1. Introduction
Batteries are an indispensable daily presence for the 21st century man; life cannot be imagined today without a phone, laptop and a multitude of other portable devices and tools powered by batteries. Disposable or rechargeable batteries, of a wide variety (lead-acid, Ni-MH, Li-ion) are the focus of researchers’ attention to improve their performance and to find economic and ecological ways of recycling them in order to valorize the useful elements they contain. The most performing batteries, intensively marketed, are those based on lead-acid, Ni-MH and Li-ion systems [
1,
2,
3]. The performances of these three types of batteries are remarkable (
Table 1)
Lead-acid batteries, although not very energy-dense, can provide very high currents at low cost, making them suitable for starting internal combustion vehicles and as backup power in telecommunications. These batteries perform well in extreme temperatures and are inexpensive. Recycling of these batteries is known and functional, although it still needs improvement. Ni/MH batteries that replaced the (toxic) Ni/Cd ones are in fierce competition with Li-ion batteries; initially they were much more expensive, but after 2010 became cheaper by reducing the content of expensive and scarce metals Co, Ni, in the composition.
Today these two types of batteries share the market: Li-ion batteries have captured the market for small devices (phones, cameras), electric vehicles e.g. LiFePO4 batteries which also offer the safest chemical configuration; Ni/MH batteries dominate the AA battery sector (produce voltages of 1.3 V compared to 3.2 – 3.8 V in the case of Li-ion batteries) and in applications that require high-current discharge rates (defibrillators). It is estimated that by 2030 Li-ion batteries will cover 95% of the market. Today, we are going through a period of intense research undertaken in order to valorize the non-ferrous metals contained in used batteries (Li-ion, Ni-MH) which, due to the high content of Ni, Co, Mn, Zn, Rare Earths, which are useful, expensive, scarce and often difficult to access, are transformed from waste into true secondary sources of raw materials [
4,
5,
6,
7,
8,
9]. Following the research undertaken in recent years, used batteries have become suppliers of materials used to obtain: colored glass, magnetic materials, graphene, electrical insulators, raw material for electroplating processes, etc.
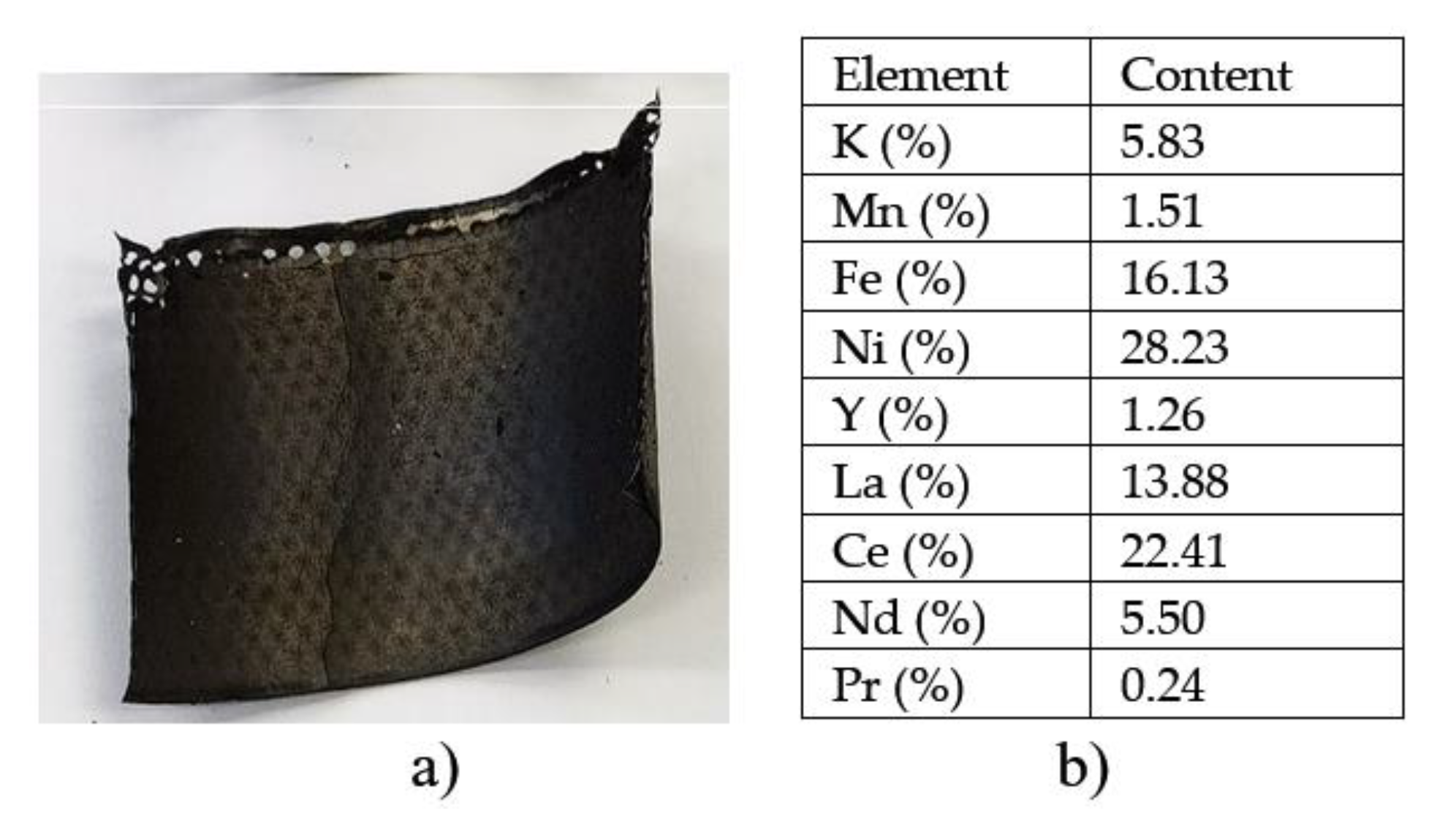
Starting in the 1980s and especially after 1990, the introduction of rare earths in Ni-MH (Nickel-Metal Hydride) batteries began, with the discovery that La, Ce, Pr and Nd, most often in the form of a mixture called mischmetal, presented superior characteristics for reversible storage of hydrogen in the anode of batteries, eliminating from the market Ni-Cd batteries that contained toxic Cd. Rare earths can absorb and then release a greater amount of hydrogen, thus resulting in a higher energy density of the battery. PR allow the acceleration of the hydrogen absorption/release processes, which leads to higher charging/discharging speeds; in addition, they extend the battery life through increased stability and resistance to degradation.
As a rule, on the labels and packaging of Ni-MH batteries, as well as Li-ion batteries, information about the capacity/voltage of the batteries, information related to operational safety, about the lifespan of the batteries can be found. The exact recipes (chemical composition, structure) of the metals and alloys found in the batteries are considered a trade secret, either to protect companies from competition or to avoid concerns caused to users who could thus find out about the ecological and social impact produced by the extraction and separation of rare earths, nickel, cobalt from ore.
Today, rare earths have become an indispensable component of modern technologies, namely communications, medical imaging equipment, production of permanent magnets, energy storage elements, lasers, etc., the recovery of these elements from used batteries is a major concern of research centers.
Table 2 shows an example of the information provided in the product data sheet by a well-known battery manufacturer (Panasonic).
It can be seen that in the product sheet, the information is incomplete, which denotes legislative gaps and ambiguous regulations; the data provided is able to discourage potential entrepreneurs willing to try recycling such batteries. Only on 28 June 2023 did the Council of the European Parliament adopt a new Legislative Act - Regulation on Batteries and Waste Batteries 2020/0353, amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and repealing Directive 2006/66/EC, which strengthens sustainability rules for batteries and waste batteries. This regulatory act stipulates, among many other measures, that batteries will have to contain documentation for recycled content. It also introduces labelling and information requirements on battery components, recycled content and a battery passport and a QR code. Moreover, the regulation establishes that by 2027, batteries incorporated in appliances should be able to be dismantled and replaced by the end user.
2. Materials and Methods
2.1. Materials
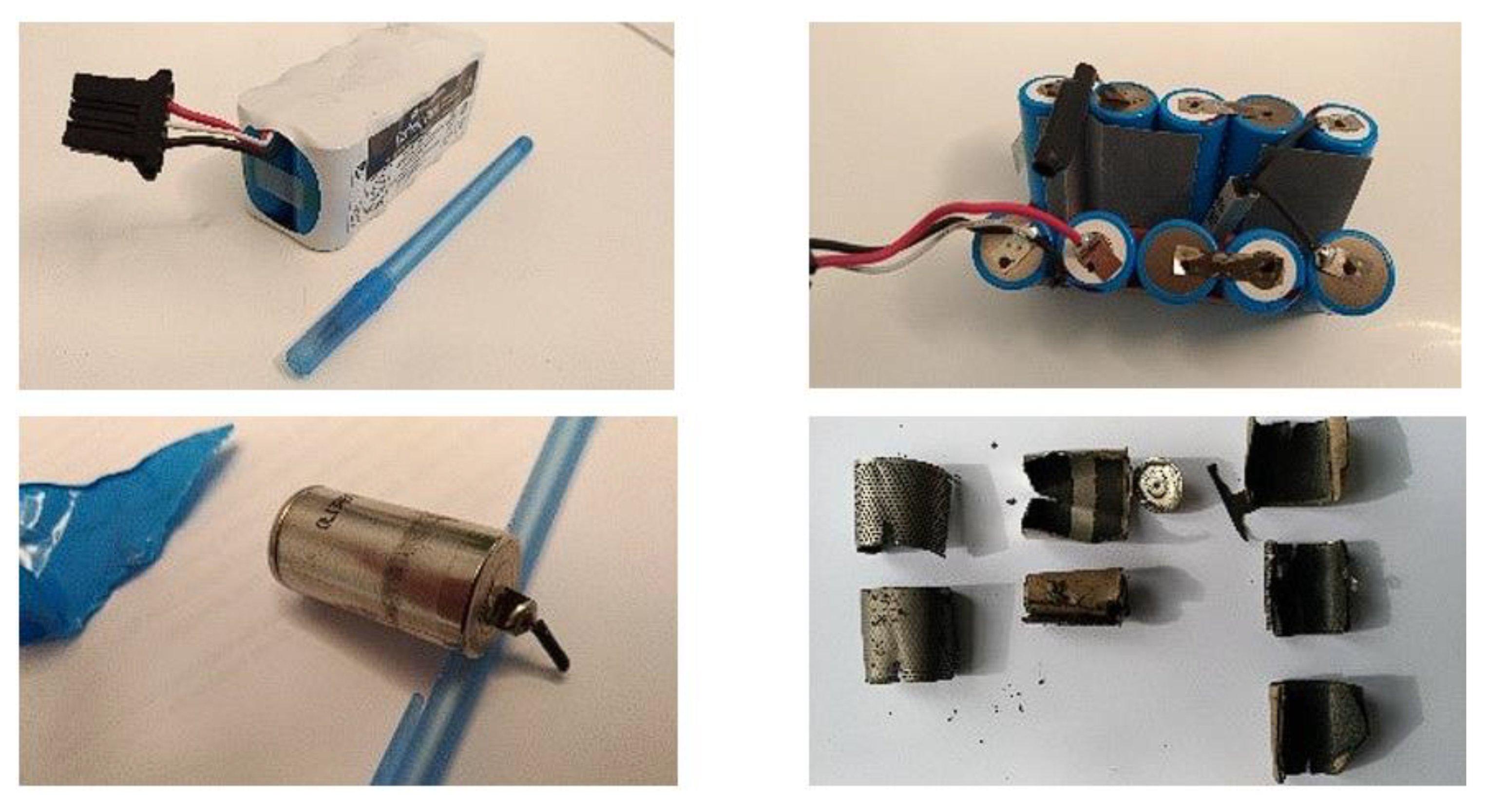
The authors focused their attention on the recovery of useful metals contained in used Ni-MH batteries. The Ni-MH batteries considered are rechargeable medical batteries used in portable defibrillators. The batteries were manually disassembled into components (
Figure 1); the anode and cathode were treated separately, aiming at the recovery of the anodic paste containing rare earths from the metal support grid and similarly of the cathodic paste containing Ni(OH)2 from the nickel support grid.
