Submitted:
18 November 2025
Posted:
19 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Test-Systems
2.3. Preparation of the Iodine-Containing Complex
2.3.1. Preparation of Dextrin Solution
2.3.2. Preparation of a Solution of Lithium Triiodide, Magnesium Triiodide
2.3.3. Preparation of Lithium Chloride Solution
2.3.4. Mixing Solutions
2.3.5. Crystallization
2.4. Quantum-Chemical Calculations
2.5. UV-Vis Spectroscopy
2.6. FT-IR
2.8. XRD
2.9. Thermogravimetric (TG) analysis
2.10. Capillary Electrophoresis
2.11. SEM-Analysis
2.12. Antimicrobial Activity Screening
- 100 µL of appropriate liquid growth medium was added to the required wells;
- 100 µL of a pre-prepared 4x stock solution of the corresponding antibiotic was added to the first wells of each row (A1, B1, C1, D1, E1, F1, H1), followed by two-fold serial dilutions across the row to create horizontal serial dilutions of the antibiotic;
- 100 µL of pre-prepared 2x dilutions of the APS compound were added vertically to generate serial dilutions along the columns;
- 20 µL of the working inoculum solution was added to each well containing 200 µL of the antibiotic and compound mixture. As a result, the final bacterial concentration in each well after inoculation was approximately 1.5×105 CFU/mL.
2.13. Cytotoxic Effect
2.13.1. Mononuclear Cells
2.13.2. Madin-Darby Canine Kidney Cells
3. Results and Discussion
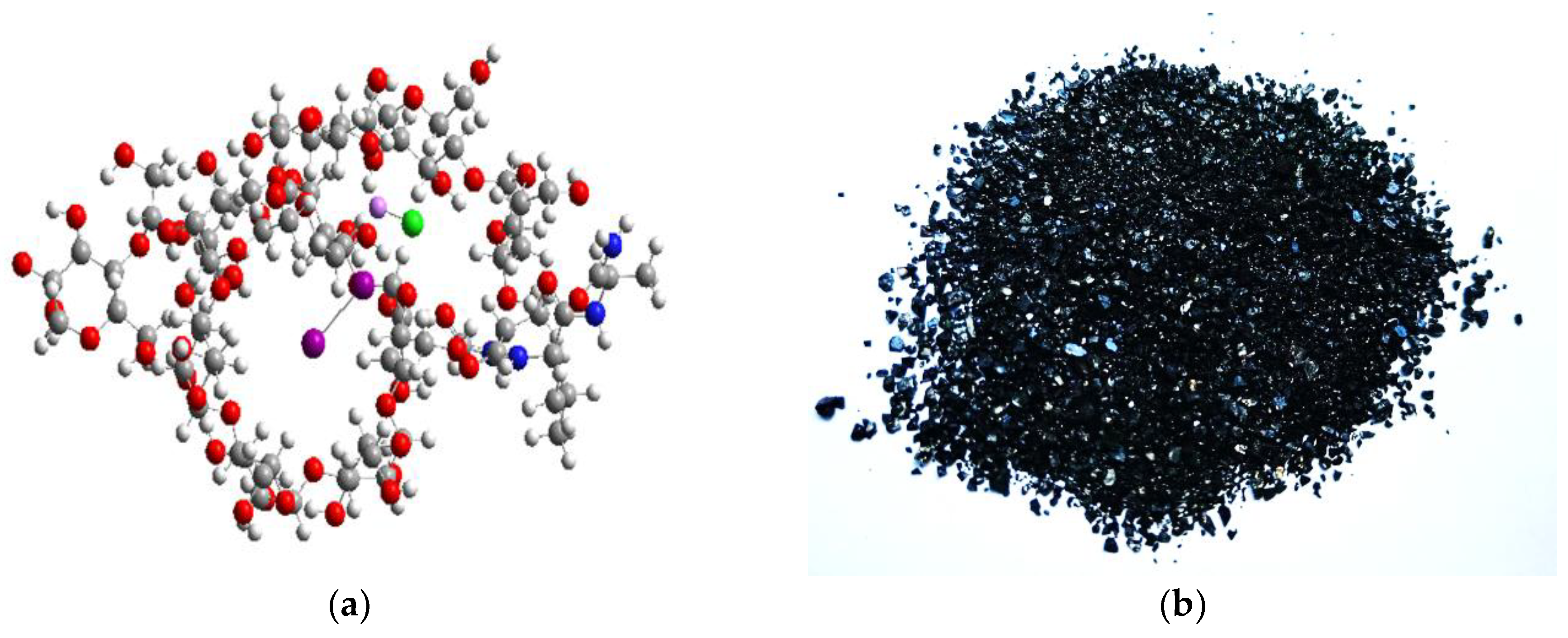
3.1. Quantum-Chemical Calculations
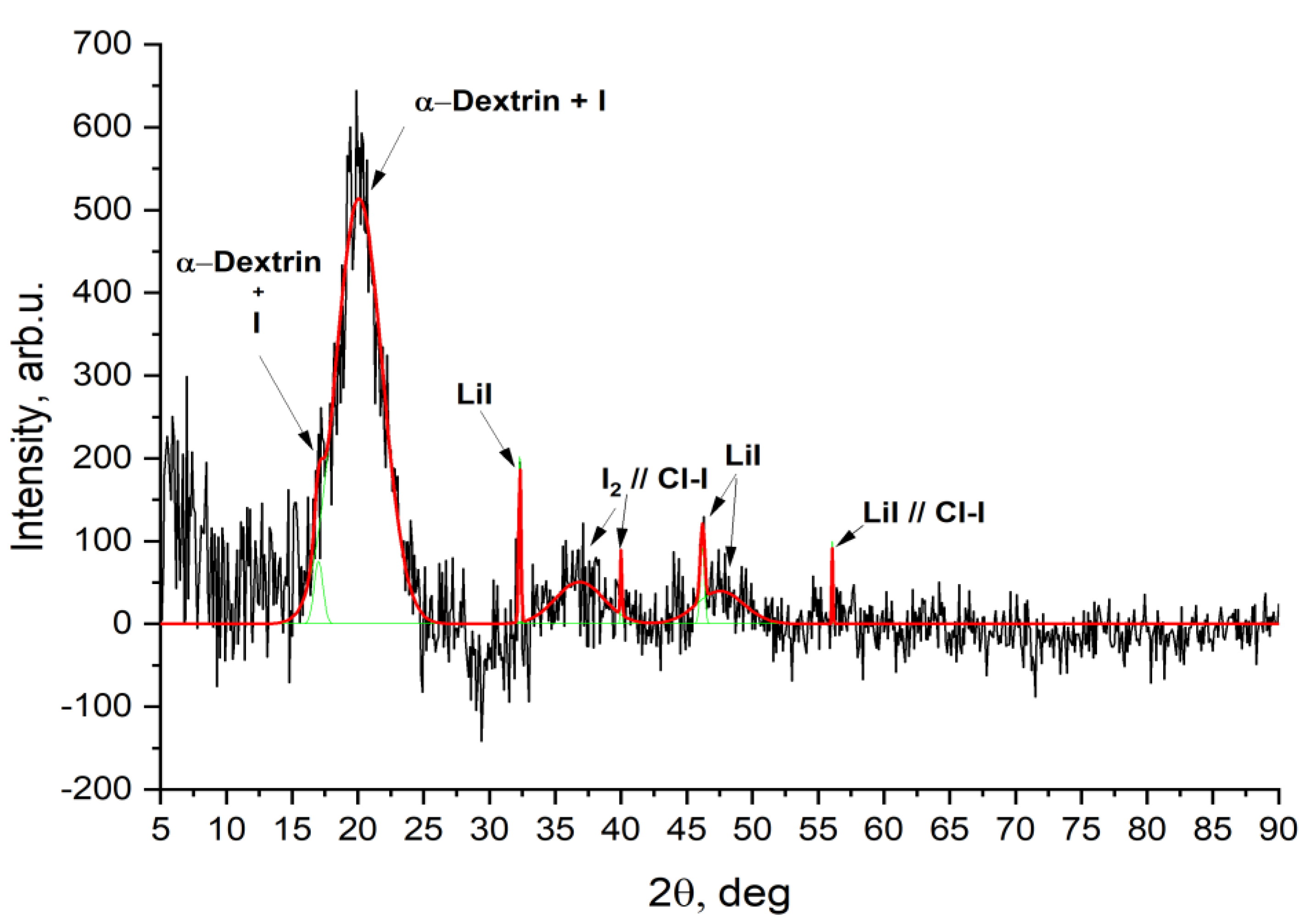
3.2. Physicochemical Properties of the Iodine-Containing Complex (IDLC)
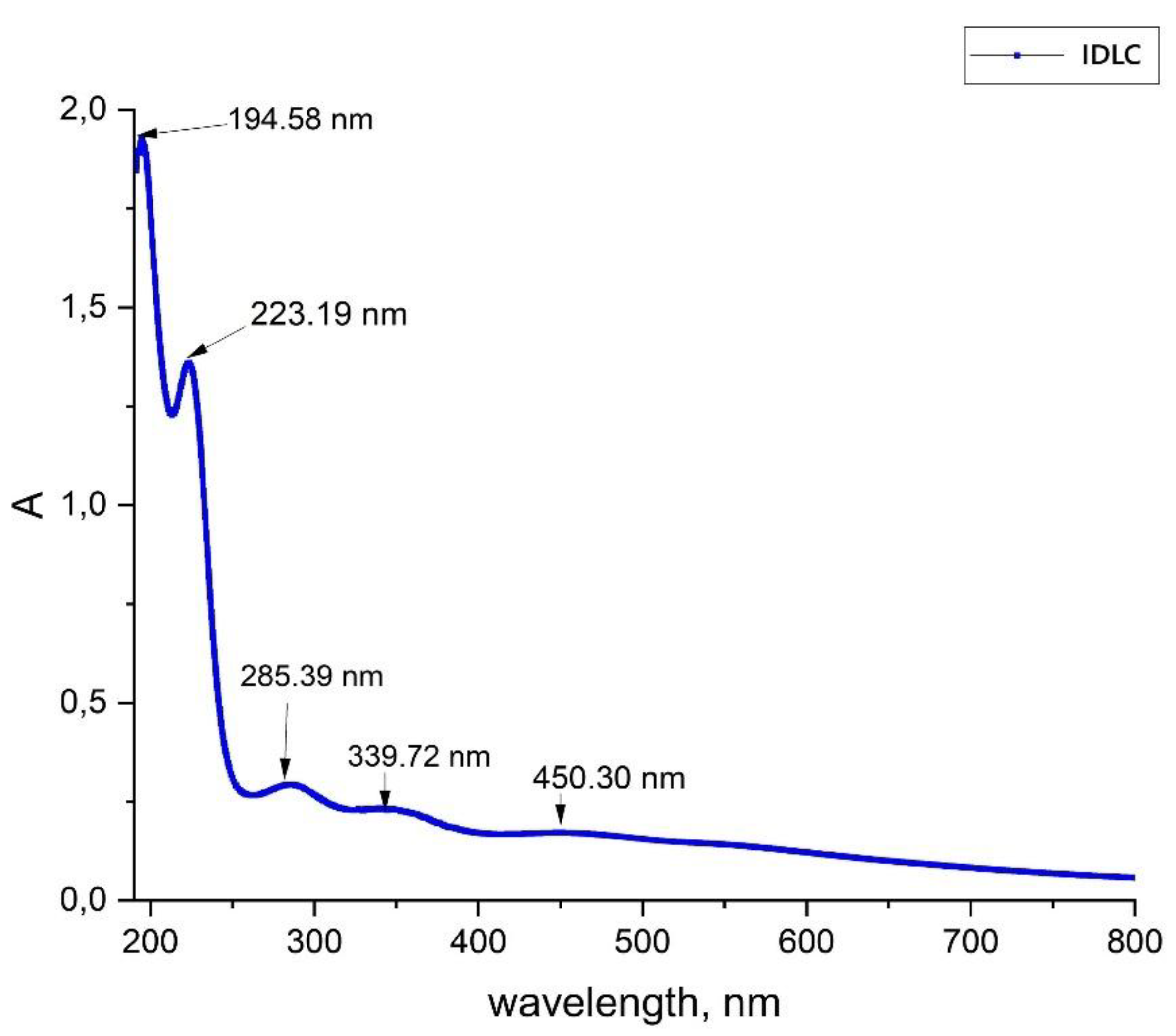
3.3. UV-Vis Spectral Analysis
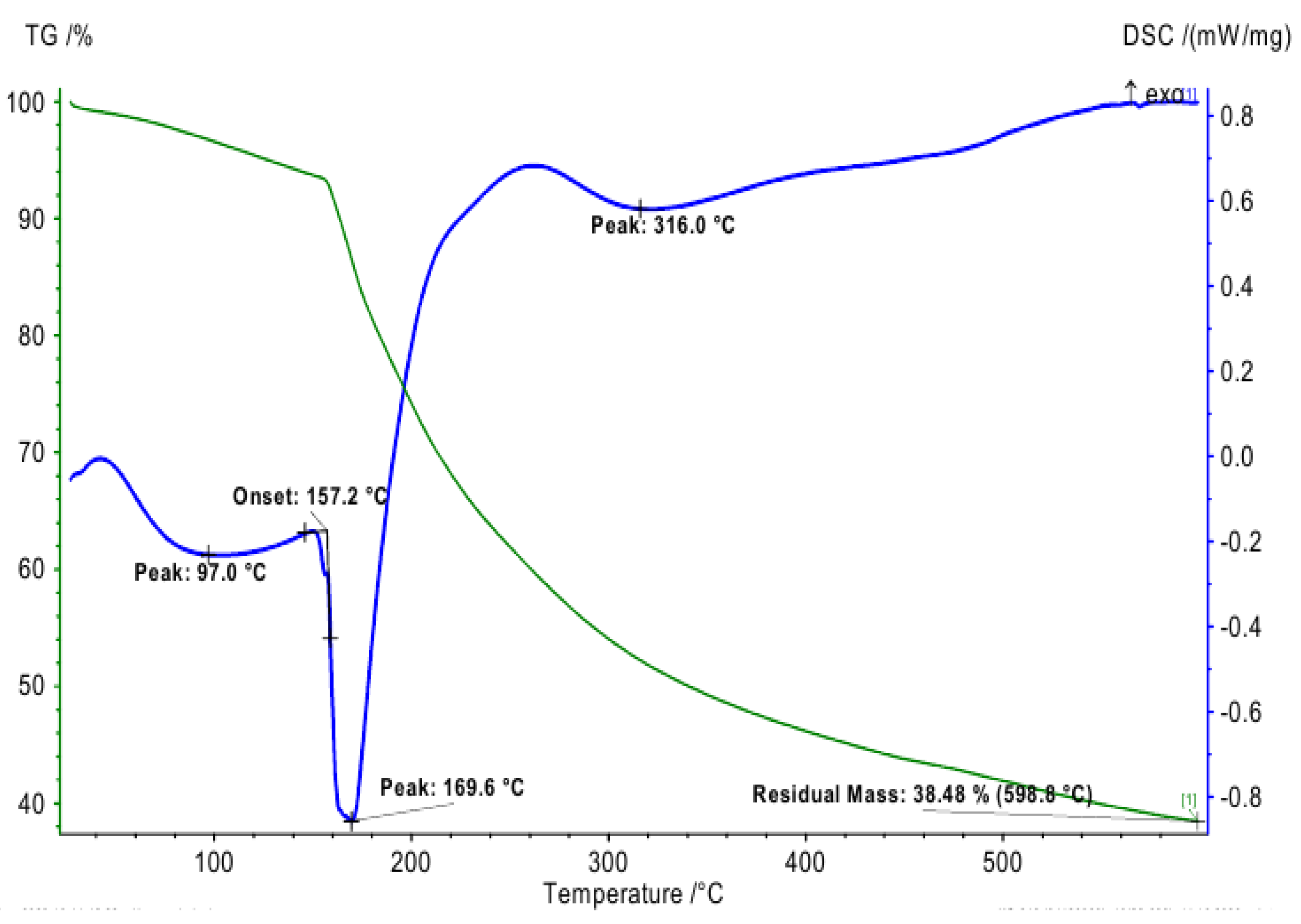
3.4. TG Analysis
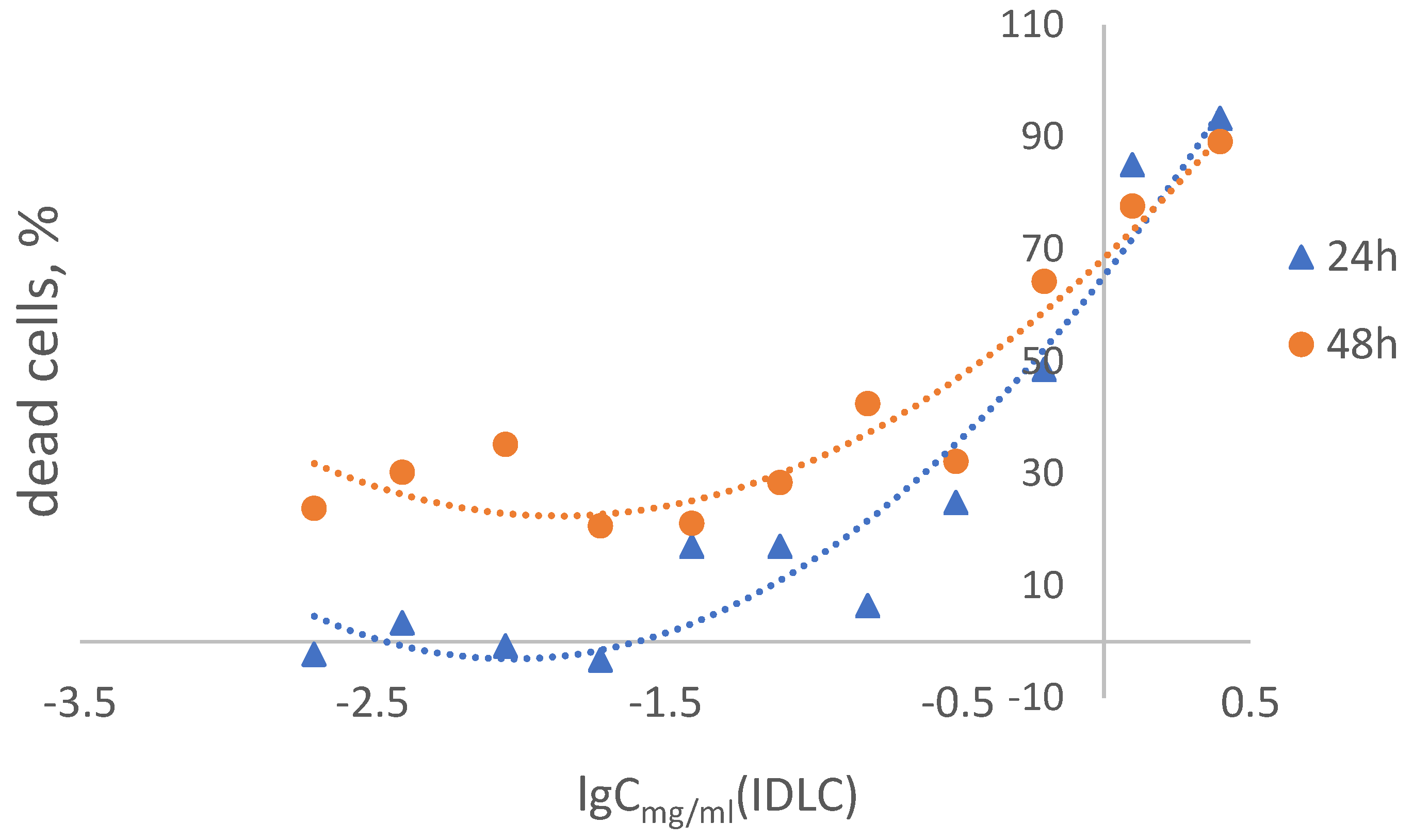
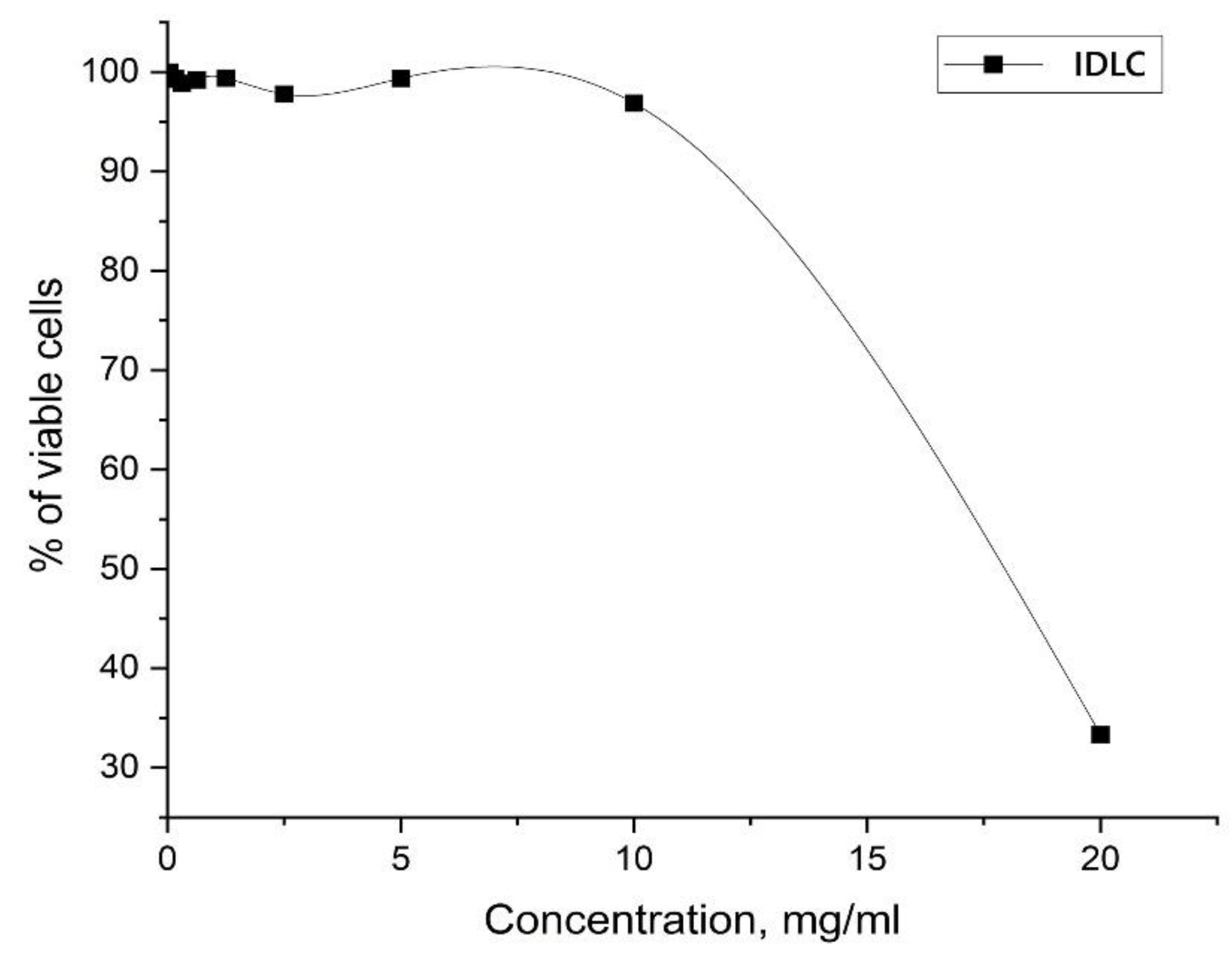
3.8. Cytotoxicity Study
3.9. Assesment of Antitumor Activity of IDLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- API active pharmaceutical ingredient
- CC50 Half cytotoxixity concntration
- DSC Differential scanning calorimetry
- IDLC iodine-dextrin-lithium complex
- MBC minimum bactericidal concentrations
- MDCK Madin-Darby Canine Kidney
