Submitted:
27 October 2025
Posted:
28 October 2025
You are already at the latest version
Abstract
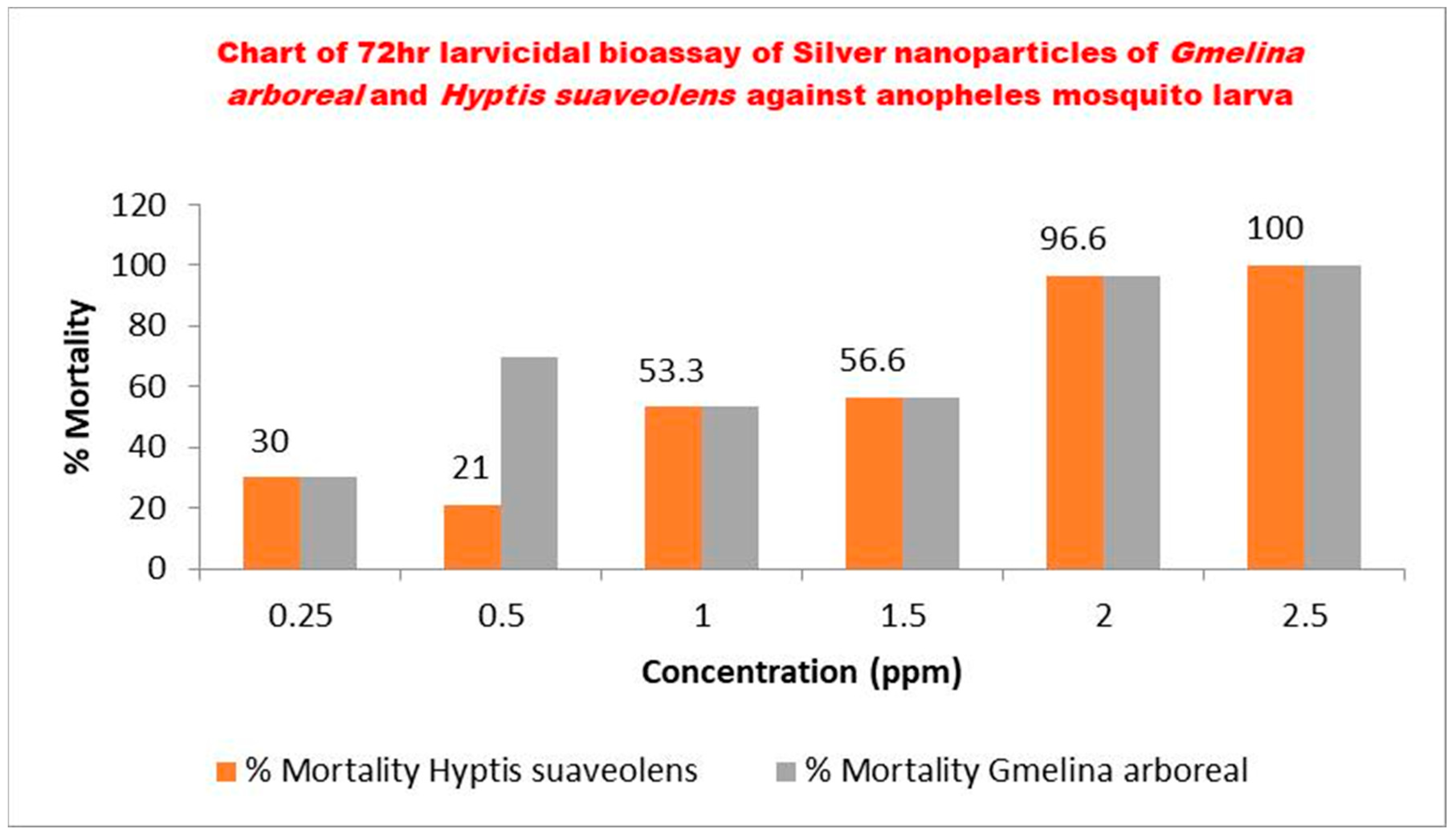
Malaria remains a leading cause of morbidity and mortality in sub-Saharan Africa, with escalating insecticide resistance among Anopheles vectors threatening the sustainability of current control interventions. The search for novel, environmentally benign larvicidal agents has increasingly focused on nanotechnology innovations derived from medicinal plants. This study assessed the larvicidal potential of silver nanoparticles (AgNPs) synthesized using aqueous leaf extracts of Gmelina arborea and Hyptis suaveolens against wild Anopheles larvae collected from Lagos State, Nigeria. Fresh plant samples were authenticated botanically, and green synthesis of AgNPs was confirmed by a characteristic honey-brown colour change and UV–visible spectrophotometric analysis. Larvicidal bioassays were performed according to WHO guidelines using six graded concentrations under controlled insectary conditions. Mortality was recorded at 24, 48, and 72 hours, and toxicological responses were analyzed using probit regression in IBM SPSS v20. After 72 hours, both AgNP formulations demonstrated a concentration-dependent increase in larval mortality, with H. suaveolens AgNPs showing greater bioefficacy than G. arborea AgNPs. Specifically, H. suaveolens AgNPs achieved LC₅₀ and LC₉₅ values of 0.292 ppm and 1.948 ppm respectively, whereas LC₅₀ = 0.469 ppm and LC₉₅ = 3.919 ppm were recorded for G. arborea AgNPs. Control mortality remained ≤10%, validating experimental reliability. The potent larvicidal activity displayed by both plant-derived AgNPs suggests that phytochemical-mediated nanoparticle synthesis may enhance toxicity against mosquito vectors while retaining environmental safety. These findings provide evidence supporting the integration of green nanotechnology into vector-control strategies to help mitigate resistance to conventional insecticides and strengthen malaria prevention efforts in endemic communities.