2.2. Laboratory Equipment Used and Working Method
Sections were cut from the anode and cathode, respectively, and weighed before and after cleaning in order to calculate the cleaning efficiency. Cleaning efficiency was calculated with the following equation:
where:
η - cleaning efficiency;
mi - initial weight of anode (cathode) piece, g;
mf - final weight of anode (cathode) piece, g.
The samples were weighed with an electronic balance type EMB 200-3, Kern, (Max.weighing weight: 200 g; Reproducibility = 0.001 g). The operation of separating the support paste was carried out in an ultrasonic bath type Emmi Emmi-12HC. The characteristics of the ultrasonic bath are: the maximum volume of the stainless-steel tank is 1.2 l, the tank having the following dimensions: 200x100x65 mm; cleaning frequency = 45 kHz; cleaning time = 1 - 60 min; the bath temperature can vary between 20 - 80ºC; maximum ultrasonic power = 100 W, ultrasonic power can be set to one of three levels: 50/75/100 W. The water temperature was measured continuously. Cusing water and a solution of about 1M citric acid as a medium were used.
The research was undertaken within the Hydrometallurgy Laboratory of the Department of Engineering and Management of Metallic Materials at POLITEHNICA University Bucharest, which has expertise in the field of recycling lead-acid and Li-ion batteries [
15,
16]. X-ray diffraction analysis was carried out using the D8-Discover diffractometer, Bruker, Germany, with Cu primary radiation (λ = 1.540598 Å), parallel geometry and 1D LynxEye detector (Bruker, Germany) on the secondary side. The diffractograms were obtained with an angular increment of 0.04°, at a scanning speed of 1 s/step in the Physical-Chemical Testing Laboratory, LI-MAT, within the National Research and Development Institute for Electrical Engineering ICPE-CA, Bucharest. The identification of the crystallographic phases was carried out using the ICDD PDF 2 Release 2022 database.
Samples from the cathode and anode of Ni-MH batteries were analyzed by scanning electron microscopy using a Quanta Inspect F50, with a field emission gun (FEG) with 1.2 nm resolution and an energy dispersive X-ray Spectrometer - EDX with resolutions of 133 eV at MnKα). An electron microscopy type Thermo Fisher Quattro S was also used.
3. Results
3.1. Separation/Recovery of Cathode Paste (Ni(OH)2) from the Support Mesh (Made of Ni).
Sections of the cathode of the used Ni-MH battery were analyzed by X-ray fluorescence before (
Figure 2) and after the ultrasonication operation in water (
Figure 3).
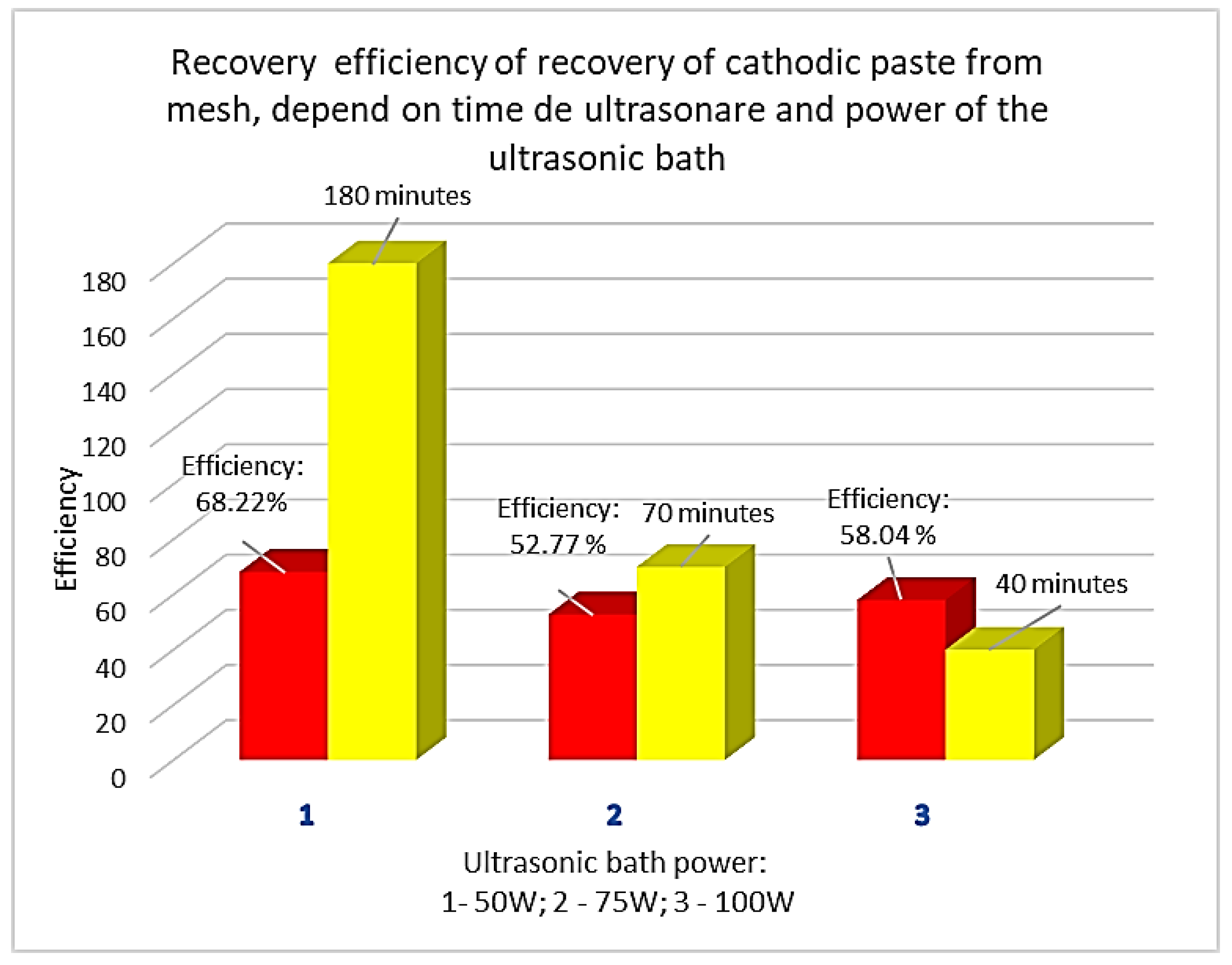
XEF analyses performed on sections cut from the cathode before ultrasonic cleaning revealed the presence of Ni, Zn, Co, Mn, Y, Cu, Fe. After ultrasonic cleaning in water, the cathodic paste was detached from the support metal mesh, made of Ni. Following XRF analysis, the following elements were identified in the recovered paste (washed and dried): NI, Zn, Co and traces of Cu and Fe. The evolution of the cleaning efficiency as a function of time and ultrasonic bath power is presented in
Figure 4.
