1. Introduction
Type 1 diabetes mellitus (T1D) is a chronic organ-specific autoimmune disease, characterized by the immune-mediated destruction of insulin-producing pancreatic β-cells [
1,
2]. This process, driven by a combination of genetic predisposition and environmental factors, results in absolute insulin deficiency. The consequent hyperglycaemia necessitates lifelong insulin replacement therapy and continuous monitoring of glycaemic status to prevent acute and chronic disease complications [
3].
The epidemiology of T1D is characterized by significant geographical variations and a steady increase in incidence worldwide, posing a significant medical and social challenge [
4]. The incidence rate ranges from approximately 1 case per 100,000 children per year in parts of Asia and South America to over 40-45 cases per 100,000 children per year in Northern European countries [
5]. The annual global increase in incidence is most notable in young children, suggesting a strengthening impact of exogenous factors that trigger the autoimmune process.. As this trend cannot be explained by genetic predisposition alone, it underscores the complex interplay between genetic susceptibility and environmental factors in the etiology of T1D [
4,
6].
Genetic factors play a significant role in the pathogenesis of T1D. The greatest contribution comes from genes of the major histocompatibility complex (MHC) class II. Specifically, the DR3-DQ2 and DR4-DQ8 haplotypes facilitate more efficient presentation of autoantigenic peptides to CD4+ T-lymphocytes compared to other alleles, thereby initiating the pathological autoimmune cascade [
7]. However, these haplotypes are neither necessary nor sufficient for disease development, underscoring the complexity of genetic predisposition [
8]. Beyond the MHC locus, genome-wide association studies have identified additional susceptibility loci, including the insulin gene regulatory region and immune regulatory genes
CTLA-4 and
PTPN22 [
9,
10]. Although their individual effects are modest, these non-HLA genes collectively confirm the polygenic architecture of T1D and provide valuable insights for elucidating disease mechanisms.
Despite extensive research, the precise contribution of exogenous factors in triggering β-cell autoimmunity remains incompletely understood. Proposed environmental triggers include viral infections, co-infections, dietary factors (such as early introduction of cow's milk or gluten and vitamin D deficiency), gut microbiota dysbiosis, and other lifestyle components [
11,
12,
13]. These factors are thought to initiate the autoimmune process through several mechanisms, including molecular mimicry, impaired intestinal barrier function, systemic inflammation, or direct β-cell damage. [
14,
15]. Ultimately, this cascade of events results in the exposure of previously sequestered autoantigens and the development of a pathological immune response.
A key event in the pathogenesis of T1D is the loss of immune tolerance to self-antigens of β-cells, leading to the activation of both humoral and cellular adaptive immunity. Autoreactive B-lymphocytes contribute to this process both as antigen-presenting cells and as sources of proinflammatory cytokines [
16]. Furthermore, they produce high-affinity circulating autoantibodies (AAbs). While these AAbs are primarily considered disease biomarkers rather than direct pathogenic factors, they serve as crucial serological markers for identifying the preclinical stage of T1D [
17,
18].
The destruction of insulin-producing β-cells in T1D is executed by two primary immune cell populations. Cytotoxic CD8+ T-lymphocytes directly recognize and eliminate target cells by inducing programmed cell death [
19]. Concurrently, CD4+ T-helper cells orchestrate the autoimmune response by activating CD8+ T-lymphocytes and secreting proinflammatory molecules that create a damaging inflammatory milieu around the islets of Langerhans [
20].
Clinical manifestation of the disease occurs only after the destruction of 40–95% of the functional β-cell mass [
2]. This prolonged preclinical stage, lasting from several months to several years, is characterized by the persistent presence of multiple autoantibodies and a progressive decline in pancreatic secretory function. This phase represents a critical "therapeutic window" for early disease diagnosis and for initiating interventions aimed at preserving residual β-cell mass [
21].
The progression of the autoimmune response is driven by the recognition of specific molecular targets, or autoantigens. Notably, the immune reaction is directed not against entire molecules, but against short, specific segments known as epitopes. The study of this epitope specificity, its temporal evolution (a phenomenon known as epitope spreading), and its correlation with clinical onset is fundamental to understanding the initiation and development of the autoimmune process [
22].
The primary clinically significant autoantigens in T1D are insulin, glutamic acid decarboxylase (GAD65), tyrosine phosphatase-like protein (IA-2/ICA512), and zinc transporter 8 (ZnT8) [
23]. Detection of high-affinity autoantibodies to these targets represents the "gold standard" for serological diagnosis and risk assessment [
24,
25,
26,
27]. Beyond these, a number of minor autoantigens are known (e.g., tetraspanin-7, ICA-69, IA-2β), though their clinical significance requires further clarification [
28]. Therefore, a detailed investigation of the molecular properties and epitopes of these autoantigens is fundamental for developing novel tools for early diagnosis, prognosis, and personalized therapy.
This review aims to systematize current scientific data on autoantigens and their immunodominant epitopes in type 1 diabetes. It provides a comprehensive synthesis of the structural and functional characteristics of major and minor autoantigens and delineates their physiological roles within pancreatic β-cells. Furthermore, the review considers the potential applications of this knowledge for developing novel approaches to diagnosis, risk prediction, and immunotherapy for the disease.
2. Major Autoantigens
The development of serological diagnostics for T1D has paralleled the chronological discovery of its key autoantigens. The first biomarker, autoantibodies to insulin (IAA), was identified in the late 1960s [
29]. This was subsequently followed in the early 1990s by the discovery of autoantibodies to glutamate decarboxylase (GAD65) (GAD65) [
30,
31]. The mid-1990s marked the next stage with the detection of autoantibodies to the pseudotyrosine phosphatase IA-2, a finding that significantly enhanced diagnostic specificity [
32,
33]. The modern diagnostic panel was completed in 2007 with the discovery of autoantibodies to zinc transporter 8 (ZnT8) [
34]. Today, the simultaneous analysis of this panel—comprising autoantibodies to insulin, GAD65, IA-2, and ZnT8—constitutes the gold standard for serological diagnosis, providing optimal sensitivity and specificity for confirming the autoimmune etiology of T1D and for assessing disease risk [
35].
The combined testing for all four autoantibodies exhibits a sensitivity of 95–98% and a specificity of 90–95% in newly diagnosed individuals, minimizing the likelihood of false-positive results [
35]. This method serves as an indispensable tool for the presymptomatic diagnosis of T1D, even prior to clinical manifestation. In apparently healthy individuals, the presence of two or more autoantibodies is associated with a lifetime risk of developing T1D that exceeds 90% within the subsequent few years [
17].
A comprehensive understanding of the mechanisms behind the autoimmune response and the generation of specific autoantibodies relies on elucidating the intracellular localization of autoantigens.
