Submitted:
06 November 2025
Posted:
06 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
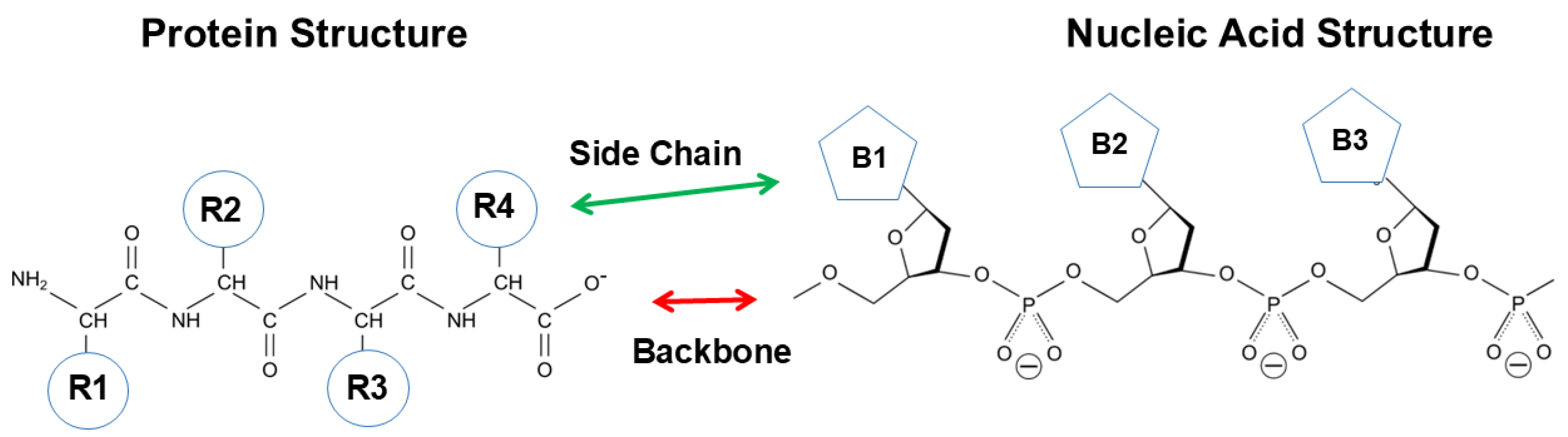
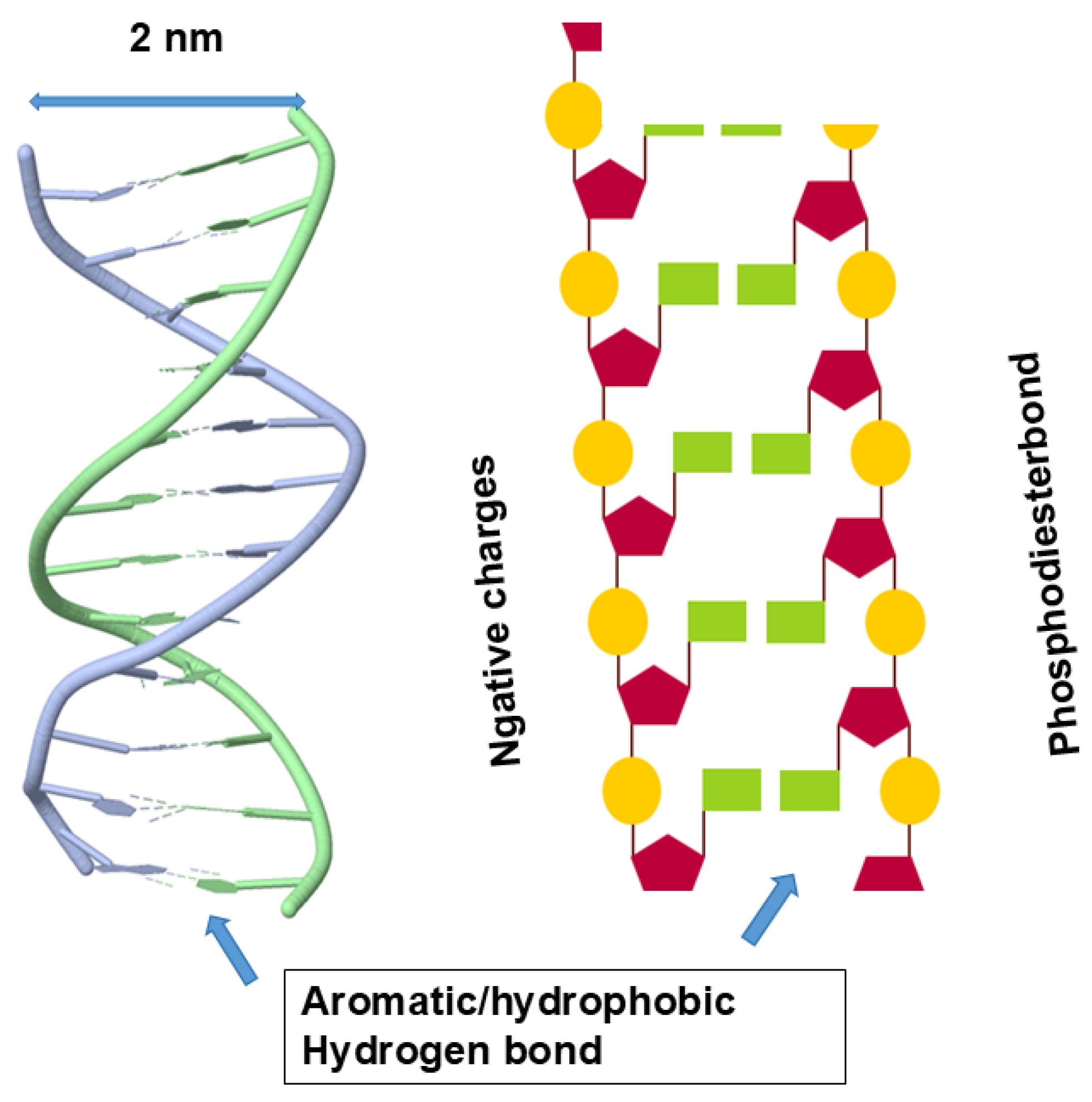
2. Structure
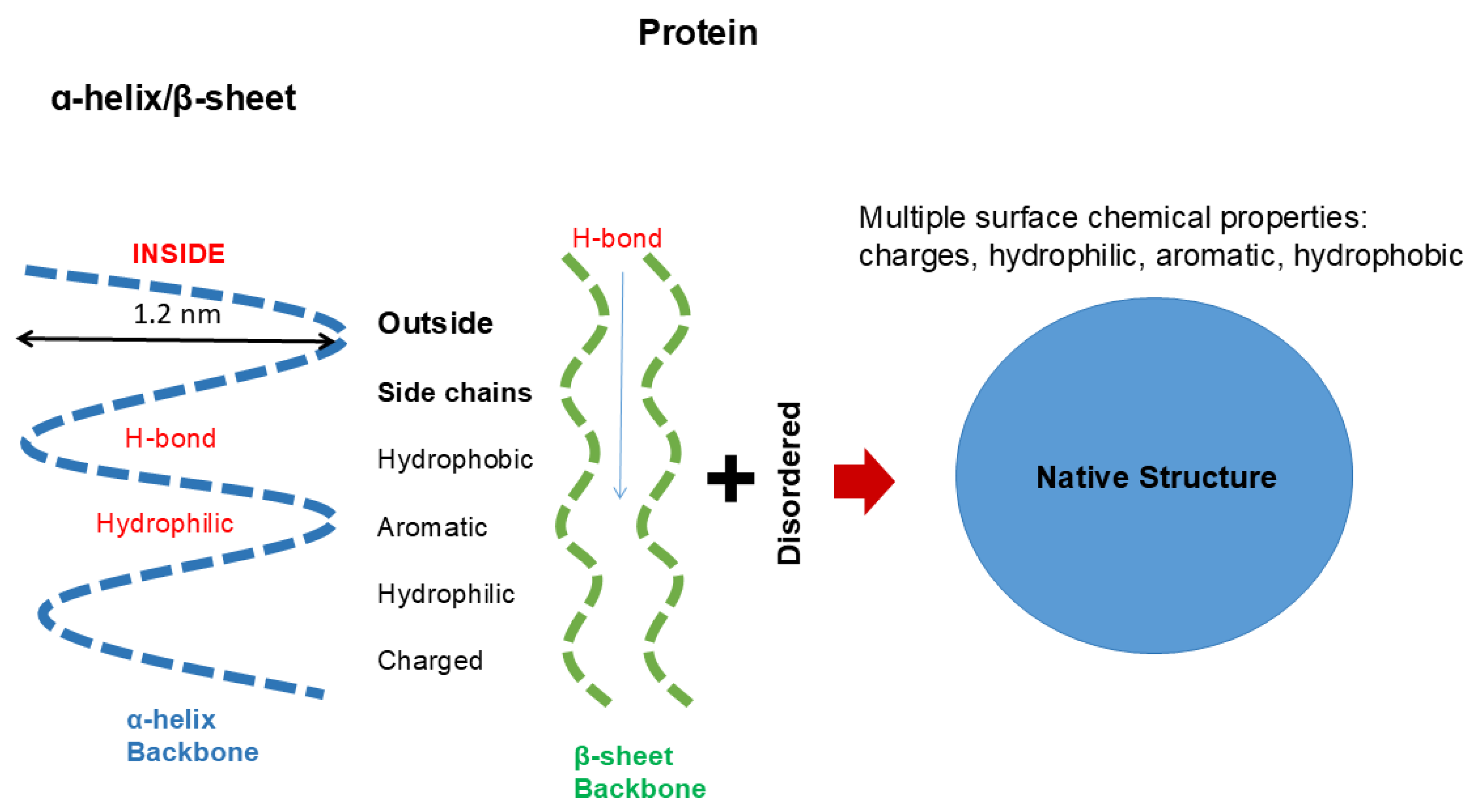
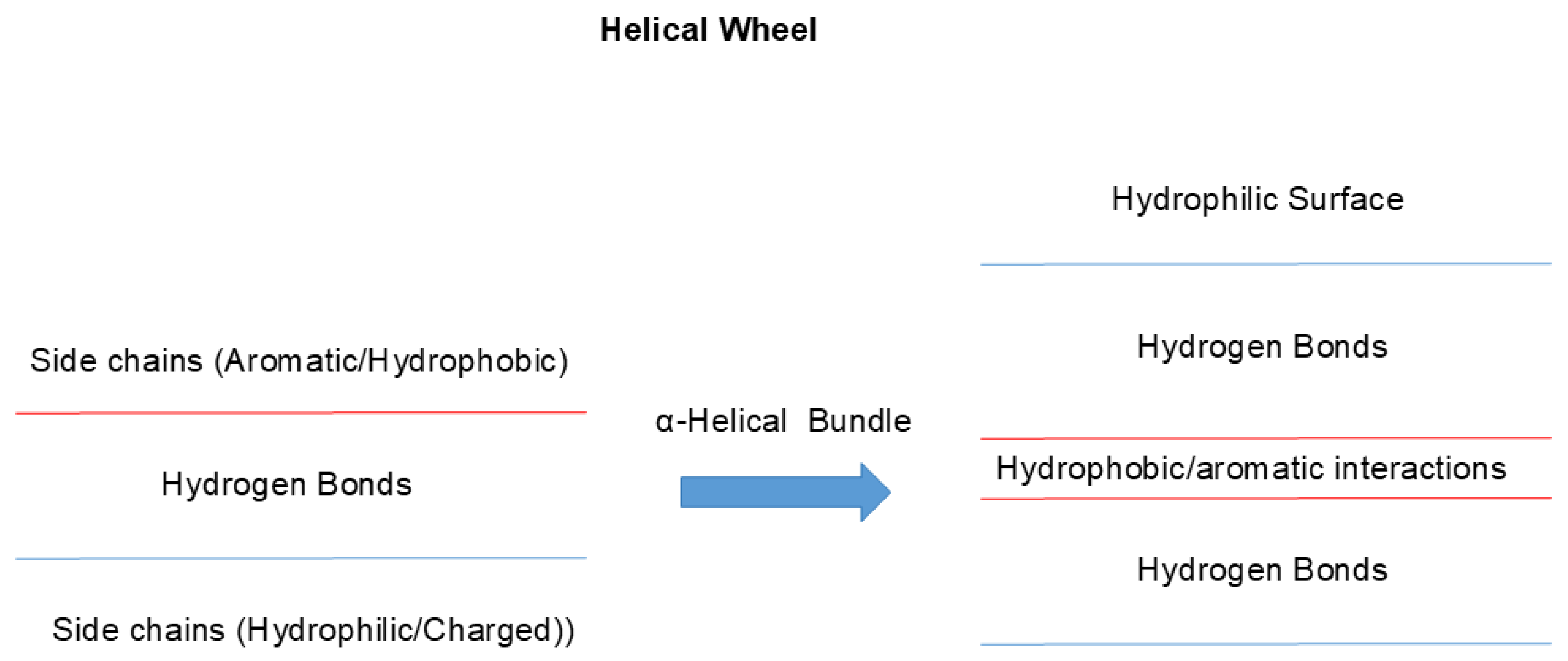
3. Interactions
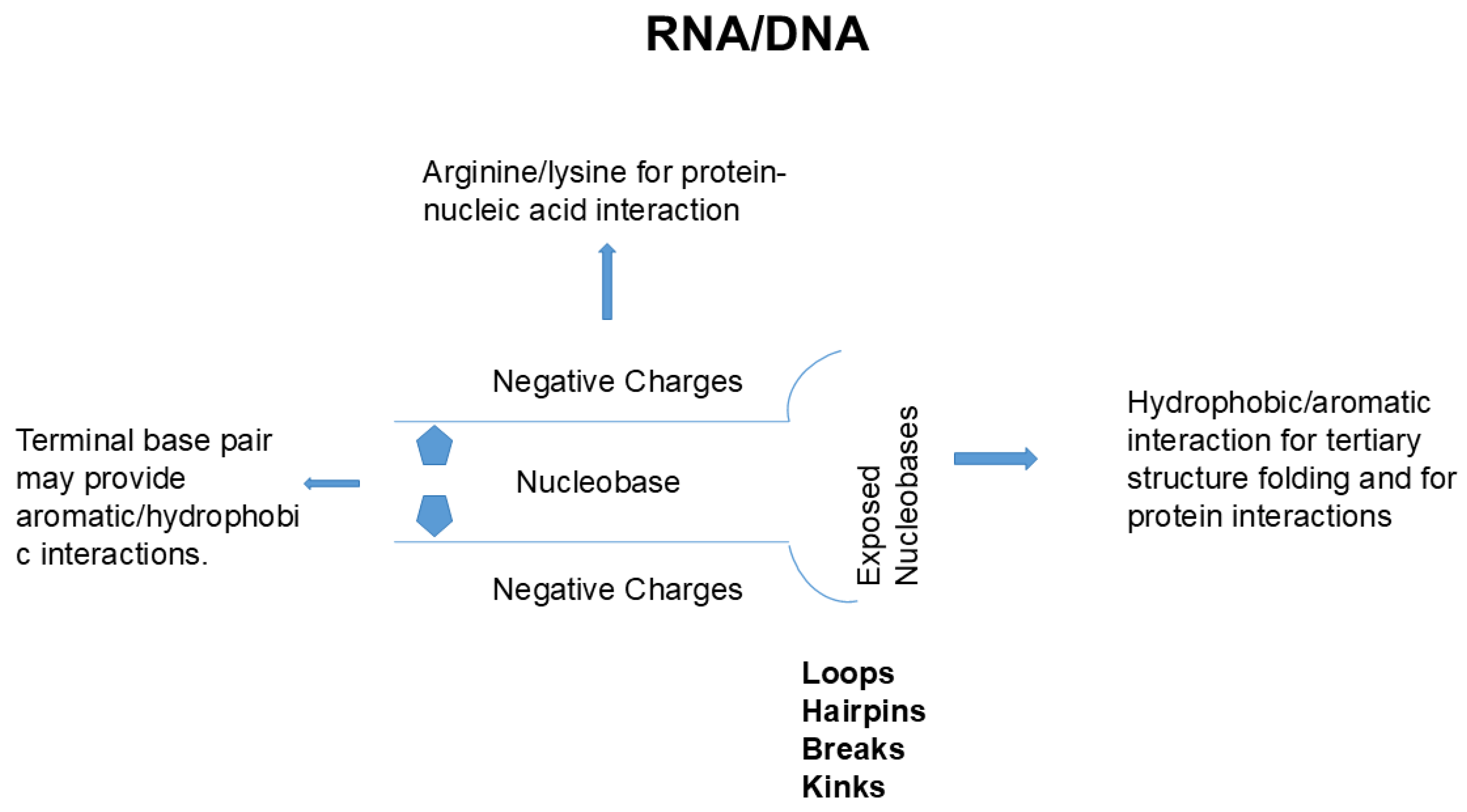
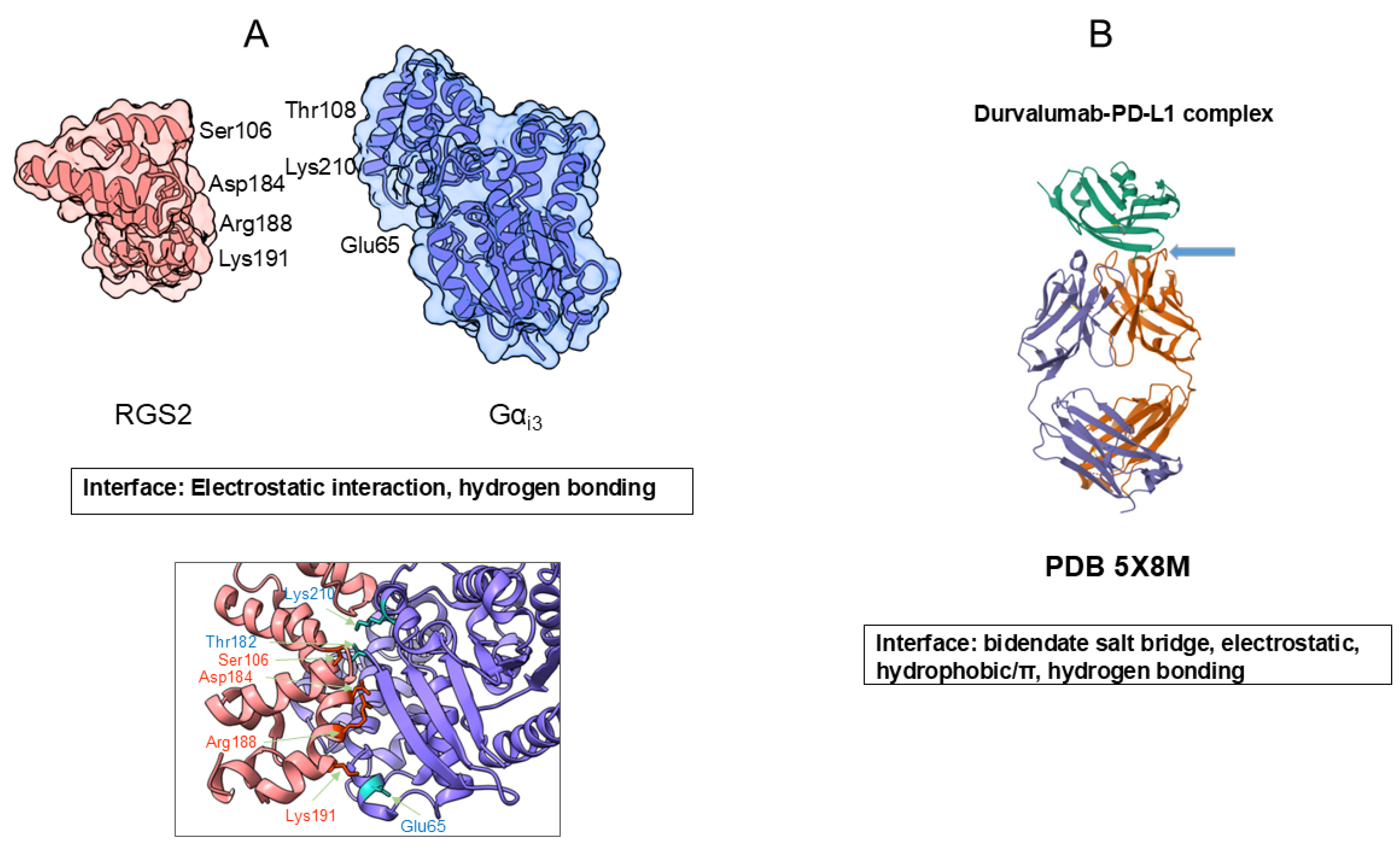
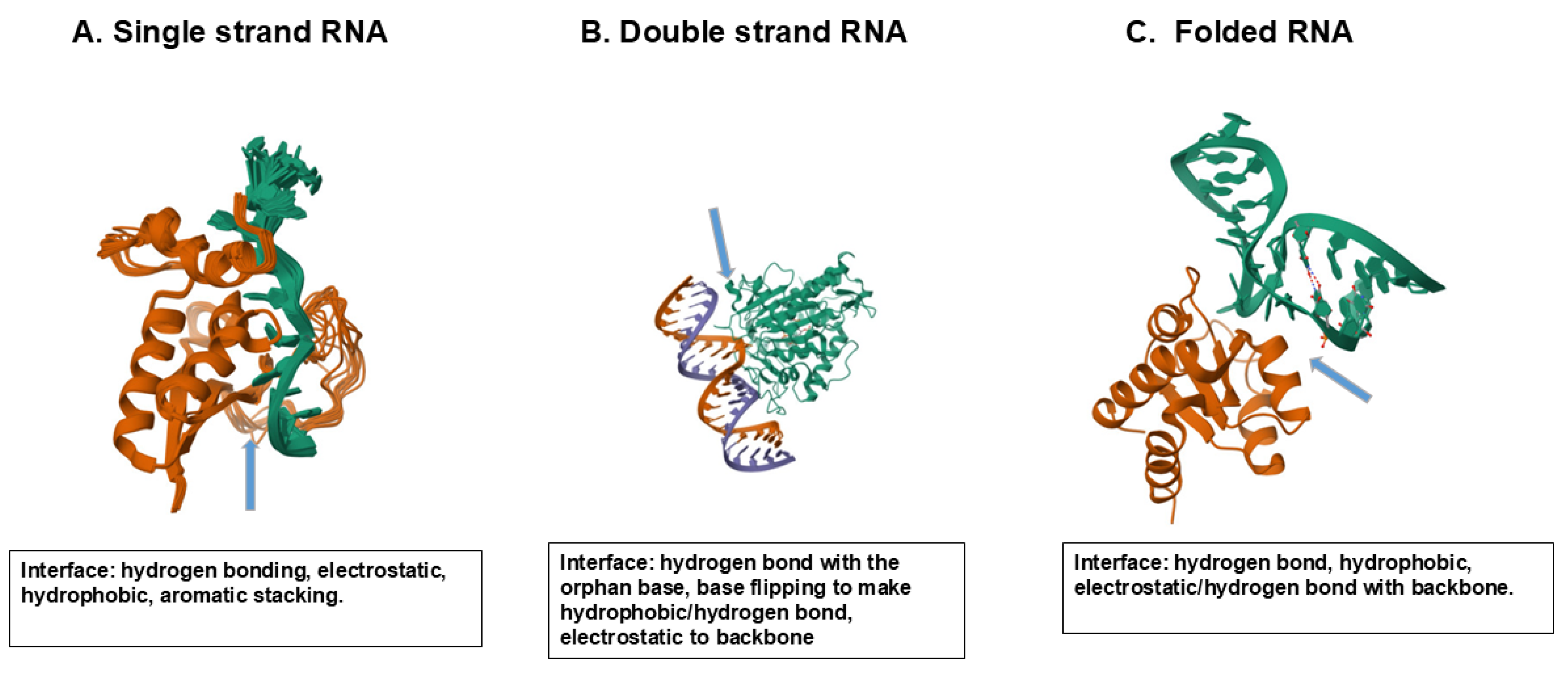
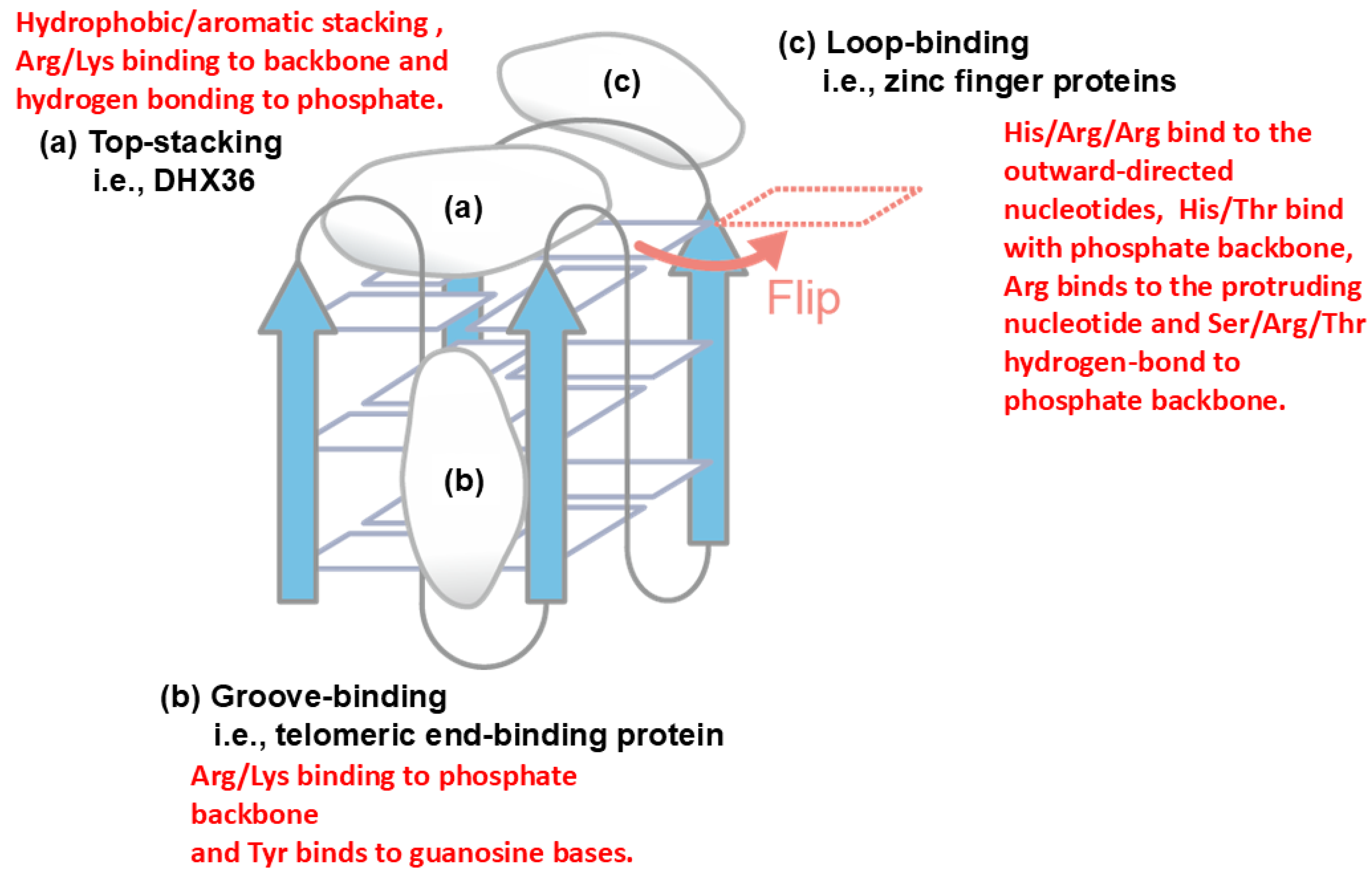
3.1. Macromolecular Interactions
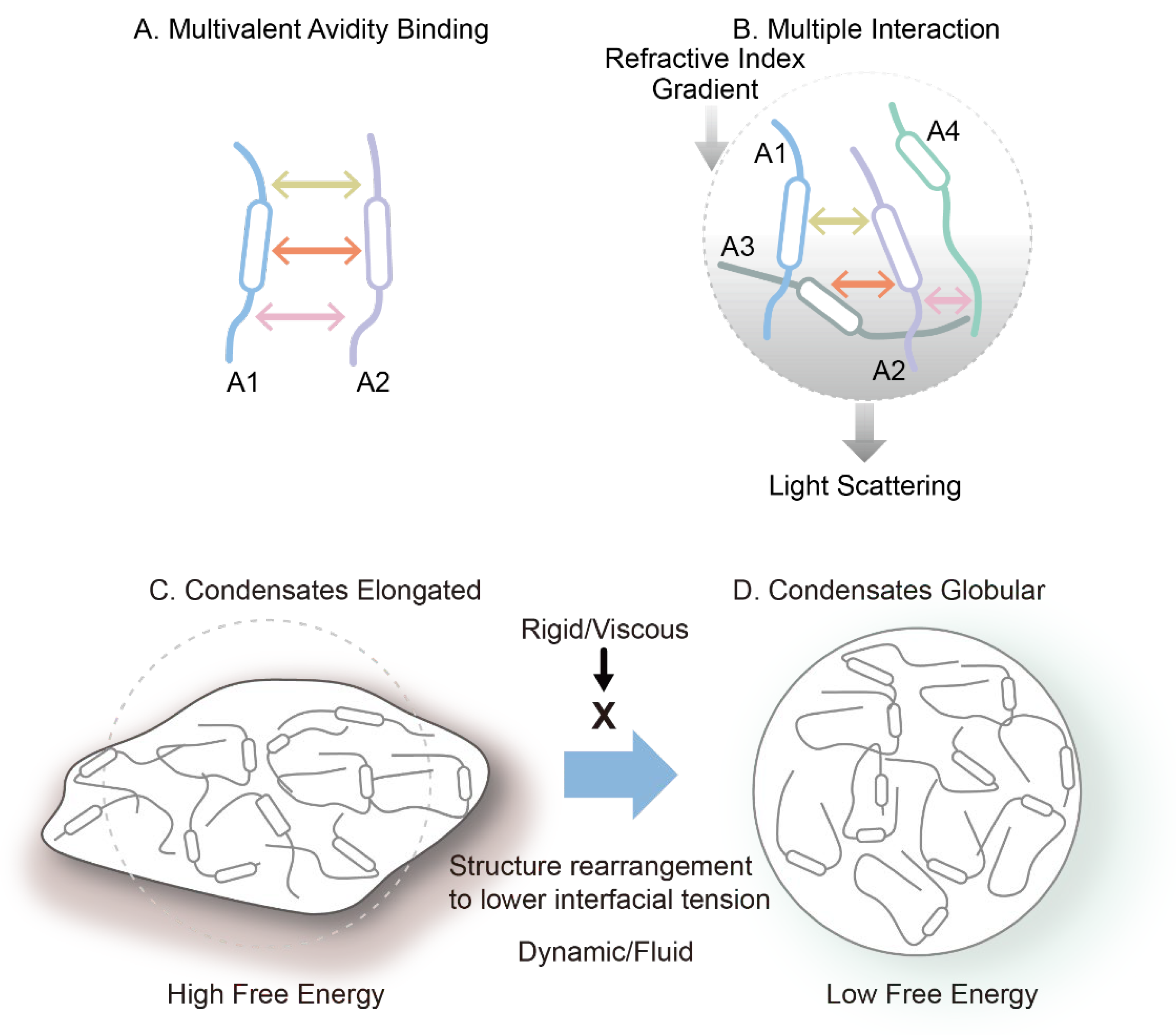
3.2. Biomolecular Condensates

3.3. Co-Solvent Interaction
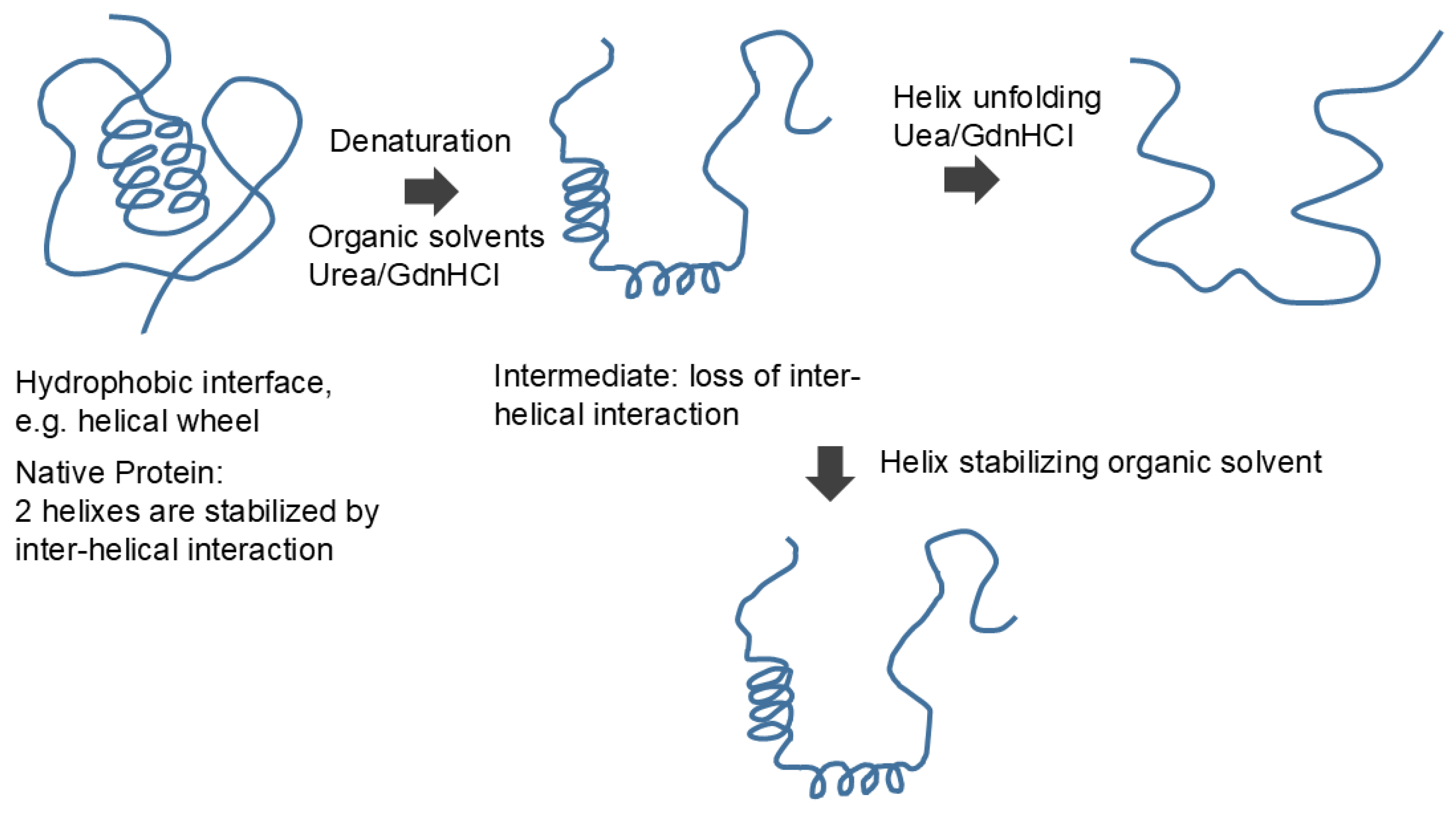
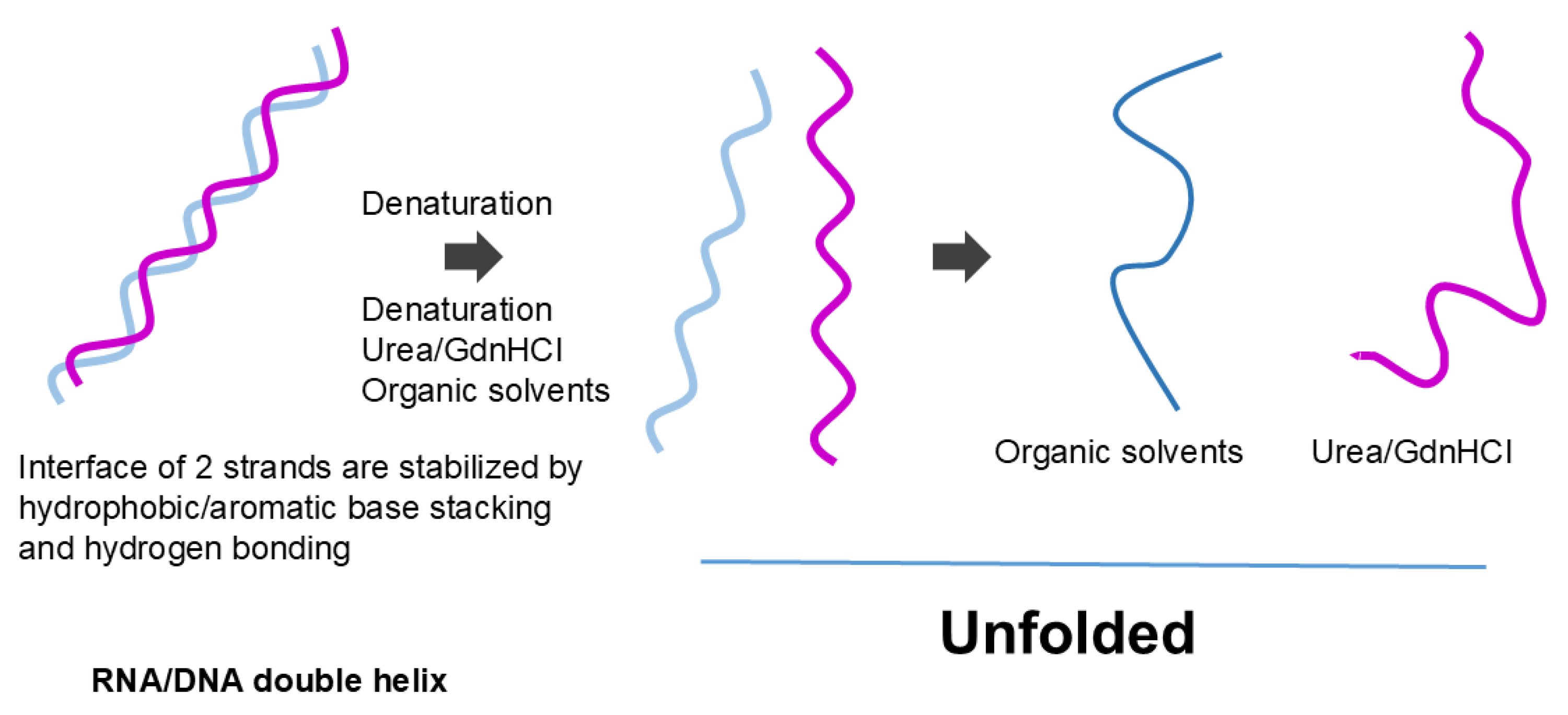
3.3.1. Organic Solvent
3.3.2. Denaturants
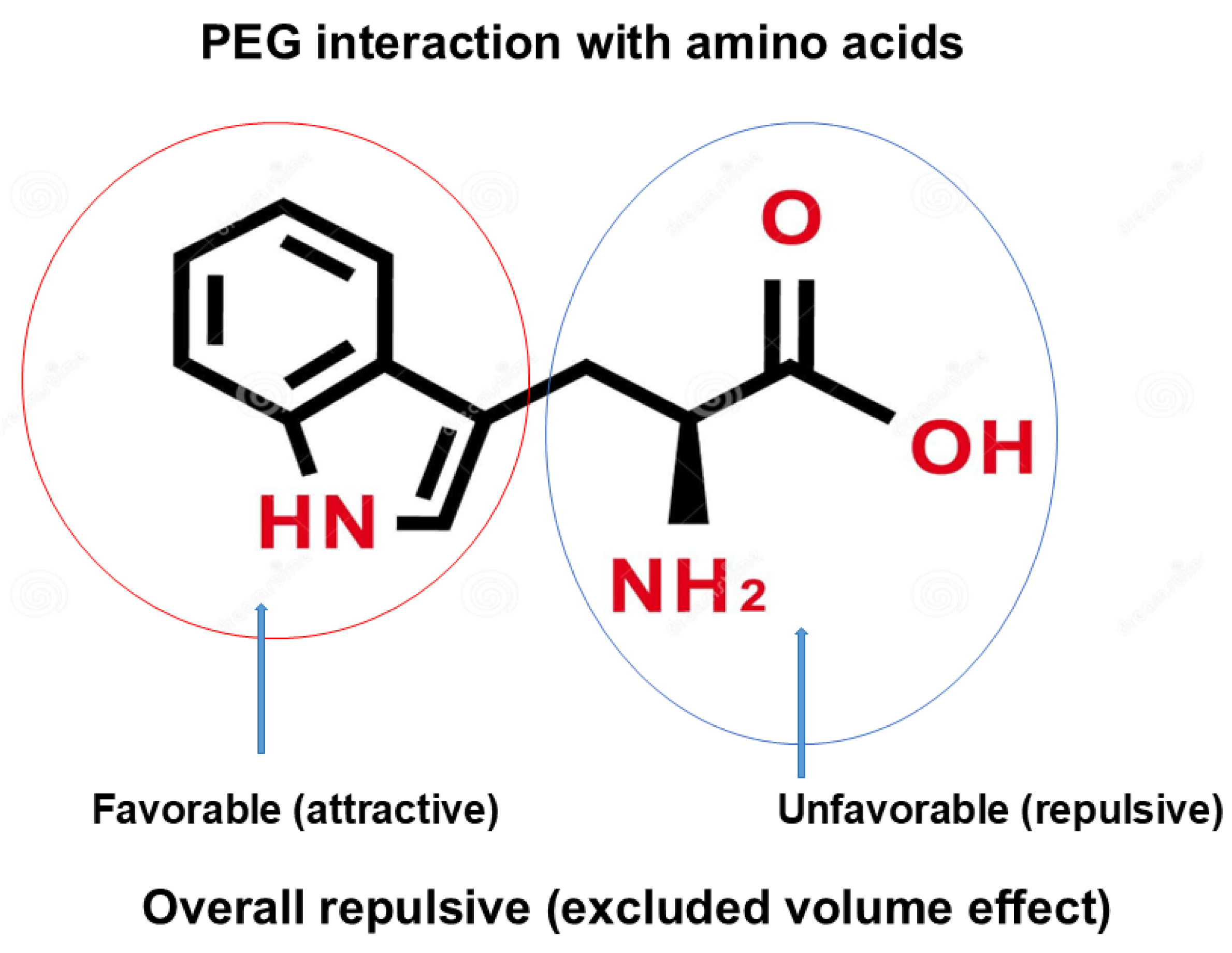
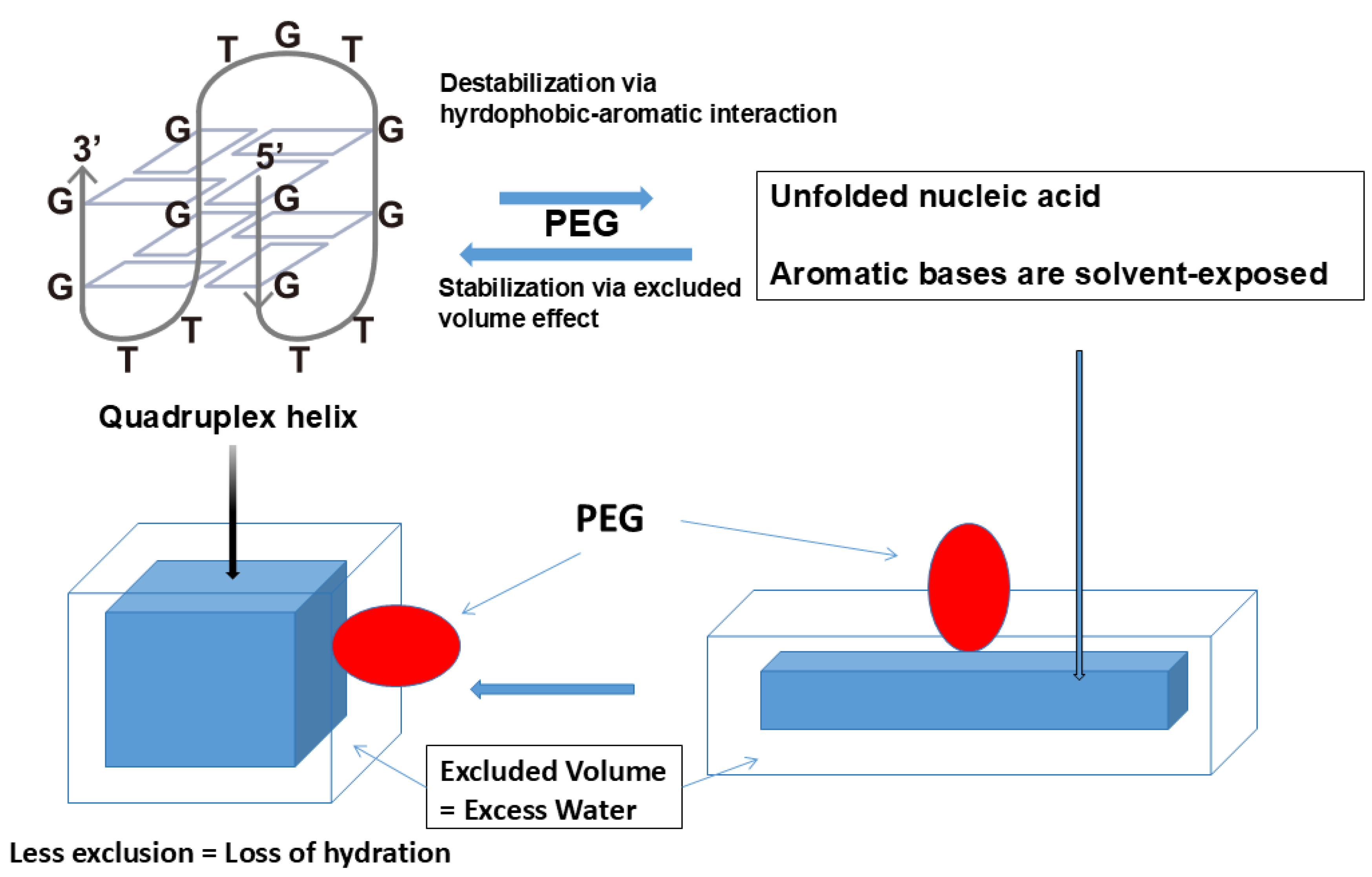
3.3.3. Polymers
4. Conclusion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | circular dichroism |
| SF1 | splicing factor 1 |
| GdnHCl | guanidine hydrochloride |
| PEG | polyethylene glycol |
References
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Danquah, M.K. Protein and peptide biopharmaceuticals: an overview. Protein Pept. Lett. 2017, 24, 94–101. [CrossRef]
- Kesik-Brodacka, M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [CrossRef]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78. [CrossRef]
- Crooke, S.T.; Liang, X.H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [CrossRef]
- Kis, Z.; Kontoravdi, C.; Shattock, R.; Shah, N. Resources, production scales and time required for producing RNA vaccines for the global pandemic demand. Vaccines (Basel) 2021, 9, 3. [CrossRef]
- Sanyal, G.; Särnefält, A.; Kumar, A. Considerations for bioanalytical characterization and batch release of COVID-19 vaccines. NPJ Vaccines 2021, 6, 1–9. [CrossRef]
- Lucas, X.; Bauzá, A.; Frontera, A.; Quiñonero, D. A thorough anion-π interaction study in biomolecules: on the importance of cooperativity effects. Chem. Sci. 2016, 7, 1038–1050. [CrossRef]
- Dougherty, D.A. The cation-π interaction. Acc. Chem. Res. 2013, 46, 885–893. [CrossRef]
