Submitted:
17 October 2025
Posted:
20 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2.1. Eligibility Criteria
- Adults aged ≥ 30 years, consistent with NHANES eligibility for full-mouth periodontal examinations.
- Participants with complete periodontal clinical data, including probing depth (PD) and clinical attachment loss (CAL).
- Participants with available data on the biomarkers of interest: white blood cell count, serum albumin, and mean corpuscular hemoglobin concentration
- Individuals with available data on key covariates: age, sex, race/ethnicity, education level, smoking, any disease.
- Participants under 30 years old, since they were not eligible for the FMPE (Full-Mouth Periodontal Examination).
- Individuals with missing periodontal examination data (non-examined or incomplete exam).
- Individuals with missing values for biomarkers (WBC, serum albumin, MCHC).
- Individuals with conditions requiring antibiotic prophylaxis prior to dental exams (excluded by NHANES protocols).
- Participants with missing key covariates (e.g., demographic or health-related confounders).
- Pregnant women (NHANES typically excludes them from some blood measures due to physiologic changes, and many biomarker analyses remove them to avoid bias).
2.2. Periodontal Examination
2.3. Definition of the Dependent Variable: Periodontal disease
2.3. Description of Independent Variable: Blood biomarkers
2.3.1. White Blood Cell Count
2.3.2. Serum Albumin
2.3.3. Mean Corpuscular Hemoglobin Concentration
2.4. Potential Confounding Variable
2.5. Statistical Methods
3. Results
4. Discussion
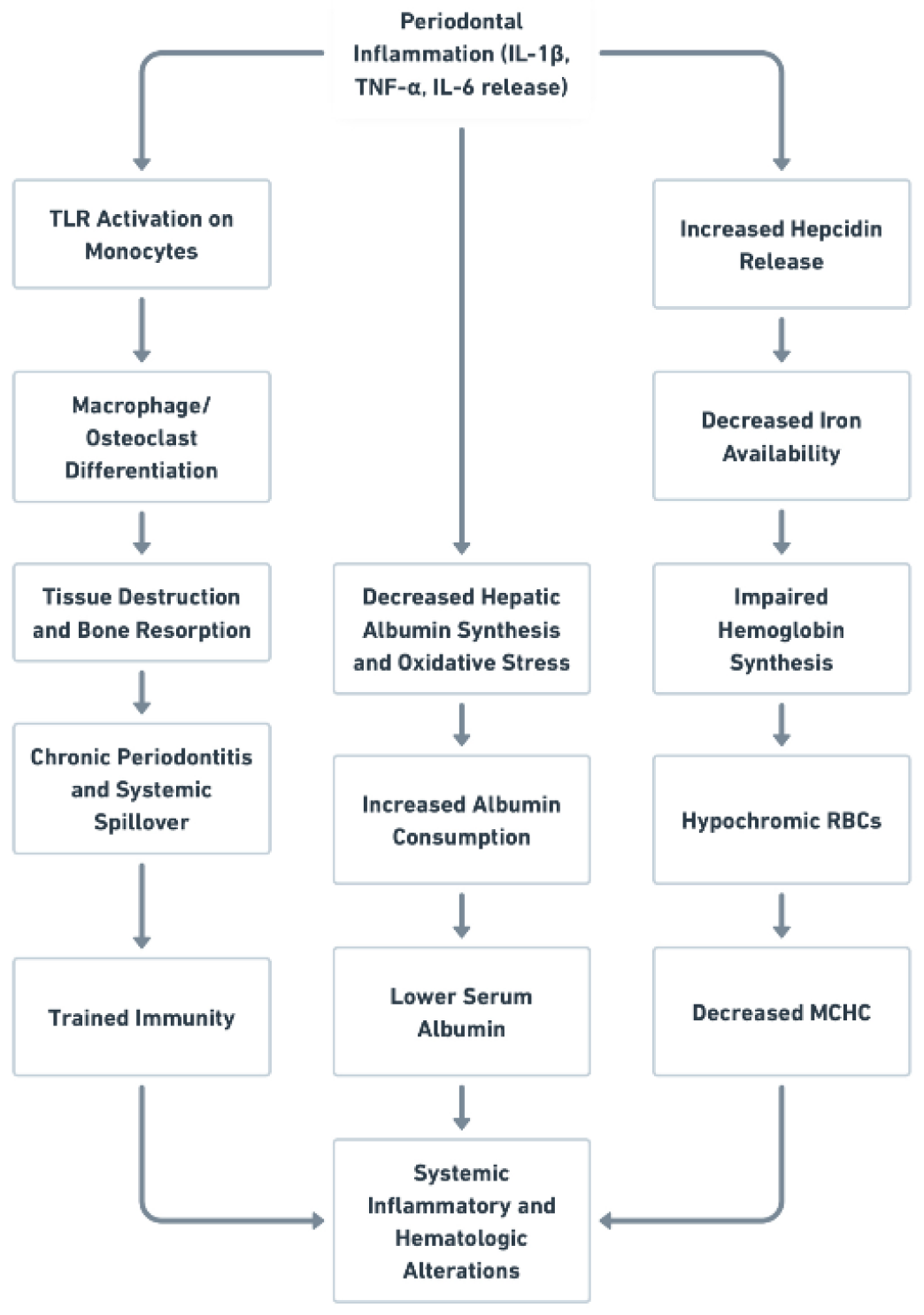
1.4. Limitation of Research
2.4. Future Perspectives
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Periodontal disease |
| WBC | White blood cell |
| MCHC | Mean corpuscular hemoglobin concentration |
| ROS | Reactive oxygen species |
References
- Hashim, N.T.; Babiker, R.; Padmanabhan, V.; Ahmed, A.T.; Chaitanya, N.C.S.K.; Mohammed, R.; Priya, S.P.; Ahmed, A.; El Bahra, S.; Islam, M.S.; et al. The Global Burden of Periodontal Disease: A Narrative Review on Unveiling Socioeconomic and Health Challenges. Int. J. Environ. Res. Public Health 2025, 22, 624. [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. https://jada.ada.org/article/S0002-8177(18)30276-9/abstract.