Keywords:
Introduction


Methods
Source and Rearing of Test Organisms
Preparation of Plant Extracts and Silver Nanoparticles
Larvicidal Bioassay
Statistical Analysis
Results
Discussion
Conclusion
References
- World Health Organization. (2023). World malaria report 2023. Geneva: World Health Organization.
- Ekpunobi, N.F.; Agu, K.C. Emergence and Re-Emergence of Arboviruses: When Old Enemies Rise Again. Cohesive J. Microbiol. Infect. Dis. 2024, 7, 1–7. [Google Scholar] [CrossRef]
- Obidi, N. Obidi, N., Chukwunwejim, C. R., Ekpunobi, N. F., Obiajulu, A. C., Obidi, N. L., & Mirabelle, O. (2025). Parasitic diseases and coinfections: Unraveling the complex web of host–pathogen interactions, challenges, and opportunities. International Journal of Medical and Pharmaceutical Research, 9(6), 1–15.
- Obidi, N.; Ekpunobi, N.F.; Chukwunwejim, C.R.; Kenechukwu, E.; Obiajulu, A.C.; Obidi, N.L.; Ogunmola, T.; Ajasa, O.S.; Mirabelle, O. Human-monkey interactions and zoonotic disease risk in an urban Nigerian community: A cross-sectional study. Magna Sci. Adv. Biol. Pharm. 2025, 15, 008–015. [Google Scholar] [CrossRef]
- Osarumwense, O.-I.I.T.; Nkechukwu, I.M.; Favour, E.N.; Izuchukwu, I.; George, C.O.; Umale, A.M.; Okechukwu, E.C. The Prevalence of Dengue Virus and Malaria Co-Infection among HIV-Infected Patients within South Eastern Nigeria. Adv. Infect. Dis. 2022, 12, 106–117. [Google Scholar] [CrossRef]
- F, E.N.; O, E.E. Secondary metabolites from endophytic fungi of moringa oleifera: antimicrobial and antioxidant properties. J. Microbiol. Exp. 2022, 10, 150–154. [Google Scholar] [CrossRef]
- Ikegbunam, M.N.; Onochie-Igbinedion, I.T.; Ekpunobi, N.F.; Harrison, A.; Emmanuel, E.; Ushie, S.N.; Adiukwu, M.U.; Esimone, C.O. Comparison of Four Diagnostic Tests Methods for the Detection of Dengue Virus Non-Structural-1 Antigen and IgM/IgG Antibodies. J. Biosci. Med. 2022, 10, 179–190. [Google Scholar] [CrossRef]
- Zohra, T.; Khalil, A.T.; Saeed, F.; Latif, B.; Salman, M.; Ikram, A.; Ayaz, M.; Murthy, H.C.A. Green Nano-Biotechnology: A New Sustainable Paradigm to Control Dengue Infection. Bioinorg. Chem. Appl. 2022, 2022, 3994340. [Google Scholar] [CrossRef]
- Ekpunobi, N.F.; Adesanoye, S.; Orababa, O.; Adinnu, C.; Okorie, C.; Akinsuyi, S. Public health perspective of public abattoirs in Nigeria, challenges and solutions. GSC Biol. Pharm. Sci. 2024, 26, 115–127. [Google Scholar] [CrossRef]
- Ekpunobi, N. F. , Mgbedo, U. G., Okoye, L. C. and Agu, K. C. (2024). Prevalence of ESBL genes in Klebsiella pneumoniae from individuals with community-acquired urinary tract infections in Lagos state, Nigeria. Journl of RNA and Genomics, 20 (2):1-6.