- Cole, C.C.; Misiura, M.; Hulgan, S.A.H.; Peterson, C.M.; Williams, J.W., 3rd; Kolomeisky, A.B. Cation-π interactions and their role in assembling collagen triple helices. Biomacromolecules 2022, 23, 4645–4654. [CrossRef]
- Gallivan, J.P.; Dougherty, D.A. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 9459–9464. [CrossRef]
- Ferrari, L.; Stucchi, R.; Konstantoulea, K.; van de Kamp, G.; Kos, R.; Geerts, W.; van der Geer, R.M.J.; Schreurs, M.A.H.; van Dam, B.H.C.; Schutten, L.H.E.et al. Arginine π-stacking drives binding to fibrils of the Alzheimer protein Tau. Nat. Commun. 2020, 11, 571. [CrossRef]
- Carter-Fenk, K.; Liu, M.; Pujal, L.; Loipersberger, M.; Tsanai, M.; Vernon, R.M.; Johnson, E.R.; Sherrill, C.D. The energetic origins of P–π contacts in proteins. J. Am. Chem. Soc. 2023, 145, 24836–24851. [CrossRef]
- Cumpstey, I.; Salomonsson, E.; Sundin, A.; Leffler, H.; Nilsson, U.J. Studies of arginine–arene interactions through synthesis and evaluation of a series of galectin-binding aromatic lactose esters. ChemBioChem 2007, 8, 1389–1398. [CrossRef]
- Herskovits, T.T.; Bowen, J.J. Solution studies of the nucleic acid bases and related compounds. Solubility in aqueous urea and amide solutions. Biochemistry 1974, 13, 5474–5483. [CrossRef]
- Herskovits, T.T.; Harrington, J.P. Solution studies of the nucleic acid bases and related model compounds. Solubility in aqueous alcohol and glycol solutions. Biochemistry 1972, 11, 4800–4811. [CrossRef]
- Hirano, A.; Tokunaga, H.; Tokunaga, M.; Arakawa, T.; Shiraki, K. The solubility of nucleobases in aqueous arginine solutions. Arch. Biochem. Biophys. 2010, 497, 90–96. [CrossRef]
- Dufour, E.; Haertlé, T. Alcohol-induced changes of β-lactoglobulin–retinol-binding stoichiometry. Protein Eng. 1990, 4, 185–190. [CrossRef]
- Shiraki, K.; Nishikawa, K.; Goto, Y. Trifluoroethanol-induced stabilization of the α-helical structure of β-lactoglobulin: implication for non-hierarchical protein folding. J. Mol. Biol. 1995, 245, 180–194. [CrossRef]
- Ferreon, A.C.M.; Deniz, A.A. Alpha-synuclein multistate folding thermodynamics: implications for protein misfolding and aggregation. Biochemistry 2007, 46, 4499–4509. [CrossRef]
