Submitted:
26 October 2025
Posted:
29 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods11/5/2025
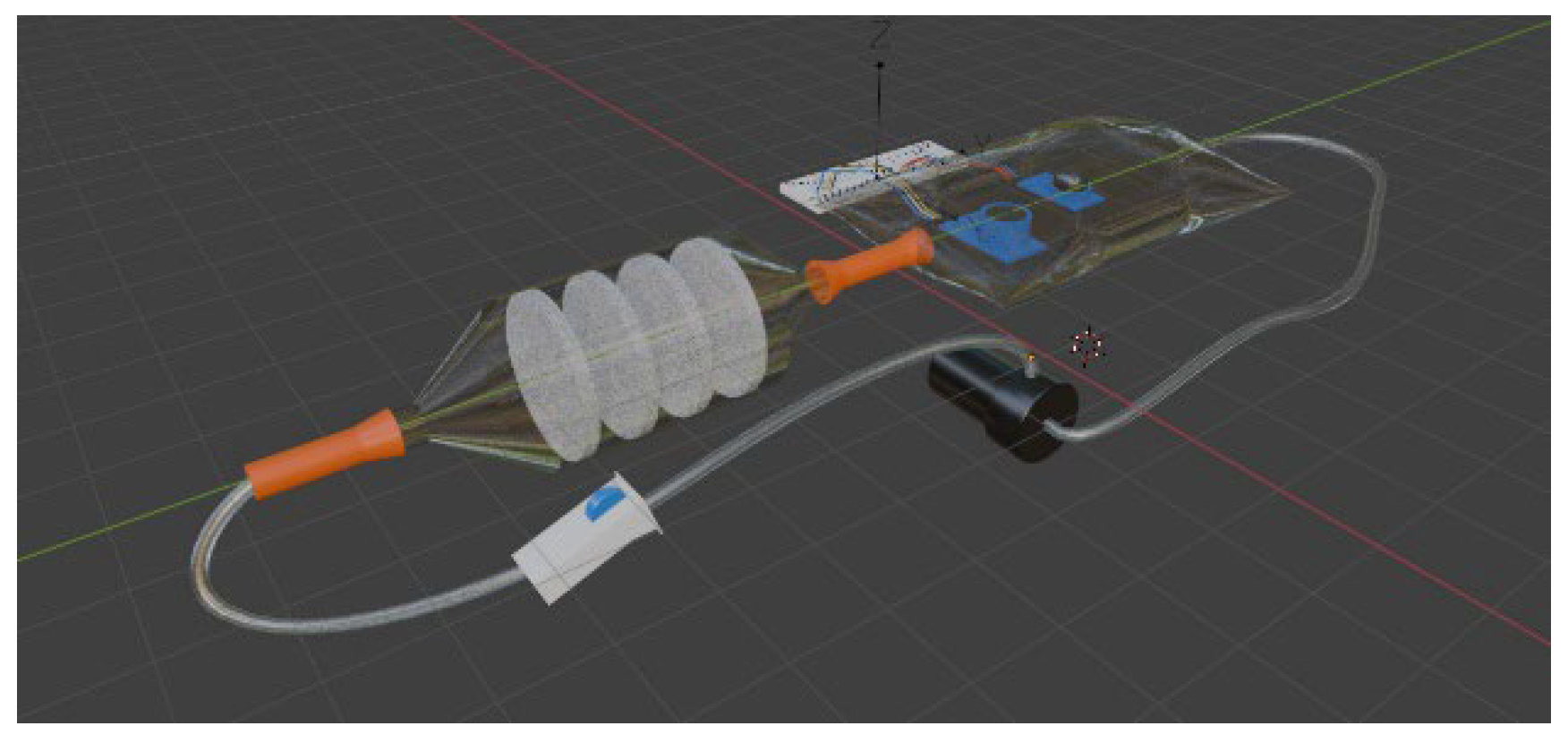
Materials
| Item | Quantity | Description | Picture |
|---|---|---|---|
| 1500 ml Plastic bottle. | 1 | A container for the filter stages. |  |
| IV infusion set | 1 | A set of tubes and valves for medical purposes. It is used as a transportation system for the air. |  |
| 2000 ml IV fluid bag | 1 | A bag that contains fluids for medical purposes. It is used as a container for the contaminated air. |  |
| Recycled plastic fibers | 2 sheets | Recycled plastic fibers from packaging. It is used as a particulate air filter that blocks particulate matter. |  |
| Recycled glass fibers | 1 sheet | Glass fibers that are recycled from conditioning systems. It is used to carry a TiO2 coating. |  |
| Titanium dioxide | 1 Kg | It is used as a photocatalyst to remove volatile organic compounds and criteria pollutants. |  |
| Recycled coal | 7 pieces | The remains of used burnt coal. It is used to clear acid combustion gases. |  |
| Recycled cotton | 4 pieces | Cotton recycled from first aid kit. It is used as a prefilter stage, to block large particles. |  |
| Arduino UNO | 1 | It is used to manage the sensors and the fan. |  |
| Air pump | 2 | It is used to pump air and regulate airflow in the system. |  |
Methods
Data Analysing
- The adsorption of activated carbon was tested by using 1.92 grams on 100 ml of 0.1 N potassium iodide solution, filtering the solution from the carbon, and titrating it with 0.1 N sodium thiosulphate to calculate the amount of iodine adsorbed. The amount of sodium thiosulphate used to titrate the solution was estimated. The titration was done depending on the color of the potassium iodide solution as an indicator.