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines2022, 10, 2659. [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [CrossRef]
- Caloian, C.S.; Șurlin, P.; Ciurea, A.; Pop, D.; Caloian, B.; Leucuța, D.C.; Țigu, A.B.; Rasperini, G.; Micu, I.C.; Stanomir, A.; et al. Exploring Periodontal Conditions, Salivary Markers, and Systemic Inflammation in Patients with Cardiovascular Diseases. Biomedicines 2024, 12, 1341. [CrossRef]
- Caloian, C.S.; Ciurea, A.; Negucioiu, M.; Roman, A.; Micu, I.C.; Picoș, A.; Soancă, A. Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review. Antibiotics 2024, 13, 359. [CrossRef]
- Dascalu, A.M.; Serban, D.; Tanasescu, D.; Vancea, G.; Cristea, B.M.; Stana, D.; Nicolae, V.A.; Serboiu, C.; Tribus, L.C.; Tudor, C.; et al. The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM). Biomedicines 2023, 11, 2106. [CrossRef]
- Kostanek, J.; Karolczak, K.; Kuliczkowski, W.; Watala, C. Red Blood Cell, White Blood Cell, and Platelet Counts as Differentiating Factors in Cardiovascular Patients with and Without Current Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 5736. [CrossRef]
- Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Managing the Systemic Impact of Periodontitis. Medicina 2022, 58, 621. [CrossRef]
- Bolyarova, T.; Stefanov, L.; Naseva, E.; Stamatov, K.; Dzhenkov, S.; Stoimenov, B.; Pancheva, R.; Dochev, N.; Ishkitiev, N. Pro-Inflammatory Markers in Serum and Saliva in Periodontitis and Hypertension. Medicina2025, 61, 1024. [CrossRef]
- Yayan, J.; Biancosino, C.; Krüger, M.; Rasche, K. Inflammation and Albumin-Based Biomarkers Are Not Independently Associated with Mortality in Critically Ill COPD Patients: A Retrospective Study. Life 2025, 15, 1371. [CrossRef]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J. Clin. Med. 2023, 12, 6017. [CrossRef]
- Xu, Z.; Karlsson, J.O.M.; Huang, Z. Modeling the Dynamics of Acute Phase Protein Expression in Human Hepatoma Cells Stimulated by IL-6. Processes 2015, 3, 50-70. [CrossRef]