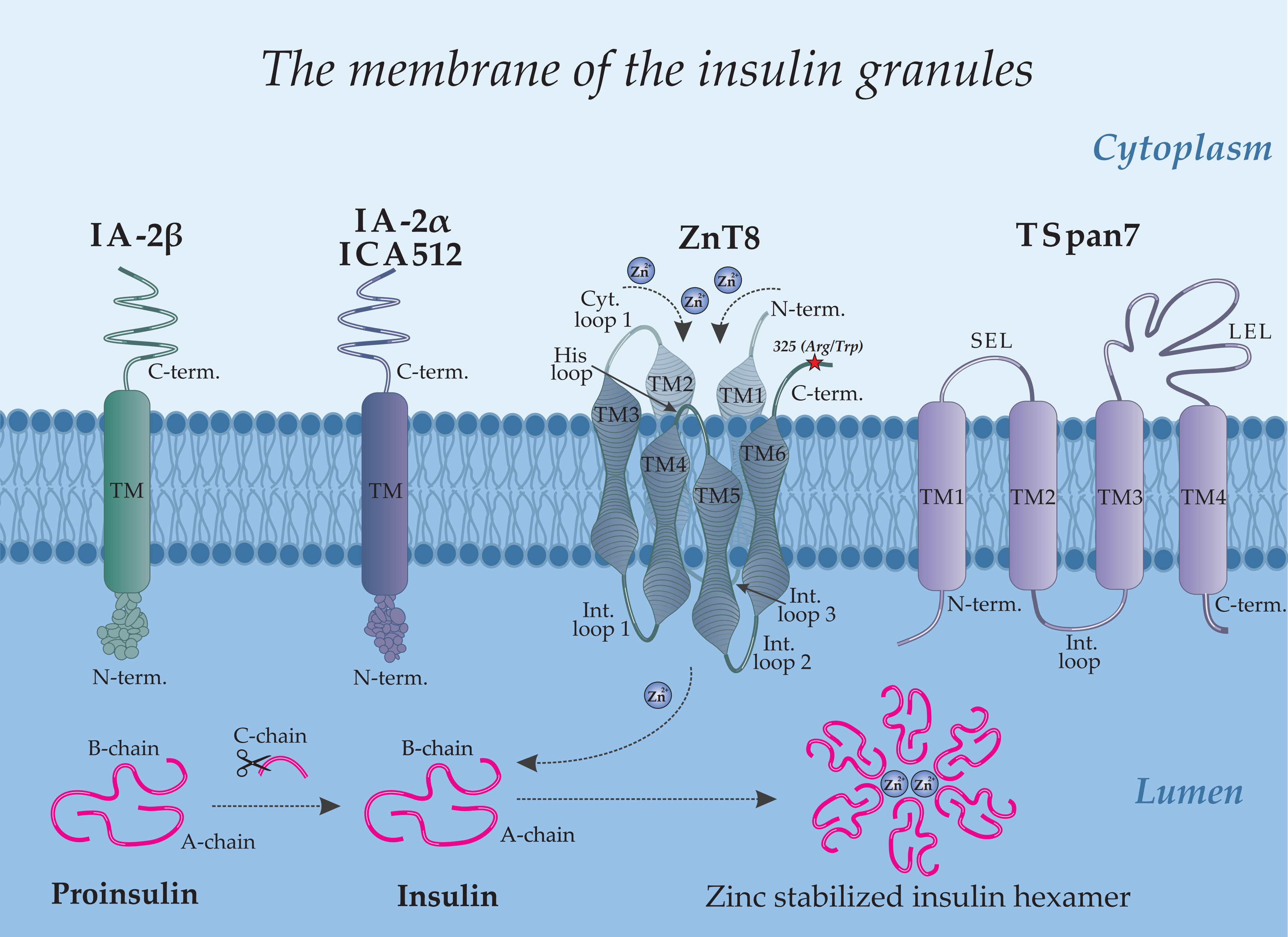
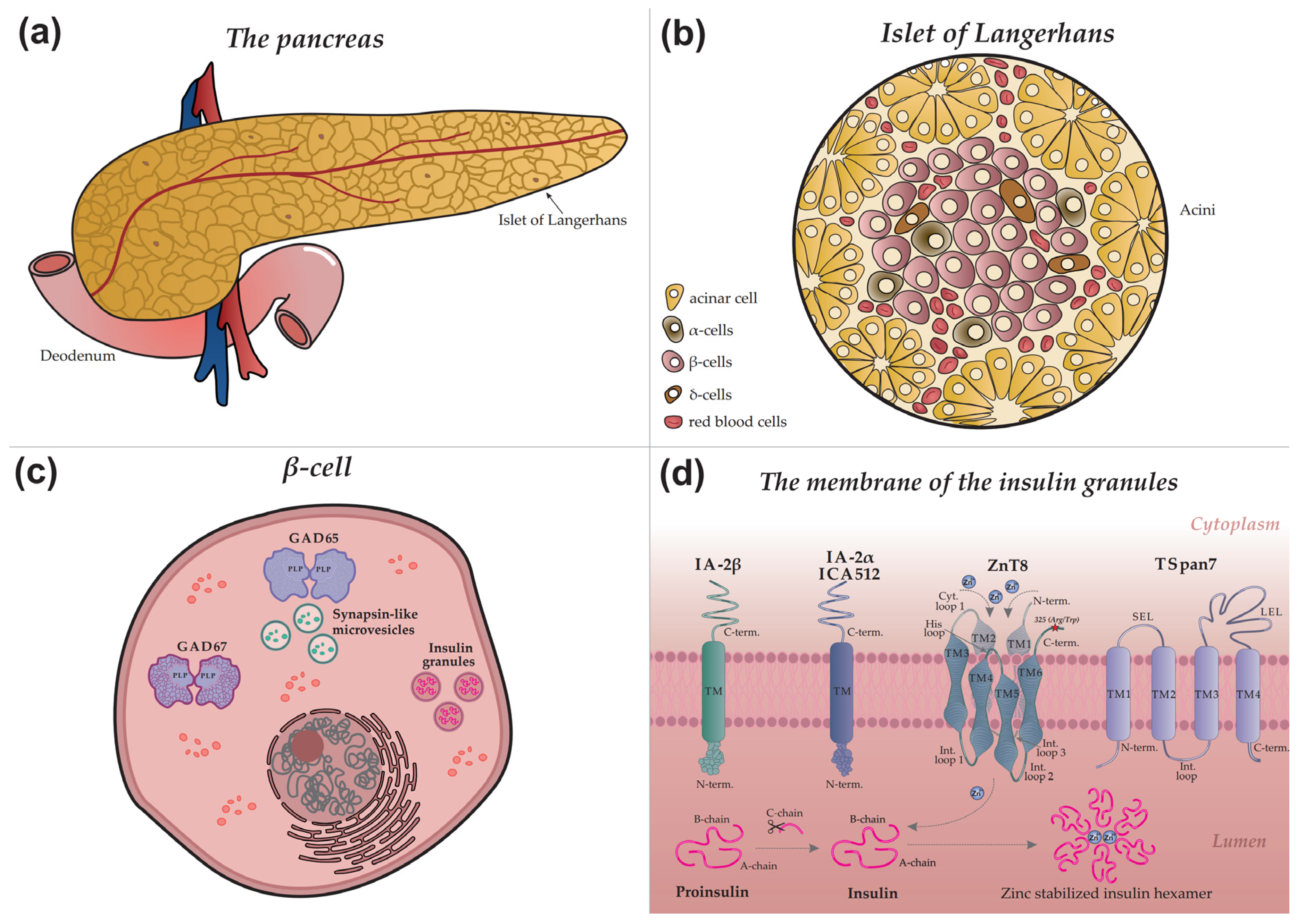
Figure 1 provides a schematic overview illustrating the architecture of the pancreas (a), an islet of Langerhans (b), a β-cell (c), and the precise intracellular localization of major and minor autoantigens within the insulin granule (d).
2.1. Insulin
Insulin, a key regulator of glucose metabolism, is stored in pancreatic β-cells within secretory granules, where it is packaged into stable hexamers (
Figure 1d) that dissociate into active monomers upon secretion. Its biosynthesis involves a multi-step pathway: preproinsulin, synthesized in the endoplasmic reticulum, is converted to proinsulin, which is subsequently cleaved during granule maturation to yield mature insulin and C-peptide. [
36,
37,
38]. The secretion of insulin into the bloodstream occurs via glucose-stimulated exocytosis, a process triggered by elevated blood glucose levels and a consequent rise in intracellular calcium concentration.
Current models propose that the autoimmune response against insulin in T1D develops through two principal mechanisms that disrupt natural immune tolerance [
39]. The first involves rapid, massive β-cell destruction, which floods the bloodstream with large quantities of insulin and its precursors. This sudden antigen exposure leads to an overt breach of immune tolerance, whereby antigen-presenting cells capture proinsulin, insulin, and C-peptide, facilitating the presentation of previously cryptic epitopes and initiating an immune response [
40]. The second mechanism is driven by chronic cellular stress, which disrupts autophagic and crinophagic pathways within β-cells. This dysregulation promotes the formation of modified protein structures, notably hybrid insulin peptides (HIPs), that are recognized by the immune system as foreign [
41]. Although initiated by distinct triggers, both pathways ultimately converge on the activation of autoreactive T-lymphocytes, autoantibody production, and progressive β-cell loss.
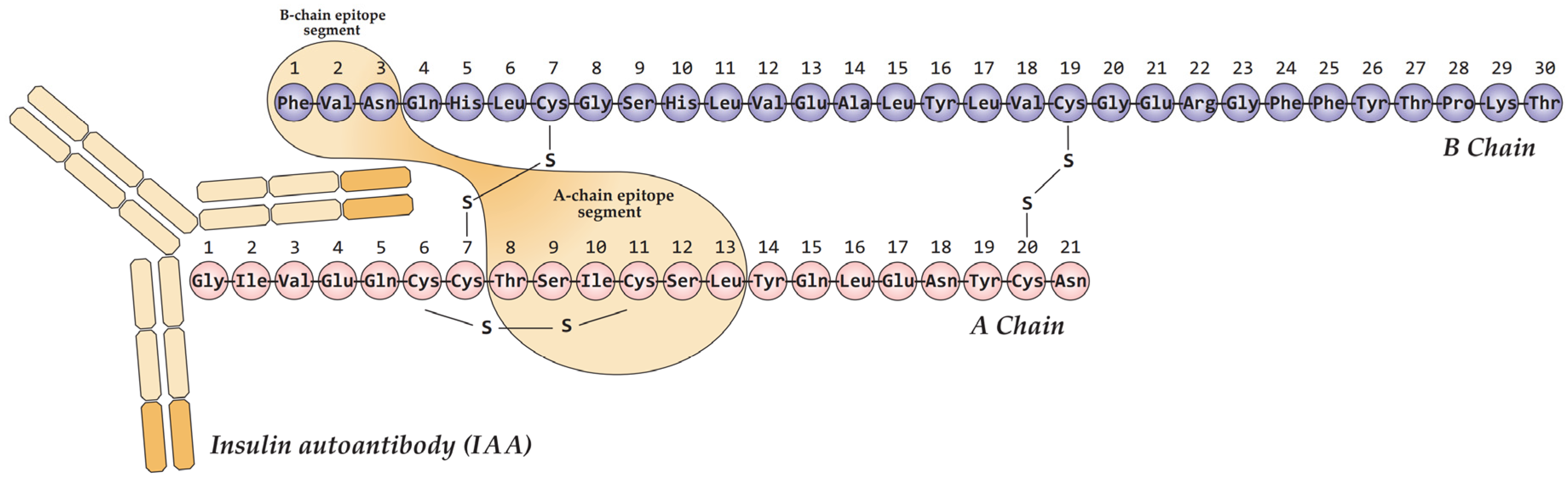
Autoantibodies to insulin (IAA) associated with T1D primarily target conformational epitopes presented by the monomeric form of the hormone [
42,
43]. Multiple such epitopes have been reported, often exhibiting partial overlap and minor variations that likely reflect methodological differences across studies. The most extensively characterized immunodominant epitope is formed by the A-chain (residues A8–A13) and B-chain (B1–B3) of mature insulin (
Figure 2) [
44,
45,
46]. Therefore, the specific tertiary structure of insulin is critical for IAA recognition in T1D, highlighting the importance of conformational epitopes over discrete linear sequences.
