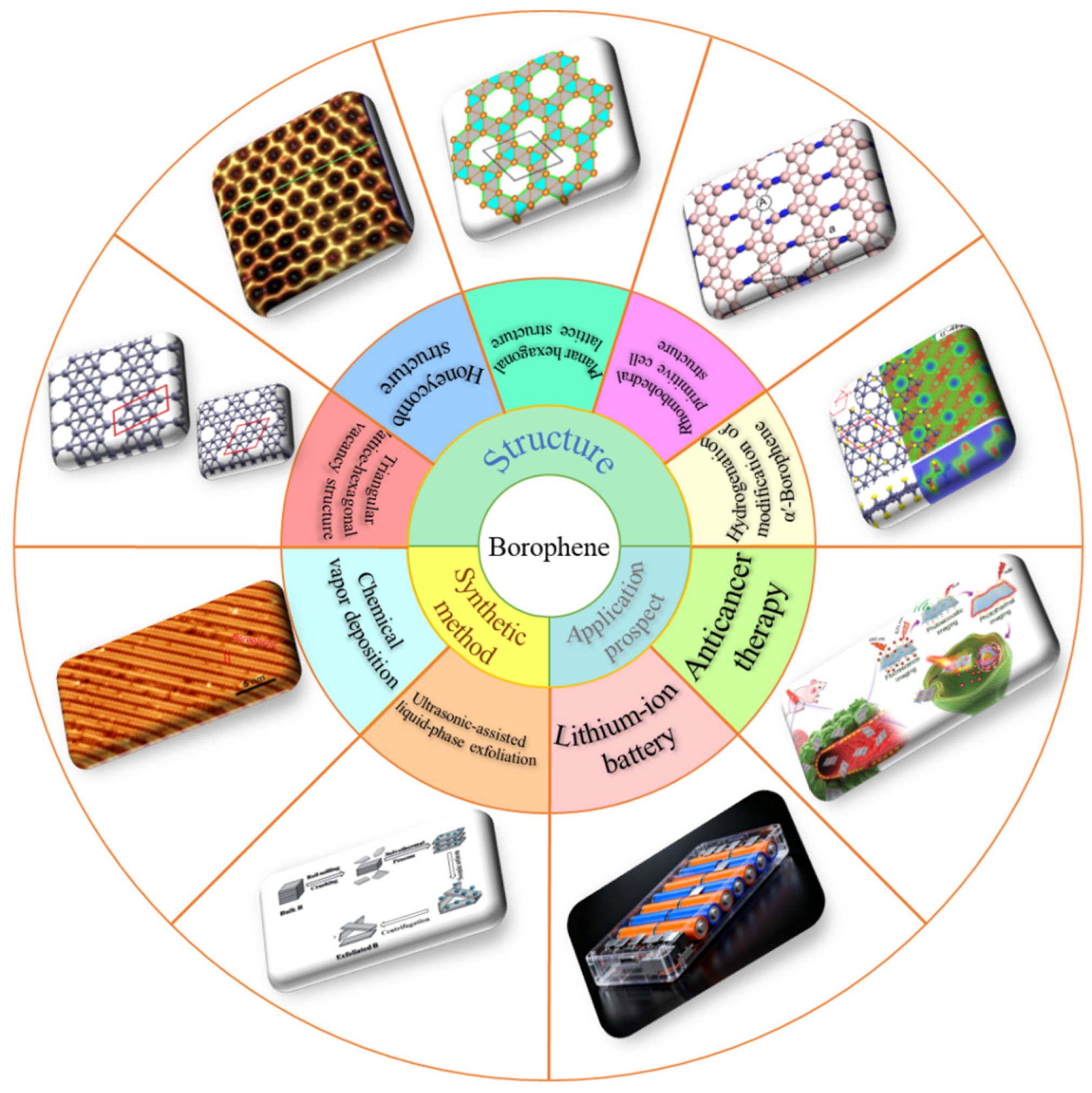
1. Introduction
Figure 1.
The figure illustrates some common structures of borophene, its synthesis methods, and applications in important fields. Reprinted with permission from ref [
1]. Copyright 2007 American Physical Society. Reprinted with permission from ref [
2]. Copyright 2018 Elsevier B.V. and Science China Press. Reprinted with permission from ref [
3]. Copyright 2024 American Chemical Society Reprinted with permission from ref [
4]. Copyright 2022 Youke Publishing Co., Ltd. Reprinted with permission from ref [
5]. Copyright 2021 arXiv (arXiv:2107.01409v1 [cond-mat.mtrl-sci]). Reprinted with permission from ref [
6]. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Reprinted with permission from ref [
7]. Copyright 2021 American Association for the Advancement of Science, AAAS Reprinted with permission from ref [
8]. Copyright 2020 Royal Society of Chemistry, RSC.
Figure 1.
The figure illustrates some common structures of borophene, its synthesis methods, and applications in important fields. Reprinted with permission from ref [
1]. Copyright 2007 American Physical Society. Reprinted with permission from ref [
2]. Copyright 2018 Elsevier B.V. and Science China Press. Reprinted with permission from ref [
3]. Copyright 2024 American Chemical Society Reprinted with permission from ref [
4]. Copyright 2022 Youke Publishing Co., Ltd. Reprinted with permission from ref [
5]. Copyright 2021 arXiv (arXiv:2107.01409v1 [cond-mat.mtrl-sci]). Reprinted with permission from ref [
6]. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Reprinted with permission from ref [
7]. Copyright 2021 American Association for the Advancement of Science, AAAS Reprinted with permission from ref [
8]. Copyright 2020 Royal Society of Chemistry, RSC.
Since the first successful synthesis of borophene on Ag(111) substrates via molecular beam epitaxy (MBE) by Mannix et al.[
9] in 2015, this two-dimensional (2D) allotrope of boron has rapidly emerged as a focal point in materials science and nanotechnology. Unlike graphene, which can be exfoliated from its naturally layered parent graphite, borophene lacks a bulk layered precursor. Its stable two-dimensional growth is therefore strongly dependent on the electronic coupling and lattice matching with metallic substrates.
Currently, the two most common approaches for borophene synthesis are molecular beam epitaxy (MBE) and chemical vapor deposition (CVD). MBE enables atomically precise growth through fine control of boron evaporation rates and substrate temperature, but the process suffers from high equipment costs and limited throughput. CVD, on the other hand, holds promise for large-scale fabrication but faces challenges such as the low decomposition efficiency of boron precursors and insufficient product purity—factors that critically restrict its scalability in practical applications.
The structural uniqueness of boron manifests both in its bulk and two-dimensional forms. The most stable bulk polymorph, β-rhombohedral boron[
10], represents a typical defect-stabilized system in which intrinsic structural vacancies are essential for maintaining stability, even at extremely low temperatures. This intrinsic complexity extends into two dimensions, forming a family of 2D boron sheets collectively known as borophene. The defining structural motif of borophene consists of a triangular boron lattice interspersed with periodically arranged hexagonal holes (HHs). Variations in the hole density (η) and their distribution lead to a rich diversity of polymorphs with distinct atomic coordination and bonding configurations.