- Tavares, L.T.R.; Saavedra-Silva, M.; López-Marcos, J.F.; Veiga, N.J.; Castilho, R.d.M.; Fernandes, G.V.d.O. Blood and Salivary Inflammatory Biomarkers Profile in Patients with Chronic Kidney Disease and Periodontal Disease: A Systematic Review. Diseases 2022, 10, 12. [CrossRef]
- Miranda-Morales, E.G.; Romero-Gutierrez, E.; Castellanos-Juárez, F.X.; Méndez-Hernández, E.M.; Salas-Leal, A.C.; La Llave-León, O.; Quiñones-Canales, G.; Sandoval-Carrillo, A.; Salas-Pacheco, J.M.; Arias-Carrión, O. Elevated Mean Corpuscular Hemoglobin Concentration as a Potential Peripheral Biomarker of Parkinson’s Disease: A Pilot Case–Control Study in a Mexican Population. Brain Sci. 2025, 15, 966. [CrossRef]
- Barshtein, G.; Livshits, L.; Gural, A.; Arbell, D.; Barkan, R.; Pajic-Lijakovic, I.; Yedgar, S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. Int. J. Mol. Sci.2024, 25, 5814. [CrossRef]
- Agarwal N, Kumar VS, Gujjari SA. Effect of periodontal therapy on hemoglobin and erythrocyte levels in chronic generalized periodontitis patients: An interventional study. J Indian Soc Periodontol. 2009;13(1):6-11. [CrossRef]
- Botelho, J.; Lyra, P.; Proença, L.; Godinho, C.; Mendes, J.J.; Machado, V. Relationship between Blood and Standard Biochemistry Levels with Periodontitis in Parkinson’s Disease Patients: Data from the NHANES 2011–2012. J. Pers. Med. 2020, 10, 69. [CrossRef]
- Blasi, A.M.; Derman, S.H.M.; Kunnel, A.; Pape, P.; Röhrig, G.; Barbe, A.G. Oral Health and the Association with Blood Parameters in Neurogeriatric Inpatients without Relevant Systemic Inflammation: An Observational Study. Geriatrics 2024, 9, 55. [CrossRef]
- Łobacz, M.; Mertowska, P.; Mertowski, S.; Kozińska, A.; Kwaśniewski, W.; Kos, M.; Grywalska, E.; Rahnama-Hezavah, M. The Bloody Crossroads: Interactions between Periodontitis and Hematologic Diseases. Int. J. Mol. Sci. 2024, 25, 6115. [CrossRef]
- Wiciński, M.; Liczner, G.; Cadelski, K.; Kołnierzak, T.; Nowaczewska, M.; Malinowski, B. Anemia of Chronic Diseases: Wider Diagnostics—Better Treatment? Nutrients 2020, 12, 1784. [CrossRef]
- National Center for Health Statistics. About the National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/nhanes/about/ (accessed on 21 September 2025).
