Submitted:
13 November 2025
Posted:
14 November 2025
You are already at the latest version
Abstract
Hippophae rhamnoides L. (sea buckthorn) is a key multipurpose shrub of Eurasia valued for its ecological resilience, nutritional properties, and economic importance. This study examines the regenerative capacity, cold tolerance, productivity, and vegetative propagation efficiency of H. rhamnoides populations originating from East Kazakhstan and maintained at the Altai Botanical Garden (ABG). Between 1981 and 2024, five natural populations (Kendyrlyk, Kaindysu, Tersayryk, Shetlasty, and Karatal Sands) were evaluated under both natural and introduction conditions. A total of 68 clonal forms were propagated and assessed for longevity, yield stability, and morphological traits. The results demonstrated high ecological plasticity and adaptation to the sharply continental climate of East Kazakhstan, withstanding winter temperatures of -38 to -44 °C without damage. Long-lived genotypes (up to 32 years) exhibited consistent productivity, yielding 3.7–14.5 kg per plant (4.6–17.5 t/ha). Large-fruited cultivars such as ‘Yubileinaya Kotukhova’, ‘Shetlastinka’, and ‘Asem’ reached fruit masses up to 95.8 g of 100 berries. Vegetative propagation by green cuttings proved highly effective, with rooting rates up to 90% when treated with HB-101, exceeding control treatments by 14.7%. Stable thickets formed by root suckers persisted for nearly four decades, confirming strong clonal stability and adaptive capacity. These findings underscore the significant potential of H. rhamnoides germplasm from East Kazakhstan for breeding cold-hardy, high-yielding cultivars suited to continental climates. The research highlights the importance of ex situ conservation at ABG and provides a foundation for further genetic, biochemical, and breeding studies aimed at enhancing the productivity and sustainability of this ecologically and economically valuable species.
Keywords:
1. Introduction
2. Materials and Methods
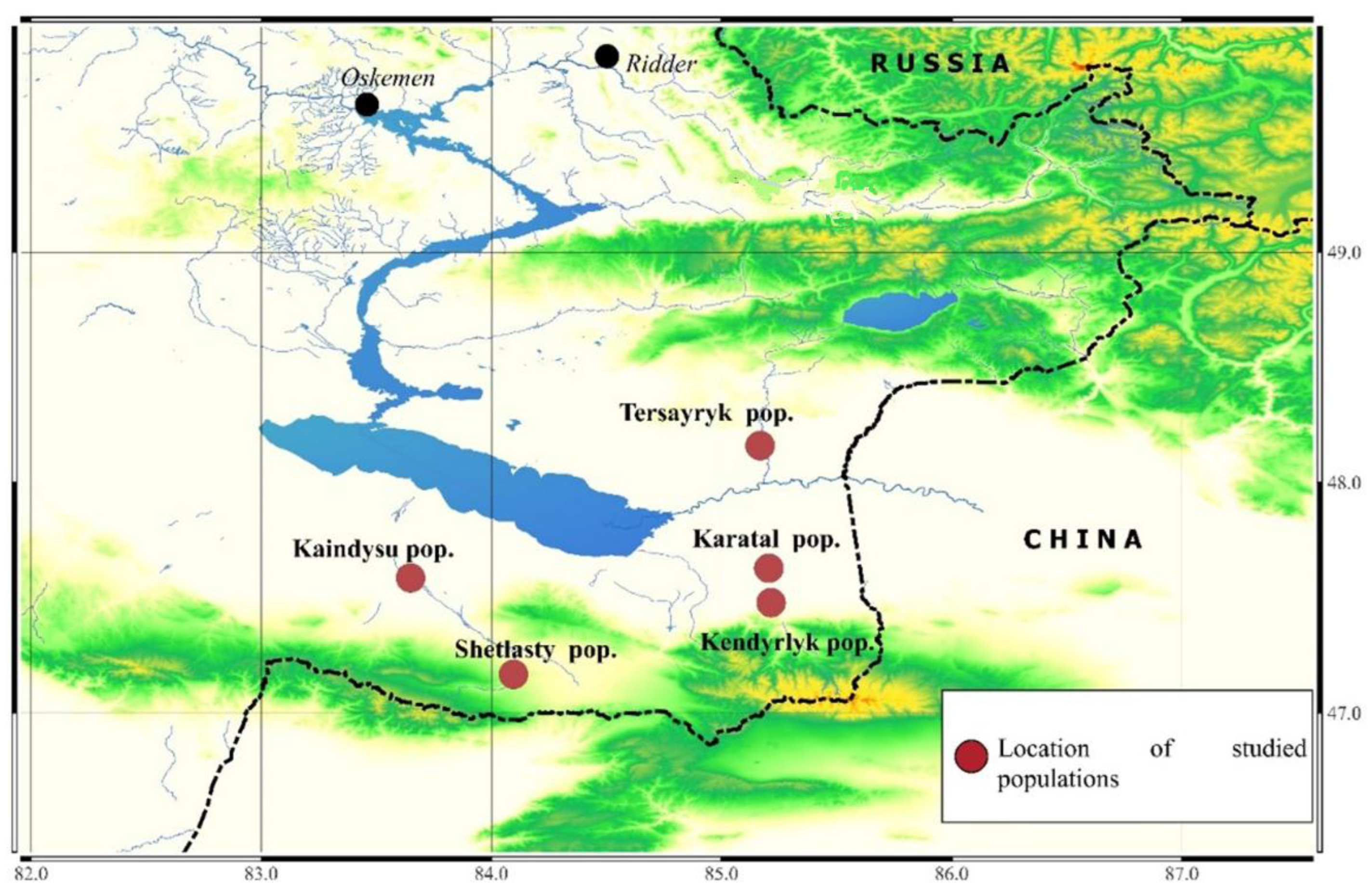
2.1. Study Sites and Plant Material
2.2. Sampling and Variability Assessment
2.3. Green Cutting Under Introduction Conditions
2.4. Statistical Analysis
3. Results
3.1. Regenerative Capacity and Cold Tolerance of H. rhamnoides Natural Populations
