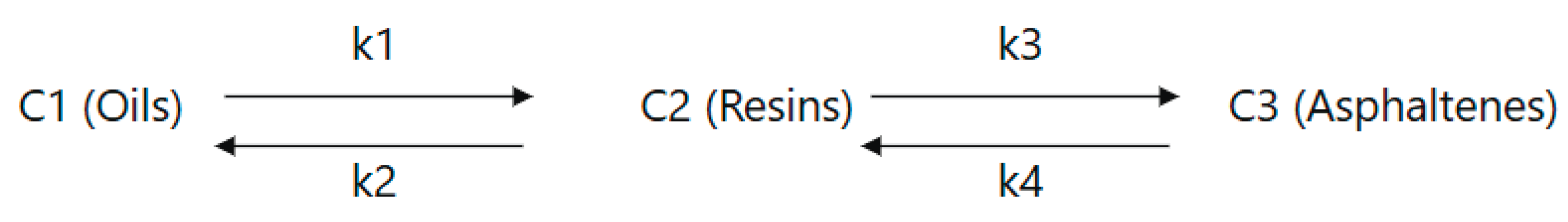1. Introduction
The growing trend of declining light oil production, as well as the growth in global energy demand, makes the issues of preliminary processing of heavy oil and unconventional oil types particularly relevant. In this regard, ultrasonic processing methods have become one of the most important areas of research [
1,
2,
3,
4]. Over the past decades, ultrasonic technology has moved from basic applications in the separation of oil and water emulsions to advanced methods of viscosity reduction, chemical transformation of heavy oil components, including desulfurization and dementalization [
5,
6,
7,
8,
9]. This area is of practical importance for improving oil recovery, optimizing transportation and improving refining processes. The economic and environmental benefits of ultrasound treatment highlight its growing importance in petroleum engineering [
10,
11].
Ultrasound causes cavitation bubbles, whose collapse generates localized energy sufficient to break carbon-carbon and carbon-heteroatom bonds in asphaltenes and resins. This process leads to molecular fragmentation, alterations in group composition, and a reduction in the size of asphaltene aggregates [
12,
13,
14]. These chemical changes correlate with reduced viscosity and improved fluidity, establishing a mechanistic relationship between ultrasonic parameters, chemical transformations, and macroscopic properties of oil [
15].
A number of authors note the predominant destructive effect of ultrasound. Thus, Razavifar et al. [
16] showed a decrease in the average molecular weight of heavy oil by ~ 7% and a decrease in the size of asphaltene aggregates, which was accompanied by a decrease in the viscosity of the system. According to Shi et al. [
17], this viscosity reduction is up to 60%. However, the presence of paraffin may complicate this effect. Ultrasonic treatment of the oil promotes paraffin crystallization, leading to an increase in viscosity, pour point, and the amount of deposited paraffin [
18]. In addition, the viscosity may initially decrease but tends to increase again over time as the oil relaxes. After a few days, a slight increase is usually observed [
5]. Studies [
6] report a contradictory effect on the content and structure of asphaltenes, which indicates an incomplete understanding of the effect of ultrasound on molecular associations. In addition, the influence of ultrasonic parameters, such as frequency, power and exposure time, on changes in chemical composition are discussed [
19]. In the article [
1] Dengaev with co-authors emphasize that, despite the widespread use of ultrasound in oil refining, the chemical transformation of hydrocarbons, especially in heavy oil, remains an understudied area.
Numerous studies have shown that ultrasonic cavitation contributes to the cracking of high molecular weight compounds, disaggregation of asphaltenes and a decrease in the viscosity of heavy oils. However, a study [
20] concluded that treatment with a high-frequency ultrasonic field significantly alters the physical and chemical characteristics of high-paraffin crude oil, leading to an increase in viscosity and related mechanical properties. This is attributed to the fact that, in the absence of stabilizing factors, radical processes under the influence of ultrasonic cavitation are accompanied by both cracking and secondary polycondensation. Radical processes can be stabilized by the addition of hydrogen, hydrogen donors, aromatic solvents, or catalysts [
13]. Integration of ultrasound and catalysts has been shown to promote the cleavage of complex molecules, resulting in lower oil viscosity and improved overall oil quality by reducing tar and asphaltenes and increasing hydrogen-to-carbon ratios [
7,
21]. Application of a Ni-skeletal catalyst together with ultrasonic treatment enhanced the quality of crude oil from the Zhanazhol field, leading to a significant increase in gasoline and diesel yields and a 49% reduction in sulfur content [
22].