- MNC Mononuclear cells
- MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- XRD X-ray diffraction analysis
References
- Lekhan, A.; Turner, R.J. Exploring Antimicrobial Interactions between Metal Ions and Quaternary Ammonium Compounds toward Synergistic Metallo-Antimicrobial Formulations. Microbiol. Spectr. 2024, 12, e0104724. [Google Scholar] [CrossRef] [PubMed]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Vergara, D.; Loza-Rodríguez, N.; Acevedo, F.; Bustamante, M.; López, O. Povidone-Iodine Loaded Bigels: Characterization and Effect as a Hand Antiseptic Agent. J. Drug Deliv. Sci. Technol. 2022, 72, 103427. [Google Scholar] [CrossRef]
- Smerdely, P.; Lim, A.; Boyages, S.C.; Waite, K.; Wu, D.; Roberts, V.; Leslie, G.; Arnold, J.; John, E.; Eastman, C.J. Topical Iodine-Containing Antiseptics and Neonatal Hypothyroidism in Very-Low-Birthweight Infants. Lancet 1989, 2, 661–664. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Wu, J.; Liu, F.; Xu, Y.; Wang, Y.; Shui, X.; Li, Q.; Zhao, B. Lithium Enhanced Plasmid-Mediated Conjugative Transfer of Antimicrobial Resistance Genes in Escherichia coli: Different Concentrations and Mechanisms. Aquat. Toxicol. 