1. Introduction
Global demand for poultry has driven a 1.2% increase in global animal feed production, reaching approximately 1.4 billion metric tonnes in 2025 [
1]. Feed accounts for 70–80% of total poultry production costs, with protein ingredients, particularly soybean meal, representing the largest share [
2,
3]. Soybean meal remains the preferred protein source due to its high nutritional quality, which can be further improved through modern processing and enzyme supplementation [
4].
Given that dietary protein is both essential for broiler growth and the most expensive feed component, protease supplementation has gained attention as a strategy to enhance amino acid digestibility, maintain growth performance, lower feed costs, and reduce nitrogen excretion [
5]. Adequate dietary amino acids, such as glutamate, glutamine, aspartate, arginine, glycine, methionine, cysteine, and threonine, are critical for maintaining intestinal integrity, mucin synthesis, immune and antioxidative responses, and microbial balance, thereby supporting gut health and nutrient utilization [
6]. For example, a
Bacillus subtilis-derived protease has been shown to improve nitrogen and crude protein digestibility, increasing amino acid availability and growth in broilers fed low-protein, condensed distillers dried grains with solubles-based diets, without adverse effects on carcass traits, energy utilization, blood biochemistry, or gut morphology [
7]. However, rising soybean meal prices, driven by climate change and trade disruptions, have intensified the search for alternative protein sources and enzyme technologies to sustain poultry productivity. Enzyme supplementation enhances the nutritional value of soybean meal feed efficiency and rising costs [
8].
Exogenous proteases are widely used to improve protein utilization, allow dietary protein reduction without compromising performance, and lower nitrogen and ammonia emissions, offering both economic and environmental benefits [
9]. Studies have shown that protease supplementation increases metabolizable energy and amino acid digestibility in broiler and swine [
10,
11]. Combined phytase and protease supplementation further enhances growth, energy utilization, and amino acid digestibility in nutrient-restricted diets [
12]. Commercial protease products have been reported to improve feed conversion, ileal digestible protein, dry matter digestibility, and energy utilization, effectively , compensating for reduced dietary lysine and energy [
13]. Nevertheless, enzyme efficacy depends on feed composition, emphasizing the need for tailored enzyme applications. Protease supplementation generally improves fat and CP digestibility and metabolizable energy across broiler diets, independent of protein or energy levels [
14]. Protease use also contributes to environmental sustainability by reducing CO₂ emissions from soybean cultivation and mitigating nitrogen-related eutrophication and acidification [
15].
In addition to improving nutrient utilization, protease supplementation enhances gut morphology, as indicated by increased villus height, reduced epithelial thickness, and improved ileal amino acid digestibility, collectively supporting feed efficiency [
16,
17]. However, excessively high protease doses may impair growth performance and nitrogen digestibility, highlighting the importance of dose optimization [
18,
19]. Inconsistent outcomes across studies are often linked to failure in integrating enzyme matrix values during diet formulation, leading to inaccurate nutrient supply estimates [
20]. Matrix-dosed protease supplementation offers a more precise strategy to reduce reliance on costly protein ingredients like soybean meal.
Despite the well-documented benefits of protease supplementation in broiler nutrition, most existing studies have evaluated single commercial products in isolation, often under non-standardized or on-top supplementation strategies. Consequently, there remains a lack of comparative evidence on how different protease formulations, particularly standard versus next-generation enzyme blends, perform when applied under a matrix-dosed design that accounts for nutrient release values. Matrix-dosed supplementation is of growing practical interest, as it allows feed cost reduction while maintaining performance; however, its success depends on the specific efficacy of the enzyme formulation used. Therefore, this study aims to evaluate the impact of protease supplementation as a partial substitute for soybean meal on growth metrics, nutrient absorption, intestinal structure, blood biochemical parameters, carcass traits, and overall economic viability in broiler chickens.
2. Materials and Methods
2.1. Ethical Statement
All animal care and experimental procedures complied with institutional animal welfare regulations. The protocol was reviewed and approved by the Animal Experimentation Ethics Committee, Faculty of Veterinary, Animal, and Biomedical Sciences, Khulna Agricultural University, Bangladesh (Approval No. AEEC/KAU/2024-1005).
2.2. Experimental Animals and Design
This study used 240 one-day-old male Cobb 500 broiler chicks, with an average initial body weight of 45.54 ± 3.67 g, sourced from a commercial hatchery. Following sex determination at hatch, the male chicks were uniformly distributed into six dietary treatment groups.
Protease X (OM Biopharma, Bangladesh) is a heat-stable serine protease blend containing acidic, neutral, and alkaline components, with an activity level of 1,000,000 units per gram. It is produced through submerged fermentation utilizing Bacillus licheniformis as the microbial source.
Protease A is a thermostable multi-protease formulation incorporating a serine protease characterized by the catalytic triad His57, Ser195, and Asp102. This advanced enzyme system facilitates rapid and efficient peptide bond cleavage, resulting in accelerated protein hydrolysis and improved protein utilization in poultry diets.
Protease F is a specialized protease designed for pelleted feed applications, formulated to withstand thermal processing while promoting rapid protein hydrolysis. It enhances nutrient digestibility and gut health, supporting cost-effective incorporation of alternative protein sources without compromising growth performance or feed efficiency.
Compared with Protease X, both Protease A and Protease F exhibit faster protein hydrolysis rates due to their optimized catalytic activity and broader substrate specificity, enabling superior nutrient release and utilization under commercial feeding conditions.
Each treatment group (T0 to T5) consisted of four replicates, with 10 chicks per replicate, totaling 40 birds per group. The dietary treatments were as follows: T0: basal diet without protease supplementation; T1: basal diet plus 250 g/tonne of Protease X as a top-up; T2: basal diet supplemented with 350 g/tonne of Protease X including matrix values; T3: basal diet plus 150 g/tonne of Protease A as a top-up; T4: basal diet with 250 g/tonne of Protease A including matrix; and T5: basal diet supplemented with 250 g/tonne of Protease F including matrix.