These data confirm the potential of the sonocatalytic approach for processing high-viscosity oils. In this regard, the aim of this research is to study the kinetics of group composition redistribution (oil, resin, asphaltenes) during ultrasonic treatment of high-viscosity oil in the presence of acid zeolite.
2. Materials and Methods
Heavy oil from the Karazhanbas field (Kazakhstan) was subjected to ultrasonic treatment. The physical and chemical properties of the oil are shown in
Table 1.
As can be seen from the data presented, the high content of resins and asphaltenes (31.2%) determines the colloidal stability of the system and forms the increased viscosity of the raw material. At the same time, the elemental composition, characterized by increased concentrations of oxygen, sulfur, nitrogen, as well as metals (V and Ni), significantly complicates catalytic processing [
23,
24]. In this regard, in modern practice, special attention is paid to the development and application of pre-activation methods for high-viscosity oils aimed at increasing the yield of light distillate fractions during subsequent processing stages [
25,
26].
Synthetic acid Y-zeolite supplied by Snabtekhmet (Almaty, Republic of Kazakhstan) was used as a catalyst for the ultrasonic treatment of high-viscosity oil. The zeolite was activated with a 20% hydrochloric acid (HCl) solution at 60–70°C. For activation, 20 g of zeolite was treated with 300 mL of the acid solution. After acid treatment, the zeolite was thoroughly washed with distilled water until a neutral was reached, separated by filtration, and dried at 105 °C for 2 h. The specific surface area and pore volume of the sample were determined by BET and STSA methods using nitrogen as an adsorbate gas on a SORBOMETER-M analyzer (Katakon, Russia).
Table 2.
Textural characteristics of zeolite.
Table 2.
Textural characteristics of zeolite.
| Sample |
SBET,
(m2/g) |
Smesoporous ,
(m2/g) |
SMICRO,
(m2/g) |
VTOTAL,
(cm3/g) |
Vmesoporous,
(cm3/g) |
VMICRO,
(cm3/g) |
Pore size, nm |
| Y-zeolite |
630.7 |
145.1 |
485.6 |
0.310 |
0.065 |
0.245 |
2.005 |
The high catalytic efficiency of zeolites is attributed to their well-developed specific surface area (631 m²/g), pronounced acidity, and high thermal stability, which are key factors for catalytic and adsorption processes.
In addition to their porous structure, the acid sites provide an additional source of active hydrogen, which enhances the cracking of high-molecular-weight components while suppressing undesired polycondensation side reactions [
27]. The acid properties of the catalysts were studied using the temperature-programmed desorption of ammonia (NH
3-TPD) method: under standardized adsorption conditions, the catalyst surface was pre-saturated with ammonia molecules; then, linear heating was carried out in an inert gas stream.
For zeolite-containing catalysts, the amount of ammonia desorbed at elevated temperatures is commonly used as an indicator of the concentration and strength of acid sites. For the studied catalyst, ammonia desorption amounted to 523.4 μmol/g at 175 °C and 1137.4 μmol/g at 245 °C. Zeolite has two types of acid sites, as evidenced by the presence of two forms of ammonia desorption on the thermodesorption spectrum: weakly acidic with a peak maximum temperature T
max = 175°C and strongly acidic with T
max = 245°C. Weak acid sites are most often referred to as Lewis acid sites, which are incompletely coordinated aluminum atoms in the zeolite crystal lattice. Zeolite acid sites with high-temperature ammonia desorption temperatures are Brønsted acid sites, which are proton-donating OH groups associated with lattice Al [
28,
29].
For experimental studies, an ultrasonic device (model TEFIC-1000D) operating at a frequency of 22 kHz and an output power of 50W was used. The duration of ultrasonic exposure varied between 3 and 11 minutes. The cavitation process was carried out in a 0.05 L glass reactor equipped with a thermostat, maintaining temperature conditions of 30, 50, and 70 °C. The weight of the high-viscosity oil sample was 30.0 g, and the catalyst content was 1.0 wt% relative to the initial oil.