2025, 279, 107263. [Google Scholar] [CrossRef] [PubMed]
- Chernova, A.; Pukhniarskaia, D.; Biryukov, M.; Plotnikov, E. Influence of Lithium Salt on Escherichia coli Growth and Viability. Ind. Biotechnol. 2022, 18, 32–37. [Google Scholar] [CrossRef]
- Vaidya, M.Y.; McBain, A.J.; Butler, J.A.; et al. Antimicrobial Efficacy and Synergy of Metal Ions against Enterococcus faecium, Klebsiella pneumoniae and Acinetobacter baumannii in Planktonic and Biofilm Phenotypes. Sci. Rep. 2017, 7, 5911. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.; Pukhnyarskaya, D.; Chernova, A. Lithium and Microorganisms: Biological Effects and Mechanisms. Curr. Pharm. Biotechnol. 2023, 24, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Pedotti, S.; Ferreri, L.; Migliore, R.; Leotta, C.G.; Pitari, G.M.; D’Antona, N.; Petralia, S.; Aleo, D.; Sgarlata, C.; Consoli, G.M.L. A Novel Cationic β-Cyclodextrin Decorated with a Choline-Like Pendant Exhibits Iodophor, Mucoadhesive and Bactericidal Properties. Carbohydr. Polym. 2024, 328, 121698. [Google Scholar] [CrossRef] [PubMed]
- Saffarionpour, S.; Diosady, L.L. Cyclodextrins and Their Potential Applications for Delivering Vitamins, Iron, and Iodine for Improving Micronutrient Status. Drug Deliv. Transl. Res. 2025, 15, 26–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, B.; Wang, L.; McClements, D.J.; Li, C. Study of Dextrin Addition on the Formation and Physicochemical Properties of Whey Protein-Stabilized Emulsion: Effect of Dextrin Molecular Dimension. Food Hydrocoll. 2022, 128, 107569. [Google Scholar] [CrossRef]
- Zisi, A.P.; Exindari, M.K.; Siska, E.K.; Koliakos, G.G. Iodine-Lithium-Alpha-Dextrin (ILαD) against Staphylococcus aureus Skin Infections: A Comparative Study of In-Vitro Bactericidal Activity and Cytotoxicity between ILαD and Povidone-Iodine. J. Hosp. Infect. 2018, 98, 134–140. [Google Scholar] [CrossRef] [PubMed]
- GOST 4159-79. Reagents. Iodine. Specifications; Standards Publishing House: Moscow, Russia, 1979; 16 pp.