The experiment was conducted in two phases: the starter phase (day 0 to 14) and the grower phase (day 15 to 33). The birds were kept in environmentally controlled enclosures, each measuring 1.2 square meters and bedded with rice husk litter. The initial brooding temperature was set at 33 °C and then decreased by 2 °C each week, reaching 24 °C by the end of the trial on day 33.
2.3. Experimental Diet
Proteases were added either as top-up (direct inclusion without nutrient adjustment) or matrix (nutrient adjustments equivalent to enzyme nutrient release). Diets were pelleted for uniform intake (starter: 2 mm × 3 mm; grower: 3 mm × 5 mm) and offered ad libitum along with fresh water. Formulations met BIS (2007) broiler nutrient requirements. Basal diet proximate composition (DM, CP, CF, EE, total ash) was analyzed using AOAC (1992) methods. Ingredient and nutrient profiles are provided in
Table 1 and
Table 2 are expressed on an as-fed basis
.
2.4. Growth Performance Parameters
Initial body weight was recorded at placement. Body weight (BW), body weight gain (BWG), and feed intake (FI) were measured on days 7, 14, 21, 28, and 33. Feed conversion ratio (FCR) was calculated as FI/BWG. Mortality was recorded daily. On day 33, final performance data were collected, and selected birds processed for further analysis.
2.5. Blood Profile
On days 21 and 33, blood (3 mL) was collected from the wing vein of one randomly selected bird per replicate (n = 4 per treatment). Samples were chilled on ice, centrifuged for serum separation, and analyzed for total protein (TP), albumin, blood urea nitrogen (BUN), creatinine, glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), and low-density lipoprotein (LDL) using commercial diagnostic kits (BioMérieux, France) and an automated analyzer (Humalyzer 300, Merck®, Japan).
2.6. Carcass Traits and Relative Organ Weights
At 33 days of age, one bird per replicate (n = 4 per treatment) was randomly selected euthanized. Carcass components including chest, wings, legs, gizzard, intestine, liver, and heart were weighed to assess carcass traits. Absolute organ and carcass weights were recorded, and data are presented as mean ± SD.
2.7. Ileal Intestinal Morphology
Ileum samples from one bird per pen (day 33) were fixed in 10% neutral buffered formalin for 72 h, dehydrated through graded ethanol (60–100%), cleared with xylene, and embedded in paraffin. Sections (4–5 µm) were prepared using a rotary microtome and stained with hematoxylin and eosin (H&E). Intact villi and crypt structures were examined under 40× magnification (Olympus BX51, Tokyo, Japan), and six well-oriented villi were selected for measurement of villus height (VH), crypt depth (CD), and VH:CD ratio by following the protocol of Lee, Oh [
21].
2.8. Nutrient Digestibility
Apparent ileal digestibility was determined on days 21 and 33 using a total collection approach. Ileal digesta from four birds per treatment were pooled by replicate and freeze-dried. Dry matter was analyzed in a hot air oven at 105 °C for 24 h; CP was determined via the Kjeldahl method [
22]. The gross energy (GE) content of both diet and excreta was measured using an adiabatic bomb calorimeter. The apparent metabolizable energy (AME) of the diet was subsequently calculated using the following formula described by [
23]:
This calculation provides an estimate of AME by accounting for the difference between energy consumed and energy excreted. Amino acid composition of the diet was determined via HPLC following hydrolysis in 6 N HCl at 110 °C for 24 h. This methodology ensures accurate quantification of nutrient digestibility for each experimental treatment.
2.9. Economical Evaluation
Economic analysis considered total cost (feed, vaccines/medications, protease) and revenue from meat sales (170 BDT/kg live weight). Feed cost (39,687.24 BDT) and vaccine cost (2,850 BDT) were constant across treatments. Protease costs were: T0 – 0 BDT, T1 – 128.98 BDT, T2 – 180.58 BDT, T3 – 79.09 BDT, T4 – 131.82 BDT, T5 – 130.40 BDT. Costs for DOC and electricity were excluded as they did not vary by treatment. Profit was calculated as revenue minus total cost, and return on investment (ROI) was derived accordingly.
2.9. Statistical Analyses
Data was analyzed using R (version 4.4.2; released 31 October 2024). Normality was tested with the Shapiro–Wilk test and homogeneity of variance with Levene’s test. As assumptions of normality were not met for several variables, treatment effects were assessed using the Kruskal–Wallis rank sum test (p < 0.05). Significant results were followed by Dunn’s post hoc test with Bonferroni correction. Orthogonal contrasts were not applied.
