Submitted:
29 October 2025
Posted:
29 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
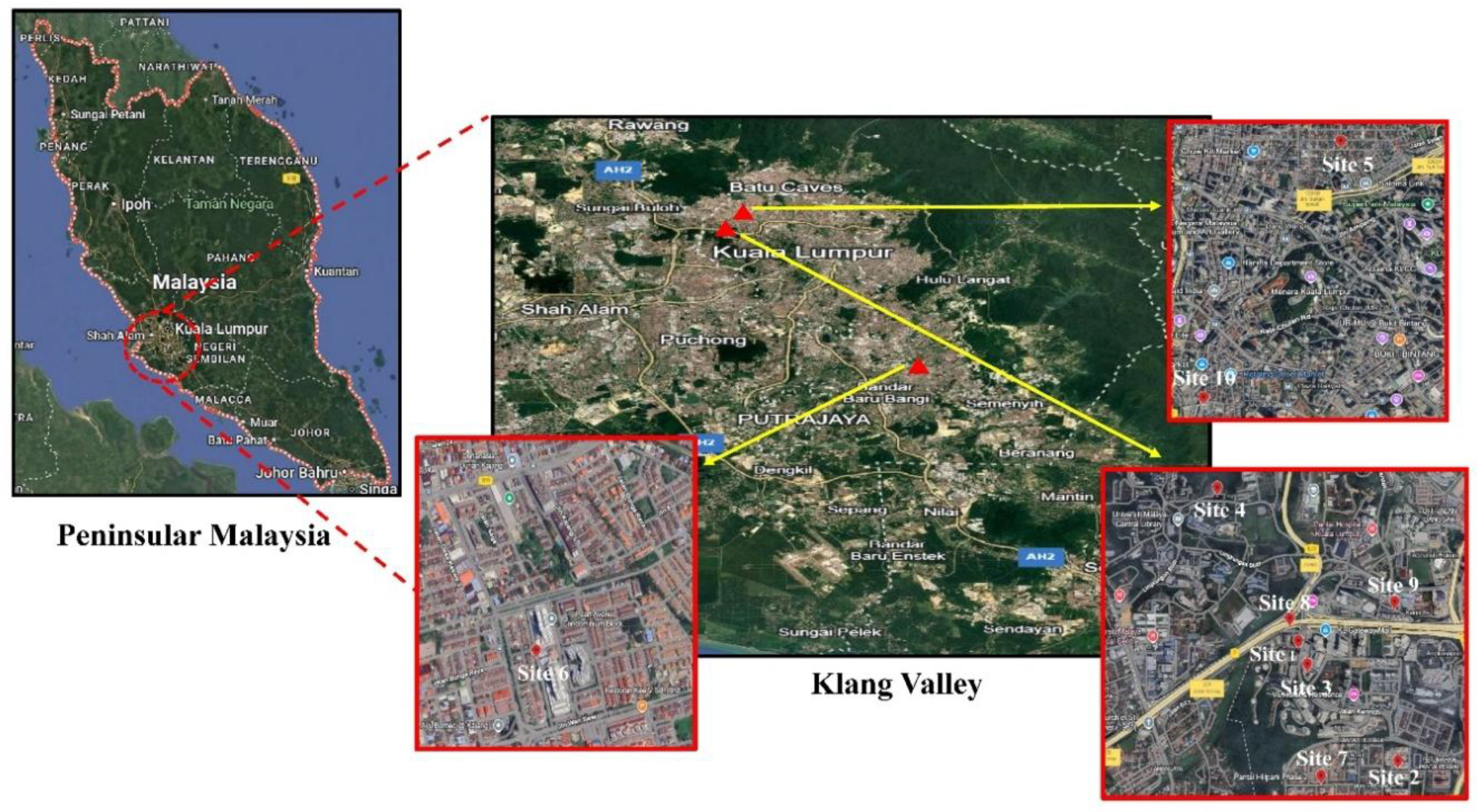
2.1. Study Area and Flea Collection
2.2. Study Area and Flea Collection
2.3. Molecular Identification of Fleas
2.4. Detection of Bartonella spp. By PCR
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Flea Collection and Prevalence