The content of oils, resins, and asphaltenes in oil was determined using a standard method [
30]. To isolate asphaltenes, the sample was diluted with a 40-fold volume of hexane, keeping for a day and filtering out the precipitate. The resulting precipitate was placed in a paper cartridge and washed with hexane from oils and resins in a Soxhlet apparatus, then asphaltenes were washed out of the cartridge with chloroform. The deasphalted samples were applied to silica gel, then were sequentially extracted in a Soxhlet apparatus into hydrocarbon components (oils) with n-hexane and resins isolated with a mixture of benzene and ethanol (1:1). Mass fractions were expressed in unit fractions of the initial oil sample.
To determine the group composition of oil fractions boiling up to 200°C and 200-300°C before and after ultrasonic treatment, chromatographic mass spectrometric analysis was performed on an Agilent Technologies 7890A gas chromatograph with a 5975C mass spectrometric detector.
3. Results and Discussions
Literature analysis has shown that the studies of the kinetics of ultrasonic processing of heavy hydrocarbons are limited. According to literature data [
31,
32], cavitation processes mainly lead to cracking and redistribution of high-molecular asphaltene-resins structures towards less condensed compounds, while deep carbonization or gasification is practically not observed. In the study [
33], a study of the kinetics of ultrasonic cavitation of the middle fraction of coal resin was carried out, where individual group of polyaromatic hydrocarbons were shown as components of the kinetic scheme. In [
32], changes in the concentration of SARA fractions with increasing ultrasonic treatment duration were demonstrated; however, in that work, kinetic conclusions were obtained using the thermogravimetry method, which makes it impossible to directly compare their results with our kinetic model. In [
32], to improve the quality of oil resins, the samples were subjected to ultrasonic treatment at a power of 800 W, a temperature of 70°C and a duration of exposure from 0 to 11 minutes. It was found that the optimal treatment time is 7 minutes, at which the viscosity of the oil decreases by 14.1% and the content of coke residue by 7.4%. At the same time, an increase in the proportion of saturated hydrocarbons and a decrease in the content of aromatic compounds, resins and asphaltenes were observed.
The proposed model describing the transformation of high-viscosity oil under ultrasonic cavitation in the presence of a catalyst is based on the interconversion of its main fractional components: oils, resins, and asphaltenes. Thus, the kinetic model of heavy oil transformations was built based on the assumption of the existence of reversible transformation pathways between the three main fractions: oils (C1), resins (C2), and asphaltenes (C3) (