3. Results
3.1. Growth Performance
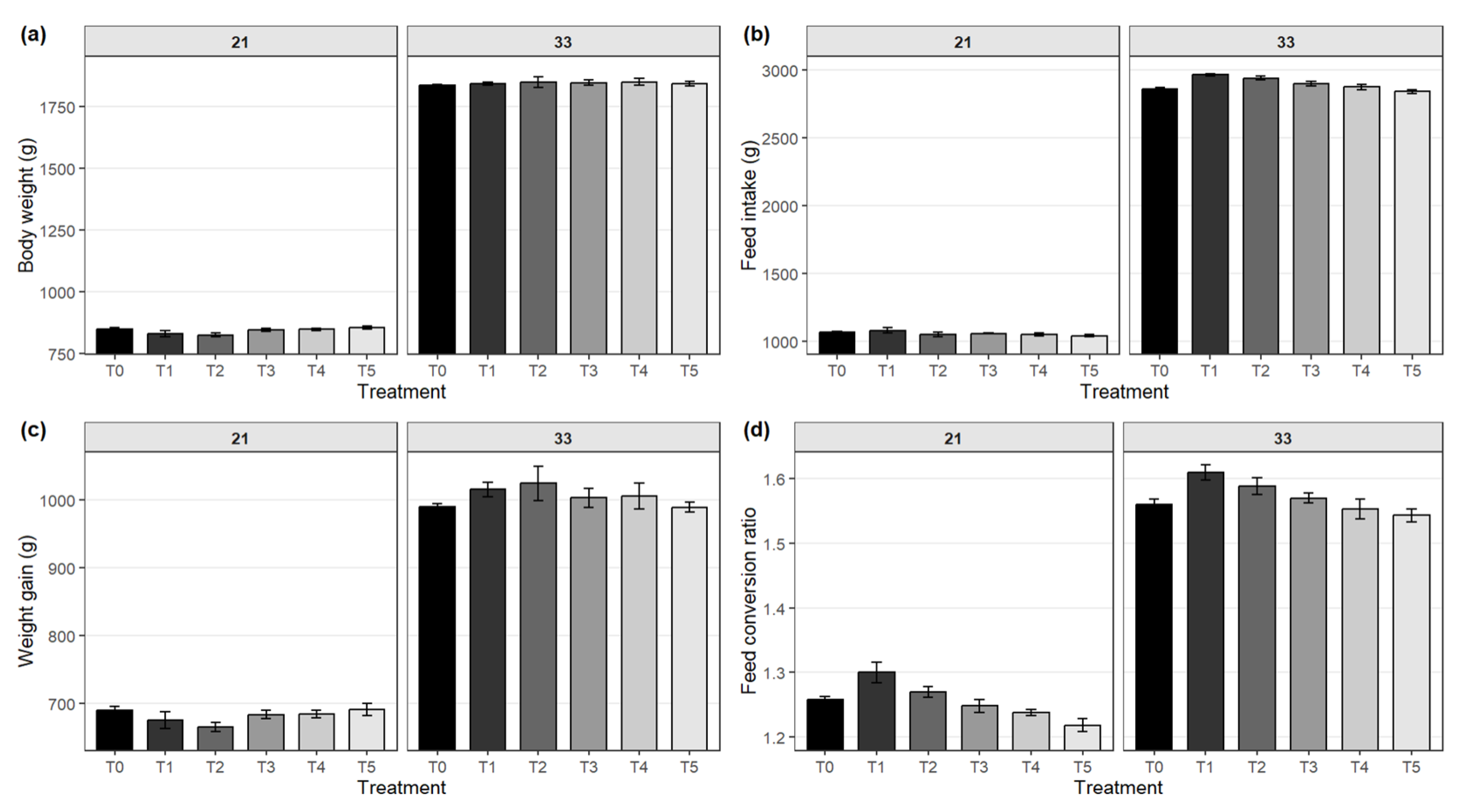
Protease supplementation significantly enhanced broiler growth, improving weight gain, feed intake, and efficiency. Higher enzyme levels produced the greatest benefits, reflecting improved nutrient utilization and growth performance (
Figure 1 and
Figure 2). At day 7, birds in the T5 group exhibited the highest body weight (163.0 ± 1.4 g), followed closely by T4 (162.5 ± 1.3 g) and T3 (162.3 ± 1.3 g), with significant differences among groups (p = 0.002). This trend continued through day 21, where the T5 group maintained the highest weight (854 ± 7 g), while the T2 group showed the lowest (825 ± 8 g; p = 0.014). However, by day 33, no significant differences in body weight were observed across groups (p = 0.400). Feed intake was also significantly affected by protease inclusion. At day 7, the highest intake was noted in T2 (136.8 ± 2.2 g; p = 0.048), while cumulative intake over 33 days was greatest in T1 (2,962.0 ± 7.0 g; p < 0.001). Body weight gain (BWG) from day 0–7 was significantly higher in protease-supplemented groups, particularly T5 (118.0 ± 1.4 g; p = 0.002), and this improvement persisted during the 8–21-day period (T5: 691 ± 9.0 g; p = 0.020) and 22–33-day period (T2: 1,024 ± 25 g; p = 0.024). Feed conversion ratio (FCR) improved significantly with protease supplementation. At day 7, FCR was lowest in T5 (0.80 ± 0.005; p = 0.001), indicating better efficiency, with continued improvements observed at day 21 (T5: 1.22 ± 0.010; p < 0.001) and day 33 (T5: 1.54 ± 0.010; p = 0.002). Overall mortality remained low and comparable among treatments, with values below 2% across all groups. Collectively, birds receiving protease-supplemented diets, particularly in the T5 group, demonstrated enhanced growth efficiency and feed utilization.