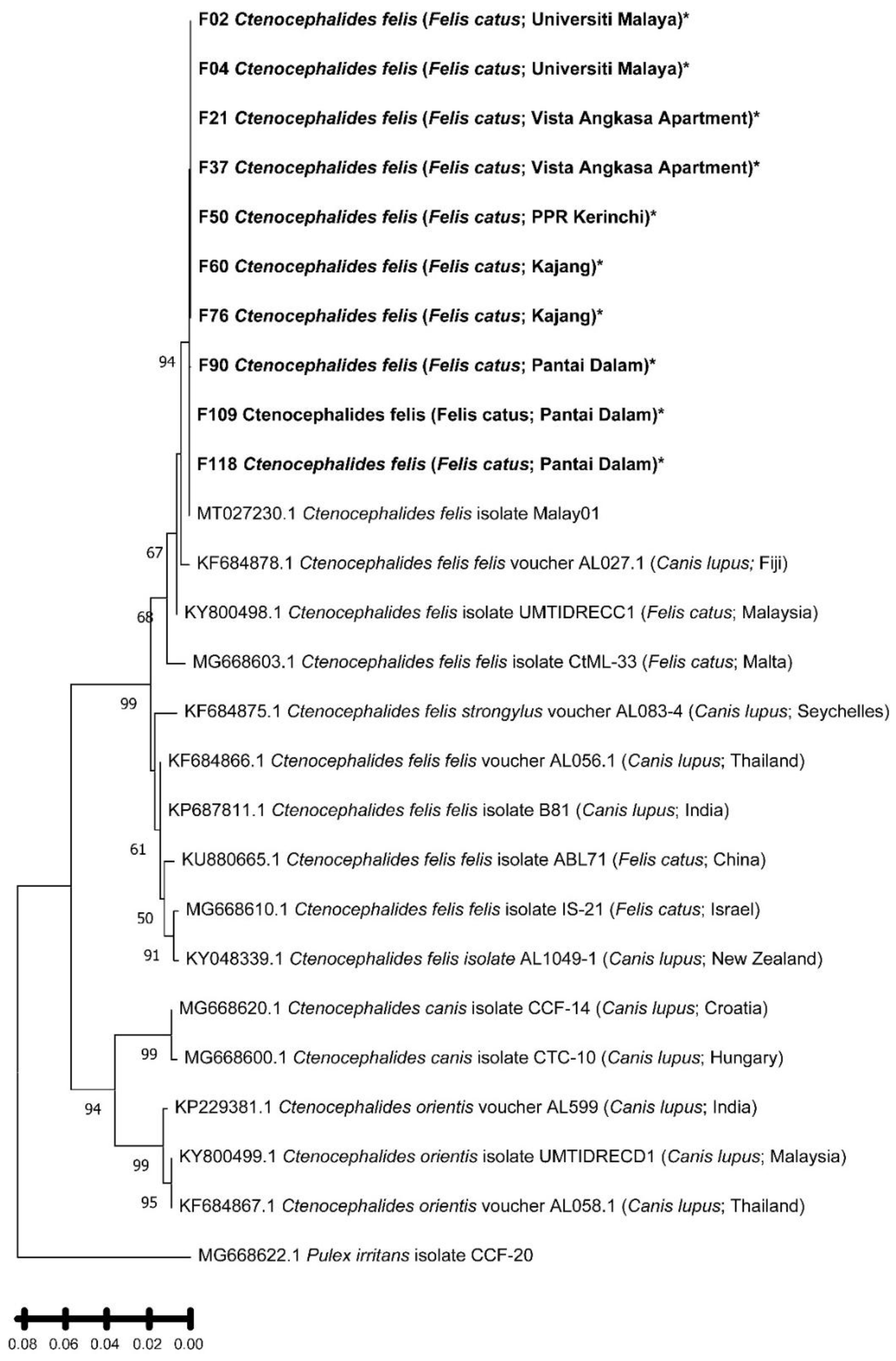
3.2. Flea Identification
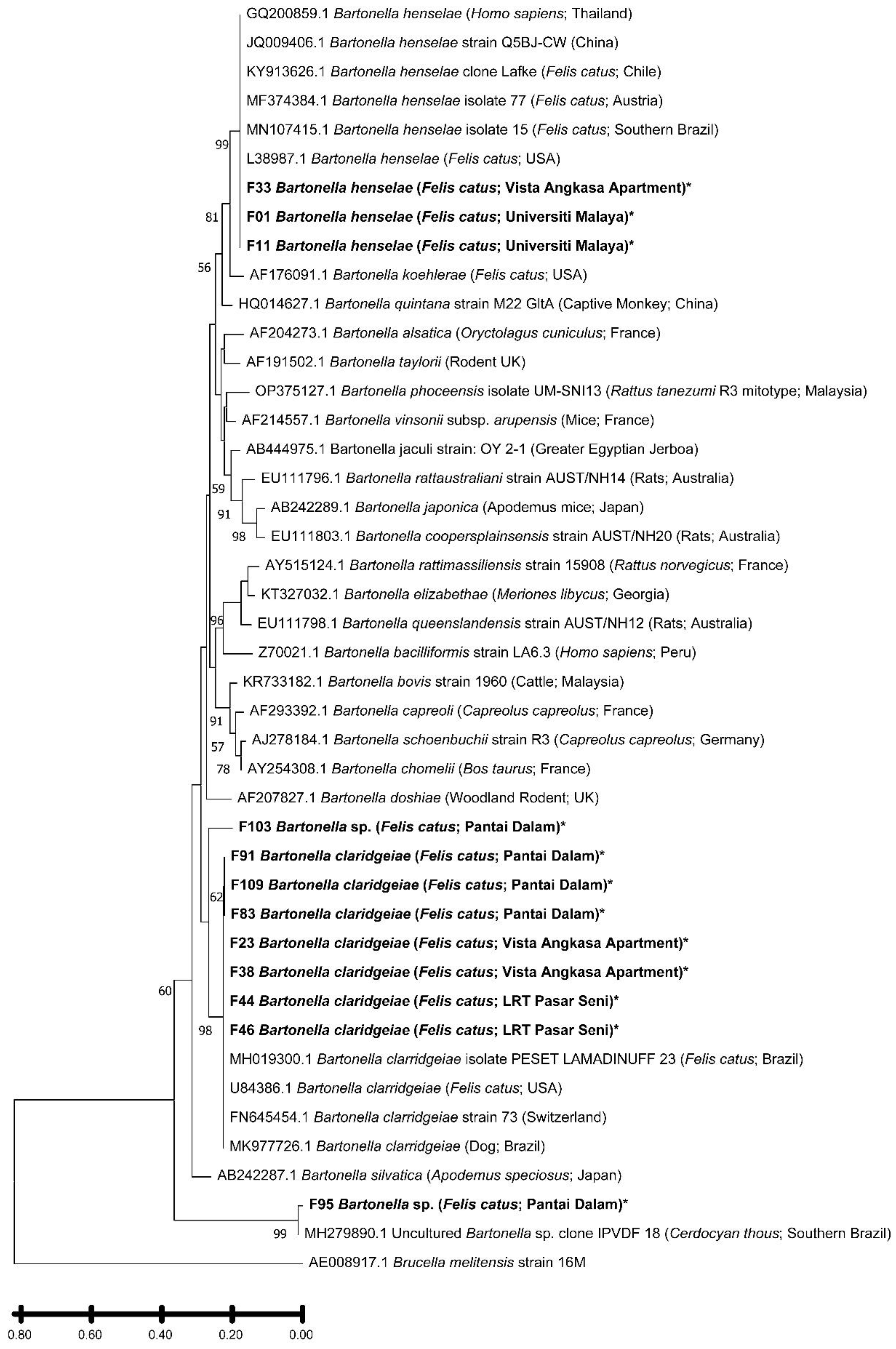
3.3. Molecular Detection of Bartonella spp.
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centonze, L. A.; Levy, J. K. Characteristics of Free-Roaming Cats and Their Caretakers. J. Am. Vet. Med. Assoc. 2002, 220, 1627–1633. [Google Scholar] [CrossRef]
- Durden, L. A.; Hinkle, N. C. Chapter 10 - Fleas (Siphonaptera). ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/B9780128140437000108.
- Linardi, P. M.; Santos, J. L. C. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): Some Issues in Correctly Identify These Species. Rev. Bras. Parasitol. Vet. 2012, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Low, V. L.; Prakash, B. K.; Tan, T. K.; Sofian-Azirun, M.; Anwar, F. H. K.; Vinnie-Siow, W. Y.; AbuBakar, S. Pathogens in Ectoparasites from Free-Ranging Animals: Infection with Rickettsia asembonensis in Ticks, and a Potentially New Species of Dipylidium in Fleas and Lice. Vet. Parasitol. 2017, 245, 102–105. [Google Scholar] [CrossRef]
- Goldstein, E. J.; Abrahamian, F. M. Diseases Transmitted by Cats. ASM Press ebook. 2016, 133–150. [Google Scholar] [CrossRef]
- Mokhtar, A. S.; Tay, S. T. Molecular Detection of Rickettsia felis, Bartonella henselae, and B. clarridgeiae in Fleas from Domestic Dogs and Cats in Malaysia. Am. J. Trop. Med. Hyg. 2011, 85(5), 931–933. [Google Scholar] [CrossRef]
- Tay, S. T.; Mokhtar, A. S.; Low, K. C.; Mohd Zain, S. N.; Jeffery, J.; Abdul Aziz, N.; Kho, K. L. Identification of Rickettsiae from Wild Rats and Cat Fleas in Malaysia. Med. Vet. Entomol. 2014, 28, 104–108. [Google Scholar] [CrossRef]