The electronic and mechanical properties of borophene further highlight its extraordinary potential. Electronically, borophene exhibits metallic conductivity, massless Dirac fermions, and charge density wave (CDW) phenomena. First-principles calculations have predicted that hydrogenated β
12-borophene could reach a superconducting transition temperature (Tc) as high as 29 K[
11] under synergistic tensile strain and hole doping. Mechanically, borophene possesses an exceptional Young’s modulus of approximately 398 GPa·nm[
12] along the a-direction—far exceeding that of graphene (42 N/m)—while maintaining outstanding flexibility. These attributes make borophene an ideal candidate for next-generation flexible electronic devices and nanoscale interconnects.
Nevertheless, several critical challenges remain before borophene can transition from laboratory research to large-scale applications. (1) Environmental instability – pristine borophene is prone to oxidation in air or moisture, forming boron oxide (B2O3); (2) High synthesis cost – MBE requires ultrahigh vacuum and high-purity solid boron sources, limiting mass production; (3) Interlayer aggregation – multilayer borophene tends to restack due to strong interlayer interactions, leading to loss of 2D characteristics. To address these issues, multi-dimensional strategies have been developed. Protective encapsulation (e.g., with h-BN), chemical modification (e.g., hydrogenation), and in silico screening through AI-assisted first-principles calculations are accelerating the discovery of stable borophene polymorphs and heterostructures. Moreover, advanced characterization techniques, such as CO-functionalized atomic force microscopy (CO-AFM)[
13], have enabled atomic-level visualization of hexagonal vacancy arrangements, providing direct experimental evidence for structure–property correlations.
This review summarizes recent progress in borophene research from three perspectives: (i) structural diversity and bonding characteristics, (ii) most common borophene synthesis methodologies, and (iii) cutting-edge applications in energy and biomedical technologies. Finally, the key challenges and future directions for achieving scalable, stable, and functional borophene are discussed.
2. Structural Characteristics of Borophene
2.1. Mixed Triangular–Hexagonal Vacancy Lattices
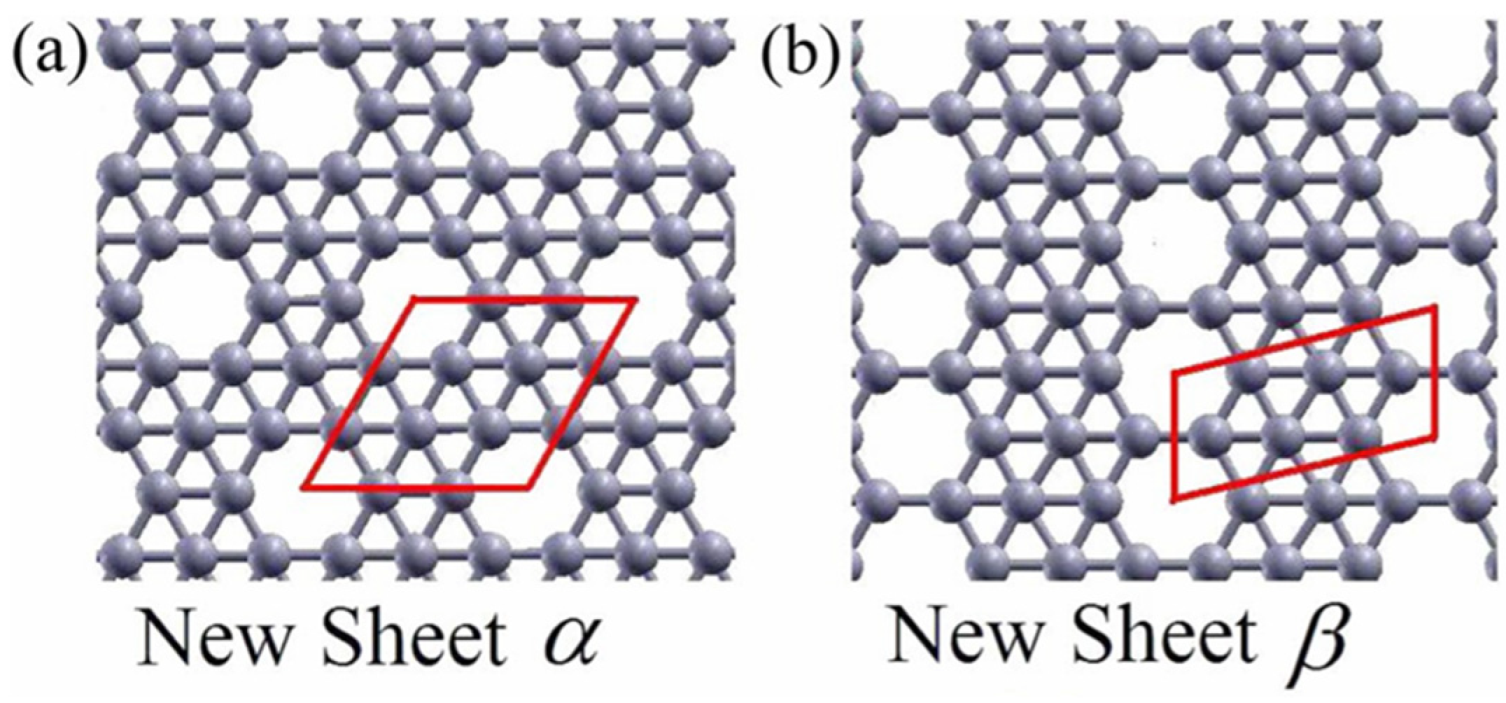
The theoretical prediction of a new, more stable boron sheet structure[
1] in 2007 marked a turning point in the understanding of 2D boron materials. Pure triangular boron lattices (η = 0) and perfect hexagonal honeycomb lattices (η = 1/3) are both intrinsically unstable. Stability is achieved by periodically introducing hexagonal vacancies (holes) into the triangular lattice, forming a hybrid structure with a tunable vacancy density (η), which defined as:
Figures 2(a) and 2(b) illustrate two representative configurations—α-sheet (η = 1/9) and β-sheet (η = 1/7)—which are entirely composed of boron atoms arranged as triangular and hexagonal motifs. These sheets can be viewed as derivatives of a triangular lattice from which atoms are periodically removed to form ordered hexagonal holes. The coexistence of triangular networks (gray atoms and bonds) and hexagonal vacancies provides mechanical robustness and electronic stability.
This structural stability arises from the competition and cooperation between two-center two-electron (2c–2e) and three-center two-electron (3c–2e) bonds. Boron, with its 2s²2p¹ valence configuration, is electron-deficient—possessing only three valence electrons but forming multiple bonds with neighboring atoms. To compensate for this deficiency, three boron atoms hybridize their sp² orbitals to form 3c–2e bonds, which delocalize electrons across the lattice and lower the total energy. Meanwhile, boron atoms located at hexagonal vacancy edges exhibit reduced coordination numbers, favoring the formation of localized 2c–2e sp² bonds similar to those in graphene.
The coexistence of these two bonding schemes yields a structurally and electronically balanced lattice: 3c–2e bonds stabilize the electron-deficient framework, whereas 2c–2e bonds reinforce local rigidity. This synergy explains why hybrid boron sheets exhibit superior planarity and thermodynamic stability compared to pure triangular boron lattices, which tend to buckle due to excessive delocalization.
