Submitted:
31 October 2025
Posted:
04 November 2025
You are already at the latest version
Abstract
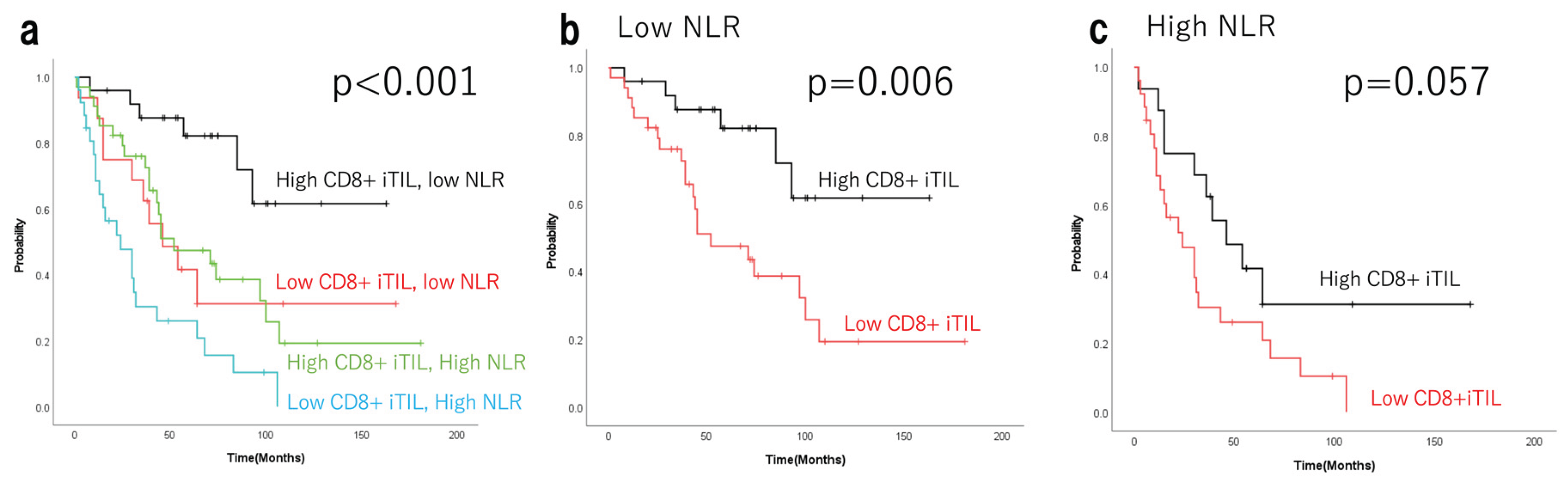
Background/Objectives: Tumour-infiltrating lymphocytes (TILs) significantly influence the prognosis of epithelial ovarian cancer (EOC). Advanced EOCs often cause neutrophilia, ascites, and malnutrition. The neutrophil-to-lymphocyte ratio (NLR) serves as a marker of systemic inflammation. This study investigated the prognostic significance of pre-treatment NLR and TILs in advanced EOCs. Methods: Overall, 101 advanced EOCs (stages III–IV, FIGO 2014) were treated between 2005 and 2020. Based on pathological findings, advanced EOCs were classified as having high or low TIL density using CD8 immunostaining. The number of marker-positive cells was counted using HALO. Progression-free survival and overall survival (OS) were compared between the high- and low-NLR groups based on CD8+ TIL levels. Results: Clinicopathological characteristics, including age, FIGO stage, histological subtype, and postoperative residual disease, did not significantly differ among the four groups defined by NLR and intra-epithelial CD8+ TILs (CD8+ iTILs). Multivariate Cox regression analysis of OS revealed that NLR and CD8+ iTILs were independent prognostic factors. The 5-year OS rates (Kaplan–Meier estimates) were 82.2% (median survival time not reached; range, 8–163 months) in the low NLR–high CD8+ iTIL group (n=25); 41.7% (46 months; range, 2–109 months) in the low NLR–low CD8+ iTIL group (n=16); 47.2% (52 months; range, 1–181 months) in the high NLR–high CD8+ iTIL group (n=34); and 26.0% (24 months, 2–106 months) in the high NLR–low CD8+ iTIL group (n=26) (p<0.001). Conclusions: In advanced EOCs, the status of tumour-localised immunity and pre-treatment systemic inflammation influenced long-term prognosis.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Immunohistochemistry
2.3. Digital Image Analysis
2.4. Statistical Analysis
2.5. Ethical Review and Participant Consent
3. Results
3.1. EOC Characteristics
3.2. Systemic Inflammatory Markers
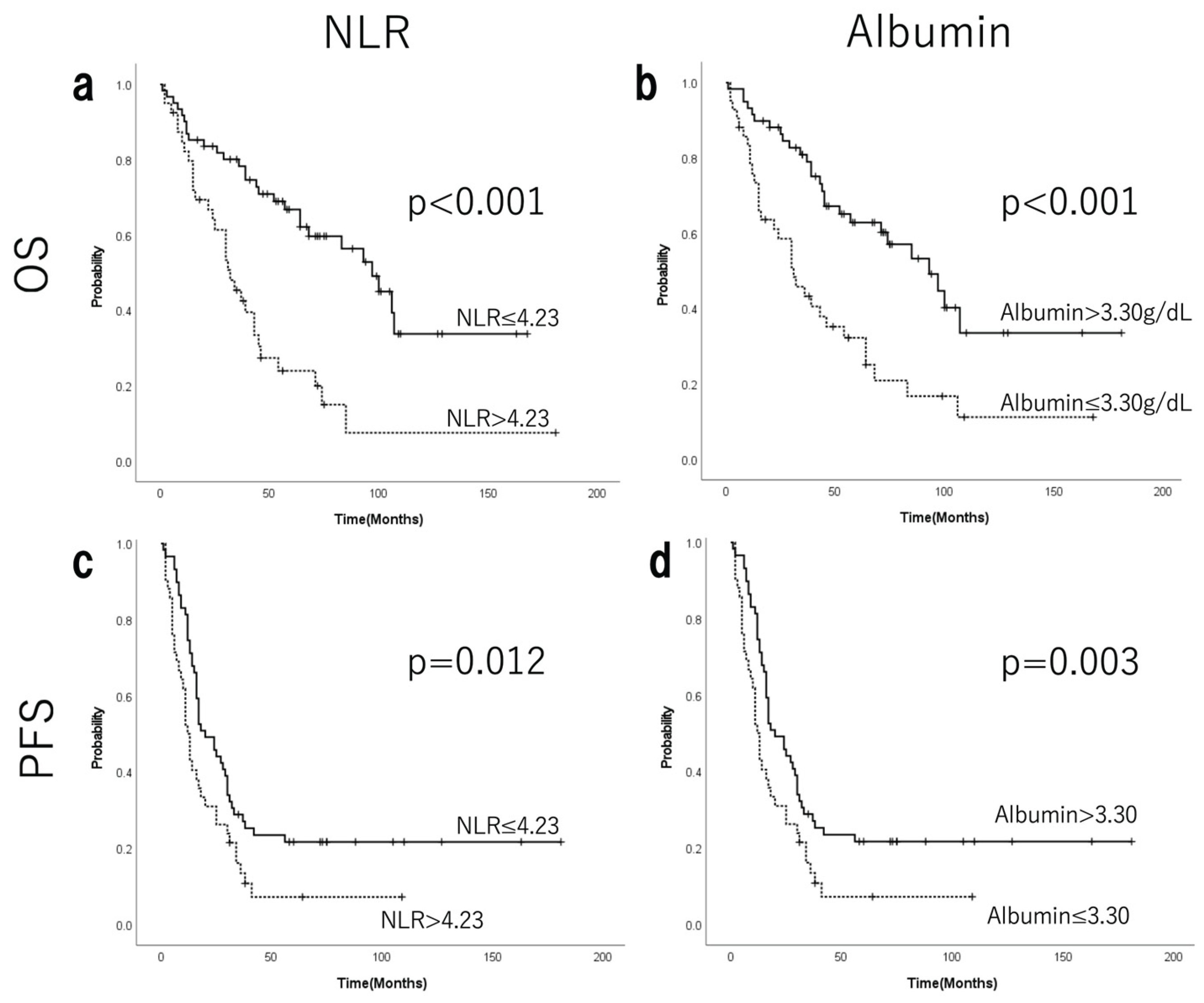
3.3. Prognostic Value of Systemic Inflammatory Markers
3.4. Density of TILs
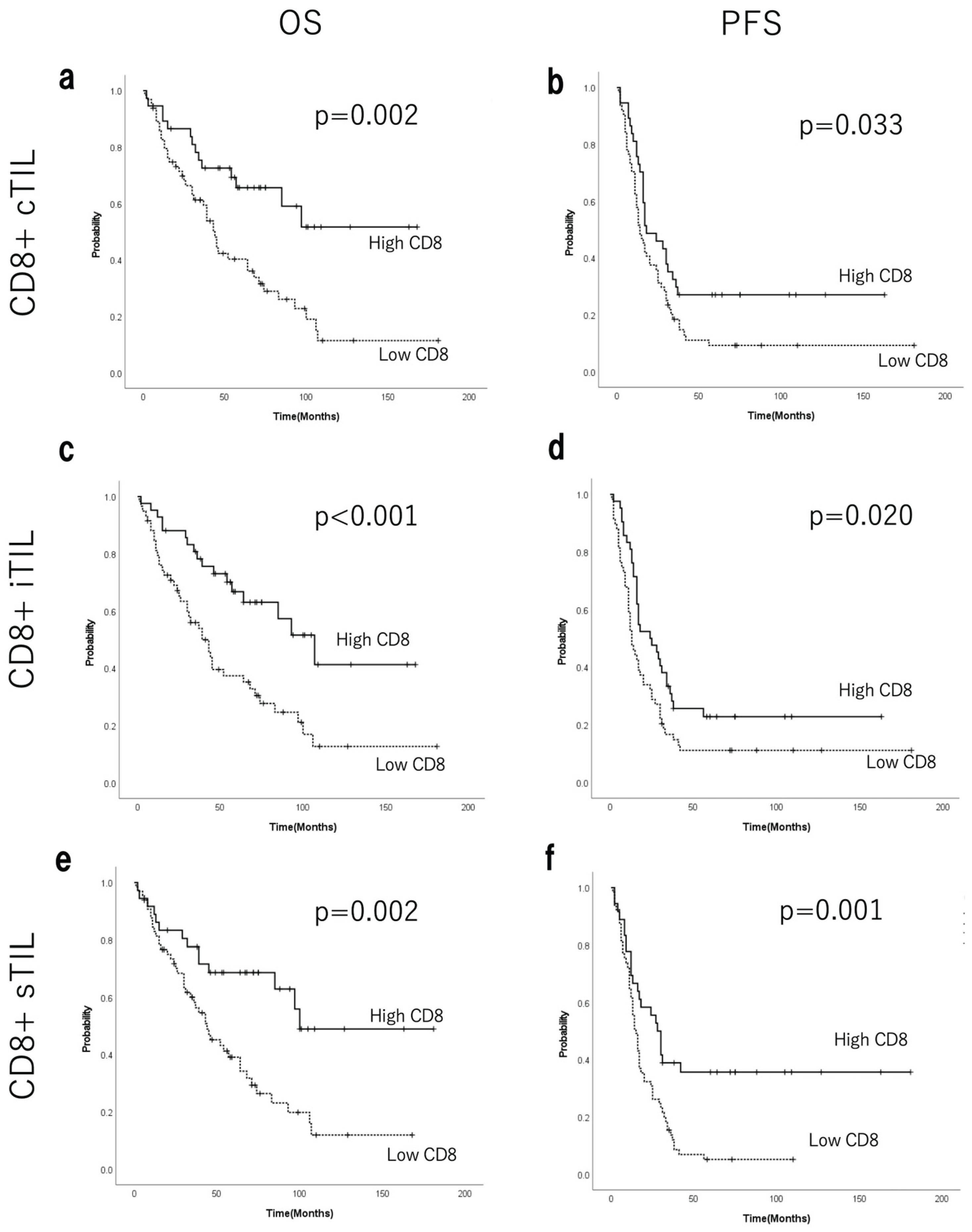
3.5. Prognostic Value of TILs
3.6. Univariate and Multivariate Survival Analyses for OS and PFS
3.7. Independence of Tils and NLR as Prognostic Factors