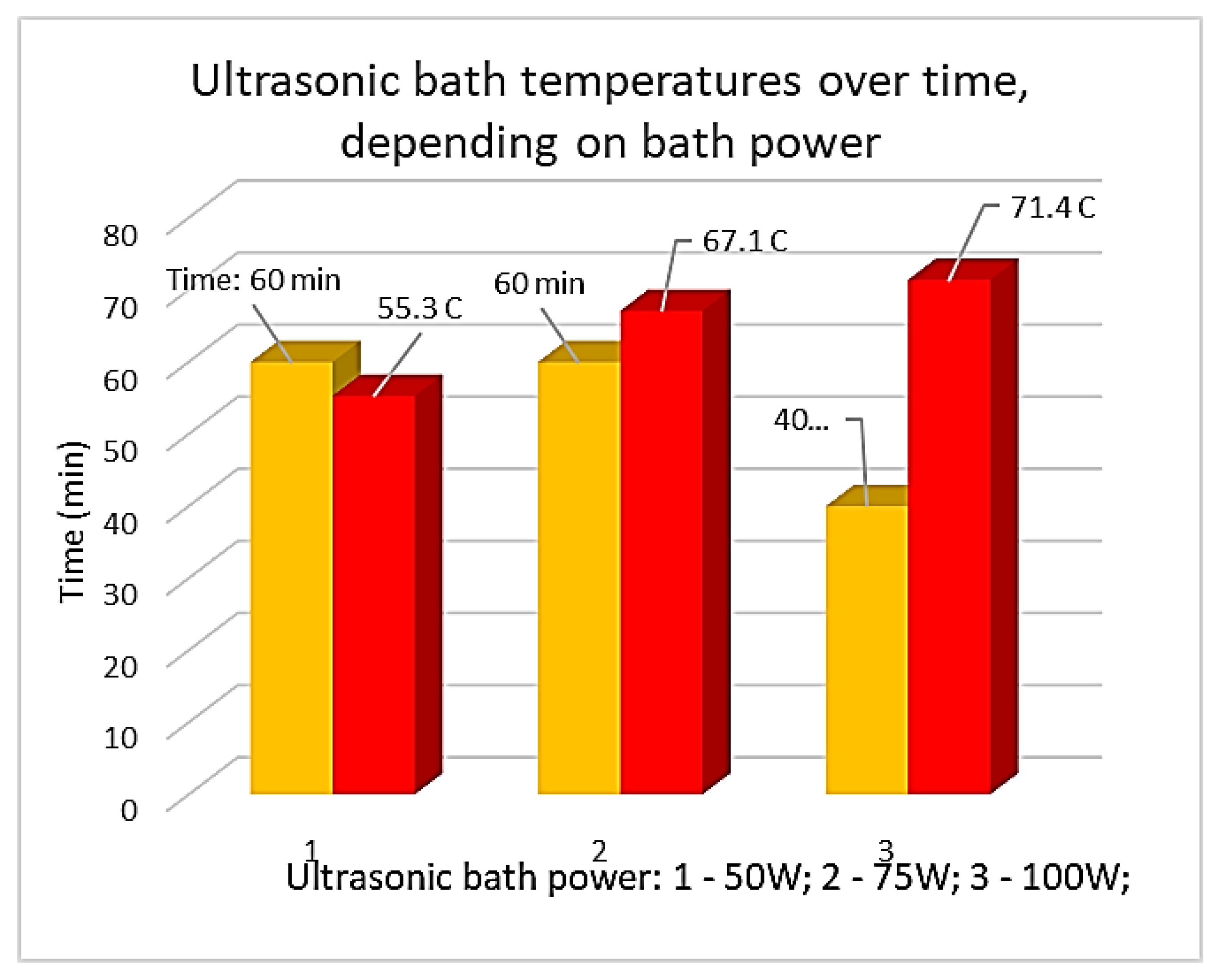
Figure 5 graphically shows the increase in water temperature in the ultrasonic bath, depending on the bath power.
From
Figure 5 it can be seen that a high temperature of the ultrasonic bath (from 55.3 to 71.4oC) is reached much faster if maximum bath power is employed (from 50W to 100W). The experiments were carried out starting from the temperature of the bed (20oC). Taking into account the conclusions drawn from the previous experiments, it was decided to perform the ultrasonication operation in water of the cathode of Ni-MH batteries by preheating the bath to 40oC. Rapid separations/cleanings (15 min) of the paste with a majority content of Ni(OH)2 from the support mesh made of Ni were obtained.
3.2. Characterization of the Cathode Support Mesh After Ultrasonication
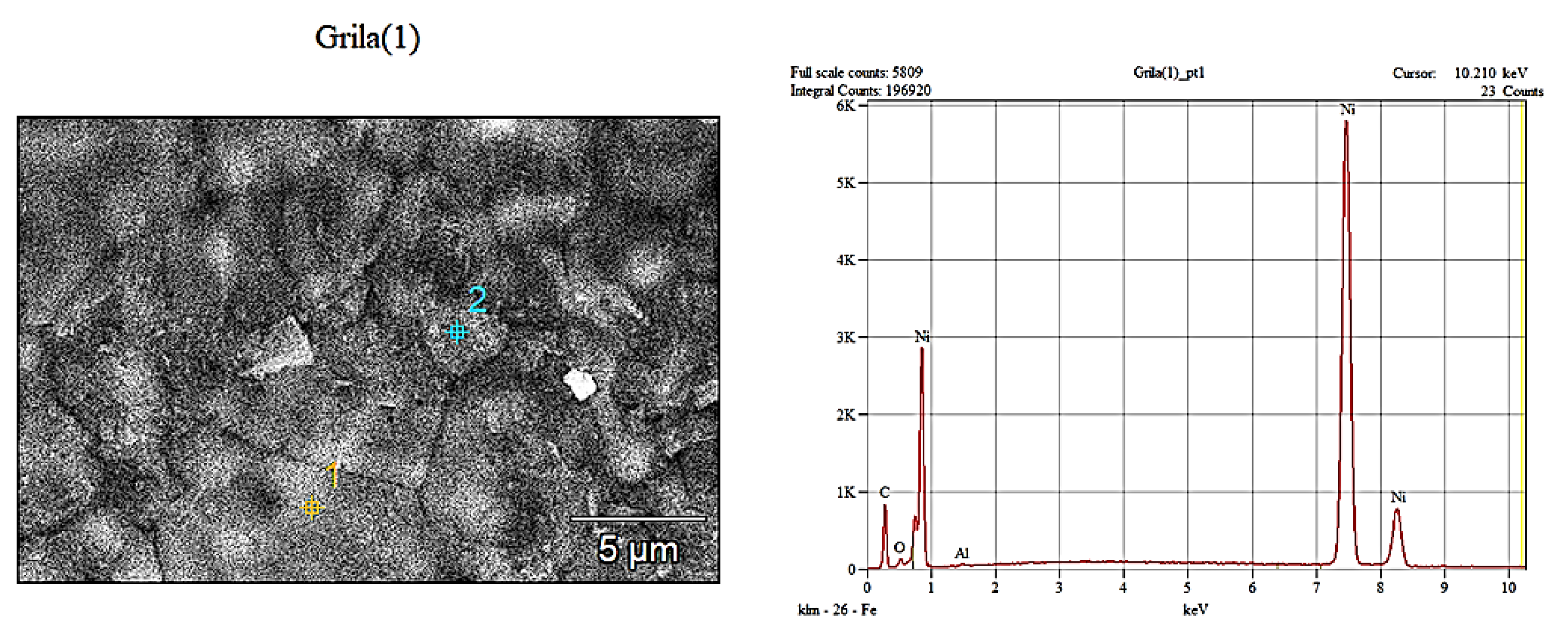
From
Figure 6.a it is observed that the support mesh presents a porous structure with open and interconnected pores with complex irregular shapes (eyes). The width of the lamellae that form the meshes of the network is between about 40 to 80 µm. The morphology of the lamellae surface presents irregularities of the order of µm.
Figure 7.
EDS analysis performed on the cathode support mesh.
Figure 7.
EDS analysis performed on the cathode support mesh.
The compositional analysis performed by the EDS method was performed at 5 points on each sample, the quantitative result presented being their average. It can be concluded that the cathode support mesh is made of nickel.
3.3. Characterization of Recovered Cathode Paste After Ultrasonication
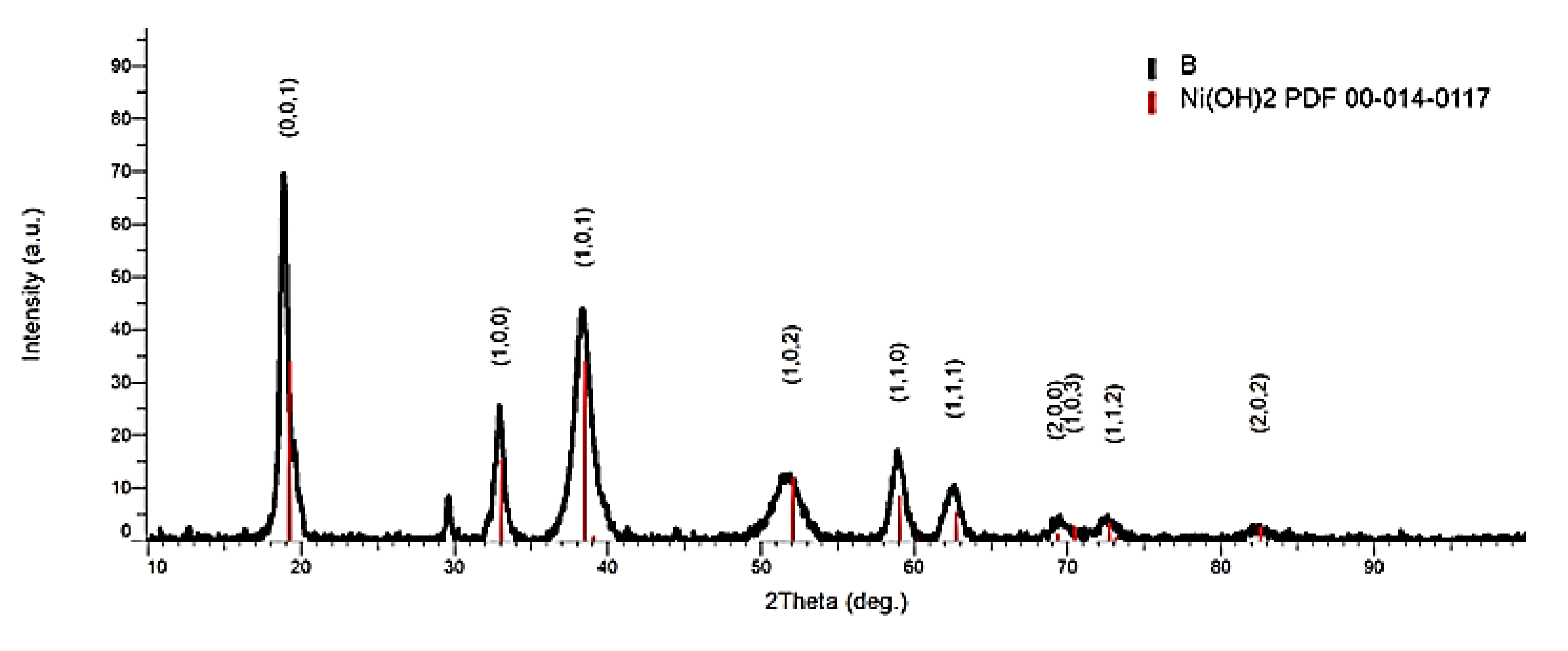
The cathode paste pressed on the nickel screen is made up of nickel hydroxide. The result of X-ray investigations on cathodic paste (
Figure 8) was obtained and presented in a previous paper.
Table 3.
Section from the X-ray diffraction index sheet for the compound Ni(OH)2 identified by the first three most important maxima (I %) recorded (PDF 00-014-0117).
Table 3.
