3.2.2. Estimation of Diffusion Length
The diffusion coefficient of the metal at a temperature just below its melting point is about 1 μm2/s so the diffusion length is estimated to be about a hundred micrometers. On the other hand, since diffusion bonding occurs at temperatures of about 50% to 80% of the metal’s melting point, metal atoms move between each other’s regions to fill voids and jump to fill atomic vacancies; as a result, the diffusion length is expected to be on the order of several micrometers.
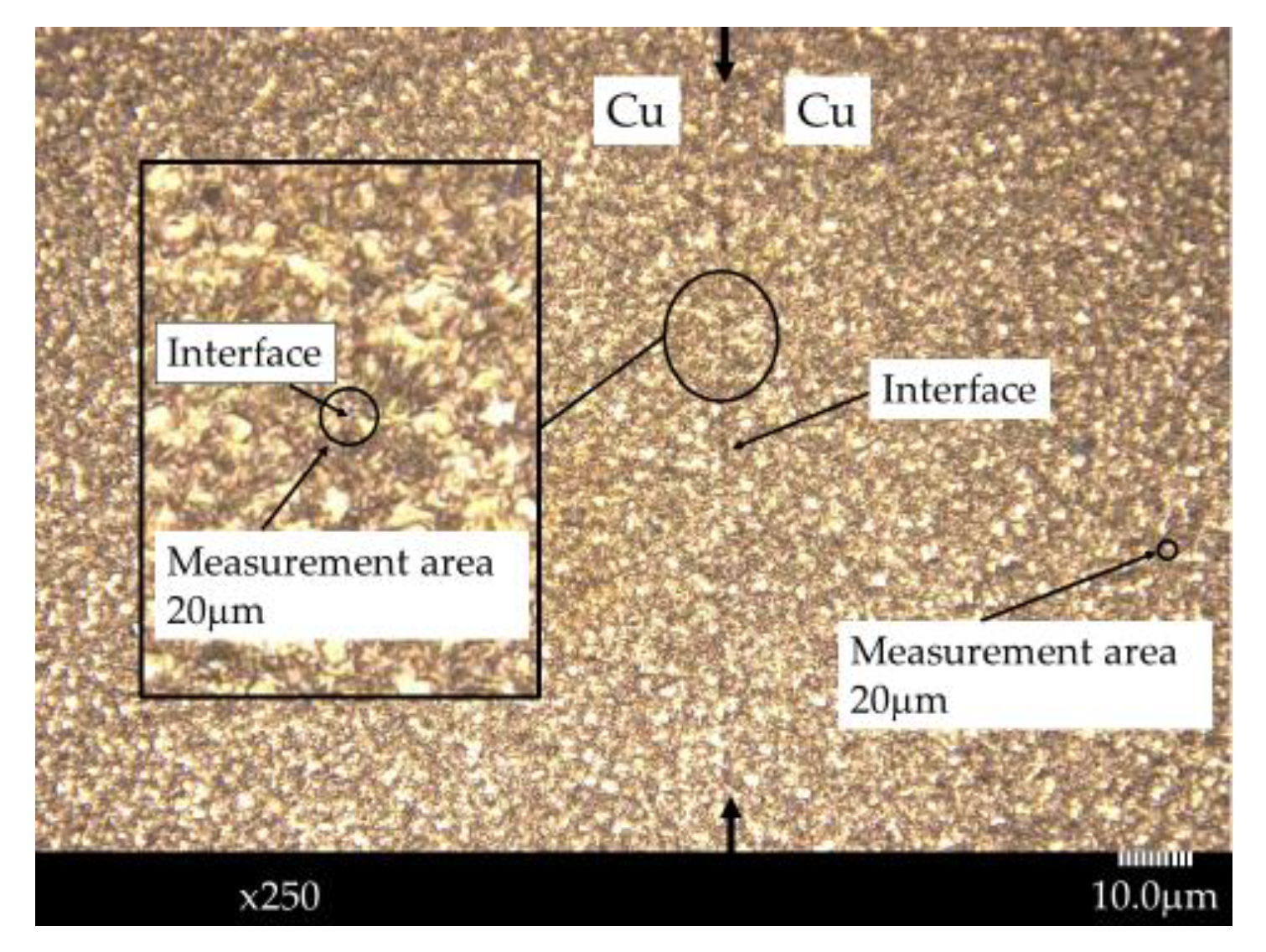
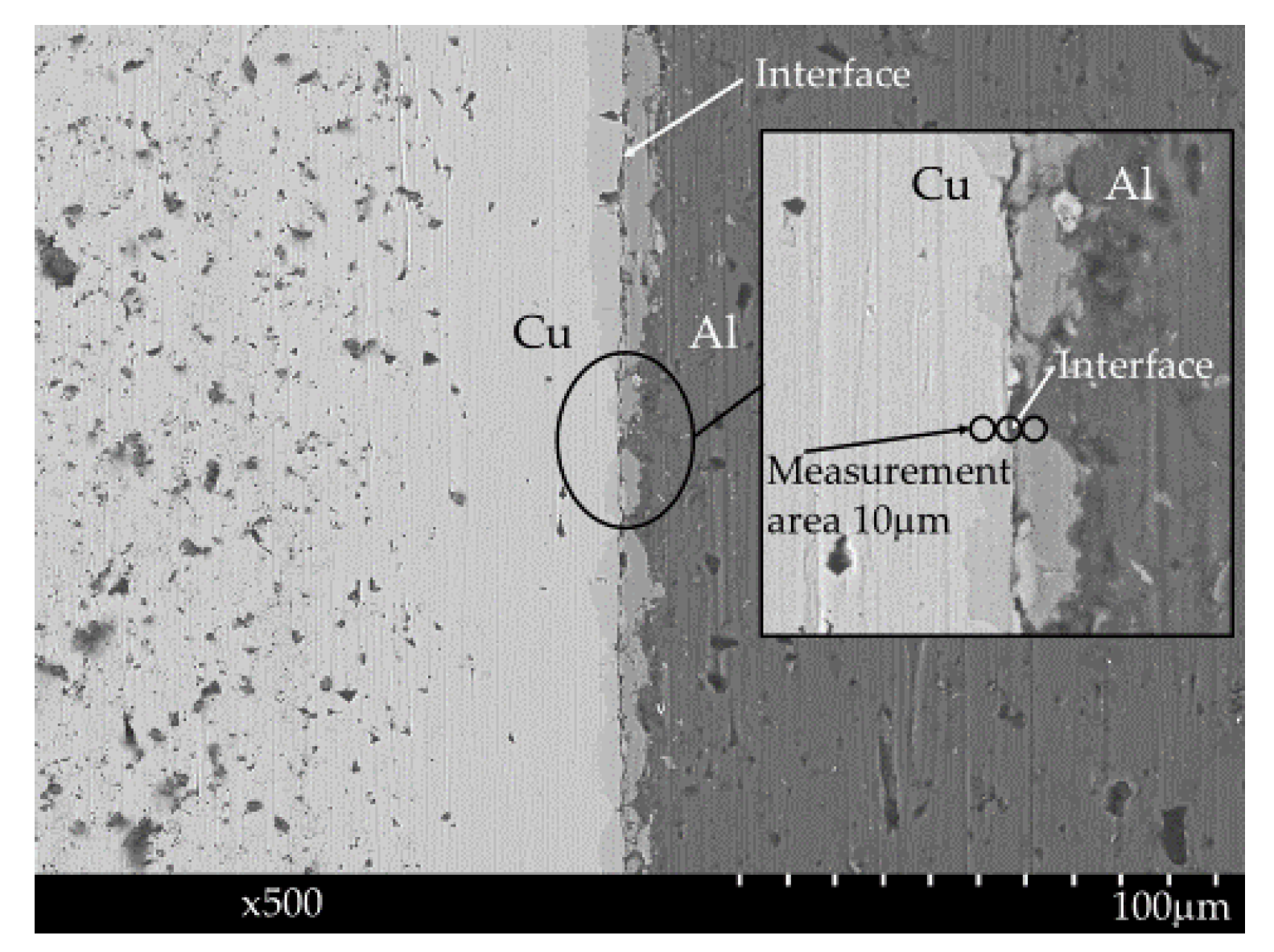
Estimating the diffusion length during diffusion bonding is useful for achieving diffusion bonded junctions with sufficient mechanical strength. In this study, we employed a heterogeneous Cu-Al direct diffusion bonded junction and conducted XPS measurements 10 μm away from the interface on both the Cu and Al sides after irradiation with characteristic AlKα X-ray beams of 10 μm diameter.