3.2. Serum Biochemical Indices
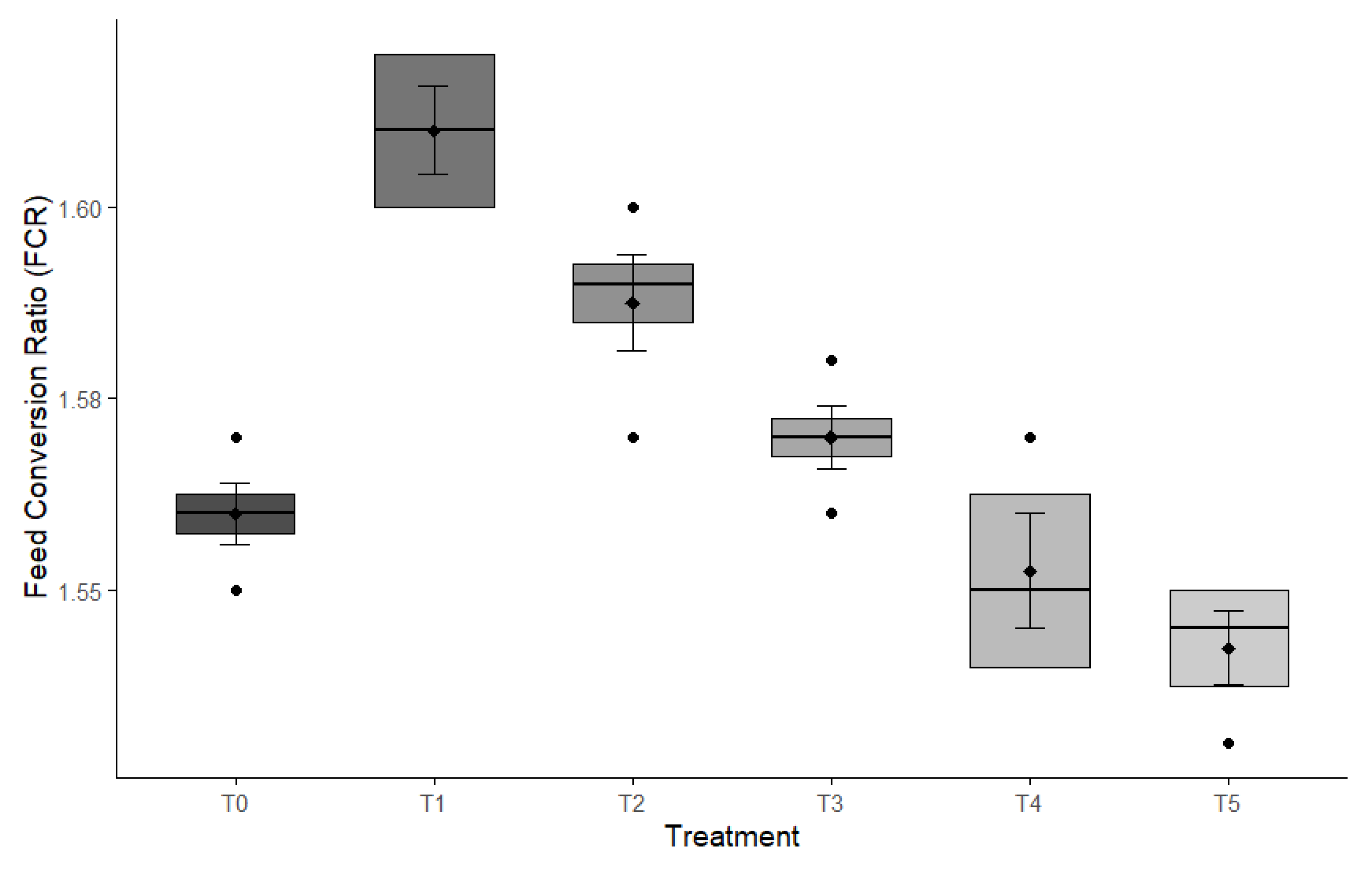
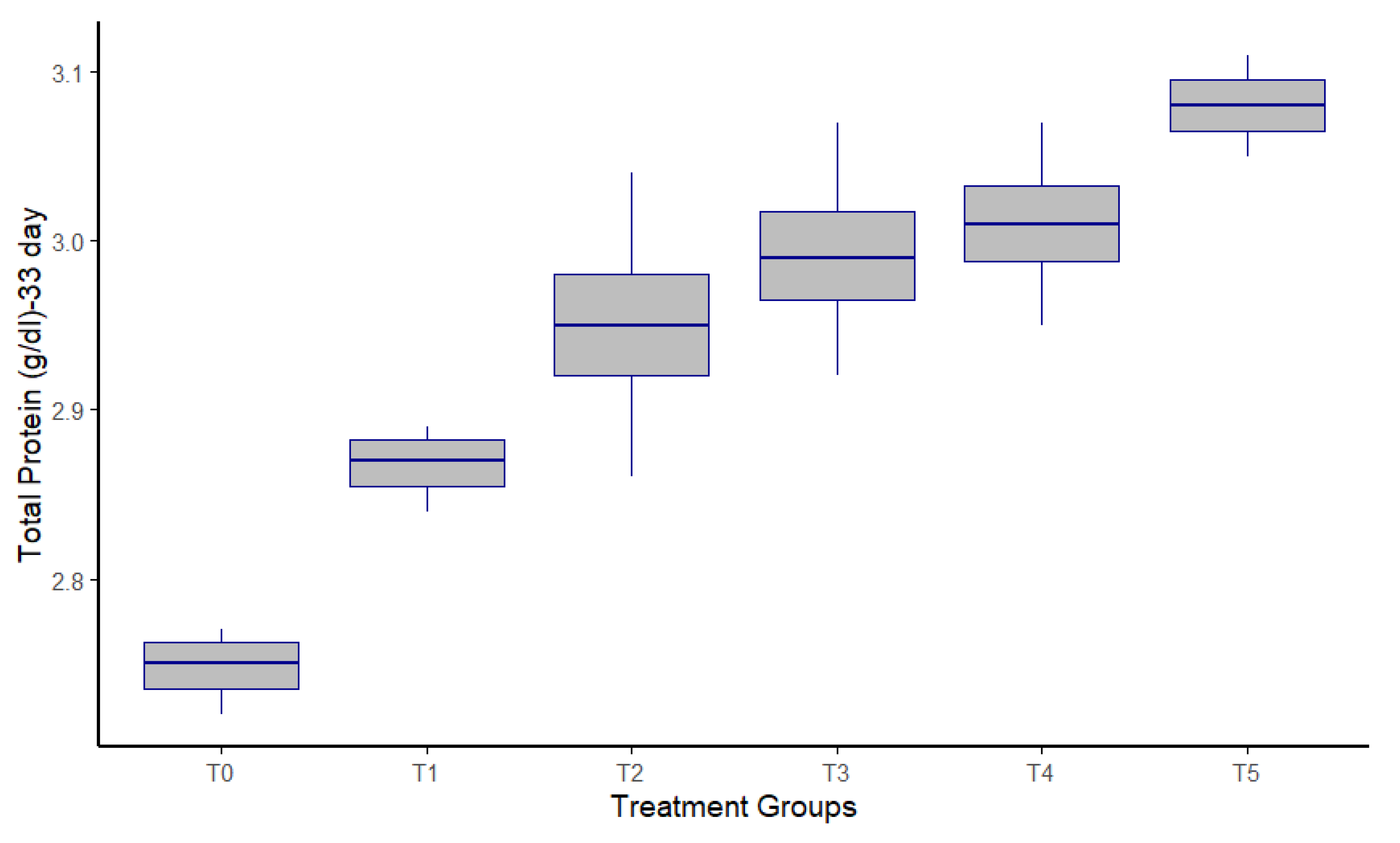
Protease supplementation significantly influenced the serum biochemical parameters of broiler chickens at both 21 and 33 days of age (
Table 3; Figure 3). Supplemented groups showed elevated TP, albumin, and creatinine concentrations, alongside reduced LDL levels, indicating improved protein metabolism and lipid regulation. TP levels increased progressively across treatments, with the highest value observed in the T5 group at both time points (p < 0.001 at day 21; p = 0.002 at day 33). Blood urea nitrogen (BUN) concentrations also showed a significant elevation with increasing protease levels, particularly at day 21 (p < 0.001), and a modest but significant variation by day 33 (p = 0.023). Creatinine levels rose consistently with enzyme supplementation, peaking in the T5 group at both ages (p < 0.001 at day 21; p = 0.006 at day 33). Similarly, albumin concentrations significantly improved in treated groups compared to the control, showing the highest values in T5 on both days (p < 0.001). Liver enzymes GOT and GPT exhibited marked changes in response to protease inclusion. GOT levels peaked in the T1 group at day 21 and in T5 at day 33 (p < 0.001 and p = 0.016, respectively), while GPT was highest in T1 at day 21 and in T5 at day 33 (p < 0.001 and p = 0.003, respectively). Interestingly, LDL concentrations decreased in higher protease-supplemented groups, especially T4 and T5, at both 21 and 33 days, indicating a possible lipid-lowering effect of protease (p < 0.001 and p = 0.002, respectively). These results suggest that dietary protease positively modulates serum protein metabolism, renal and hepatic function, and lipid profiles in broiler chickens.