3.2. Yield, Longevity of Productivity, and Fruit Characteristics of Introduced H. rhamnoides
3.3. Formation and Stability of Clonal Thickets in the Introduced Population of H. rhamnoides
3.4. Vegetative Propagation of H. rhamnoides by Green Cutting Under Introduction Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGB | Altai Botanical Garden |
| ANOVA | Analysis of variance |
| KA | Kaindysu population |
| KE | Kendyrlyk population |
| KS | Karatal Sands population |
| SH | Shetlasty population |
| T | Tersayryk population |
References
- Jubayer, M.F.; Mazumder, M.A.R.; Nayik, G.A.; Ansari, M.J.; Ranganathan, T.V. Hippophae rhamnoides L.: Sea buckthorn. In Immunity Boosting Medicinal Plants of the Western Himalayas; Springer Nature: Singapore, 2023; pp. 463–491.
- Liu, L.; Guo, Y.; Liu, X.; Yao, Y.; Qi, W. Relationship between the roots of Hippophae rhamnoides at different stump heights and the root microenvironment in feldspathic sandstone areas. PeerJ 2023, 11, e14819. [CrossRef]
- Yao, Y.; Tigerstedt, P.M. Geographical variation of growth rhythm, height, and hardiness, and their relations in Hippophae rhamnoides. Silva Fennica 1995, 29, 147–156. [CrossRef]
- Li, T.S.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): a multipurpose plant. HortTechnology 1996, 6(4), 370–380. [CrossRef]
- Enescu, C.M. Sea-buckthorn: a species with a variety of uses, especially in land reclamation. Dendrobiology 2014, 72, 71–76. [CrossRef]
- He, C.; Gao, G.; Zhang, J.; Duan, A.; Luo, H. Proteome profiling reveals insights into cold-tolerant growth in sea buckthorn. Proteome Science 2016, 14(1), 14. [CrossRef]
- Liu, J.; Zhang, R.; Zhang, G.; Guo, J.; Dong, Z. Effects of soil drought on photosynthetic traits and antioxidant enzyme activities in Hippophae rhamnoides seedlings. Journal of Forestry Research 2017, 28(2), 255–263. [CrossRef]
- Vdovina, T.A.; Isakova, E.A.; Lagus, O.A.; Sumbembayev, A.A. Selection assessment of promising forms of natural Hippophae rhamnoides (Elaeagnaceae) populations and their offspring in the Kazakhstan Altai Mountains. Biodiversitas Journal of Biological Diversity 2024, 25(4), 1–10. [CrossRef]
- Bruvelis, A. Sea buckthorn Hippophaë rhamnoides L.—taxonomy, distribution and introduction in Baltic States. Vegetation of Latvia 2007, 13, 33–38.