Figure 2.
(a),(b)Two examples of ourBS (top view). Red solid lines show the unit cells. Reprinted with permission from ref 1. Copyright 2007 American Physical Society.
Figure 2.
(a),(b)Two examples of ourBS (top view). Red solid lines show the unit cells. Reprinted with permission from ref 1. Copyright 2007 American Physical Society.
2.2. Honeycomb Borophene
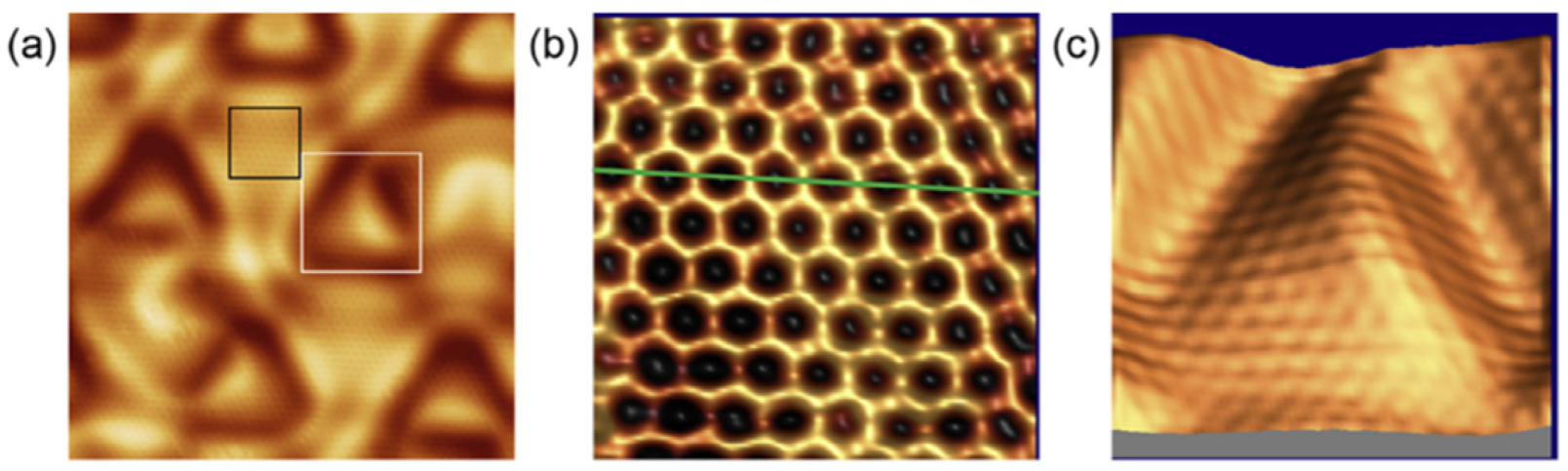
In 2018, Li et al.[
2] experimentally confirmed that borophene grown on Al(111) under ultrahigh vacuum conditions possesses a nearly perfect honeycomb lattice. However, long-range interactions between borophene and the substrate induce strain relaxation, giving rise to periodic triangular corrugations observable in scanning tunneling microscopy (STM) images.
As shown in
Figure 3(a), the surface morphology exhibits quasi-periodic triangular ripples, a direct manifestation of surface strain modulation. The high-resolution STM image in
Figure 3(b) reveals a flat honeycomb lattice superimposed on the corrugated background, with identical brightness at A and B sites, confirming that all boron atoms reside within the same plane. The 3D STM reconstruction in
Figure 3(c) further demonstrates that the honeycomb atomic lattice is embedded atop macroscopic triangular undulations.
This discovery provided the first direct evidence of planar sp²-hybridized boron in a graphene-like configuration, highlighting the possibility of achieving purely covalent boron sheets with exceptional in-plane order when lattice matching and substrate coupling are optimized.
2.3. δ5-Borophene: Planar Hexagonal Network
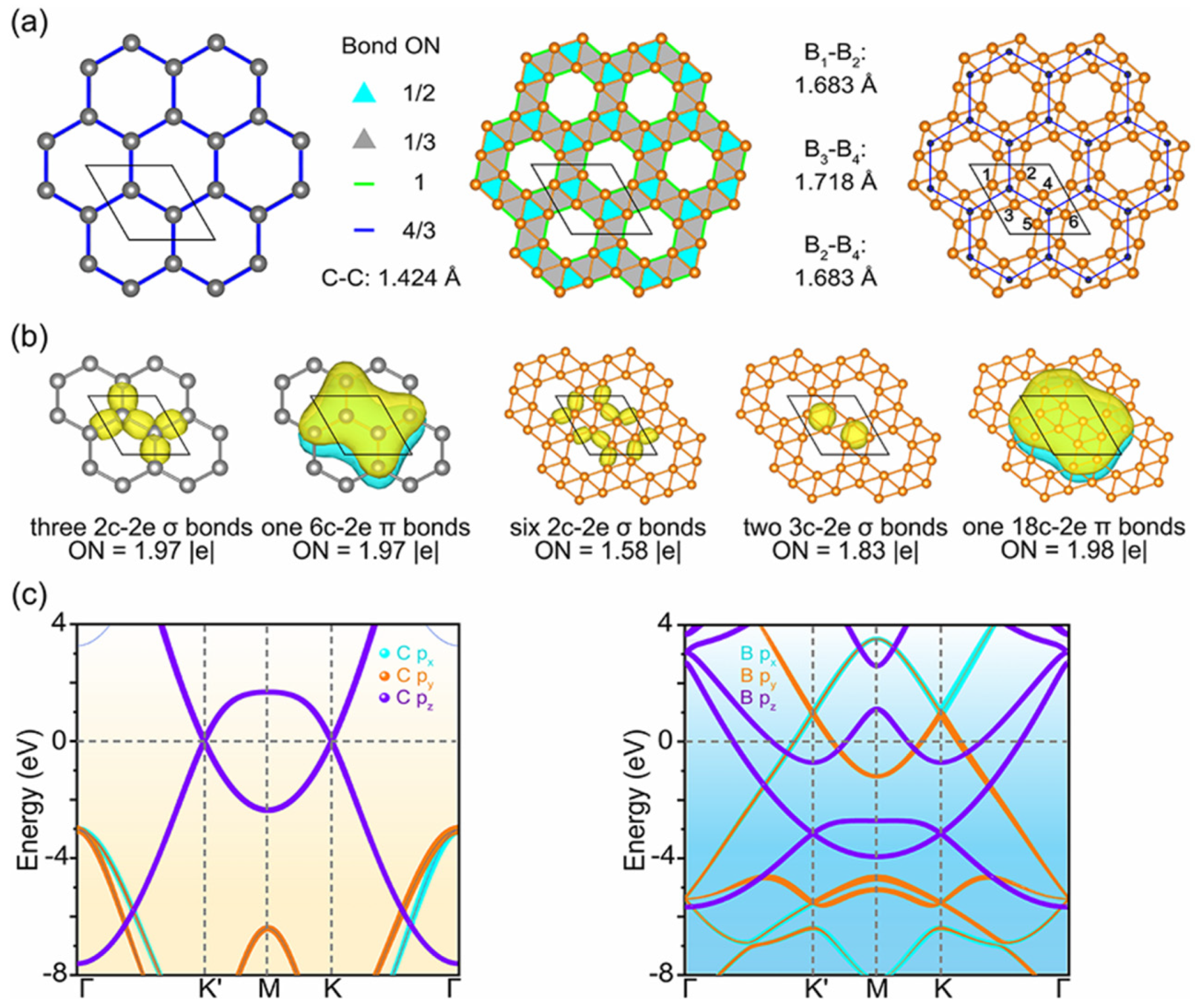
Among over 600 theoretically predicted van der Waals-stacked bilayer configurations, δ5-borophene stands out as one of the few that forms a stable multilayered structure.[
14] As shown in
Figure 4(a), δ5-borophene adopts a planar hexagonal grid (lattice constant 4.48 Å, average B–B distance 1.69 Å) belonging to the P6/m (C6h) space group. All boron atoms exhibit fivefold coordination with negligible out-of-plane distortion (<0.1 Å).