3.3. Carcass Traits and Organ Weights
Protease supplementation had minimal impact on overall carcass composition in 33-day-old broilers, with most carcass and organ weights remaining unaffected. Notably, gizzard weight increased significantly in the highest supplementation group, suggesting enhanced digestive organ development and potential improvements in feed utilization. The effects of protease supplementation on the carcass traits of 33-day-old Cobb 500 broiler chickens are summarized in
Table 4. No significant differences (p > 0.05) were observed among treatment groups for most carcass parameters, including carcass weight, chest, wings, legs, intestine, liver, and heart weights, indicating that dietary protease had minimal influence on these traits. However, a statistically significant difference (p = 0.020) was noted in gizzard weight, with the highest value observed in the T5 group (38.0 ± 0.63 g), suggesting a potential impact of protease supplementation on gizzard development. These findings imply that while protease did not markedly affect overall carcass composition, it may enhance certain organ developments, such as the gizzard, possibly reflecting improved digestive activity or feed utilization.
3.4. Intestinal Morphology
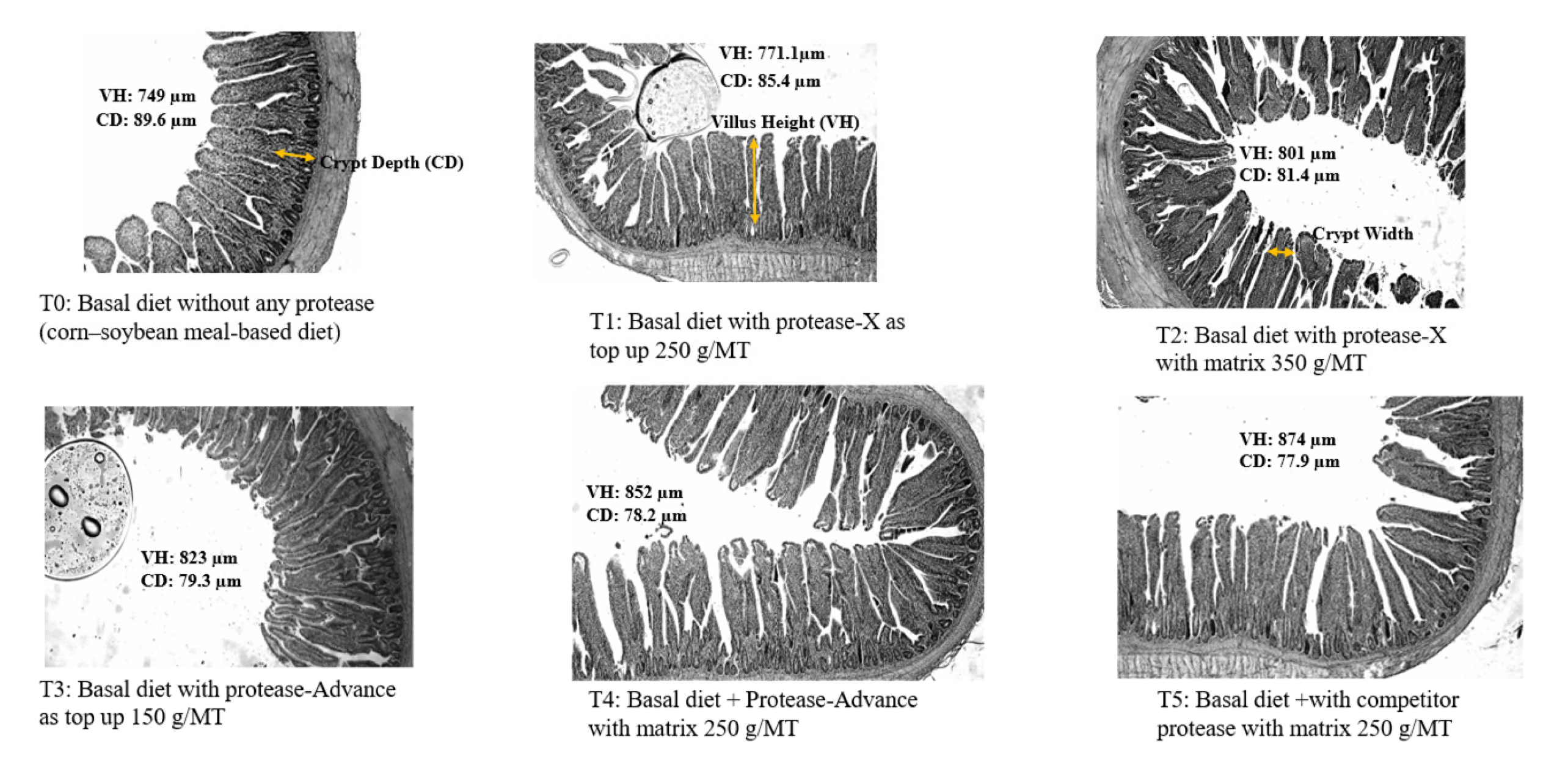
Protease supplementation markedly enhanced intestinal morphology, improving villus height, reducing crypt depth, and increasing the villus-to-crypt ratio. These changes indicate a more efficient absorptive surface, suggesting improved nutrient uptake and intestinal health in broilers. The effects on ileal morphology were consistently observed on both 21 and 33 days (
Table 5; Figure 4). Results revealed significant improvements across all measured parameters (p < 0.001). At day 21, villus height increased progressively with protease supplementation, ranging from 749.0 ± 1.0 µm in the control group (T0) to 874.0 ± 1.0 µm in the highest supplemented group (T5). Concurrently, crypt depth decreased from 89.6 ± 0.4 µm in T0 to 77.9 ± 0.1 µm in T5. Consequently, the villus height to crypt depth (VH:CD) ratio improved significantly, increasing from 8.36 ± 0.04 in T0 to 11.23 ± 0.02 in T5. By day 33, a similar trend was observed, with villus height increasing from 814.0 ± 1.0 µm (T0) to 931.0 ± 1.0 µm (T5), while crypt depth showed a marked reduction from 86.4 ± 0.3 µm to 74.0 ± 0.1 µm. The VH:CD ratio continued to rise with supplementation, reaching 12.59 ± 0.01 in T5 compared to 9.42 ± 0.03 in T0. Although several intestinal morphology parameters, including villus height and VH:CD ratio, differed significantly among treatments (p < 0.001), some absolute changes were relatively small. Therefore, while statistically significant, these differences may have limited biological impact, and interpretations have been adjusted to focus on meaningful functional trends rather than minor numerical variations. These findings suggest that dietary protease supplementation enhances intestinal morphology by promoting villus development and reducing crypt depth, thereby potentially improving nutrient absorption efficiency in broilers.