- As shown in Figure 5, the efficiency of the filter was tested by passing an air sample from the combustion of paper that contained CO, VOCs, and particulate matter through the filter and using the values given by the sensors to calculate the efficiency.
3. Results
- The amount of sodium thiosulphate used to titrate the solution was 39.5 ± 0.1 ml, after plugging it in the equation the iodine number equals 354 ± 0.2 mg/g, by substituting in Equation 1 & 2:
- 2.
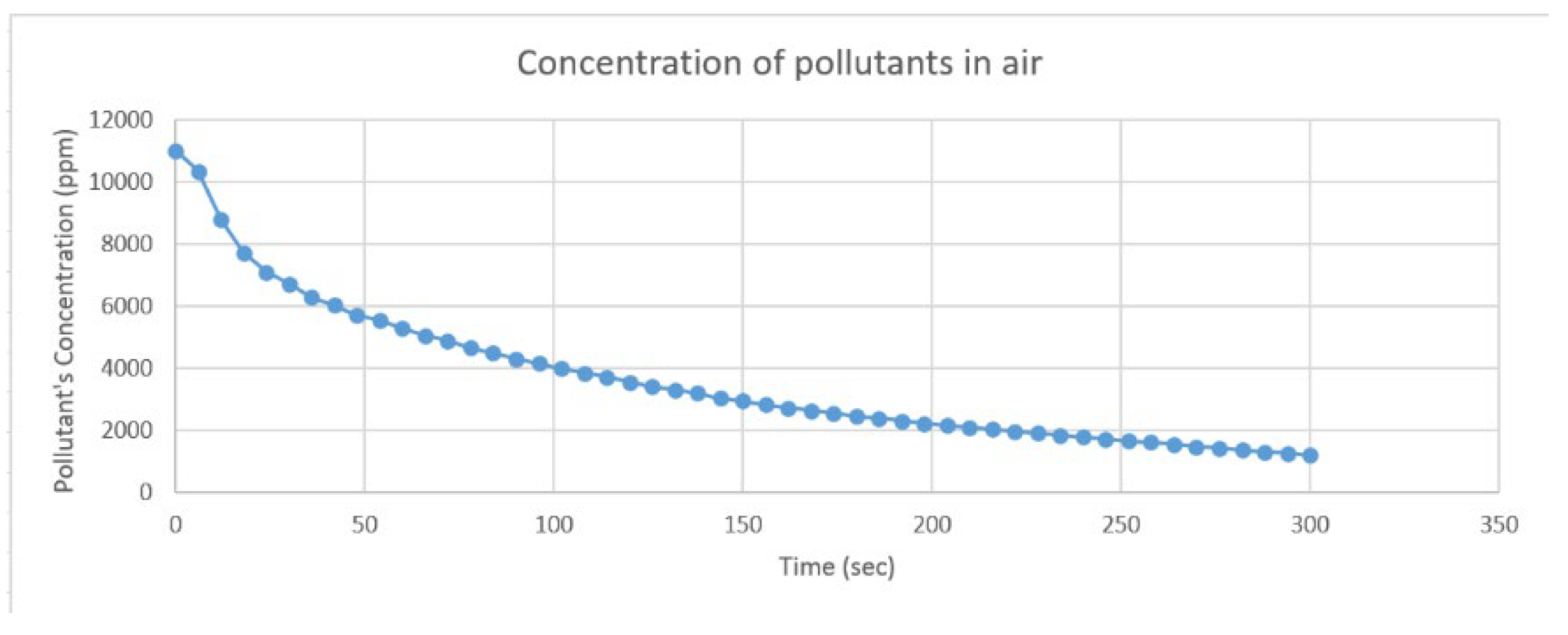
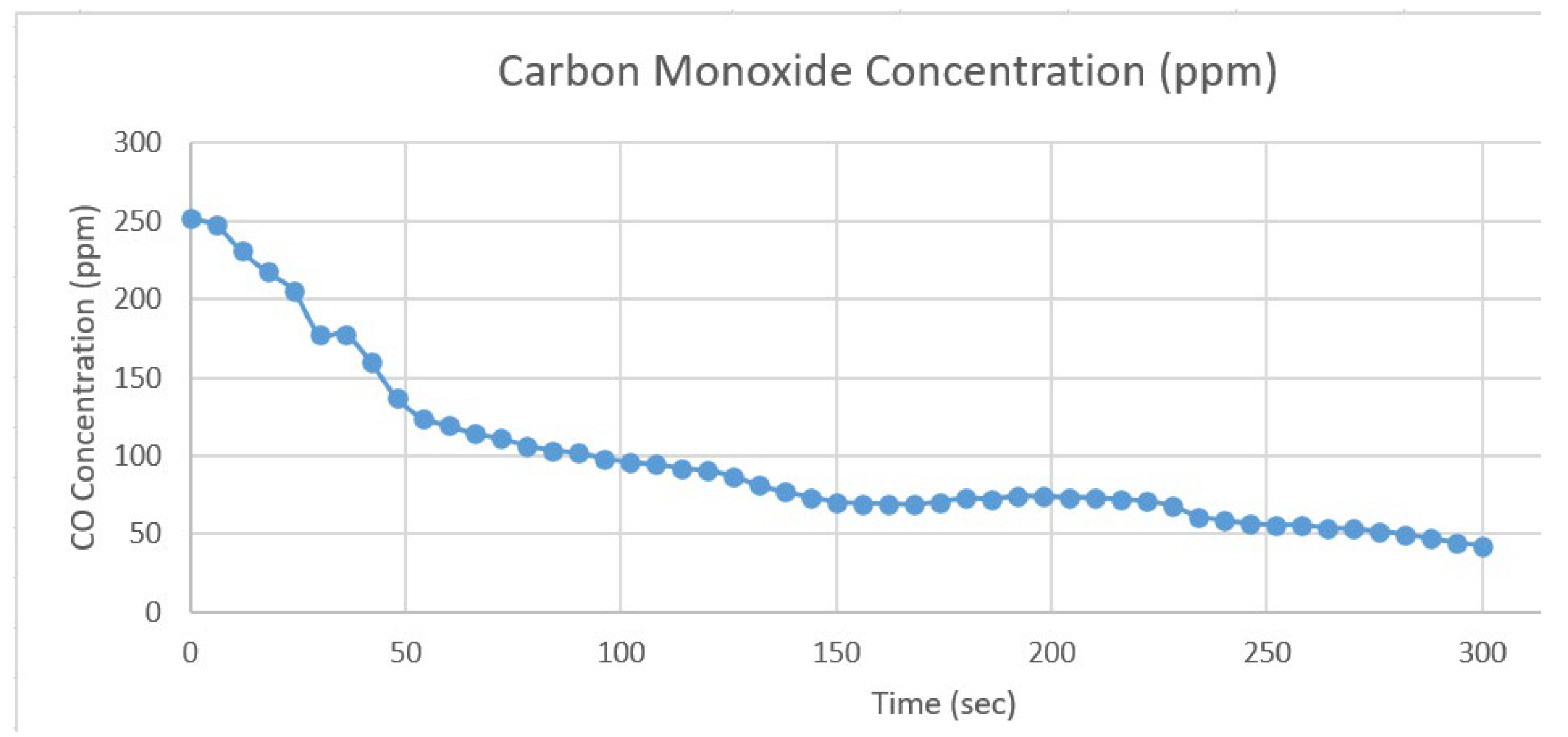
- An amount of air with a pollutant concentration of 11001.72 particles per million (ppm) includes carbon monoxide (CO) concentration of 251.68 ppm was added to the filter, and it was able to get rid of about 89.08% of pollutants, as shown in Figure 6 and Table 2, and about 83.23% of carbon monoxide (CO) in the air, as shown in Figure 7 and Table 3, in only 5 minutes.