- Sunmonu, A.B.; Ekpunobi, N.F. Larvicidal potential of silver nanoparticles synthesized from Ocimum gratissimum leaf extracts against anopheles’ mosquito. GSC Biol. Pharm. Sci. 2023, 25, 041–048. [Google Scholar] [CrossRef]
- Ekpunobi, N.; Markjonathan, I.; Olanrewaju, O.; Olanihun, D. Idiosyncrasies of COVID-19; A Review. Iran. J. Med Microbiol. 2020, 14, 290–296. [Google Scholar] [CrossRef]
- Ekpunobi, N.; Akinsuyi, O.; Ariri, T.; Ogunmola, T. The Reemergence of Monkeypox in Nigeria. Challenges 2023, 14, 22. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiology Control. 2017, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Favour, E.N.; Isaac, A.A. Phenotypic characterization of biofilm formation and efflux pump activity in multi-drug resistant staphylococcus species isolated from asymptomatic students. J. Microbiol. Exp. 2020, 8, 223–229. [Google Scholar] [CrossRef]
- Montero, J. C. (2019). Larvicidal activity of Gmelina arborea fruit extract against dengue vector Aedes aegypti mosquito. International Journal of Biosciences, 15(5), 577-586.
- Oyinke, G. N. , Ogidi, O. C. ( 6(2), 36–39.
- Okoye, L.C.; Ugwu, M.C.; Oli, A.N.; Okoye, E.C.S.; Ekpunobi, N.F.; Okezie, U.M.; Mgbedo, U.G. Genotypic detection of metallo-Beta-Lactamases among multidrug resistant Klebsiella pneumoniae isolated from urine samples of UTI patients. GSC Biol. Pharm. Sci. 2024, 29, 248–255. [Google Scholar] [CrossRef]
- Ekpunobi, N.F.; Obidi, N.; Idowu, O.; Okoye, L.C.; Agu, C.K.; Ogunsanya, T.; Ogbodo, U.C.; Uwanta, L.I. Knowledge and awareness of breast cancer symptoms, risk factors, and early detection methods among Nigerian university students. 2025. [CrossRef]
- Animashaun, T. , Ekpunobi, N., Amukele, T., Ogunsanya, T., Aina, T., Idowu. O., Olayemi, A. and Obidi, N. (2024). Evaluation of the biological characteristics of breast cancer in women from Nigeria: A non-intervational pilot study vol. 1. Accessed from: https://metaphorlaboratory.com/wp-content/uploads/2025/01/Metaphor-Dec-2024-Research-Article.pdf. [CrossRef]
- Gillies, M. T. and Coetzee, M. (1987). A supplement to the Anophelinae of Africa south of the Sahara (Afro- An annotated checklist and bibliography of the mostropical Region). Johannesburg: South African Institute for Medical Research. Interior Health, 2009. Pest management plan for control of mosquito larvae that are potential West Nile virus vectors.
- Umoren, S. A., Obot, I. B., & Gasem, Z. M. (2014). Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J. Mater. Environ. Sci, 5(3), 907-914.
- Obidi, N.O.; Ekpunobi, N.F. A narrative review exploring phage therapy as a sustainable alternative solution to combat antimicrobial resistance in Africa: Applications, challenges and future directions. Afr. J. Clin. Exp. Microbiol. 2025, 26, 106–113. [Google Scholar] [CrossRef]
- Chukwunwejim, C.R.; Ekpunobi, N.F.; Ogunmola, T.; Obidi, N.; Ajasa, O.S.; Obidi, N.L. Molecular identification of multidrug-resistant bacteria from eggshell surfaces in Nigeria: A growing threat to public health. Magna Sci. Adv. Biol. Pharm. 2025, 15, 021–028. [Google Scholar] [CrossRef]
- Ekpunobi, N.F.; Ugwu, M.C.; Agu, K.C.; Chukwunwejim, C.R.; Nedum, C.H.; Okoye, L.C.; Mgbedo, U.C. Prevalence and molecular detection of ESBL genes in uropathogens isolated from children and adolescents in a General Hospital in Lagos, Nigeria. Afr. J. Clin. Exp. Microbiol. 2025, 26, 263–273. [Google Scholar] [CrossRef]
- Ekpunobi, N.; Chukwunwejim, C.R.; Okoye, S.C.; Oghonyon, E.I.; Obidi, N.; Agu, K.C. Gut microbiome dynamics and their emerging role in breast and colorectal cancer: implications for diagnosis and therapy. J. Microbiol. Exp. 2025, 13, 84–90. [Google Scholar] [CrossRef]