The structural stability originates from a combination of multi-center σ bonds and delocalized π bonds, as revealed by solid-state adaptive natural density partitioning (SSAdNDP)[
15] analysis [
Figure 4(b)]. The bonding network features fractional occupancy: σ bonds within B₃ triangles (ON ≈ 1.83 |e|), inter-triangle 2c–2e σ bonds (ON ≈ 1.58 |e|), and a delocalized 18c–2e π bond (ON ≈ 1.98 |e|) that extends throughout the entire lattice. This complex bonding system compensates for boron’s electron deficiency and maintains in-plane rigidity.
The orbital-projected band structure [
Figure 4(c)] reveals linear Dirac-like dispersion near the K/K′ points contributed by px/py orbitals, while the pz orbitals form deep-lying π bands located 3.6 eV below the Fermi level. These features confer metallic conductivity and suggest potential phonon-mediated superconductivity with a predicted critical temperature around 22 K[
16]. The synergy of Dirac fermion behavior, metallicity, and structural flatness positions δ5-borophene as a promising candidate for high-speed electronic and superconducting applications.
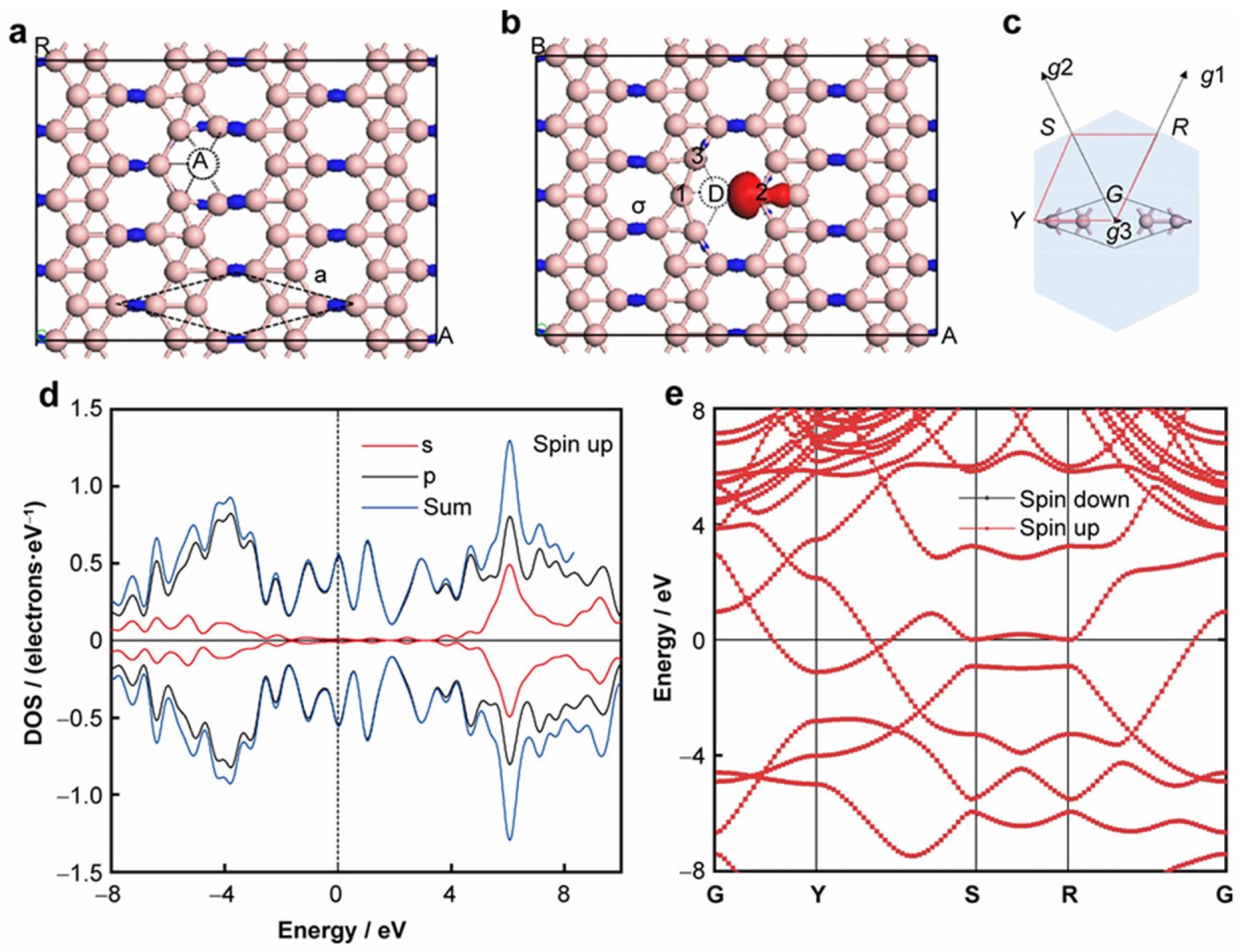
2.4. χ3-Borophene: Rhombic Lattice and Magnetic Defects
As illustrated in
Figure 5(a), χ
3-borophene crystallizes in the C2mm space group with a rhombic unit cell (a = 0.445 nm). The lattice comprises two types of boron sites: five-coordinated A atoms serving as topological junctions, and four-coordinated D atoms acting as bridging units between lattice segments. This coordination asymmetry dictates the material’s bonding behavior and defect response.[
17]
Differential charge density maps [
Figure 5(b)] show strong localization of σ-bond electrons between D-site atoms (blue isosurfaces, 300 e·nm⁻³), which are essential for maintaining the material’s in-plane mechanical strength. The overlapping pz orbitals form a delocalized π system that complements the localized σ bonds, producing a mixed bonding framework[
18].
When vacancy defects are introduced, unpaired σ electrons emerge at D2 edge atoms, leading to localized spin polarization with a magnetic moment of ~1 μB per defect. This defect-induced magnetism originates from broken σ bonds and local electronic degeneracy at vacancy edges, providing a potential route for magnetism engineering in borophene[
4].