- Tian, L.; Wu, W.; Zhou, X.; Zhang, D.; Yu, Y.; Wang, H.; Wang, Q. The ecosystem effects of sand-binding shrub Hippophae rhamnoides in alpine semi-arid desert in the northeastern Qinghai–Tibet plateau. Land 2019, 8(12), 183. [CrossRef]
- Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Skłodowska, M.; Ionov, M. Hippophae rhamnoides L. leaf and twig extracts as rich sources of nutrients and bioactive compounds with antioxidant activity. Scientific Reports 2022, 12(1), 1095. [CrossRef]
- Vdovina, T.; Lagus, O.; Vinokurov, A.; Aimenova, Z.; Sumbembayev, A. Assessment of biochemical composition of fruits of Hippophae rhamnoides (Elaeagnaceae juss.), Viburnum opulus (Viburnaceae raf.) and Lonicera caerulea subsp. altaica (Caprifoliaceae juss.). Metabolites 2025, 15(4), 256. [CrossRef]
- He, N.; Wang, Q.; Huang, H.; Chen, J.; Wu, G.; Zhu, M.; Ma, Q. A comprehensive review on extraction, structure, detection, bioactivity, and metabolism of flavonoids from sea buckthorn (Hippophae rhamnoides L.). Journal of Food Biochemistry 2023, 2023(1), 4839124. [CrossRef]
- Wang, Z.; Zou, J.; Shi, Y.; Zhang, X.; Zhai, B.; Guo, D.; Luan, F. Extraction techniques, structural features and biological functions of Hippophae rhamnoides polysaccharides: A review. International Journal of Biological Macromolecules 2024, 263, 130206. [CrossRef]
- Teng, H.; He, Z.; Hong, C.; Xie, S.; Zha, X. Extraction, purification, structural characterization and pharmacological activities of polysaccharides from sea buckthorn (Hippophae rhamnoides L.): A review. Journal of Ethnopharmacology 2024, 324, 117809. [CrossRef]
- Ranjith, A.; Kumar, K.S.; Venugopalan, V.V.; Arumughan, C.; Sawhney, R.C.; Singh, V. Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoides, H. salicifolia, and H. tibetana) grown in the Indian Himalayas. Journal of the American Oil Chemists' Society 2006, 83(4), 359–364. [CrossRef]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochemical Systematics and Ecology 2004, 32(9), 809–816. [CrossRef]
- Tiitinen, K.M.; Yang, B.; Haraldsson, G.G.; Jonsdottir, S.; Kallio, H.P. Fast analysis of sugars, fruit acids, and vitamin C in sea buckthorn (Hippophae rhamnoides L.) varieties. Journal of Agricultural and Food Chemistry 2006, 54(7), 2508–2513. [CrossRef]
- Sabir, S.M.; Maqsood, H.; Hayat, I.; Khan, M.Q.; Khaliq, A. Elemental and nutritional analysis of sea buckthorn (Hippophae rhamnoides ssp. turkestanica) berries of Pakistani origin. Journal of Medicinal Food 2005, 8(4), 518–522. [CrossRef]
- Upadhyay, N.K.; Kumar, M.Y.; Gupta, A. Antioxidant, cytoprotective and antibacterial effects of sea buckthorn (Hippophae rhamnoides L.) leaves. Food and Chemical Toxicology 2010, 48(12), 3443–3448. [CrossRef]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxidative Medicine and Cellular Longevity 2016, 2016(1), 4692486. [CrossRef]