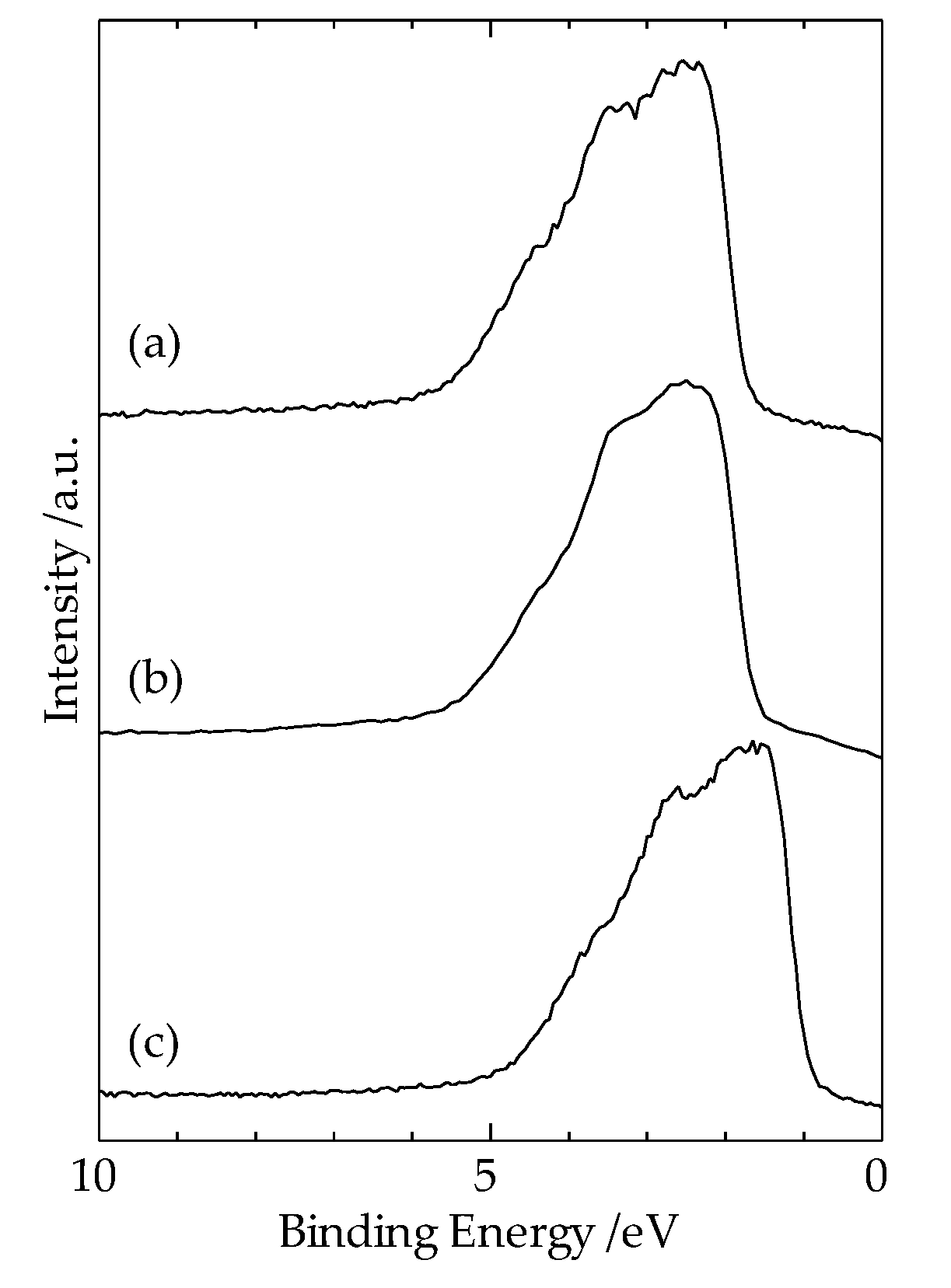
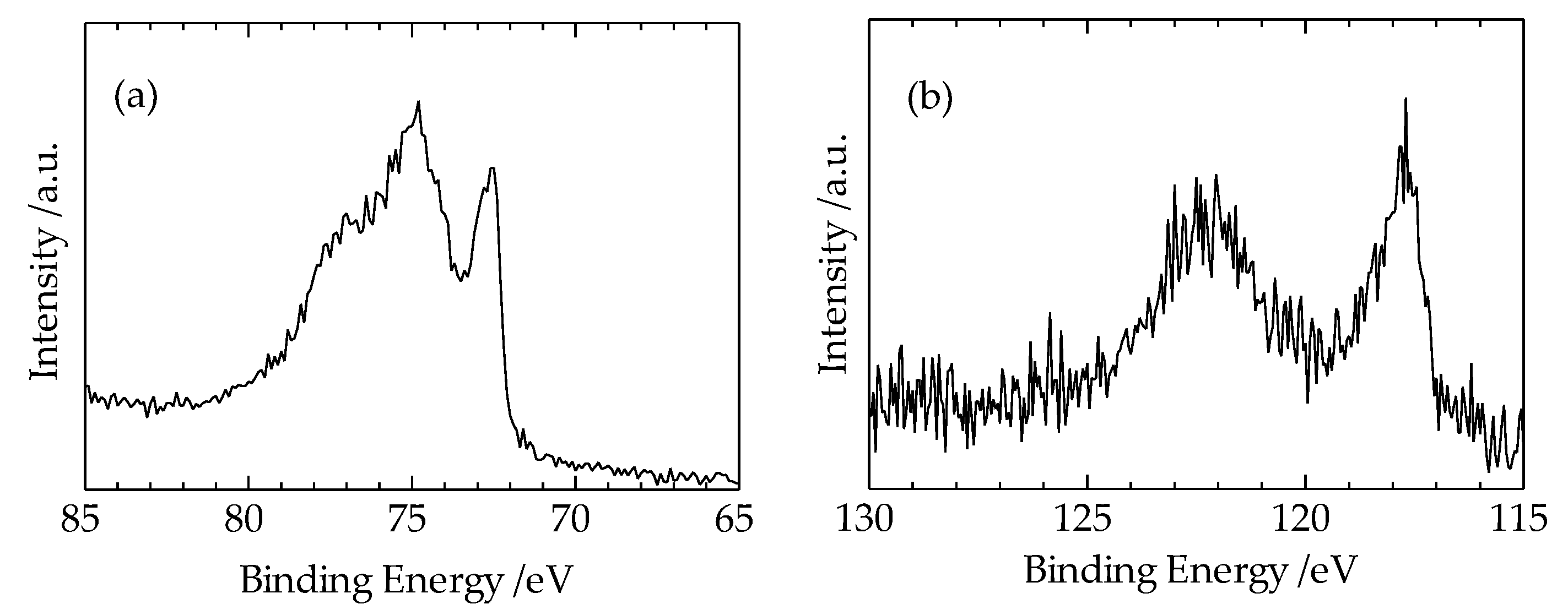
Figure 5a,b show the XPS measurement results of Cu3p, Al2p spectra, and Al2s and Cu3s spectra, respectively, taken from the Cu region 10 μm away from the interface of the heterogeneous Cu-Al direct diffusion-bonded junction. In
Figure 5(a), the spectrum from 75 eV to 77 eV corresponds to Cu3p, and the spectrum at 72.5 eV corresponds to Al2p. In
Figure 5(b), the spectrum at 117.5 eV corresponds to Al2s, and the spectrum at 122.5 eV corresponds to Cu3s. These results suggest that the diffusion length of Al atom in the Cu region is about 10 μm.