3.5. Nutrient Digestibility
Protease supplementation markedly improved nutrient digestibility in broilers, enhancing protein and energy utilization. Dry matter and crude protein levels in digesta increased, while apparent metabolizable energy and amino acid availability were also elevated, reflecting more efficient feed utilization across treatments. The dry matter content of the diet on day 33 significantly differed among treatments, ranging from 88.14% in T1 to 89.52% in T2 (
Table 6; p < 0.001). Similarly, the DM content in digesta showed a significant variation (p < 0.001), with values ranging from 77.13% in T0 to 77.61% in T5. Crude protein levels in the diet also varied significantly (p < 0.001), decreasing from 22.11% in T0 to a minimum of 21.09% in T3. In contrast, the CP content in digesta increased significantly (p < 0.001) across treatments, with the highest value (20.10%) observed in T5. Apparent metabolizable energy (AME) in the diet was consistent across groups, averaging around 3,130 kcal/kg, but AME in the digesta increased significantly (p < 0.001) from 2,768 kcal/kg in T0 to 2,803 kcal/kg in T5. Furthermore, amino acid content in the diet showed significant differences (p = 0.001), with the lowest value recorded in T3 (2.190%) and the highest in T0 (2.260%). These findings indicate that protease supplementation has a significant impact on nutrient digestibility parameters, particularly enhancing energy and protein utilization in broiler chickens.
3.6. Economic Evaluation
Protease supplementation enhanced economic efficiency, with higher-supplemented groups achieving the greatest profit and return, while the control and lower-supplemented groups showed comparatively lower gains. Overall, the economic analysis across treatments revealed notable variations in profit and return on investment (ROI). All groups incurred a uniform total feed cost of BDT 39,687.24 and a medicine/vaccine cost of BDT 2,850. However, the additional cost of protease varied among the supplemented groups, ranging from BDT 79.09 in T3 to BDT 180.58 in T2. Consequently, the total production cost was highest in T2 (BDT 54,217.78) and lowest in control group T0 (BDT 54,037.20), which received no protease. Despite having similar total incomes of BDT 57,776 across all groups, net profit and ROI differed. The T5 group, supplemented with BDT 130.40 worth of protease, achieved the highest profit (BDT 3,800.90) and ROI (7.01%), indicating superior economic efficiency. This was followed by T4 (ROI 6.89%), T2 (6.83%), and T3 (6.74%). In contrast, the control group (T0) yielded the lowest ROI (6.67%), while T1, with a moderate protease cost, recorded the lowest profit (BDT 3,550.50) and ROI (6.55%) among the treated groups. These findings in
Table 7 suggest that strategic supplementation with protease can enhance economic returns, particularly as observed in the T5 group.
4. Discussion
The present study demonstrated that dietary protease supplementation significantly enhanced broiler growth performance during the early rearing phase, as reflected by higher body weight at 7 and 21 days of age. These improvements can be attributed to enhanced protein digestibility and more efficient amino acid absorption [
24]. Previous studies have similarly reported that broilers receiving a combination of acid and neutral protease exhibited improved body weight gain and nitrogen retention compared with negative controls [
25]. Moreover, increasing dietary protease levels from 0% to 0.09% has been shown to yield linear improvements in average daily gain and nutrient digestibility [
26]. Supplementation with non-starch polysaccharide enzymes combined with protease further enhanced growth rate and crude protein digestibility [
27]. Protease supplementation has also been linked to the upregulation of genes associated with nutrient transport and growth regulation, thereby supporting improved growth performance. Nonetheless, inconsistent effects observed in some parameters are in agreement with previous reports, where variability in enzyme efficacy has been attributed to factors such as bird age, enzyme source, dosage, and diet composition [
28].
Intestinal morphology was markedly improved in birds receiving protease, particularly through increased villus height, which enlarges the absorptive surface area and enhances nutrient uptake efficiency. These findings align with earlier studies demonstrating that protease supplementation improves jejunal villus architecture, supporting greater intestinal integrity and digestive capacity [29-32]. Such morphological adaptations are crucial for sustaining growth performance, as they directly contribute to improved nutrient absorption and gastrointestinal health.