Section from the X-ray diffraction index sheet for the compound Ni(OH)2 identified by the first three most important maxima (I %) recorded (PDF 00-014-0117).
| 2θ |
D (Å) |
I |
h |
k |
l |
| 19.258 |
4.605 |
100 |
0 |
0 |
1 |
| 33.064 |
2.707 |
45 |
1 |
0 |
0 |
| 38.541 |
2.334 |
100 |
1 |
0 |
1 |
| 39.098 |
2.302 |
2 |
0 |
0 |
2 |
| 52,100 |
1.754 |
35 |
1 |
0 |
2 |
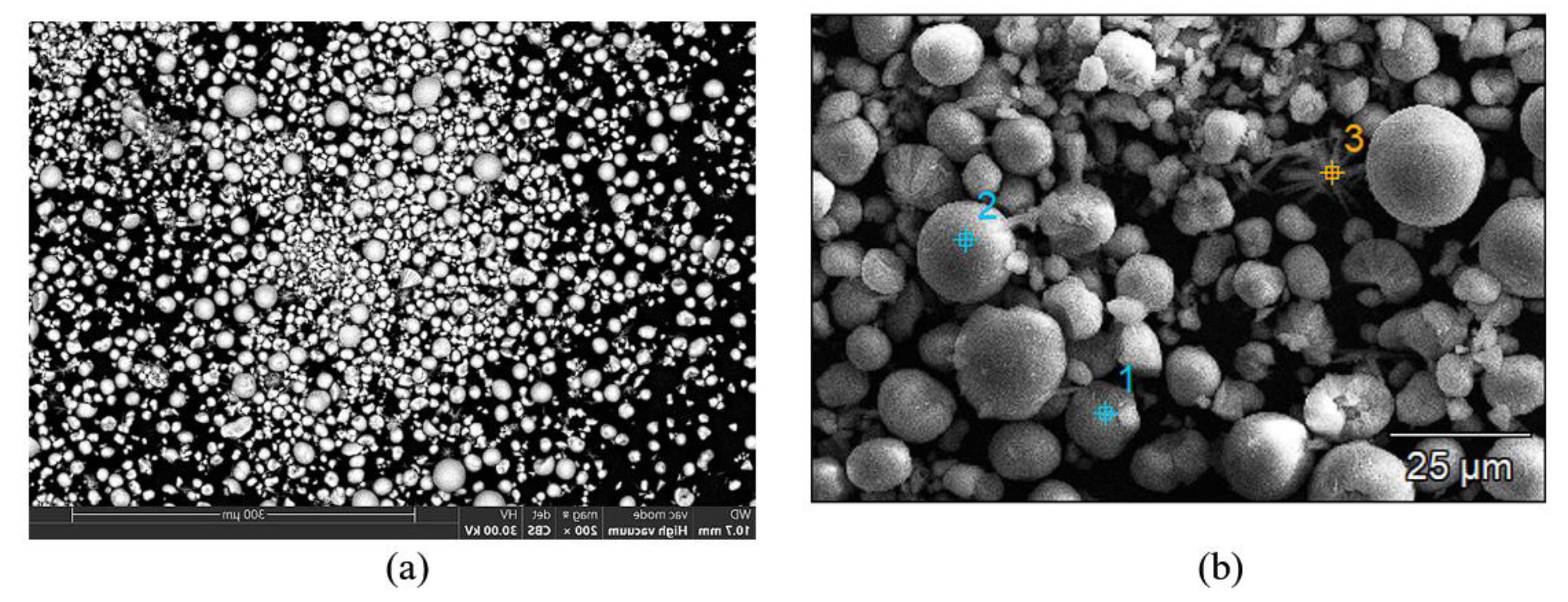
The electron microscopy (EM) analysis performed on the cathodic powder resulting from ultrasonication reveals the particle morphology: predominantly globular particles with dimensions between 10-20µm (
Figure 9). The globular particles have approximately identical compositions with small variations in elemental concentrations (
Figure 10).
3.4. Separation/Recovery of Rare Earth Containing anode Paste from the SUPPORT grid (Made of Ni Alloy)
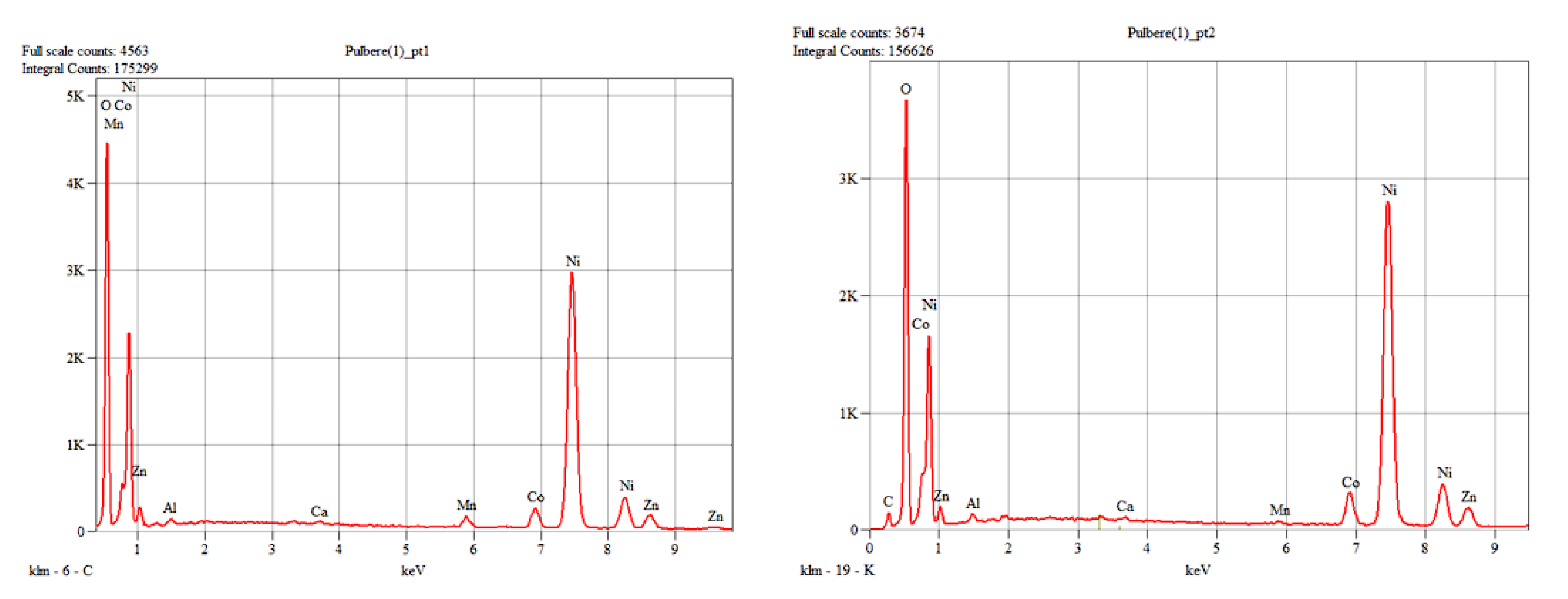
The anode used in Ni-MH batteries consists of a support metal grid made of a Ni-Fe alloy on which an anode paste containing rare earths is compacted. The image of the analyzed section of the anode of used Ni-MH batteries before ultrasonication accompanied by the chemical composition (XRF analysis) are presented in
Figure 11.
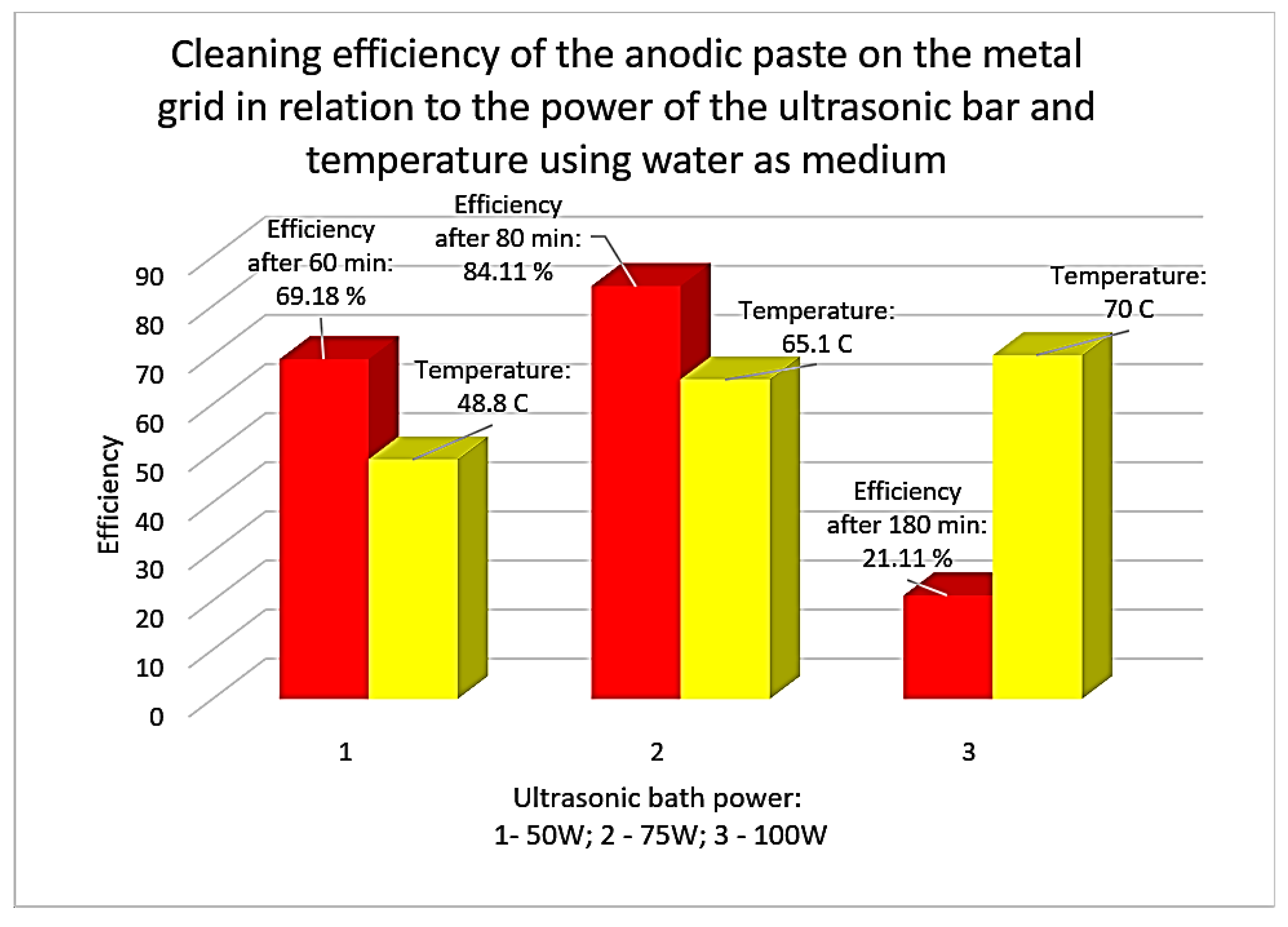
As an ultrasonication medium for cleaning/separating the rare earth containing anode paste from the support grid, drinking water was used first and then 1M citric acid solution. The results of the research using water as the ultrasonic medium are presented in the diagram in
Figure 12. Starting the research with water preheated to 40 °C, it can be seen that the efficiency varies between 21.11% and 84.11%. The maximum efficiency was reached after 80 minutes of ultrasonication at a bath power of 75W. An increase in the ultrasonic power to 100W decreases the efficiency and only has the effect of increasing the water temperature to 70 °C after 180 minutes.