- Che Kamaruddin, N.; Adrus, M.; Wan Ismail, W. N. Prevalence of Ectoparasites on a Stray Cat Population from “Town of Knowledge” Kota Samarahan, Sarawak, Malaysian Borneo. Turk. J. Vet. Anim. Sci. 2020, 44(6), 1212–1221. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M. B.; Hoeh, W. R.; Lutz, R. A.; Vrijenhoek, R. C. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. PubMed 1994, 3, 294–299. [Google Scholar]
- Norman, A.; Regnery, R. L.; Jameson, P. H.; Greene, C. E.; Krause, D. C. Differentiation of Bartonella-like Isolates at the Species Level by PCR-Restriction Fragment Length Polymorphism in the Citrate Synthase Gene. J. Clin. Microbiol. 1995, 33, 1797–1803. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, Y.; Li, L.-Y.; Chen, S.-Y.; Liu, G.-H.; Liu, W. Population Genetics and Genetic Variation of Ctenocephalides felis and Pulex irritans in China by Analysis of Nuclear and Mitochondrial Genes. Parasites Vectors 2022, 15. [Google Scholar] [CrossRef]
- Frank, H. K.; Boyd, S. D.; Hadly, E. A. Global Fingerprint of Humans on the Distribution of Bartonella Bacteria in Mammals. PLoS Negl. Trop. Dis. 2018, 12(11), e0006865–e0006865. [Google Scholar] [CrossRef]
- Krasnov, B.; Khokhlova, I.; Shenbrot, G. The Effect of Host Density on Ectoparasite Distributions: An Example of a Rodent Parasitized by Fleas. Ecol. 2002, 83, 164–175. [Google Scholar] [CrossRef]
- Hii, S. F.; Kopp, S. R.; Abdad, M. Y.; Thompson, M. F.; O’Leary, C. A.; Rees, R. L.; Traub, R. J. Molecular Evidence Supports the Role of Dogs as Potential Reservoirs for Rickettsia felis. Vector-Borne Zoonotic Dis. 2011, 11, 1007–1012. [Google Scholar] [CrossRef]
- Scola, B. L.; Zeaiter, Z.; Khamis, A.; Raoult, D. Gene-Sequence-Based Criteria for Species Definition in Bacteriology: The Bartonella Paradigm. Trends Microbiol. 2003, 11, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Azrizal-Wahid, N.; Sofian-Azirun, M.; Low, V. L. Risk Factors Associated with Flea Infestation on Cats. Trop. Biomed. 2019, 36, 810–821. [Google Scholar] [PubMed]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Pathogens in Fleas Collected from Cats and Dogs: Distribution and Prevalence in the UK. Parasites Vectors 2019, 12. [Google Scholar] [CrossRef]