- Ekpunobi, N.F.; Ugwu, M.C.; Agu, K.C.; Chukwunwejim, C.R.; Oghonyon, E.I.; Ogunmola, T.; Ajasa, O.S.; Okoye, S.C.; Obidi, N. Extended-Spectrum Beta-Lactamase-Producing Uropathogens in Pediatric UTIs: Molecular Characteristics, Clinical Impact and Public Health Consequences. J. Adv. Biol. Biotechnol. 2025, 28, 691–709. [Google Scholar] [CrossRef]
- Okoye, S.C.; Chukwunwejim, C.R.; Ekpunobi, N.F.; Ajasa, O.S.; Ojuade, I.O.; Obidi, N.; Agu, K.C. Prevalence of Multidrug-Resistant Escherichia coli O157:H7 in Poultry Faeces from Live Bird Markets in Lagos, Nigeria. GSC Biol. Pharm. Sci. 2025, 32, 025–030. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Bagavan, A.; Jayaseelan, C.; Zahir, A.A.; Elango, G.; Kamaraj, C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2010, 108, 693–702. [Google Scholar] [CrossRef]
- Azarudeen, R.M.S.T.; Govindarajan, M.; AlShebly, M.M.; AlQahtani, F.S.; Amsath, A.; Senthilmurugan, S.; Vijayan, P.; Benelli, G. Size-controlled biofabrication of silver nanoparticles using the Merremia emarginata leaf extract: Toxicity on Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) and non-target mosquito predators. J. Asia-Pacific Èntomol. 2017, 20, 359–366. [Google Scholar] [CrossRef]
- Govindarajan, M.; Kadaikunnan, S.; Alharbi, N.S.; Benelli, G. Single-step biological fabrication of colloidal silver nanoparticles using Hugonia mystax: larvicidal potential against Zika virus, dengue, and malaria vector mosquitoes. Artif. Cells, Nanomedicine, Biotechnol. 2016, 45, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Aina, D.A.; Owolo, O.; Lateef, A.; O Aina, F.; Hakeem, A.S.; Adeoye-Isijola, M.; Okon, V.; Asafa, T.B.; Elegbede, J.A.; Olukanni, O.D.; et al. Biomedical Applications of Chasmanthera dependens stem extract mediated silver nanoparticles as Antimicrobial, Antioxidant, Anticoagulant, thrombolytic, and Larvicidal agents. Karbala Int. J. Mod. Sci. 2019, 5, 2. [Google Scholar] [CrossRef]
- Benelli, G.; Kadaikunnan, S.; Alharbi, N.S.; Govindarajan, M. Biophysical characterization of Acacia caesia-fabricated silver nanoparticles: effectiveness on mosquito vectors of public health relevance and impact on non-target aquatic biocontrol agents. Environ. Sci. Pollut. Res. 2017, 25, 10228–10242. [Google Scholar] [CrossRef]
- Mondal, N.K.; Mondal, A.; Hajra, A.; Shaikh, W.A.; Chakraborty, S. Synthesis of silver nanoparticle with Colocasia esculenta (L.) stem and its larvicidal activity against Culex quinquefasciatus and Chironomus sp. Asian Pac. J. Trop. Biomed. 2019, 9, 510–517. [Google Scholar] [CrossRef]
- Muthukumaran, U. , Govindarajan, M., Rajeswary, M. & Hoti, S. L.(2015). Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitology Research,114(5):1817-1827.
| Silver Nanoparticles | Concentration (ppm) |
Mortality (%) ±SE | Lethal Concentration |
|---|---|---|---|
| Control | 0 | 0 |
72h LC50(ppm)(LCL-UCL) 0.292(0.043-0.515) |
|
Hyptis suaveolens |
0.25 | 30±0.344 | |
| 0.5 | 21±0.344 | ||
| 1.0 | 53.3±0.344 | 72h LC95(ppm)(LCL-UCL) 1.948(1.069-17.152) |
|
| 1.5 | 56.6±0.344 | ||
| 2.0 | 96.6±0.344 | ||
| 2.5 | 100±0.344 |
| Silver Nanoparticles | Concentration(ppm) | Mortality (%)±SE | Lethal Concentration |
|---|---|---|---|
| Control | 0 | 0 |
LC50 (ppm) (LCL-UCL) 0.469(-) |
|
Gmelina arboreal |
0.25 | 30±0.311 | |
| 0.5 | 70 ±0.311 | ||
| 1.0 | 53.3±0.311 |
LC95 (ppm) (LCL-UCL) 3.919(-) |
|
| 1.5 | 53.3±0.311 | ||
| 2.0 | 96.6±0.311 | ||
| 2.5 | 100±0.311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