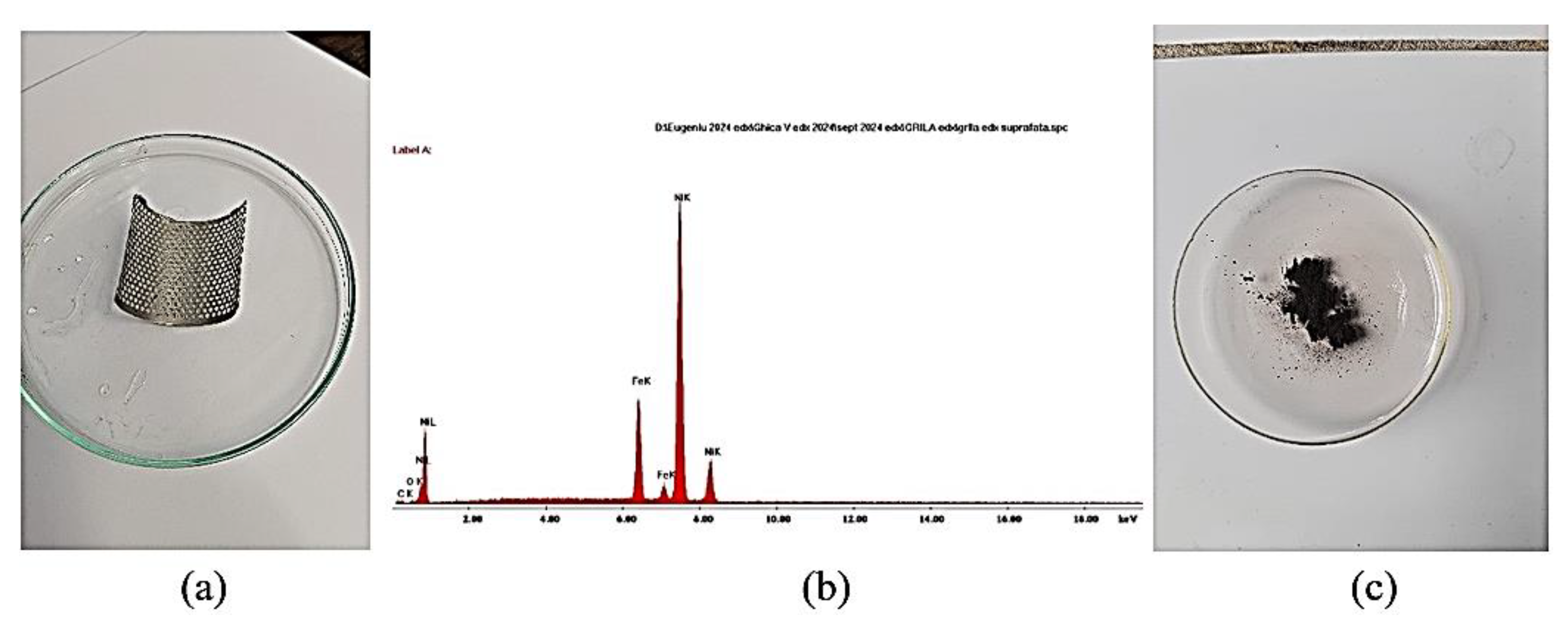
The ultrasonication time is high even in the case of achieving a high separation efficiency (over 80%). Next, the metal grid cleaned of the anodic paste (
Figure 13a) was analyzed by EDX analysis (
Figure 13b), which confirmed that it is made of a Ni-Fe alloy; the resulting paste (
Figure 13c) was analyzed on the electron microscope (
Figure 14,
Figure 15).
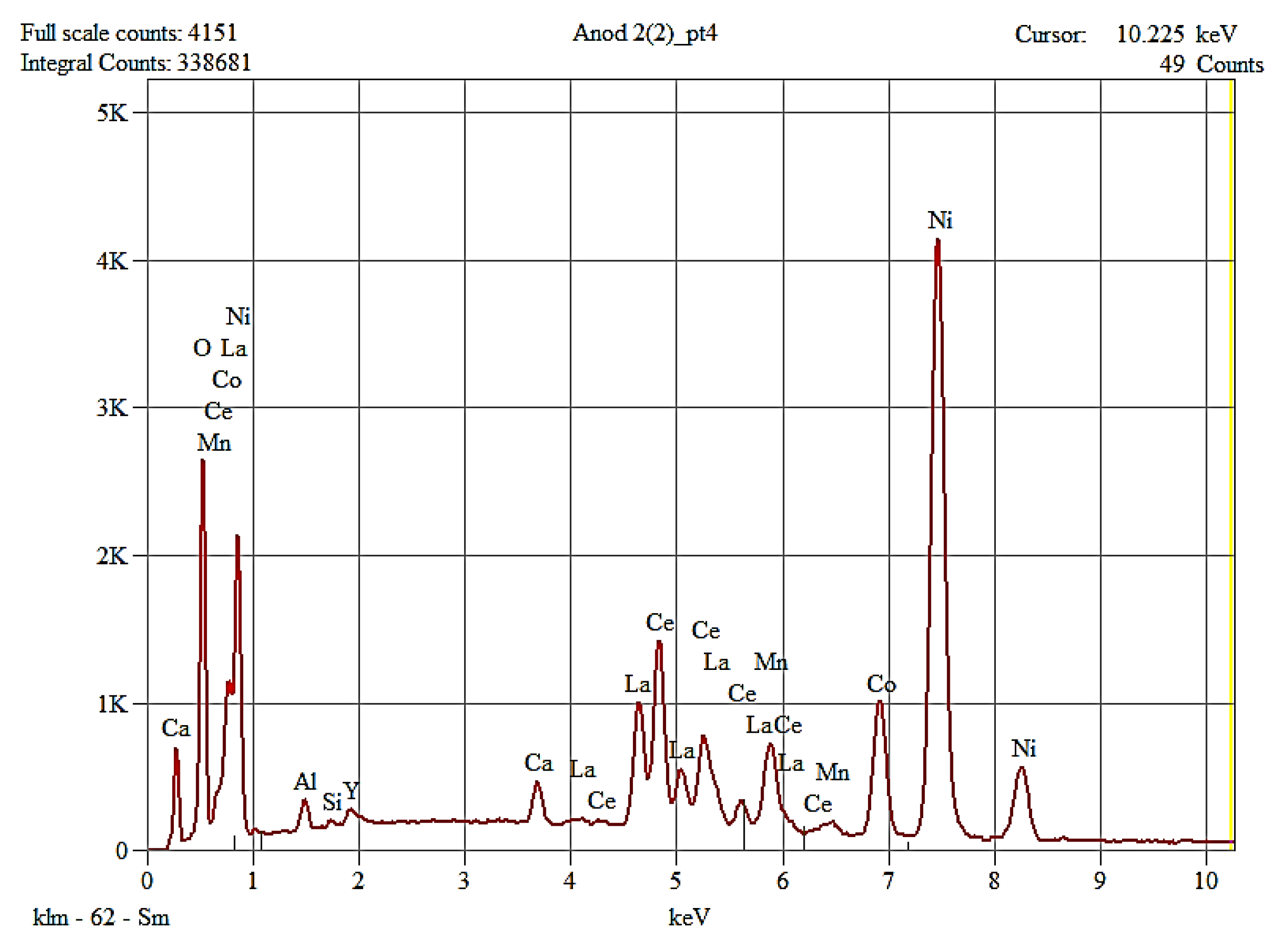
The morphology of the recovered anode paste is represented by agglomerations of angular particles with dimensions ranging from 5-25 µm, which were analyzed by EDX analysis, which highlighted the presence of rare earths (Ce, La, Y) along with Ni, Co, Mn, elements originating from the anode support grid.