Figure 1).
Based on
Figure 1, a system of ordinary differential equations of the first order was formed, which describes the rate of change of concentrations over time.
where,
C1- oil fraction,
C2 – resin fraction
C3 – asphaltene fraction.
k – rate constants
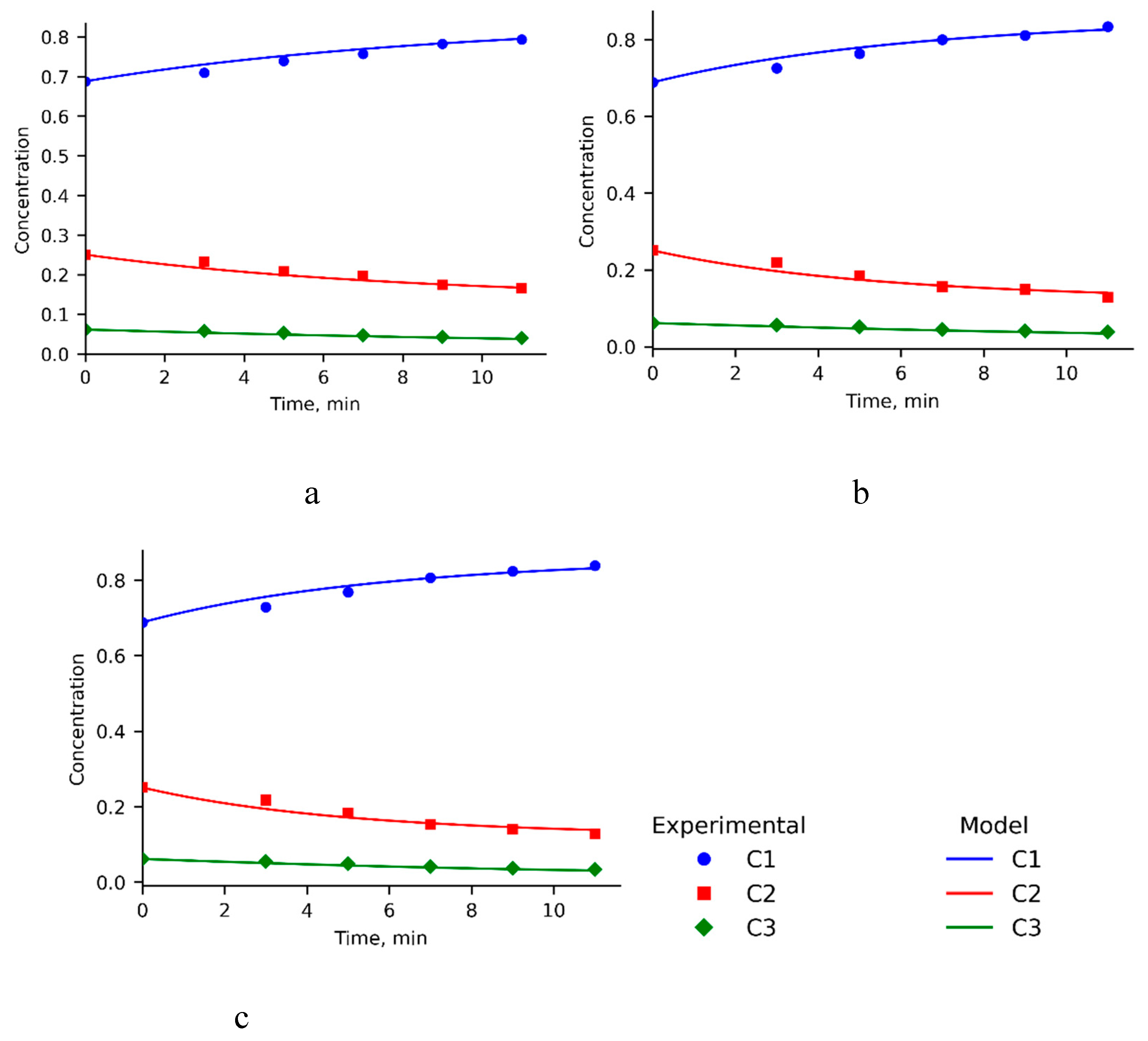
To determine the kinetic parameters, kinetic curves of the process of ultrasonic treatment of high-viscosity oil at 30, 50 and 70°C were taken in the time interval from 3 to 11 minutes (
Figure 2).
A consistent increase in the proportion of oils (C1) was observed, accompanied by a parallel decrease in the concentrations of resins (C2) and asphaltenes (C3). The initial proportions of these components in the dehydrated oil are presented in
Table 1. During ultrasonic treatment of high-viscosity oil for 11 minutes, it was found that an increase in the process temperature contributes to more pronounced structural changes. Thus, at 30°C the content of the oil fraction increases by 10.4%, at 50°C by 14.3%, and at 70°C it reaches 14.8%. Simultaneously, a decrease in the concentration of resinous substances was observed: by 8.4%, 12.1% and 12.2%, respectively, for these temperature conditions. The proportion of asphaltenes also decreases, but less significantly compared to resins: by 2.0%, 2.2% and 2.6%. The experimental error in determining the yields of oils, resins, and asphaltenes from high-viscosity oil was ±3.4%. The observed increase in the oil content indicates partial radical cracking of high-molecular-weight compounds, leading to the formation of lighter fractions. Under these conditions, resins primarily act as intermediates: their accumulation does not occur; rather, significant depletion is observed due to rapid subsequent transformation into oils. The preferential degradation of resin molecules under ultrasonic treatment has been demonstrated in previous studies, highlighting the effectiveness of ultrasonic waves in altering the chemical structure of these compounds [
1]. It is likely that the acid sites of the zeolite catalyst play a key role in proton activation and the subsequent breakdown of resin–asphaltene aggregates. In addition, cavitation effects induced by ultrasonic exposure, including microjets, localized shear, and transient heating, contribute to the rupture of weak intermolecular bonds and the disaggregation of supramolecular structures. The average discrepancies between the experimentally measured concentrations of oil components and the values predicted by the model were 0.78% at 30 °C, 1.30% at 50 °C, and 1.37% at 70 °C.