A novel class of insulin epitopes, termed neoepitopes, has been identified in recent years. Their generation is linked to the unique biochemical environment of insulin secretory granules, which promotes the formation of hybrid insulin peptides [
47,
48]. These unique compounds are created through the covalent fusion of insulin fragments with other granule peptides, such as chromogranin A (forming HIP 2.5) or amylin (forming HIP 6.9) [
49]. The resulting modified structures exhibit enhanced immunogenicity and are believed to play a pivotal role in both initiating and maintaining the autoimmune process, as they are recognized by the immune system as foreign entities.
2.2. Glutamic Acid Decarboxylase 65 (GAD65)
Glutamic acid decarboxylase 65 (GAD65) is a key enzyme in pancreatic β-cells, catalyzing the synthesis of the primary inhibitory neurotransmitter γ-aminobutyric acid (GABA) from glutamate [
50,
51]. Its activity is dependent on the cofactor pyridoxal-5'-phosphate (PLP) [
52].
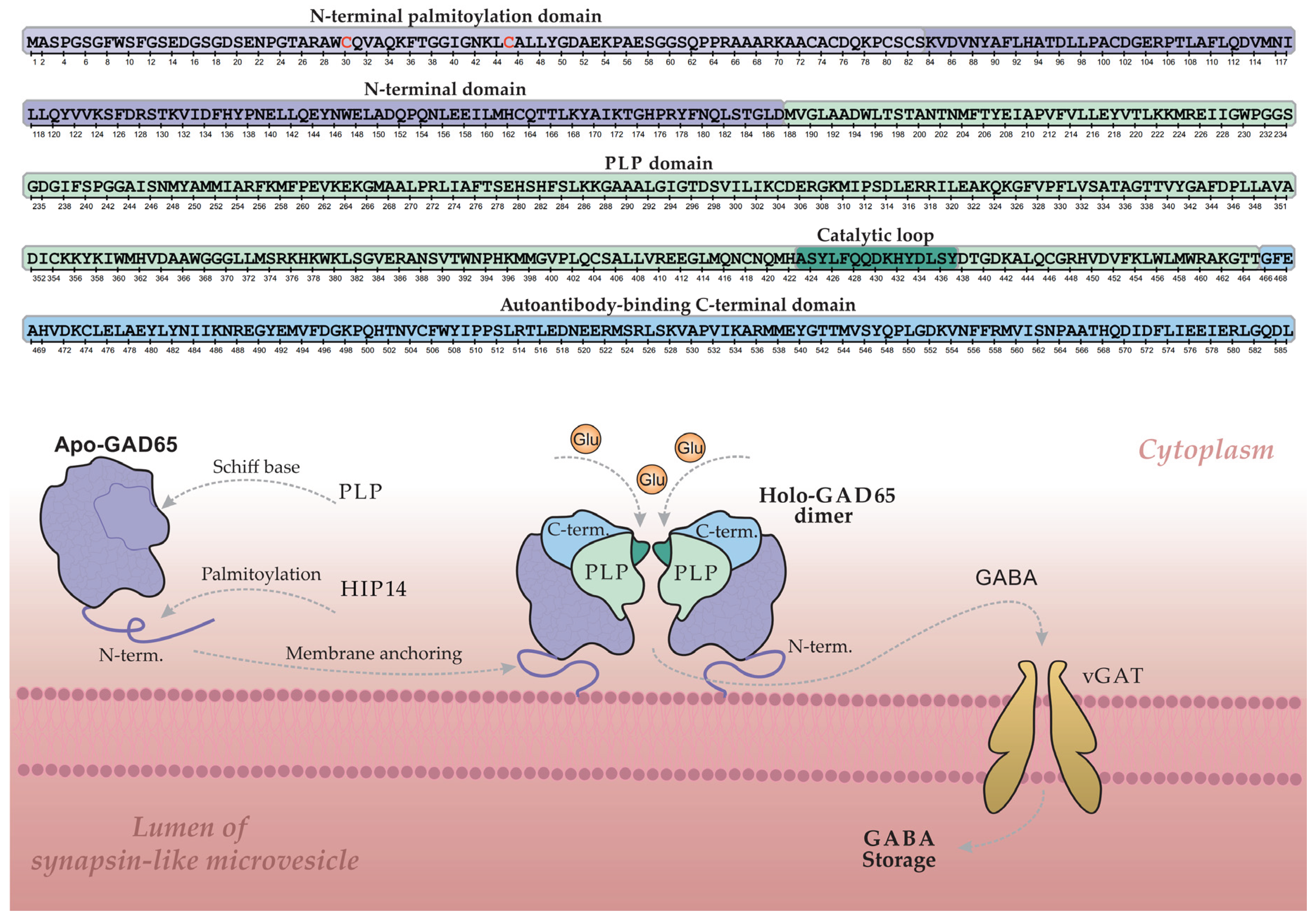
Figure 3 illustrates the GABA synthesis cycle involving GAD65 within a β-cell, depicting the enzyme's dynamic transition between its inactive, cytoplasmic apo-form (lacking PLP) and its active, membrane-bound holo-form (a PLP-bound homodimer).
The GAD65 molecule comprises three structural domains: an N-terminal domain, a PLP-binding domain, and a C-terminal domain. The N-terminal domain contains palmitoylation sites (Cys30 and Cys45) that, upon modification, serve as a hydrophobic membrane anchor. This palmitoylation is catalyzed by the palmitoyltransferase HIP14 (ZDHHC17), as illustrated in
Figure 3 [
53,
54]. Following palmitoylation, GAD65 is firmly anchored to the membrane of synapse-like microvesicles within the β-cell, where it converts to the active holo-form and facilitates local GABA synthesis. Newly synthesized GABA is then transported into the vesicular lumen via the vesicular GABA transporter (vGAT) for storage and subsequent release. Regulation of enzymatic activity and GABA output is mediated by a catalytic loop within the PLP-binding domain [
55,
56].
The C-terminal domain of GAD65 is of particular interest, as it constitutes an immunological "hotspot" in T1D and represents the primary target for GAD65 autoantibodies (GADA) [
57,
58,
59]. GADA predominantly recognize conformational epitopes dependent on the protein's three-dimensional structure, and less commonly target linear epitopes. The spectrum of GAD65 epitopes is notably broad and demonstrates considerable heterogeneity across patients [
60,
61]. Reported variations in GADA epitope specificity may also reflect methodological differences and diversity in the patient cohorts studied, highlighting the importance of standardized approaches for consistent epitope mapping.
The principal, well-characterized immunodominant region of GAD65, located within the C-terminal domain (approximately residues 466-585), is highlighted in blue in
Figure 3 (C-term.) [
62]. A second, less frequently targeted immunogenic region encompasses the segment between the C-terminal and PLP-binding domains (approx. residues 244-450). Notably, key conformational epitopes often depend on the spatial proximity between these two domains, an interaction particularly stabilized in the dimeric form of the protein. This underscores the critical role of GAD65's tertiary and quaternary structure in its recognition by autoantibodies [
52].
The N-terminal region of GAD65 (
Figure 3), while not a direct target for most autoantibodies in T1D, is critical for the proper formation of conformational epitopes elsewhere in the molecule [
63]. Intriguingly, studies demonstrate that an N-terminally truncated GAD65 isoform exhibits enhanced and more specific binding to T1D autoantibodies [
64,
65,
66]. The structural mechanisms underlying this enhanced immunoreactivity remain to be fully elucidated and warrant further investigation.
In summary, the immune response against GAD65 in T1D exhibits complex topography, targeting a broad spectrum of epitopes. A detailed understanding of GAD65 autoantibody epitope specificity and its clinical implications remains a critical research goal. Although these autoantibodies are also present in other conditions like autoimmune polyglandular syndrome type I (APS-1) and stiff-person syndrome, their epitope profiles can differ substantially from those in T1D [
67]. This disparity highlights the importance of precise epitope mapping for GAD65, not only to clarify the pathogenesis of T1D but also to elucidate the distinct autoimmune mechanisms operating across different diseases.
2.3. Tyrosine Phosphatase-like Protein (IA-2/ICA512)
IA-2 (Islet Antigen 2), also known as ICA512, is localized to the membrane of insulin secretory granules in β-cells (
Figure 1d). Although it belongs to the receptor-like protein tyrosine phosphatase (PTP) family, IA-2 is classified as a pseudophosphatase, having lost its catalytic activity during evolution due to key amino acid substitutions in its active site while retaining the characteristic structural organization of the family [
68,
69]. The exact function of IA-2 is still being elucidated; however, it has been identified as a key regulator of insulin granule stability and pool size. Its absence accelerates granule degradation by enhancing autophagy and crinophagy. Furthermore, IA-2 plays a role in regulating the exocytosis of insulin-secreting granules, highlighting its critical role in insulin secretion mechanisms [
70,
71].
The molecular structure of IA-2 includes a cleavable signal peptide and three principal domains: an N-terminal domain oriented toward the insulin granule lumen, a transmembrane (TM) domain, and a large cytoplasmic C-terminal domain housing the pseudophosphatase segment (
Figure 1d) [
23].