4. Discussion
4.1. Prefilters
4.2. UV-C Light
4.3. Photocatalysis
4.4. Filtration Using Activated Carbon
5. Conclusions
6. Recommendations
- 1.
- Nanocellulose fibers:
- 2.
- Electrostatic precipitators:
References
- A Jawale, S. (2019). INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH. Intravenous-c-band-ultraviolet-light-therapy-ivuvlt-as-a-treatment-forbacterial- And-viral-infections-including-covid-19 May 2020 1588420002 6424994. [CrossRef]
- Abbasi-Kangevari, M., Malekpouret al. (2023). Effect of air pollution on disease burden, mortality, and life expectancy in North Africa and the Middle East: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Planetary Health, 7(5), e358–e369. [CrossRef]
- Ao, C., & Lee, S. (2004). Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chemical Engineering Science, 60(1), 103–109. [CrossRef]
- ASTM D4607-14, (2021), Standard Test Method for Determination of Iodine Number of Activated Carbon. American Society for Testing and Materials, . [CrossRef]
- Bhave, P. P., & Yeleswarapu, D. (2019). Removal of indoor air pollutants using Activated Carbon—A review. In Lecture notes on multidisciplinary industrial engineering (pp. 65–75). [CrossRef]
- Bilici, Z., Bouchareb, R., Sacak, T., Yatmaz, H. C., & Dizge, N. (2020). Recycling of TiO2-containing waste and utilization by photocatalytic degradation of a reactive dye solution. Water Science & Technology, 83(5), 1242–1249. [CrossRef]
- Blue Pure 211i Max | Air purifier for up to 635 ft2 | Blueair. (n.d.-b). https://www.blueair.com/us/air-purifiers/blue-pure-211i-max/3541.html?cgid=air-purifiers#start=1.
- BlueAir Support. (n.d.). https://support.blueair.com/faq/s/article/Are-Blueair-purifiers-energy-efficient-agent?language=en_US.
- BlueAir Support. (n.d.-b). https://support.blueair.com/faq/s/article/My-Protect-air-purifier-makes-an-unexpected-noise?language=en_US.
- EnviroKlenz. (2023, December 4). What is a HEPA filter, and how does it work? EnviroKlenz. https://enviroklenz.com/how-does-hepa-filter-work/.
- EnviroKlenz. (2023a, November 27). Air Scrubber vs UV Light Air Filtration. EnviroKlenz. https://enviroklenz.com/air-scrubber-vs-uv-light-air-filtration/.
- EnviroKlenz. (2023a, November 27). Air Scrubber vs UV Light Air Filtration. EnviroKlenz. https://enviroklenz.com/air-scrubber-vs-uv-light-air-filtration/.
- EnviroKlenz. (2024, November 21). EnviroKlenz Air System – EnviroKlenz. https://enviroklenz.com/product/enviroklenz-mobile/.
- EnviroKlenz. (2024a, October 23). EnviroKlenz replacement air cartridge. https://enviroklenz.com/product/enviroklenz-14x14-replacement-cartridge-250-cfm-exchange-unit/.
- Ganjoo, R., Sharma, S., Kumar, A., & Daouda, M. M. A. (2023). Activated Carbon: Fundamentals, classification, and properties. In The Royal Society of Chemistry eBooks (pp. 1–22). [CrossRef]
- Gómez-López, V. M., Jubinville et al. (2021). Inactivation of foodborne viruses by UV light: a review. Foods, 10(12), 3141. [CrossRef]
- Gómez-López, V. M., Jubinville, et al. Inactivation of Foodborne Viruses by UV Light: A Review. Foods, 10(12), 3141. [CrossRef]
- Haider, A., Al-Anbari, R., Kadhim, G., & Jameel, Z. (2018). Synthesis and photocatalytic activity for TiO2 nanoparticles as air purification. MATEC Web of Conferences, 162, 05006. [CrossRef]
- Han, S., Kim, J., & Ko, S. (2021). Advances in air filtration technologies: structure-based and interaction-based approaches. Materials Today Advances, 9, 100134. [CrossRef]
- Hetaba, A., McNally, C., & Habersky, E. (n.d.). REFUGEE ENTITLEMENTS IN EGYPT. AUC Knowledge Fountain. https://fount.aucegypt.edu/faculty_journal_articles/4985/.