- Cao, Y.; Wang, D.; Zhou, P.; Zhao, Y.; Sun, Y.; Wang, J. Influence of conventional surfactants on the self-assembly of a bola-type amphiphilic peptide. Langmuir 2017, 33, 5446–5455. [CrossRef]
- Arakawa, T.; Tokunaga, M.; Kita, Y.; Niikura, T.; Baker, R.W.; Reimer, J.M.; Vaughn, D.F.; Roberts, C.J.; Joshi, P.K. Structure analysis of proteins and peptides by difference circular dichroism spectroscopy. Protein J. 2021, 40, 867–875. [CrossRef]
- Ahari, D.; Sahil, K.; Kaushal, S.; Sharma, A.; Rangan, L.; Swaminathan, R. Structural transitions of dehydrin in response to temperature, the presence of trifluoroethanol and sodium dodecyl sulfate, and its protective role in heat and cold stress. Biochemistry 2025, 64, 3045–3062. [CrossRef]
- Yang, J.F.; Wang, F.; Wang, M.Y.; Wang, D.; Zhou, Z.S.; Hao, G.F.; Liu, Y.; Chen, X.; Zhao, Q. CIPDB: A biological structure databank for studying cation and π interactions. Drug Discov. Today 2023, 28, 103546. [CrossRef]
- Chiang, C.H.; Horng, J.C. Cation–π interaction induced folding of AAB-type collagen heterotrimers. J. Phys. Chem. B 2016, 120, 1205–1211. [CrossRef]
- Schiffer, M.; Edmundson, A.B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys. J. 1967, 7, 121–135. [CrossRef]
- Brindley, M.A.; Suter, R.; Schestak, I.; Kiss, G.; Wright, E.R.; Plemper, R.K. A stabilized headless measles virus attachment protein stalk efficiently triggers membrane fusion. J. Virol. 2013, 87, 11693–11703. [CrossRef]
- Phuong, H.B.T.; Phuong, N.T.; Bui, L.M.; Thi, H.P.; Tran, T.T.P.; Quoc, T.N.; Nguyen, L.T.; Le, P.; Nguyen, D.T. Optimizing amphipathic antimicrobial peptides via helical wheel rotation. ChemMedChem 2025, e202500316. [CrossRef]
- López, J.C.; Sánchez, R.; Blanco, S.; Alonso, J.L. Microsolvation of 2-azetidione: a model for the peptide group–water interactions. Phys. Chem. Chem. Phys. 2015, 17, 2054–2066. [CrossRef]
- Scoppola, E.; Sodo, A.; McLain, S.E.; Ricci, M.A.; Bruni, L. Water–peptide site-specific interactions: a structural study on the hydration of glutathione. Biophys. J. 2014, 106, 1701–1709. [CrossRef]