Table 3 shows the kinetic parameters (rate and activation energy constants) for the conversion of components to high viscosity oil.
The following parameters were used in the calculation of all rate constants: Nmax = 100, step accuracy = 1.00×10⁻⁶, and criterion accuracy = 1.00×10⁻⁷. The average error at each point was 9.96×10⁻⁸ for the rate constants at 30 °C, 9.88×10⁻⁸ at 50 °C, and 9.94×10⁻⁸ at 70 °C. Analysis of the temperature dependences of the reaction rates shows an increase in the constants k1-k4 with an increase in temperature from 30 to 70°C. The highest absolute values are characteristic of k2 and k4, which indicates the dominant role of resin-oil (C2 → C1) and asphaltene-resin (C3 → C2) transitions in the overall transformation scheme.
The calculation of activation energies using the Arrhenius equation revealed values in the range of 7-10 kJ/mol. It should be emphasized that these values are significantly lower than typical activation energies for thermal processes of cracking of resin-asphaltene structures [
34]. The results obtained may indicate the combined catalytic and cavitation nature of the ongoing transformations. Local "hot spots" generated from cavitation, together with the acid sites of the zeolite catalyst, probably create conditions for more efficient reactions at relatively low energy barriers.
A comparison of activation energies shows that the highest energy barrier (≈10.4 kJ/mol) corresponds to the conversion of resins into oils (described by the rate constant k₂), which may represent the key limiting step in the degradation of resins to form oils. In contrast, lower activation energies for k₃ and k₄ (7.2 and 7.0 kJ/mol, respectively) indicate that transformations between resins and asphaltenes proceed relatively easily.
For a general qualitative analysis of the oil fraction composition, gas chromatography–mass spectrometry (GC–MS) was performed on the light and middle fractions of high-viscosity oil before and after ultrasonic treatment at 70°C for 7 minutes in the presence of a zeolite catalyst.
Table 4 presents the group composition of fractions boiling below 200°C and in the 200–300 °C range.
A comparative analysis of the group composition of light (<200 °C) and middle (200–300 °C) fractions of high-viscosity oil before and after ultrasonic treatment showed significant changes in the distribution of hydrocarbon and heteroatom-containing compounds. The most noticeable change is an increase in the proportion of paraffin hydrocarbons: in the light fraction from 13.42 to 22.48%, and in the middle fraction from 26.03 to 63.44%. This indicates the cracking of heavier structures and the redistribution of products towards the paraffin phase.
The proportion of non-condensed naphthenes also increased (from 31.82 to 37.17% in the light fraction and from 4.85 to 5.06% in the middle fraction), which indicates partial hydronaphthenization and stabilization of the products. At the same time, the concentration of condensed naphthenes increases even more significantly: from 4.97 to 11.88% and from 3.68 to 7.79%, respectively, which can be associated with the restructuring of polycyclic structures.
Aromatic compounds exhibited multidirectional changes. The benzene content increased slightly (from 6.09 to 7.11% and from 3.78 to 4.36%), while naphthalenes decreased sharply - from 8.96 to 1.32% in the light fraction and from 14.87 to 5.43% in the midle fraction. This redistribution indicates the breakdown of more condensed aromatic structures and their partial conversion into less condensed or aliphatic products.
The content of oxygen-containing compounds decreased several times: from 14.01 to 3.85% and from 23.19 to 4.85%. Similarly, nitrogenous (2.91 and 3.74%) and sulfurous (2.02 and 3.59%) compounds completely disappeared, which indicates deep deasphaltenization and cracking of resinous-asphaltene components.
Finally, the proportion of olefins increased - from 3.12 to 7.81% and from 1.97 to 6.21% - which may result from partial dearomatization and the formation of unsaturated hydrocarbons during ultrasonic processing. At the same time, alkynes practically disappear: their concentration in the light fraction decreases from 1.29 to an undetectable level. Only trace amounts of no more than 0.22% appear in the middle fraction.
Thus, the main trends include an increase in the proportion of paraffinic and olefinic compounds, a decrease in the content of heteroatom-containing components and a decrease in the concentration of polycyclic aromatic structures.
4. Conclusions
The results of this study confirmed the effectiveness of ultrasonic treatment combined with a zeolite catalyst for processing heavy (high-viscosity) oil to produce light fractions.