- Cantó, G. J.; Guerrero, R. I.; Olvera-Ramírez, A. M.; Milián, F.; Mosqueda, J.; Aguilar-Tipacamú, G. Prevalence of Fleas and Gastrointestinal Parasites in Free-Roaming Cats in Central Mexico. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- S. Jittapalapong; A. Sangvaranond; T. Inpankaew; N. Pinyopanuwat; W. Chimnoi; C. Kengradomkij; S. Saengow; S. Wongnakphet. Ectoparasites of Stray Cats in Bangkok Metropolitan Areas, Thailand. Agric. Nat. Res. 2008, 42, 463–470. [Google Scholar]
- ElSeify, M.; Aggour, M.; Sultan, K.; Marey, N. Ectoparasites in Stray Cats in Alexandria Province, Egypt: A Survey Study. Alex. J. Vet. Sci. 2016, 48. [Google Scholar] [CrossRef]
- Nasser Hajipour; Mojtaba Keighobadi; Minas, A. ; Abad, R.; Mostafa Golabi; Badali, A. Prevalence of Flea Infestation in Stray Cats in North West of Iran, Iran. Biol. Forum. 2015, 7, 575–580. [Google Scholar]
- Kreppel, K. S.; Telfer, S.; Rajerison, M.; Morse, A.; Baylis, M. Effect of Temperature and Relative Humidity on the Development Times and Survival of Synopsyllus fonquerniei and Xenopsylla cheopis, the Flea Vectors of Plague in Madagascar. Parasites Vectors 2016, 9. [Google Scholar] [CrossRef]
- Salant, H.; Mumcuoglu, K. Y.; Baneth, G. Ectoparasites in Urban Stray Cats in Jerusalem, Israel: Differences in Infestation Patterns of Fleas, Ticks and Permanent Ectoparasites. Med. Vet. Entomol. 2013, 28, 314–318. [Google Scholar] [CrossRef]
- Farrell, S.; McGarry, J.; Noble, P. M.; Pinchbeck, G. J.; Cantwell, S.; Radford, A. D.; Singleton, D. A. Seasonality and Other Risk Factors for Fleas Infestations in Domestic Dogs and Cats. Med. Vet. Entomol. 2023, 37. [Google Scholar] [CrossRef]