Since the spectra of Cu3p and Al2p are clearly observed in
Figure 5(a), we attempted to estimate the diffusion length of Al atoms in the Cu region using the XPS spectra measurement results shown in
Table 2. The method we adopted below is intended to estimate the approximate diffusion length, as obtaining an accurate diffusion length through quantitative analysis based on XPS spectrum measurement is difficult.
Below, we estimated the diffusion lengths using a one-dimensional model. The x coordinate is set as follows: the interface is at x=0, x>0 is the Cu region, and x<0 is the Al region.
Similarly, when the normalized number of Al atoms (
) as a function of
x, in the Al region sufficiently far from the interface is set to 1,
is equal to 0.5 at the interface. The following equation holds for any value of
x:
The numbers of Cu atoms and Al atoms in the Cu region are proportional to the numbers of photoelectrons of each atom obtained by XPS measurement. The shape of the photoelectron spectrum is precisely given by the well-known formula of Doniach-Sunjic-Shirley, but here, for simplicity, the photoelectron spectrum is approximated by the Lorentz distribution function [
9]. Then, the following formula holds:
where
,
,
,
n and
are the number of atoms in the analysis region, the peak height in the measured spectrum, the spectral FWHM, the number of electrons in the orbital (state), for example,
n=6 in the p state, and the photoionization cross-section of the orbital, respectively.
Using
Table 2, the following formula holds:
This formula represents the ratio of Al atoms to Cu atoms at a specific point. Therefore, the following result is obtained using equation (1):
This result indicates the concentration of Al atoms at the given point.
In the Cu region, the solution of the diffusion equation (Fick’s second law) is assumed to follow the formula when thermal diffusion stops after a certain time has passed since the diffusion bonding was formed:
Here
represents the diffusion length of an Al atom in the Cu region. The diffusion length (
) is estimated as
μm.
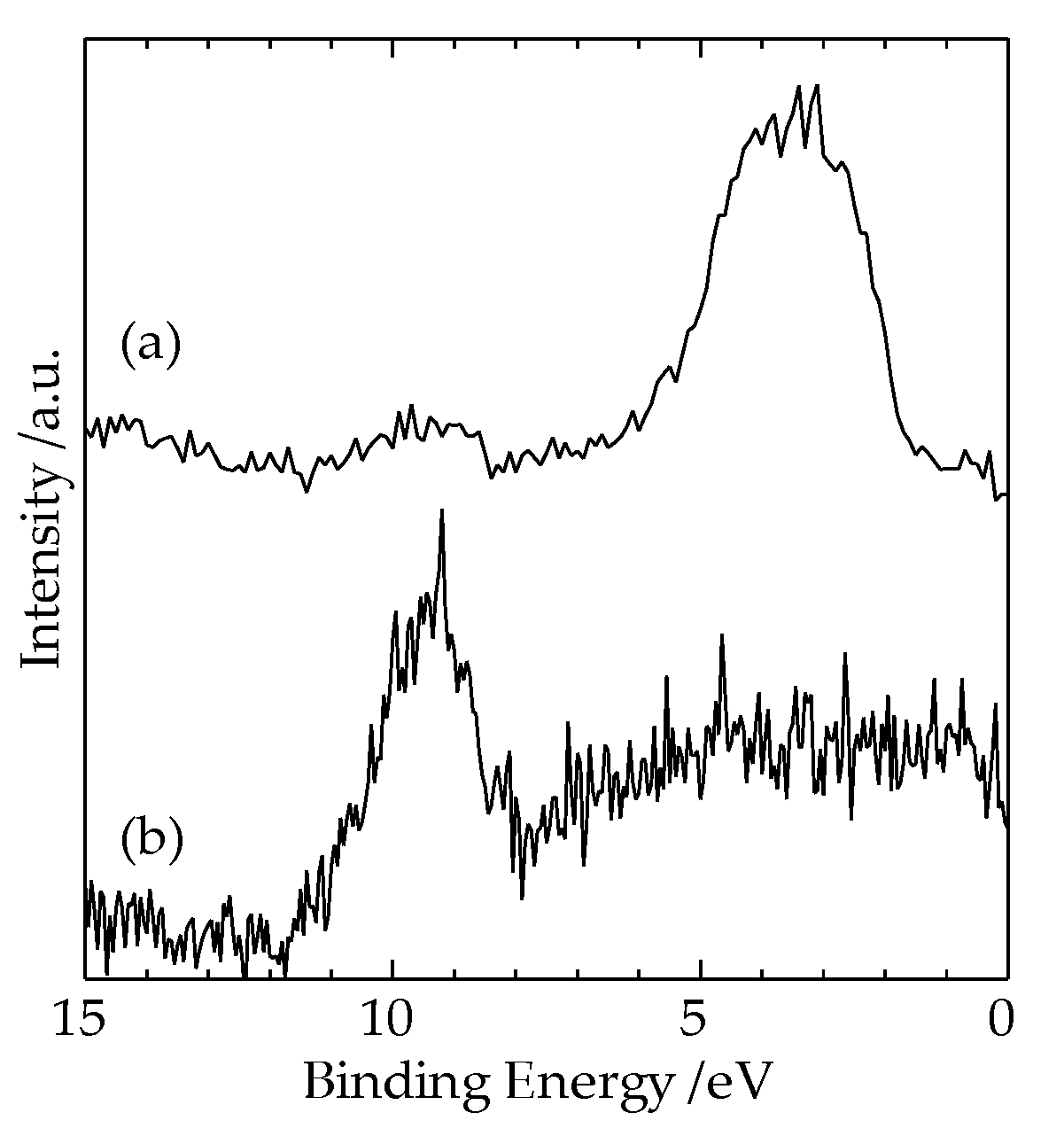
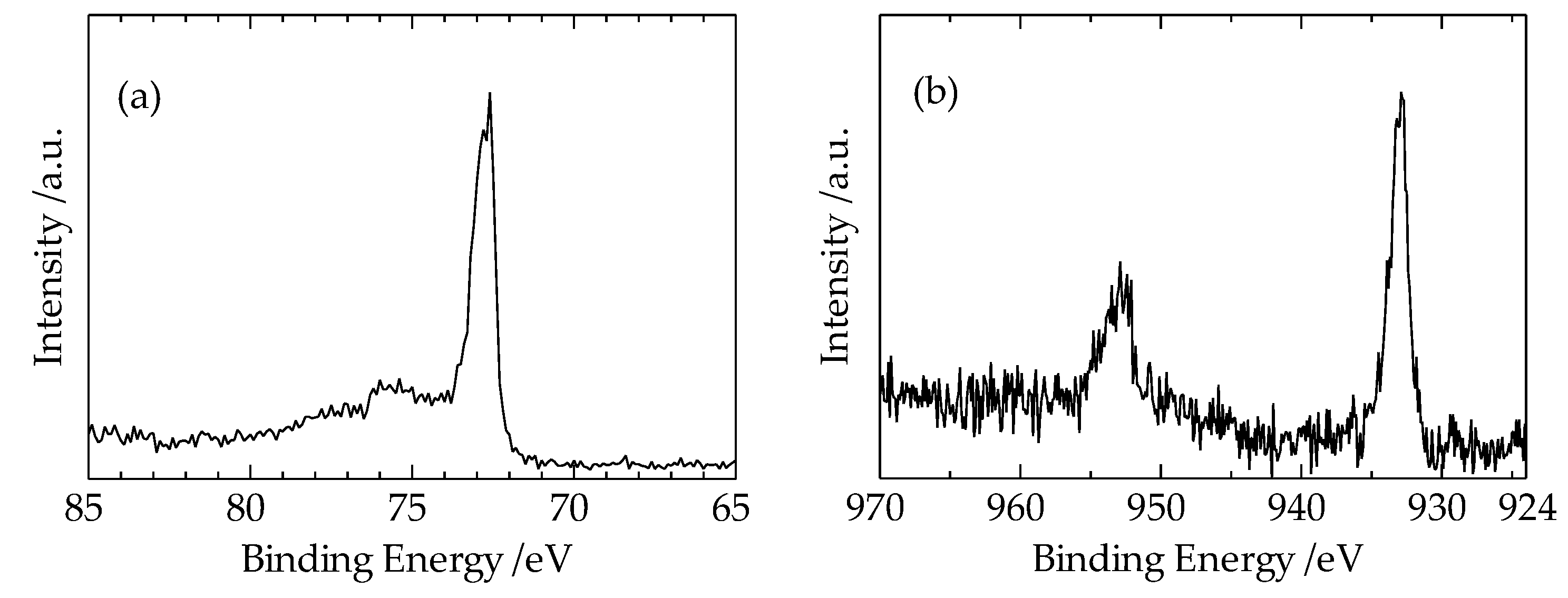
Figure 6a,b show the results of the XPS measurements of Al2p, Cu3p, and Cu2p spectra in the Al region 10 μm away from the interface of the heterogeneous Cu-Al direct diffusion bonded junction, respectively. In
Figure 6(a), the spectrum from 75 eV to 77 eV corresponds to Cu3p, and the spectrum at 72.5 eV corresponds to Al2p. The spectra at 933 eV and 952 eV in
Figure 6(b) correspond to Cu2p3/2 and Cu2p1/2, respectively.
It is found in
Figure 6(a) that the Al2p spectrum is clearly observed, while Cu3p spectrum is not clearly observed.
Figure 6(b) also shows that Cu2p spectrum can be observed in the Al region, even 10 μm away from the interface. These results suggest that the diffusion length of Cu atom in the Al region is about several micrometers.
Since the spectra of Al2p and Cu2p are clearly observed in
Figure 6a,b , respectively, we estimated the diffusion length (
) of a Cu atom in the Al region using the XPS spectra measurement results shown in
Table 3 as well as
.
Using
Table 3, the following formula holds:
This formula represents the ratio of Cu atoms to Al atoms at a specific point. Therefore, the following result is obtained using equation (1):
This result indicates the concentration of Cu atoms at the given point.
If the following formula holds in the Al region as well as in the Cu region:
The diffusion length (
) is estimated as
μm.
3.2.3. Estimation of Diffusion Coefficient
Inter-diffusion between two metals is also called chemical diffusion and is theoretically considered using Gibbs free energy. The diffusion phenomenon in the chemical field occurs in the liquid or gas phase. The temperature during diffusion bonding is relatively lower than the melting point of each material, so the diffusion phenomenon does not occur in the liquid phase. The explanation for inter-diffusion between two metals using free energy is that free energy decreases when two materials diffuse into each other.