- Home | Air purifiers. (n.d.). Blueair. https://www.blueair.com/us/.
- INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH. (2019). INTRAVENOUS C BAND ULTRAVIOLET LIGHT THERAPY (IVUVLT) AS a TREATMENT FOR BACTERIAL AND VIRAL INFECTIONS INCLUDING COVID 19. [CrossRef]
- Kim, M., Jeong, S., Park, J., & Lee, J. (2021). Assessment of pre-filter systems to control indoor inflow of particulate matter. Journal of Building Engineering, 43, 103052. [CrossRef]
- Kim, M., Jeong, S., Park, J., & Lee, J. (2021). Assessment of pre-filter systems to control indoor inflow of particulate matter. Journal of Building Engineering, 43, 103052. [CrossRef]
- Limmongkon, Y., Johns, J., & Charerntanyarak, L. (2013). Preparation of a TiO2-coated photocatalytic air filter for use with an electrostatic air filter pack for xylene removal. ScienceAsia, 39(3), 284. [CrossRef]
- Limmongkon, Y., Johns, J., & Charerntanyarak, L. (2013). Preparation of a TiO2-coated photocatalytic air filter for use with an electrostatic air filter pack for xylene removal. ScienceAsia, 39(3), 284. [CrossRef]
- Mahmoud, A., Hefny, R., & Elnasser, R. (2022). IMPACT OF POPULATION GROWTH ON THE MOST IMPORTANT ECONOMIC VARIABLES IN EGYPT. Sinai Journal of Applied Sciences (Print), 0(0), 0. [CrossRef]
- Mo, J., Zhang, Y., Xu, Q., Lamson, J. J., & Zhao, R. (2009). Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmospheric Environment, 43(14), 2229–2246. [CrossRef]
- Mo, J., Zhang, Y., Xu, Q., Lamson, J. J., & Zhao, R. (2009). Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmospheric Environment, 43(14), 2229–2246. [CrossRef]
- Perry, J. L., Agui, J. H., & Vijayakimar, R. (2016). Submicron and Nanoparticulate Matter Removal by HEPA-Rated Media Filters and Packed Beds of Granular Materials. In https://ntrs.nasa.gov/ (NASA/TM-2016-218224). Marshall Space Flight Center. https://ntrs.nasa.gov/api/citations/20170005166/downloads/20170005166.pdf.
- Rana, A. K., Mostafavi, E., Alsanie, W. F., Siwal, S. S., & Thakur, V. K. (2023). Cellulose-based materials for air purification: A review. Industrial Crops and Products, 194, 116331. [CrossRef]
- Ren, H., Koshy, P., Chen, W., Qi, S., & Sorrell, C. C. (2016). Photocatalytic materials and technologies for air purification. Journal of Hazardous Materials, 325, 340–366. [CrossRef]
- Salman, A., Al-Tayib, M., Hag-Elsafi, S., Zaidi, F. K., & Al-Duwarij, N. (2021). Spatiotemporal assessment of air quality and heat island effect due to industrial activities and urbanization in southern Riyadh, Saudi Arabia. Applied Sciences, 11(5), 2107. [CrossRef]
- Salman, D. (2011). Industrial development and the trade-off toenvironment: measurement techniques, meanings and outcomes in the context of water poverty in Egypt. International Journal of Green Economics, 5(1), 87. [CrossRef]
- Statista. (2022, November 15). International migrants in Egypt 2020, by country of origin and gender. https://www.statista.com/statistics/1237901/stock-of-international-migrants-in-egypt-by-country-of-origin-and-gender/.
- Statista. (2022b, December 16). International migrants in Egypt 2020, by country of origin. https://www.statista.com/statistics/1237888/stock-of-international-migrants-in-egypt-by-country-of-origin/.