- Mohd Zain, S. N.; Sahimin, N.; Pal, P.; Lewis, J. W. Macroparasite Communities in Stray Cat Populations from Urban Cities in Peninsular Malaysia. Vet. Parasitol. [CrossRef]
- Xhaxhiu, D.; Kusi, I.; Rapti, D.; Visser, M.; Knaus, M.; Lindner, T.; Rehbein, S. Ectoparasites of Dogs and Cats in Albania. Parasitology Res. J. 2009, 105, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Zouari, S.; Khrouf, F.; M’ghirbi, Y.; Bouattour, A. First Molecular Detection and Characterization of Zoonotic Bartonella Species in Fleas Infesting Domestic Animals in Tunisia. Parasites Vectors. [CrossRef]
- Jamal; Rabah, S. ; Yacoub, H.; Hajrah, N. H.; Atef, A.; Al-Matary, M.; Edris, S.; Alharbi, M. G.; Magdah Ganash; Jazem Mahyoub; Al-Hindi, R. R.; Al-Ghamdi, K. M.; Hall, N.; Bahieldin, A.; Kamli, M. R.; Rather, I. A. Molecular Evolution of Cytochrome c Oxidase-I Protein of Insects Living in Saudi Arabia. PLOS ONE 2019, 14, e0224336–e0224336. [Google Scholar] [CrossRef]
- Azrizal-Wahid, N.; Sofian-Azirun, M.; Low, V. L. Flea-Borne Pathogens in the Cat Flea Ctenocephalides Felis and Their Association with MtDNA Diversity of the Flea Host. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101621. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.; Franc, M.; Davoust, B.; Didier Raoult. Molecular Detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in Cat Fleas, France. Emerg. Infect. Dis. 2003, 9, 338–342. [Google Scholar] [CrossRef]
- Lappin, M. R.; Griffin, B.; Brunt, J. D.; Riley, A.; Burney, D. P.; Hawley, J. R.; Brewer, M.; Jensen, W. Prevalence of Bartonella Species, Haemoplasma Species, Ehrlichia Species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the Blood of Cats and Their Fleas in the United States. J. Feline. Med. Surg. 2006, 8, 85–90. [Google Scholar] [CrossRef]