Within the cytoplasmic domain, two major epitopes have been identified in regions 604-776 and 771-979 aa [
72]. Autoantibodies in T1D patients predominantly target the 771-979 region, a preference attributed to its unique tertiary structure stabilized by disulfide bridges among five conserved cysteine residues (C839, C849, C909, C919, C941) [
73]. Thus, these disulfide bonds are essential for both structural integrity and the formation of a key conformational epitope. This specific architecture may facilitate cross-reactivity with homologous tyrosine phosphatases, thereby expanding the range of potential autoimmune targets in T1D—a phenomenon that constitutes an active research focus [
74]. Another autoantigen from the tyrosine phosphatase family, IA-2β (phogrin), classified as minor, will be discussed in
Section 3.2.
2.4. Zinc Transporter 8 (ZnT8)
Zinc transporter 8 (ZnT8), encoded by the
SLC30A8 gene, is a member of the SLC30 family and is predominantly expressed in pancreatic β-cells, where it localizes to the membrane of secretory insulin granules (
Figure 1d) [
75]. Its primary function is to actively transport zinc ions (Zn²⁺) from the cytosol into the granule lumen. The accumulated zinc plays a critical structural role by promoting insulin packaging: two zinc ions coordinate six insulin monomers to form a stable hexameric complex. This organization is essential for maintaining insulin's conformational stability and enables its regulated secretion in response to elevated blood glucose levels [
76,
77,
78].
The molecular structure of ZnT8 is characterized by homodimerization on the secretory granule membrane, a prerequisite for zinc transport. Each monomer integrates into the lipid bilayer via six transmembrane domains (TM1–TM6), with both N- and C-termini facing the cytosol (
Figure 1d). The protein's architecture features five connecting loops: three intragranular (IL1–IL3) and two cytoplasmic, including a short loop and a histidine-rich loop. The latter, situated between TM4 and TM5, is a key functional element that directly coordinates zinc ions [
79]. Particular interest lies in the C-terminal domain, whose unique structure and surface accessibility establish it as the primary immunodominant epitope in T1D.
Autoantibody specificity against the C-terminal region of ZnT8 is determined by a single nucleotide polymorphism (rs13266634) in the
SLC30A8 gene (
Figure 1d; Arg325Trp polymorphism indicated by a red star). The amino acid substitution of arginine for tryptophan at position 325 gives rise to two principal autoantigen variants: ZnT8-Arg (encoded by the C allele) and ZnT8-Trp (encoded by the T allele). The autoimmune response in T1D exhibits a striking allele-specific pattern; approximately 97% of C-allele carriers possess antibodies directed against ZnT8-325Arg, whereas T-allele carriers typically target ZnT8-325Trp [
80,
81]. Consequently, this high allelic specificity necessitates the inclusion of antigens corresponding to both variants in diagnostic panels to achieve optimal diagnostic sensitivity.
3. Minor Autoantigens
3.1. Glutamic Acid Decarboxylase 67 (GAD67)
In contrast to the major autoantigen GAD65, GAD67 is a cytoplasmic isoform uniformly distributed in the β-cell cytosol and does not associate with vesicle membranes (
Figure 1c). Unlike the reversibly regulated GAD65, GAD67 is constitutively active, predominantly existing as a PLP-bound holo-dimer responsible for maintaining basal GABA levels. The GAD67 molecule comprises an N-terminal dimerization domain, a PLP-binding catalytic domain, and a C-terminal domain. Although autoantibodies to GAD67 are detected in approximately 60–70% of T1D patients, they typically result from cross-reactivity with GAD65 due to high inter-isoenzyme homology, thus defining GAD67 as a minor autoantigen. [
51].
3.2. Phogrin (IA-2β)
IA-2β (phogrin), encoded by the
PTPRN2 gene, is a homolog of IA-2/ICA512 (PTPRN1). In contrast to IA-2, phogrin localizes not only to insulin granule membranes but also to secretory vesicles in other endocrine cell types. Its molecular structure comprises an N-terminal pseudocatalytic domain, homologous to tyrosine phosphatases but catalytically inactive, a transmembrane domain (TM), and a cytoplasmic C-terminal domain (C-term.) (
Figure 1d). The C-terminal domain, stabilized by disulfide bridges between conserved cysteine residues, serves as the primary target for autoantibodies. Autoantibodies to IA-2β are detected in approximately 40–60% of newly diagnosed T1D patients, typically in conjunction with anti-IA-2 antibodies—a finding attributable to their extensive structural homology. Consequently, IA-2β is classified as a minor autoantigen. [
82].
3.3. Tetraspanin 7 (TSpan7)
Tetraspanin-7 (TSpan7), a member of the tetraspanin family, is localized in β-cells primarily on insulin granule membranes and, to a lesser extent, on the plasma membrane. Identified relatively recently as a T1D-associated autoantigen, data on the prevalence of corresponding autoantibodies remain limited. Anti-TSpan7 autoantibodies are detected relatively infrequently, in approximately 5–10% of newly diagnosed T1D patients, underscoring its status as a minor autoantigen [
83]. Tetraspanins organize specialized membrane microdomains (tetraspanin networks) hypothesized to participate in intercellular adhesion and exocytosis regulation. The TSpan7 structure features short intracellular N- and C-termini, four transmembrane domains, and two extracellular loops—a small (SEL) and a large (LEL) loop (
Figure 1d). The LEL contains six cysteine residues forming stabilizing disulfide bonds. Its complex tertiary structure and surface accessibility establish the LEL as the primary target for autoantibodies in T1D [
84].
3.4. ICA1 (Islet Cell Autoantigen 1, ICA69)
Islet Cell Autoantigen 1 (ICA1, or ICA69) is a 69 kDa cytoplasmic protein expressed in pancreatic β-cells and other neuroendocrine tissues. It is uniformly distributed throughout the cytosol and associates with membranes of immature secretory granules in the Golgi region. A key structural feature is its N-terminal BAR domain, which mediates heterodimerization with the BAR domain of PICK1. This functional complex plays a critical role in insulin granule biogenesis and maturation [
85]. Autoantibodies to ICA69 are detected in approximately 15–20% of newly diagnosed T1D patients, thus supporting its classification as a minor autoantigen [
86].
4. Current Diagnostic Methods and Therapeutic Prospects for T1D
4.1. Multiplex Assays for the Detection of Diabetes-Associated Autoantibodies
The detection of preclinical T1D has undergone a significant evolution in recent decades, marked by a paradigm shift from monoplex to multiplex platforms. These advanced systems enable a comprehensive profiling of the autoimmune response by simultaneously assessing antibodies against multiple autoantigens and their specific epitopes. This transition is driven by the clinical need for enhanced accuracy in early disease detection, improved risk stratification, and identification of concomitant autoimmune conditions. The adoption of multiplex technologies thus represents a natural progression in the refinement of T1D diagnostics.
Traditional monoplex immunoassays — including radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescent immunoassay (CLIA) — remain the gold standard for confirming T1D autoimmunity and assessing disease risk [
87]. These methods allow for the detection of autoantibodies against key antigens: insulin, GAD65, IA-2, and ZnT8 [
28]. However, the requirement for separate analysis of each marker renders large-scale screening labor-intensive and costly, posing a significant limitation for comprehensive serological profiling. Although the prognostic value of a single autoantibody remains uncertain in asymptomatic individuals, the presence of two or more autoantibodies constitutes an unequivocal marker of high risk for progression to overt T1D.
4.1.1. Array-ELISA
Array-ELISA represents a significant advance in T1D serodiagnostics, offering substantial improvements over conventional mono- and multiplex platforms [
88]. This method enables the simultaneous yet discrete detection of five major autoantibodies (GADA, IA-2A, ICA, ZnT8-A, and IAA), thereby eliminating the requirement for supplementary confirmatory testing. A notable practical advantage is its compatibility with standard 96-well plates and routine laboratory instrumentation, ensuring accessibility for widespread clinical implementation. Consequently, Array-ELISA provides an optimal balance of high diagnostic sensitivity, specificity, and practical feasibility, making it particularly suitable for large-scale screening programs.
4.1.2. Protein Microarrays
Protein microarray-based multiplex immunoassay represents a promising diagnostic approach for T1D, enabling simultaneous detection of a broad autoantibody profile. This encompasses both organ-specific antibodies (e.g., anti-GAD65, anti-IA-2, anti-ICA, anti-TPO, anti-TG, anti-21-OH) and markers specific for autoimmune polyglandular syndrome type I (e.g., anti-IFN-ω, anti-IFN-α2a, anti-IL-22) within a single assay [
89]. Validation studies demonstrate high analytical reliability, with coefficients of concordance between the microarray platform and reference monoplex ELISA ranging from 0.75 to 0.92 for autoantibodies to GAD65, IA-2, and ICA. The overall agreement rate of 88-97% confirms the platform's strong performance and suitability for comprehensive serological profiling.
4.1.3. ADAP (Antibody Detection by Agglutination-PCR)
ADAP is a highly sensitive immunoassay that utilizes DNA barcoding technology. The technique involves covalently coupling T1D autoantigens or their epitopes to unique DNA oligonucleotides. Following incubation with a patient sample and specific binding to autoantibodies, these DNA tags are amplified by PCR, enabling exceptional detection sensitivity and specificity. This platform permits the simultaneous identification of autoantibodies against GAD65, IA-2, ZnT8, insulin, and thyroglobulin in a single reaction, making it a highly promising tool for large-scale screening of pediatric populations at elevated risk for T1D [
90,
91]. Nevertheless, this method remains experimental and has not yet been translated into routine clinical practice.