3.8. Evaluation of PD-L1 Expression (TPS)
3.9. Density of TILs and the Prognostic Value of TILs in HGSC and Non-HGSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TILs | Tumour-infiltrating lymphocytes |
| EOC | Epithelial ovarian cancer |
| NLR | Neutrophil-to-lymphocyte ratio |
| FIGO | International Federation of Gynaecology and Obstetrics |
| PFS | Progression-free survival |
| OS | Overall survival |
| HGSC | High-grade serous carcinoma |
| PDS | Primary debulking surgery |
| NACT | Neoadjuvant chemotherapy |
| IDS | Interval debulking surgery |
| PARPi | Poly (ADP-ribose) polymerase inhibitors |
| cTILs | Combined intraepithelial and stromal TILs |
| sTILs | Stromal TILs |
| iTILs | Intraepithelial TILs |
| TPS | Tumour proportion score |
| ROC | Receiver operating characteristic |
| ICIs | Immune checkpoint inhibitors |
| PD-L1 | Programmed death-ligand 1 |
References
- Kehoe, S. FIGO staging in ovarian carcinoma and histological subtypes. J. Gynecol. Oncol. 2020, 31, e70. [Google Scholar] [CrossRef]
- Smolarz, B.; Biernacka, K.; Lukasiewicz, H.; Samulak, D.; Piekarska, E.; Romanowicz, H.; Makowska, M. Ovarian Cancer-Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds. Int. J. Mol. Sci. 2025, 26, 4611. [Google Scholar] [CrossRef]
- Sellers, T.A.; Peres, L.C.; Hathaway, C.A.; Tworoger, S.S. Prevention of Epithelial Ovarian Cancer. Cold Spring Harb. Perspect. Med. 2023, 13, a038216. [Google Scholar] [CrossRef]
- Rajan, S.; Dhamija, E.; Malhotra, N.; Vats, D.; Singh, A.; Singhal, S. Cachexia in gynecologic cancers: The role of biomarkers and cachexia index. Int. J. Gynaecol. Obstet. 2025, ahead of print. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lang, J. The prognostic and clinical value of neutrophil-to-lymphocyte ratio (NLR) in ovarian cancer: A systematic review and meta-analysis. J. Med. Biochem. 2024, 43, 323–333. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Chap, B.S.; Rayroux, N.; Grimm, A.J.; Ghisoni, E.; Dangaj Laniti, D. Crosstalk of T cells within the ovarian cancer microenvironment. Trends Cancer 2024, 10, 1116–1130. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.P.; Balmaceda, C.; Bravo, M.L.; Kato, S.; Villarroel, A.; Owen, G.I.; Roa, J.C.; Cuello, M.A.; Ibanez, C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Mendiola, M.; Hernando, B.; Berjon, A.; Cadiz, A.; Chaves-Urbano, B.; Heredia-Soto, V.; Spagnolo, E.; Hernandez Gutierrez, A.; Hardisson, D.; et al. Prognostic markers of inflammation in endometrioid and clear cell ovarian cancer. Int. J. Gynecol. Cancer 2022, 32, 1009–1016. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; Gonzalez-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Gonzalez-Martin, A.; Lorusso, D.; Gourley, C.; Mirza, M.R.; Kurtz, J.E.; Okamoto, A.; Moore, K.; Kridelka, F.; McNeish, I.; et al. Clinical research in ovarian cancer: Consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022, 23, e374–e384. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef]
- Honda, M.; Kawamura, H.; Kobayashi, H.; Takiguchi, K.; Muto, A.; Yamazaki, S.; Teranishi, Y.; Shiraso, S.; Kono, K.; Hori, S.; et al. An ascites grading system for predicting the prognosis of gastric cancer with peritoneum dissemination. Ann. Gastroenterol. Surg. 2020, 4, 660–666. [Google Scholar] [CrossRef]
- Xu, M.; Liu, D.; Wang, L.; Sun, S.; Liu, S.; Zhou, Z. Clinical implications of CT-detected ascites in gastric cancer: Association with peritoneal metastasis and systemic inflammatory response. Insights Imaging 2024, 15, 237. [Google Scholar] [CrossRef]
- Harada, H.; Hachisuga, T.; Harada, Y.; Shibahara, M.; Murakami, M.; Nuratdinova, F.; Higami, S.; Tohyama, A.; Kinjo, Y.; Ueda, T.; et al. Intra-Tumoral Lymphocytic Infiltration Is Associated with Favorable Prognosis in Suboptimal Surgery in High-Grade Serous Ovarian Carcinoma. Diagnostics 2025, 15, 422. [Google Scholar] [CrossRef]
- Collet, L.; Ardin, M.; Venet, D.; Berthet, J.; Ghamry-Barrin, S.; Treilleux, I.; Noel, J.C.; Leheurteur, M.; Meunier, J.; Bengrine Lefevre, L.; et al. Unraveling the Tumor Microenvironment and PD-L1 Expression across Tissue Types in High-Grade Serous Ovarian Cancer in the NeoPembrOV/GINECO Phase II Randomized Trial. Clin. Cancer Res. 2025, 31, 3317–3331. [Google Scholar] [CrossRef]
- Nakamura, Y.; Saldajeno, D.P.; Kawaguchi, K.; Kawaoka, S. Progressive, multi-organ, and multi-layered nature of cancer cachexia. Cancer Sci. 2024, 115, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Winata, I.G.S.; Pradnyana, I.; Yusrika, M.U.; Pradnyaandara, I.; Pradnyadevi, P.A.S. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as an Early Prognostic Marker in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2024, 25, 1921–1927. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- John-Olabode, S.O.; Okunade, K.S.; Olorunfemi, G.; Soibi-Harry, A.; Rimi, G.; Osunwusi, B.; Okunowo, A.; Amaeshi, L.; Anorlu, R. Pretreatment Neutrophil-to-Lymphocyte Ratio: A Prognostic Biomarker of Survival in Patients With Epithelial Ovarian Cancer. Cureus 2021, 13, e16429. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, I.; Chung, Y.S.; Nam, E.; Kim, S.; Kim, S.W.; Kim, Y.T.; Lee, J.Y. Pretreatment neutrophil-to-lymphocyte ratio and its dynamic change during neoadjuvant chemotherapy as poor prognostic factors in advanced ovarian cancer. Obstet. Gynecol. Sci. 2018, 61, 227–234. [Google Scholar] [CrossRef]
- Liontos, M.; Andrikopoulou, A.; Koutsoukos, K.; Markellos, C.; Skafida, E.; Fiste, O.; Kaparelou, M.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. Neutrophil-to-lymphocyte ratio and chemotherapy response score as prognostic markers in ovarian cancer patients treated with neoadjuvant chemotherapy. J. Ovarian Res. 2021, 14, 148. [Google Scholar] [CrossRef]
- Yanazume, S.; Kitazono, I.; Togami, S.; Tanimoto, A.; Kobayashi, H. Potential biomarkers for predicting the efficacy of a pembrolizumab-containing regimen in advanced cervical cancer: A real-world analysis. Turk. J. Obstet. Gynecol. 2025, 22, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Nonatelli, F.; Villanueva, H.; Barainka, M.; Zheleva, A.; van Santen, H.M.; Pastor, F. Modulating T Cell Responses by Targeting CD3. Cancers 2023, 15, 1189. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Hudry, D.; Le Guellec, S.; Meignan, S.; Becourt, S.; Pasquesoone, C.; El Hajj, H.; Martinez-Gomez, C.; Leblanc, E.; Narducci, F.; Ladoire, S. Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints. Cancers 2022, 14, 5332. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Chen, H.; Molberg, K.; Strickland, A.L.; Castrillon, D.H.; Carrick, K.; Jiang, Q.; Niu, S.; Rivera-Colon, G.; Gwin, K.; Hinson, S.; et al. PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Different Types of Tubo-ovarian Carcinoma and Their Prognostic Value in High-grade Serous Carcinoma. Am. J. Surg. Pathol. 2020, 44, 1050–1060. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.W.; Nam, K.H.; Han, S.H.; Kim, J.W.; Ahn, S.H.; Park, D.J.; Lee, K.W.; Lee, H.S.; Kim, H.H. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 2017, 20, 602–611. [Google Scholar] [CrossRef]