Analysis of the kinetics of oil, resin, and asphaltene redistribution in high-viscosity Karazhanbas oil allowed the determination of the conditions under which the process proceeds with maximum efficiency. Thus, with an increase in temperature from 30 to 70 °C and a treatment duration of 3 to 11 minutes, a consistent increase in oil content was accompanied by a simultaneous decrease in the concentrations of resins and asphaltenes. The highest rate of oil formation occurred within the first 7 minutes of treatment.
The conversion schemes developed during the study ("oils → resins" and "resins → asphaltenes") allowed the identification of the dominant reaction pathways. he conversion of resins into oils occurred at the highest rate (k₂ = 0.1148–0.1860 min⁻¹). The cracking reaction of asphaltenes into resins (k4=0.1023-0.1413 min-1) is the second fastest conversion process. Condensation reactions of oils into resins (k1 = 0.0175-0.0252 min-1) and resins into asphaltenes (k3 = 0.0139-0.0194 min-1) proceed significantly more slowly, which confirms the focus of ultrasonic exposure on the cracking of high-viscosity oil structure.
The calculated activation energies (7.0-10.4 kJ/mol) showed that cavitation treatment of high-viscosity oil in the presence of a catalyst allows the heavy oil to be processed at low energy costs. This indicates that the destructive transformations of the main components of high-viscosity oil occur mainly under the influence of the physicochemical effects of cavitation, and not due to thermal decomposition at high temperatures.
A group analysis of light and middle oil fractions (after cavitation treatment at 70°C for 7 minutes in the presence of 1.0% zeolite) revealed an increase in the content of paraffinic, naphthenic, benzene and olefinic hydrocarbons while reducing the proportion of naphthalene and heteroatomic compounds. he results obtained highlight new environmentally friendly opportunities for producing light fractions and confirm the effectiveness of ultrasonic treatment in the presence of a zeolite catalyst for processing high-viscosity oil. The kinetic parameters investigated in this study provide a basis for controlling the production process, thereby enhancing its economic efficiency.
Author Contributions
Conceptualization, D.A. and M.B.; methodology, D.A. and M.B..; software, Y.K. and N.R.; validation, D.A., M.B. and Y.K.; formal analysis, D.A., M.B., A.A., N.B., S.T., Z.A., N.R, and Y.K.; investigation, D.A., M.B., A.A., N.B., S.T., Z.A., N.R, and Y.K.; resources, M.B., N.R, and Y.K. ; data curation, D.A., N.R., Y.K.; writing—original draft preparation, D.A..; writing—review and editing, D.A.; visualization, A.A. ,N.B., S.T.; supervision, D.A. and Y.K; project administration, D.A.; funding acquisition, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP22684288 Destruction of the Karazhanbas field high-viscosity oil by ultrasonic action in the presence of a catalyst).
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dengaev, A.V.; Khelkhal, M.A.; Getalov, A.A.; Baimukhametov, G.F.; Kayumov, A.A.; Vakhin, A.V.; Gafurov, M.R. Innovations in Oil Processing: Chemical Transformation of Oil Components through Ultrasound Assistance. Fluids 2023, 8, 108. [CrossRef]
- Zhu, S.; Liu, X; Zhang Zh. Experimental Investigation of the Effect of Ultrasonic Wave on the Saturated Hydrocarbons in Castilla Crude Oil; In: Springer eBooks; 2022; pp. 126–134. [CrossRef]
- Gao, J.; Wu, P.; Li, C.; Xu, D.; Wang, X. Influence and mechanism study of ultrasonic electric power input on heavy oil viscosity. Energies 2022, 16(1), 79. https://doi=10.3390/en16010079.