4.1.4. Phage Immunoprecipitation Sequencing (PhIP-Seq)
PhIP-Seq represents a powerful approach for multiplex serological profiling in T1D research [
92]. This high-throughput technology immobilizes proteome-wide peptide libraries on a unified platform, enabling comprehensive characterization of antibody interactions within patient sera. The method facilitates global serological analysis by simultaneously screening thousands of peptide sequences representing diverse autoantigens [
93]. While PhIP-Seq has proven invaluable for
de novo autoantigen discovery, its primary limitation lies in preferential detection of antibodies targeting linear epitopes, making it less suitable for studying conformational epitopes that require intact tertiary protein structures.
4.1.5. Three Screen Islet Cell Autoantibody Assay (ICA)
The Three Screen ICA™ system (RSR Limited, Cardiff, U.K.) became one of the first commercially available solutions for the simultaneous detection of autoantibodies to GAD65, IA-2, and ZnT8 [
94]. While it substantially accelerates analysis throughput, this method has a fundamental limitation: a positive result does not identify antibody specificity, necessitating confirmation with monoplex assays. Furthermore, the panel's exclusion of insulin autoantibodies (IAA) reduces its diagnostic sensitivity, particularly for early-stage T1D and in pediatric populations. These constraints highlight the necessity for further development of this diagnostic approach.
4.1.6. Multiplex Electrochemiluminescence (ECL)
Multiplex ECL immunoassays, pioneered by the research group of Liping Yu at the Barbara Davis Center for Diabetes (University of Colorado), constitute an advanced platform for T1D diagnostics [
95]. This technology enables the simultaneous quantification of autoantibodies to insulin, GAD65, and IA-2, alongside antibodies to tissue transglutaminase (tTG), facilitating concurrent risk assessment for T1D and asymptomatic celiac disease [
96]. The clinical utility of this multiplex ECL platform has been robustly validated in large-scale epidemiological studies, including the Autoimmunity Study in Kids (ASK), where it demonstrated high diagnostic accuracy and reproducibility in a cohort of 20,000 children [
97].
In summary, contemporary T1D diagnostics are advancing towards integrated, multiparameter strategies that consolidate information from multiple autoantigens, their fragments, and epitopes. Established technologies such as protein microarrays, Three Screen ICA, Array-ELISA, multiplex ECL, ADAP, and PhIP-Seq now provide the foundation for high-precision screening, early detection, and predictive risk assessment. These platforms facilitate the comprehensive detection of autoantibody repertoires while maintaining sensitivity and specificity comparable to traditional monoplex assays.
Despite these advances, persistent challenges include a lack of standardization, limited autoantigen panels, and the need for validation of novel biomarkers. A promising future direction involves generating personalized diagnostic profiles through epitope-specific mapping of individual immune responses — from full-length autoantigens to discrete epitopes. Such a strategy would unlock new potential not only for early T1D diagnosis but also for individualized prognosis and the development of targeted immunotherapies.
4.2. Antigen-Specific Immunotherapy (ASI)
In recent years, antigen-specific immunotherapy (ASI) has emerged as a promising strategy aimed at the selective induction of regulatory T-lymphocytes (Tregs) and the restoration of immune tolerance to pancreatic β-cells. The primary targets for ASI in T1D are key autoantigens, including insulin, GAD65, IA-2, and ZnT8. A principal challenge in developing these therapies is the substantial heterogeneity of the autoimmune response among patients, requiring robust stratification strategies to achieve therapeutic efficacy. While numerous clinical trials have demonstrated acceptable safety profiles for various ASI approaches, their clinical efficacy remains under investigation in later-phase clinical trials [
98].
4.2.1. Plasmid DNA Immunization
Plasmid DNA immunization represents a promising strategy for suppressing the autoimmune response against β-cell antigens while preserving systemic immune competence. This approach involves the in vivo delivery of plasmid vectors encoding target autoantigens or their immunodominant epitopes, facilitating endogenous synthesis of proteins in their native conformation. This enables antigen presentation under non-inflammatory conditions ("immune ignorance"), thereby promoting the induction of peripheral immune tolerance [
98,
99]. An advanced iteration of this strategy involves the co-expression of autoantigens with immunomodulatory cytokines such as IL-10. This engineered co-expression promotes the differentiation of naïve T-cells into a Treg phenotype while concurrently inducing anergy in autoreactive effector T-lymphocytes [
100].
4.2.2. Peptide-Based Therapy
Peptide-based therapy employs synthetic fragments of autoantigens for precision modulation of the immune response. Unlike DNA immunization, which relies on endogenous antigen synthesis, this approach involves the exogenous administration of pre-formed peptides. The primary mechanism of action entails their uptake and presentation by immature dendritic cells in the absence of co-stimulatory signals, thereby promoting immune tolerance via the induction of regulatory T-cells (Treg) and/or clonal deletion of autoreactive T-lymphocytes [
101]. n alternative strategy utilizes altered peptide ligands (APLs)—modified sequences with enhanced binding affinity for MHC class II molecules. These engineered ligands are designed to selectively stimulate regulatory T-cell populations rather than activating pathogenic effector responses [
102].
4.2.3. Intranasal Administration
Intranasal administration of autoantigens represents one of the most extensively investigated ASI approaches, exploiting the principle of mucosal tolerance induction [
103]. Insulin has been the most thoroughly studied antigen in this context, with other T1D-associated antigens explored to a lesser extent. Following intranasal delivery, autoantigens are captured by specialized antigen-presenting cells in the nasal mucosa and the associated nasal-associated lymphoid tissue (NALT). This process triggers local activation and expansion of antigen-specific regulatory T-cells (Tregs), which subsequently enter systemic circulation, migrate to the pancreas, and initiate restoration of immune tolerance directly at the site of autoimmune injury. Despite encouraging results in preclinical models and a favorable safety profile, large-scale clinical trials—including the Intranasal Insulin Trials (INIT I/II)—have not demonstrated significant efficacy in preventing T1D onset in at-risk children [
104].
The therapeutic strategies discussed — plasmid DNA immunization, peptide-based therapy, and intranasal antigen delivery — constitute promising approaches that nevertheless require optimization of dosing regimens, investigation of potential cross-reactivity, and validation in large-scale clinical studies. The success of such immunotherapy is contingent upon a specific "therapeutic window," characterized by the presence of multiple autoantibodies alongside preserved functional β-cell mass. This patient population represents the primary target for ASI. Thus, the efficacy of antigen-specific therapy is directly dependent on early and precise diagnosis, underscoring the critical need for integrated programs that combine autoantibody screening, immune monitoring, and timely intervention. A personalized approach, tailored to individual autoantibody profiles and disease stage, represents a fundamental requirement for successfully re-establishing immune tolerance and maintaining residual insulin secretion.
5. Conclusions
Type 1 diabetes mellitus arises from a complex interplay of genetic susceptibility, immune dysregulation, and environmental triggers, culminating in the loss of immune tolerance and the destruction of pancreatic β-cells. The autoimmune response targets a well-defined set of β-cell autoantigens, with insulin, GAD65, IA-2, and ZnT8 constituting the primary targets.
In recent years, the analysis of autoantibody epitope specificity has proven crucial for elucidating T1D pathogenesis. It is now established that immunodominant epitopes for most of these autoantigens are conformational and often localized to C-terminal regions, which become exposed during β-cell degradation. Detailed epitope mapping has significantly advanced our understanding of the autoimmune process, particularly the phenomenon of epitope spreading, and helps delineate the progression from preclinical stages to clinical manifestation.
The introduction of modern, highly sensitive multiplex platforms for detecting T1D-associated autoantibodies into clinical practice provides a dual benefit: preventing acute complications like ketoacidosis and creating a unique opportunity for early diagnosis. This early detection opens a critical "therapeutic window" for intervention. At this preclinical stage, antigen-specific immunotherapy aimed at restoring tolerance to key autoantigens represents the most promising therapeutic strategy. The continued development of personalized diagnostic and therapeutic approaches, integrated with in-depth epitope mapping, is forging a new paradigm in T1D management. This strategy shifts the focus from managing insulin deficiency to actively halting the autoimmune process itself, with the ultimate goal of preserving a functional β-cell mass.