- Kireev, S.V.; Simanovsky, I.G.; Shnyrev, S.L. Investigation of the Absorption Spectra of Molecular Iodine in Liquid Media in the UV and Visible Regions. In Scientific Session of MEPhI; 2007; Volume 4, pp. 49. [CrossRef]
- Brown, G.D.; Bauer, J.; Osborn, H.M.; Kuemmerle, R. A Solution NMR Approach to Determine the Chemical Structures of Carbohydrates Using the Hydroxyl Groups as Starting Points. ACS Omega 2018, 3, 17957–17975. [Google Scholar] [CrossRef] [PubMed]
- Bodach, A.; Ortmeyer, J.; Herrmann, B.; Felderhoff, M. Amino–Organolithium Compounds and Their Aggregation for the Synthesis of Amino–Organoaluminium Compounds. Eur. J. Inorg. Chem. 2021, 23, 2248–2256. [Google Scholar] [CrossRef]
- Cardoso, M.V.C.; Sabadini, E. Before and Beyond the Micellization of n-Alkyl Glycosides: A Water-¹H NMR Relaxation Study. Langmuir 2013, 29, 15778–15786. [Google Scholar] [CrossRef] [PubMed]
- Sabitov, A.; Turganbay, S.; Kerimkulova, A.; Doszhanov, Y.; Saurykova, K.; Atamanov, M.; Zhumazhanov, A.; Bolatova, D. 1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley. Appl. Sci. 2025, 15, 10752. [Google Scholar] [CrossRef]
- European Pharmacopoeia; 7th ed. ; Council of Europe: Strasbourg, France, 2011; Volume 1, pp. 77–82.
- Pracher, L.; Zeitlinger, M. Preclinical and Clinical Studies in the Drug Development Process of European Medicines Agency-Approved Non-HIV Antiviral Agents: A Narrative Review. Clin. Microbiol. Infect. 2025, 31, 931–940. [Google Scholar] [CrossRef]
- OECD. Guideline for the Testing of Chemicals No. 487 – In Vitro Mammalian Cell Micronucleus Test; OECD: Paris, France, 2016. [Google Scholar]