However, inter-diffusion occurs at the diffusion-bonded interface when the two materials are not in the liquid phase. The XPS analysis revealed that the diffusion length of Al into the Cu region () was 11.8 μm, and the diffusion length of Cu () into the Al region was 7.85 μm. The reason why the diffusion length () is greater than that of is qualitatively considered below.
Diffusion- bonded junctions are prepared at temperatures below the melting point of the metals used. For example, in the case of Cu-Al direct diffusion bonding, the temperature during production is at most 550 °C. As shown in
Table 4, the melting points of Cu and Al are 1085 °C and 660.3 °C, respectively. Therefore, the temperature during production is lower than the melting points of both metals, and the inter-diffusion phenomenon at the bonded interface occurs in the solid phase, not in the liquid phase. At the beginning of the diffusion bonding process, voids exist at the end faces of the two metal materials used at the bonding interface, and diffusion and recrystallization occur to fill those voids. Thereafter, inter-diffusion is thought to proceed to fill lattice vacancies present in each region. The diffusion length (
) obtained in this study was about 50% larger than that of
. The diffusion length we estimated is the value at which inter-diffusion stops after the Cu-Al direct diffusion bond is formed and the junction is cooled during annealing. It is challenging to determine the exact temperature at which inter-diffusion ceased. However, due to the rapid decrease in the diffusion coefficient of metals as temperature decreases, it is assumed that inter-diffusion halted before the temperature reached approximately 400 °C.