- Tian, H.; Ling, N.; Guo, C.; Gao, M.; Wang, Z.; Liu, B.; Li, W. Immunostimulatory activity of sea buckthorn polysaccharides via TLR2/4-mediated MAPK and NF-κB signaling pathways in vitro and in vivo. International Journal of Biological Macromolecules 2024, 283, 137678. [CrossRef]
- Melnikova, N.V.; Arkhipov, A.A.; Zubarev, Y.A.; Novakovskiy, R.O.; Turba, A.A.; Pushkova, E.N.; Dmitriev, A.A. Genetic diversity of Hippophae rhamnoides varieties with different fruit characteristics based on whole-genome sequencing. Frontiers in Plant Science 2025, 16, 1542552. [CrossRef]
- Nawaz, M.A.; Krutovsky, K.V.; Mueller, M.; Gailing, O.; Khan, A.A.; Buerkert, A.; Wiehle, M. Morphological and genetic diversity of sea buckthorn (Hippophae rhamnoides L.) in the Karakoram mountains of northern Pakistan. Diversity 2018, 10(3), 76. [CrossRef]
- Zhou, W.; Wang, Y.; Zhang, G.; Luan, G.; Chen, S.; Meng, J.; Suo, Y. Molecular sex identification in dioecious Hippophae rhamnoides L. via RAPD and SCAR markers. Molecules 2018, 23(5), 1048. [CrossRef]
- Jhajhariya, M.; Mangla, Y.; Chandra, A.; Goel, S.; Tandon, R. Variable resource allocation pattern, biased sex-ratio, and extent of sexual dimorphism in subdioecious Hippophae rhamnoides. PLOS ONE 2024, 19(4), e0302211. [CrossRef]
- Nybom, H.; Ruan, C.; Rumpunen, K. The systematics, reproductive biology, biochemistry, and breeding of sea buckthorn—A review. Genes 2023, 14(12), 2120. [CrossRef]
- Dolkar, P.; Dolkar, D.; Angmo, S.; Srivastava, R.B.; Stobdan, T. An improved method for propagation of seabuckthorn (Hippophae rhamnoides L.) by cuttings. National Academy Science Letters 2016, 39(5), 323–326. [CrossRef]
- Shah, S.R.U.; Plaksina, T.; Sriskandarajah, S.; Lundquist, P.O. Shoot organogenesis from roots of seabuckthorn (Hippophaë rhamnoides L.): structure, initiation and effects of phosphorus and auxin. Trees 2015, 29(6), 1989–2001. [CrossRef]
- Vdovina, T.; Lagus, O.; Isakova, E. Peculiarities of the root-suckering ability of Hippophae rhamnoides L. plants (East Kazakhstan region). Fundamental and Experimental Biology 2025, 11930(3), 38–47. [CrossRef]
- Sriskandarajah, S.; Lundquist, P.O. High frequency shoot organogenesis and somatic embryogenesis in juvenile and adult tissues of seabuckthorn (Hippophae rhamnoides L.). Plant Cell, Tissue and Organ Culture (PCTOC) 2009, 99(3), 259–268. [CrossRef]
- Liu, C.Q.; Xia, X.L.; Yin, W.L.; Zhou, J.H.; Tang, H.R. Direct somatic embryogenesis from leaves, cotyledons and hypocotyls of Hippophae rhamnoides. Biologia Plantarum 2007, 51(4), 635–640. [CrossRef]
- Zubarev, Y.A.; Gunin, A.V.; Vorobjeva, A.V. Rooting green cuttings of Altai seabuckthorn cultivars in industrial-scale experiment. RUDN Journal of Agronomy and Animal Industries 2022, 17(2), 131–145. [CrossRef]
- Güneş, M.; Alkaç, O.S.; Öcalan, O.N. Propagation of some sea buckthorn (Hippophae rhamnoides) cultivars by semi-hardwood cuttings. Journal of New Results in Science 2020, 9(2), 32–38.