- Turganbay, S.; Ilin, A.; Sabitov, A.; Askarova, D.; Jumagaziyeva, A.; Iskakbayeva, Z.; Seisembekova, A.; Bukeyeva, T.; Azembayev, A. Dextrin/Polyvinyl Alcohol/Iodine Complexes: Preparation, Characterization, and Antibacterial, Cytotoxic Activity. Eng. Sci. 2025, 35, 1595. [Google Scholar] [CrossRef]
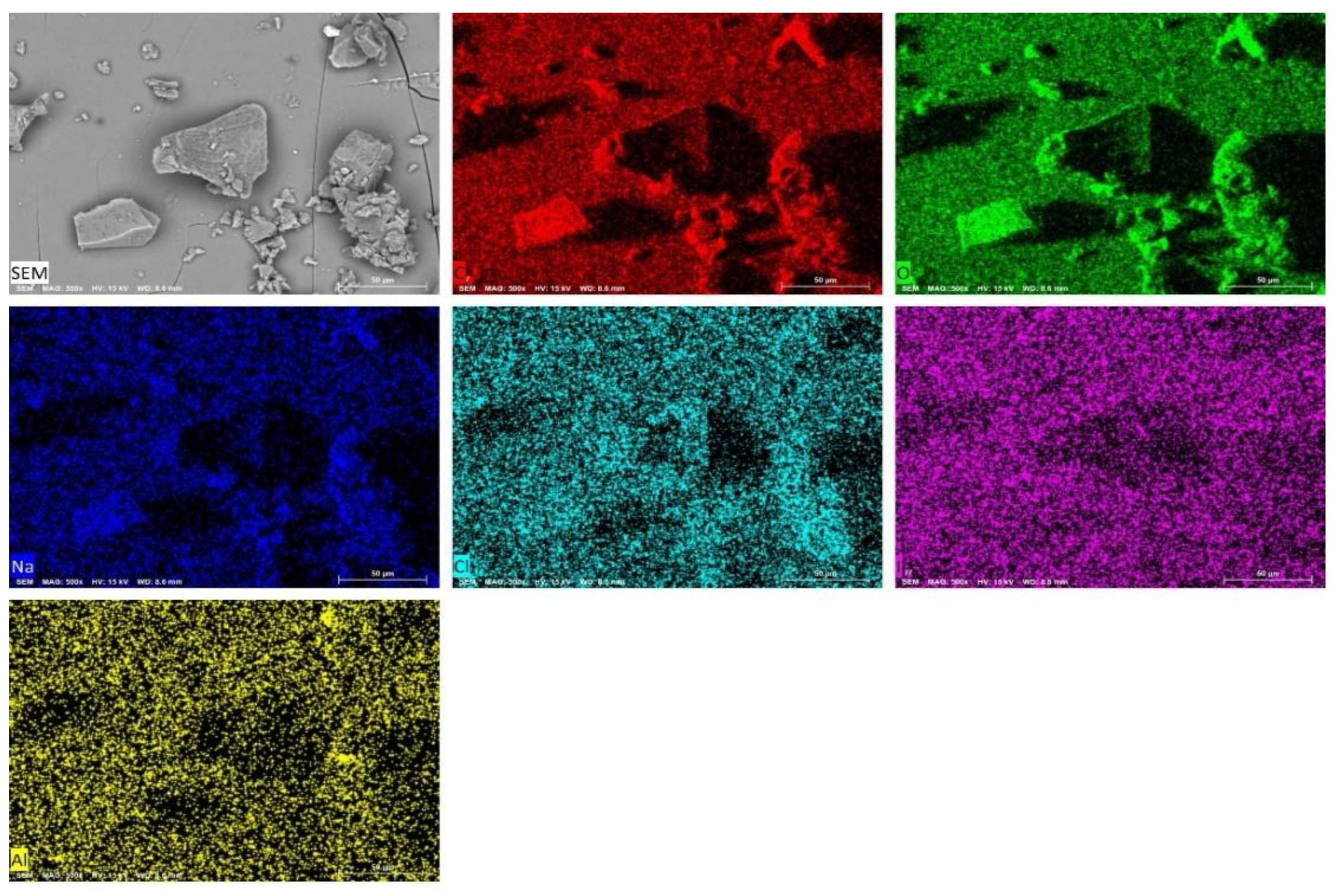
| Compound | Solubility in water | рН | Melting point, 0С | Quantitative determination of iodine, g/kg |
|---|---|---|---|---|
| IDLC | 1 g / 20 ml, soluble | 4,83 ± 0,01 | 146-148± 1 | 50,71 ± 0,01 |
| Compound | I-, mg/l (CE) | Li+, mg/l (CE) | ||
| exp. | theo. | exp. | theo. | |
| IDLC | 43,25 ± 0,08 | 47,5 | 6,78 ± 0,01 | 8,2 |
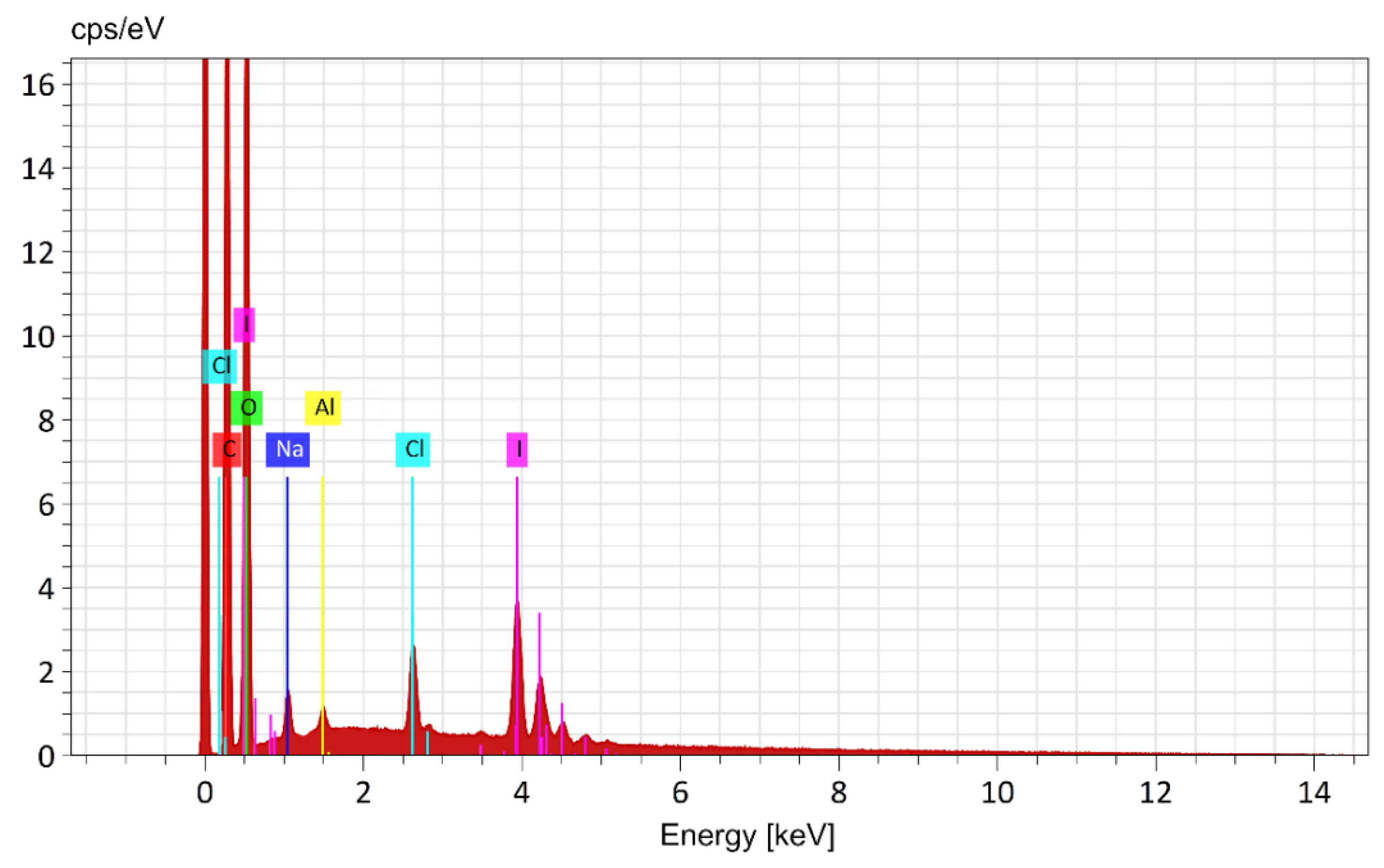
| Element | Mass % (theory) C124H248O122NI4K2. |
Mass % (detected) |
Line type |
|---|---|---|---|
| C | 34,70 | 34.62 ± 0.15 | K Series |
| O | 45,52 | 47.68 ± 0.33 | K Series |
| К | 1,82 | 1.55 ± 0.05 | K Series |
| N | 0,33 | 0,30 ± 0.02 | - |
| I | 11,84 | 10.15 ± 0.16 | L Series |
| H* | 5.78 | - | - |
| Totals | 100 | 94.3 |
| Compound name |
Name of the test strains | ||||||
| S. aureus ATCC 6538-P | S. aureus ATCC BAA-39 | E. coli ATCC 8739 | E. coli ATCC 196 | P.aeruginosa ATCC 9027 | P.aeruginosa TA2 | A.baumannii ATCC BAA-1790 | |
| Value of the minimum bactericidal concentration (MBC), mcg/ml in terms of substance | |||||||
| IDLC | 15,63 | 1,95 | 15,63 | 7,81 | 7,81 | 3,91 | 3,91 |
| Concentration of IDLC, mg/ml | % of viable cells (Mean ± SD) | |
|---|---|---|
| 24 h | 48 h | |
| Negative control | 100 ± 13,4 | 100 ± 13,0 |