3.5. Ultrasonication in Citric acid Medium of an Anode Grid Section
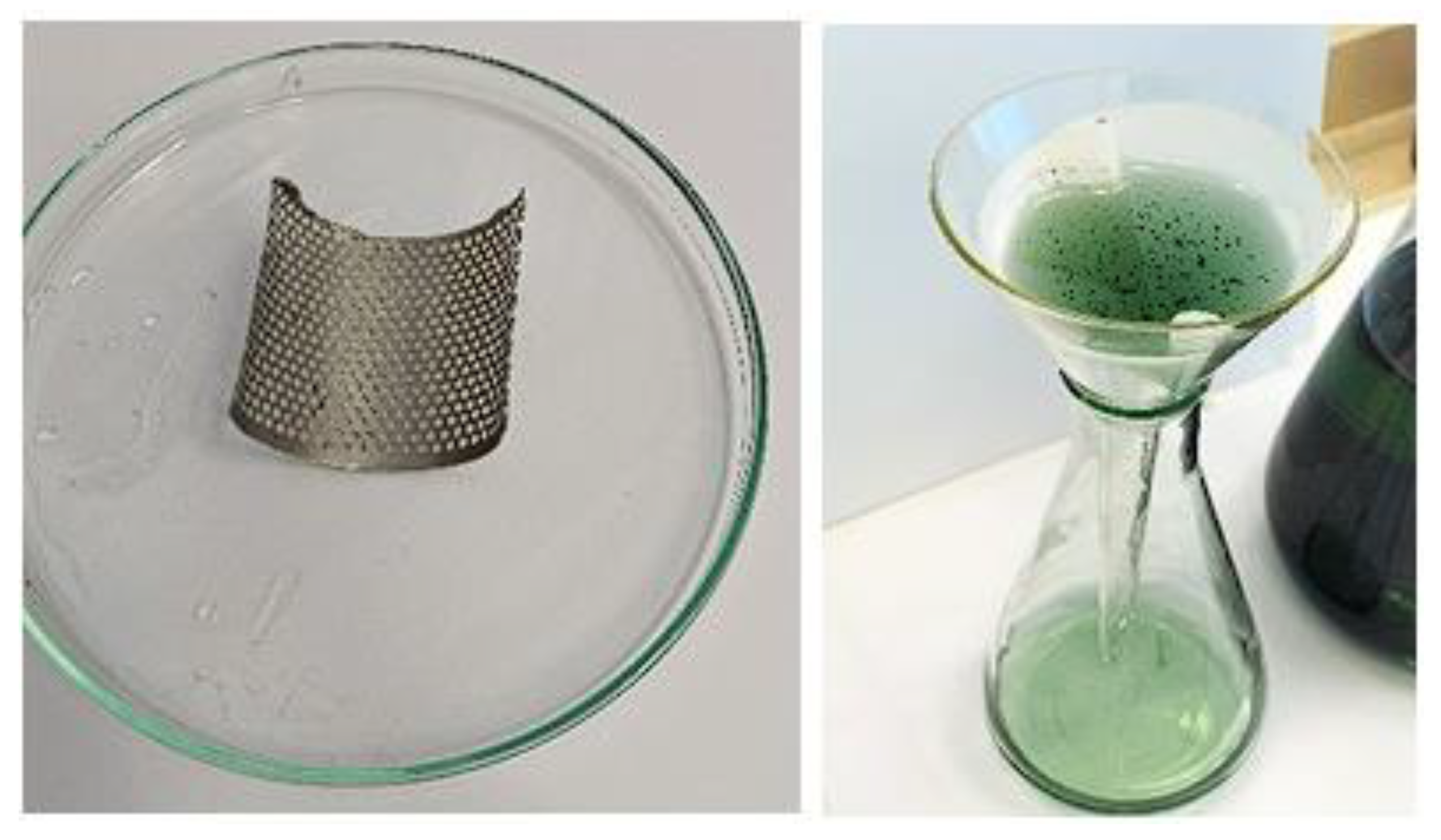
Using ultrasonication in a citric acid environment (about 1%M) (see
Figure 16), the following were observed: if we start the cleaning process with a solution of about 1 M citric acid (C
6H
8O
7) at room temperature (T-20
oC) and a maximum power of the ultrasonic bath (P=100W), the cleaning time of the anode grid is between 20-40 min.
The weakly acidic solution (1M citric acid) completely dissolves the anode paste (rare earths pass into solution). The result was expected, Brazilian [
18] researchers proposed leaching the anode material solution with citric acid in order to obtain metallo-organic precursors that, when heated, lead to a mixture of nickel and rare earth oxides. The process is slow; however, dissolution occurs within 24 hours under continuous stirring. The use of ultrasound accelerates the dissolution reaction, shortening the duration to minutes.
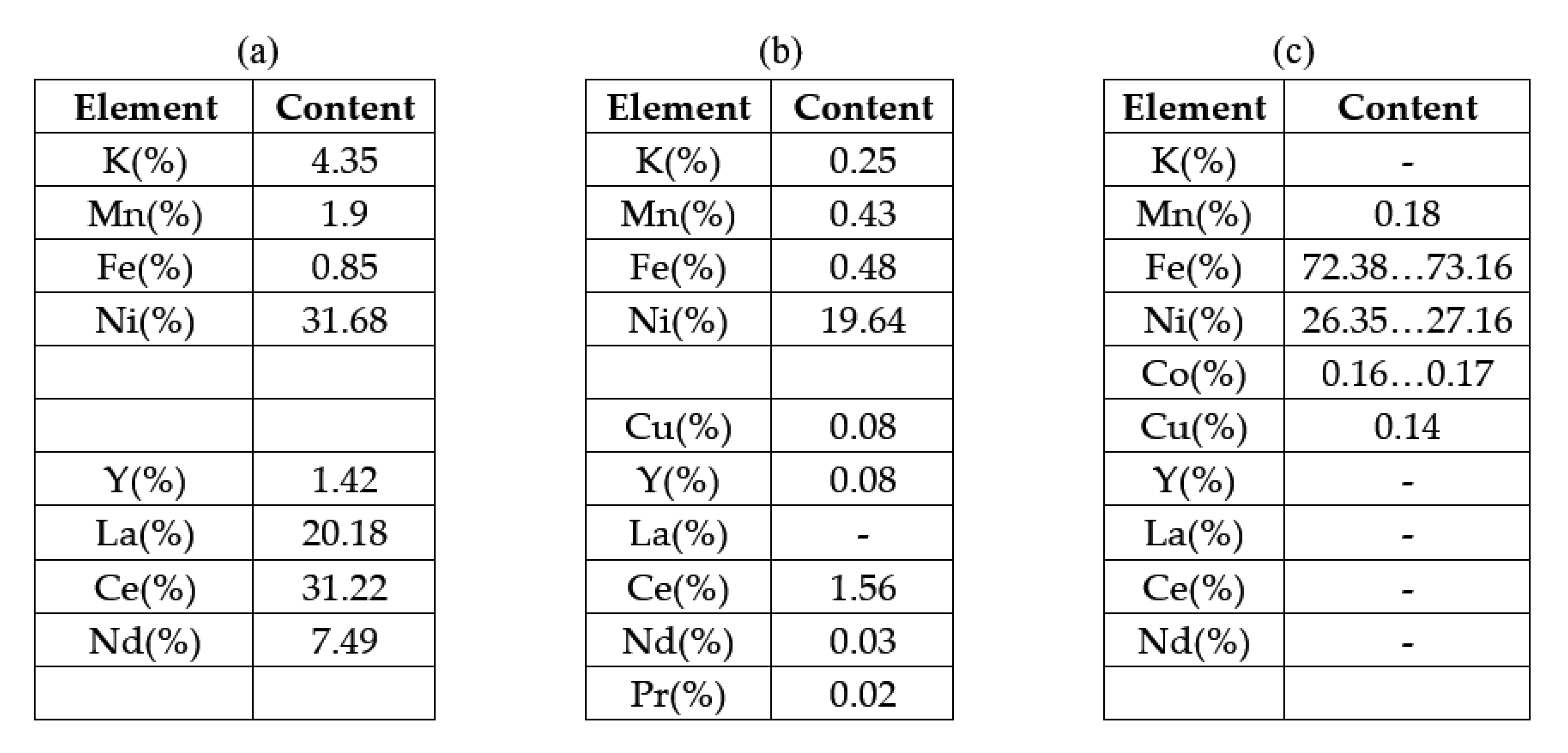
As can be seen from
Figure 17, the entire amount of Lanthanum and most of Ce and Nd went into solution; traces of Nd remained and traces of Pr were also identified.
4. Conclusions
The high content of non-ferrous metals such as Ni, Co, Zn, Rare Earths, present in used Ni-MH batteries, justifies research on the recovery of these metals; these metals constitute a secondary source of raw materials, and their reuse in the economic circuit represents an economic opportunity and an ecological obligation.
After a manual separation into components (cathode, anode, electrical connections, casing) of Li-MH batteries used in medical applications, research focused on finding a cheap and low-polluting technology for ultrasonic separation in water intended for human consumption, respectively in a 1M citric acid solution, of the cathode/anode paste on the cathode screen/anode grid support in order to identify useful metals that can be recovered.
The cathode was ultrasonicated in a stainless-steel bath in drinking water; the support screen – made of Ni was cleaned of the cathode paste. The cleaning efficiency slowly decreases from 68.22% to 58.04% as the ultrasonic bath power increases but with a significant reduction in the time required for cleaning (from 180 minutes to 40 minutes). If maximum bath power is used, namely 100W, a high temperature of the ultrasonic bath, i.e. over 70oC, is reached in short time.
There is a good reason to separate the cathode paste from the support mesh:
if Ni is to be recovered and melt in an electric arc furnace, the cathode support mesh made of Ni, (Tmelting = 1455oC) together with the paste (Ni(OH)2), then Ni(OH)2 would decompose at 300-400oC, releasing oxygen and transforming into NiO (temperature melting of NiO, T melting = 1995oC) which would impure the nickel.
being separated from the cathode mesh, Ni(OH)2 could be used as a precursor for the NiO product: Ni(OH)
2 heated at 400 °C for 1 hour in air leads to its decomposition into NiO and water.
After that, NiO can be used for various electronic and electrochemical applications [
19,
20,
21].
Following electron microscopy analyses, it was found that the support mesh is made of nickel and has a porous structure with open and interconnected pores, with complex irregular shapes, meshes with a lamella width of approximately 40 to 80 µm. Cathode paste, which is pressed on the nickel mesh, is made of globular particles of nickel hydroxide, particles with dimensions between 10-20 µm. The anode support grid is made of a Ni-Fe alloy. The recovery of the anode paste was performed by ultrasonication in drinking water and 1M citric acid solution.
In the first case (drinking water as the ultrasonication medium), the maximum cleaning/recovery efficiency of the paste containing rare earths was 84.11%, a value reached after 80 minutes and at an ultrasonic bath power of 75W. An increase in the purity of the ultrasonic bath does not help (recovery efficiency decreases) and increases the temperature to undesirable values. The recovered anode paste contains a mixture of rare earths (La, Ce,), Yttrium and nickel detached from the support grid. The particle sizes resulting from ultrasonication in water are smaller than 25 microns.
In the case of performing the separation/recovery operation of the anode paste in 1M citric acid solution, the rare earths passed into the solution in about 20 to 40 minutes, the paste identifying elements detached from the support grid and traces of Ce.
Today, the search for ecological and cheap solutions for the recovery of useful metals contained in used Ni-MH batteries is of great diversity [
19,
20,
21]. Future research will continue in several directions:
finding a solution for mechanized dismantling and sorting of used Ni-MH batteries into components;
optimizing the process of recovering useful metals from used Ni-MH batteries by ultrasonication in a citric acid environment, because the process is cheap and environmentally friendly;
advantageous utilization of expensive and scarce non-ferrous metals and rare earths contained in used Ni-MH batteries.