- Nasereddin, A.; Risheq, A.; Harrus, S.; Azmi, K.; Ereqat, S.; Baneth, G.; Salant, H.; Mumcuoglu, K. Y.; Abdeen, Z. Bartonella Species in Fleas from Palestinian Territories: Prevalence and Genetic Diversity. J. Vector. Ecol. 2014, 39, 261–270. [Google Scholar] [CrossRef]
- Persichetti, M.-F.; Solano-Gallego, L.; Serrano, L.; Altet, L.; Reale, S.; Masucci, M.; Pennisi, M.-G. Detection of Vector-Borne Pathogens in Cats and Their Ectoparasites in Southern Italy. Parasites Vectors 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Saengsawang, P.; Kaewmongkol, G.; Phoosangwalthong, P.; Chimnoi, W.; Inpankaew, T. Detection of Zoonotic Bartonella Species in Ticks and Fleas Parasitizing Free-Ranging Cats and Dogs Residing in Temples of Bangkok, Thailand. Vet. Parasitol. Reg. Stud. Reports. 2021, 25, 100612. [Google Scholar] [CrossRef] [PubMed]
- Schott, D.; Souza, U. A.; B. Dall'Agnol; Webster, A.; Doyle, R.; Peters, F.; M. Favarini; F. Mazim; Rosa, A. O.; Jardim, A.; Trigo, T. C.; Reck, J. Detection of Rickettsia spp. And Bartonella spp. In Ctenocephalides felis Fleas from Free-Ranging Crab-Eating Foxes (Cerdocyon thous). Med. Vet. Entomol. 2019, 33, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Weinborn-Astudillo, R. M.; Pau, N.; Tobar, B. Z.; Jaffe, D. A.; Boulouis, H.-J.; Sepúlveda, P.; Müller, A.; Chomel, B. B. Bartonella Infection in Stray Dogs from Central and Southern Chile (Linares and Puerto Montt). Vector-Borne Zoonotic Dis. 2020, 20, 187–192. [Google Scholar] [CrossRef]
- WHO. WHO Results Report 2023 Shows Notable Health Achievements and Calls for Concerted Drive toward Sustainable Development Goals. www.who.int. https://www.who.int/news/item/07-05-2024-who-results-report-2023-shows-notable-health-achievements-and-calls-for-concerted-drive-toward-sustainable-development-goals.