Author Contributions
Conceptualization, I.K. and E.S.; writing-original draft preparation, I.K.; writing-review and editing, E.S. and D.G.; visualization, A.K. and E.L; supervision, E.S and D.G.; project administration, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation to the EIMB Center for Precision Genetic Technologies in Medicine, agreement number 075-15-2025-519
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAb |
Autoantibody |
| ADAP |
Antibody Detection by Agglutination-PCR |
| APLs |
Altered Peptide Ligands |
| ASI |
Antigen-Specific Immunotherapy |
| ASK |
Autoimmunity Study in Kids |
| CLIA |
Chemiluminescent Immunoassay |
| ECL |
Electrochemiluminescence |
| ELISA |
Enzyme-Linked Immunosorbent Assay |
| ENDIT |
European Nicotinamide Diabetes Intervention Trial |
| GABA |
Gamma-Aminobutyric Acid |
| GAD65 |
Glutamic Acid Decarboxylase 65 |
| GAD67 |
Glutamic Acid Decarboxylase 67 |
| GADA |
GAD65 Autoantibodies |
| HLA |
Human Leukocyte Antigen |
| HIP14 |
Huntingtin-interacting protein 14 |
| IA-2 |
Islet Autoantigene 2 |
| IAA |
Insulin Autoantibodies |
| ICA1 |
Islet Cell Autoantigen 1 |
| LEL |
Large Extracellular Loop |
| MHC |
Major histocompatibility complex |
| NALT |
Nasal-Associated Lymphoid Tissue |
| PCR |
Polymerase Chain Reaction |
| PhIP-Seq |
Phage Immunoprecipitation sequencing |
| PICK1 |
Protein Interacting with C Kinase 1 |
| PLP |
Pyridoxal-5'-phosphate |
| RIA |
Radioimmunoassay |
| SEL |
Small Extracellular Loop |
| T1D |
Type 1 diabetes |
| TM |
Transmembrane domain |
| Treg |
Regulatory T-lymphocytes |
References
- Roep, B.O.; Thomaidou, S.; Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nature reviews. Endocrinology 2021, 17, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Mirmira, R.G. The pathogenic "symphony" in type 1 diabetes: A disorder of the immune system, beta cells, and exocrine pancreas. Cell metabolism 2023, 35, 1500–1518. [Google Scholar] [CrossRef]
- Mauvais, F.X.; Endert, P.M. Type 1 Diabetes: A Guide to Autoimmune Mechanisms for Clinicians. Diabetes, obesity & metabolism 2025, 27 (Suppl. S6), 40–56. [Google Scholar]
- Bell, K.J.; Lain, S.J. The Changing Epidemiology of Type 1 Diabetes: A Global Perspective. Diabetes, obesity & metabolism 2025, 27 (Suppl. S6), 3–14. [Google Scholar]
- Gomber, A.; Ward, Z.J.; Ross, C.; Owais, M.; Mita, C.; Yeh, J.M.; Reddy, C.L.; Atun, R. Variation in the incidence of type 1 diabetes mellitus in children and adolescents by world region and country income group: A scoping review. PLOS global public health 2022, 2, e0001099. [Google Scholar] [CrossRef]
- Mittal, R.; Camick, N.; Lemos, J.; Hirani, K. Gene-environment interaction in the pathophysiology of type 1 diabetes. Frontiers in endocrinology 2024, 15, 1335435. [Google Scholar] [CrossRef]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA region in the prediction of type 1 diabetes. Current diabetes reports 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Tay, G.K.; Al Naqbi, H.; Mawart, A.; Baalfaqih, Z.; Almaazmi, A.; Deeb, A.; Alsafar, H. Segregation Analysis of Genotyped and Family-Phased, Long Range MHC Classical Class I and Class II Haplotypes in 5 Families With Type 1 Diabetes Proband in the United Arab Emirates. Frontiers in genetics 2021, 12, 670844. [Google Scholar] [CrossRef] [PubMed]
- Brand, O.; Gough, S.; Heward, J. HLA, CTLA-4 and PTPN22 : the shared genetic master-key to autoimmunity? Expert reviews in molecular medicine 2005, 7, 1–15. [Google Scholar] [CrossRef]
- Houcken, J.; Degenhart, C.; Bender, K.; Konig, J.; Frommer, L.; Kahaly, G.J. PTPN22 and CTLA-4 Polymorphisms Are Associated With Polyglandular Autoimmunity. The Journal of clinical endocrinology and metabolism 2018, 103, 1977–1984. [Google Scholar] [CrossRef]
- Pei, J.; Wei, S.; Pei, Y.; Wu, H.; Wang, D. Role of Dietary Gluten in Development of Celiac Disease and Type I Diabetes: Management Beyond Gluten-Free Diet. Current medicinal chemistry 2020, 27, 3555–3576. [Google Scholar] [CrossRef]
- Tomasics, G.; Schandl, L.; Polyak, A.; Winkler, G. Diabetes mellitus and the intestinal microbiome. Orvosi hetilap 2023, 164, 981–987. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Chiles, K.; Huber, J.; Ziehl, A.; Turke, M.; Pietrowicz, M. Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kohil, A.; Al-Asmakh, M.; Al-Shafai, M.; Terranegra, A. The Interplay Between Diet and the Epigenome in the Pathogenesis of Type-1 Diabetes. Frontiers in nutrition 2020, 7, 612115. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.R.N.; Hirani, K.; von Herrath, M. Immunological and virological triggers of type 1 diabetes: insights and implications. Frontiers in immunology 2023, 14, 1326711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Li, R.; Huang, Y.; Chen, H.; Nie, H.; Liu, L.; Zou, X.; Zhong, J.; Zheng, B.; Gong, Q. The role of B cells in the pathogenesis of type 1 diabetes. Frontiers in immunology 2024, 15, 1450366. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama 2013, 309, 2473–2479. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef]
- Mallone, R.; Martinuzzi, E.; Blancou, P.; Novelli, G.; Afonso, G.; Dolz, M.; Bruno, G.; Chaillous, L.; Chatenoud, L.; Bach, J.M.; et al. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 2007, 56, 613–621. [Google Scholar] [CrossRef]
- Walker, L.S.; von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clinical and experimental immunology 2016, 183, 16–29. [Google Scholar] [CrossRef]
- Insel, R.A.; Dunne, J.L.; Atkinson, M.A.; Chiang, J.L.; Dabelea, D.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Krischer, J.P.; Lernmark, A.; et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care 2015, 38, 1964–1974. [Google Scholar] [CrossRef]
- Delong, T.; Nakayama, M. Epitope Hierarchy in Type 1 Diabetes Pathogenesis. Cold Spring Harbor perspectives in medicine 2024. [Google Scholar] [CrossRef]
- Franke, B.; Galloway, T.S.; Wilkin, T.J. Developments in the prediction of type 1 diabetes mellitus, with special reference to insulin autoantibodies. Diabetes/metabolism research and reviews 2005, 21, 395–415. [Google Scholar] [CrossRef]
- Lampasona, V.; Liberati, D. Islet Autoantibodies. Current diabetes reports 2016, 16, 53. [Google Scholar] [CrossRef]
- Battaglia, M. Experiments by nature: lessons on type 1 diabetes. Tissue antigens 2014, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wenzlau, J.M.; Hutton, J.C. Novel diabetes autoantibodies and prediction of type 1 diabetes. Current diabetes reports 2013, 13, 608–615. [Google Scholar] [CrossRef]
- Arvan, P.; Pietropaolo, M.; Ostrov, D.; Rhodes, C.J. Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harbor perspectives in medicine 2012, 2. [Google Scholar] [CrossRef]
- Kawasaki, E. Anti-Islet Autoantibodies in Type 1 Diabetes. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef]
- Pal, S.; Chaturvedi, U.C.; Mehrotra, R.M.; Gupta, N.N.; Sircar, A.R. Insulin "auto-antibodies" in diabetes mellitus. Indian journal of medical sciences 1969, 23, 598–601. [Google Scholar] [PubMed]
- Velloso, L.A.; Kampe, O.; Hallberg, A.; Christmanson, L.; Betsholtz, C.; Karlsson, F.A. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type 1 diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. The Journal of clinical investigation 1993, 91, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Hagopian, W.A.; Karlsen, A.E.; Gottsater, A.; Landin-Olsson, M.; Grubin, C.E.; Sundkvist, G.; Petersen, J.S.; Boel, E.; Dyrberg, T.; Lernmark, A. Quantitative assay using recombinant human islet glutamic acid decarboxylase (GAD65) shows that 64K autoantibody positivity at onset predicts diabetes type. The Journal of clinical investigation 1993, 91, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.S.; Wasserfall, C.; Maclaren, N.K.; Notkins, A.L. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America 1996, 93, 6367–6370. [Google Scholar] [CrossRef] [PubMed]
- Passini, N.; Larigan, J.D.; Genovese, S.; Appella, E.; Sinigaglia, F.; Rogge, L. The 37/40-kilodalton autoantigen in insulin-dependent diabetes mellitus is the putative tyrosine phosphatase IA-2. Proceedings of the National Academy of Sciences of the United States of America 1995, 92, 9412–9416. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Juhl, K.; Yu, L.; Moua, O.; Sarkar, S.A.; Gottlieb, P.; Rewers, M.; Eisenbarth, G.S.; Jensen, J.; Davidson, H.W.; et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 17040–17045. [Google Scholar] [CrossRef]
