1. Introduction
Neurogenesis, the process by which new neurons are formed within the brain, occurs both during embryonic development and, to a more limited extent, in the adult brain. For decades, the prevailing view held that neurons do not multiply after development. The first indication to the contrary came in 1962, when Altman reported autoradiographic evidence of neuronal proliferation in adult rats [
1].
Two decades later, Bayer et al. provided additional evidence for neuronal proliferation in the adult mammalian brain [
2]. Although recent literature remains divided, with some studies supporting [
3,
4,
5,
6,
7,
8,
9] and others questioning [
10,
11,
12] the extent and significance of human adult neurogenesis (for review, see [
13]), its potential contribution to human brain function and to the pathogenesis of neuropsychiatric disorders cannot be dismissed. Among brain regions where neurogenic activity has been reported, the hippocampus consistently exhibits the highest rates [
14].
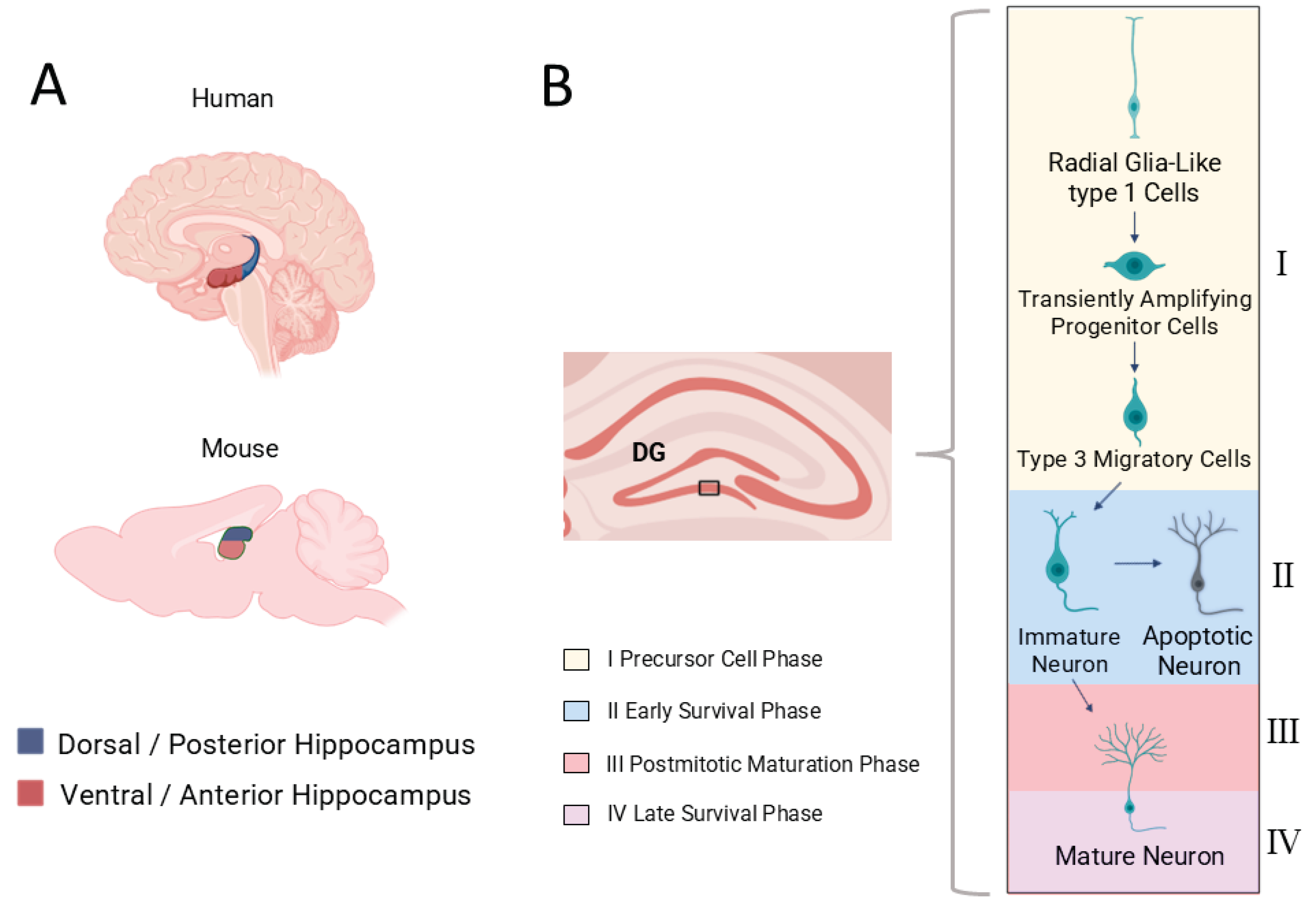
The hippocampus subserves a wide range of functions, including spatial navigation and cognition [
15,
16], learning and memory consolidation [
15,
17] and emotional processing [
18]. AHN occurs in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), where newborn granule cells are generated and integrated into existing hippocampal circuits [
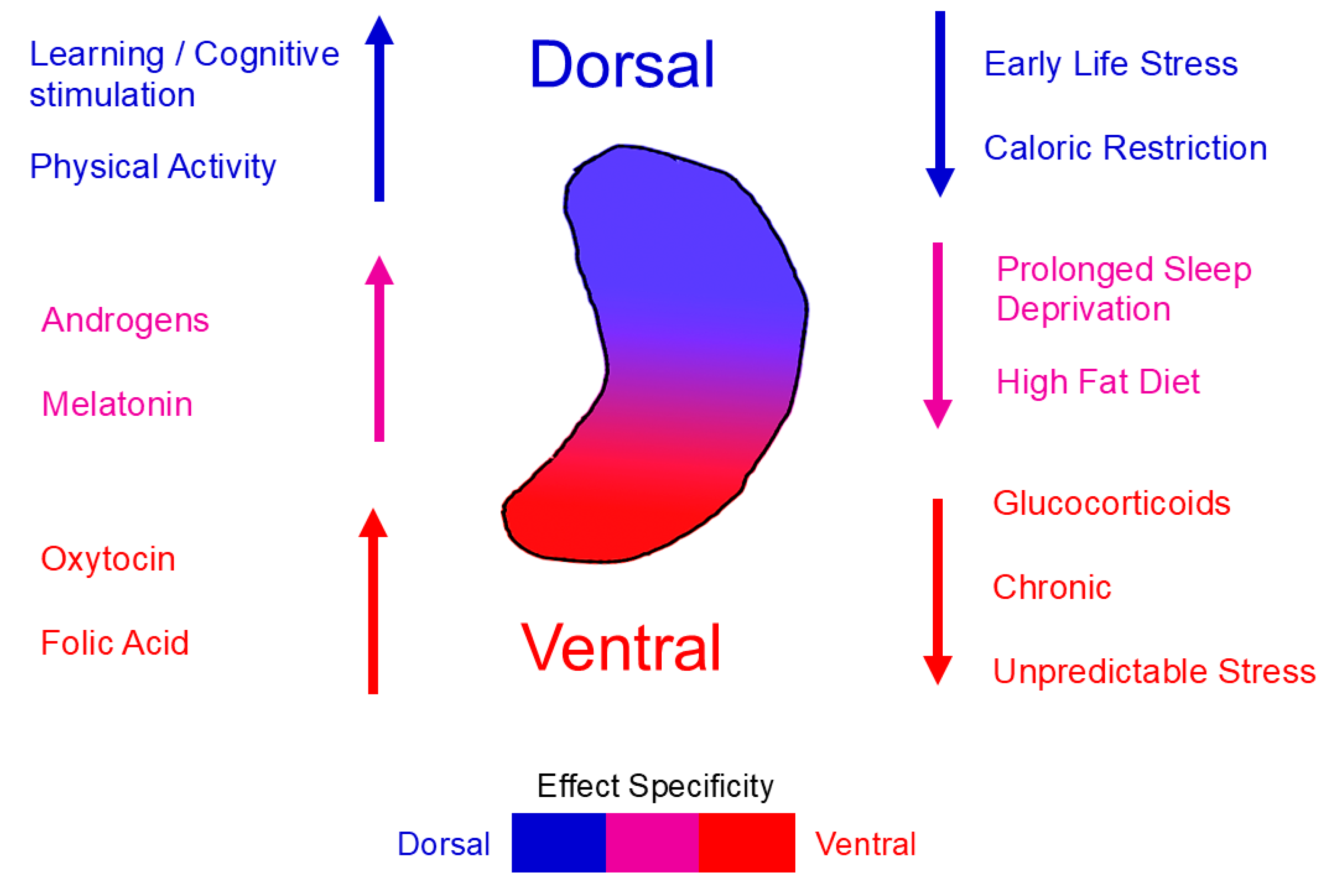
19], linking experience to structural plasticity. Importantly, the hippocampus is not functionally uniform. It displays a longitudinal (dorsoventral or septotemporal) specialization, with the dorsal portion being more involved in spatial and cognitive processing, and the ventral portion contributing to emo-tional and stress-related regulation [
20]. Growing evidence suggests that AHN also exhibits region-specific regulatory mechanisms and behavioral outcomes [
21]. These dorsoventral distinctions, discussed in detail in
Section 4, provide an anatomical framework for understanding how diverse physiological, behavioral, and pathological factors exert distinct influences on cognition and emotion.
The discovery of AHN and its capacity to reshape neural plasticity has prompted intensive investigation into how intrinsic and extrinsic factors modulate its levels. Modifiable influences, such as hormonal environment, behavior and diet are of particular interest due to their potential to reveal molecular mechanisms through which lifestyle impacts brain function. Notably, several studies indicate that some of these factors appear to differentially affect AHN along the hippocampal axis, suggesting that both baseline neurogenesis and regional plasticity may determine the direction and magnitude of their effects. Recognizing these dorsoventral distinctions provides a framework for understanding how diverse physiological, behavioural, and pathological factors exert distinct influences on cognition and emotion.
While numerous reviews have summarized modulators of AHN [
22,
23,
24] , this subregional perspective is largely overlooked. The goal of this review is therefore to synthesize evidence on the most widely studied modifiers of AHN in animal models and humans, with explicit attention to subregion-specific effects along the hippocampal dorsoventral axis. We further examine how these mechanisms relate to neuropsychiatric disease, highlighting their potential translational and therapeutic implications.
2. Adult Neurogenesis in the Hippocampus: Brief Historical Overview and Basic Mechanisms
Despite the increasing interest and research being conducted on adult neurogenesis over the past decades, there is still notable controversy on whether its presence is significant enough in humans, with some claiming that it is purely an evolutionary remnant, especially in organisms with high cognitive function [
12]. However, there have been some studies which support that a considerable level of adult neurogenesis does in fact exist in humans. The first indication of neurogenic activity taking place in the adult human hippocampus came into light in 1998, when Eriksson et al, administered a birth-dating marker, Bromodeoxyuridine (BrdU) in 5 middle-aged patients and observed newly born neurons (BrdU +) expressing factors such as calbindin in the postmortem evaluation of their hippocampi [
25]. Following this report, many studies explored the extent of adult neurogenesis in humans. One of the most striking and controversial studies that have been conducted to this end measured the quantity of the isotope
14C in hippocampal neurons following the above-ground nuclear bomb tests between 1945 and 1963 [
26]. In the aftermath of these tests,
14C was released in the atmosphere and was absorbed by plants. Humans consumed products from these plants or meat from animals feeding on them which contained
14C and this isotope was absorbed and used by dividing cells in their adult organisms. Spalding et al, found large quantities of
14C in the hippocampal DG that were consistent with 700 new neurons being born in each hippocampus per day and an annual turnover of 1.75% of the hippocampal neurons within the renewing fraction [
26]. These groundbreaking results have faced criticism with some authors arguing that postmitotic neurons can integrate
14C through other mechanisms than cell division, such as DNA repair or methylation [
12]. However, Spalding et al, claim that the amount of
14C they detected in the adult hippocampus is too high to be explained just by these mechanisms alone and that an underlying neurogenic activity more accurately reflects these findings [
26]. Nevertheless, even if levels of AHN in humans are minimal, clarifying the underlying mechanisms could still yield insights with potential translational relevance.
AHN has been reported to be mediated by Neural Stem Cells (NSCs) that reside in niches mainly in the SGZ of the hippocampal DG, where they remain quiescent. It has been proposed that AHN can be divided into four phases: The precursor cell phase (I), the early survival phase (II), the postmitotic maturation phase (III) and the late survival phase (IV) [
27] (
Figure 1). Different cell types are present in each phase which can be identified by specific protein-markers they express. In the precursor cell phase, radial glia-like stem cells of three types can be identified. Radial glia-like type 1 cells (expressing the markers GFAP, Nestin, BLBP, Sox2) give rise to radial glia-like type 2a (expressing the markers GFAP
+/-, Nestin, BLBP, Sox2) and 2b (expressing the markers Nestin, DCX, PSA-NCAM, NeuroD, Prox1) cells. Type 2 cells, also known as Transiently Amplifying Progenitor cells (TAPs), exhibit high proliferative activity, in contrast to their successors, type 3 cells (expressing DCX, PSA-NCAM, NeuroD and Prox1), which are mainly involved in migration into the granular layer. In the early survival phase, new-born neurons (expressing Calretinin, NeuN, DCX, PSA-NCAM, NeuroD and Prox1) either survive or undergo apoptosis [
27]. The fate of these newly born neurons can be influenced by multiple factors which we will be discussing later in this review. The postmitotic maturation phase follows the early survival phase and facilitates synaptogenesis. New neurons in this phase have a lower threshold for long term potentiation than their older equivalents and exhibit increased synaptic plasticity. Finally, the late survival stage refers to the morphological maturation of the newborn neurons who have emerged from the thousands of neuroblasts originally generated and have established functional connections within the existing circuitry [
27]. Interestingly, several studies have reported differing sensitivities of the dorsal and ventral DG to various stimuli that induce or impair AHN.
3. Factors Modifying Hippocampal Neurogenesis
3.1. Hormones
Hormones are soluble chemical messengers produced and secreted by endocrine glands in multicellular organisms. They contribute to homeostasis by acting in multiple organs, such as the brain. Their action is mediated through intracellular or extracellular receptors which are expressed at certain target cells. Research over the past years has underscored the neurogenic impact of various hormones in the hippocampus, which could involve stimulatory, inhibitory or neuroprotective outcomes. Although circulating hormone levels are not directly modifiable, they can be easily influenced either by other factors such as diet and behavior or by extrinsic administration
3.1.1. Cortisol / Corticosterone
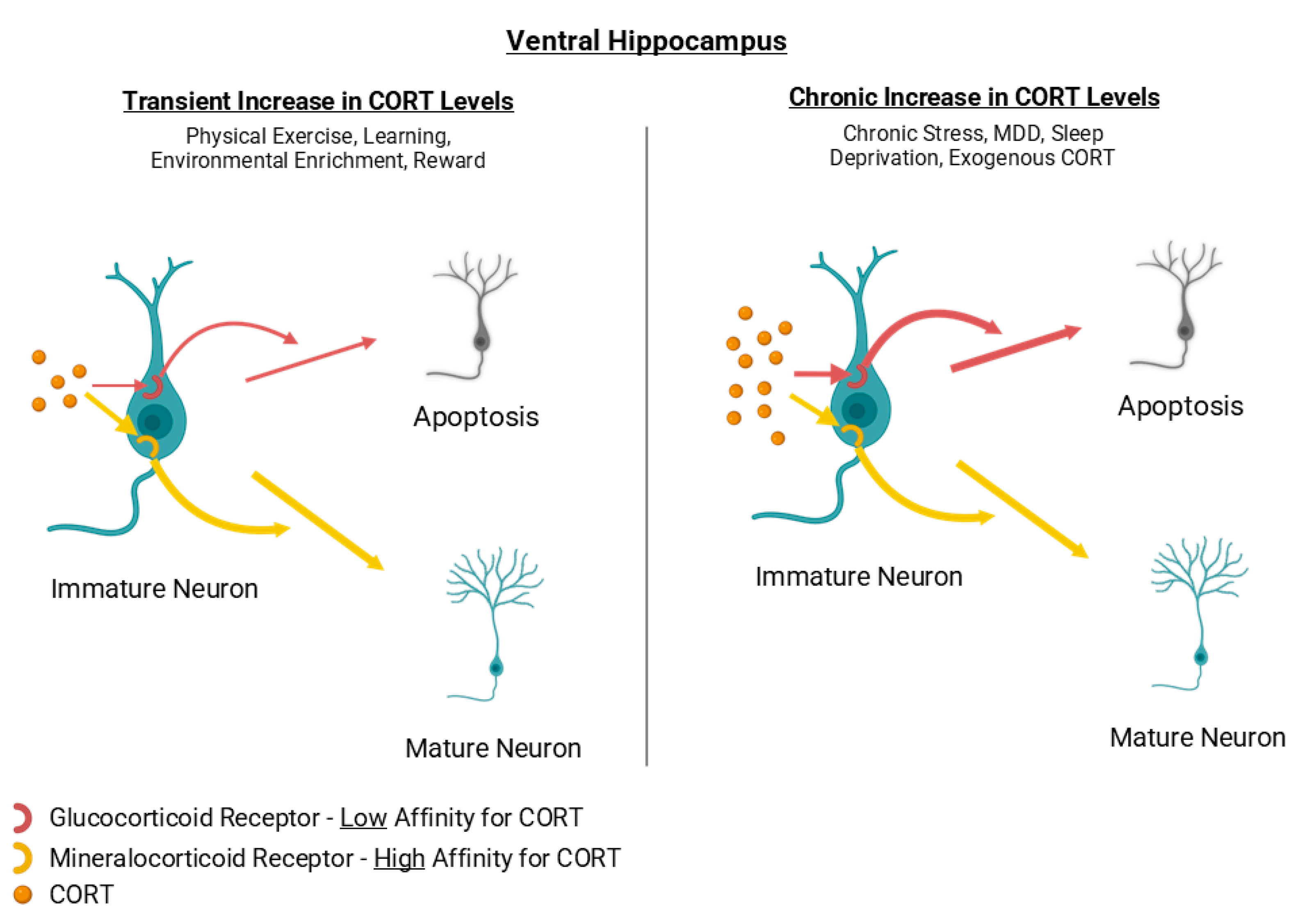
Cortisol in human and corticosterone in rodents, collectively referred to as CORT, are steroid hormones synthesized by the zona fasciculata of the adrenal glands. CORT is involved in multiple functions such as immunosuppression and the metabolism of proteins, lipids and carbohydrates. Its secretion is regulated by the Hypothalamic-Pituitary-Adrenal (HPA) axis and typically increases in response to stress. As a steroid hormone, CORT mainly exerts its action through intracellular glucocorticoid receptors (GRs) or mineralocorticoid receptors (MRs). The cortisol-receptor complex binds to specific element DNA motifs or interacts with transcription factors (e.g. NF-κB, STAT3 and AP-1) in the target cell’s nucleus to modulate gene expression.
MR and GR are abundantly expressed in hippocampal neurons, with MRs exhibiting ~10-fold higher affinity for CORT than GRs [
28,
29]. Pharmacological studies report that MR agonists and GR antagonists have been associated with a positive effect on hippocampal neurogenesis [
30,
31,
32]. Conversely, prolonged CORT exposure has been shown to reduce hippocampal neurogenesis [
33,
34,
35] and the ventral hippocampus seems to have an increased intrinsic sensitivity to CORT than its dorsal counterpart [
36]. Recently, Wang et. al (2024) have suggested that p21 (kinase inhibitor promoting cycle arrest) overexpression with subsequent accumulation of ROS might be the culprit mechanism behind the CORT-induced deficits in hippocampal neurogenesis [
33].
At the same time, many authors argue that moderate-to-high circulating CORT levels are also associated with an increase in hippocampal neurogenesis. Physical exercise, enriched environment, sexual experience as well as learning have all been correlated with both elevated neurogenic rates and higher circulating CORT levels [
23,
37,
38,
39,
40,
41,
42,
43] . However, it has been suggested that the rewarding nature of these activities may lead to the activation of mechanisms that overcome the negative effects of high CORT levels resulting in an overall increase in neurogenesis [
30]. Other authors suggest that there is an optimal level of circulating CORT that favors neurogenic activity, and any factor that either increases or decreases this level could lead to diminished rates of AHN [
31].