- Gawinski, C.; Michalski, W.; Mroz, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Tumor-Infiltrating Lymphocytes (TILs) in Left-Sided Colorectal Cancer Patients. Biology 2022, 11, 385. [Google Scholar] [CrossRef]
- Giro, A.; Passildas-Jahanmohan, J.; Kossai, M.; Bidet, Y.; Molnar, I.; Bernadach, M.; Penault-Llorca, F.; Abrial, C.; Durando, X.; Radosevic-Robin, N. Comparison of the Predictive and Prognostic Capacities of Neutrophil, Lymphocyte and Platelet Counts and Tumor-infiltrating Lymphocytes in Triple-negative Breast Cancer: Preliminary Results of the PERCEPTION Study. Anticancer. Res. 2024, 44, 4983–4994. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Suzawa, K.; Hashimoto, K.; Tanaka, S.; Shien, K.; Miyoshi, K.; Yamamoto, H.; Okazaki, M.; Sugimoto, S.; Toyooka, S. Utility of neutrophil-to-lymphocyte ratio as an indicator of tumor immune status in non-small cell lung cancer. Jpn. J. Clin. Oncol. 2024, 54, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R.; et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Kourilovitch, M.; Galarza-Maldonado, C. Could a simple biomarker as neutrophil-to-lymphocyte ratio reflect complex processes orchestrated by neutrophils? J. Transl. Autoimmun. 2023, 6, 100159. [Google Scholar] [CrossRef]
- Li, G.P.; Zhang, D.; Li, M.H.; Yuan, F.F.; Hou, X.J.; He, D.J.; Wei, X.D.; Fu, Y.W. Association between the neutrophil-to-lymphocyte ratio and cancer in adults from NHANES 2005-2018: A cross-sectional study. Sci. Rep. 2024, 14, 23678. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Hamada, K.; Murakami, R.; Ueda, A.; Kashima, Y.; Miyagawa, C.; Taki, M.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Minamiguchi, S.; et al. A Deep Learning-Based Assessment Pipeline for Intraepithelial and Stromal Tumor-Infiltrating Lymphocytes in High-Grade Serous Ovarian Carcinoma. Am. J. Pathol. 2024, 194, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef]
- Rimel, B.J.; Pujade-Lauraine, E.; Moore, K.; Pfisterer, J.; Han, S.; Cibula, D.; Reyners, A.; Redondo, A.; Papadimitriou, C.; Eitan, R.; et al. Phase 3 Clinical Trials Evaluating Poly(ADP-Ribose) Polymerase Inhibition Plus Immunotherapy for First-Line Treatment of Advanced Ovarian Cancer. Oncologist 2025, 30, oyaf270. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Cvek, J.; Randall, L.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 404, 1321–1332. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- You, B.; Purdy, C.; Copeland, L.J.; Swisher, E.M.; Bookman, M.A.; Fleming, G.; Coleman, R.; Randall, L.M.; Tewari, K.S.; Monk, B.J.; et al. Identification of Patients With Ovarian Cancer Experiencing the Highest Benefit From Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study. J. Clin. Oncol. 2022, 40, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Darb-Esfahani, S.; Kunze, C.A.; Kulbe, H.; Sehouli, J.; Wienert, S.; Lindner, J.; Budczies, J.; Bockmayr, M.; Dietel, M.; Denkert, C.; et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016, 7, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016, 141, 293–302. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Lee, Y.J.; Kim, S.H.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 414–421. [Google Scholar] [CrossRef] [PubMed]
| N=101 | N(%) | |
|---|---|---|
| Age(years) | < 50 | 23(22.8%) |
| ≥ 50 | 78(77.2%) | |
| Stage | III | 72(72.3%) |
| IV | 29(28.7%) | |
| Lymph node metastasis | Yes | 29(28.7%) |
| No | 29(28.7%) | |
| Unknown | 43(42.6%) | |
| Chemotherapy | Adjuvant | 60(59.4%) |
| Neo-adjuvant | 29(28.7%) | |
| First-line | 12(11.9%) | |
| Residual disease | Complete(R=0) | 20(19.8%) |
| Optimal(≤1cm) | 38(37.6%) | |
| Suboptimal(>1cm) | 31(30.7%) | |
| Exploratory laparotomy | 12(11.9%) | |
| Histological subtype | HGSC | 70 (69.3%) |
| Clear cell | 19(18.8%) | |
| Endometrioid | 9(8.9%) | |
| Mucinous | 3(3.0%) | |
| NLR | ≤ 4.23 | 59(58.4%) |
| > 4.23 | 42(41.6%) | |
| Albumin | ≤ 3.30 g/dL | 40(39.6%) |
| > 3.30 g/dL | 61(60.3%) | |
| Ascites | Grade1 | 21(20.8%) |
| Grade2 | 34(33.7%) | |
| Grade3 | 46(45.5%) | |
| Recurrence | Yes | 84(83.2%) |
| No | 17(16.8%) | |
| Prognosis | NED+AWD | 42(41.6%) |
| DOD | 59(58.4%) |
| Clinicopathological Association with NLR | NLR≤4.23 (N=59) |
NLR>4.23 (N=42) |
p-Value | |
|---|---|---|---|---|
| Age | <50 | 13 | 10 | 0.834 |
| ≥ 50 | 46 | 32 | ||
| Stage | III | 46 | 26 | 0.079 |
| IV | 13 | 16 | ||
| Histological subtype | HGSC | 44 | 26 | 0.355 |
| Clear cell | 10 | 9 | ||
| Endometrioid | 3 | 6 | ||
| Mucinous | 2 | 1 | ||
| Lymph node metastasis | Yes | 21 | 8 | 0.192 |
| No | 15 | 14 | ||
| Chemotherapy | Adjuvant | 40 | 20 | 0.076 |
| Neo-adjuvant | 12 | 17 | ||
| First-line | 7 | 5 | ||
| Residual disease | Complete(R=0) | 13 | 7 | 0.913 |
| Optimal(≤1cm) | 22 | 16 | ||
| Suboptimal(>1cm) | 17 | 14 | ||
| Exploratory laparotomy | 7 | 5 | ||
| Albumin | ≤ 3.30 g/dL | 45 | 16 | 0.095 |