- Scognamiglio, P.L.; Platella, C.; Napolitano, E.; Musumeci, D.; Roviello, G.N. From prebiotic chemistry to supramolecular biomedical materials: exploring the properties of self-assembling nucleobase-containing peptides. Molecules 2021, 26, 3558. [CrossRef]
- Lund, M.; Jönsson, B.; Woodward, C.E. Implications of a high dielectric constant in proteins. J. Chem. Phys. 2007, 126, 225103. [CrossRef]
- Kimple, A.J.; Soundararajan, M.; Hutsell, S.Q.; Roos, A.K.; Urban, D.J.; Setola, V.; Sucic, M.B.; Tesmer, K.K. Structural determinants of G-protein alpha subunit selectivity by regulator of G-protein signaling 2 (RGS2). J. Biol. Chem. 2009, 284, 19402–19411. [CrossRef]
- Lee, H.T.; Lee, J.Y.; Lim, H.; Lee, S.H.; Moon, Y.J.; Pyo, H.J.; Kim, K.S.; Kim, D.H. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 2017, 7, 5532. [CrossRef]
- Tsumoto, K.; Ogasahara, K.; Ueda, Y.; Watanabe, K.; Yutani, K.; Kumagai, I. Role of Tyr residues in the contact region of anti-lysozyme monoclonal antibody HyHEL-10 for antigen binding. J. Biol. Chem. 1995, 270, 18551–18557. [CrossRef]
- Tsumoto, K.; Yokota, A.; Tanaka, Y.; Ui, M.; Tsumuraya, T.; Fujii, I.; Kato, M.; Kobayashi, S. Critical contribution of aromatic rings to specific recognition of polyether rings: the case of ciguatoxin CTX3C-ABC and its specific antibody 1C49. J. Biol. Chem. 2008, 283, 12259–12266. [CrossRef]
- Akiba, H.; Tsumoto, K. Thermodynamics of antibody–antigen interaction revealed by mutation analysis of antibody variable regions. J. Biochem. 2015, 158, 1–11. [CrossRef]
- Liu, Z.; Luyten, I.; Bottomley, M.J.; Messias, A.C.; Houngninou-Molango, S.; Sprangers, R.; Lam, R.F.; de Almeida, M.S.; Abrahams, J.P.; Steinmetz, M.O. Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science 2001, 294, 1098–1102. [CrossRef]
- Matthews, M.M.; Thomas, J.M.; Zheng, Y.; Tran, K.; Phelps, K.J.; Scott, A.I.; Lam, R.F.; Roberts, C.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; Bowman, G.R.; Hall, K.B.; Soranno, A.; Holehouse, A.S. Structures of human ADAR2 bound to dsRNA reveal base-flipping mechanism and basis for site selectivity. Nat. Struct. Mol. Biol. 2016, 23, 426–433. [CrossRef]