Taken together, chronically elevated CORT, due to persistent stress or exogenous administration, tends to suppress AHN, with a ventral-biased vulnerability, while moderate to high CORT levels have been reported to bolster AHN [
36]. Considering MR/GR pharmacology and receptor affinity, low-to-moderate CORT levels likely support AHN primarily through MR-dominant signalling, whereas sustained high CORT levels inhibit AHN via GR-dominant signalling [
44]. Activities that produce transient CORT elevations [
23,
37,
38,
39,
40,
41,
42,
43], may enhance AHN by maintaining favourable MR/GR balance, whereas conditions that produce chronic CORT elevations [
45] can impair AHN through GR saturation and downstream anti-proliferative pathways [
28,
29,
30,
31,
32,
44] (
Figure 2) . Further work should delineate the exact impact of CORT levels on hippocampal neuron proliferation in both the dorsal and ventral hippocampus. In summary, glucocorticoid-induced suppression of AHN is not uniform along the dorsoventral axis of the hippocampus. The ventral DG, which is more responsive to stress and emotional regulation, displays greater vulnerability to corticosterone elevation, whereas the dorsal segment shows comparatively milder and more transient effects.
3.1.2. Estrogens
Estrogens are a group of steroid hormones that are known for their major role in the development the female reproductive system. Outside of this traditional function, estrogens also have considerable effects on multiple organs and tissues, such as the bone, liver, blood vessels, skin, fat and central nervous system. The three most dominant types of estrogen are 17β-estradiol, estrone and estriol. 17β-estradiol is mainly produced by the ovaries, estriol is produced by the placenta and estrone is produced in adipose tissue via aromatization. Estrogens primarily exert their action through intracellular nuclear receptors (ERα and ERβ) but also through membrane-bound receptors G protein-coupled estrogen receptor 1 (GPER1), ER-X and Gq-mER [
46].
ERα and ERβ and GPER1 receptors are expressed widely in the brain, with ERβ mainly being found in the hippocampal DG [
47]. GPER1 deficiency has been shown to impair neurogenesis in the hippocampus, indicating that GPER1 could play a role in the induction of hippocampal neurogenesis [
48]. Multiple researchers have noted that estradiol stimulates AHN and cell proliferation of adult female rats in a time and dose-dependent manner. The dose of estradiol that has been most associated with positive effects in hippocampal neurogenesis is 10 μg. Acute administration of estradiol has been correlated with increased cell proliferation and survival in the hippocampus, while chronic administration has been associated with the opposite effects [
49,
50,
51]. Research regarding estrogen’s impact on the male hippocampus, however, has been scarce. There are indications that survival of young granule neurons in the male DG is increased after estradiol administration [
52]. Many authors also highlight the fact that even though most experiments are being conducted with estradiol, which is a potent estrogen, different types of estrogen can also have different effects on neurogenesis. Estrone administration, for example, has been associated by many with a decreased neurogenic potential, especially when it comes to cell proliferation [
49,
51]. In addition, aromatase inhibition has been reported to augment AHN levels in middle-aged female mice [
53].
Regarding the regional impact of estrogens on AHN, available evidence remains limited. In one study, the phytoestrogen daidzein enhanced neurogenesis in both the ventral and dorsal hippocampus, with more prominent effects dorsally [
54]. Overall, estrogens tend to transiently enhance AHN in female rodents, whereas prolonged or chronic exposure may reduce hippocampal cell proliferation, suggesting a complex, time-dependent regulation. The negative impact of chronic estrone administration also needs further evaluation, as this hormone is elevated in obesity due to increased aromatization of androgens in adipose tissue [
55]. Taken together, these findings indicate that estrogens exert regionally distinct influences on AHN along the hippocampal longitudinal axis. Experimental data suggest that estrogen-induced proliferation and neuronal differentiation are generally stronger in the dorsal DG, consistent with its cognitive specialization, whereas ventral neurogenesis appears less robust or more transiently modulated, potentially reflecting differences in ER distribution and stress-related signaling between the two poles of the hippocampus.
3.1.3. Androgens
Androgens are a group of steroid hormones responsible for the development of the male reproductive system. They also have anabolic actions in the musculoskeletal system. The main androgens found in mammals are testosterone and Dihydrotestosterone. Testosterone is produced and secreted by Leydig cells in the testis and can be converted to DHT by 5α-reductase in peripheral tissue. Androgens mainly exert their actions by binding to intracellular Androgen Receptors (AR) and modulating the expression of multiple genes in the target cells.
ARs are expressed in multiple regions of the brain, such as the hippocampus [
56]. Many researchers report a positive correlation trend between long-derm androgen administration and the survival of new hippocampal neurons in both the dorsal and the ventral hippocampus, with statistically significant benefit being observed only in young male rats [
56,
57,
58]. On the contrary, anabolic steroid androgens, which are commonly used among athletes, have been shown to abolish the positive neurogenic effects of exercise [
59]. Overall, androgens, with the exception of anabolic steroids, seem to be beneficial to AHN in an age and sex specific pattern, favouring only young males and only if administered chronically.
Evidence on the dorsoventral specificity of androgen effects on AHN remains limited, although some findings suggest that androgenic modulation may exhibit regional differences. Testosterone and its metabolites appear to enhance cell proliferation and neuronal survival predominantly in the dorsal DG [
57,
60], consistent with its role in cognitive and spatial processes, whereas effects in the ventral region are weaker or context-dependent [
61] This gradient may reflect variations in AR density, local aromatization to estrogens, and the distinct functional circuitry of the dorsal and ventral hippocampus. For instance, AR expression is somewhat higher in the dorsal hippocampus [
61,
62], suggesting greater responsiveness in this region. Conversely, alterations in androgen/estrogen balance, such as those induced by aromatase inhibition, can enhance ventral neurogenesis [
53]. Together, these findings indicate that androgenic modulation acts in a region-dependent and context-sensitive manner along the hippocampal longitudinal axis.
3.1.4. Oxytocin
Oxytocin is a peptide hormone mainly synthesized in the hypothalamus and secreted into the bloodstream by the posterior pituitary gland. Acting through its transmembrane G-protein-coupled receptor (OXTR), oxytocin regulates multiple physiological and behavioral functions, including parturition, uterine contraction, milk ejection, sexual behavior, and social interaction [
63,
64]
OXTR is abundantly expressed in the hippocampus of rodents but appears much less prevalent in the human hippocampus [
65,
66].
Research on the impact of oxytocin on AHN remains limited, though several studies indicate that chronic intranasal or peripheral oxytocin administration increases neurogenic activity, particularly in the ventral DG [
67,
68]. Peripheral oxytocin treatment can even counteract the inhibitory effects of stress and glucocorticoids on hippocampal cell proliferation [
64]. However, other studies reported reduced neurogenesis following oxytocin administration under acute treatment paradigms [
69], suggesting that the duration and route of administration critically determine its neurogenic outcome.
Evidence on the dorsoventral specificity of oxytocin’s effects on AHN remains limited. In adult rats, systemic or intracerebral oxytocin administration promotes social interaction and stress resilience but exerts modest or inconsistent effects on hippocampal cell proliferation overall [
69]. Nevertheless, the behavioral domains influenced by oxytocin, such as social bonding, anxiety regulation, and affective processing, are functions predominantly mediated by the ventral hippocampus [
70,
71]. Neurogenesis in the ventral DG has been linked to the modulation of stress-related and emotional behaviors [
72], suggesting that oxytocin’s neuromodulatory actions may preferentially influence this hippocampal segment. Although direct comparisons of dorsal and ventral neurogenic responses to oxytocin are scarce, current evidence supports a model in which ventral AHN is particularly sensitive to oxytocin-mediated regulation, consistent with the peptide’s broader role in emotional adaptation and social cognition.
3.1.5. Thyroid Hormones
Thyroid hormones (THs), primarily triiodothyronine (T3) and thyroxine (T4), are peptide hormones synthesized and secreted by the thyroid gland. They exert their effects through intracellular and membrane-associated thyroid hormone receptors (TRs), including TRα1, TRα2, and TRβ1, which are widely expressed in the adult brain [
73,
74]. THs play essential roles in regulating metabolism, homeostasis, and neurodevelopment.
Experimental models indicate that hypothyroid states reduce the survival and differentiation of adult DG cell progenitors in both mice and rats [
75,
76,
77]. In contrast, hyperthyroidism has been associated with enhanced neuronal differentiation and postmitotic progenitor survival in mice [
76], though such effects were not replicated in rats [
75], suggesting species-dependent variability. To date, no studies have explicitly examined the impact of THs on neurogenesis along the dorsoventral hippocampal axis. Nevertheless, indirect evidence suggests potential region-specific effects. TRα and TRβ are differentially distributed across hippocampal subregions [
78].
Moreover, TH signaling interacts with neurotrophic and glucocorticoid pathways [
79,
80], both of which show stronger regulatory impact in the ventral hippocampus. This raises the possibility that TH modulation could influence dorsal neurogenesis more directly through receptor-mediated transcriptional control, while exerting indirect or stress-linked effects ventrally via endocrine cross-talk.
In summary, hypothyroidism appears to impair, and hyperthyroidism to potentially enhance, AHN, but species differences and methodological variability complicate interpretation. Future research should clarify how TH receptor distribution and signaling intersect with dorsoventral specialization to determine regional susceptibility or plasticity within the adult hippocampus, as well as their relevance to human neurogenesis.
3.1.6. Melatonin
Melatonin is an indoleamine hormone primarily synthesized and secreted by the pineal gland, typically in response to darkness. It regulates circadian rhythm, core body temperature, energy metabolism, and immune function, and also exerts potent antioxidant and anti-inflammatory effects. Melatonin acts mainly through its G-protein–coupled receptors MT1 and MT2, which are distributed throughout the mammalian brain, including the hippocampus. MT1 receptors are abundantly expressed across the hippocampus, whereas MT2 receptors show higher density in the CA3 region [
81].
Several studies have investigated melatonin’s role in AHN. Pinealectomy in rats markedly decreases the number of newborn hippocampal neurons, an effect reversed—and even surpassed—by chronic melatonin supplementation [
82]. Consistent findings demonstrate that melatonin primarily enhances neuronal survival and differentiation, rather than cell proliferation, thereby potentiating the effects of other pro-neurogenic stimuli such as physical exercise [
83,
84,
85,
86]. Melatonin’s neuroprotective role is further supported by studies showing attenuation of AHN deficits induced by hyperglycemia, glucocorticoid exposure, or manganese toxicity [
87,
88,
89]. Additionally, melatonin administration improves recovery after ischemic injury, promoting neuronal regeneration possibly through MT2-mediated signaling [
90,
91]. Collectively, these findings establish melatonin as a robust enhancer of hippocampal neurogenesis and neuronal resilience. Its beneficial effects appear to stem largely from antioxidant and anti-inflammatory actions that improve survival and maturation of newborn neurons.
Regarding dorsoventral specificity, evidence points to a ventral predominance of melatonin’s neurogenic and behavioral effects. In rodents, melatonin treatment ameliorates stress-induced anxiety and depression-like behaviors, functions mediated primarily by ventral hippocampal circuits [
71,
92]. Moreover, activation of MT2 receptors has been shown to promote AHN and antidepressant-like outcomes through upregulation of Brain Derived Neurotrophic Factor (BDNF) and modulation of glucocorticoid signaling [
93,
94]. Although direct dorsal–ventral comparisons remain limited, these data suggest that melatonin preferentially enhances ventral DG plasticity, consistent with its role in emotional regulation and stress resilience. Future work should aim to clarify whether melatonin’s pro-neurogenic actions extend equivalently to dorsal hippocampal domains involved in cognitive processes.
3.1.7. Growth Hormone, IGF-1
Growth Hormone (GH) is a peptide hormone synthesized, stored and secreted by somatotropic cells in the anterior pituitary gland. It plays a major role in somatic growth, regulating bone and organ development as well as lipid and mineral metabolism. Although GH acts directly on tissues through growth hormone receptors (GHRs), many of its effects are mediated indirectly by insulin-like growth factor-1 (IGF-1). GH stimulates the synthesis of IGF-1 in the liver and peripheral tissues, and IGF-1 in turn promotes tissue growth via its membrane-bound receptor tyrosine kinase (IGF1R).
Both GHRs and IGF1Rs are expressed in multiple brain regions, including the hippocampus [
95,
96]. Peripheral administration of GH or IGF-1 increases cell proliferation in the hippocampal formation [
97,
98], and several studies suggest that exercise-induced neurogenesis is partly mediated by upregulation of circulating GH and IGF-1 [
99,
100]. These trophic hormones enhance neurogenesis by promoting angiogenesis, synaptic plasticity, and neuronal survival through BDNF- and Vascular Endothelial Growth Factor (VEGF)-dependent pathways [
101,
102,
103]
To date, no studies have directly examined the impact of GH or IGF-1 on neurogenesis across the dorsoventral axis of the hippocampus. However, indirect evidence suggests region-specific modulation. Exercise and growth factor signaling preferentially engage ventral hippocampal circuits associated with stress resilience and emotional regulation [
71,
92], implying that GH/IGF-1–mediated neurogenesis may be more dynamically regulated in this region. Conversely, the dorsal hippocampus, enriched in GH receptor expression, could support GH-driven enhancement of spatial and cognitive processes [
104,
105]
Collectively, GH and IGF-1 appear to positively modulate hippocampal cell proliferation and survival, but their region-specific influence along the hippocampal longitudinal axis remains to be determined. Future studies should address whether these hormones differentially regulate ventral (emotional) and dorsal (cognitive) neurogenesis, particularly in the context of exercise and aging.
3.1.8. Erythropoietin
Erythropoietin (EPO) is a peptide hormone that is mainly synthesized in the kidneys in response to hypoxia and stimulates erythropoiesis (proliferation and differentiation of erythrocyte precursors). It exerts its actions via its membrane receptors (EPO-R) which are expressed at target cells.
EPO-R expression is observed in various brain cell types, including hippocampal neural progenitor cells [
106]. There are reports indicating that EPO enhances neurogenesis and inhibits apoptosis of new-born cells in the hippocampal DG [
107,
108]. Other researchers have observed increased rates of neurogenesis correlated with increased levels of EPO due to cognitive challenge-induced hypoxia in hippocampal neurons [
109]. Furthermore, the impact of EPO in neurogenesis could suggest another mechanism through which the positive neurogenic effects of exercise are mediated [
110]. Nevertheless, to our knowledge, there have not been reports yet exploring EPO’s effect on neurogenesis in the ventral compared to the dorsal hippocampus.
Overall, there has been some evidence that supports a positive neurogenic effect of EPO in the hippocampus, but further research could explore its exact action along the hippocampal longitudinal axis.
3.2. Behavior
3.2.1. Physical Activity
Physical activity has long been associated with improved brain health and cognitive performance. Light aerobic exercise enhances multitasking and executive function in young adults [
111], while chronic intermittent voluntary running in mice produces long-lasting benefits on learning and memory [
112]. These findings have prompted extensive investigation into the molecular mechanisms underlying the neurogenic and cognitive effects of exercise.
Human imaging studies reveal that regular aerobic activity in late adulthood increases anterior hippocampal gray matter volume, a region enriched in adult neurogenesis, suggesting that the beneficial cognitive effects of exercise may be mediated by increased neuronal production [
113,
114]. In both rodents and humans, physical activity elevates circulating growth factors such as IGF-1, BDNF, and VEGF [
115,
116], which together promote synaptic plasticity, angiogenesis, and neurogenesis in the hippocampal formation [
38,
100]. More recently, irisin, a myokine secreted by skeletal muscle during exercise, has been shown to exert anti-inflammatory and neuroprotective effects, contributing to activity-induced hippocampal neurogenesis [
117,
118]. The adipokine adiponectin may similarly support the pro-neurogenic and cognitive benefits of physical exercise [
119]. Furthermore, ablation of newborn neurons following running abolishes the typical exercise-induced improvements in cognition and memory [
120], demonstrating that AHN plays a causal role in mediating exercise-driven brain plasticity. It should be noted, however, that excessive or prolonged voluntary running can sometimes reduce neurogenesis, possibly due to increased physiological stress and glucocorticoid release [
121].
Regarding the distribution of exercise-induced neurogenesis along the hippocampal dorsoventral axis, most studies report stronger effects in the dorsal DG, consistent with its role in spatial learning and cognitive processing [
38,
83,
122]. For example, it has been demonstrated that environmental enrichment combined with voluntary exercise preferentially enhanced dorsal neurogenesis, which correlated with improved spatial performance, whereas ventral neurogenesis contributed more to stress resilience and affective behavior [
123]. Other studies, however, observed increases in both dorsal and ventral subregions, suggesting that exercise engages distinct neurogenic pools depending on intensity, duration, and hormonal context. Overall, physical activity enhances AHN through systemic and local trophic factors, with dorsal neurogenesis predominantly supporting cognitive gains and ventral neurogenesis mediating emotional regulation and stress adaptation. Clarifying the parameters that shift this balance may help optimize exercise-based interventions for cognitive and affective disorders.
3.2.2. Sleep
Sleep is divided into Rapid Eye Movement (REM) and non-Rapid Eye Movement Non-REM (NREM) sleep. REM sleep comprising 25% of total sleep time, is characterized by random rapid movement of the eyes with beta waves being recognized on electroencephalogram (EEG) recordings. NREM is further divided into Stage 1 (N1), Stage 2 (N2) and Stage 3 (N3). N1 is the lightest sleep stage and comprises 5% of total sleep time with theta waves being observed on EEG. N2 sleep is deeper than N1, comprises 45% of total sleep time and sleep spindles along with K complexes can be seen on EEG. Finally, N3 is the deepest non-REM sleep, comprises 25% of total sleep time and the EEG at this stage consists of delta waves which have the lowest frequency and the highest amplitude [
124].
Adequate sleep is essential for optimal brain function, influencing cognition, memory, and mood regulation [
125,
126,
127]. Since these processes are closely linked to AHN, numerous studies have examined how sleep and neurogenesis interact. Sleep deprivation, fragmentation, or restriction consistently reduce neurogenic potential, impairing newborn neuron proliferation, survival, and differentiation (for review see [
24]). Prolonged or repeated sleep loss has been shown to decrease neurogenesis and cell proliferation in both the dorsal and ventral DG [
128]. Furthermore, the decrease in DCX-labeled cells was more pronounced in the ventral hippocampus than in the dorsal region [
128]
However, as sleep deprivation also constitutes a potent stressor, elevated glucocorticoid levels may mediate part of the observed neurogenic suppression [
129]. Distinguishing direct effects of disrupted sleep from stress-related mechanisms therefore remains an important experimental challenge.
Beyond general suppression, sleep and its disruption appear to influence AHN differentially along the dorsoventral hippocampal axis. While studies in rodents have shown that sleep deprivation preferentially impairs neurogenesis in the ventral DG, corresponding with increases in anxiety-like behaviors [
130], it has also been reported that REM sleep deprivation can also reduce cell proliferation in the dorsal DG and impair hippocampal-dependent spatial memory [
131].
These findings suggest that sleep disturbances may predominantly affect ventral neurogenesis [
128,
129], consistent with the ventral hippocampus’s sensitivity to stress and emotional regulation. This is further supported by findings presented by O’Leary and Cryan [
71], as well as by Tanti and Belzung [
132], who provide comprehensive reviews demonstrating that stress affects multiple stages of AHN preferentially in the ventral rather than the dorsal hippocampus. Additionaly, Perera et al. [
133] showed in non-human primates that chronic stress reduced immature neurons in the anterior (ventral equivalent) but not posterior hippocampus, and this effects was correlated with stress-induced anhedonia.
Future studies should employ experimental designs that disentangle the direct neurogenic effects of sleep architecture from those mediated by stress hormones. Such differentiation is crucial, as therapeutic strategies targeting glucocorticoid signaling may protect ventral neurogenesis, while interventions enhancing sleep quality and REM integrity could preferentially restore dorsal and ventral neurogenic balance.
3.2.3. Learning
The process of learning has long been associated with the hippocampal formation, and since high neurogenic activity occurs in this region, AHN has been proposed as a mechanism supporting learning and memory.
The relationship between learning and neurogenesis appears to be bidirectional. Inhibition of neurogenic potential in animal models disrupts contextual learning and memory performance [
134,
135], while specific learning tasks enhance AHN by increasing the survival of newborn granule cells [
136]. Training paradigms such as trace conditioning, stimulus contiguity conditioning, long-delay conditioning, and the Morris water maze are associated with elevated neurogenic rates [
137]. Moreover, the complexity of the task and the degree of hippocampal engagement correlate positively with the survival and integration of new neurons [
137,
138,
139].