| > 3.30 g/dL | 14 | 26 | ||
| Ascites | Grade1 | 17 | 4 | <0.001* |
| Grade2 | 25 | 9 | ||
| Grade3 | 17 | 29 | ||
| Clinicopathological Association with eCD8+ TIL | Low CD8+ iTIL | High CD8+ iTIL | p-Value | |
|---|---|---|---|---|
| Age | <50 | 14 | 9 | 0.81 |
| ≥50 | 45 | 33 | ||
| Stage | III | 42 | 30 | 1 |
| IV | 17 | 12 | ||
| Histological subtype | HGSC | 35 | 35 | 0.041* |
| Clear cell | 16 | 3 | ||
| Endometrioid | 5 | 4 | ||
| Mucinous | 3 | 0 | ||
| Lymph node metastasis | Yes | 17 | 12 | 0.43 |
| No | 13 | 16 | ||
| Chemotherapy | Adjuvant | 39 | 21 | 0.024* |
| Neo-adjuvant | 11 | 18 | ||
| Initial | 9 | 3 | ||
| Residual disease | Complete(R=0) | 10 | 10 | 0.245 |
| Optimal(≤1cm) | 19 | 19 | ||
| Suboptimal(>1cm) | 21 | 10 | ||
| Exploratory laparotomy | 9 | 3 | ||
| NLR | ≤ 4.23 | 33 | 26 | 0.68 |
| > 4.23 | 26 | 16 | ||
| Albumin | ≤ 3.30 g/dL | 32 | 29 | 0.097 |
| > 3.30 g/dL | 27 | 13 | ||
| Ascites | Grade1 | 9 | 12 | 0.13 |
| Grade2 | 24 | 10 | ||
| Grade3 | 26 | 20 | ||
| OS | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | ||
| Age | <50 vs. 50≤ | 1.03(0.5545-1.912) | 0.92 | ||
| Stage | III vs. IV | 1.474(0.8629-2.517) | 0.15 | ||
| Lymph node metastasis | Yes vs. No | 0.7439(0.3573-1.549) | 0.42 | ||
| Chemotherapy | Adjuvant vs. neo-adjuvant + initial | 0.9788(0.5536-1.731) | 0.94 | ||
| Residual disease | Complete + optimal vs. suboptimal + exploratory laparotomy | 1.765(1.056-2.952) | 0.03* | 1.430(0.823-2.486) | 0.205 |
| Albumin | >3.3 vs. 3.3≥ | 3.051(1.792-5.196) | <0.001* | 2.183(1.166-4.085) | 0.015* |
| NLR | ≤4.23 vs. 4.23< | 2.612(1.557-4.383) | <0.001* | 2.056(1.142-3.702) | 0.016* |
| Ascites | Grade1+2 vs. Grade3 | 2.423(1.425-4.12) | 0.001* | 1.297(0.681-2.471) | 0.429 |
| CD8+ cTILs | High vs. low | 0.3944(0.2159-0.7202) | 0.002* | 1.360(0.538-3.440) | 0.516 |
| CD8+ iTILs | High vs. low | 0.3915(0.2222-0.6897) | 0.001* | 0.430(0.199-0.933) | 0.033* |
| CD8+ sTILs | High vs. low | 0.3895(0.2125-0.7139) | 0.002* | 0.599(0.279-1.283) | 0.187 |
| CD4+ cTILs | High vs. low | 0.3355(0.1915-0.5877) | <0.001* | 0.592(0.313-1.121) | 0.108 |
| CD4+ iTILs | High vs. low | 0.6187(0.3662-1.045) | 0.072† | ||
| CD4+ sTILs | High vs. low | 0.6163(0.3605-1.054) | 0.076† | ||
| TPS | ≥1% vs. <1% | 0.9279(0.4912-1.753) | 0.81 | ||
| PFS | Univariate analysis | Multivariate analysis | |||
| HR(95%CI) | p-Value | HR(95%CI) | p-Value | ||