- Hamma, T.; Ferré-D’Amaré, A.R. Structure of protein L7Ae bound to a K-turn derived from an archaeal box H/ACA sRNA at 1.8 Å resolution. Structure 2004, 12, 893–903. [CrossRef]
- Moore, T.; Zhang, Y.; Fenley, M.O.; Li, H. Molecular basis of box C/D RNA–protein interactions: cocrystal structure of archaeal L7Ae and a box C/D RNA. Structure 2004, 12, 807–818. [CrossRef]
- Meier-Stephenson, V. G4-quadruplex-binding proteins: review and insights into selectivity. Biophys. Rev. 2022, 14, 635–654. [CrossRef]
- Chen, M.C.; Tippana, R.; Demeshkina, N.A.; Murat, P.; Balasubramanian, S.; Myong, S.; Yu, H.; Gross, J.T.; Zhao, K. Structural basis of G-quadruplex unfolding by the DEAH/RHA helicase DHX36. Nature 2018, 558, 465–469. [CrossRef]
- Horvath, M.P.; Schultz, S.C. DNA G-quartets in a 1.86 Å resolution structure of an Oxytricha nova telomeric protein–DNA complex. J. Mol. Biol. 2001, 310, 367–377. [CrossRef]
- Ladame, S.; Schouten, J.A.; Roldan, J.; Redman, J.E.; Neidle, S.; Balasubramanian, S. Exploring the recognition of quadruplex DNA by an engineered Cys₂–His₂ zinc finger protein. Biochemistry 2006, 45, 1393–1399. [CrossRef]
- Chavali, S.S.; Cavender, C.E.; Mathews, D.H.; Wedekind, J.E. Arginine forks are a widespread motif to recognize phosphate backbones and guanine nucleobases in the RNA major groove. J. Am. Chem. Soc. 2020, 142, 19835–19839. [CrossRef]
- MacDougall, G.; Anderton, R.S.; Trimble, A.; Mastaglia, F.L.; Knuckey, N.W.; Meloni, B.P. Poly-arginine-18 (R18) confers neuroprotection through glutamate receptor modulation, intracellular calcium reduction, and preservation of mitochondrial function. Molecules 2020, 25, 2977. [CrossRef]
- Rodriguez-Casado, A.; Molina, M.; Carmona, P. Core protein–nucleic acid interactions in hepatitis C virus as revealed by Raman and circular dichroism spectroscopy. Appl. Spectrosc. 2007, 61, 1219–1224. [CrossRef]