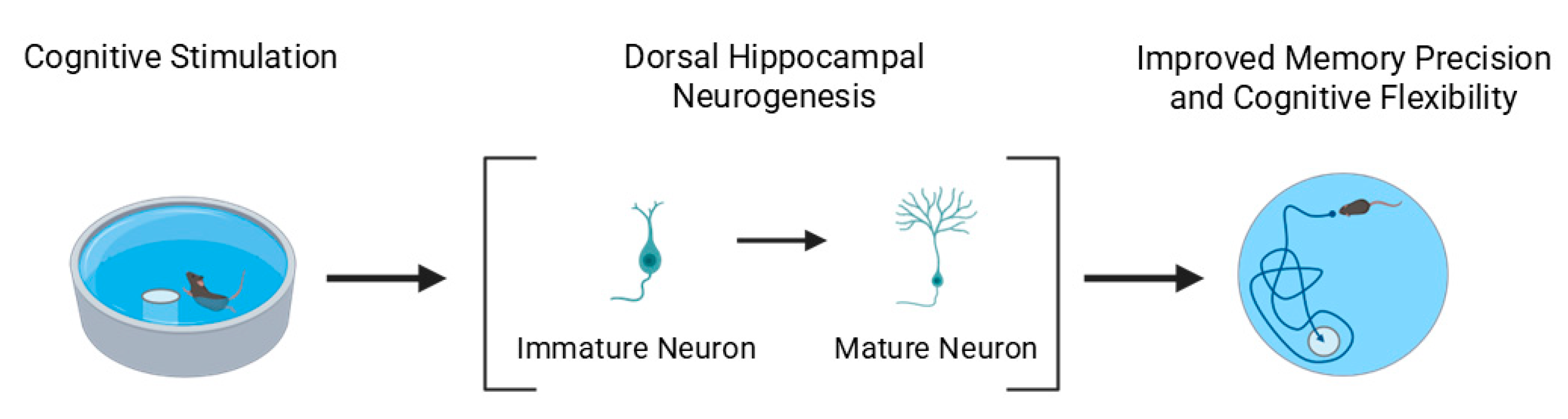
The increased survival of newborn hippocampal neurons has been linked to improved memory precision and cognitive flexibility. For example, in the Morris water maze, mice with enhanced neurogenesis exhibit more spatially precise navigation and greater adaptability when the hidden platform is relocated, reflecting improved memory updating and reduced perseveration [
140].
Although most data derive from rodent studies, evidence suggests similar adaptive processes in humans. Neuroimaging and neuropsychological assessments of London taxi drivers, who undergo extensive spatial training, revealed increased posterior hippocampal gray matter volume compared with controls and bus drivers [
141,
142]. This structural enlargement corresponded to superior performance in spatial navigation and landmark proximity judgments but reduced recall of complex non-spatial visual patterns, indicating a potential functional specialization and trade-off in hippocampal processing. These findings support the concept that persistent cognitive demands can induce hippocampal remodeling—possibly involving AHN—favoring memory improvements in domain-relevant circuits.
Regarding the dorsoventral axis, converging evidence indicates that learning-related increases in neurogenesis are more pronounced in the dorsal hippocampus, which is primarily involved in spatial and contextual learning [
123,
143] (
Figure 3). In contrast, ventral hippocampal neurogenesis appears to contribute more to emotionally charged or reward-based learning [
144]. For instance, learning paradigms with strong emotional valence or stress components, such as fear conditioning or reward anticipation, recruit ventral DG circuits where neurogenesis facilitates adaptive behavioral responses [
145]. Thus, dorsal neurogenesis supports cognitive precision and spatial mapping, whereas ventral neurogenesis underlies motivational and affective learning, reflecting complementary but distinct roles along the hippocampal longitudinal axis.
3.2.4. Rewarding Experience
The reward system comprises a network of interconnected brain regions responsible for evaluating, predicting, and anticipating rewarding stimuli, as well as motivating behavior and generating the sensation of pleasure. The orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) compute the expected value of potential rewards and the effort required to obtain them. This information is integrated by the ventromedial (vmPFC) and dorsolateral prefrontal cortex (dlPFC) to guide decision-making on reward pursuit [
146]. When rewards are obtained, dopaminergic neurons in the ventral tegmental area (VTA) signal deviations between expected and actual outcomes (reward prediction errors) [
147,
148]. If outcomes are as good as or better than predicted, these neurons increase firing toward limbic and cortical targets, including the prefrontal cortex, cingulate gyrus, nucleus accumbens (NAc), hippocampus, and amygdala. Dopaminergic input to the amygdala and cingulate gyrus generates the emotional experience of pleasure, while projections to the hippocampus facilitate long-term memory consolidation of rewarding events, enabling their future recall [
148].
Although research directly investigating the effects of rewarding experiences on AHN is still limited, available data suggest that repeated engagement in rewarding behaviors can enhance neurogenic activity. For example, sexual experience, which robustly activates the reward system [
149], increases neuronal proliferation in the hippocampal DG of both young and middle-aged rats [
150]. These effects partially restore age-related declines in neurogenesis and improve performance in cognitive tasks [
151].
Beyond behavioral experiences, dopamine, the principal neurotransmitter in reward processing, exerts direct neurogenic effects. Dopaminergic stimulation enhances the proliferation of neural precursor cells and the survival of newborn neurons in the DG [
152,
153], likely via D1/D2 receptor–mediated modulation of BDNF and CREB signaling cascades. This suggests that reward-related dopaminergic activity may couple motivational states to hippocampal structural plasticity.
Although no studies have systematically examined dorsoventral differences in reward-related AHN, converging evidence supports a ventral predominance. The ventral hippocampus is functionally connected to the NAc and amygdala, and neurogenesis in the ventral DG has been shown to modulate reward sensitivity, stress resilience, and affective regulation [
144,
145]In contrast, dorsal hippocampal neurogenesis contributes more to cognitive flexibility and spatial memory, which can also influence reward learning indirectly [
123]. These findings collectively suggest that rewarding experiences, as well as their dopaminergic reinforcement, may primarily recruit ventral hippocampal neurogenic circuits, aligning with the emotional and motivational functions of this subregion. Future research should explore how distinct rewarding stimuli (e.g., social interaction, novelty seeking, or food-related rewards) differentially influence dorsal and ventral neurogenesis and whether these effects contribute to adaptive versus maladaptive motivational behaviors.
3.2.5. Stress
Stress is defined as a physiological response to demanding environmental or psychosocial conditions which can cause uncertainty about future situations or events. Normally, its induction better prepares the organism to overcome these challenges and to reachieve homeostasis. A balanced stress response is essential for the optimal functionality of these organisms but conditions which cause extreme acute or chronic stress can lead to various negative effects.
Stress exerts profound effects on brain structure and function, and the hippocampus is one of the primary neural targets of stress-induced remodeling. The impact of stress on AHN depends on its duration, intensity, and controllability. Acute stress may transiently suppress progenitor proliferation, whereas chronic or unpredictable stress paradigms consistently produce long-lasting reductions in AHN [
154,
155]. The underlying mechanisms involve sustained activation of the HPA axis, resulting in elevated glucocorticoid levels that impair neuronal proliferation, differentiation, and survival [
156,
157]. In addition, stress-induced inflammatory cytokines, oxidative stress, and decreased trophic signaling—particularly BDNF downregulation—further contribute to reduced neurogenesis [
72,
158]
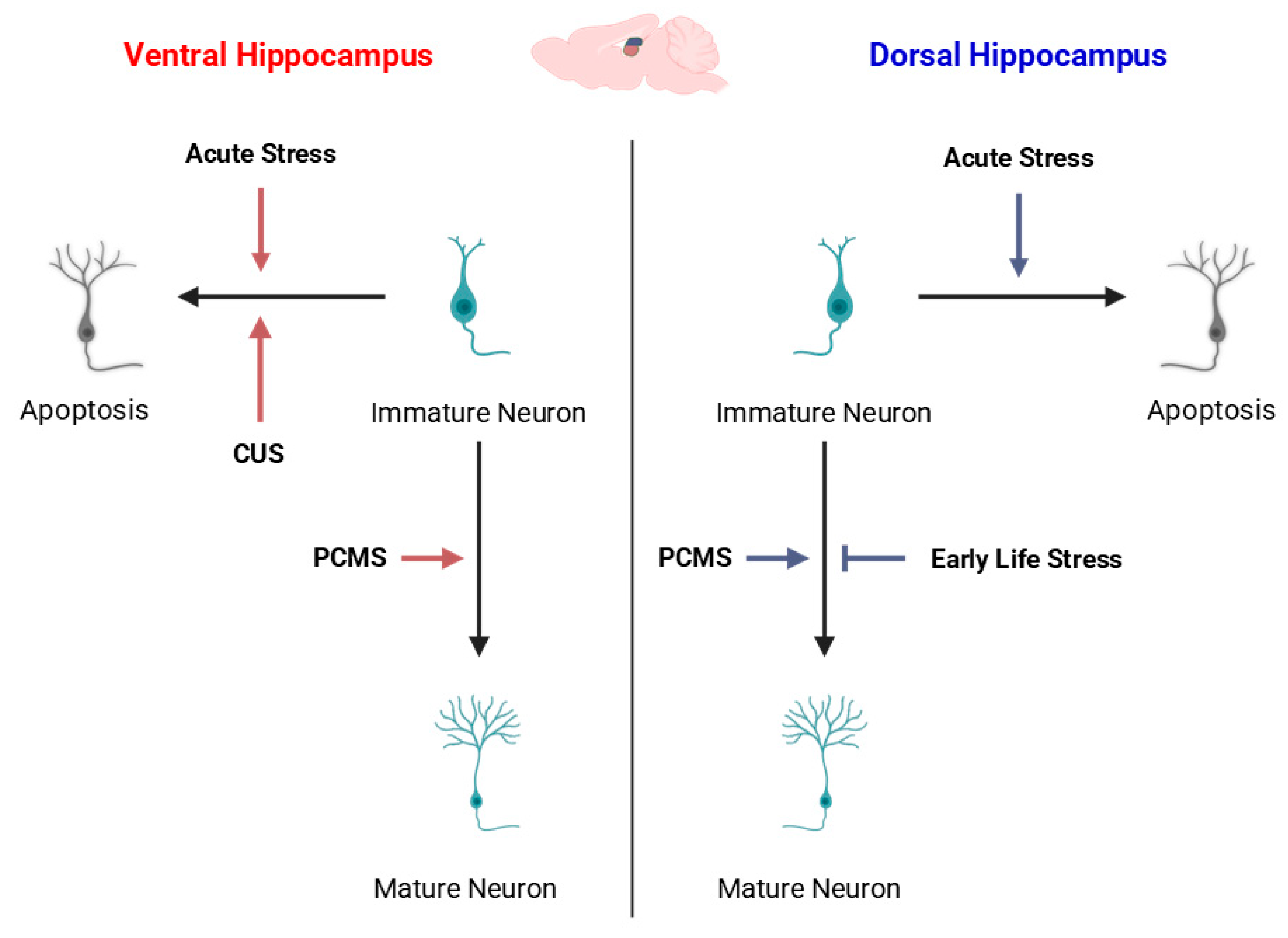
Experimental models such as chronic restraint stress, chronic unpredictable mild stress (CUMS), and social defeat have consistently shown diminished cell proliferation and neuronal differentiation within the DG [
150,
159] (
Figure 4). These reductions in AHN are accompanied by behavioral correlates of depression and anxiety, suggesting that decreased neurogenesis contributes to stress-related psychopathology [
152]. Pharmacological or behavioral interventions that reduce HPA axis hyperactivity or increase trophic signaling (e.g., antidepressants, environmental enrichment, voluntary exercise) can partially restore AHN and alleviate these behavioral deficits [
155]
The effects of stress are not uniform along the hippocampal dorsoventral axis
. A growing body of evidence demonstrates that ventral hippocampal neurogenesis is particularly sensitive to stress exposure, whereas dorsal neurogenesis is relatively more resilient. Chronic stress paradigms preferentially suppress cell proliferation and reduce immature neuron numbers in the ventral DG [
72,
157]. Conversely, the dorsal hippocampus, which is primarily involved in spatial and cognitive functions, exhibits smaller or transient changes in proliferative activity following similar stress conditions [
71]. This regional asymmetry is in line with functional specialization since the ventral hippocampus regulates emotional and stress-related responses, while the dorsal hippocampus supports cognitive processing and memory.
Moreover, recovery of ventral neurogenesis following chronic stress correlates closely with antidepressant efficacy and behavioral normalization, emphasizing its role as a key mediator of emotional resilience [
92,
145]. In contrast, dorsal neurogenesis appears to contribute more to the restoration of cognitive flexibility after stress cessation. Overall, stress primarily targets the ventral AHN, reflecting its integration into limbic–endocrine circuits. Future studies should aim to delineate the molecular determinants of this regional vulnerability and to clarify how interventions that selectively restore ventral AHN might enhance emotional resilience without perturbing cognitive function.
3.3. Diet and Nutritional Modifiers of AHN
The relationship between nutrition and brain health has been thoroughly studied and it is now widely accepted that a balanced diet throughout an organism’s lifetime is of great importance in order to achieve optimal brain function. The World Health Organization (WHO) considers a healthy diet to be a principal preventative factor for the development of cognitive decline and dementia [
160]. To better understand the impact of nutrition on mental health, researchers have studied the molecular mechanisms involved. Evidence suggests that synaptic plasticity and hippocampal neurogenesis are significantly affected by both the amount and the type of food consumed [
22]. Many of these dietary and hormonal factors exert region-specific influences along the hippocampal axis, a distinction further examined in the following section.
3.3.1. Caloric Restriction & High Fat Diet
The restriction of caloric intake without the induction of malnutrition, known as caloric restriction (CR), has been associated with multiple health benefits and extended longevity [
161]. In the hippocampus, CR influences the adult neurogenic process, though results remain inconsistent across studies. Some authors report that CR increases the number of newborn cells in the DG [
162,
163], possibly through the enhanced release of BDNF [
164]. Others, however, suggest that CR may decrease the neurogenic potential of neural progenitor cells [
165,
166,
167]. Age appears to be an important moderating factor: younger animals may be more susceptible to CR-induced reductions in neurogenesis, whereas older individuals often show maintained or improved neurogenic capacity under CR conditions [
165].
Staples et al. [
167] provided important insights into the phase- and region-specific effects of CR along the hippocampal dorsoventral axis. Their findings indicate that CR enhances the proliferation of neural progenitors while simultaneously reducing the survival of newborn neurons, and that these effects are confined to the ventral DG. In contrast, the same study found a reduction in total granule cell number restricted to the dorsal DG, whereas the ventral subregion remained unaffected. This apparent bidirectional impact underlines the need to assess AHN not only by cell counts but also by the functional integration of newborn neurons into hippocampal circuits. Region-specific vulnerability suggests that dietary interventions may exert paradoxical effects on cognition versus mood regulation, thereby enhancing emotional adaptability via ventral neurogenesis while potentially impairing dorsal, cognition-related plasticity.
Diets rich in saturated fats represent the opposite metabolic condition and are established risk factors for obesity, coronary artery disease, and several cancers [
160]. Mounting evidence indicates that high-fat diets (HFDs) also impair brain health, leading to cognitive deficits and accelerated neurodegenerative processes such as dementia and Alzheimer’s disease (AD) [
168,
169,
170,
171]. The neurobiological mechanisms underlying these effects involve significant disruption of hippocampal neurogenesis, with the ventral hippocampus showing greater susceptibility to diet-induced damage than other subregions [
172,
173,
174]. This inhibition of AHN is believed to arise from neuroinflammatory processes, mediated by activation of M1-type microglia, which release pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [
173,
174,
175]. Microglia express Toll-like receptor 4 (TLR4), which normally recognizes bacterial lipopolysaccharides (LPS), but can also be activated by oxidized low-density lipoproteins (LDL) generated during lipid metabolism [
176,
177,
178]. Activation of this pathway promotes microglial polarization toward the M1 pro-inflammatory phenotype, producing an inflammatory environment that is unfavorable to neurogenesis, leading to decreased proliferation and differentiation of newborn neurons [
179].
Collectively, these findings reveal that caloric restriction and high-fat diet exert opposing effects on hippocampal neurogenesis, and that these effects are regionally distinct along the dorsoventral axis. While CR may differentially modulate proliferation and survival between dorsal and ventral hippocampus, HFDs predominantly compromise ventral neurogenic integrity through inflammatory mechanisms. Understanding how energy balance interacts with hippocampal subregional specialization will be critical for designing dietary interventions aimed at optimizing both cognitive and emotional brain health.
3.3.2. Vitamins and Other Nutritional Factors
Vitamins and micronutrients are essential for neurodevelopment, neuronal maintenance, and neurotransmission, and their deficiencies are major contributors to central and peripheral nervous system disorders. Several vitamins play particularly important roles in AHN by regulating cell proliferation, differentiation, and survival.
B-complex vitamins, including vitamin B12 and folic acid, are critical for DNA synthesis and methylation, and therefore indispensable for NSC proliferation. Folic acid deficiency has been associated with reduced neurogenic rates—especially in the ventral hippocampus, where emotional and stress-regulatory circuits are more sensitive to metabolic disturbances [
180]. Vitamin B1 (thiamine) deficiency, which causes Wernicke encephalopathy, dramatically decreases the survival of newborn hippocampal neurons [
181]. This reduction likely contributes to the cognitive and memory deficits seen in Wernicke’s pathology, due to the strong interconnectivity between the mammillary bodies and hippocampus involved in episodic memory formation and confabulation [
181,
182].
Vitamin A (retinol), through its active metabolite retinoic acid (RA), functions as both an antioxidant and a regulator of gene expression. Vitamin A deficiency reduces hippocampal neurogenesis, likely through impaired cell proliferation, whereas RA supplementation restores it [
183]. Conversely, chronic RA administration, as used in some dermatological treatments, has been reported to suppress AHN [
184], underscoring the need for precise dose regulation.
Vitamin C (ascorbic acid), a potent antioxidant and enzymatic cofactor, enhances hippocampal neurogenesis and counteracts D-galactose–induced brain aging by promoting newborn neuron maturation [
185]. Postnatal vitamin C deficiency reduces total hippocampal neuron counts in guinea pigs [
186]. Overall, vitamin C appears to support neurogenesis not only in the hippocampus but also in other neurogenic niches (for review, see [
187]).
Among trace elements, zinc is particularly important for neurogenic integrity. Zinc supplementation promotes survival of neuronal progenitor cells, likely through the inhibition of apoptosis [
188]. Similarly, selenium supports AHN by increasing neural progenitor survival and reducing neuroinflammation. Its role is mediated via PI3K/Akt/Nrf2 and BDNF/TrkB signaling pathways [
189]. Although regional differences have not yet been systematically explored, selenium’s neuroprotective role may extend preferentially to the ventral hippocampus, where oxidative and inflammatory stress are more prominent.
Omega-3 polyunsaturated fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are essential for maintaining neuronal membrane fluidity and synaptic function. DHA supplementation increases hippocampal neurogenesis by enhancing newborn neuron survival [
190], while long-term dietary EPA/DHA intake reverses age-related declines in neurogenesis [
191]. These effects may be more pronounced in the ventral hippocampus, consistent with the antidepressant and anxiolytic effects of omega-3 fatty acids and the ventral region’s role in emotional regulation.
Flavonoids, a broad class of plant-derived polyphenols, have received considerable attention for their antioxidant, anti-inflammatory, and neuroprotective effects. They modulate oxidative stress and neuroinflammation and may act directly on neuronal progenitors to promote proliferation and differentiation (for review, see [
192]). Curcumin, the yellow polyphenolic pigment found in Curcuma longa (turmeric), shares similar properties. It enhances AHN by rescuing newborn neurons from apoptosis and activating the Wnt/β-catenin pathway while increasing BDNF expression [
193,
194].
Collectively, these findings highlight the essential role of vitamins, trace elements, and bioactive dietary compounds in maintaining AHN. While evidence for dorsoventral specificity remains limited, deficiencies in vitamins such as folate or nutrients affecting oxidative and inflammatory homeostasis appear to impair ventral neurogenesis preferentially, potentially leading to emotional dysregulation. In contrast, nutrients that enhance synaptic and trophic signaling (e.g., omega-3 fatty acids, vitamin A, curcumin) may also strengthen dorsal neurogenesis linked to cognitive resilience. Future work should address how specific micronutrient pathways interact with hippocampal subregional specialization to support both cognitive and emotional health.
Table 1.
A summary of the most important nutritional factors affecting AHN along with examples of foods that contain them in high concentrations.
Table 1.