Figure 5(c), as the first Brillouin zone containing high-symmetry points, serves as a key spatial framework for analyzing the electronic structure of χ
3-borophene [
19]. The high-symmetry points (such as Γ, Y, S, R) and corresponding paths (Γ→Y→S→R→Γ) provide a periodic spatial reference for the calculations of density of states in
Figure 5(d) and band structure in
Figure 5(e). The shape of this Brillouin zone and the distribution of high-symmetry points strictly match the rhombic unit cell of χ
3-borophene.
Electronic density of states (DOS) and band structure calculations [Figures 5(d)–(e)] confirm that pristine χ3-borophene is metallic and nonmagnetic, with symmetric spin-up and spin-down channels. Upon defect formation, the degeneracy is lifted, resulting in spin-split states that enable controlled tuning of local magnetic moments—crucial for spintronic applications.
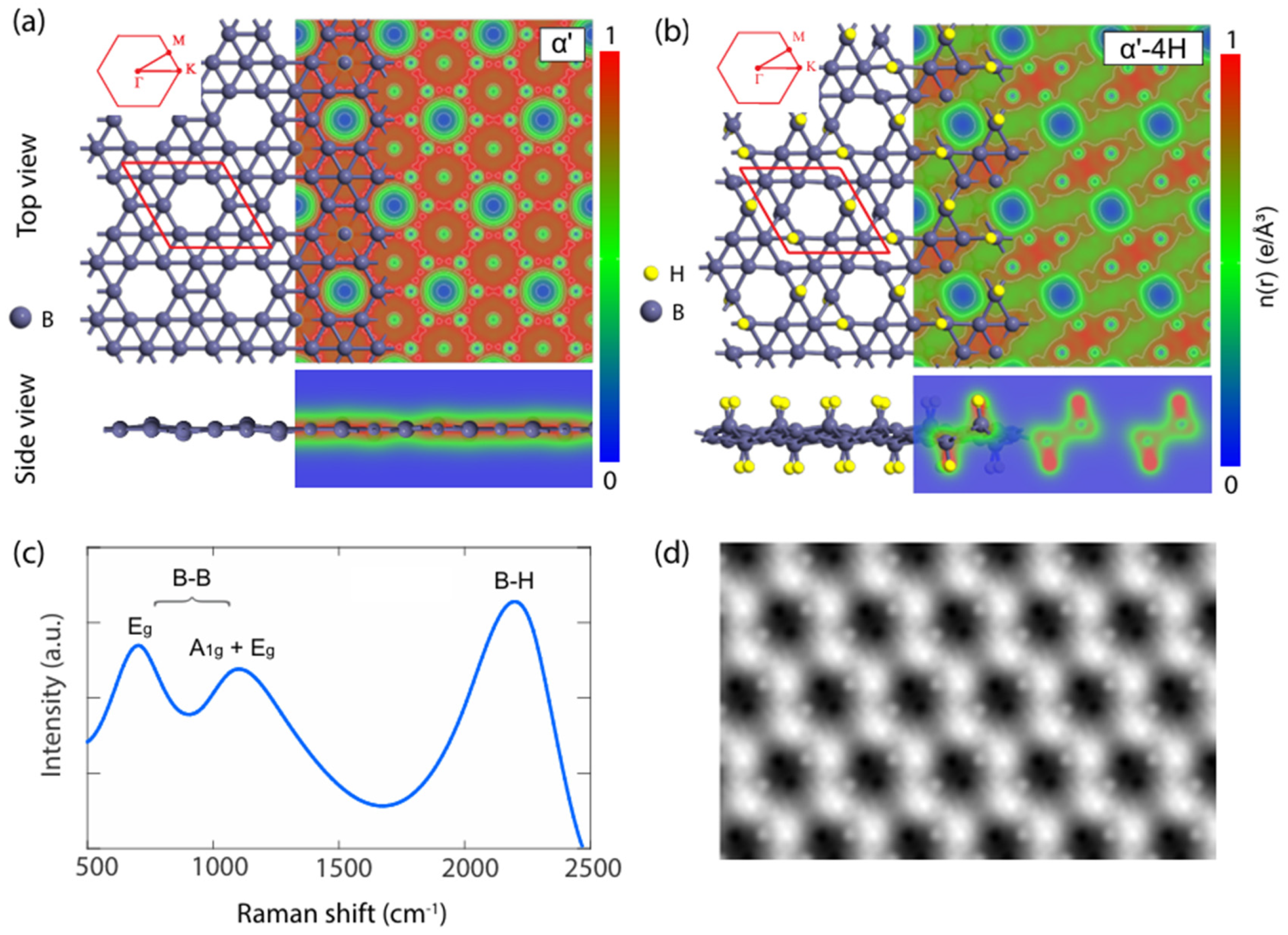
2.5. α′-Borophene and Hydrogenation Effects
In the rich crystal structure system of borophene, the α' phase and its derivatives [
5] have attracted significant attention due to their unique structural characteristics and tunability. As a typical porous two-dimensional boron structure, the fully relaxed configuration of α'-borophene is nearly planar (as shown in
Figure 6(a)), with a buckling height of only 0.37 Å, exhibiting significant isotropic characteristics. The lattice constant is approximately 5.05 Å, with uniform B–B bond lengths of 1.68 Å, and a hexagonal hole (HHs) concentration of η = 1/8. This structural feature is an important foundation for its stable existence. Electron density cross-sectional analysis reveals a uniformly distributed electron sea in α'-borophene, which directly confirms its metallic nature. This electron density distribution characteristic is also clearly identifiable in
Figure 6(a), consistent with the characterization results of borophene's metallic properties by angle-resolved photoemission spectroscopy (ARPES) and scanning tunneling microscopy (STM).
Hydrogen modification is an effective strategy for regulating the structure and electronic properties of borophene. As a typical hydrogenated derivative of α'-borophene, α'-4H-borophene undergoes significant structural changes due to the formation of B–H bonds. Hydrogenation increases the buckling height of borophene to 0.88 Å, which is a direct result of the attractive interaction between boron and hydrogen atoms, while also disrupting the lattice isotropy and differentiating the B–B bond lengths into 1.69 Å and 1.77 Å. The lattice constant remains at approximately 5.05 Å. These structural parameters and configuration changes are clearly presented in
Figure 6(b). The electron density cross-sectional map (
Figure 6(b)) shows that electrons in α'-4H-borophene significantly aggregate around hydrogen atoms, leading to a reduction in in-plane carrier density and a substantial decrease in electronic conductivity compared to α'-borophene. This transformation in electronic structure is closely related to the semiconductor characteristics of hydrogenated borophene [
20].
Structural characterization techniques provide key evidence for the structural authenticity of α'-4H-borophene. Simulated Raman spectroscopy (
Figure 6(c)) shows three characteristic peaks at 700 cm⁻¹, 1100 cm⁻¹, and 2200 cm⁻¹, corresponding to the Eg vibration mode of B–B clusters, the A
1g+E
g vibration mode, and the combined vibration mode of B–H bonds, respectively. These vibration modes highly match the structural characteristics of chemically modified borophene. Meanwhile, the simulated STM image of α'-4H-borophene (
Figure 6(d)) highly coincides with experimental observations. Combined with the verification role of Raman spectroscopy (
Figure 6(c)), it further confirms the structural consistency between the theoretically constructed α'-4H-borophene model and the actual synthesized product, which is consistent with the strategy of combining STM and density functional theory (DFT) to verify the structure in multilayer borophene research.