- Ham, S.; Lee, C. Exploring the impact of nucleic acids on protein stability in bacterial cell lysate. Biochim. Biophys. Acta 2023, 1867, 130445. [CrossRef]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Struchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.; Bowman, G.R.; Hall, K.B.; Soranno, A.; Holehouse, A.S. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021, 12, 1936. [CrossRef]
- Vos, J.D.; Aguilar, P.P.; Köppl, C.; Fischer, A.; Grünwald-Gruber, C.; Dürkop, M.; Becker, S.; Fuchs, R.; Müller, S.; Schneider, T.M. Production of full-length SARS-CoV-2 nucleocapsid protein from Escherichia coli optimized by native hydrophobic interaction chromatography hyphenated to multi-angle scattering detection. Talanta 2021, 235, 122691. [CrossRef]
- Carlson, C.R.; Asfaha, J.B.; Ghent, C.M.; Howard, C.J.; Hartooni, N.; Safari, M.; Gerber, R.A.; Gardner, D.A.; Williamson, E.M. Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol. Cell 2020, 80, 1092–1103. [CrossRef]
- Lu, S.; Ye, Q.; Singh, D.; Cao, Y.; Diedrich, J.K.; Yates, J.R., 3rd; Guttman, S.H.; Smith, R.R. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat. Commun. 2021, 12, 502. [CrossRef]
- Laue, T.M.; Shire, S.J. The molecular interaction process. J. Pharm. Sci. 2019, 307, 1. [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [CrossRef]
- Garabedian, M.V.; Su, Z.; Dabdoub, J.; Tong, M.; Deiters, A.; Hammer, D.A.; Good, M.C. Protein condensate formation via controlled multimerization of intrinsically disordered sequences. Biochemistry 2022, 61, 2237–2246. [CrossRef]
- Martin, E.W.; Holehouse, A.S.; Peran, I.; Farag, M.; Incicco, J.J.; Bremer, A.; Grace, C.R.; Soranno, A.; Pappu, R.V.; Mittag, T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 2020, 367, 694–699. [CrossRef]
- Vieregg, J.R.; Lueckheide, M.; Marciel, A.; Leon, D.M.; Tirrell, K.L. Oligonucleotide–peptide complexes: Phase control by hybridization. J. Am. Chem. Soc. 2018, 140, 1632–1638. [CrossRef]
- Wadsworth, G.; Srinivasan, S.; Lai, L.; Datta, M.; Gopalan, V.; Banerjee, P.R. RNA-driven phase transitions in biomolecular condensates. Mol. Cell 2024, 85, 3670–3682. [CrossRef]
- Zhang, Y.; You, R.; Zhao, W.; Zhou, J.; Chen, J. G-quadruplex structures trigger RNA phase separation. Nucleic Acids Res. 2019, 47, 11746–11754. [CrossRef]