A summary of the most important nutritional factors affecting AHN along with examples of foods that contain them in high concentrations.
| Nutritional Factor |
Effect on AHN |
Examples of Foods with High Concentrations |
References |
| High-Fat |
Decrease |
Processed meat, Butter, Full-Fat dairy products |
[172]
[195] |
| Folic Acid |
Increase |
Leafy green vegetables, legumes, whole grains, egg yolk, liver, citrus fruit |
[180]
[196] |
| Vitamin B1 |
Increase |
Brown rice, whole grains, pork, poultry, soybeans, nuts, peas, dried beans |
[181]
[197] |
| Vitamin E |
Increase |
Wheat germ oil, sunflower seeds, almonds, hazelnuts, peanuts, broccoli, spinach |
[198]
[199]
|
| Vitamin A |
Increase |
Pumpkin, carrot, squash, sweet potato, mango, papaya, poultry, fish, red grapefruit, collards |
[183]
[200] |
| Vitamin C |
Increase |
Citrus fruits, berries, tomatoes, potatoes, green leafy vegetables |
[187]
[201] |
| Zinc |
Increase |
Beef, legumes, poultry, cereals, pork |
[188]
[202] |
| Omega-3 Fatty Acids |
Increase |
Fish, poultry, dairy |
[191]
[203] |
| Flavonoids |
Increase |
Red wine, tea, peppers, blueberries, strawberries, apples, spinach, onions |
[192]
[204] |
| Curcumin |
Increase |
Turmeric, yellow curry |
[193] |
| Selenium |
Increase |
Seafood, meats, eggs and dairy products |
[189] |
4. AHN in a Dorsoventral Perspective
The hippocampus exhibits a remarkable functional and structural differentiation along its dorsoventral axis, which extends from the dorsal (septal) to the ventral (temporal) pole. This organization is accompanied by distinct, yet interrelated, behavioural and cognitive functions subserved by each region: the dorsal hippocampus is primarily engaged in spatial and cognitive processing, whereas the ventral hippocampus is preferentially involved in emotion, stress regulation, and affective behaviour [
205,
206], which are accompanied by specializations at the molecular and cellular levels [
20,
206,
207,
208]. Correspondingly, AHN also displays regional heterogeneity in baseline activity, regulation, and functional relevance [
145,
209,
210].
4.1. Dorsoventral Heterogeneity of Neurogenesis
Early studies suggested that cell proliferation and immature neuron density (marked by doublecortin expression) were higher in the dorsal DG of rodents, implying a predominance of neurogenesis related to cognitive functions [
54,
143]. However, subsequent research revealed that mature adult-born neurons are more numerous in the ventral DG, indicating that overall neuronal addition may actually favor the ventral region. This contradiction could be the result of increased newborn neuron survival in the ventral hippocampus and be dependent on species, age, and environmental conditions.
In marmosets, for example, a relatively homogeneous distribution of doublecortin (DCX)-positive neurons was observed along the septotemporal axis [
210], whereas in rodents, dorsal enrichment in proliferation coexists with stronger neurogenic modulation in the ventral domain under specific physiological or stress conditions [
123,
211]. Furthermore, this apparent paradox suggests that regional differences in survival rates and maturation kinetics are critical determinants of final neurogenic output.
In general, baseline levels of progenitor proliferation and immature neuron density are higher in the dorsal DG, and cognitive stimulation through environmental enrichment and learning paradigms tends to preferentially enhance dorsal neurogenesis [
123,
143] supporting its tonic role in information encoding, spatial learning, and pattern separation in the dorsal hippocampus [
54,
143]. In contrast, the ventral DG typically exhibits lower baseline neurogenesis, but it shows increased sensitivity to environmental, hormonal, and emotional stimuli. Chronic stress paradigms, such as social defeat and unpredictable chronic stress, preferentially suppress ventral neurogenesis, while antidepressant treatments predominantly restore neurogenesis in this same region. Neurogenesis in the ventral hippocampus is preferentially affected by stress, antidepressants, and neuromodulators [
71,
72,
92](O’Leary et al., 2014; Alvarez-Contino et al., 2023; Liu et al., 2023). For instance, chronic stress or elevated Tau expression selectively suppresses ventral neurogenesis and promotes anxiety-like behaviour [
72], whereas antidepressant treatments such as fluoxetine or GALR2/Y1R agonists restore neurogenesis primarily in the ventral DG and alleviate depressive phenotypes [
92,
212]. Thus, while the dorsal hippocampus maintains stable, tonic neurogenesis supporting cognitive operations, the ventral hippocampus is dynamically regulated by stress hormones, immune signals, and antidepressant treatments, reflecting its emotional and adaptive plasticity. This regional specialization aligns with the established functional dichotomy with the dorsal hippocampus involved mainly in spatial/cognitive processing and the ventral hippocampus more involved in emotional/stress regulation.
4.2. Molecular Mechanisms Underlying Dorsoventral Differences
The molecular mechanisms underlying dorsoventral differences in neurogenesis are increasingly understood. For instance, the miR-17-92 cluster, is more highly expressed in the ventral DG, promoting neurogenesis and exerting antidepressant-like effects [
213]. Similarly, region-specific signalling via neuregulin-1 and β₂-adrenergic or GABAergic pathways selectively promote ventral neurogenesis and emotional resilience [
92,
214,
215,
216]. Conversely, dorsal neurogenesis is preferentially modulated by learning and cognitive stimulation, with higher increases in the dorsal hippocampus following environmental enrichment, physical activity, and training tasks [
123,
143]. Transcription factors such as AP2γ or JNK1 influence proliferation and neuronal differentiation with predominant effects in the dorsal hippocampus, consistent with its cognitive specialization [
216,
217], reinforcing a distinction between cognitive-tonic and emotional-plastic neurogenic segmentation of the hippocampus. On the other hand, the miR-17-92 cluster shows higher expression in the ventral hippocampus and promotes neurogenesis with antidepressant-like effects [
213,
218]. In addition, JNK1 inhibition produces preferential enhancement of neurogenesis in the ventral hippocampus [
216]. Also, the secreted frizzled-related protein 3 (sfrp3) exhibits a dorsoventral gradient that could potentially contribute to differential adult NSC activity between dorsal and ventral regions [
219,
220].
Norepinephrine and potassium chloride treatment significantly preferentially enhance neurosphere formation in temporal (ventral-equivalent) hippocampal progenitors regions compared with septal (dorsal-equivalent) ones, indicating higher intrinsic excitability and responsiveness of ventral neural stem cells [
221,
222].
4.3. Functional Implications
Selective regulation of neurogenesis along this axis produces behaviourally dissociable outcomes, thereby supporting a dual neurogenic system. Neurogenesis in the dorsal hippocampus contributes to pattern separation, spatial learning, and memory precision, whereas ventral-born neurons integrate into circuits regulating affective and stress responses [
144,
145]. Suppression of ventral neurogenesis increases anxiety and stress susceptibility [
72], while enhancement of ventral hippocampus neurogenesis confers stress resilience and antidepressant-like effects [
145,
212]. Importantly, neurogenic responses to environmental or pharmacological modulation, including exercise, enriched environments, or antidepressant drugs, show regionally distinct outcomes. Manipulations that potentiate dorsal neurogenesis correlates with cognitive flexibility and spatial memory performance [
123,
143]. Environmental enrichment or voluntary exercise can upregulate neurogenesis in both hippocampal segments but contributes differentially to behavioural improvement: dorsal neurogenesis supports cognitive recovery, whereas ventral neurogenesis appears to mediate emotional normalization [
123]. Interestingly, adult-born neurons in both dorsal and ventral hippocampus contribute to contextual fear conditioning, but likely through distinct mechanisms. Dorsal adult-born neurons may contribute to the acquisition and recall of context representations, while ventral adult-born neurons may modulate the ability of these representations to activate fear via hippocampal projections to the amygdala [
223,
224,
225,
226]. These findings indicate that behavioural outcomes depend on the spatial locus of neurogenesis activation.
4.4. Integrative Perspective
Taken together, these findings support a bidirectional model in which dorsal and ventral neurogenesis function as complementary systems for adaptive behaviour. The dorsal hippocampus functions as a stable, cognition-oriented generator with higher tonic neurogenic output, and the ventral hippocampus, characterized by lower baseline activity but greater modulatory plasticity, may function as a flexible regulator of affective behaviour, supporting emotional adaptation to environmental and physiological challenges. As illustrated in
Figure 5, modulatory influences exhibit distinct dorsoventral profiles, highlighting their predominant regional effects and directionality on AHN. This spatial specialization may have evolved to allow parallel yet integrated regulation of cognition and emotion via distinct neurogenic domains. Variability in dorsoventral neurogenesis patterns across species [
210,
227] and experimental models add further complexity to this framework, and suggest that region-specific susceptibility to stress, hormones, or disease factors could underlie differential neuropsychiatric vulnerabilities across species, thereby pointing to important clinical implications. For instance, understanding that ventral neurogenesis preferentially responds to antidepressant treatments while dorsal neurogenesis supports cognitive enhancement could inform precision medicine approaches for psychiatric and neurological conditions. The clinical implications of AHN will be further discussed in
Section 5.
Table 2.
Summary of factors affecting AHN that have been studied along the hippocampal longitudinal axis.
Table 2.
Summary of factors affecting AHN that have been studied along the hippocampal longitudinal axis.
| Factor |
Effect on Ventral AHN |
Effect on Dorsal AHN |
References |
| Learning |
No change / Decrease |
Increase |
[143,228] |
| Chronic Unpredictable Stress |
Decrease |
No change |
[156] |
| Early Life Stress |
No change |
Decrease |
[158] |
| Physical Activity |
No change / Increase |
Increase |
[38,83,122] |
| Prolonged Sleep Deprivation |
Decrease |
Decrease |
[128] |
| Caloric Restriction |
Increase / Decrease |
Decrease |
[167] |
| High-Fat Diet |
Decrease |
Decrease |
[173] |
| Folic Acid |
Increase |
No change / Increase |
[180] |
| Cortisol |
Decrease* |
No change / Decrease* |
[36] |
| Estrogens |
Increase / No change /
Decrease ** |
Increase / Decrease ** |
[49,54] |
| Androgens |
Increase |
Increase |
[58] |
| Oxytocin |
Increase |
No change |
[68] |
| Melatonin |
Increase |
Increase |
[83] |
5. Neuropsychiatric Implications
Many of the factors that influence the rates of adult Neurogenesis AHN have also been associated with multiple neuropsychiatric disorders, characterized by cognitive, emotional, and motivational dysregulation, such as Major Depressive Disorder (MDD), Schizophrenia and AD. This observation led to the assumption that adult Neurogenesis AHN may play a role in the pathogenesis and prognosis of these diseases with recent evidence further supporting this theory [
229]. The dorsoventral differentiation of AHN provides a valuable anatomical and functional framework to interpret these alterations. Disorders with predominant cognitive impairments may involve dysfunction of dorsal neurogenesis, whereas those characterized by emotional dysregulation, anxiety, or motivational disturbances are more likely associated with ventral deficits. Reduced ventral neurogenesis is a recurring feature across mood and stress-related disorders, whereas symptoms associated with dorsal deficits are often prodromal in conditions marked by cognitive impairment such as AD.
5.1. Major Depressive Disorder & Anxiety Disorders
MDD is a psychiatric disorder classically characterized by persistent depressed mood and loss of interest in activities that were previously enjoyed by the diseased individual (anhedonia). When symptoms are severe, patients might experience suicidal ideation and / or resort to self-harm [
230]. Anxiety disorders are either chronic or episodic/situational states characterized primarily by excessive fear, worry, or nervousness that is disproportionate to the situation and interferes with daily functioning. Evidence shows reduced AHN in models of MDD and anxiety, particularly within the ventral hippocampus, which integrates stress, serotonergic, and neuroendocrine inputs [
72,
145].
Multiple studies have been conducted to explore structural differences in patients with MDD with evidence indicating that the hippocampi of the diseased are usually characterized by reduced gray matter volume. This finding is positively correlated with the duration of the illness [
231,
232]. However, evidence is not sufficient to support causality.
In addition, acute and severe forms of MDD are associated with an elevated cortisol response [
45] which is detrimental to newborn neuron proliferation. These findings indicate that reduced rates of AHN could explain a part of MDD pathogenesis [
233]. This hypothesis is further supported by the fact that the same factors which have been found to be beneficial for the mood of MDD patients, such as healthy diet and lifestyle, are also shown to have positive effects in neurogenic activity. Moreover, anti-depressants used for the treatment of MDD, such as Selective Serotonin Reuptake Inhibitors (SSRIs), Tricyclic Antidepressants (TCAs) and Monoamine oxidase Inhibitors (MAOIs) have been shown to significantly increase the rates of AHN through an increase in the number and maturation neural progenitor cells [
212,
234,
235,
236,
237]. These neurogenic enhancements seem to in fact be essential for the positive effect of anti-depressants, since mice with abolished neurogenic potential fail to show behavioral improvement following chronic anti-depressant administration [
238,
239]. Chronic stress, glucocorticoid excess, and pro-inflammatory states suppress ventral proliferation and neuronal differentiation, effects that can be reversed by antidepressant treatment or by stimulation of neurotrophic signaling [
71,
92,
212]. Antidepressants such as SSRIs, Agomelatine or GALR2/Y1R agonists preferentially restore neurogenesis in the ventral domain, paralleling the normalization of emotional behavior [
212,
240,
241]. The neurobiological mechanism through which the neurogenic benefits of anti-depressants are mediated is potentially related to the activation of the Wnt pathway in neural progenitor cells, induced by the increase of Serotonin levels and the subsequent activation of 5-hydroxytryptamine (5-HT) receptors and increased BDNF release. [
242,
243]. The activation of the Wnt pathway through this mechanism has particularly been observed in the newborn neurons located in the Ventral Hippocampus [
243]. Another proposed mechanism through which antidepressants and serotonin enhance AHN levels is through the increase of hippocampal BDNF levels [
244,
245].
However, whether enhanced neurogenesis is a cause or consequence of antidepressant efficacy remains unresolved. Longitudinal imaging or biomarker studies could clarify if AHN recovery precedes mood improvement, thereby testing the neurogenesis hypothesis of depression more directly.
Furthermore, it should be noted that Nonpharmacologic treatments of MDD, such as Electroconvulsive Therapy (ECT) and vagus nerve stimulation, have also been associated with significantly enhanced AHN [
246].
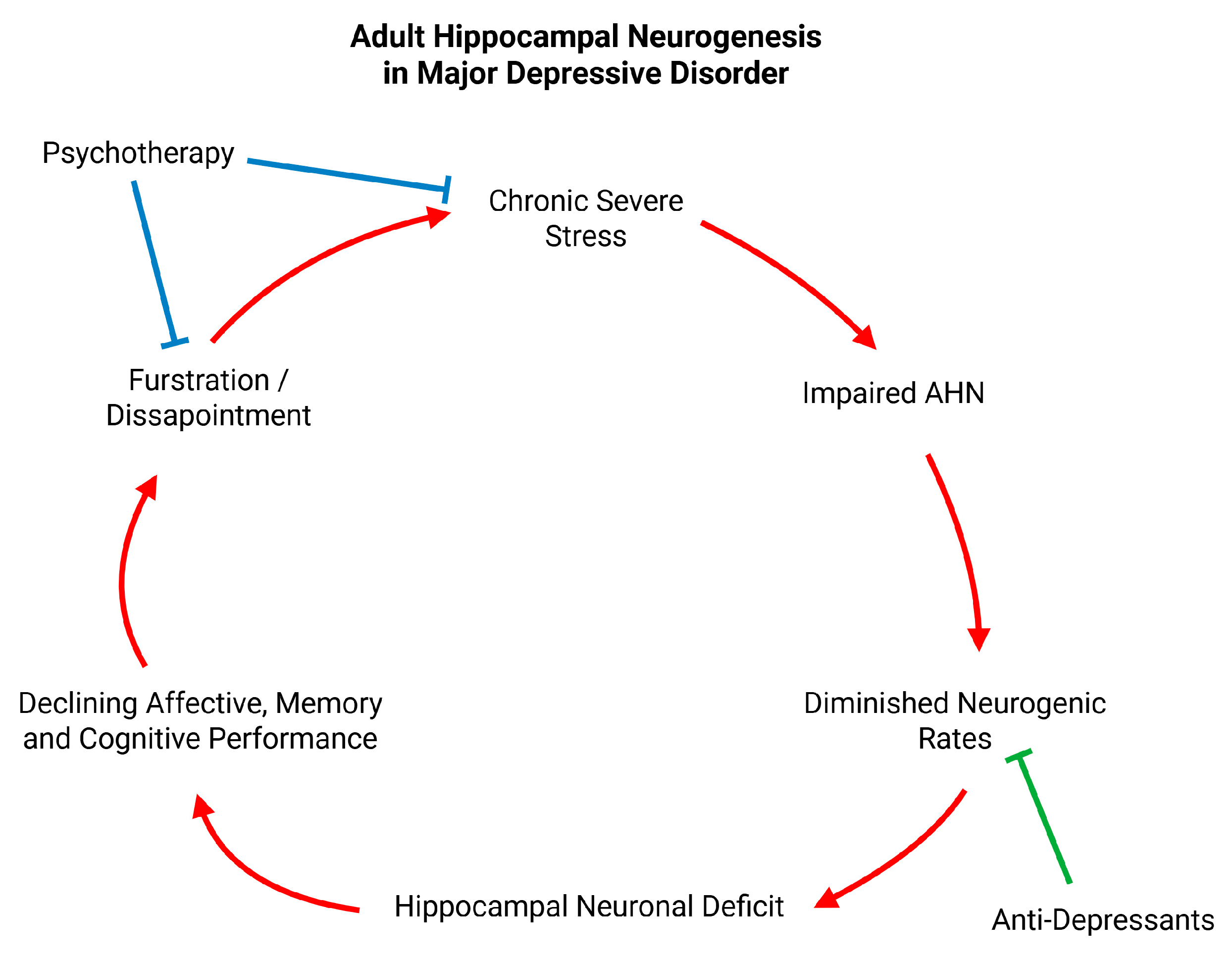
From a more psychological and clinical perspective, a potential mechanism through which these neuronal deficits seen in the hippocampus of untreated MDD patients can lead to the expression of MDD symptomatology is the dysfunction of the existing and constantly diminishing circuitry to provide emotional variability to the patient. This mechanism could partly account for the persistent depressive mood and anhedonia observed in patients with MDD (
Figure 6). The birth of new neurons could enhance the function of the ventral hippocampus and offer the patient an increase of emotional diversity apart from the persistent depressed mood MDD is characterized by. Additionally, researchers have shown that the neuronal deficits in the dorsal hippocampus of MDD patients can also lead to a diminished memory performance [
247]. This subpar performance can interfere with the patient’s daily life and can lead to their frustration and disappointment which can further negatively impact their mood. This cycle of events can confine MDD patients in a depressive state and psychiatric intervention at this stage should be deemed necessary (
Figure 6).
It should be noted, however, that although decreased neurogenic rates can not solely lead to depressive behaviors [
248], we propose that decreased neurogenic rates can diminish the ability of an individual to psychologically overcome daily psychosocial stressors and can therefore predispose to the development and unfavorable prognosis of MDD. In addition to depression, reduced AHN has also been observed in chronic anxiety models, consistent with the ventral hippocampus’ role in affective regulation [
155]. While the evidence base is smaller, these findings suggest that impaired neurogenesis may contribute more broadly to stress-related psychopathology.
5.2. Schizophrenia
Schizophrenia is a chronic neuropsychiatric disorder, in which the patient experiences positive symptoms, such as hallucinations, delusions, disorganized speech and behaviour, as well as negative symptoms, such as impaired cognitive performance, social isolation, reduced motivation and flat affect. These symptoms cause patients to face significant disability, impairing their ability to function in daily life and can reduce their life expectancy by approximately 13-15 years [
249].
The brains of patients suffering from Schizophrenia have been well studied by researchers with results indicating that the hippocampi are among the key regions affected. More specifically, a meta-analysis comparing patients suffering from chronic schizophrenia and healthy controls showed that the average hippocampal volume was significantly smaller in schizophrenia patients [
250]. In addition, altered AHN has also been described in schizophrenia, though evidence is more heterogeneous. Neurodevelopmental models and patient studies suggest reduced proliferation and impaired integration of new granule cells in the DG [
246,
251]. There have been reports of decreased expression of cell proliferation markers and impaired maturation of newborn neurons in the adult hippocampus of schizophrenia patients, indicating that the structural differences mentioned above could be attributed to low neurogenic potential [
246]. Weissleder et. al. have explored the pathological origin of these observations and suggested that an increase in immune cell and macrophage population could explain this neurogenic impairment [
252]. It should be noted, however, that since Schizophrenia has strong heritability and usually presents at a young age, the genetic component of the disease cannot be dismissed. Many genes have been associated with an increased risk for schizophrenia development with DISC1, Dgcr8, FEZ1, NPAS3, SNAP-25 and a-CaMKII being of great interest. Notably, all of these genes not only predispose to schizophrenia but are also significantly important in the neurogenic process, playing roles in NSC proliferation and newborn neuron maturation [
246]. Moreover, a recent study showed that a mutation in the Neuregulin 1 Nuclear Signaling Pathway can cause a dysregulation of schizophrenia susceptibility genes and lead to significantly reduced synaptic plasticity NSC proliferation, even further supporting the existence of an association between Schizophrenia and Neurogenesis [
253].