Author Contributions
Conceptualization, Valeriu Ghica, Narcis Daniel Saftere and Eugenia Tanasă; Data curation, Florin Miculescu, Ana Vasile, Narcis Daniel Saftere, Mircea Ionut Petrescu and Eugenia Tanasă; Formal analysis, Florin Miculescu; Funding acquisition, Valeriu Ghica and Şener Karabulut; Investigation, Valeriu Ghica, Ana Vasile, Angelos Markopoulos, Mircea Ionut Petrescu, Eugenia Tanasă and Anca Icleanu; Methodology, Florin Miculescu, Mircea Ionut Petrescu, Eugenia Tanasă and Anca Icleanu; Resources, Valeriu Ghica, Angelos Markopoulos and Şener Karabulut; Software, Ana Vasile and Anca Icleanu; Supervision, Valeriu Ghica, Angelos Markopoulos and Şener Karabulut; Validation, Narcis Daniel Saftere, Angelos Markopoulos and Şener Karabulut; Visualization, Ana Vasile, Mircea Ionut Petrescu and Eugenia Tanasă; Writing – original draft, Florin Miculescu, Narcis Daniel Saftere, Mircea Ionut Petrescu and Anca Icleanu; Writing – review & editing, Angelos Markopoulos and Şener Karabulut.
Funding
This research was funded by ERASMUS+ Programme Key Action 2 Cooperation Partnerships for Higher Education (KA220-HED) Titled “Advanced Higher Education for Battery Lifecycle and Sustainability (BTRexpert)” (2024-I-PL01-KA220-HED-000249112); Cod SMIS/ID: 2024-I-PL01-KA220-HED-000249112.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alluraiah, C.N.; Nandogopal, V.; Veeramanikandan, P.; Godfrey, D.; Meena, S.; Bridha, G. Comparison of SoC in Ni-MH and Lithium-Ion Battery for E Vehicle. Int. J. Electr. Electron. Res. (IJEER) 2024, 12(4), 1258–1263. [CrossRef]
- Ourici, A. Battery Technologies Comparison for Electric Vehicles. Indian J. Sci. Technol. 2023, 16(20), 1461–1468. [CrossRef]
- Mars, N.; Krouz, F.; Louar, F.; Sbita, L. Comparison Study of Different Dynamic Battery Models. In Proceedings of the 2017 International Conference on Green Energy Conversion Systems (GECS); IEEE: Hammamet, Tunisia, 2017; pp. 1–6. [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a Recycling Process for Li-Ion Batteries. J. Power Sources 2012, 207, 173–182. [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A Promising Approach for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Power Sources 2017, 351, 192–199. [CrossRef]
- Müller, T.; Friedrich, B. Development of a Recycling Process for Nickel-Metal Hydride Batteries. J. Power Sources 2006, 158, 1498–1509. [CrossRef]
- Innocenzi, V.; Veglio, F. Separation of Manganese, Zinc and Nickel from Leaching Solution of Nickel-Metal Hydride Spent Batteries by Solvent Extraction. Hydrometallurgy 2012, 129–130, 50–58. [CrossRef]
- Innocenzi, V.; Ippolito, N.A.; De Michelis, I.; Prisciandaro, M.; Medici, F.; Veglio, F. A Review of the Processes and Lab-Scale Techniques for the Treatment of Spent Rechargeable NiMH Batteries. J. Power Sources 2017, 362, 202–218. [CrossRef]
- Rueda, H.; Arenas, M.; Delvasto, P. Production of a Nickel-Based Catalyst for Urea Electrooxidation Using Spent Batteries as Raw Material: Electrochemical Synthesis and Implications from a Circular Economy Standpoint. Sustain. Mater. Technol. 2021, 29, e00296. [CrossRef]
- Panasonic Energy Co. Technical Downloads. https://energy.panasonic.com/na/business/downloads/psds (accessed on 14 October 2025).
- Innocenzi, V.; Veglio, F. Recovery of Rare Earths and Base Metals from Spent Nickel-Metal Hydride Batteries by Sequential Sulphuric Acid Leaching and Selective Precipitations. J. Power Sources 2012, 211, 184–191. [CrossRef]
- Constantine, J.; Lie, J.; Liu, J.C. Recovery of Rare Earth Elements from Spent NiMH Batteries Using Subcritical Water Extraction with Citric Acid. J. Environ. Chem. Eng. 2022, 10(3), 108000. [CrossRef]
- Lie, J.; Liu, J.C. Selective Recovery of Rare Earth Elements (REEs) from Spent NiMH Batteries by Two-Stage Acid Leaching. J. Environ. Chem. Eng. 2021, 9(5), 106084. [CrossRef]
- Liu, H.; Zhang, S.; Pan, D.; Tian, J.; Yang, M.; Wu, M. Rare Earth Elements: Recycling from Waste Phosphor by Dual Hydrochloric Acid Dissolution. J. Hazard. Mater. 2014, 272, 96–101. [CrossRef]
- Badanoiu, G.; Buzatu, T.; Ghica, V.G.; Buzatu, M.; Iacob, G.; Petrescu, M.I. Study of PbSO₄ Solubilisation in NaOH Solution for Treatment of Oxide–Sulphate Pastes Obtained from Dismembered Lead–Acid Batteries. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76(1), 209–214.
- Ghica, V.G.; Toma, C.M.; Buzatu, M.; Petrescu, M.I.; Iacob, G.; Antoniac, I.V.; Vasile, E.; Veglio, F. Recovery of Active Cathode Material Containing Co and Li from Waste Li-Ion Batteries. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2017, 79(3), 75–86.
- Vasile, A.; Ghica, V.G.; Iacob, G.; Petrescu, M.I.; Mihailov, E.; Patroi, D. Recycling Ni-MH Batteries Used in Medical Devices. Part I – Investigations on the Contents of Ni-MH Batteries. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2024, 86(2), 223–234.
- Marins, A.A.L.; Boasquevisque, L.M.; Muri, E.J.B.; Freitas, M.B.J.G. Environmentally Friendly Recycling of Spent Ni–MH Battery Anodes and Electrochemical Characterization of Nickel and Rare Earth Oxides Obtained by Sol–Gel Synthesis. Mater. Chem. Phys. 2022, 280, 125821. [CrossRef]
- Li, T.; Okada, T.; Ichimura, M. Drop-Dry Deposition of Ni(OH)₂ Precursor for Fabrication of NiO Thin Films. Materials 2022, 15(13), 4513. [CrossRef]
- Li, L.; Xu, S.; Ju, Z.; Wu, F. Recovery of Ni, Co and Rare Earths from Spent Ni–Metal Hydride Batteries and Preparation of Spherical Ni(OH)₂. Hydrometallurgy 2009, 100(1–2), 41–46. [CrossRef]
- Díaz-López, J.C.; Angarita, J.; Vargas-Angarita, C.Y.; Blanco, S.; Delvasto, P. Electrolytic Recovery of Nickel and Cobalt as Multi-Elemental Coatings: An Option for the Recycling of Spent Ni-MH Batteries. J. Phys. Conf. Ser. 2018, 1119, 012003. [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical Recycling of Li-Ion, Ni–Cd and Ni–MH Batteries: A Minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [CrossRef]
- Pham, H.D.; Krishnan, S.G.; Wang, T.; Fernando, J.F.S.; Padwal, C.; Golberg, D.V.; Dubal, D.P. Upcycling of Nickel Oxide from Spent Ni-MH Batteries as Ultra-High Capacity and Stable Li-Based Energy Storage Devices. Sustain. Mater. Technol. 2023, 36, e00602. [CrossRef]
Figure 1.
Manual disassembly of used Ni-MH batteries.
Figure 1.
Manual disassembly of used Ni-MH batteries.
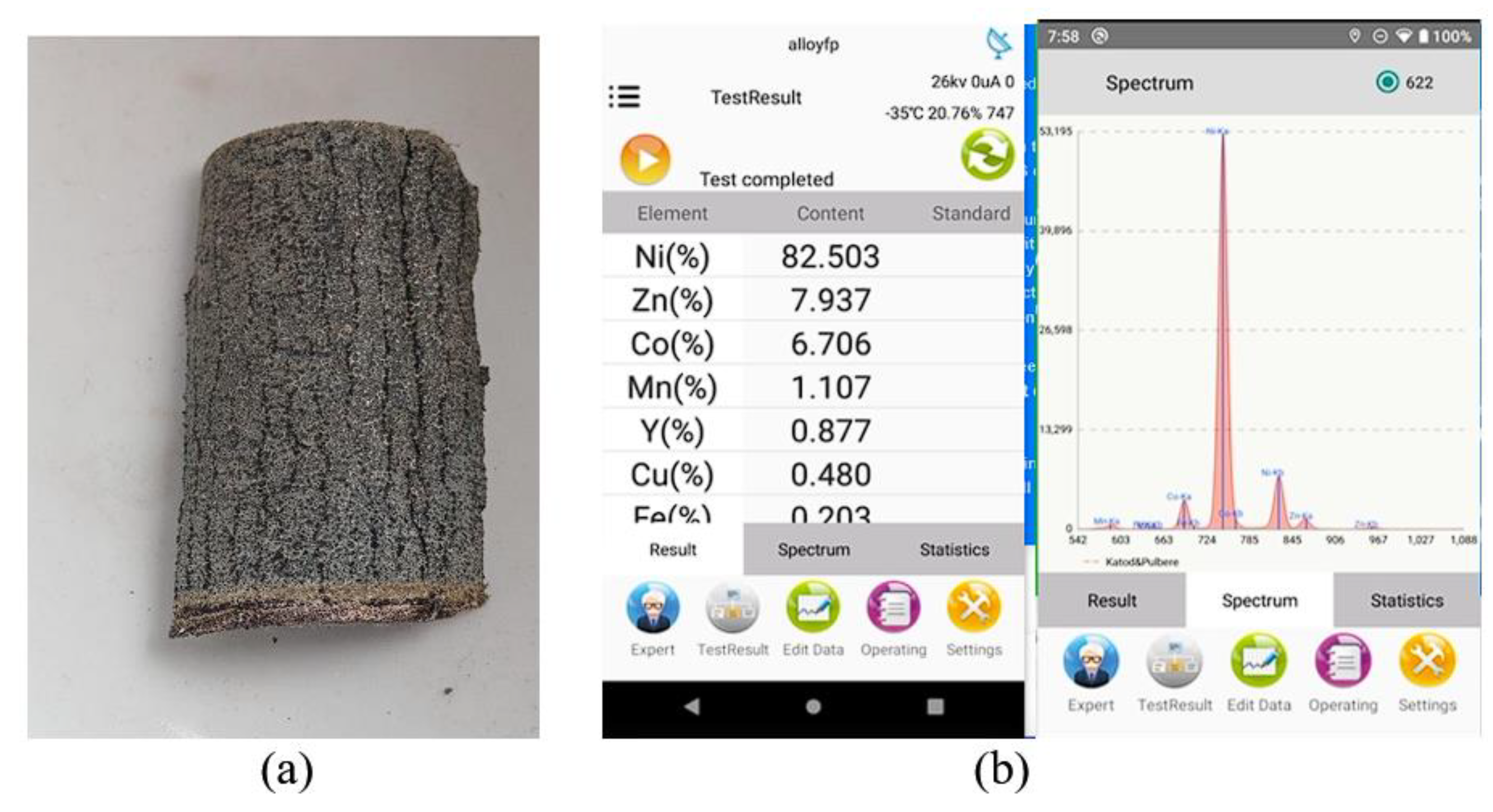
Figure 2.