- Malani, R.S. Role of Sonication in the Upgradation of Heavy Crude Oil. Ultrasound Technology for Fuel Processing. Bentham Science Publishers, 2023; pp. 237–252;. [CrossRef]
- Baimukhametov, G.F.; Dengaev, A.V.; Safiullina, E.; Kayumov, A.A.; Drozdov, I.; Shishulin, V.; Boushra, A.; Vakhin, A.V.; Sargin, B.V.; Sidibe, M.S. Relaxation process in crude oil after ultrasonic treatment. Int. J. Eng. Trans. B: Appl. 2024, 37(5), 896–903. [CrossRef]
- Nguele, R.; Okawa, H.; Sasaki, K. Insights into bitumen viscosity reduction using ultrasound-assisted EOR. SPE Western Regional Meeting, Anchorage, Alaska, USA, 22-25 May 2023. [CrossRef]
- Li, H.; Wang, Y.; Qin, S.; Cao, S.; Yu, B.; Sun, D. Study on viscosity reduction mechanism for offshore heavy oil under the synergistic action of nanocatalyst and ultrasound. Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 4-6 November 2020. [CrossRef]
- Ibrahim, N.K.; Noori, W.A.; Khasbag, J.M. Ultrasound-Assisted Oxidative Desulfurization of Diesel. J. Eng. 2016, 22(11), 55–67. Available online: https://joe.uobaghdad.edu.iq/index.php/main/article/download/121/107.
- Mohapatra, D.P.; Kirpalani, D.M. Bitumen heavy oil upgrading by cavitation processing: effect on asphaltene separation, rheology, and metal content. Appl. Petrochem. Res. 2016, 6(2), 107–115. [CrossRef]
- Liu, J.; Yang, F.; Junyong, X.; Wu, F.; Pu, C. Mechanism of ultrasonic physical-chemical viscosity reduction for different heavy oils. ACS Omega 2021, 6(3), 2276–2283. [CrossRef]
- Moldabayeva, G.Zh.; Suleimenova, R.T.; Turdiyev, M.F.; Shayakhmetova, Zh.B.; Karimova, A.S. Scientific and Technical Substantiation of Reducing Oil Viscosity. Int. J. Eng. Res. Technol. 2020, 13(5), 967 972. [CrossRef]
- Azeez, A.W.; Hussein, H.Q. Effects of Ultrasonic Treatment and Hydrogen Donor Addition on the Viscosity of Iraqi Heavy Crude Oil. Baghdad Sci. J. 2024, 22 (3), 746-755. [CrossRef]
- Qajar, J.; Razavifar, M.; Riazi, M. A mechanistic study of the synergistic and counter effects of ultrasonic and solvent treatment on the rheology and asphaltene structure of heavy crude oil. Chem. Eng. Prcess. 2023, 195, 109619 . [CrossRef]
- Dengaev, A.V.; Kayumov, A.A.; Getalov, A.A.; Aliev, F.A.; Baimukhametov, G.F.; Sargin, B.V.; Maksimenko, A.F.; Vakhin, A.V. Chemical viscosity reduction of heavy oil by multi-frequency ultrasonic waves with the main harmonics of 20–60 kHz. Fluids 2023, 8(4), 136. [CrossRef]
- Baimukhametov, G.F.; Kayumov, A.A.; Dengaev, A.V.; Maksimenko, A.F.; Marakov, D.A.; Shishulin, V.A.; Drozdov, I.M.; Samuylova, L.V.; Getalov, A.A.; Aliev, F.A.; Vakhin, A.V. Unveiling the potential of cavitation erosion-induced heavy crude oil upgrading. Fluids 2023, 8, 274. [CrossRef]
- Razavifar, M.; Yunusov, T.; Mukhametdinova, A.; Bakulin, D.; Qajar, J.; Cheremisin, A.; Riazi, M. Improving oil recovery with ultrasound: mitigating asphaltene-induced formation damage. J. Pet. Explor. Prod. Technol. 2025, 15, 78. [CrossRef]
- Shi, J.; Li, T.; Sun, L.; Jiang, T.; Yu, X.; Yu, K.; Lu, S.; Xu, W. Molecular dynamics simulation on the process of ultrasonic viscosity reduction. Processes 2024, 12(12), 2803. [CrossRef]
- Volkova, G.I.; Morozova, A.V. The influence of ultrasonic treatment on the properties of oil systems. J. Phys.: Conf. Ser. 2020, 1611(1), 012018. [CrossRef]
- Sister, V.G.; Gridneva, E.S.; Abramov, O.V. Ultrasound-induced change in chemical properties of petroleum products. Chem. Pet. Eng. 2009, 45, 3–6. [CrossRef]