Cognitive and negative symptoms, including working-memory deficits and contextual disorganization, are likely associated with dorsal hippocampal dysfunction, given the dorsal domain’s role in cognitive mapping and mnemonic precision. Conversely, positive and affective symptoms, such as stress-sensitive hallucinations or emotional dysregulation, may reflect ventral hippocampal hyperactivity and aberrant coupling with limbic regions [
254]. In rodent models, ventral hippocampal hyperexcitability drives dopaminergic dysregulation in the VTA and NAc, a hallmark of psychosis [
255]. If adult-born ventral neurons normally function to modulate or buffer this excitatory output, impaired ventral AHN could exacerbate the limbic overdrive underlying positive symptoms. Conversely, deficient dorsal AHN could impair contextual binding and contribute to cognitive disorganization.
In summary, while Schizophrenia symptoms cannot solely be attributed to low neurogenic rates, the disease’s underlying pathophysiology could lead to impaired AHN which could explain some of the structural differences and the symptoms that the patients present with, especially the ones related to cognitive and affective functions. The genes that have been implicated to predispose individuals to schizophrenia could play such an important role in the birth and maturation of neurons, that both the developing and developed brain are affected. A possibility that can also not be excluded, however, is that these adult hippocampal neurogenic deficits observed in schizophrenia patients, could also be resultant from the emotional flattening and stress that these individuals experience. In addition, schizophrenia may involve a bidirectional imbalance along the dorsoventral axis of the hippocampus: ventral hippocampus overactivity with reduced inhibitory neurogenic modulation, and dorsal hippocampus under-recruitment impairing cognitive integration. Further research needs to be conducted to determine the exact role of the known predisposing genes in the neurogenic process and the precise role of dorsal and ventral hippocampus neurogenesis in the development of schizophrenia symptoms.
5.3. Alzheimer’s Disease
AD is a neurodegenerative disorder and the most common form of dementia, a condition characterized by cognitive decline which significantly impairs an individual’s ability to meet their basic needs independently. AD patients experience a progressive deterioration in memory performance, attention, reasoning ability, speech production and comprehension [
256].
A characteristic finding in brains of AD patients is significant brain atrophy and neuronal death in many brain areas, including the hippocampal formation. This degeneration is thought to be caused by the impaired clearance and accumulation of senile plaques and neurofibrillary tangles in the affected brain tissue [
257,
258]. Strikingly, many of the non-genetic risk and protective factors for AD have also been associated with impairment and enhancement of AHN respectively [
Table 3]. This observation has led many researchers to further explore the relationship between AHN and AD with evidence indicating that neurogenic potential is greatly diminished in AD patients compared to healthy adults [
5]. Furthermore, other conditions that have been associated with low adult hippocampal neurogenic rates, such as MDD, have also been considered as risk factors for the development of AD [
259].
Though the decline in AHN in AD is widespread, is, however, particularly evident in the dorsal hippocampus, consistent with the dominance of cognitive and mnemonic impairments in the disease [
260]. In addition, loss of progenitor proliferation and impaired neuronal differentiation parallel the progression of hippocampal atrophy and memory deficits. While both hippocampal segments eventually deteriorate, the selective vulnerability of the dorsal hippocampus corresponds to the early predominance of spatial and episodic memory decline, whereas the ventral hippocampus contribution may become more evident in later stages, when anxiety and affective disturbances emerge.
Collectively, the above findings certainly suggest a connection between AHN and AD. Although low neurogenic rates cannot solely explain the pathogenesis of AD, they could play a decisive role in the development and prognosis of the disease in combination with the other associated factors, such as the accumulation of senile plaques and neurofibrillary tangles. In addition, avoiding factors that are detrimental to AHN, such as a high-fat diet and stress, and engaging in activities that enhance neurogenic potential, such as physical exercise and learning, which promote dorsal neurogenesis and have been associated with partial cognitive benefits, further reinforces the functional link between normal AHN in the dorsal hippocampus and mnemonic flexibility. These activities contribute to the formation of a strong cognitive reserve, which has been shown to play an important role in preventing AD and other neurodegenerative disorders [
261,
262]. Collectivelly, the accumulation of neutoxic molecules in combination with low AHN rates, influenced by environmental factors, could have an additive effect which is detrimental to the aging brain’s resilience against neurodegenerative diseases, such as AD.
Table 3.
A summary of acquired factors discussed in this review and their impact on the development of Alzheimer’s Disease (AD) in comparison to their effect on AHN.
Table 3.
A summary of acquired factors discussed in this review and their impact on the development of Alzheimer’s Disease (AD) in comparison to their effect on AHN.
| Factor |
Protective / Risk Factor for AD |
Effect on AHN |
References |
| Learning / Education |
Protective |
Increase |
[136,261] |
| Physical Activity |
Protective |
Increase |
[38,263] |
| High-Fat diet |
Risk Factor |
Decrease |
[172,174,264] |
| Sleep Deprivation |
Risk Factor |
Decrease |
[128,265] |
| Stress / Depression |
Risk Factor |
Decrease |
[154,155,233,266] |
5.4. Addiction
Substance and Nonsubstance addiction are neuropsychiatric disorders in which patients have a strong and repeated desire to seek and consume a certain substance or perform certain activities. This desire is usually characterized by tolerance, with patients each time seeking a greater amount or an increased exposure to the substance or activity they are addicted to. The individuals who fail to satisfy this need can develop withdrawal symptoms which makes abstinence from the addicting factor even more challenging [
267].
The neurobiology of addiction involves the aberrant activation of the brain’s dopaminergic reward system, in which neurons from the midbrain’s VTA project to multiple areas including the frontal cortex, NAc and the hippocampus. The increased activation of Dopamine receptors especially in the NAc is implicated for the development of addiction’s symptomatology. The hippocampal formation also plays a vital role in the pathophysiology of addiction through efferent and afferent neuronal projections towards and from the VTA and NAc, and is responsible for the storing of contextual memories related to the addictive factor and possibly for initiating the driving force towards its acquirement [
246,
268,
269]. In particular, addictive behaviors engage neural circuits linking the hippocampus to reward and motivation centers, particularly the NAc and VTA, which receive strong input from the ventral hippocampus. Ventral hippocampal projections provide contextual information that can bias reward seeking and drug craving [
270].
Regarding substance addiction, the vast majority of addictive drugs, including Nicotine, Opioids, Cocaine, Amphetamine and 3,4-Methylenedioxymethamphetamine (MDMA), has been associated with either decreased NSC proliferation or decreased survival of newborn neurons in the hippocampi of rodents (for review see [
271]). The susceptibility to addiction has also been associated with AHN, as individuals suffering with other conditions that are considered to decrease neurogenic rates, such as MDD, Stress and Schizophrenia, are also more likely to become addiction victims [
272,
273,
274]. This claim is further supported by the fact that ablation of AHN makes rat models more susceptible to cocaine addiction [
275]. Notably, another study demonstrated that blocking ventral AHN levels led rats to preferring immediate small rewards over greater delayed ones, a finding consistent with addictive behaviour [
144]. Furthermore, activities that are known to increase neurogenic rates, such as physical exercise, have been found to be protective against relapse [
276,
277]. These observations led many researchers to believe that AHN plays a vital role in the prevention as well as treatment of addiction [
278,
279] and activities that further enhance it, such as physical activity, healthy diet, sufficient sleep and stress relief, could be advised to patients to further minimize their chances of relapse and succumbing to addiction in the first place. Interestingly, experimental disruption of AHN enhances cue-induced reinstatement of drug seeking, whereas restoration of neurogenesis reduces relapse susceptibility [
278]. The ventral hippocampus, through its connectivity with limbic-reward circuits, may act as a contextual gate, linking environmental cues to motivational drive. Thus, ventral AHN could serve a homeostatic role, dampening hyper-reactivity of reward circuits and promoting behavioral flexibility. In contrast, dorsal AHN likely contributes to the cognitive control of reward-related learning and extinction, processes such as discriminating safe from drug-associated contexts or updating maladaptive memories. A dorsoventral imbalance, with ventral suppression and dorsal rigidity, may therefore underlie both emotional vulnerability and impaired cognitive regulation during addiction.
6. Discussion
The existence and functional significance of AHN in humans remain debated, yet accumulating evidence suggests it persists at low but functionally relevant levels across life. While early reports provided compelling evidence for ongoing neuronal birth in the adult hippocampus, subsequent studies have questioned its prevalence, proposing that neurogenesis may decline to negligible levels in adulthood. Recent findings, however, suggest a more nuanced picture: AHN may persist throughout life but at levels that vary with age, disease state, and methodological approach. In particular, studies using sensitive markers and rigorous tissue preservation consistently detect immature neurons even in aged individuals, whereas negative findings often arise from postmortem samples with compromised integrity. Beyond the presence vs absence dichotomy, the critical question is whether the observed levels are functionally relevant. Evidence linking reduced AHN with MDD, AD, and stress vulnerability argues that even modest neurogenesis could shape plasticity in human circuits. Resolving this debate will require standardized methodologies and, crucially, the development of non-invasive tools to quantify neurogenic activity in the living human brain.
The growing interest in AHN and decades of research have led to significant progress in understanding the neurobiological mechanisms underlying this process. Additionally, notable studies have not only identified factors that influence AHN but have also examined which stages of neurogenesis they affect. Some studies have even explored whether these factors exert subregion-specific effects within the hippocampal formation [
Table 2]. This detail, however, is commonly overlooked, as most studies assess AHN levels in the hippocampus as a whole. Given the undeniable evidence that the dorsal/posterior and ventral/anterior hippocampus differ not only functionally but also electrophysiologically [
20,
208], we urge future research to present data separately for these two structures, treating them as distinct entities. From our review of the literature, we consider worth highlighting the increased sensitivity of the ventral hippocampus to glucocorticoids [
36], which are closely associated with stress, and the heightened plasticity of the dorsal/posterior hippocampus in response to learning challenges [
141,
142,
143]. Given the role of the ventral hippocampus in affective functions and the role of the dorsal hippocampus in cognitive functions, these findings suggest the possibility of a region-specific recruitment of newborn neurons by the hippocampal DG in accordance to the brain’s functional demands. From a cellular perspective, this recruitment could be mediated through an increased survival and maturation to apoptosis ratio of newborn immature neurons during the early survival phase of the neurogenic process in response to stimuli from the affective or cognitive challenges. This hypothesis is of great interest and should be further explored.
Furthermore, we demonstrated findings suggesting a negative correlation between the levels of AHN and the development of neuropsychiatric disease, such as MDD and AD. However, while current evidence does not establish causality, the possibility that low AHN levels may predispose individuals to these conditions and influence their prognosis warrants further investigation. Experiments have been conducted involving mice with evidence indicating that AHN enhancement can promote resilience to stress and cognitive impairment [
280,
281]. Therefore, modulation of AHN levels through utilization of the easily modifiable factors affecting them could be explored in clinical practice, as an attempt to conservatively enhance AHN rates may complement existing treatments, potentially improving prognosis and treatment efficacy for neuropsychiatric diseases. Moreover, given the importance of AHN levels in the prognosis and their potential in the treatment of mental disease, AHN rates have been proposed as a potential biomarker for mental health, although reliable non-invasive quantification methods are still lacking.
Future methods enabling the quantification of neurogenic activity in living humans may predict the development of neuropsychiatric diseases long before symptom onset, allowing physicians to make early interventions to delay disease progression and thus significantly improve the quality of the patient’s life in the long term. Bridging rodent and human data remains a key challenge due to species-specific differences in hippocampal architecture and behavioral correlates. Future work integrating imaging, stem-cell models, and computational approaches may clarify AHN’s translational relevance.
Collectively, research on AHN in humans is still in its early stages. Scepticism regarding its functional and clinical significance, along with challenges in studying its levels in living humans, has discouraged further exploration. Yet, many questions remain unresolved. Could the stress resilience linked to neurogenic enhancement in rodents also apply to humans? Can the benefits of activities like physical exercise in alleviating neuropsychiatric symptoms be attributed to an enhancement of AHN rates? Could the utilization of modifiable factors that increase AHN rates in animals lead to clinical improvements or slow the progression of neurodegenerative diseases in humans? Addressing these questions could reignite scientific interest in AHN and unlock its clinical potential in preventing, diagnosing, and treating neuropsychiatric disease, while also taking us one step closer in understanding the neurobiology behind emotion, cognition and brain function as a whole. Moreover, recognizing the region-specific plasticity of AHN may ultimately refine our understanding of cognition–emotion interactions and inform region-targeted interventions for neuropsychiatric disease.
Author Contributions
Investigation, I.E., Supervision, C.P. Writing-original draft preparation, I.E.; writing-review and editing, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Acknowledgments
During the preparation of this manuscript, the authors used BioRender and EndNote 21 for the purposes of figure creation and reference management. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI |
Multidisciplinary Digital Publishing Institute |
| DOAJ |
Directory of open access journals |
| TLA |
Three letter acronym |
| LD |
Linear dichroism |
| AHΝ |
Adult Hippocampal Neurogenesis |
| BrdU |
Bromodeoxyuridine |
| NSC |
Neural Stem Cell |
| TAP |
Transiently Amplifying Progenitor |
| SGZ |
Subgranular Zone |
| DG |
Dentate Gyrus |
| CORT |
Cortisol / Corticosterone |
| HPA |
Hypothalamic-Pituitary-Adrenal |
| GR |
Glucocorticoid Receptor |
| MR |
Mineralocorticoid Receptor |
| ER |
Estrogen Receptor |
| GPER1 |
G Protein-coupled Estrogen Receptor 1 |
| AR |
Androgen Receptor |
| OXTR |
Oxytocin Receptor |
| TH |
Thyroid Hormone |
| TR |
Thyroid Hormone Receptor |
| GH |
Growth Hormone |
| IGF-1 |
Insulin-like Growth Factor – 1 |
| IGF1R |
Insulin Growth Factor 1 Receptor |
| GHR |
Growth Hormone Receptor |
| EPO |
Erythropoietin |
| EPO-R |
Erythropoietin Receptor |
| BDNF |
Brain Derived Neurotrophic Factor |
| VEGF |
Vascular Endothelial Growth Factor |
| REM |
Rapid Eye Movement |
| NREM |
Non-Rapid Eye Movement |
| EEG |
Electroencephalogram |
| OFC |
Orbitofrontal Cortex |
| ACC |
Anterior Cingulate Cortex |
| vmPFC |
Ventromedial Prefrontal Cortex |
| dlPFC |
Dorsolateral Prefrontal Cortex |
| VTA |
Ventral Tegmental Area |
| NaC |
Nucleus Accumbens |
| CRH |
Corticotropin-Releasing Hormone |
| ACTH |
Adrenocorticotropic Hormone |
| CUS |
Chronic Unpredictable Stress |
| IL-1 |
Interleukin-1 |
| PCMS |
Predictable Chronic Mild Stress |
| WHO |
World Health Organization |
| IL-6 |
Interleukin-6 |
| TNF-α |
Tumor Necrosis Factor – α |
| TLR |
Toll-Like Receptor |
| LPS |
Lipopolysaccharides |
| LDL |
Low-Density Lipoprotein |
| RA |
Retinoic Acid |
| DHA |
Docosahexaenoic Acid |
| EPA |
Eicosapentaenoic Acid |
| MDD |
Major Depressive Disorder |
| SSRI |
Selective Serotonin Reuptake Inhibitor |
| TCA |
Tricyclic Antidepressants |
| MAOI |
Monoamine Oxidase Inhibitor |
| 5-HT |
5-Hydroxytryptamine |
| ECT |
Electroconvulsive Therapy |
| AD |
Alzheimer’s Disease |
| MDMA |
3,4-Methylenedioxymethamphetamine |
References
- Altman, J. Are New Neurons Formed in the Brains of Adult Mammals? Science 1962, 135, doi:10.1126/science.135.3509.1127.
- Bayer, S.A.; Yackel, J.W.; Puri, P.S. Neurons in the Rat Dentate Gyrus Granular Layer Substantially Increase During Juvenile and Adult Life. Science 1982-5-21, 216, doi:10.1126/science.7079742.
- Boldrini M., F.C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J., Hen R., Mann J.J. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, doi:10.1016/j.stem.2018.03.015.
- Tobin M.K., M.K., Disouky A., Shetti A., Bheri A., Honer W.G., Kim N., Dawe R.J., Bennett D.A., Arfanakis K., Lazarov O. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, doi:10.1016/j.stem.2019.05.003.
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M.; Moreno-Jiménez, E.P.; Flor-García, M.; et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature Medicine 2019 25:4 2019-03-25, 25, doi:10.1038/s41591-019-0375-9.
- Zhou, Y.; Su, Y.; Li, S.; Kennedy, B.C.; Zhang, D.Y.; Bond, A.M.; Sun, Y.; Jacob, F.; Lu, L.; Hu, P.; et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 2022 607:7919 2022-07-06, 607, doi:10.1038/s41586-022-04912-w.
- Wang, W.; Wang, M.; Yang, M.; Zeng, B.; Qiu, W.; Ma, Q.; Jing, X.; Zhang, Q.; Wang, B.; Yin, C.; et al. Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell Research 2022 Jun 24, 32, doi:10.1038/s41422-022-00678-y.
- EB, P.; MB, D.; IP, I.; VG, P.; SG, D.; DS, K. Effect of acute hypoxic shock on the rat brain morphology and tripeptidyl peptidase I activity. Acta histochemica 2016 Jun, 118, doi:10.1016/j.acthis.2016.05.003.
- Dumitru, I.; Paterlini, M.; Zamboni, M.; Ziegenhain, C.; Giatrellis, S.; Saghaleyni, R.; Björklund, Å.; Alkass, K.; Tata, M.; Druid, H.; et al. Identification of proliferating neural progenitors in the adult human hippocampus. Science 2025-07-03, 389, doi:10.1126/science.adu9575.
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018 555:7696 2018-03-07, 555, doi:10.1038/nature25975.
- Seki, T.; Hori, T.; Miyata, H.; Maehara, M.; Namba, T.; Seki, T.; Hori, T.; Miyata, H.; Maehara, M.; Namba, T. Analysis of proliferating neuronal progenitors and immature neurons in the human hippocampus surgically removed from control and epileptic patients. Scientific Reports 2019, 9, doi:10.1038/s41598-019-54684-z.
- Duque, A.; Arellano, J.I.; Rakic, P.; Duque, A.; Arellano, J.I.; Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Molecular Psychiatry 2021 27:1 2021-10-19, 27, doi:10.1038/s41380-021-01314-8.
- G, Z.; PA, S.; AK, S. Neural stem cells persist to generate new neurons in the hippocampus of adult and aged human brain - Fiction or accurate? Ageing research reviews 2023 Dec, 92, doi:10.1016/j.arr.2023.102133.
- Jurkowski, M.P.; Bettio, L.; K. Woo, E.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Frontiers in Cellular Neuroscience 2020/09/29, 14, doi:10.3389/fncel.2020.576444.
- Bird, C.M.; Burgess, N.; Bird, C.M.; Burgess, N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience 2008 9:3 2008/03, 9, doi:10.1038/nrn2335.
- JD, S. Hippocampal function in cognition. Psychopharmacology 2004 Jun, 174, doi:10.1007/s00213-004-1795-9.
- Voss, J.L.; Bridge, D.J.; Cohen, N.J.; Walker, J.A. A closer look at the hippocampus and memory. Trends in cognitive sciences 2017 Jun 15, 21, doi:10.1016/j.tics.2017.05.008.
- Zhu, Y.; Gao, H.; Tong, L.; Li, Z.; Wang, L.; Zhang, C.; Yang, Q.; Yan, B. Emotion Regulation of Hippocampus Using Real-Time fMRI Neurofeedback in Healthy Human. Frontiers in Human Neuroscience 2019/07/16, 13, doi:10.3389/fnhum.2019.00242.
- Sahay, A.; Hen, R.; Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nature Neuroscience 2007 10:9 2007-08-28, 10, doi:10.1038/nn1969.
- Fanselow, M.S.; Dong, H.-W. Are The Dorsal and Ventral Hippocampus functionally distinct structures? Neuron 2010 Jan 14, 65, doi:10.1016/j.neuron.2009.11.031.
- Kheirbek, M.A.; Hen, R. Dorsal vs Ventral Hippocampal Neurogenesis: Implications for Cognition and Mood. Neuropsychopharmacology 2011 36:1 2010-11-30, 36, doi:10.1038/npp.2010.148.