2.6. Summary of Structural Diversity
The differences and correlations in the aforementioned structural characteristics fully reflect the tunability of borophene's structure and its structure–property relationship with electronic properties. The polymorphism of borophene encompasses numerous experimentally realized and theoretically predicted phases, including α, β, β12, δ5, χ3, χ6, and α′ configurations[
21]. These structures differ significantly in vacancy density (η), atomic coordination number (CN), and lattice symmetry, leading to a wide range of electronic behaviors—from metallic and semiconducting to superconducting. This rich structural diversity underpins borophene’s versatility as a multifunctional material platform, offering tunable properties through atomic-scale design and surface modification.
3. Synthesis Strategies for Borophene
The synthesis of borophene remains one of the most technically challenging aspects of 2D materials research, primarily due to the absence of a naturally layered precursor and the complex bonding characteristics of boron. Current synthesis methods can be broadly categorized into bottom-up and top-down strategies. The former includes molecular beam epitaxy (MBE) and chemical vapor deposition (CVD), which build the structure atom-by-atom, whereas the latter relies on liquid-phase exfoliation from bulk precursors. Both approaches aim to achieve high-quality, large-area, and structurally controllable borophene suitable for device applications.
3.1. Bottom-Up Growth: Chemical Vapor Deposition (CVD)
The CVD method offers a scalable and controllable route to borophene synthesis but faces two intrinsic challenges: the lack of efficient boron precursors and the chemical instability of the resulting films under ambient conditions. Traditional physical vapor deposition approaches yield atomically thin films but fail to produce uniform large-area layers[
22], while early CVD routes suffered from inefficient boron source decomposition and uncontrolled layer thickness.
A significant breakthrough was reported by Cuxart
et al[
7]. (2021), who demonstrated a simple and controllable CVD process capable of producing high-quality monolayer borophene. In their approach, diborane (B
2H
6) served as the sole boron precursor in an ultrahigh-vacuum chamber. By exploiting the trace amounts of B
2H
6 naturally present in commercial borazine (B
3N
3H
6), a liquid-nitrogen cold trap was employed for repeated freeze–thaw purification, yielding a pure B
2H
6 gas stream. This reactive gas was then introduced into pre-treated single-crystal Ir(111) and Cu(111) substrates, whose atomically flat surfaces were prepared by sputtering–annealing cycles.
The substrate temperature played a crucial role: deposition on Ir(111) at ~1233 K and on Cu(111) at ~773 K enabled catalytic dehydrogenation of B2H6, producing highly mobile boron adatoms that self-assembled into a single-layer borophene sheet. The growth self-terminated after reaching approximately one monolayer, ensuring uniform film thickness. By adjusting the lattice matching between the borophene and the underlying metal, distinct structural phases could be selectively formed: χ6-borophene with wavy stripes on Ir(111), and χ3-borophene with rectangular unit cells on Cu(111). Both exhibited micrometer-scale single-crystal domains with low defect densities and epitaxial alignment along the <110> direction of the substrate.
A particularly elegant aspect of this work lies in the co-synthesis of borophene and h-BN heterostructures using the same precursor system. By sequentially toggling the cold trap, B3N3H6 and B2H6 could be introduced to grow either h-BN or borophene layers. For example, partial nucleation of h-BN islands followed by borophene growth yielded atomically sharp lateral heterojunctions, whereas reversing the sequence produced vertical h-BN/borophene stacks. In situ X-ray photoelectron spectroscopy confirmed that h-BN layers effectively encapsulate the underlying borophene, preventing oxidation. After exposure to 900 L of O2, the B 1s peak of h-BN-protected samples remained unchanged, while unprotected borophene displayed strong oxidation peaks (192–194 eV).
This integrated CVD process thus provides a scalable and flexible route for producing wafer-level borophene and its van der Waals heterostructures, avoiding the need for high-temperature solid boron evaporation required in MBE. The ability to control layer thickness, orientation, and interfacial chemistry marks a critical step toward the practical application of borophene-based electronic and energy devices.
3.2. Top-Down Exfoliation: Solvothermal-Assisted Liquid-Phase Exfoliation
Liquid-phase exfoliation (LPE) is a cost-effective and scalable approach widely used for producing 2D materials such as graphene and transition-metal dichalcogenides. However, the absence of a layered boron crystal and the strong covalent B–B bonds make borophene exfoliation particularly challenging. Conventional ultrasonication methods often result in fragmented, amorphous products with poor crystallinity and irregular thickness.
To overcome these limitations, Zhang
et al. [
8] (2020) developed a solvothermal-assisted liquid-phase exfoliation (S-LPE) method that significantly enhances exfoliation efficiency and flake quality. The process begins with high-energy ball milling of bulk boron powder in acetone, reducing the particle size and facilitating solvent penetration. The milled powder is then subjected to solvothermal treatment at 200 °C, during which acetone molecules intercalate between boron layers and weaken interatomic interactions. Subsequent probe ultrasonication provides mechanical shear forces that successfully delaminate the pre-swollen boron particles into few-layer borophene nanosheets.
Among various tested solvents, acetone proved optimal due to its surface tension and compatibility with boron, yielding borophene flakes with an average thickness of ~3.5 nm and lateral size up to 5 μm. Structural characterization confirmed that the crystallinity was largely preserved, although partial surface oxidation occurred due to air exposure.
The innovation of the S-LPE approach lies in the synergistic combination of solvothermal swelling and ultrasonic shear, which mitigates the fragmentation issues common in direct sonication and enables the production of large, uniform borophene nanosheets. This top-down method complements bottom-up CVD growth, offering a promising pathway for producing processable borophene dispersions suitable for ink-based fabrication, composite reinforcement, and biomedical nanodevices.
3.3. Summary of Synthesis Strategies
In summary, significant progress has been achieved in the synthesis of borophene over the past decade. MBE provides atomic-scale precision for fundamental studies, CVD enables scalable fabrication and heterostructure engineering, and liquid-phase exfoliation offers a low-cost route to dispersible nanosheets. Nevertheless, critical challenges remain — notably environmental instability, oxidation sensitivity, and interlayer aggregation. Future efforts should focus on the development of oxidation-resistant encapsulation methods, low-cost precursor chemistries, and machine-learning-assisted growth control, paving the way for industrial-scale production and application of borophene in next-generation electronic, energy, and biomedical systems.
4. Energy and Biomedical Applications of Borophene
With its outstanding electronic conductivity, high mechanical strength, rich surface chemistry, and tunable structural polymorphism, borophene has demonstrated remarkable potential across a wide range of technological fields. Recent advances highlight two particularly promising directions: biomedical applications (notably in cancer diagnosis and therapy) and energy storage systems (such as lithium-ion and sodium-ion batteries).