- Amoroso, M.; Achenbach, P.; Powell, M.; Coles, R.; Chlebowska, M.; Carr, L.; Furmaniak, J.; Scholz, M.; Bonifacio, E.; Ziegler, A.G.; et al. 3 Screen islet cell autoantibody ELISA: A sensitive and specific ELISA for the combined measurement of autoantibodies to GAD(65), to IA-2 and to ZnT8. Clinica chimica acta; international journal of clinical chemistry 2016, 462, 60–64. [Google Scholar] [CrossRef]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Experimental & molecular medicine 2016, 48, e219. [Google Scholar]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nature reviews. Molecular cell biology 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Rohli, K.E.; Boyer, C.K.; Blom, S.E.; Stephens, S.B. Nutrient Regulation of Pancreatic Islet beta-Cell Secretory Capacity and Insulin Production. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Carre, A.; Mallone, R. Making Insulin and Staying Out of Autoimmune Trouble: The Beta-Cell Conundrum. Frontiers in immunology 2021, 12, 639682. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Faridi, P.; Lim, J.J.; Ting, Y.T.; Onwukwe, G.; Bhattacharjee, P.; Jones, C.M.; Tresoldi, E.; Cameron, F.J.; La Gruta, N.L.; et al. T cell receptor recognition of hybrid insulin peptides bound to HLA-DQ8. Nature communications 2021, 12, 5110. [Google Scholar] [CrossRef]
- Potter, K.N.; Wilkin, T.J. The molecular specificity of insulin autoantibodies. Diabetes/metabolism research and reviews 2000, 16, 338–353. [Google Scholar] [CrossRef]
- Padoa, C.J.; Crowther, N.J.; Thomas, J.W.; Hall, T.R.; Bekris, L.M.; Torn, C.; Landin-Olsson, M.; Ortqvist, E.; Palmer, J.P.; Lernmark, A.; et al. Epitope analysis of insulin autoantibodies using recombinant Fab. Clinical and experimental immunology 2005, 140, 564–571. [Google Scholar] [CrossRef]
- Achenbach, P.; Koczwara, K.; Knopff, A.; Naserke, H.; Ziegler, A.G.; Bonifacio, E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. The Journal of clinical investigation 2004, 114, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.R.; Thomas, J.W.; Padoa, C.J.; Torn, C.; Landin-Olsson, M.; Ortqvist, E.; Hampe, C.S. Longitudinal epitope analysis of insulin-binding antibodies in type 1 diabetes. Clinical and experimental immunology 2006, 146, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kanatsuna, N.; Papadopoulos, G.K.; Moustakas, A.K.; Lenmark, A. Etiopathogenesis of insulin autoimmunity. Anatomy research international 2012, 2012, 457546. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Gu, Y.; Michels, A.; Rewers, M.; Haskins, K.; Yu, L. Identification of Autoantibodies to a Hybrid Insulin Peptide in Type 1 Diabetes. Diagnostics 2023, 13. [Google Scholar] [CrossRef]
- Groegler, J.; Callebaut, A.; James, E.A.; Delong, T. The insulin secretory granule is a hotspot for autoantigen formation in type 1 diabetes. Diabetologia 2024, 67, 1507–1516. [Google Scholar] [CrossRef]
- Wiles, T.A.; Powell, R.; Michel, R.; Beard, K.S.; Hohenstein, A.; Bradley, B.; Reisdorph, N.; Haskins, K.; Delong, T. Identification of Hybrid Insulin Peptides (HIPs) in Mouse and Human Islets by Mass Spectrometry. Journal of proteome research 2019, 18, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Bergado-Acosta, J.R.; Muller, I.; Richter-Levin, G.; Stork, O. The GABA-synthetic enzyme GAD65 controls circadian activation of conditioned fear pathways. Behavioural brain research 2014, 260, 92–100. [Google Scholar] [CrossRef]
- Fenalti, G.; Law, R.H.; Buckle, A.M.; Langendorf, C.; Tuck, K.; Rosado, C.J.; Faux, N.G.; Mahmood, K.; Hampe, C.S.; Banga, J.P.; et al. GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nature structural & molecular biology 2007, 14, 280–286. [Google Scholar]
- Stander, S.H.D.; Reboul, C.F.; Le, S.N.; Williams, D.E.; Chandler, P.G.; Costa, M.G.S.; Hoke, D.E.; Jimma, J.D.T.; Fodor, J.; Fenalti, G.; et al. Structure and dynamics of GAD65 in complex with an autoimmune polyendocrine syndrome type 2-associated autoantibody. Nature communications 2025, 16, 2275. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Patterson, G.; Schaufele, F.; Lippincott-Schwartz, J.; Baekkeskov, S. A palmitoylation cycle dynamically regulates partitioning of the GABA-synthesizing enzyme GAD65 between ER-Golgi and post-Golgi membranes. Journal of cell science 2008, 121, 437–449. [Google Scholar] [CrossRef]
- Christgau, S.; Aanstoot, H.J.; Schierbeck, H.; Begley, K.; Tullin, S.; Hejnaes, K.; Baekkeskov, S. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic beta-cells by palmitoylation in the NH2-terminal domain. The Journal of cell biology 1992, 118, 309–320. [Google Scholar] [CrossRef]
- Ragan, C.M.; Ahmed, E.I.; Vitale, E.M.; Linning-Duffy, K.; Miller-Smith, S.M.; Maguire, J.; Lonstein, J.S. Postpartum State, but Not Maternal Caregiving or Level of Anxiety, Increases Medial Prefrontal Cortex GAD(65) and vGAT in Female Rats. Frontiers in global women's health 2021, 2, 746518. [Google Scholar] [CrossRef]
- Kass, I.; Hoke, D.E.; Costa, M.G.; Reboul, C.F.; Porebski, B.T.; Cowieson, N.P.; Leh, H.; Pennacchietti, E.; McCoey, J.; Kleifeld, O.; et al. Cofactor-dependent conformational heterogeneity of GAD65 and its role in autoimmunity and neurotransmitter homeostasis. Proceedings of the National Academy of Sciences of the United States of America 2014, 111, E2524–E2529. [Google Scholar] [CrossRef]
- Keshavarzi, E.; Noveiry, B.B.; Rezaei, N. The relationship between GAD65 autoantibody and the risk of T1DM onset. Journal of diabetes and metabolic disorders 2022, 21, 1935–1942. [Google Scholar] [CrossRef]
- Krause, S.; Landherr, U.; Agardh, C.D.; Hausmann, S.; Link, K.; Hansen, J.M.; Lynch, K.F.; Powell, M.; Furmaniak, J.; Rees-Smith, B.; et al. GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes care 2014, 37, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Guyer, K.M.; Dong, F.; Jiang, L.; Steck, A.K.; Rewers, M.; Eisenbarth, G.S.; Yu, L. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 2013, 62, 4174–4178. [Google Scholar] [CrossRef]
- Peng, Y.; Li, X.; Xiang, Y.; Yan, X.; Zhou, H.; Tang, X.; Cheng, J.; Niu, X.; Liu, J.; Ji, Q.; et al. GAD65 Antibody Epitopes and Genetic Background in Latent Autoimmune Diabetes in Youth (LADY). Frontiers in immunology 2022, 13, 836952. [Google Scholar] [CrossRef]
- Bansal, N.; Hampe, C.S.; Rodriguez, L.; O'Brian Smith, E.; Kushner, J.; Balasubramanyam, A.; Redondo, M.J. DPD epitope-specific glutamic acid decarboxylase (GAD)65 autoantibodies in children with Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association 2017, 34, 641–646. [Google Scholar] [CrossRef]
- Padoa, C.J.; Banga, J.P.; Madec, A.M.; Ziegler, M.; Schlosser, M.; Ortqvist, E.; Kockum, I.; Palmer, J.; Rolandsson, O.; Binder, K.A.; et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes 2003, 52, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.C.; Brigatti, C.; Liberati, D.; Grace, S.L.; Gillard, B.T.; Long, A.E.; Marzinotto, I.; Shoemark, D.K.; Chandler, K.A.; Achenbach, P.; et al. The first 142 amino acids of glutamate decarboxylase do not contribute to epitopes recognized by autoantibodies associated with Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association 2018, 35, 954–963. [Google Scholar] [CrossRef]
- Pollanen, P.M.; Harkonen, T.; Ilonen, J.; Toppari, J.; Veijola, R.; Siljander, H.; Knip, M. Autoantibodies to N-terminally Truncated GAD65(96-585): HLA Associations and Predictive Value for Type 1 Diabetes. The Journal of clinical endocrinology and metabolism 2022, 107, e935–e946. [Google Scholar] [CrossRef]
- Hampe, C.S.; Radtke, J.R.; Wester, A.; Carlsson, A.; Cedervall, E.; Jonsson, B.; Ivarsson, S.A.; Elding Larsson, H.; Larsson, K.; Lindberg, B.; et al. Reduced display of conformational epitopes in the N-terminal truncated GAD65 isoform: relevance for people with stiff person syndrome or DQ8/8-positive Type 1 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association 2019, 36, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Wester, A.; Skarstrand, H.; Lind, A.; Ramelius, A.; Carlsson, A.; Cedervall, E.; Jonsson, B.; Ivarsson, S.A.; Elding Larsson, H.; Larsson, K.; et al. An Increased Diagnostic Sensitivity of Truncated GAD65 Autoantibodies in Type 1 Diabetes May Be Related to HLA-DQ8. Diabetes 2017, 66, 735–740. [Google Scholar] [CrossRef]
- Galli, J.R.; Austin, S.D.; Greenlee, J.E.; Clardy, S.L. Stiff person syndrome with Anti-GAD65 antibodies within the national veterans affairs health administration. Muscle & nerve 2018, 58, 801–804. [Google Scholar]