- Melgar-Locatelli, S.; Ceglia, M.d.; Mañas-Padilla, M.C.; Rodriguez-Pérez, C.; Castilla-Ortega, E.; Castro-Zavala, A.; Rivera, P. Nutrition and adult neurogenesis in the hippocampus: Does what you eat help you remember? Frontiers in Neuroscience 2023 Feb 23, 17, doi:10.3389/fnins.2023.1147269.
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Experimental Neurology 2012/01/01, 233, doi:10.1016/j.expneurol.2011.01.008.
- Navarro-Sanchis, C.; Brock, O.; Winsky-Sommerer, R.; Thuret, S. Modulation of Adult Hippocampal Neurogenesis by Sleep: Impact on Mental Health. Frontiers in Neural Circuits 2017/10/12, 11, doi:10.3389/fncir.2017.00074.
- PS, E.; E, P.; T, B.-E.; AM, A.; C, N.; DA, P.; FH, G. Neurogenesis in the adult human hippocampus. Nature medicine 1998 Nov, 4, doi:10.1038/3305.
- Spalding, Kirsty L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, Hagen B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, Bruce A.; et al. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell 2013/06/06, 153, doi:10.1016/j.cell.2013.05.002.
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harbor Perspectives in Biology 2015 Sep, 7, doi:10.1101/cshperspect.a018812.
- Kloet, E.R.D.; Reul, J.M.H.M. Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 1987/01/01, 12, doi:10.1016/0306-4530(87)90040-0.
- Menuet, D.L.; Lombès, M. The neuronal mineralocorticoid receptor: From cell survival to neurogenesis. Steroids 2014/12/01, 91, doi:10.1016/j.steroids.2014.05.018.
- Saaltink, D.-J.; Vreugdenhil, E.; Saaltink, D.-J.; Vreugdenhil, E. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cellular and Molecular Life Sciences 2014 71:13 2014-02-13, 71, doi:10.1007/s00018-014-1568-5.
- Gass, P.; Kretz, O.; Wolfer, D.P.; Berger, S.; Tronche, F.; Reichardt, H.M.; Kellendonk, C.; Lipp, H.P.; Schmid, W.; Schütz, G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO reports 2000-11-01, 1, doi:10.1093/embo-reports/kvd088.
- Gesmundo, I.; Villanova, T.; Gargantini, E.; Arvat, E.; Ghigo, E.; Granata, R. The Mineralocorticoid Agonist Fludrocortisone Promotes Survival and Proliferation of Adult Hippocampal Progenitors. Frontiers in Endocrinology 2016/06/16, 7, doi:10.3389/fendo.2016.00066.
- Wang, G.; Cao, L.; Li, S.; Zhang, M.; Li, Y.; Duan, J.; Li, Y.; Hu, Z.; Wu, J.; Li, T.; et al. Corticosterone Impairs Hippocampal Neurogenesis and Behaviors through p21-Mediated ROS Accumulation. Biomolecules 2024, Vol. 14, Page 268 2024-02-23, 14, doi:10.3390/biom14030268.
- HS, S.; SH, L.; HJ, M.; YH, S.; HJ, J.; KH, L.; C, A.; EM, J. Prolonged stress response induced by chronic stress and corticosterone exposure causes adult neurogenesis inhibition and astrocyte loss in mouse hippocampus. Brain research bulletin 2024 Mar, 208, doi:10.1016/j.brainresbull.2024.110903.
- S, B.; LA, G. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 07/14/2010, 168, doi:10.1016/j.neuroscience.2010.04.023.
- BR, L.; MG, C.; GM, M.; YM, N.; JF, C.; OF, O.L. Adult-born neurons from the dorsal, intermediate, and ventral regions of the longitudinal axis of the hippocampus exhibit differential sensitivity to glucocorticoids. Molecular psychiatry 2021 Jul, 26, doi:10.1038/s41380-020-0848-8.
- SK, D.; A, G.; S, U.; MB, M.; AC, L.; JM, R. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology 2003 Jul, 144, doi:10.1210/en.2003-0097.
- Bednarczyk, M.R.; Aumont, A.; Décary, S.; Bergeron, R.; Fernandes, K.J.L. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus 2009/10/01, 19, doi:10.1002/hipo.20621.
- F, M.; R, D.; BB, J.; D, J. Effect of environmental enrichment on stress related systems in rats. Journal of neuroendocrinology 2004 May, 16, doi:10.1111/j.1365-2826.2004.01173.x.
- J, V.; BN, S.; TR, R.; BS, S.R. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neuroscience letters 05/22/2009, 455, doi:10.1016/j.neulet.2009.03.059.
- A, T.; Q, R.; F, M.; A, S.; C, B. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology 2012 Sep, 63, doi:10.1016/j.neuropharm.2012.04.022.
- H, B.-J.; G, V.-P.; M, A.-S.; S, R.-M. Hormonal responses to different sexually related conditions in male rats. Hormones and behavior 2006 Mar, 49, doi:10.1016/j.yhbeh.2005.08.005.
- Leuner, B.; Mendolia-Loffredo, S.; Kozorovitskiy, Y.; Samburg, D.; Gould, E.; Shors, T.J. Learning Enhances the Survival of New Neurons beyond the Time when the Hippocampus Is Required for Memory. Journal of Neuroscience 2004-08-25, 24, doi:10.1523/JNEUROSCI.0204-04.2004.
- Odaka, H.; Adachi, N.; Numakawa, T. Impact of glucocorticoid on neurogenesis. Neural Regeneration Research 2017 Jul, 12, doi:10.4103/1673-5374.211174.
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder—Translating Findings From Humans to Animal Models and Back. Frontiers in Psychiatry 2020 Jan 22, 10, doi:10.3389/fpsyt.2019.00974.
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Hormones and behavior 2015 Jun 27, 74, doi:10.1016/j.yhbeh.2015.06.010.
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Edvinsson, J.C.A.; Edvinsson, L.; Haanes, K.A. Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects. The Journal of Headache and Pain 2020 Nov 10, 21, doi:10.1186/s10194-020-01197-0.
- C, Z.; JG, N.; XR, K.; XJ, M.; Q, L.; FF, C.; WF, R.; J, L. G protein-coupled estrogen receptor 1 deficiency impairs adult hippocampal neurogenesis in mice with schizophrenia. Journal of chemical neuroanatomy 2023 Oct, 132, doi:10.1016/j.jchemneu.2023.102319.
- Yagi, S.; Mohammad, A.; Wen, Y.; Burrowes, A.A.B.; Blankers, S.A.; Galea, L.A.M. Estrogens dynamically regulate neurogenesis in the dentate gyrus of adult female rats. Hippocampus 2024/11/01, 34, doi:10.1002/hipo.23633.
- Tanapat, P.; Hastings, N.B.; Gould, E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. Journal of Comparative Neurology 2005/01/17, 481, doi:10.1002/cne.20385.
- RE, M.; CK, B.; LA, G. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Hormones and behavior 2013 Jan, 63, doi:10.1016/j.yhbeh.2012.09.011.
- BK, O.; TT, L.; LA, G. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience 2004, 128, doi:10.1016/j.neuroscience.2004.06.039.
- Chaiton JA, W.S., Galea LA. Chronic aromatase inhibition increases ventral hippocampal neurogenesis in middle-aged female mice. Psychoneuroendocrinology 2019.
- Yamada, J.; Hatabe, J.; Tankyo, K.; Jinno, S. Cell type- and region-specific enhancement of adult hippocampal neurogenesis by daidzein in middle-aged female mice. Neuropharmacology 2016/12/01, 111, doi:10.1016/j.neuropharm.2016.08.036.
- HK, K.; T, D.; H, P.; HL, K. Enhanced conversion of androstenedione to estrogens in obese males. The Journal of clinical endocrinology and metabolism 1980 Nov, 51, doi:10.1210/jcem-51-5-1128.
- Duarte-Guterman, P.; Lieblich, S.E.; Wainwright, S.R.; Chow, C.; Chaiton, J.A.; Watson, N.V.; Galea, L.A.M. Androgens Enhance Adult Hippocampal Neurogenesis in Males but Not Females in an Age-Dependent Manner. Endocrinology 2019/09/01, 160, doi:10.1210/en.2019-00114.
- Spritzer M.D., G.L.A.M. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Developmental Neurobiology 2007, 67, doi:10.1002/dneu.20457.
- Swift-Gallant, A.; Duarte-Guterman, P.; Hamson, D.K.; Ibrahim, M.; Monks, D.A.; Galea, L.A.M. Neural androgen receptors affect the number of surviving new neurones in the adult dentate gyrus of male mice. Journal of Neuroendocrinology 2018/04/01, 30, doi:10.1111/jne.12578.
- FG, N.G.; J, F.; D, V.C.; RC, C.; GM, V.; V, D.A.; MK, d.M.R.; PI, B.; EA, C.; RM, A. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014 Dec, 50, doi:10.1016/j.psyneuen.2014.08.009.
- Hamson DK, W.S., Taylor JR,Jones BA,Watson NV,Galea LA. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology 2013.
- Galea LA, W.S., Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. Journal of neuroendocrinology 2013.
- Tabori NE, S.L., Znamensky V,Romeo RD,Alves SE,McEwen BS,Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience 2005.
- Gimpl G, F.F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews 2001.
- Robert C Froemke, L.J.Y. Oxytocin, Neural Plasticity, and Social Behavior. Annual review of neuroscience 2021.
- Yoshimura R., K.H., Kimura T., Araki T., Maeno H., Tanizawa O., Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology 1993.
- Boccia, M.L.; Petrusz, P.; Suzuki, K.; Marson, L.; Pedersen, C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013/12/03, 253, doi:10.1016/j.neuroscience.2013.08.048.
- Monari, P.K.; Herro, Z.J.; Bymers, J.; Marler, C.A. Chronic intranasal oxytocin increases acoustic eavesdropping and adult neurogenesis. Hormones and Behavior 2023/11/01, 156, doi:10.1016/j.yhbeh.2023.105443.
- Leuner, B.; Caponiti, J.M.; Gould, E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus 2012/04/01, 22, doi:10.1002/hipo.20947.
- Duarte-Guterman, P.; Lieblich, S.E.; Qiu, W.; Splinter, J.E.J.; Go, K.A.; Casanueva-Reimon, L.; Galea, L.A.M. Oxytocin has sex-specific effects on social behaviour and hypothalamic oxytocin immunoreactive cells but not hippocampal neurogenesis in adult rats. Hormones and Behavior 2020/06/01, 122, doi:10.1016/j.yhbeh.2020.104734.
- Anacker C, H.R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nature Reviews 2017.
- O’Leary OF, C.J. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends in pharmacological sciences 2014.
- Liu, H., Yang, Zhi,Yu, Chunyan,Dong, Hao,Wang, Shiyan,Wang, Gang,Wang, Denian. Tau aggravates stress-induced anxiety by inhibiting adult ventral hippocampal neurogenesis in mice. Cerebral Cortex 2023.
- Cook C.B., K.R.J. Expression of erbAα and β mRNAs in regions of adult rat brain. Molecular and Cellular Endocrinology 1990, 70, doi:10.1016/0303-7207(90)90054-C.
- Wallis, K.; Dudazy, S.; Hogerlinden, M.v.; Nordström, K.; Mittag, J.; Vennström, B.r. The Thyroid Hormone Receptor α1 Protein Is Expressed in Embryonic Postmitotic Neurons and Persists in Most Adult Neurons. Molecular Endocrinology 2010/10/01, 24, doi:10.1210/me.2010-0175.
- Desouza, L.A.; Ladiwala, U.; Daniel, S.M.; Agashe, S.; Vaidya, R.A.; Vaidya, V.A. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Molecular and Cellular Neuroscience 2005/07/01, 29, doi:10.1016/j.mcn.2005.03.010.
- Kapoor, R.; Desouza, L.A.; Nanavaty, I.N.; Kernie, S.G.; Vaidya, V.A. Thyroid Hormone Accelerates the Differentiation of Adult Hippocampal Progenitors. Journal of Neuroendocrinology 2012/09/01, 24, doi:10.1111/j.1365-2826.2012.02329.x.
- Kapoor, R.; Hogerlinden, M.v.; Wallis, K.; Ghosh, H.; Nordstrom, K.; Vennstrom, B.; Vaidya, V.A. Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010 Aug 13, 24, doi:10.1096/fj.10-161802.
- J, B. Thyroid hormone receptors in brain development and function Nature clinical practice. Endocrinology & metabolism 2007.
- Pia D. Bagamasbad, J.E.C.E., Joseph R. Knoedler,Arasakumar Subramani,Ariel J. Harden,Robert J. Denver. Coordinated transcriptional regulation by thyroid hormone and glucocorticoid interaction in adult mouse hippocampus-derived neuronal cells. PLOS ONE 2019.
- Muriel Rigolet, N.B., Marylou Scharwatt,Evelyne Duvernois-Berthet,Daniel R. Buchholz,Laurent M. Sachs,Rigolet, Muriel,Buisine, Nicolas,Scharwatt, Marylou,Duvernois-Berthet, Evelyne,Buchholz, Daniel R.,Sachs, Laurent M. Crosstalk between Thyroid Hormone and Corticosteroid Signaling Targets Cell Proliferation in Xenopus tropicalis Tadpole Liver. International Journal of Molecular Sciences 2022.
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G.; Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Structure and Function 2017 222:7 2017-05-06, 222, doi:10.1007/s00429-017-1439-6.
- K, R.; M, D.B.; BA, P. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. Journal of pineal research 2009 Nov, 47, doi:10.1111/j.1600-079X.2009.00716.x.
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. Journal of pineal research 2012 Nov 28, 54, doi:10.1111/jpi.12023.
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G.; Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin Modulates Cell Survival of New Neurons in the Hippocampus of Adult Mice. Neuropsychopharmacology 2009 34:9 2009-05-06, 34, doi:10.1038/npp.2009.46.
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. Journal of Pineal Research 2011/01/01, 50, doi:10.1111/j.1600-079X.2010.00802.x.
- Ramírez-Rodríguez, G.B.; Palacios-Cabriales, D.M.; Ortiz-López, L.; Estrada-Camarena, E.M.; Vega-Rivera, N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal- and Ventral- Dentate Gyrus Concomitantly with Its Antidepressant-Like Effect in Male Balb/C Mice. International Journal of Molecular Sciences 2020 Mar 3, 21, doi:10.3390/ijms21051724.
- Wongchitrat, P.; Lansubsakul, N.; Kamsrijai, U.; Sae-Ung, K.; Mukda, S.; Govitrapong, P. Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochemistry International 2016/11/01, 100, doi:10.1016/j.neuint.2016.09.006.
- Ruksee, N.; Tongjaroenbuangam, W.; Mahanam, T.; Govitrapong, P. Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. The Journal of Steroid Biochemistry and Molecular Biology 2014/09/01, 143, doi:10.1016/j.jsbmb.2014.02.011.
- He, W.; Wang, X.; Yang, X.; Zhang, G.; Zhang, J.; Chen, L.; Niu, P.; Chen, T. Melatonin mitigates manganese-induced neural damage via modulation of gut microbiota-metabolism in mice. Science of The Total Environment 2024/05/01, 923, doi:10.1016/j.scitotenv.2024.171474.
- Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Hwang, I.K.; Kwon, Y.-G.; Kim, Y.-M.; Won, M.-H. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. Journal of Neuroscience Research 2010/09/01, 88, doi:10.1002/jnr.22430.
- Chern, C.-M.; Liao, J.-F.; Wang, Y.-H.; Shen, Y.-C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radical Biology and Medicine 2012/05/01, 52, doi:10.1016/j.freeradbiomed.2012.01.030.
- Jose Erik Alvarez-Contino, E.D.-S., Marina Mirchandani-Duque,Jose Andrés Sánchez-Pérez,Miguel A. Barbancho,Alexander López-Salas,Natalia García-Casares,Kjell Fuxe,Dasiel O. Borroto-Escuela,Manuel Narváez. GALR2 and Y1R agonists intranasal infusion enhanced adult ventral hippocampal neurogenesis and antidepressant-like effects involving BDNF actions. Journal of Cellular Physiology 2023.
- Joseph Wai-Hin Leung, K.-K.C., Shirley Pui-Ching Ngai,Hector Wing-Hong Tsang,Benson Wui-Man Lau,Leung, Joseph Wai-Hin,Cheung, Kwok-Kuen,Ngai, Shirley Pui-Ching,Tsang, Hector Wing-Hong,Lau, Benson Wui-Man. Protective Effects of Melatonin on Neurogenesis Impairment in Neurological Disorders and Its Relevant Molecular Mechanisms. International Journal of Molecular Sciences 2020.
- Boiko, D.I., Shkodina, Anastasiia D.,Hasan, Mohammad Mehedi,Bardhan, Mainak,Kazmi, Syeda Kanza,Chopra, Hitesh,Bhutra, Prerna,Baig, Atif Amin,Skrypnikov, Andrii M.,Boiko, Dmytro I.,Shkodina, Anastasiia D.,Hasan, Mohammad Mehedi,Bardhan, Mainak,Kazmi, Syeda Kanza,Chopra, Hitesh,Bhutra, Prerna,Baig, Atif Amin,Skrypnikov, Andrii M. Melatonergic Receptors (Mt1/Mt2) as a Potential Additional Target of Novel Drugs for Depression. Neurochemical Research 2022.
- Wasinski, F.; Klein, M.O.; Bittencourt, J.C.; Metzger, M.; Donato, J. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Research 2021/01/15, 1751, doi:10.1016/j.brainres.2020.147189.
- Russo, V.C.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The Insulin-Like Growth Factor System and Its Pleiotropic Functions in Brain. Endocrine Reviews 2005/12/01, 26, doi:10.1210/er.2004-0024.
- N, D.A.; J, L.; J, I.; H, G.K. Peripheral growth hormone induces cell proliferation in the intact adult rat brain. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society 2010 Jun, 20, doi:10.1016/j.ghir.2009.12.003.
- MA, A.; ND, A.; H, H.; J, O.; PS, E. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 04/15/2000, 20, doi:10.1523/JNEUROSCI.20-08-02896.2000.
- Blackmore, D.G.; Vukovic, J.; Waters, M.J.; Bartlett, P.F. GH Mediates Exercise-Dependent Activation of SVZ Neural Precursor Cells in Aged Mice. PLoS ONE 2012 Nov 27, 7, doi:10.1371/journal.pone.0049912.
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006/01/01, 140, doi:10.1016/j.neuroscience.2006.02.084.
- José Luis Trejo, E.C., Ignacio Torres-Alemán. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. Journal of Neuroscience 2001.
- Yasunari Matsuzaka, R.Y., Matsuzaka, Yasunari,Yashiro, Ryu. Molecular Regulation and Therapeutic Applications of Brain-Derived Neurotrophic Factor–Tropomyosin-Related Kinase B Signaling in Major Depressive Disorder Though Its Interaction with Vascular Endothelial Growth Factor and N-Methyl-D-Aspartic Acid Receptors: A Narrative Review. Biologics 2025.
- Hiroshi Udo, Y.Y., Takako Kino,Koichiro Ohnuki,Wataru Mizunoya,Takao Mukuda,Hiroyuki Sugiyama. Enhanced Adult Neurogenesis and Angiogenesis and Altered Affective Behaviors in Mice Overexpressing Vascular Endothelial Growth Factor 120. Journal of Neuroscience 2008.
- Sonia Sanchez-Bezanilla, D.J.B., Rebecca J. Hood,N. David Åberg,Patricia Crock,Frederick R. Walker,Michael Nilsson,Jörgen Isgaard,Lin Kooi Ong. Growth Hormone Increases BDNF and mTOR Expression in Specific Brain Regions after Photothrombotic Stroke in Mice. Neural Plasticity 2022.
- Kamilla G. Haugland, A.O., Andreas Lande,Kirsten B. Kjelstrup,Vegard H. Brun. Hippocampal growth hormone modulates relational memory and the dendritic spine density in CA1. Learning & Memory 2020.
- Noguchi, C.T.; Asavaritikrai, P.; Teng, R.; Jia, Y. Role of erythropoietin in the brain. Critical reviews in oncology/hematology 2007 May 4, 64, doi:10.1016/j.critrevonc.2007.03.001.
- Arabpoor, Z.; Hamidi, G.; Rashidi, B.; Shabrang, M.; Alaei, H.; Sharifi, M.R.; Salami, M.; Dolatabadi, H.R.D.; Reisi, P. Erythropoietin improves neuronal proliferation in dentate gyrus of hippocampal formation in an animal model of Alzheimer’s disease. Advanced Biomedical Research 2012 Aug 28, 1, doi:10.4103/2277-9175.100157.