X-ray fluorescence analysis on a cathode section before ultrasonication. (a) Photo section of the cathode of the Ni-MH battery, (b) XRF Analysis Whole cathode (mesh/screen metal + paste).
Figure 2.
X-ray fluorescence analysis on a cathode section before ultrasonication. (a) Photo section of the cathode of the Ni-MH battery, (b) XRF Analysis Whole cathode (mesh/screen metal + paste).
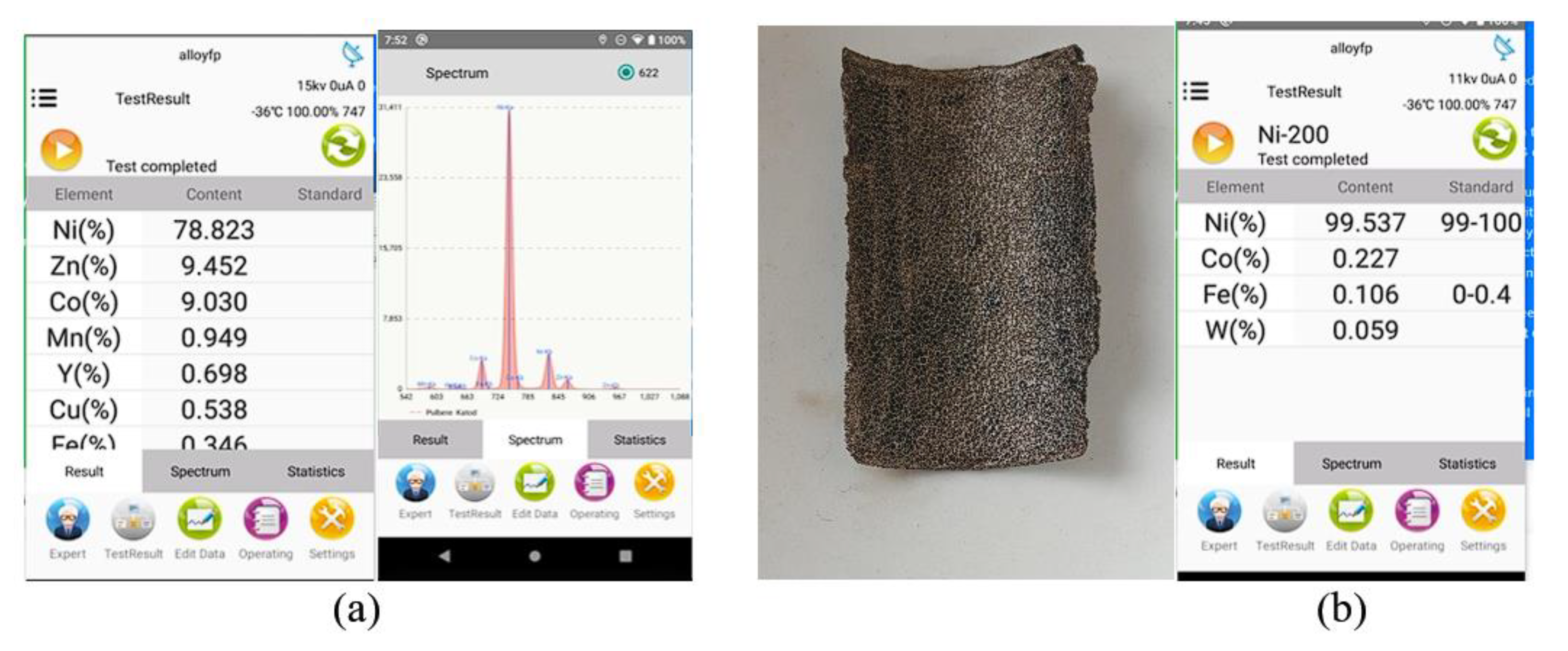
Figure 3.
X-ray fluorescence analysis on a cathodic section after ultrasonication. (a) XRF Analysis Paste Cathodic paste, (b) Photo and XRF Analysis of mesh/screen metallic support of cathode.
Figure 3.
X-ray fluorescence analysis on a cathodic section after ultrasonication. (a) XRF Analysis Paste Cathodic paste, (b) Photo and XRF Analysis of mesh/screen metallic support of cathode.
Figure 4.
Cleaning efficiency depending on time and power of the ultrasonic bath.
Figure 4.
Cleaning efficiency depending on time and power of the ultrasonic bath.
Figure 5.
Increase in ultrasonic bath temperature over time, depending on bath power.
Figure 5.
Increase in ultrasonic bath temperature over time, depending on bath power.
Figure 6.
(a) Structure of the cathode support mesh, (b) cathode support mesh lamella.
Figure 6.
(a) Structure of the cathode support mesh, (b) cathode support mesh lamella.
Figure 8.
X-ray investigations on cathodic paste [
17].
Figure 8.
X-ray investigations on cathodic paste [
17].
Figure 9.
Morphology of globular particles obtained after ultrasonication of the cathode of used Ni-MH batteries (a) and EDX analysis performed on globular particles obtained after ultrasonication of the cathode of used Ni-MH batteries.
Figure 9.
Morphology of globular particles obtained after ultrasonication of the cathode of used Ni-MH batteries (a) and EDX analysis performed on globular particles obtained after ultrasonication of the cathode of used Ni-MH batteries.
Figure 10.
Chemical composition of two globular particles (X-ray emission spectrum); besides the well-defined presence of nickel, traces of other elements (Co, Zn, Mn) are also recorded.
Figure 10.
Chemical composition of two globular particles (X-ray emission spectrum); besides the well-defined presence of nickel, traces of other elements (Co, Zn, Mn) are also recorded.
Figure 11.
(a) Section of the anode grid, (b)chemical analysis of the support metal grid.
Figure 11.
(a) Section of the anode grid, (b)chemical analysis of the support metal grid.
Figure 12.
The cleaning efficiency of the anodic paste on the support grid resulting from ultrasonication in water, correlated with the power of the ultrasonic bath and the temperature of the water in the ultrasonication tank.
Figure 12.
The cleaning efficiency of the anodic paste on the support grid resulting from ultrasonication in water, correlated with the power of the ultrasonic bath and the temperature of the water in the ultrasonication tank.
Figure 13.
(a) Support grid after cleaning, (b) EDX analysis identifies the presence of Ni and Fe, (c) anodic paste recovered after ultrasonication in water.
Figure 13.
(a) Support grid after cleaning, (b) EDX analysis identifies the presence of Ni and Fe, (c) anodic paste recovered after ultrasonication in water.
Figure 14.
The morphology of the anodic paste recovered by ultrasonication in water. .
Figure 14.
The morphology of the anodic paste recovered by ultrasonication in water. .
Figure 15.
Chemical composition of the powder resulting from ultrasonication in water of a section of the anode of the urated Ni-MH battery (X-ray emission spectrum).
Figure 15.
Chemical composition of the powder resulting from ultrasonication in water of a section of the anode of the urated Ni-MH battery (X-ray emission spectrum).
Figure 16.
The cleaned anode grid and the resulting solution after ultrasonication in 1M citric acid.
Figure 16.
The cleaned anode grid and the resulting solution after ultrasonication in 1M citric acid.
Figure 17.
Results of XRF analysis performed on: (a) paste detached from the support grid, before ultrasonication in 1M citric acid solution, (b) powder recovered after filtration of the aqueous solution ultrasonicated in 1M citric acid solution, (c) metal support grid recovered after ultrasonication in 1M citric acid solution.
Figure 17.
Results of XRF analysis performed on: (a) paste detached from the support grid, before ultrasonication in 1M citric acid solution, (b) powder recovered after filtration of the aqueous solution ultrasonicated in 1M citric acid solution, (c) metal support grid recovered after ultrasonication in 1M citric acid solution.
Table 1.
Comparison between the performances of lead-acid, Ni/MH and Li-ion batteries.
Table 1.
Comparison between the performances of lead-acid, Ni/MH and Li-ion batteries.
| |
Lead-acid |
Ni/MH |
Li-ion |
| Voltage |
2.1 V |
1.3 V |
3.8 V Volume energy |
| Weight energy |
30-50 Wh/kg |
70-80 Wh/kg (2001) –
100-500 Wh/kg (2023) |
120 Wh/kg (2001) –
300 Wh/kg (2001) |
| Volume energy |
80-90 Wh/dm3 (2001) –
400 Wh/dm3 (2023) |
150-200 Wh/dm3 (2001) – 300 Wh/dm3 (2023) |
150 Wh/dm3(2001) –
700 Wh/dm3 (2023) |
| Power |
180 W/kg (2001) –
<1000 W/kg (2001) |
200-300 Wh/kg (2021)
100-500 Wh/kg (2023) |
500 Wh/kg (2021)
500-700 Wh/kg (2023) |
| Cycle life |
<350 |
1000 (2001)
– 2000 (2023) |
1500 (2001) –
3000 (2023) |
| Cost |
50-150 $/KWh |
330 $/KWh (2001) –
139 $/KWh (2023) |
800 $ $/KWh (2001) -
75-259 $/KWh (2023) |
Table 2.
Composition and ingredient information [
10].
Table 2.
Composition and ingredient information [
10].
| Common Chemical name |
CAS number |
Concentration/
Percentage Range |
Nickel Hydroxide
Cobalt Hydroxide |
12054-48-7
21041-93-0 |
15-25% (15-30%)*
1-5% |
| Hydrogen absorbing alloy |
7440-02-0(Ni)
7440-48-4(Co)
7439-96-5(Mn)
7429-90-5(Al) |
20-35% (20-40%)* |
| Nickel |
7440-02-0(Ni) |
3-10% (3-10%)* |
| Iron |
7439-89-6(Fe) |
10-25% (15-40%)* |
Potassium Hydroxide
Sodium Hydroxide
Lithium Hydroxide |
1310-58-3
1310-73-2
1310-65-2 |
0-15% (0-15%)* |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).