- Blasi, A.M.; Derman, S.H.M.; Kunnel, A.; Pape, P.; Röhrig, G.; Barbe, A.G. Oral Health and the Association with Blood Parameters in Neurogeriatric Inpatients without Relevant Systemic Inflammation: An Observational Study. Geriatrics 2024, 9 55. [CrossRef]
- Beydoun, H.A.; Hossain, S.; Beydoun, M.A.; Weiss, J.; Zonderman, A.B.; Eid, S.M. Periodontal Disease, Sleep Duration, and White Blood Cell Markers in the 2009 to 2014 National Health and Nutrition Examination Surveys. J. Periodontol. 2019, 91(5), 582–595. [CrossRef]
- Shirmohammadi A, Faramarzi M, Salari A, Sadighi Shamami M, Babaloo AR, Mousavi Z. Effect of non-surgical periodontal treatment on serum albumin levels in patients with chronic periodontitis. J Adv Periodontol Implant Dent. 2018;10(1):18-23. Published 2018 Jun 20. [CrossRef]
- Pola, N.M.; Orcina, B.d.F.; Lima, B.D.; Colussi, P.R.G.; Muniz, F.W.M.G. Impact of the Association Between Nutritional Status and Oral Health-Related Quality of Life in Older Adults from Two Cities in Southern Brazil: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2025, 22, 1083. [CrossRef]
- Parihar, S.; Sharma, N.K.; Bhatnagar, A.; Kishore, D.; Parihar, A.V.; Rahman, F. Comparison of Hematological Parameters for Signs of Anemia among Participants with and without Chronic Periodontitis: A Cross-Sectional Study. J. Indian Assoc. Public Health Dent. 2019, 17(1), 4–7. [CrossRef]
- Anumolu, V.N.; Srikanth, A.; Paidi, K. Evaluation of the Relation between Anemia and Periodontitis by Estimation of Blood Parameters: A Cross-Sectional Study. J. Indian Soc. Periodontol. 2016, 20(3), 265–272. [CrossRef]
- Sulaiman, Y.; Pacauskienė, I.M.; Šadzevičienė, R.; Anuzyte, R. Oral and Gut Microbiota Dysbiosis Due to Periodontitis: Systemic Implications and Links to Gastrointestinal Cancer: A Narrative Review. Medicina 2024, 60, 1416. [CrossRef]
- Ferrara, E.; Mastrocola, F. Pattern Recognition Receptors in Periodontal Disease: Molecular Mechanisms, Signaling Pathways, and Therapeutic Implications. J. Mol. Pathol. 2024, 5, 497-511. [CrossRef]
- Gu, Y.; Han, X. Toll-Like Receptor Signaling and Immune Regulatory Lymphocytes in Periodontal Disease. Int. J. Mol. Sci. 2020, 21, 3329. [CrossRef]
- Bassani, B.; Cucchiara, M.; Butera, A.; Kayali, O.; Chiesa, A.; Palano, M.T.; Olmeo, F.; Gallazzi, M.; Dellavia, C.P.B.; Mortara, L.; Parisi, L.; Bruno, A. Neutrophils’ Contribution to Periodontitis and Periodontitis-Associated Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24(20), 15370. [CrossRef]
- Magán-Fernández, A.; Rasheed Al-Bakri, S.M.; O’Valle, F.; Benavides-Reyes, C.; Abadía-Molina, F.; Mesa, F. Neutrophil Extracellular Traps in Periodontitis. Cells 2020, 9, 1494. [CrossRef]
- Huang, J.; Cai, X.; Ou, Y.; Zhou, Y.; Wang, Y. Resolution of Inflammation in Periodontitis: A Review. Int. J. Clin. Exp. Pathol. 2018, 11(9), 4283–4295.
- Gamonal, J.; Sanz, M.; O’Connor, A.; Acevedo, A.; Suarez, I.; Sanz, A.; Martínez, B.; Silva, A. Delayed Neutrophil Apoptosis in Chronic Periodontitis Patients. J. Clin. Periodontol. 2003, 30(7), 616–623. [CrossRef]
- Zhang, M., Liu, Y., Afzali, H., & Graves, D. T. (2024). An update on periodontal inflammation and bone loss. Frontiers in immunology, 15, 1385436. [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [CrossRef]
- Ferrara, E.; D’Albenzio, A.; Bassignani, J.; Di Tanna, I.; Murmura, G.; Balice, G. The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 7710. [CrossRef]
- Fageeh, H.I.; Fageeh, H.N.; Patil, S. Monocyte Differentiation into Destructive Macrophages on In Vitro Administration of Gingival Crevicular Fluid from Periodontitis Patients. J. Pers. Med. 2021, 11, 555. [CrossRef]
- Yin, L., Li, X., & Hou, J. (2022). Macrophages in periodontitis: A dynamic shift between tissue destruction and repair. The Japanese dental science review, 58, 336–347. [CrossRef]