- Tauber, D.; Tauber, G.; Parker, R. Mechanisms and regulation of RNA condensation in RNP granule formation. Trends Biochem. Sci. 2020, 45, 764–778. [CrossRef]
- Maity, H.; Nguyen, H.; Hori, N.; Thirumalai, D. Odd–even disparity in the population of slipped hairpins in RNA repeat sequences with implications for phase separation. Proc. Natl. Acad. Sci. U.S.A. 2023, 120, e2301409120. [CrossRef]
- Shil, S.; Tsuruta, M.; Kawauchi, K.; Miyoshi, D. Factors Affecting Liquid-Liquid Phase Separation of RGG Peptides with DNA G-Quadruplex. ChemMedChem. 2025, 20, e202400460. [CrossRef]
- Tsuruta, M.; Shil, S.; Taniguchi, S.; Kawauchi, K.; Miyoshi, D. The role of cytosine methylation in regulating the topology and liquid-liquid phase separation of DNA G-quadruplexes. Chem Sci. 2025, 16, 4213-4225. [CrossRef]
- Amato, J.; Madanayake, T.W.; Iaccarino, N.; Novellino, E.; Randazzo, A.; Hurley, L.H.; Pagano, B. HMGB1 binds to the KRAS promoter G-quadruplex: a new player in oncogene transcriptional regulation? Chem Commun (Camb). 2018, 54, 9442-9445. [CrossRef]
- Chen, L.; Dickerhoff, J.; Zheng, K.W.; Erramilli, S.; Feng, H.; Wu, G.; Onel, B.; Chen, Y.; Wang, K.B.; Carver, M. et al. Structural basis for nucleolin recognition of MYC promoter G-quadruplex. Science. 2025, 388, eadr1752. [CrossRef]
- Sahoo, B.R.; Deng, X.; Wong, E.L.; Clark, N.; Yang, H.J.; Kovalenko, A.; Subramanian, V.; Guzman, B.B.; Harris, S.E.; Dehury, B. et al. Visualization of liquid-liquid phase transitions using a tiny G-quadruplex binding protein. Nat Commun. 2025,16, 8578. [CrossRef]
- Yoo, W.; Song, Y.W.; Bansal, V.; Kim, K.K. G-quadruplex-dependent transcriptional regulation by molecular condensation in the Bcl3 promoter. Nucleic Acids Res. 2025, 53, gkaf827. [CrossRef]
- Nozaki, Y.; Tanford, C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J. Biol. Chem. 1971, 246, 2211–2217. [CrossRef]
- Trikulenko, A.V. Thermodynamic and stereochemical parameters to evaluate stability of hydrophobic cores of globular proteins. Biochemistry (Mosc.) 1998, 63, 1290–1293. https://pubmed.ncbi.nlm.nih.gov/9864468/.