4.1. Borophene in Cancer Diagnosis and Therapy
Ji et al. [
6] have demonstrated the value of ultra-thin boron nanosheets (BNSs) prepared via a top-down approach (as shown in
Figure 7(a), (b), (c), which confirmed the synthesis of polyethylene glycol (PEG)-modified BNSs) in cancer therapy. These nanosheets not only serve as drug delivery carriers but also act as efficient photothermal converters (as shown in
Figure 7(d)) and multimodal imaging probes, enabling a closed-loop of diagnosis-treatment integration.
After PEG modification, the BNSs exhibit strong broadband absorption at 808 nm (as shown in
Figure 7(e)). When irradiated with near-infrared (NIR) laser for 5 minutes, the temperature of the solution increases significantly by 37 °C, with a high photothermal conversion efficiency of 42.5% (as shown in
Figure 7(f)). Moreover, five cycles of stability tests confirm their excellent stability, proving that they can serve as reliable photothermal therapeutic agents (as shown in
Figure 7(g)).
Meanwhile, this strong optical absorption property endows the material with excellent photoacoustic (PA) signals in vivo. At 24 hours after tail vein injection into mice, the PA intensity in the tumor region increases by more than four times, and the signal intensity shows a good linear relationship with the nanosheet concentration. This provides a precise navigation basis for real-time image-guided therapy.
In addition, boron-based nanosheets, as drug carriers, possess high loading capacity and controllable loading performance (as shown in
Figure 7(h), (i)). In terms of therapy, the high specific surface area of BNSs enables a saturated doxorubicin (DOX)-loading capacity of 114% (mass fraction) (as shown in
Figure 7(j)). Under the dual stimulation of an acidic environment and NIR laser, a drug release rate of 77.6% is achieved within 24 hours (as shown in
Figure 7(k)), which effectively utilizes the acidic and thermosensitive properties of the tumor microenvironment to realize controlled chemo-photothermal synergistic therapy at specific time points and specific spatial locations.
In the MCF-7 tumor-bearing mouse model experiment, the experimental group that received both DOX-loaded BNSs and NIR laser-induced photothermal therapy achieved complete tumor regression within 14 days without recurrence. The therapeutic effect was significantly better than that of the single therapy groups. Meanwhile, the body weight and serum biochemical indicators of the experimental animals remained normal, verifying the good biosafety of this therapeutic system.
In conclusion, the thermal oxidation etching-liquid phase exfoliation method can be used for large-scale preparation of BNSs with a thickness of approximately 3 nm. After PEG modification, a multifunctional nanoplatform integrating drug delivery, photothermal therapy, and multimodal imaging is successfully constructed. This provides a scalable technical route for the clinical translation of BNS-based diagnosis-treatment integration nanoplatforms.
4.2. Borophene for Energy Storage Applications
Beyond biomedicine, borophene has attracted extensive attention in the field of electrochemical energy storage due to its metallic conductivity, high carrier mobility, and strong interaction with alkali metal ions. These properties make it a highly promising anode material for next-generation lithium-ion (LIBs), sodium-ion (SIBs), and magnesium-ion batteries (MIBs), as well as for hydrogen storage and supercapacitors.
4.2.1. Lithium- and Sodium-Ion Batteries
First-principles calculations have shown that borophene possesses multiple favorable features for high-performance anodes. Its ultrahigh theoretical specific capacity (up to 1984 mAh·g⁻¹ for Li and 1240 mAh·g⁻¹ for Na[
23]) surpasses most known 2D materials, including graphene and phosphorene. The metallic nature of borophene ensures excellent electronic conductivity, while its open hexagonal vacancies provide abundant adsorption sites and short ion diffusion pathways.
For instance, Zhang et al. (2016) demonstrated that lithium (Li) and sodium (Na) atoms can stably adsorb on the surface of borophene, with binding energies of approximately −1.77 eV and −1.49 eV, respectively, without causing significant lattice distortion. The calculation results show that the diffusion barrier of Na is as low as 0.33 eV, and that of Li is about 0.66 eV[
23], indicating that the material possesses excellent ion transport performance. Furthermore, the flexible structure of borophene exhibits a volume change of less than 2.2% during the Li/Na intercalation process, which enables it to accommodate large ion fluxes without mechanical degradation. This not only achieves high energy density but also ensures excellent cycling stability.
4.2.2. Hydrogen Storage and Supercapacitors
Borophene, leveraging its lightweight nature and large specific surface area, emerges as an ideal candidate for hydrogen storage materials. Theoretical studies indicate that functionalization with lithium metal atoms significantly enhances H₂ adsorption capacity through polarization and charge transfer effects, achieving a specific capacity exceeding 13.7%[
24], which fully meets the U.S. Department of Energy (DOE) targets for onboard hydrogen storage systems.Furthermore, boronene combines high electrical conductivity with mechanical flexibility, making it suitable as an electrode material for flexible supercapacitors. Its high surface charge density facilitates rapid ion adsorption and desorption, resulting in exceptional power performance. The integration of boronene with conductive polymers or MXene-like materials can further improve capacitance retention and mechanical robustness over repeated charge-discharge cycles.
4.3. Summary and Outlook
In summary, borophene exhibits outstanding potential in both biomedical and energy-related applications due to its structural versatility and superior physical–chemical properties. Yet, practical deployment still faces substantial barriers, including air instability, large-scale synthesis difficulties, and incomplete understanding of its biotoxicity and degradation behavior. Addressing these challenges will require interdisciplinary collaboration that bridges materials science, computational modeling, chemistry, and biomedical engineering.
Future research directions should prioritize:
- (1)
development of oxidation-resistant and biocompatible functionalization methods;
- (2)
machine-learning-guided structural screening for stable borophene derivatives;
- (3)
integration into flexible electronic and energy systems;
- (4)
comprehensive toxicological and pharmacological studies for biomedical applications.
These advances will ultimately pave the way toward scalable, safe, and multifunctional borophene materials, bridging the gap between theoretical prediction and real-world technological implementation.
5. Conclusions and Future Perspectives
As an emerging member of the two-dimensional material family, borophene has captivated researchers with its extraordinary structural diversity, metallic conductivity, and unique mechanical and chemical properties. Over the past decade, remarkable progress has been made in both theoretical exploration and experimental synthesis, transforming borophene from a theoretical curiosity into a tangible and multifunctional nanomaterial.
From a structural viewpoint, borophene exhibits rich polymorphism governed by the periodic arrangement of hexagonal vacancies within a triangular lattice, leading to a continuum of phases with tunable electronic, mechanical, and optical characteristics. Such versatility enables borophene to surpass traditional 2D materials in various respects, including anisotropic elasticity, high carrier mobility, and multi-valent adsorption capability, thereby expanding its potential across multiple technological fields.
In terms of synthesis, significant strides have been achieved through molecular beam epitaxy (MBE), chemical vapor deposition (CVD), and solvothermal-assisted liquid-phase exfoliation (S-LPE). MBE has enabled atomic-scale control and phase identification, while CVD provides a scalable route for large-area borophene and van der Waals heterostructures. The development of solution-based exfoliation methods has further opened pathways for flexible and printable borophene-based composites. Nevertheless, the high synthesis cost, environmental instability, and limited structural control remain key obstacles hindering industrial translation.