We will subsequently analyze the variation in thermodynamic binding energy (
δH) [
10]. Consider a scenario where Al atoms jump and diffuse into lattice vacancies within the Cu region. The temperature at which Cu-Al direct diffusion bonding samples are processed does not exceed 550 °C, which is below the melting points of Cu and Al as shown in
Table 4, with the single vacancy mechanism being dominant. [11–13Here, an impurity-vacancy pair comprising one vacancy and one impurity atom is considered, with the interaction between the lattice vacancy of the Cu crystal and the Al impurity atom assumed to be electrostatic. Among the changes in free energy, if we focus on the change in binding energy (
δH), it is proportional to the screening potential created by the impurity atoms [
10]. If the Thomas-Fermi potential [
11,
12,
13] is employed as the screening potential, the following equation applies:
Here
and
are the numbers of valence electrons in the outermost shells of Cu and Al atoms, respectively. Additionally,
,
d , and
e are the dielectric constant of vacuum, the shortest interatomic distance (jump distance), and the absolute value of the electron charge, respectively. In equation (11),
λ is the Thomas-Fermi screening constant, which is given by the following equation:
Hereandare the Fermi energy and the electron density, respectively.
Table 5 presents the Fermi energy and the screening constant calculated using the free-electron model. The calculated values of binding energy (
δH) are also presented in
Table 5. Since the crystal structure of both Cu and Al is FCC, the shortest interatomic distance (
d) is given by
Here the lattice constant is denoted as a.
In this discussion, the key point is not the precision of δH’s value, but its sign. Specifically, when Al atoms jump and diffuse as impurity atoms into lattice vacancies in the Cu region, δH becomes negative, leading to a decrease in the crystal system’s free energy, thus enhancing stability.
Cu atoms, when acting as impurity atoms, tend to have a positive change in binding energy (δH) when diffusing into lattice vacancies in the Al region, as shown in equation (11). This results in a smaller decrease in free energy compared to the diffusion of Al atoms into the Cu region. Consequently, Al atoms more readily diffuse into the Cu region, whereas Cu atoms do not easily diffuse into the Al region, resulting in a longer diffusion length () compared to.
Fujikawa, S. and Hirano, K. reported that the diffusion coefficient of Cu atom as an impurity in Al was 1.40 × 10
-13 m
2/s at 550 °C through precise measurements using radioactive Cu isotopes [
14]. Liu, K., Yu, H., Li, X., and Wu, S fabricated a diffusion bonded Al-Cu junction at 540 °C for 48 hours and carried out experiments for the formation of intermetallic compounds between Al and Cu near the junction interface, reporting that θ(Al
2Cu) grew on the Al side of the bonded interface, and γ
1(Al
4Cu
9) grew on the Cu side [
15,
16]. They also reported that the inter-diffusion coefficients of θ(Al
2Cu) and γ
1(Al
4Cu
9) were 3.14 × 10
-13 m
2/s and 1.48 × 10
-12 m
2/s, respectively [
15].
Recently, Luu, H.T. and Merkert, N. analyzed the inter-diffusion phenomenon at the Cu-Al diffusion bonding interface using molecular dynamics simulations [
17]. They found that the diffusion coefficients of Cu atom and Al atom at 577 °C are nearly the same, at approximately 2.0 × 10
-16 m
2/s. They also reported that this diffusion coefficient value agrees with the experimental results of Matsuo, N. and Oikawa, H. [
18].
In our experiment, we assumed that the time from stopping heating until the inter-diffusion phenomenon stopped was
τ=5 hours. We then estimated the time-average diffusion coefficient (
) using following equation:
Here L denotes the diffusion length.
It was found that the time-average diffusion coefficients of the Al atom in the Cu region and the Cu atom in the Al region at 550 °C are approximately 1.84 × 10
-15 m
2/s and 8.45 × 10
-16 m
2/s, respectively. The values obtained for the diffusion coefficient agree with experimentally determined diffusion coefficients of Cu-Al solid solutions that are within the order of magnitude, 10
-15 m
2/s, [
18].