- Statista. (2024, February 28). Youth unemployment rate in Egypt in 2022. https://www.statista.com/statistics/811968/youth-unemployment-rate-in-egypt/.
- Statistics and Graph 1: EGYPT population (2024) - Worldometer. (n.d.). https://www.worldometers.info/world-population/egypt-population/.
- Test Method for Determination of Iodine Number of Activated Carbon. (2006). [CrossRef]
- Thiagarajan, V., & Thiyagarajan, S. (2023). Dye-Sensitized titanium dioxide photocatalysis – a novel solution for personal air filtration. Journal of Student Research, 11(1). [CrossRef]
- Zaleska, A., Hanel, A., & Nischk, M. (2010). Photocatalytic air purification. Recent Patents on Engineering, 4(3), 200–216. [CrossRef]



|
Time (sec) |
Pollutants Concentration (ppm) |
Time (sec) |
Pollutants Concentration (ppm) |
Time (sec) |
Pollutants Concentration (ppm) |
Time (sec) |
Pollutants Concentration (ppm) |
|---|---|---|---|---|---|---|---|
| 0 | 11001.72 | 156 | 2827.50 | 78 | 4657.80 | 234 | 1854.66 |
| 6 | 10331.34 | 162 | 2717.63 | 84 | 4488.98 | 240 | 1785.94 |
| 12 | 8767.99 | 168 | 2634.75 | 90 | 4307.88 | 246 | 1719.50 |
| 18 | 7719.67 | 174 | 2542.62 | 96 | 4150.01 | 252 | 1662.92 |
| 24 | 7085.93 | 180 | 2442.57 | 102 | 3981.03 | 258 | 1608.04 |
| 30 | 6707.29 | 186 | 2388.05 | 108 | 3849.48 | 264 | 1562.02 |
| 36 | 6272.27 | 192 | 2293.15 | 114 | 3706.66 | 270 | 1480.78 |
| 42 | 6028.24 | 198 | 2221.13 | 120 | 3553.12 | 276 | 1423.65 |
| 48 | 5701.57 | 204 | 2161.11 | 126 | 3405.35 | 282 | 1361.65 |
| 54 | 5543.74 | 210 | 2092.87 | 132 | 3290.60 | 288 | 1308.51 |
| 60 | 5282.67 | 216 | 2026.51 | 138 | 3192.99 | 294 | 1256.88 |
| 66 | 5052.81 | 222 | 1961.55 | 144 | 3031.73 | 300 | 1200.86 |
| 72 | 4871.89 | 228 | 1907.43 | 150 | 2940.68 |
|
Time (sec) |
Carbon Monoxide Concentration (ppm) |
Time (sec) |
Carbon Monoxide Concentration (ppm) |
Time (sec) |
Carbon Monoxide Concentration (ppm) |
Time (sec) |
Carbon Monoxide Concentration (ppm) |
|---|---|---|---|---|---|---|---|
| 0 | 251.68 | 156 | 69.30 | 78 | 106.49 | 234 | 61.14 |
| 6 | 247.65 | 162 | 69.30 | 84 | 103.47 | 240 | 58.55 |
| 12 | 230.75 | 168 | 68.54 | 90 | 101.74 | 246 | 56.37 |
| 18 | 217.32 | 174 | 70.44 | 96 | 98.34 | 252 | 55.38 |
| 24 | 205.66 | 180 | 72.56 | 102 | 95.73 | 258 | 55.54 |
| 30 | 177.83 | 186 | 72.17 | 108 | 94.32 | 264 | 53.60 |
| 36 | 177.83 | 192 | 73.94 | 114 | 92.01 | 270 | 52.95 |
| 42 | 159.85 | 198 | 73.73 | 120 | 90.19 | 276 | 51.37 |
| 48 | 137.04 | 204 | 73.35 | 126 | 86.86 | 282 | 49.67 |
| 54 | 123.96 | 210 | 72.95 | 132 | 80.85 | 288 | 46.81 |
| 60 | 119.20 | 216 | 71.98 | 138 | 76.73 | 294 | 44.19 |
| 66 | 114.04 | 222 | 70.82 | 144 | 73.45 | 300 | 42.21 |
| 72 | 111.13 | 228 | 67.80 | 150 | 70.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