| 2,5 | 6,7 ± 4,7 | 10,8 ± 1,8 |
| 1,25 | 15,0 ± 4,5 | 22,3 ± 29,8 |
| 0,625 | 51,5 ± 15,5 | 35,8 ± 5,5 |
| 0,312 | 75,2 ± 22,2 | 57,9 ± 14,2 |
| 0,156 | 83,6 ± 9,7 | 59,6 ± 4,8 |
| 0,078 | 83,0 ± 12,5 | 71,6 ± 13,1 |
| 0,039 | 83,0 ± 22,4 | 78,9 ± 17,1 |
| 0,019 | 103,3 ± 22,6 | 79,4 ± 15,9 |
| 0,009 | 100,8 ± 23,0 | 64,8 ± 6,6 |
| 0,004 | 96,6 ± 15,9 | 69,8 ± 7,5 |
| 0,002 | 122,3 ± 29,0 | 76,2 ± 15,5 |
| CC50 | 0,23 mg/ml | 0,48 mg/ml |
| Concentration, mg/mL | % of viable cells (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| HepG2 | HeLa | AGS | К562 | Н9 | MeT-5A | |
| Negative control | 100 ± 6,5 | 100 ± 3,7 | 100 ± 3,8 | 100,0 ± 3,0 | 100,0 ± 2,3 | 100,0 ± 4,6 |
| 5 | 28,7 ± 2,5 | 50,6 ± 2,7 | 3,9 ± 0,6 | 11,8 ± 2,4 | 9,8 ± 0,5 | 34,9 ± 2,8 |
| 2,5 | 74,9 ± 9,7 | 102,4 ± 3,3 | 28,0 ± 0,4 | 108,2 ± 4,6 | 38,1 ± 4,1 | 50,0 ± 0,8 |
| 1,25 | 158,6 ± 8,6 | 182,9 ± 5,3 | 88,9 ± 0,6 | 98,6 ± 13,9 | 92,3 ± 9,8 | 81,7 ± 3,0 |
| 0,625 | 154,4 ± 2,7 | 142,1 ± 4,3 | 101,6 ± 3,3 | 97,5 ± 4,1 | 95,4 ± 4,7 | 123,1 ± 3,0 |
| 0,312 | 130,2 ± 11,4 | 136,0 ± 2,9 | 101,6 ± 3,3 | 103,3 ± 3,6 | 95,1 ± 8,1 | 117,8 ± 6,9 |
| 0,156 | 129,0 ± 6,4 | 129,9 ± 1,4 | 111,7 ± 4,0 | 101,2 ± 5,9 | 103,8 ± 10,5 | 110,9 ± 1,9 |
| 0,08 | 124,0 ± 4,7 | 116,1 ± 1,7 | 102,4 ± 0,7 | 95,8 ± 11,9 | 87,4 ± 6,9 | 114,5 ± 5,4 |
| 0,04 | 125,9 ± 8,4 | 136,5 ± 1,7 | 106,3 ± 3,6 | 89,9 ± 8,2 | 98,0 ± 17,7 | 113,1 ± 4,3 |
| 0,02 | 149,0 ± 31,2 | 113,5 ± 9,3 | 98,8 ± 7,0 | 85,6 ± 3,7 | 95,7 ± 4,0 | 99,3 ± 16,9 |
| CC50, mg/mL | ~2,440 | 1,201 | 1,765 | 3,533 | 2,003 | 1,370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