- Volkova, G.I.; Anufriev, R.V. Structural and mechanical properties of highly paraffinic crude oil processed in high-frequency acoustic field. Key Eng. Mater. 2015, 670, 55–61. [CrossRef]
- Montes, D.; Cortés, F.B.; Franco, C.A. Reduction of heavy oil viscosity through ultrasound cavitation assisted by NiO nanocrystals-functionalized SiO₂ nanoparticles. Dyna 2018, 85(207), 153–160. [CrossRef]
- Kairbekov, Zh.K.; Anisimov, A.V.; Myltykbaeva, Zh.K.; Kanseitova, D.K.; Rakhmanov, E.V.; Seisembekova, A.B. Sonocatalytic oxidative desulfurization of oil from the Zhanazhol oilfield. Moscow Univ. Chem. Bull. 2017, 72(1), 29–33. [CrossRef]
- Bodykov, D.U.; Seilkhanov, T.M.; Nazhipkyzy, M.; Toylybayev, A.S.; Salakhov, R. NMR-Spectrometric determination of the fragmented oil composition from the Karazhanbas and Zhangurshi oil deposits. Eurasian Chem.-Technol. J. 2018, 20(3), 229–233. [CrossRef]
- Yedrissov, A.T.; Aitbekova, D.E.; Tusipkhan, A.; Tateyeva, A.B.; Baikenova, G.G.; Baikenov, M.I.; Kaikenov, D.A. TGA-based thermokinetics of high-viscosity oil decomposition in the presence of nanocatalysts, catalytic additives, and polymers. Petrol. Chem. 2021, 61(4), 431–437. [CrossRef]
- Khamidullin, R.F.; Kharlampidi, Kh.E.; Nikulin, R.M.; Sitalo, A.V.; Sharaf, F.A. Increasing the yield of light distillates by activation of oil stock. Chem. Technol. Fuels Oils 2017, 52(6), 670–678. [CrossRef]
- Lin, W.; Wu, Y.; Su, G.; Xiao, J.; Wang, S. Ultrasound irradiation for upgrading vacuum residue: a comprehensive study on its effects on rheological, structural, thermal behavior, and catalytic hydrocracking performance. Ind. Eng. Chem. Res. 2023, 62(49), 21120-21129 . [CrossRef]
- Muldakhmetov, Z.M.; Ordabaeva, A.T.; Meiramov, M.G.; Gazaliev, A.M.; Kim, S.V. Catalytic hydrogenation of anthracene on binary (bimetallic) composite catalysts. Catalysts 2023, 13(6), 957. [CrossRef]
- Vosmerikov, A.A.; Vosmerikova, L.N.; Vosmerikov, A.V. Acidic and catalytic properties of Mg-containing zeolite catalyst in the propane conversion to olefinic hydrocarbons. ChemChemTech [Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol.]2023, 66(11), 42–49. [CrossRef]
- Zhai, D.; Liu, Y.; Zheng, H.; Zhao, L.; Gao, J.; Xu, C.; Shen, B. A first-principles evaluation of the stability, accessibility, and strength of Brønsted acid sites in zeolites. J. Catal. 2017, 352, 627–637. [CrossRef]
- Dmitriev, D.E.; Golovko, A.K. Transformations of resins and asphaltenes during the thermal treatment of heavy oils. Petrol. Chem. 2010, 50(2), 106–113. [CrossRef]
- Mansouri, H.; Mohammadidoust, A.; Mohammadi, F. An optimization study on quality promotion of heavy crude oil exposed ultrasonic waves and magnetic nanoparticles addition. Chem. Eng. Process. - Process Intensif. 2021, 167, 108542. [CrossRef]
- Maye, P.E.E.; Jingyi, Y.; Taoyan, Y.; Xinru, X. The effects of ultrasonic treatment on the molecular structure of residual oil. China Petrol. Process. Petrochem. Technol. 2017, 4, 82–88.
- Balpanova, N. Z.; Tusipkhan, A.; Gyulmaliev, A. M.; Ma, F., Kyzkenova, A. Z.; Aitbekova, D. E.; Baikenov, M. I. Kinetics of cavitation of an intermediate fraction of coal tar. Solid Fuel Chemistry. 2020, 54(4), 208-213. [CrossRef]
- Zhao, Y.; Gray, M.R.; Chung, K.H. Molar kinetics and selectivity in cracking of Athabasca asphaltenes. Energy Fuels. 2001, 15(3), 751–755. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).