- Leconte, C.; Bihel, E.; Lepelletier, F.-X.; Bouët, V.; Saulnier, R.; Petit, E.; Boulouard, M.; Bernaudin, M.; Schumann-Bard, P. Comparison of the effects of erythropoietin and its carbamylated derivative on behaviour and hippocampal neurogenesis in mice. Neuropharmacology 2011/02/01, 60, doi:10.1016/j.neuropharm.2010.09.025.
- Wakhloo, D.; Scharkowski, F.; Curto, Y.; Butt, U.J.; Bansal, V.; Steixner-Kumar, A.A.; Wüstefeld, L.; Rajput, A.; Arinrad, S.; Zillmann, M.R.; et al. Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nature Communications 2020 Mar 9, 11, doi:10.1038/s41467-020-15041-1.
- RR, K.; BR, K. The effects of peripheral hormone responses to exercise on adult hippocampal neurogenesis. Frontiers in endocrinology 11/24/2023, 14, doi:10.3389/fendo.2023.1202349.
- Morris, T.P.; Fried, P.J.; Macone, J.; Stillman, A.; Gomes-Osman, J.; Costa-Miserachs, D.; Muñoz, J.M.T.; Santarnecchi, E.; Pascual-Leone, A. Light Aerobic Exercise Modulates Executive Function and Cortical Excitability. The European journal of neuroscience 2019 Nov 1, 51, doi:10.1111/ejn.14593.
- Tour, S.L.; Shaikh, H.; Beardwood, J.H.; Augustynski, A.S.; Wood, M.A.; Keiser, A.A. The weekend warrior effect: Consistent intermittent exercise induces persistent cognitive benefits. Neurobiology of Learning and Memory 2024/10/01, 214, doi:10.1016/j.nlm.2024.107971.
- Eisenstein, T.; Giladi, N.; Hendler, T.; Havakuk, O.; Lerner, Y. Physically Active Lifestyle Is Associated With Attenuation of Hippocampal Dysfunction in Cognitively Intact Older Adults. Frontiers in Aging Neuroscience 2021/10/06, 13, doi:10.3389/fnagi.2021.720990.
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 2011 Jan 31, 108, doi:10.1073/pnas.1015950108.
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wójcicki, T.R.; Mailey, E.L.; et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, Behavior, and Immunity 2013/02/01, 28, doi:10.1016/j.bbi.2012.10.021.
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences 2007/09/01, 30, doi:10.1016/j.tins.2007.06.011.
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025/07/01, 24, doi:10.1111/acel.70061.
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nature Metabolism 2021 3:8 2021-08-20, 3, doi:10.1038/s42255-021-00438-z.
- SY, Y.; A, L.; A, X.; KF, S. Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment? Neural regeneration research 2015 Jan, 10, doi:10.4103/1673-5374.150637.
- XA, Z.; DG, B.; J, Z.; FA, N.; X, T.; ND, K.; A, C.; KY, V.; KH, C.; T, J.; et al. Neurogenic-dependent changes in hippocampal circuitry underlie the procognitive effect of exercise in aging mice. iScience 11/16/2021, 24, doi:10.1016/j.isci.2021.103450.
- Aztiria, E.; Capodieci, G.; Arancio, L.; Leanza, G. Extensive training in a maze task reduces neurogenesis in the adult rat dentate gyrus probably as a result of stress. Neuroscience Letters 2007/04/12, 416, doi:10.1016/j.neulet.2007.01.069.
- Nishijima, T.; Kawakami, M.; Kita, I. Long-Term Exercise Is a Potent Trigger for ΔFosB Induction in the Hippocampus along the dorso–ventral Axis. PLoS ONE 2013 Nov 25, 8, doi:10.1371/journal.pone.0081245.
- Zheng J, J.Y., Xu LC,Ma LY,Liu FY,Cui S,Cai J,Liao FF,Wan Y,Yi M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. The Journal of Neuroscience 2017.
- Patel AK, R.V., Shumway KR, et al. Physiology, Sleep Stages. StatPearls 2024.
- Diekelmann, S.; Born, J.; Diekelmann, S.; Born, J. The memory function of sleep. Nature Reviews Neuroscience 2010 11:2 2010-01-04, 11, doi:10.1038/nrn2762.
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P.; Krause, A.J.; Simon, E.B.; Mander, B.A.; et al. The sleep-deprived human brain. Nature Reviews Neuroscience 2017 18:7 2017-05-18, 18, doi:10.1038/nrn.2017.55.
- Perlis, M.L.; Giles, D.E.; Buysse, D.J.; Tu, X.; Kupfer, D.J. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of Affective Disorders 1997/02/01, 42, doi:10.1016/S0165-0327(96)01411-5.
- Murata, Y.; Oka, A.; Iseki, A.; Mori, M.; Ohe, K.; Mine, K.; Enjoji, M. Prolonged sleep deprivation decreases cell proliferation and immature newborn neurons in both dorsal and ventral hippocampus of male rats. Neuroscience Research 2018/06/01, 131, doi:10.1016/j.neures.2017.08.008.
- Mirescu, C.; Peters, J.D.; Noiman, L.; Gould, E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America 2006 Nov 29, 103, doi:10.1073/pnas.0608644103.
- Fernandes, C., Rocha, Nuno Barbosa F. ,Rocha, Susana ,Herrera-Solís, Andrea ,Salas-Pacheco, José ,García-García, Fabio ,Murillo-Rodríguez, Eric ,Yuan, Ti-Fei ,Machado, Sergio ,Arias-Carrión, Oscar Detrimental role of prolonged sleep deprivation on adult neurogenesis. Frontiers in Cellular Neuroscience 2015.
- Soto-Rodriguez, S., Lopez-Armas, Gabriela ,Luquin, Sonia ,Ramos-Zuñiga, Rodrigo ,Jauregui-Huerta, Fernando ,Gonzalez-Perez, Oscar ,Gonzalez-Castañeda, Rocio E. . Rapid Eye Movement Sleep Deprivation Produces Long-Term Detrimental Effects in Spatial Memory and Modifies the Cellular Composition of the Subgranular Zone. Frontiers in Cellular Neuroscience 2016.
- Tanti A, B.C. Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? . Neuroscience 2013.
- Perera TD, D.A., Keegan KA,Thirumangalakudi L,Lipira CM,Joyce N,Lange C,Higley JD,Rosoklija G,Hen R,Sackeim HA,Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PloS one 2011.
- Hernández-Rabaza, V.; Llorens-Martín, M.; Velázquez-Sánchez, C.; Ferragud, A.; Arcusa, A.; Gumus, H.G.; Gómez-Pinedo, U.; Pérez-Villalba, A.; Roselló, J.; Trejo, J.L.; et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 2009/03/03, 159, doi:10.1016/j.neuroscience.2008.11.054.
- Winocur, G.; Wojtowicz, J.M.; Sekeres, M.; Snyder, J.S.; Wang, S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 2006/01/01, 16, doi:10.1002/hipo.20163.
- TJ, S. Saving new brain cells. Scientific American 2009 Mar, 300, doi:10.1038/scientificamerican0309-46.
- Shors, T.J. From Stem Cells to Grandmother Cells: How Neurogenesis Relates to Learning and Memory. Cell Stem Cell 2008/09/11, 3, doi:10.1016/j.stem.2008.08.010.
- Daniel M Curlik, I.; Shors, T.J. Learning Increases the Survival of Newborn Neurons Provided That Learning Is Difficult to Achieve and Successful. Journal of Cognitive Neuroscience 2010 Oct 18, 23, doi:10.1162/jocn.2010.21597.
- HM, S.; AL, G.; TJ, S. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learning & memory (Cold Spring Harbor, N.Y.) 05/10/2007, 14, doi:10.1101/lm.488707.
- Berdugo-Vega, G.; Lee, C.-C.; Garthe, A.; Kempermann, G.; Calegari, F. Adult-born neurons promote cognitive flexibility by improving memory precision and indexing. Hippocampus 2021/10/01, 31, doi:10.1002/hipo.23373.
- EA, M.; K, W.; HJ, S. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus 2006, 16, doi:10.1002/hipo.20233.
- K, W.; EA, M. Acquiring “the Knowledge” of London’s layout drives structural brain changes. Current biology : CB 12/20/2011, 21, doi:10.1016/j.cub.2011.11.018.
- S, T.; A, V.; SK, J. Training on an Appetitive Trace-Conditioning Task Increases Adult Hippocampal Neurogenesis and the Expression of Arc, Erk and CREB Proteins in the Dorsal Hippocampus. Frontiers in cellular neuroscience 04/17/2020, 14, doi:10.3389/fncel.2020.00089.
- Seib, D.R., Espinueva, Delane F.,Princz-Lebel, Oren,Chahley, Erin,Stevenson, Jordann,O’Leary, Timothy P.,Floresco, Stan B.,Snyder, Jason S.,Seib, Désirée R.,Espinueva, Delane F.,Princz-Lebel, Oren,Chahley, Erin,Stevenson, Jordann,O’Leary, Timothy P.,Floresco, Stan B.,Snyder, Jason S. Hippocampal neurogenesis promotes preference for future rewards. Molecular Psychiatry 2021.
- Anacker C, L.V., Stevens GS,Millette A,Shores R,Jimenez JC,Chen B,Hen R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018.
- Grabenhorst, F.; Rolls, E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences 2011/02/01, 15, doi:10.1016/j.tics.2010.12.004.
- JY, C.; S, H.; L, V.; BB, L.; N, U. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 01/18/2012, 482, doi:10.1038/nature10754.
- Speranza, L.; Porzio, U.d.; Viggiano, D.; Donato, A.d.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021 Mar 26, 10, doi:10.3390/cells10040735.
- Komisaruk, B.R.; Cerro, M.C.R.d. How Does Our Brain Generate Sexual Pleasure? International Journal of Sexual Health 2021 Oct 13, 33, doi:10.1080/19317611.2021.1989534.
- Leuner, B.; Glasper, E.R.; Gould, E. Sexual Experience Promotes Adult Neurogenesis in the Hippocampus Despite an Initial Elevation in Stress Hormones. PLOS ONE 14 Ιουλ 2010, 5, doi:10.1371/journal.pone.0011597.
- Glasper, E.R.; Gould, E. Sexual experience restores age-related decline in adult neurogenesis and hippocampal function. Hippocampus 2013 Mar 5, 23, doi:10.1002/hipo.22090.
- Takamura, N.; Nakagawa, S.; Masuda, T.; Boku, S.; Kato, A.; Song, N.; An, Y.; Kitaichi, Y.; Inoue, T.; Koyama, T.; et al. The effect of dopamine on adult hippocampal neurogenesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2014/04/03, 50, doi:10.1016/j.pnpbp.2013.12.011.
- Rachele Salvi, T.S., Johannes C M Schlachetzki,Elisabeth Waldmann,Stefan Schwab,Beate Winner,Jürgen Winkler,Zacharias Kohl. Distinct Effects of Chronic Dopaminergic Stimulation on Hippocampal Neurogenesis and Striatal Doublecortin Expression in Adult Mice. Frontiers in Neuroscience 2016.
- Thomas, R.M.; Hotsenpiller, G.; Peterson, D.A. Acute Psychosocial Stress Reduces Cell Survival in Adult Hippocampal Neurogenesis without Altering Proliferation. Journal of Neuroscience 2007-03-14, 27, doi:10.1523/JNEUROSCI.3849-06.2007.
- Simon, M.; Czéh, B.; Fuchs, E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Research 2005/07/12, 1049, doi:10.1016/j.brainres.2005.05.006.
- Hawley, D.F.; Leasure, J.L. Region-specific response of the hippocampus to chronic unpredictable stress. Hippocampus 2012/06/01, 22, doi:10.1002/hipo.20970.
- Andrea Du Preez, D.O., Inez Eiben,Ksenia Musaelyan,Martin Egeland,Patricia A. Zunszain,Cathy Fernandes,Sandrine Thuret,Carmine M. Pariante. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain, Behavior, and Immunity 2021.
- Ruiz, R.; Roque, A.; Pineda, E.; Licona-Limón, P.; Valdéz-Alarcón, J.J.; Lajud, N. Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 2018/10/01, 96, doi:10.1016/j.psyneuen.2018.07.012.
- Mirescu C, G.E. Stress and adult neurogenesis. Hippocampus 2006.
- W.H.O. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. 2019.
- Roth, L.W.; Polotsky, A.J. Can we live longer by eating less? A review of caloric restriction and longevity. Maturitas 2012/04/01, 71, doi:10.1016/j.maturitas.2011.12.017.
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P.; Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. Journal of Molecular Neuroscience 2001 15:2 2000/10, 15, doi:10.1385/JMN:15:2:99.
- Kim, Y.; Kim, S.; Kim, C.; Sato, T.; Kojima, M.; Park, S. Ghrelin is required for dietary restriction-induced enhancement of hippocampal neurogenesis: lessons from ghrelin knockout mice. Endocrine Journal 2015, 62, doi:10.1507/endocrj.EJ14-0436.
- Z, K.; K, A.-D.; A, K.; H, D.; Ş, B.; G, Ü. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain research 08/27/2015, 1618, doi:10.1016/j.brainres.2015.05.041.
- Cardoso, A.; Marrana, F.; Andrade, J.P. Caloric restriction in young rats disturbs hippocampal neurogenesis and spatial learning. Neurobiology of Learning and Memory 2016/09/01, 133, doi:10.1016/j.nlm.2016.07.013.
- Yanai S., O.H., Sugioka K. Dietary restriction inhibits spatial learning ability and hippocampal cell proliferation in rats. Japanese Psychological Research 2008, 50, doi:10.1111/j.1468-5884.2007.00360.x.
- Staples, M.C.; Fannon-Pavlich, M.J.; Mysore, K.K.; Dutta, R.R.; Ongjoco, A.T.; Quach, L.W.; Kharidia, K.M.; Somkuwar, S.S.; Mandyam, C.D. Dietary Restriction reduces hippocampal neurogenesis and granule cell neuron density without affecting the density of mossy fibers. Brain research 2017 Mar 8, 1663, doi:10.1016/j.brainres.2017.02.028.
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary Fats and the Risk of Incident Alzheimer Disease. Archives of Neurology 2003/02/01, 60, doi:10.1001/archneur.60.2.194.
- Greenwood, C.E., & Winocur, G. . Cognitive impairment in rats fed high-fat diets: A specific effect of saturated fatty-acid intake. Behavioral Neuroscience 1996, 110, doi:10.1037/0735-7044.110.3.451.
- Greenwood, C.E.; Winocur, G. Learning and memory impairment in rats fed a high saturated fat diet. Behavioral and Neural Biology 1990/01/01, 53, doi:10.1016/0163-1047(90)90831-P.
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.M.; Hofman, A.; Breteler, M.M.B. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Annals of Neurology 1997/11/01, 42, doi:10.1002/ana.410420514.
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. European Journal of Neurology 2006/12/01, 13, doi:10.1111/j.1468-1331.2006.01500.x.
- Yao, X.; Yang, C.; Wang, C.; Li, H.; Zhao, J.; Kang, X.; Liu, Z.; Chen, L.; Chen, X.; Pu, T.; et al. High-Fat Diet Consumption in Adolescence Induces Emotional Behavior Alterations and Hippocampal Neurogenesis Deficits Accompanied by Excessive Microglial Activation. International Journal of Molecular Sciences 2022 Jul 27, 23, doi:10.3390/ijms23158316.
- Granholm, A.-C.; Bimonte-Nelson, H.A.; Moore, A.B.; Nelson, M.E.; Freeman, L.R.; Sambamurti, K.; Ann-Charlotte Granholm, H.A.B.-N., Alfred B. Moore, Matthew E. Nelson, Linnea R. Freeman, Kumar Sambamurti. Effects of a Saturated Fat and High Cholesterol Diet on Memory and Hippocampal Morphology in the Middle-Aged Rat. Journal of Alzheimer’s disease 2008-06-04, 14, doi:10.3233/JAD-2008-14202.
- Freeman, L.R.; Haley-Zitlin, V.; Rosenberger, D.S.; Granholm, A.-C. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms. Nutritional neuroscience 2013 Nov 26, 17, doi:10.1179/1476830513Y.0000000092.
- Miller Y.I., V.S., Binder C.J., Feramisco J.R., Kirkland T.N., Witztum J.L. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. Journal of Biological Chemistry 2003, 278, doi:10.1074/jbc.M209634200.
- Hodgkinson C.P., L.R.C., Patel K., Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2008, 28, doi:10.1161/ATVBAHA.108.175992.
- Miller, Y.I. Toll-like Receptors and Atherosclerosis: Oxidized Ldl As an Endogenous Toll-like Receptor Ligand. Future Cardiology 2005-11-1, 10.2217/14796678.1.6.785, doi:10.2217/14796678.1.6.785.
- Connolly, M.G.; Yost, O.L.; Potter, O.V.; Giedraitis, M.E.; Kohman, R.A. Toll-like receptor 4 differentially regulates adult hippocampal neurogenesis in an age- and sex-dependent manner. Hippocampus 2020 Apr 28, 30, doi:10.1002/hipo.23209.
- W, Q.; AR, G.; Y, W.; J, A.; LAM, G. Folic acid, but not folate, regulates different stages of neurogenesis in the ventral hippocampus of adult female rats. Journal of neuroendocrinology 2019 Oct, 31, doi:10.1111/jne.12787.
- Zhao, N.; Zhong, C.; Wang, Y.; Zhao, Y.; Gong, N.; Zhou, G.; Xu, T.; Hong, Z. Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine deficiency at early pre-pathological lesion stage. Neurobiology of Disease 2008/02/01, 29, doi:10.1016/j.nbd.2007.08.014.
- Bateman, J.R.; Ferguson, M.A.; Anderson, C.A.; Arciniegas, D.B.; Gilboa, A.; Berman, B.D.; Fox, M.D. Network Localization of Spontaneous Confabulation. The Journal of Neuropsychiatry and Clinical Neurosciences 2024, 36, 45–52, doi:10.1176/appi.neuropsych.20220160.
- Bonnet, E.; Touyarot, K.; Alfos, S.; Pallet, V.; Higueret, P.; Abrous, D.N. Retinoic Acid Restores Adult Hippocampal Neurogenesis and Reverses Spatial Memory Deficit in Vitamin A Deprived Rats. PLoS ONE 2008 Oct 22, 3, doi:10.1371/journal.pone.0003487.
- Crandall, J.; Sakai, Y.; Zhang, J.; Koul, O.; Mineur, Y.; Crusio, W.E.; McCaffery, P.; Crandall, J.; Sakai, Y.; Zhang, J.; et al. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proceedings of the National Academy of Sciences 2004-4-6, 101, doi:10.1073/pnas.0306336101.
- Nam, S.M.; Seo, M.; Seo, J.-S.; Rhim, H.; Nahm, S.-S.; Cho, I.-H.; Chang, B.-J.; Kim, H.-J.; Choi, S.-H.; Nah, S.-Y.; et al. Ascorbic Acid Mitigates D-galactose-Induced Brain Aging by Increasing Hippocampal Neurogenesis and Improving Memory Function. Nutrients 2019, Vol. 11, Page 176 2019-01-15, 11, doi:10.3390/nu11010176.
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. The American Journal of Clinical Nutrition 2009/09/01, 90, doi:10.3945/ajcn.2009.27954.
- Salazar, K.; Jara, N.; Ramírez, E.; Lima, I.d.; Smith-Ghigliotto, J.; Muñoz, V.; Ferrada, L.; Nualart, F. Role of vitamin C and SVCT2 in neurogenesis. Frontiers in Neuroscience 2023 Jun 22, 17, doi:10.3389/fnins.2023.1155758.
- RS, C.; NM, T.; J, H.; G, S.; CW, L. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain research 10/27/2008, 1237, doi:10.1016/j.brainres.2008.08.040.
- Arian Daneshpour, M.E.N.L., Karl-Heinz Wagner,Shaun Sabico,Nasser M. Al-Daghri,Dara Aldisi,Daniel König,José Francisco López Gil,Brendon Stubbs. Selenium and brain aging: A comprehensive review with a focus on hippocampal neurogenesis. Ageing Research Reviews 2025.
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006/01/01, 139, doi:10.1016/j.neuroscience.2006.01.021.
- Dyall, S.C.; Michael, G.J.; Michael-Titus, A.T. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. Journal of Neuroscience Research 2010/08/01, 88, doi:10.1002/jnr.22390.
- Davinelli, S.; Medoro, A.; Ali, S.; Passarella, D.; Intrieri, M.; Scapagnini, G. Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging. Current Neuropharmacology 2023 Mar 8, 21, doi:10.2174/1570159X21666221031103909.
- Kim, S.J.; Son, T.G.; Park, H.R.; Park, M.; Kim, M.-S.; Kim, H.S.; Chung, H.Y.; Mattson, M.P.; Lee, J. Curcumin Stimulates Proliferation of Embryonic Neural Progenitor Cells and Neurogenesis in the Adult Hippocampus. The Journal of Biological Chemistry 2008 May 23, 283, doi:10.1074/jbc.M708373200.