- WOAH. Vector-borne diseases surveillance: a global health imperative, /: World Organisation for Animal Health. https.
- Cruz, T. N. D. A. O.; Gonçalves, L. R.; Eduarda, M.; Marcos Rogério André; Alexandre Diaz Munhoz; Santiago, R. ; Fabiana Lessa Silva. Threat under Cats’ Claws: Molecular Detection and Risk Factors for Zoonotic Bartonella Species in Blood and Claw Samples from Cats in Brazil. Acta. Trop. 2022, 232, 106496–106496. [Google Scholar] [CrossRef]
- Deak, G.; Safarov, A.; Xie, X. C.; Wang, R.; Mihalca, A. D.; Šlapeta, J. Fleas from the Silk Road in Central Asia: Identification of Ctenocephalides canis and Ctenocephalides orientis on Owned Dogs in Uzbekistan Using Molecular Identification and Geometric Morphometrics. Parasites Vectors 2022, 15. [Google Scholar] [CrossRef]
| Gene | Primer Name | Sequence (5′- 3′) | Direction | Detection | Reference |
| cox1 | LCO | GGT CAA CAA ATC ATA AAG ATA TTG G | Forward | Flea species | [9] |
| Cff-R | GAA GGG TCA AAG AAT GAT GT | Reverse | |||
| gltA | BhCS.781p | GGG GAC CAG CTC ATG GTG G | Forward | Bartonella | [10] |
| BhCS.1137n | AAT GCA AAA AGA ACA GTA AAC A | Reverse |
| Gene | Initial Denaturation | Denaturation | Annealing | Extension | Final Extension | Cycle | Amplicon size (bp) | Reference |
| cox1 | 95 °C 1 min |
95 °C 15 s |
55 °C 15 s |
72 °C 10 s |
72 °C 5 min |
35 | 550 | [9] |
| gltA | 94 °C 10 min |
94 °C 30 s |
51 °C 45 s |
72 °C 30 s |
72 °C 7 min |
35 | 379 | [10] |
| Location type | Location Code | Location Name | Number of cats examined | Number of cats infested with fleas (Prevalence) | Total fleas collected |
| Housing Area | Site 1 | Vista Angkasa Apartment | 14 | 7 (50.0) | 53 |
| Site 2 | PPR Kerinchi | 7 | 1 (14.2) | 3 | |
| Site 3 | Pangsapuri 17 Tingkat Kerinchi | 4 | 2 (50.0) | 3 | |
| Eatery Area | Site 4 | Universiti Malaya | 26 | 6 (23.1) | 17 |
| Site 5 | Kampung Baru | 7 | 0 (0.0) | 0 | |
| Site 6 | Kajang | 5 | 3 (60.0) | 25 | |
| Site 7 | Pantai Dalam | 21 | 11 (52.4) | 94 | |
| Public Transportation Area | Site 8 | LRT Universiti Station | 2 | 2 (100.0) | 2 |
| Site 9 | LRT Kerinchi Station | 1 | 1 (100.0) | 3 | |
| Site 10 | LRT Pasar Seni Station | 2 | 2 (100.0) | 4 | |
| Total | 89 | 35 | 204 |
| Risk Factor | Variable | Flea-infested cat | Total fleas | Prevalence of Infestation (%) |
| Area | Housing area (n = 25) | 10 | 59 | 40.0 |
| Eatery area (n = 59) | 20 | 136 | 33.9 | |
| Public transportation area (n = 5) | 5 | 9 | 100.0 | |
| Gender | Male (n = 20) | 8 | 35 | 40.0 |
| Female (n = 69) | 27 | 169 | 39.0 | |
| Age | Adult (n = 84) | 33 | 200 | 39.0 |
| Juvenile (n = 5) | 2 | 4 | 40.0 |