- Gunin, A.V.; Panteleeva, E.I.; Zubarev, Yu.A.; Pugach, V.A.; Vorobyeva, A.V. Evaluation of sea buckthorn varieties and hybrids by indicators affecting harvesting efficiency. Vestnik of Altai State Agrarian University 2018, 7(165), 70–76. (in Russian).
- Sumbembayev, A.A.; Kotukhov, Y.A.; Danilova, A.N.; Aitzhan, M. Endemic and endangered vascular flora of Kazakhstan’s Altai Mountains: A baseline for sustainable biodiversity conservation. Sustainability 2025, 17(16), 7283. [CrossRef]
- Almerekova, S.; Yermagambetova, M.; Sumbembayev, A.; Imanbayeva, A.; Turuspekov, Y. DNA barcoding of Hippophae rhamnoides L. collected from natural and introduced populations in Kazakhstan. Eurasian Journal of Applied Biotechnology 2024, 3, 9–19. [CrossRef]
- Kondrashov, V.T. Methodology for describing wild forms of sea buckthorn. Plant Resources 1977, 13(1), 140–144. (in Russian).
- Danusevicius, D.; Lindgren, D. Efficiency of selection based on phenotype, clone and progeny testing in long-term breeding. Silvae Genetica 2002, 51(1), 19–25.
- Dragavtseva, I.A.; Bandurko, I.A.; Efimova, I.L. Limiting environmental factors determining the productivity of perennial garden plantations. New Technologies 2013, 2, 110–114. (in Russian).
- Filipchenko, Yu.A. Variability and Methods of Its Study; Nauka: Moscow, Russia, 1978; p. 236. (in Russian).
- Sinskaya, E.N. The species and its structural parts at various levels of the organic world. Bulletin of VIR 1979, 91, 7–24. (in Russian).
- Iroshnikov, A.I. et al. Methodology for studying intraspecific variability of tree species; Nauka: Moscow, Russia, 1973; p. 31. (in Russian).
- Dale, A.; Galić, D. Repetitive vegetative propagation of first-year sea buckthorn (Hippophae rhamnoides L.) cuttings. Canadian Journal of Plant Science 2017, 98(3), 609–615. [CrossRef]
- Wang, B.L.; Zhao, Y.; Han, X.Y. Effect of different hormone treatments and matrix formulations on rooting of micro-cutting of Hippophae rhamnoides. Acta Horticulturae Sinica 2023, 50, 101–110.
- Lan, D.; Xing, Z.; Xing, J. Research on rooting cuttings of hard branch of Hippophae rhamnoides. Agricultural Science & Technology 2015, 16(6), 1306.
- Zenkova, M.; Pinchykova, J. Chemical composition of sea-buckthorn and highbush blueberry fruits grown in the Republic of Belarus. Food Science and Applied Biotechnology 2019, 2(2), 121–129. [CrossRef]
- Wang, H.; Liu, H.; Yang, M.; Bao, L.; Ge, J. Phylogeographic study of Chinese seabuckthorn (Hippophae rhamnoides subsp. sinensis Rousi) reveals two distinct haplotype groups and multiple microrefugia on the Qinghai-Tibet Plateau. Ecology and Evolution 2014, 4(22), 4370–4379. [CrossRef]
- Bedareva, O.M. et al. Conservation and rational use of populations of sea buckthorn (Hippophae rhamnoides L.) in the Kaliningrad region. Agrarian Russia 2014, 2, 39–41. (in Russian).
- Shkolnikova, M.N.; Rozhnov, E.D.; Chugunova, O.V. Distinctive features of the biochemical composition of fruits of Altai sea buckthorn varieties. Life of Genomes 2022, 3, 36. (in Russian).
- Bogomolova, N.I.; Lupin, M.V. Biological productivity potential of sea buckthorn in natural and industrial plantations of Russia. Vestnik of Agricultural Science 2021, 6(93), 62–67. (in Russian).
- Trineeva, O.V.; Rudaya, M.A.; Slivkin, A.I. Study of the carotenoid composition of fruits of different sea buckthorn varieties using thin-layer chromatography. Chemistry of Plant Raw Material 2020, 1, 223–228. (in Russian).