| Age | <50 vs. 50≤ | 1.03(0.5545-1.912) | 0.29 | ||
| Stage | III vs. IV | 1.474 (0.8629-2.517) | 0.09† | ||
| Lymph node metastasis | Yes vs. No | 0.7439(0.3573-1.549) | 0.57 | ||
| Chemotherapy | Adjuvant vs. neo-adjuvant + initial | 0.9788(0.5536-1.731) | 0.69 | ||
| Residual disease | Complete + optimal vs. suboptimal + exploratory laparotomy | 1.765(1.056-2.952) | 0.002* | 1.712(1.061-2.763) | 0.028* |
| Albumin | >3.3 vs. 3.3≤ | 3.051(1.792-5.196) | 0.003* | 1.400(0.836-2.346) | 0.201 |
| NLR | ≤4.23 vs. 4.23< | 2.612(1.557-4.383) | 0.015* | 1.432(0.863-2.378) | 0.165 |
| Ascites | Grade1+2 vs. Grade3 | 2.423(1.425-4.12) | 0.008* | 1.160(0.671-2.007) | 0.594 |
| CD8+ cTILs | High vs. low | 0.3944(0.2159-0.7202) | 0.038* | 1.493(0.714-3.124) | 0.287 |
| CD8+ iTILs | High vs. low | 0.3915(0.2222- 0.6897) | 0.024* | 0.549(0.282-1.068) | 0.077† |
| CD8+ sTILs | High vs. low | 0.3895(0.2125-0.7139) | 0.001* | 0.592(0.330-1.060) | 0.078† |
| CD4+ cTILs | High vs. low | 0.3355(0.1915-0.5877) | 0.002* | 0.838(0.452-1.554) | 0.575 |
| CD4+ iTILs | High vs. low | 0.6187(0.3662-1.045) | 0.52 | ||
| CD4+ sTILs | High vs. low | 0.6163(0.3605-1.054) | 0.007* | 0.726(0.406-1.296) | 0.297 |
| TPS | ≥1% vs. <1% | 0.9279(0.4912- 1.753) | 0.59 |
| NLR <4.23 | NLR ≥4.23 | |||||
|---|---|---|---|---|---|---|
| N=101 | High CD8+iTIL (N=25) |
Low CD8+iTIL (N=16) |
High CD8+iTIL (N=34) |
Low CD8+iTIL (N=26) |
p-Value | |
| Age | <50 | 5 | 4 | 8 | 6 | 0.983 |
| ≥ 50 | 20 | 12 | 26 | 20 | ||
| Stage | III | 18 | 11 | 28 | 15 | 0.218 |
| IV | 7 | 5 | 6 | 11 | ||
| Histological subtype | HGSC | 22 | 12 | 24 | 16 | 0.553 |
| Clear cell | 2 | 1 | 7 | 5 | ||
| Endometrioid | 1 | 2 | 2 | 4 | ||
| Mucinous | 0 | 1 | 1 | 1 | ||
| Lymph node metastasis | Yes | 7 | 8 | 8 | 6 | 0.267 |
| No | 9 | 3 | 12 | 5 | ||
| Unknown | 9 | 5 | 14 | 15 | ||
| Chemotherapy | Adjuvant | 16 | 4 | 24 | 16 | 0.001* |
| Neo-adjuvant | 6 | 12 | 6 | 5 | ||
| Initial | 3 | 0 | 4 | 5 | ||
| Residual disease | Complete(R=0) | 5 | 5 | 8 | 2 | 0.407 |
| Optimal(≤1cm) | 10 | 8 | 12 | 8 | ||
| Suboptimal(>1cm) | 7 | 3 | 10 | 11 | ||
| Exploratory laparotomy | 3 | 0 | 4 | 5 | ||
| Albumin | ≤ 3.30 g/dL | 4 | 9 | 10 | 17 | <0.001* |
| > 3.30 g/dL | 21 | 7 | 24 | 9 | ||
| Ascites | Grade1 | 11 | 1 | 6 | 3 | <0.001* |
| Grade2 | 8 | 2 | 17 | 7 | ||
| Grade3 | 6 | 13 | 11 | 16 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