- Solimena, M.; Dirkx, R., Jr.; Hermel, J.M.; Pleasic-Williams, S.; Shapiro, J.A.; Caron, L.; Rabin, D.U. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. The EMBO journal 1996, 15, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jeong, D.G.; Jeong, S.K.; Yoon, T.S.; Ryu, S.E. Crystal structure of the major diabetes autoantigen insulinoma-associated protein 2 reveals distinctive immune epitopes. Diabetes 2007, 56, 41–48. [Google Scholar] [CrossRef]
- Seissler, J.; Nguyen, T.B.; Aust, G.; Steinbrenner, H.; Scherbaum, W.A. Regulation of the diabetes-associated autoantigen IA-2 in INS-1 pancreatic beta-cells. Diabetes 2000, 49, 1137–1141. [Google Scholar] [CrossRef]
- Trajkovski, M.; Mziaut, H.; Schubert, S.; Kalaidzidis, Y.; Altkruger, A.; Solimena, M. Regulation of insulin granule turnover in pancreatic beta-cells by cleaved ICA512. The Journal of biological chemistry 2008, 283, 33719–33729. [Google Scholar] [CrossRef]
- Leslie, R.D.; Atkinson, M.A.; Notkins, A.L. Autoantigens IA-2 and GAD in Type I (insulin-dependent) diabetes. Diabetologia 1999, 42, 3–14. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, B.; Matsumoto, Y.; Li, Q.; Notkins, A.L.; Lan, M.S. Autoantibodies to IA-2 and IA-2 beta in insulin-dependent diabetes mellitus recognize conformational epitopes: location of the 37- and 40-kDa fragments determined. Journal of immunology 1997, 159, 3662–3667. [Google Scholar] [CrossRef]
- Torii, S. Expression and function of IA-2 family proteins, unique neuroendocrine-specific protein-tyrosine phosphatases. Endocrine journal 2009, 56, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, Q.; Su, Y.; Sun, W.; Huang, Q.; Wei, W. Zinc and its regulators in pancreas. Inflammopharmacology 2019, 27, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Long, A.E. What has zinc transporter 8 autoimmunity taught us about type 1 diabetes? Diabetologia 2019, 62, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Du, J.; Merriman, C.; Gong, Z. Genetic, Functional, and Immunological Study of ZnT8 in Diabetes. International journal of endocrinology 2019, 2019, 1524905. [Google Scholar] [CrossRef]
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nature genetics 2019, 51, 1596–1606. [Google Scholar] [CrossRef]
- Daniels, M.J.; Jagielnicki, M.; Yeager, M. Structure/Function Analysis of human ZnT8 (SLC30A8): A Diabetes Risk Factor and Zinc Transporter. Current research in structural biology 2020, 2, 144–155. [Google Scholar] [CrossRef]
- Kawasaki, E. ZnT8 and type 1 diabetes. Endocrine journal 2012, 59, 531–537. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Frisch, L.M.; Hutton, J.C.; Davidson, H.W. Mapping of conformational autoantibody epitopes in ZNT8. Diabetes/metabolism research and reviews 2011, 27, 883–886. [Google Scholar] [CrossRef]
- Noguera, M.E.; Primo, M.E.; Jakoncic, J.; Poskus, E.; Solimena, M.; Ermacora, M.R. X-ray structure of the mature ectodomain of phogrin. Journal of structural and functional genomics 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Walther, D.; Eugster, A.; Jergens, S.; Gavrisan, A.; Weinzierl, C.; Telieps, T.; Winkler, C.; Ziegler, A.G.; Bonifacio, E. Tetraspanin 7 autoantibodies in type 1 diabetes. Diabetologia 2016, 59, 1973–1976. [Google Scholar] [CrossRef]
- Eugster, A.; Kraus, G.; Lidzba, V.; Muller, D.; Jolink, M.; Ziegler, A.G.; Bonifacio, E. Cytoplasmic ends of tetraspanin 7 harbour epitopes recognised by autoantibodies in type 1 diabetes. Diabetologia 2019, 62, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Mao, Z.; Kam, C.; Xiao, N.; Cao, X.; Shen, C.; Cheng, K.K.; Xu, A.; Lee, K.M.; Jiang, L.; et al. PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance. PLoS biology 2013, 11, e1001541. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Kardorf, J.; Schulte, B.; Lampeter, E.F.; Gries, F.A.; Melchers, I.; Wagner, R.; Bertrams, J.; Roep, B.O.; Pfutzner, A. Autoantibodies to the islet antigen ICA69 occur in IDDM and in rheumatoid arthritis. Diabetologia 1995, 38, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Manfredi, M.; Garrafa, E.; Pancani, S.; Lechiara, A.; Mobilia, E.M.; Grossi, V.; Lari, B.; Bizzaro, N.; Pesce, G. A comparison of current methods to measure antibodies in type 1 diabetes. Clinical chemistry and laboratory medicine 2025, 63, 2104–2112. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, J.; Hu, J. Improved diagnosis of type-1 diabetes mellitus using multiplexed autoantibodies ELISA array. Analytical biochemistry 2022, 649, 114722. [Google Scholar] [CrossRef]
- Savvateeva, E.N.; Yukina, M.Y.; Nuralieva, N.F.; Filippova, M.A.; Gryadunov, D.A.; Troshina, E.A. Multiplex Autoantibody Detection in Patients with Autoimmune Polyglandular Syndromes. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Lind, A.; Freyhult, E.; de Jesus Cortez, F.; Ramelius, A.; Bennet, R.; Robinson, P.V.; Seftel, D.; Gebhart, D.; Tandel, D.; Maziarz, M.; et al. Childhood screening for type 1 diabetes comparing automated multiplex Antibody Detection by Agglutination-PCR (ADAP) with single plex islet autoantibody radiobinding assays. EBioMedicine 2024, 104, 105144. [Google Scholar] [CrossRef]
- Lind, A.; de Jesus Cortez, F.; Ramelius, A.; Bennet, R.; Robinson, P.V.; Seftel, D.; Gebhart, D.; Tandel, D.; Maziarz, M.; Agardh, D.; et al. Multiplex agglutination-PCR (ADAP) autoantibody assays compared to radiobinding autoantibodies in type 1 diabetes and celiac disease. Journal of immunological methods 2022, 506, 113265. [Google Scholar] [CrossRef]
- Larman, H.B.; Laserson, U.; Querol, L.; Verhaeghen, K.; Solimini, N.L.; Xu, G.J.; Klarenbeek, P.L.; Church, G.M.; Hafler, D.A.; Plenge, R.M.; et al. PhIP-Seq characterization of autoantibodies from patients with multiple sclerosis, type 1 diabetes and rheumatoid arthritis. Journal of autoimmunity 2013, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gunarathne, S.M.S.; Liu, W.; Zhou, Y.; Jiang, Y.; Li, S.; Huang, J. PhIP-Seq: methods, applications and challenges. Frontiers in bioinformatics 2024, 4, 1424202. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Takahashi, Y.; Komeda, T.; Sakuma, M. Evaluation of Biochemical Characteristics and Performance of the 3 Screen ICA ELISA Kit. International journal of molecular sciences 2024, 25. [Google Scholar] [CrossRef]
- Yu, L. Islet Autoantibody Detection by Electrochemiluminescence (ECL) Assay. Methods in molecular biology 2016, 1433, 85–91. [Google Scholar]
- Jia, X.; He, L.; Miao, D.; Zhang, C.; Rewers, M.; Yu, L. A High-throughput Multiplexed Screening for Type 1 Diabetes, Celiac Diseases, and COVID-19. Journal of visualized experiments : JoVE 2022. [Google Scholar] [CrossRef]
- He, L.; Jia, X.; Rasmussen, C.G.; Waugh, K.; Miao, D.; Dong, F.; Frohnert, B.; Steck, A.K.; Simmons, K.M.; Rewers, M.; et al. High-Throughput Multiplex Electrochemiluminescence Assay Applicable to General Population Screening for Type 1 Diabetes and Celiac Disease. Diabetes technology & therapeutics 2022, 24, 502–509. [Google Scholar]
- Kreiner, F.F.; von Scholten, B.J.; Coppieters, K.; von Herrath, M. Current state of antigen-specific immunotherapy for type 1 diabetes. Current opinion in endocrinology, diabetes, and obesity 2021, 28, 411–418. [Google Scholar] [CrossRef]
- Thomas, R.; Carballido, J.M.; Wesley, J.D.; Ahmed, S.T. Overcoming Obstacles in the Development of Antigen-Specific Immunotherapies for Type 1 Diabetes. Frontiers in immunology 2021, 12, 730414. [Google Scholar] [CrossRef]
- Brusko, M.A.; Stewart, J.M.; Posgai, A.L.; Wasserfall, C.H.; Atkinson, M.A.; Brusko, T.M.; Keselowsky, B.G. Immunomodulatory Dual-Sized Microparticle System Conditions Human Antigen Presenting Cells Into a Tolerogenic Phenotype In Vitro and Inhibits Type 1 Diabetes-Specific Autoreactive T Cell Responses. Frontiers in immunology 2020, 11, 574447. [Google Scholar] [CrossRef]
- Smith, E.L.; Peakman, M. Peptide Immunotherapy for Type 1 Diabetes-Clinical Advances. Frontiers in immunology 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Van Rampelbergh, J.; Achenbach, P.; Leslie, R.D.; Ali, M.A.; Dayan, C.; Keymeulen, B.; Owen, K.R.; Kindermans, M.; Parmentier, F.; Carlier, V.; et al. First-in-human, double-blind, randomized phase 1b study of peptide immunotherapy IMCY-0098 in new-onset type 1 diabetes. BMC medicine 2023, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.M.; Lemos, J.R.N.; Hirani, K.; von Herrath, M. Type 1 diabetes: immune pathology and novel therapeutic approaches. Diabetology international 2024, 15, 761–776. [Google Scholar] [CrossRef]
- Jacobsen, L.M.; Schatz, D.A. Insulin immunotherapy for pretype 1 diabetes. Current opinion in endocrinology, diabetes, and obesity 2021, 28, 390–396. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).