oplopenone | C₁₅H₂₆O₂ | 238.37 | - | - | - | - | - | - | - | - | - | X | - | - |
| Neophytadiene | C₂₀H₃₈ | 278.5 | X | X | - | - | - | - | - | - | - | - | - | - |
| Phytol | C₂₀H₄₀O | 296.5 | - | - | - | - | - | - | - | - | - | X | - | - |
| Heptacosane | C₂₇H₅₆ | 380.7 | - | - | - | - | - | - | - | - | - | X | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