The digestibility trial corroborated these outcomes, revealing significant improvements in dry matter, crude protein, and apparent metabolizable energy utilization, especially in birds receiving higher protease inclusion (T5 group). These results are consistent with prior reports showing enhanced ileal digestibility and nitrogen retention in broilers supplemented with protease [30, 33]. Specifically, supplementation with
Bacillus licheniformis-derived protease at 300–400 mg/kg has been shown to increased protein digestibility, jejunal trypsin activity, and v[29–32illus height, thereby improving nutrient assimilation [
32]. Similarly, supplementation with 50 g/tonne protease improved amino acid digestibility, gut morphology, and cecal microbiota balance [
31]. Notably, protease efficacy appears to be influenced by dietary protein content, with benefits being more pronounced under optimal protein levels, while diets with markedly reduced crude protein may limit protease effects [
30,
33]. Collectively, enhanced nutrient utilization likely underpins the improvements in growth performance and feed efficiency observed in the current study.
Protease supplementation also elicited favorable metabolic responses, as evidenced by increased concentrations of lysine, methionine, and threonine in breast muscle, indicative of more efficient protein utilization and synthesis [
34]. Concurrently, reductions in LDL cholesterol alongside increases in HDL suggest beneficial modulation of lipid metabolism, consistent with improved physiological status and overall health in broilers [
21]. These metabolic changes further highlight the role of protease in optimizing nutrient assimilation and systemic functions beyond growth performance.
Despite improvements in growth performance, feed intake, and feed conversion ratio, protease supplementation did not significantly alter carcass yield or relative organ weights. This observation aligns with previous research indicating that early growth advantages do not necessarily translate into changes in carcass composition at market age [
21,
35]. Such outcomes may reflect compensatory growth mechanisms, whereby early gains are balanced over the course of the rearing period, resulting in comparable carcass characteristics across treatments.
From an economic standpoint, protease supplementation improved profitability by enhancing feed efficiency and nutrient utilization without substantially increasing feed costs. These improvements translated into higher returns on investment and profit margins in supplemented groups. Such outcomes are consistent with previous reports that highlighted enzyme supplementation as a cost-effective strategy to improve productivity in intensive poultry production systems [
21,
36].
In summary, protease supplementation in broiler diets offers multiple benefits, including improved nutrient utilization, gut morphology, metabolism, and economic efficiency. However, variable outcomes across studies indicate that enzyme source, diet composition, and bird age must be carefully considered when formulating enzyme-based feeding strategies.
5. Conclusions
Matrix-dosed protease supplementation improved broiler growth, nutrient digestibility, gut morphology, and profitability. Protease F at 250 g/tonne, a rapidly protein-hydrolyzing enzyme, achieved the highest body weight, feed efficiency, CP digestibility, and AME, offering the greatest economic benefit under the study conditions. Protease X at 350 g/tonne also enhanced performance, particularly in the finisher phase. These findings indicate that matrix-dosed proteases, especially those enabling rapid protein hydrolysis, are more effective than on-top supplementation, supporting their practical use to improve productivity and cost-effectiveness in broiler production, while acknowledging that economic gains are modest and context-dependent.
Author Contributions
Conceptualization, methodology, funding, and data analysis, S.K.N.; Software and draft preparation, M.S.I.; Data curation and investigation, M.T.H.; Resources and project administration, M.M.I.; Conceptual input and resources, R.A.D; Field investigation and data curation, T.S.; Resources and technical support, M.S.H.; Supervision, review, and editing, S.S.; Review, editing, and validation, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding from funding agencies.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Experimentation Ethics Committee of the Faculty of Veterinary, Animal, and Biomedical Sciences, Khulna Agricultural University, Bangladesh (protocol # AEEC/KAU/2024-1005; date of approval: 10 November 2024). All animal care and experimental procedures were conducted in accordance with institutional animal welfare regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
Acknowledgments
The authors gratefully acknowledge the support and cooperation of the staff from the Department of Animal Nutrition, Khulna Agricultural University. Special thanks are also extended to Rossari Biotech Limited, India, for their technical support and contributions to the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tony Mcdougal. Demand for poultry meat drives feed production. 2025.
- Katu, J.K., T. Tóth, and L. Varga, Enhancing the nutritional quality of low-grade poultry feed ingredients through fermentation: A review. Agriculture 2025, 15, 476. [Google Scholar] [CrossRef]
- Yaqoob, M.U.; et al. Effect of partially replacing soybean meal with sunflower meal with supplementation of multienzymes on growth performance, carcass characteristics, meat quality, ileal digestibility, digestive enzyme activity and caecal microbiota in broilers . Animal bioscience 2022, 35, 1575. [Google Scholar] [CrossRef]
- Erdaw, M. Bhuiyan, and P. Iji, Enhancing the nutritional value of soybeans for poultry through supplementation with new-generation feed enzymes. World’s Poultry Science Journal 2016, 72, 307–322. [Google Scholar] [CrossRef]
- Qiu, K.; et al. Effects of dietary crude protein and protease levels on performance, immunity capacity, and AA digestibility of broilers . Agriculture 2023, 13, 703. [Google Scholar] [CrossRef]
- Yang, Z. and S.F. Liao, Physiological effects of dietary amino acids on gut health and functions of swine. Frontiers in veterinary science 2019, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Shad, A.; et al. Effects of a novel protease from bacillus subtilis K-5 in low protein corn distiller dried grains with solubles (cDDGS) based diets on performance and nutrients digestibility in broiler chickens. Brazilian Journal of Poultry Science 2022, 24, eRBCA-2021-1542. [Google Scholar] [CrossRef]
- Woyengo, T. Beltranena, and R. Zijlstra, Nonruminant nutrition symposium: controlling feed cost by including alternative ingredients into pig diets: a review. Journal of Animal Science 2014, 92, 1293–1305. [Google Scholar] [CrossRef]
- Philipps-Wiemann, P. , Proteases—animal feed, in Enzymes in human and animal nutrition. 2018, Elsevier. 279-297.
- da Silva, J.M.S.; et al. Effect of protease supplementation on the digestibility of amino acids in animal-origin meals for broiler diets. 2021.