In the realm of applications, borophene has exhibited great promise in both biomedicine and energy storage. Its high photothermal conversion efficiency and large boron content make it an ideal platform for cancer diagnosis and therapy, particularly in photothermal, photodynamic, and boron neutron capture treatments. Concurrently, its outstanding electronic conductivity and high theoretical specific capacity render it a leading candidate for lithium/sodium-ion batteries, hydrogen storage, and flexible supercapacitors. Despite these advances, comprehensive understanding of its long-term stability, biocompatibility, and interfacial behavior remains incomplete.
Looking ahead, several key directions are expected to guide future borophene research:
(i)Exploration of stable borophene derivatives — Through chemical functionalization, alloying, or surface passivation (e.g., BN, graphene, or polymer encapsulation), oxidation resistance and environmental stability can be substantially improved.
(ii)Integration with artificial intelligence and machine learning — AI-assisted in silico screening will accelerate the discovery of stable polymorphs and optimize synthesis conditions by correlating structural features with thermodynamic and kinetic stability.
(iii)Expansion of interdisciplinary applications — The combination of borophene with flexible electronics, biointerfaces, and quantum devices could unlock novel functionalities that extend beyond conventional materials.
(iv)Establishment of standardized characterization and safety protocols — Systematic evaluation of borophene’s physicochemical properties, cytotoxicity, and environmental impact will be essential for regulatory approval and biomedical deployment.
In conclusion, borophene stands at the forefront of next-generation 2D materials, bridging fundamental science and applied technology. Its unique combination of atomic-level tunability, outstanding electronic performance, and chemical versatility promises a transformative impact on energy, electronics, and biomedicine. With continuous advancements in synthesis, theoretical modeling, and interdisciplinary collaboration, borophene is poised to evolve from a laboratory material into a cornerstone of future nanotechnology.
References
- Tang H and Ismail-Beigi S 2007 Novel precursors for boron nanotubes: The competition of two-center and three-center bonding in boron sheets Phys. Rev. Lett. 99 115501.
- Li W, Kong L, Chen C, Gou J, Sheng S, Zhang W, Li H, Chen L, Cheng P and Wu K 2018 Experimental realization of honeycomb borophene Science Bulletin 63 282–6.
- Wang M, Yi W, Song H, Wu F, Fu Y, Liu X and Cui Z 2024 Build borophite from borophenes: A boron analogue graphite Nano Lett. 24 3448–55.
- Lin Q-L, Liang H, Zhou C-Q, Qian Z-F, Sun Y-L, Wang X-Y and Wang R-H 2022 Defect-induced magnetism in χ3 borophene Rare Met. 41 3486–94.
- 2021; 5. Mohebpour M A, Mozvashi S M, Vishkayi S I and Tagani M B 2021 Electronic and excitonic properties of semi-hydrogenated borophene sheets.
- Ji X, Kong N, Wang J, Li W, Xiao Y, Gan S T, Zhang Y, Li Y, Song X, Xiong Q, Shi S, Li Z, Tao W, Zhang H, Mei L and Shi J 2018 A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy Advanced Materials 30 1803031.
- Cuxart M G, Seufert K, Chesnyak V, Waqas W A, Robert A, Bocquet M-L, Duesberg G S, Sachdev H and Auwärter W 2021 Borophenes made easy Sci. Adv. 7 eabk1490. [CrossRef]
- Zhang F, She L, Jia C, He X, Li Q, Sun J, Lei Z and Liu Z-H 2020 Few-layer and large flake size borophene: Preparation with solvothermal-assisted liquid phase exfoliation RSC Adv. 10 27532–7. [CrossRef]
- Mannix A J, Zhou X-F, Kiraly B, Wood J D, Alducin D, Myers B D, Liu X, Fisher B L, Santiago U, Guest J R, Yacaman M J, Ponce A, Oganov A R, Hersam M C and Guisinger N P 2015 Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs Science 350 1513–6.
- Prasad D L V K, Balakrishnarajan M M and Jemmis E D 2005 Electronic structure and bonding of β -rhombohedral boron using cluster fragment approach Phys. Rev. B 72 195102.
- Šoškić B N, Bekaert J, Sevik C and Milošević M V 2024 Enhanced superconductivity of hydrogenated β12 borophene Nano Lett. 24 12650–7.
- Wang H, Li Q, Gao Y, Miao F, Zhou X-F and Wan X G 2016 Strain effects on borophene: ideal strength, negative possion’s ratio and phonon instability New J. Phys. 18 73016. [CrossRef]
- Liu X, Wang L, Li S, Rahn M S, Yakobson B I and Hersam M C 2019 Geometric imaging of borophene polymorphs with functionalized probes Nat Commun 10 1642.
- Tang H and Ismail-Beigi S 2010 First-principles study of boron sheets and nanotubes Phys. Rev. B 82 115412.
- Galeev T R, Dunnington B D, Schmidt J R and Boldyrev A I 2013 Solid state adaptive natural density partitioning: a tool for deciphering multi-center bonding in periodic systems Phys. Chem. Chem. Phys. 15 5022. [CrossRef]
- Zhao Y, Zeng S and Ni J 2016 Phonon-mediated superconductivity in borophenes Appl. Phys. Lett. 108 242601. [CrossRef]
- Zhu M-H, Weng X-J, Gao G, Dong S, Lin L-F, Wang W-H, Zhu Q, Oganov A R, Dong X, Tian Y, Zhou X-F and Wang H-T 2019 Magnetic borophenes from an evolutionary search Phys. Rev. B 99 205412.
- Zhang Y, Ju W, Li T and Li H 2020 Band engineering of borophene superlattice based on zigzag nanoribbons: a DFT study Mod. Phys. Lett. B 34 2050359. [CrossRef]
- 2022; 19. Tsujikawa Y, Shoji M, Hamada M, Takeda T, Mochizuki I, Hyodo T, Matsuda I and Takayama A 2022 Structure of χ3-borophene studied by total-reflection high-energy positron diffraction (TRHEPD) Molecules 27 4219.
- Li Q, Kolluru V S C, Rahn M S, Schwenker E, Li S, Hennig R G, Darancet P, Chan M K Y and Hersam M C 2021 Synthesis of borophane polymorphs through hydrogenation of borophene Science 371 1143–8. [CrossRef]
- Anju R S and Shiju N R 2025 On the 10th anniversary of borophene: Birth, growth and status quo Materials Today 88 393–414.
- Feng B, Zhang J, Zhong Q, Li W, Li S, Li H, Cheng P, Meng S, Chen L and Wu K 2016 Experimental realization of two-dimensional boron sheets Nature Chem 8 563–8.
- Zhang X, Hu J, Cheng Y, Yang H Y, Yao Y and Yang S A 2016 Borophene as an extremely high capacity electrode material for li-ion and na-ion batteries Nanoscale 8 15340–7.
- Li L, Zhang H and Cheng X 2017 The high hydrogen storage capacities of li-decorated borophene Comput. Mater. Sci. 137 119–24. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).