| Flea in this study | Location | Closest GenBank match | Maximum Identity (%) | Query cover (%) | E-value | Origin |
| F02 | University Malaya | C. felis isolate Malay01 (MT027230.1) | 99.83 | 99 | 0.0 | East and Southeast Asia |
| F04 | University Malaya | C. felis isolate Malay01 (MT027230.1) | 97.83 | 98 | 0.0 | East and Southeast Asia |
| F21 | Vista Angkasa Apartment | C. felis isolate Malay01 (MT027230.1) | 100.00 | 98 | 0.0 | East and Southeast Asia |
| F37 | Vista Angkasa Apartment | C. felis isolate Malay01 (MT027230.1) | 100.00 | 98 | 0.0 | East and Southeast Asia |
| F50 | PPR Kerinchi | C. felis isolate Malay01 (MT027230.1) | 100.00 | 100 | 0.0 | East and Southeast Asia |
| F60 | Kajang | C. felis isolate Malay01 (MT027230.1) | 100.00 | 100 | 0.0 | East and Southeast Asia |
| F76 | Kajang | C. felis isolate Malay01 (MT027230.1) | 100.00 | 98 | 0.0 | East and Southeast Asia |
| F90 | Pantai Dalam | C. felis isolate Malay01 (MT027230.1) | 100.00 | 100 | 0.0 | East and Southeast Asia |
| F109 | Pantai Dalam | C. felis isolate Malay01 (MT027230.1) | 100.00 | 100 | 0.0 | East and Southeast Asia |
| F118 | Pantai Dalam | C. felis isolate Malay01 (MT027230.1) | 99.83 | 98 | 0.0 | East and Southeast Asia |
| Sample ID | Location | Closest GenBank match | Maximum Identity (%) | Query cover (%) | E-value | Origin |
| F01 | University Malaya | B. henselae isolate 15 (MN107415.1) | 99.70 | 100 | 5e-172 | Brazil |
| F11 | University Malaya | B. henselae isolate 15 (MN107415.1) | 99.70 | 100 | 5e-172 | Brazil |
| F23 | Vista Angkasa Apartment | B. claridgeiae isolate PESET LAMADINUFF 23 (MH019300.1) | 99.41 | 100 | 2e-170 | Brazil |
| F33 | Vista Angkasa Apartment | B. henselae isolate 15 (MN107415.1) | 98.53 | 100 | 2e-166 | Brazil |
| F38 | Vista Angkasa Apartment | B. claridgeiae isolate PESET LAMADINUFF 23 (MH019300.1) | 99.41 | 100 | 2e-170 | Brazil |
| F44 | LRT Pasar Seni | B. claridgeiae isolate PESET LAMADINUFF 25 (MH019301.1) | 99.69 | 96 | 6e-166 | Brazil |
| F46 | LRT Pasar Seni | B. claridgeiae isolate PESET LAMADINUFF 23 (MH019300.1) | 99.70 | 100 | 2e-171 | Brazil |
| F83 | Pantai Dalam | B. claridgeiae isolate PESET LAMADINUFF 25 (MH019301.1) | 99.11 | 100 | 1e-167 | Brazil |
| F91 | Pantai Dalam | B. claridgeiae isolate PESET LAMADINUFF 25 (MH019301.1) | 98.24 | 100 | 1e-163 | Brazil |
| F95 | Pantai Dalam | Uncultured Bartonella sp. clone IPVDF_18 (MH279890.1) | 98.48 | 98 | 2e-160 | Brazil |
| F103 | Pantai Dalam | Bartonella sp. isolate Dog_9 (MN233800.1) | 92.51 | 99 | 4e-128 | Chile |
| F109 | Pantai Dalam | B. claridgeiae isolate PESET LAMADINUFF 23 (MH019300.1) | 99.41 | 100 | 8e-170 | Brazil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