- S, L.; D, G.; M, Y.; Q, Q.; J, L.; T, C. Curcumin Improves Neurogenesis in Alzheimer’s Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. International journal of molecular sciences 05/08/2024, 25, doi:10.3390/ijms25105123.
- O’Sullivan, T.A.; Hafekost, K.; Mitrou, F.; Lawrence, D. Food Sources of Saturated Fat and the Association With Mortality: A Meta-Analysis. American Journal of Public Health 2013 Sep, 103, doi:10.2105/AJPH.2013.301492.
- M, K.-K.; M, V.; P, B. Seasonal folate serum concentrations at different nutrition. Central European journal of public health 2013 Mar, 21, doi:10.21101/cejph.a3785.
- Martel JL, K.C., Doshi H, et al. Vitamin B1 (Thiamine). StatPearls [Internet] 2024.
- Ciaroni, S.; Cecchini, T.; Ferri, P.; Cuppini, R.; Ambrogini, P.; Santi, S.; Benedetti, S.; Grande, P.D.; Papa, S. Neural precursor proliferation and newborn cell survival in the adult rat dentate gyrus are affected by vitamin E deficiency. Neuroscience Research 2002/12/01, 44, doi:10.1016/S0168-0102(02)00157-8.
- Zaaboul, F.; Liu, Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Comprehensive Reviews in Food Science and Food Safety 2022/03/01, 21, doi:10.1111/1541-4337.12924.
- Karlsson, O.; Kim, R.; Hasman, A.; Subramanian, S.V. Consumption of Vitamin-A-Rich Foods and Vitamin A Supplementation for Children under Two Years Old in 51 Low- and Middle-Income Countries. Nutrients 2021 Dec 31, 14, doi:10.3390/nu14010188.
- Abdullah M, J.R., Attia FN. Vitamin C (Ascorbic Acid). StatPearls [Internet] 2023.
- J, M.; NM, B. Zinc and copper intakes and their major food sources for older adults in the 1994-96 continuing survey of food intakes by individuals (CSFII). The Journal of nutrition 2000 Nov, 130, doi:10.1093/jn/130.11.2838.
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: public health risks and benefits | British Journal of Nutrition | Cambridge Core. British Journal of Nutrition 2012/06, 107, doi:10.1017/S0007114512001456.
- Ivey, K.L.; Jensen, M.K.; Hodgson, J.M.; Eliassen, A.H.; Cassidy, A.; Rimm, E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. The British journal of nutrition 2017 Jun 13, 117, doi:10.1017/S0007114517001325.
- Bannerman DM, S.R., Sanderson DJ,McHugh SB,Rawlins JN,Monyer H,Seeburg PH. Hippocampal synaptic plasticity, spatial memory and anxiety. Nature reviews. Neuroscience 2014.
- Strange, B.A., Witter, Menno P.,Lein, Ed S.,Moser, Edvard I.,Strange, Bryan A.,Witter, Menno P.,Lein, Ed S.,Moser, Edvard I. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 2014.
- Thompson CL, P.S., Jeromin A,Ng LL,MacPherson CR,Mortrud MT,Cusick A,Riley ZL,Sunkin SM,Bernard A,Puchalski RB,Gage FH,Jones AR,Bajic VB,Hawrylycz MJ,Lein ES. Genomic anatomy of the hippocampus. Neuron 2008.
- C, P. Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Frontiers in bioscience (Landmark edition) 01/01/2018, 23, doi:10.2741/4584.
- Jason S Snyder, J.S.C., Meredith A Clifford,Sara I Jeurling,Patrick Hurley,Ashly Brown,J Frances Kamhi,Heather A Cameron. Adult-Born Hippocampal Neurons Are More Numerous, Faster Maturing, and More Involved in Behavior in Rats than in Mice. The Journal of Neuroscience 2009.
- I, A. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harbor perspectives in biology 2015.
- O’Leary OF, O.C.R., Cryan JF. Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice. Neuropharmacology 2012.
- Roberta Gioia, T.S., Tamara Diamanti,Stefania Fimmanò,Marina Vitale,Henrik Ahlenius,Zaal Kokaia,Felice Tirone,Laura Micheli,Stefano Biagioni,Giuseppe Lupo,Arianna Rinaldi,Antonella De Jaco,Emanuele Cacci. Adult hippocampal neurogenesis and social behavioural deficits in the R451C Neuroligin3 mouse model of autism are reverted by the antidepressant fluoxetine. Journal of Neurochemistry 2023.
- Jin J, K.H., Sun T,Kim SN. Distinct function of miR-17-92 cluster in the dorsal and ventral adult hippocampal neurogenesis. Biochemical and biophysical research communications 2018.
- Mahar I, T.S., Davoli MA,Dominguez-Lopez S,Qiang C,Rachalski A,Turecki G,Mechawar N. Subchronic peripheral neuregulin-1 increases ventral hippocampal neurogenesis and induces antidepressant-like effects. PloS one 2011.
- Bortolotto V, B.H., Cuccurazzu B,Rinaldi M,Canonico PL,Grilli M. Salmeterol, a β2 Adrenergic Agonist, Promotes Adult Hippocampal Neurogenesis in a Region-Specific Manner. Frontiers in pharmacology 2019.
- Mohammad H, M.F., Ortega-Martinez S,Hollos P,Eerola K,Komulainen E,Kulesskaya N,Freemantle E,Fagerholm V,Savontaus E,Rauvala H,Peterson BD,van Praag H,Coffey ET. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Molecular psychiatry 2018.
- Mateus-Pinheiro A, A.N., Patrício P,Machado-Santos AR,Loureiro-Campos E,Silva JM,Sardinha VM,Reis J,Schorle H,Oliveira JF,Ninkovic J,Sousa N,Pinto L. AP2γ controls adult hippocampal neurogenesis and modulates cognitive, but not anxiety or depressive-like behavior. Molecular psychiatry 2017.
- Jin J, K.S., Liu X,Zhang H,Zhang C,Seo JS,Kim Y,Sun T. miR-17-92 Cluster Regulates Adult Hippocampal Neurogenesis, Anxiety, and Depression. Cell reports 2016.
- Sun J, B.M., Jun H,Guo JU,Sun GJ,Will B,Yang Z,Jang MH,Song H,Ming GL,Christian KM. A septo-temporal molecular gradient of sfrp3 in the dentate gyrus differentially regulates quiescent adult hippocampal neural stem cell activation. Molecular brain 2015.
- Jang MH, B.M., Kitabatake Y,Sun J,Song J,Kang E,Jun H,Zhong C,Su Y,Guo JU,Wang MX,Sailor KA,Kim JY,Gao Y,Christian KM,Ming GL,Song H. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 2013.
- Berg, D.A., Bond, Allison M.,Ming, Guo-li,Song, Hongjun,Daniel A. Berg,Allison M. Bond,Guo-li Ming,Hongjun Song. Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Research 2018.
- Liang, Z., Jin, Nuomeng,Guo, Weixiang,Liang, Ziqi,Jin, Nuomeng,Guo, Weixiang. Neural stem cell heterogeneity in adult hippocampus. Cell Regeneration 2025.
- Huckleberry KA, S.F., Copeland T,Chitwood RA,Yin W,Drew MR. Dorsal and ventral hippocampal adult-born neurons contribute to context fear memory. Neuropsychopharmacology 2018.
- Ritger, A.C., Parker, Rachel K. ,Trask, Sydney ,Ferrara, Nicole C. . Elevated fear states facilitate ventral hippocampal engagement of basolateral amygdala neuronal activity. Frontiers in Behavioral Neuroscience 2024.
- Marco N. Pompili, N.H., Sidney I. Wiener. Integration of fear learning and fear expression across the dorsoventral axis of the hippocampus. bioRxiv 2024.
- Carolina Irurita Ballesteros, B.d.O.G., Silvia Maisonette,J. Landeira-Fernandez. Effect of Dorsal and Ventral Hippocampal Lesions on Contextual Fear Conditioning and Unconditioned Defensive Behavior Induced by Electrical Stimulation of the Dorsal Periaqueductal Gray. PLOS ONE 2014.
- van Dijk RM, W.F., Wolfer DP,Slomianka L,Amrein I. Consistent within-group covariance of septal and temporal hippocampal neurogenesis with behavioral phenotypes for exploration and memory retention across wild and laboratory small rodents. Behavioural brain research 2019.
- DF, H.; K, M.; BR, C.; JL, L. Differential response of hippocampal subregions to stress and learning. PloS one 2012, 7, doi:10.1371/journal.pone.0053126.
- Márquez-Valadez, B.; Gallardo-Caballero, M.; Llorens-Martín, M. Human adult hippocampal neurogenesis is shaped by neuropsychiatric disorders, demographics, and lifestyle-related factors. Cell Stem Cell 2025/09/15, 10.1016/j.stem.2025.08.010, doi:10.1016/j.stem.2025.08.010.
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M.; Marx, W.; Penninx, B.W.J.H.; Solmi, M.; et al. Major depressive disorder. Nature Reviews Disease Primers 2023 9:1 2023-08-24, 9, doi:10.1038/s41572-023-00454-1.
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neuroscience & Therapeutics 2018 Mar 5, 24, doi:10.1111/cns.12835.
- Margaret C. McKinnon, K.Y., Anthony Nazarov and Glenda M. MacQueen. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience 2009.
- Jacobs, B.L.; van Praag, H.; Gage, F.H.; Jacobs, B.L.; van Praag, H.; Gage, F.H. Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular Psychiatry 2000 5:3 2000-06-28, 5, doi:10.1038/sj.mp.4000712.
- JE, M.; AJ, E.; EJ, N.; RS, D. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 12/15/2000, 20, doi:10.1523/JNEUROSCI.20-24-09104.2000.
- Boldrini, M.; Santiago, A.N.; Hen, R.; Dwork, A.J.; Rosoklija, G.B.; Tamir, H.; Arango, V.; John Mann, J.; Boldrini, M.; Santiago, A.N.; et al. Hippocampal Granule Neuron Number and Dentate Gyrus Volume in Antidepressant-Treated and Untreated Major Depression. Neuropsychopharmacology 2013 38:6 2013-01-07, 38, doi:10.1038/npp.2013.5.
- Boldrini, M.; Underwood, M.D.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; John Mann, J.; Arango, V.; Boldrini, M.; Underwood, M.D.; Hen, R.; et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 2009 34:11 2009-07-15, 34, doi:10.1038/npp.2009.75.
- Ribeiro, I.; Silveira-Rosa, T.; Martins-Macedo, J.; Marques-Ferraz, L.; Dourado, A.R.; Martins-Ferreira, G.; Farrugia, F.; Rodrigues, A.J.; Abrous, D.N.; Alves, N.D.; et al. Chronic stress and cytogenesis ablation disrupt hippocampal neuron connectivity, with fluoxetine restoring function with sex-specific effects. Neurobiology of Stress 2025/07/01, 37, doi:10.1016/j.ynstr.2025.100743.
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biological Psychiatry 2008/08/15, 64, doi:10.1016/j.biopsych.2008.02.022.
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003-8-8, 301, doi:10.1126/science.1083328.
- Banasr M, S.A., Hery M,Mocaër E,Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biological psychiatry 2006.
- Tanti A, W.W., Girault V,Brizard B,Devers S,Leguisquet AM,Surget A,Belzung C. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 2013.
- QG, Z.; D, L.; EJ, R.; H, S. Regional-specific effect of fluoxetine on rapidly dividing progenitors along the dorsoventral axis of the hippocampus. Scientific reports 10/19/2016, 6, doi:10.1038/srep35572.
- Zhou, W.-J.; Xu, N.; Kong, L.; Sun, S.-C.; Xu, X.-F.; Jia, M.-Z.; Wang, Y.; Chen, Z.-Y. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Translational Psychiatry 2016 Sep 13, 6, doi:10.1038/tp.2016.122.
- Matthew E. Szapacs, T.A.M., Lino Tessarollo,W. Ernest Lyons,Laura A. Mamounas,Anne M. Andrews. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. Journal of Neuroscience Methods 2004.
- Leschik J, G.A., Cicek C,Péron S,Tevosian M,Beer A,Radyushkin K,Bludau A,Ebner K,Neumann I,Singewald N,Berninger B,Lessmann V,Lutz B. Brain-derived neurotrophic factor expression in serotonergic neurons improves stress resilience and promotes adult hippocampal neurogenesis. Progress in neurobiology 2022.
- Kang, E.; Wen, Z.; Song, H.; Christian, K.M.; Ming, G.-l. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harbor Perspectives in Biology 2016 Sep, 8, doi:10.1101/cshperspect.a019026.
- Shelton, D.J., & Kirwan, C. B. A possible negative influence of depression on the ability to overcome memory interference. Behavioural Brain Research 2013, 256, doi:10.1016/j.bbr.2013.08.016.
- RD, A.; LA, M.; M, R.; Y, G.; H, C.; K, D. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science (New York, N.Y.) 08/10/2007, 317, doi:10.1126/science.1144400.
- Hany M, R.B., Rizvi A, et al. Schizophrenia. StatPearls 2024.
- Kuo, S.S.; Pogue-Geile, M.F. Variation in Fourteen Brain Structure Volumes in Schizophrenia: A Comprehensive Meta-Analysis of 246 Studies. Neuroscience and biobehavioral reviews 2019 Jan 4, 98, doi:10.1016/j.neubiorev.2018.12.030.
- Reif A, F.S., Finger M,Strobel A,Lauer M,Schmitt A,Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Molecular psychiatry 2006.
- Weissleder, C.; North, H.F.; Bitar, M.; Fullerton, J.M.; Sager, R.; Barry, G.; Piper, M.; Halliday, G.M.; Webster, M.J.; Shannon Weickert, C.; et al. Reduced adult neurogenesis is associated with increased macrophages in the subependymal zone in schizophrenia. Molecular Psychiatry 2021 26:11 2021-05-31, 26, doi:10.1038/s41380-021-01149-3.
- Rajebhosale, P.; Jone, A.; Johnson, K.R.; Hofland, R.; Palarpalar, C.; Khan, S.; Role, L.W.; Talmage, D.A. Neuregulin1 Nuclear Signaling Influences Adult Neurogenesis and Regulates a Schizophrenia Susceptibility Gene Network within the Mouse Dentate Gyrus. Journal of Neuroscience 2024-10-23, 44, doi:10.1523/JNEUROSCI.0063-24.2024.
- Kandilakis CL, P.C. Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia. International journal of molecular sciences 2025.
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature reviews. Neuroscience 2016.
- Kumar A, S.J., Lui F, et al. Alzheimer Disease. StatPearls 2024.
- Ávila-Villanueva, M.; Dolado, A.M.; Gómez-Ramírez, J.; Fernández-Blázquez, M. Brain Structural and Functional Changes in Cognitive Impairment Due to Alzheimer’s Disease. Frontiers in Psychology 2022 Jun 21, 13, doi:10.3389/fpsyg.2022.886619.
- Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An english translation of alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”. Clinical Anatomy 1995/01/01, 8, doi:10.1002/ca.980080612.
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; 3rd, C.F.R. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. The British Journal of Psychiatry 2013/05, 202, doi:10.1192/bjp.bp.112.118307.
- Moreno-Jiménez EP, T.-R.J., Flor-García M,Rábano A,Llorens-Martín M. Evidences for Adult Hippocampal Neurogenesis in Humans. The Journal of Neuroscience 2021.
- Stern, Y.; Gurland, B.; Tatemichi, T.K.; Tang, M.X.; Wilder, D.; Mayeux, R. Influence of Education and Occupation on the Incidence of Alzheimer’s Disease. JAMA 1994/04/06, 271, doi:10.1001/jama.1994.03510370056032.
- Disouky, A.; Lazarov, O. Adult hippocampal neurogenesis in Alzheimer’s disease. Progress in molecular biology and translational science 2020 Oct 16, 177, doi:10.1016/bs.pmbts.2020.09.002.
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological Medicine 2009/01, 39, doi:10.1017/S0033291708003681.
- Valentin-Escalera, J.; Leclerc, M.; Calon, F. High-Fat Diets in Animal Models of Alzheimer’s Disease: How Can Eating Too Much Fat Increase Alzheimer’s Disease Risk? Journal of Alzheimer’s Disease 2024 Jan 30, 97, doi:10.3233/JAD-230118.
- Lv, Y.-N.; Cui, Y.; Zhang, B.; Huang, S.-M. Sleep deficiency promotes Alzheimer’s disease development and progression. Frontiers in Neurology 2022 Dec 14, 13, doi:10.3389/fneur.2022.1053942.
- Wallensten, J.; Ljunggren, G.; Nager, A.; Wachtler, C.; Bogdanovic, N.; Petrovic, P.; Carlsson, A.C.; Wallensten, J.; Ljunggren, G.; Nager, A.; et al. Stress, depression, and risk of dementia – a cohort study in the total population between 18 and 65 years old in Region Stockholm. Alzheimer’s Research & Therapy 2023 15:1 2023-10-02, 15, doi:10.1186/s13195-023-01308-4.
- Fluyau D, H.M., Charlton TE. Drug Addiction. StatPearls 2024.
- Uhl, G.R.; Koob, G.F.; Cable, J. The neurobiology of addiction. Annals of the New York Academy of Sciences 2019 Jan 15, 1451, doi:10.1111/nyas.13989.
- Peyton, L.; Oliveros, A.; Choi, D.-S.; Jang, M.-H.; Peyton, L.; Oliveros, A.; Choi, D.-S.; Jang, M.-H. Hippocampal regenerative medicine: neurogenic implications for addiction and mental disorders. Experimental & Molecular Medicine 2021 53:3 2021-03-30, 53, doi:10.1038/s12276-021-00587-x.
- Belujon P, G.A. Dopamine System Dysregulation in Major Depressive Disorders. The international journal of neuropsychopharmacology 2017.
- Chambers, R.A. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug and Alcohol Dependence 2013/06/01, 130, doi:10.1016/j.drugalcdep.2012.12.005.
- L, D.; A, U.; JM, N.; E, F. Major depression and comorbid substance use disorders. Current opinion in psychiatry 2008 Jan, 21, doi:10.1097/YCO.0b013e3282f32408.
- Sinha, R. Chronic Stress, Drug Use, and Vulnerability to Addiction. Annals of the New York Academy of Sciences 2008 Oct, 1141, doi:10.1196/annals.1441.030.
- Ward, H.B.; Nemeroff, C.B.; Carpenter, L.; Grzenda, A.; McDonald, W.M.; Rodriguez, C.I.; Kraguljac, N.V. Substance use disorders in schizophrenia: Prevalence, etiology, biomarkers, and treatment. Personalized Medicine in Psychiatry 2023/07/01, 39-40, doi:10.1016/j.pmip.2023.100106.
- Noonan M.A., B.S.E., Fuller D.C., Eisch A.J. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. The Journal of Neuroscience 2010, 30, doi:10.1523/JNEUROSCI.4256-09.2010.
- Ussher, M.H.; Taylor, A.; Faulkner, G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews 2008, 10.1002/14651858.CD002295.pub3, doi:10.1002/14651858.CD002295.pub3.
- Brown, R.A.; Abrantes, A.M.; Read, J.P.; Marcus, B.H.; Jakicic, J.; Strong, D.R.; Oakley, J.R.; Ramsey, S.E.; Kahler, C.W.; Stuart, G.; et al. Aerobic Exercise for Alcohol Recovery. Behavior Modification 2008-07-15, 33, doi:10.1177/0145445508329112.
- O, D.; LF, V.; JE, S.; L, D.-A.; CJ, Y.; JC, S.; O, G.; GF, K.; CD, M. Hippocampal neurogenesis protects against cocaine-primed relapse. Addiction biology 2014 Jul, 19, doi:10.1111/adb.12019.
- Castilla-Ortega, E.; Santín, L.J. Adult hippocampal neurogenesis as a target for cocaine addiction: a review of recent developments. Current Opinion in Pharmacology 2020/02/01, 50, doi:10.1016/j.coph.2019.10.002.
- Levone, B.R.; Cryan, J.F.; O’Leary, O.F. Role of adult hippocampal neurogenesis in stress resilience. Neurobiology of Stress 2015/01/01, 1, doi:10.1016/j.ynstr.2014.11.003.
- Planchez, B.; Lagunas, N.; Guisquet, A.-M.L.; Legrand, M.; Surget, A.; Hen, R.; Belzung, C. Increasing Adult Hippocampal Neurogenesis Promotes Resilience in a Mouse Model of Depression. Cells 2021 Apr 21, 10, doi:10.3390/cells10050972.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).