- Mamedova, Sh.M.; Novruzov, E.N. Content and qualitative composition of carotenoids in the fruits of some forms of sea buckthorn (Hippophae rhamnoides L.) growing in Northern Azerbaijan. Bulletin of Moscow Region State University. Series: Geographical Environment and Living Systems 2016, 3, 33–41. (in Russian).
- Khovalyg, N. Bioresource potential of sea buckthorn phytocenes in the conditions of Southern Siberia. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; 524, 02013.


| Population | Age category | Number of plants per ha (count.) | % of total |
|---|---|---|---|
| Kendyrlyk (KE) | Juvenile (1–5 years) | 1740 | 65.0 |
| Intermediate (6–10 years) | 482 | 18.0 | |
| Pre-mature (10–15 years) | 148 | 5.5 | |
| Mature (16–20 years) | 106 | 4.1 | |
| Senescent (21–25 years) | 197 | 7.4 | |
| Total | 2673 | 100 | |
| Kaindysu (KA) | Juvenile (1–5 years) | 540 | 30.7 |
| Intermediate (6–10 years) | 415 | 23.6 | |
| Pre-mature (10–15 years) | 605 | 34.4 | |
| Mature (16–20 years) | 135 | 7.7 | |
| Senescent (21–25 years) | 65 | 3.6 | |
| Total | 1760 | 100 | |
| Tersayryk (T) | Juvenile (1–5 years) | 2360 | 72.6 |
| Intermediate (6–10 years) | 380 | 11.7 | |
| Pre-mature (10–15 years) | 368 | 11.3 | |
| Mature (16–20 years) | 86 | 2.6 | |
| Senescent (21–25 years) | 53 | 1.8 | |
| Total | 3247 | 100 | |
| Shetlasty (SH) | Juvenile (1–5 years) | 5173 | 79.8 |
| Intermediate (6–10 years) | 556 | 8.6 | |
| Pre-mature (10–15 years) | 483 | 7.5 | |
| Mature (16–20 years) | 123 | 1.9 | |
| Senescent (21–25 years) | 143 | 2.2 | |
| Total | 6478 | 100 | |
| Karatal Sands (KS) | Juvenile (1–5 years) | 81 | 22 |
| Intermediate (6–10 years) | 59 | 16 | |
| Pre-mature (10–15 years) | 36 | 10 | |
| Mature (16–20 years) | 191 | 52 | |
| Senescent (21–25 years) | 367 | 100 |
| Form, cultivar | Non-treated (control) | Heteroauxin, concentration of 0.015% | Ecogel, concentration of 0.01% | НВ – 101, concentration of 0.02% |
| Rooting, % | ||||
| Yubileynaya Kotukhova | 69.5 | 78.7 | 87.0 | 90.2 |
| Pamyati Baytulina | 64.2 | 68.6 | 67.3 | 69.6 |
| Shetlastinka | 71.2 | 69.2 | 68.4 | 90.3 |
| Plakuchaya | 60.7 | 76.2 | 83.4 | 89.7 |
| Fakel | 67.7 | 75.6 | 78.5 | 82.2 |
| Feyyerverk | 65.6 | 73.0 | 72.5 | 80.1 |
| Asem | 62.4 | 81.9 | 84.1 | 86.4 |
| Solnyshko (1-18) | 70.8 | 80.4 | 79.7 | 83.9 |
| Nesravnennaya (SH-9-81(3-27) | 68.9 | 80.2 | 83.7 | 82.7 |
| Krasnoplodnaya KE-14-81(4-27) | 63.8 | 62.7 | 64.1 | 70.1 |
| Gustoy tumanT-2-82 (1-24) | 72.0 | 70.1 | 79.4 | 81.8 |
| Krasavchik KE-8-82 (2-20) | 66.3 | 67.1 | 72.3 | 78.3 |
| Bogatyr T-17-82 (1-21) | 61.9 | 72.7 | 71.9 | 71.2 |
| Mean values | 66.5±3.8 | 73.5±5.7 | 76.4±7.3 | 81.2±7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