- Xavier Junior, M.d.L.; et al. Metabolizable energy and amino acid digestibility of soybean meal from different sources for broiler chickens supplemented with protease . Animals 2024, 14, 782. [Google Scholar] [CrossRef]
- Bernardes, R.D. , Effect of the combination of phytase and protease on the performance of broilers and on the values of metabolizable energy and amino acid digestibility of diets. 2022.
- Vieira, S.L.; et al. Growth performance and nutrient digestibility of broiler chickens as affected by a novel protease. Frontiers in Animal Science 2023, 3, 1040051. [Google Scholar] [CrossRef]
- Freitas, D.; et al. Performance and nutrient utilization of broilers fed diets supplemented with a novel mono-component protease . Journal of Applied Poultry Research 2011, 20, 322–334. [Google Scholar] [CrossRef]
- Leinonen, I. and A.G. Williams, Effects of dietary protease on nitrogen emissions from broiler production: a holistic comparison using Life Cycle Assessment. Journal of the Science of Food and Agriculture 2015, 95, 3041–3046. [Google Scholar] [CrossRef]
- Cowieson, A.; et al. The effect of a mono-component exogenous protease and graded concentrations of ascorbic acid on the performance, nutrient digestibility and intestinal architecture of broiler chickens. Animal Feed Science and Technology 2018, 235, 128–137. [Google Scholar] [CrossRef]
- Kamel, N.; et al. Effects of a monocomponent protease on performance parameters and protein digestibility in broiler chickens. Agriculture and Agricultural Science Procedia 2015, 6, 216–225. [Google Scholar] [CrossRef]
- Flores, C.; et al. Direct-fed microbial and its combination with xylanase, amylase, and protease enzymes in comparison with AGPs on broiler growth performance and foot-pad lesion development . Journal of Applied Poultry Research 2016, 25, 328–337. [Google Scholar] [CrossRef]
- Walk, C.; et al. Evaluation of novel protease enzymes on growth performance and nutrient digestibility of poultry: enzyme dose response . Poultry Science 2019, 98, 5525–5532. [Google Scholar] [CrossRef]
- Ndazigaruye, G.; et al. Effects of low-protein diets and exogenous protease on growth performance, carcass traits, intestinal morphology, cecal volatile fatty acids and serum parameters in broilers . Animals 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; et al. Effects of exogenous protease on performance, economic evaluation, nutrient digestibility, fecal score, intestinal morphology, blood profile, carcass trait, and meat quality in broilers fed normal diets and diets considered with matrix value . Poultry Science 2023, 102, 102565. [Google Scholar] [CrossRef] [PubMed]
- Chromý, V.; et al. The Kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. A review of Kjeldahl methods adopted by laboratory medicine . Critical reviews in analytical chemistry 2015, 45, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R. Wiltafsky-Martin, and V. Ravindran, Application of apparent metabolizable energy versus nitrogen-corrected apparent metabolizable energy in poultry feed formulations: a continuing conundrum. Animals 2021, 11, 2174. [Google Scholar] [CrossRef]
- Cowieson, A.; et al. Interactive effects of dietary protein source and exogenous protease on growth performance, immune competence and jejunal health of broiler chickens . Animal Production Science 2016, 57, 252–261. [Google Scholar] [CrossRef]
- Mahmood, T.; et al. Effect of different exogenous proteases on growth performance, nutrient digestibility, and carcass response in broiler chickens fed poultry by-product meal-based diets. Livestock Science 2017, 200, 71–75. [Google Scholar] [CrossRef]
- Park, J.H. S.I. Lee, and I.H. Kim, The effect of protease on growth performance, nutrient digestibility, and expression of growth-related genes and amino acid transporters in broilers. J Anim Sci Technol 2020, 62, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; et al. Effects of protease and non-starch polysaccharide enzyme on performance, digestive function, activity and gene expression of endogenous enzyme of broilers . PLoS One 2017, 12, e0173941. [Google Scholar] [CrossRef] [PubMed]
- Radhi, K.S.; et al. Growth performance of broiler chickens fed diets supplemented with amylase and protease enzymes individually or combined . Open veterinary journal 2023, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; et al. Effects of antibiotic growth promoter and dietary protease on growth performance, apparent ileal digestibility, intestinal morphology, meat quality, and intestinal gene expression in broiler chickens: a comparison . Journal of animal science 2020, 98, skaa254. [Google Scholar] [CrossRef]
- Song, X.; et al. Effects of reduced-protein diets with protease supplementation on growth, carcass yield, intestinal morphology, organ development, nutrient digestibility, and blood biochemical of broiler chickens . Translational Animal Science 2023, 7, txad098. [Google Scholar] [CrossRef]
- Huyan, L.; et al. Effects of protease supplementation on growth performance, organ development, gut morphology, and microbial profile of broiler chicken. Acta Agriculturae Scandinavica, Section A—Animal Science 2022, 71, 40–50. [Google Scholar] [CrossRef]
- Yi, W.; et al. Effect of a novel alkaline protease from Bacillus licheniformis on growth performance, carcass characteristics, meat quality, antioxidant capacity, and intestinal morphology of white feather broilers . Journal of the Science of Food and Agriculture 2024, 104, 5176–5185. [Google Scholar] [CrossRef]
- Jabbar, A.; et al. Interactive effect of exogenous protease enzyme and dietary crude protein levels on growth and digestibility indices in broiler chickens during the starter phase . Tropical Animal Health and Production 2021, 53, 23. [Google Scholar] [CrossRef]
- Saleh, A.A.; et al. Effect of supplemental serine-protease from Bacillus licheniformis on growth performance and physiological change of broiler chickens . Journal of Applied Animal Research 2020, 48, 86–92. [Google Scholar] [CrossRef]
- Park, J.H. S.I. Lee, and I.H. Kim, The effect of protease on growth performance, nutrient digestibility, and expression of growth-related genes and amino acid transporters in broilers. Journal of Animal Science and Technology 2020, 62, 614. [Google Scholar] [CrossRef]
- Wealleans, A.; et al. Performance and cost-benefit improvements following supplementation with a combination of direct-fed microbials and enzymes to broiler chickens raised with or without ionophores . Journal of Applied Poultry Research 2018, 27, 23–32. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).