- Kita, Y.; Arakawa, T.; Lin, T.Y.; Timasheff, S.N. Contribution of the surface free energy perturbation to protein–solvent interactions. Biochemistry 1994, 33, 15178–15189. [CrossRef]
- Nozaki, Y.; Tanford, C. The solubility of amino acids and related compounds in aqueous ethylene glycol solutions. J. Biol. Chem. 1965, 240, 3568–3575. [CrossRef]
- Arakawa, T.; Kita, Y.; Timasheff, S.N. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem. 2007, 131, 62–70. [CrossRef]
- Pitts, E.P.; Timasheff, S.N. Interaction of ribonuclease A with aqueous 2-methyl-2,4-pentanediol at pH 5.8. Biochemistry 1978, 17, 615–623. [CrossRef]
- Xu, R.; Zheng, M.; Farajtabar, A.; Zhao, H. Solubility modelling and preferential solvation of adenine in solvent mixtures of N,N-dimethylformamide, N-methyl pyrrolidone, propylene glycol and dimethyl sulfoxide plus water. J. Chem. Thermodyn. 2018, 125, 225–234. [CrossRef]
- Zhou, Y.; Han, D.; Tao, T.; Zhang, S.; Wang, J.; Gong, J.; Li, L.; Sun, H. Solubility measurement, thermodynamic correlation and molecular simulations of uracil in alcohol + water binary solvents at 233.15–318.15 K. J. Mol. Liq. 2020, 318, 114259. [CrossRef]
- Herskovits, T.T. Conformation of proteins and polypeptides. I. Extension of the solvent perturbation technique of difference spectroscopy to the study of proteins and polypeptides in organic solvents. J. Biol. Chem. 1965, 240, 628–638. https://www.jbc.org/article/S0021-9258(17)45221-5/pdf.
- Alonso, D.O.; Dill, K.A. Solvent denaturation and stabilization of globular proteins. Biochemistry 1991, 30, 5947–5985. [CrossRef]
- Khan, J.M.; Malik, A.; Sharma, P.; Fatima, S. Anionic surfactants cause dual conformational changes in insulin. Int. J. Biol. Macromol. 2023, 247, 125790. [CrossRef]
- Luo, P.; Baldwin, R.L. Mechanism of helix induction by trifluoroethanol: a framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 1997, 36, 8413–8421. [CrossRef]
- Kumari, N.K.P.; Jagannadham, M.V. Deciphering the molecular structure of cryptolepain in organic solvents. Biochimie 2011, 94, 310–317. [CrossRef]
- Srisailam, S.; Kumar, T.K.; Simathi, T.; Yu, C. Influence of backbone conformation on protein aggregation. J. Am. Chem. Soc. 2002, 124, 1884–1888. [CrossRef]
- Sinanoglu, O.; Abdulnur, S. Effect of water and other solvents on the structure of biopolymers. Fed. Proc. 1965, 24, S12–S23. https://documentsdelivered.com/source/032/901/032901967.php.
- Geiduschek, E.P.; Herskovits, T.T. Nonaqueous solutions of DNA. Reversible and irreversible denaturation in methanol. Arch. Biochem. Biophys. 1961, 95, 114–129. [CrossRef]
- Herskovits, T.T.; Singer, S.J.; Geiduschek, E.P. Nonaqueous solutions of DNA. Denaturation in methanol and ethanol. Arch. Biochem. Biophys. 1961, 94, 99–114. [CrossRef]
- Yoshizawa, S.; Arakawa, T.; Shiraki, K. Dependence of ethanol effects on protein charges. Int. J. Biol. Macromol. 2014, 68, 169–172. [CrossRef]
- Yoshikawa, H.; Hirano, A.; Arakawa, T.; Shiraki, K. Mechanistic insights into protein precipitation by alcohol. Int. J. Biol. Macromol. 2012, 50, 865–871. [CrossRef]
- Yoshikawa, H.; Hirano, A.; Arakawa, T.; Shiraki, K. Effects of alcohol on the solubility and structure of native and disulfide-modified bovine serum albumin. Int. J. Biol. Macromol. 2012, 50, 1286–1291. [CrossRef]
- van Oss, C.J. On the mechanism of the cold ethanol precipitation method of plasma protein fractionation. J. Protein Chem. 1989, 8, 661–668. [CrossRef]
- Klemm, W.R. Dehydration: a new alcohol theory. Alcohol 1990, 7, 49–59. [CrossRef]
- Peng, L.; Dai, H.; Wang, H.; Zhu, H.; Ma, L.; Yu, Y.; Zhang, X.; Li, M. Effect of different dehydration methods on the properties of gelatin films. Food Chem. 2022, 374, 131814. [CrossRef]
- Herskovits, T.T. Nonaqueous solutions of DNA; denaturation by urea and its methyl derivatives. Biochemistry 1963, 2, 335–340. [CrossRef]
- Misawa, S.; Kumagai, I. Refolding of therapeutic proteins produced in Escherichia coli as inclusion bodies. Biopolymers 1999, 51, 297–307. [CrossRef]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [CrossRef]
- Upadhyyay, V.; Singh, A.; Panda, A.K. Purification of recombinant ovalbumin from inclusion bodies of Escherichia coli. Protein Expr. Purif. 2016, 117, 52–58. [CrossRef]
- Nozaki, Y.; Tanford, C. The solubility of amino acids and related compounds in aqueous urea solutions. J. Biol. Chem. 1963, 238, 4074–4081. [CrossRef]
- Nozaki, Y.; Tanford, C. The solubility of amino acids, diglycine, and triglycine in aqueous guanidine hydrochloride solutions. J. Biol. Chem. 1970, 245, 1648–1652. [CrossRef]
- Goyal, S.; Chattopadhyay, A.; Kasavajhala, K.; Priyakumar, U.D. Role of urea-aromatic stacking interactions in stabilizing the aromatic residues of the protein in urea-induced denatured state. J. Am. Chem. Soc. 2017, 139, 14931–14946. [CrossRef]
- Raghunathan, S.; Jaganade, T.; Priyakumar, U.D. Urea-aromatic interactions in biology. Biophys. Rev. 2020, 12, 65–84. [CrossRef]
- Lee, J.C.; Timasheff, S.N. Partial specific volumes and interactions with solvent components of proteins in guanidine hydrochloride. Biochemistry 1974, 13, 257–265. [CrossRef]
- Alodia, N.; Jaganade, T.; Priyakumar, U.D. Quantum mechanical investigation of the nature of nucleobase-urea stacking interaction, a crucial driving force in RNA unfolding in aqueous urea. J. Chem. Sci. 2018, 130, 158. [CrossRef]
- Sarkar, S.; Singh, P.C. Sequence specific hydrogen bond with DNA with denaturants affects its stability: Spectroscopic and simulation studies. Biochim. Biophys. Acta 2021, 1865, 129735. [CrossRef]
- Shkel, I.A.; Knowles, B.; Record, M.T., Jr. Separating chemical and excluded volume interactions of polyethylene glycols with native proteins: comparison with PEG effects on DNA helix formation. Biopolymers 2015, 103, 517–527. [CrossRef]
- Arakawa, T.; Timasheff, S.N. Mechanism of poly(ethylene glycol) interaction with proteins. Biochemistry 1985, 24, 6756–6762. [CrossRef]
- Lee, L.L.; Lee, J.C. Thermal stability of proteins in the presence of poly(ethylene glycols). Biochemistry 1987, 26, 7813–7819. [CrossRef]
- Lee, J.C.; Lee, L.L. Preferential solvent interactions between proteins and polyethylene glycols. J. Biol. Chem. 1981, 256, 625–631. [CrossRef]
- Schellman, J.A. Protein stability in mixed solvents: a balance of contact interaction and excluded volume. Biophys. J. 2003, 85, 108–125. [CrossRef]
- Santos, A.; de Haro, M.L.; Fiumara, G.; Saija, F. The effective colloid interaction in the Asakura-Oosawa model. Assessment of non-pairwise terms from virial expansion. J. Chem. Phys. 2015, 142, 224903. [CrossRef]
- Minton, A.P. Macromolecular crowding. Curr. Biol. 2006, 16, R269–R271. [CrossRef]
- Rivas, G.; Minton, A.P. Macromolecular crowding in vitro, in vivo and in between. Trends Biochem. Sci. 2016, 41, 970–981. [CrossRef]
- Hirano, A.; Shiraki, K.; Arakawa, T. Polyethylene glycol behaves like weak organic solvent. Biopolymers 2012, 97, 117–122. [CrossRef]
- Nakano, S.; Karimata, H.; Ohmichi, T.; Kawakami, J.; Sugiyama, N. The effect of molecular crowding with nucleotide length and cosolute structure on DNA duplex stability. J. Am. Chem. Soc. 2004, 126, 14330–14331. [CrossRef]
- Miyoshi, D.; Nakao, A.; Sugimoto, N. Molecular crowding regulates the structural switch of the DNA G-quadruplex. Biochemistry 2002, 41, 15017–15024. [CrossRef]
- Miyoshi, D.; Karimata, H.; Sugimoto, N. Hydration regulates thermodynamics of G-quadruplex formation under molecular crowding conditions. J. Am. Chem. Soc. 2006, 128, 7957–7963. [CrossRef]
- Baldwin, R.L.; Rose, G.D. How the hydrophobic factor drives protein folding. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 12462–12466. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