- Han, N.; Liu, Y.; Du, J.; Xu, J.; Guo, L.; Liu, Y. Regulation of the Host Immune Microenvironment in Periodontitis and Periodontal Bone Remodeling. Int. J. Mol. Sci. 2023, 24, 3158. [CrossRef]
- Hajishengallis, G., Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 21, 426–440 (2021). [CrossRef]
- Schenkein, H. A., & Loos, B. G. (2013). Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. Journal of clinical periodontology, 40 Suppl 14(0 14), S51–S69. [CrossRef]
- Noz, M. P., Plachokova, A. S., Smeets, E. M. M., Aarntzen, E. H. J. G., Bekkering, S., Vart, P., Joosten, L. A. B., Netea, M. G., & Riksen, N. P. (2021). An Explorative Study on Monocyte Reprogramming in the Context of Periodontitis In Vitro and In Vivo. Frontiers in immunology, 12, 695227. [CrossRef]
- Hajishengallis, G., Li, X., Divaris, K., & Chavakis, T. (2022). Maladaptive trained immunity and clonal hematopoiesis as potential mechanistic links between periodontitis and inflammatory comorbidities. Periodontology 2000, 89(1), 215–230. [CrossRef]
- Tanaka, T., Narazaki, M., & Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology, 6(10), a016295. [CrossRef]
- Chen, J., Li, Y., Chen, X. et al. The albumin-globulin ratio is associated with periodontitis in American adults: results from the NHANES 2009–2014. Sci Rep 15, 27626 (2025). [CrossRef]
- Mahmood, M. K., Kurda, H. A., Qadir, B. H., Tassery, H., Lan, R., Tardivo, D., & Abdulghafor, M. A. (2024). Implication of serum and salivary albumin tests in the recent oral health related epidemiological studies: A narrative review. The Saudi dental journal, 36(5), 698–707. [CrossRef]
- Al-Kalisi, M., Al-Hajri, M., & Al-Rai, S. (2022). Relationship between Undernutrition and Periodontal Diseases among a Sample of Yemeni Population: A Cross-Sectional Study. International journal of dentistry, 2022, 7863531. [CrossRef]
- Azzolino, D., Passarelli, P. C., De Angelis, P., Piccirillo, G. B., D'Addona, A., & Cesari, M. (2019). Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients, 11(12), 2898. [CrossRef]
- Roche, M., Rondeau, P., Singh, N. R., Tarnus, E., & Bourdon, E. (2008). The antioxidant properties of serum albumin. FEBS letters, 582(13), 1783–1787. [CrossRef]
- Zheng, Z., Xie, X., Wang, L., Xu, M., He, J., Deng, Y., & Yu, K. (2025). Association between neutrophil-percentage-to-albumin ratio and periodontitis: insights from a population-based study. Frontiers in nutrition, 12, 1551349. [CrossRef]
- Liu, W.; Guo, D. Oxidative Stress in Periodontitis and the Application of Antioxidants in Treatment: A Narrative Review. Front. Physiol. 2025, 16, 1485367. [CrossRef]
- Beddhu, S.; Kaysen, G.A.; Yan, G.; Ornt, D.; Cheung, A.K.; HEMO Study Group. Association of Serum Albumin and Atherosclerosis in Chronic Hemodialysis Patients. Am. J. Kidney Dis. 2002, 40(5), 965–972. [CrossRef]
- Angjelova, A., Jovanova, E., Polizzi, A., Laganà, L., Santonocito, S., Ragusa, R., & Isola, G. (2024). Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. Journal of clinical medicine, 13(13), 3781. [CrossRef]
- Chen, K.; Ma, S.; Deng, J.; Jiang, X.; Ma, F.; Li, Z. Ferroptosis: A New Development Trend in Periodontitis. Cells 2022, 11, 3349. [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 Mediates Hypoferremia of Inflammation by Inducing the Synthesis of the Iron Regulatory Hormone Hepcidin. J. Clin. Invest. 2004, 113(9), 1271–1276. [CrossRef]
- Han, Y., Luo, Z., Yue, Z. G., Miao, L. L., Xv, M., Chang, S., Zhan, Y., & Hou, J. (2023). The tendency of anemia of inflammation in periodontal diseases. Clinical science (London, England : 1979), 137(3), 251–264. [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019, 286(5), 826–854. [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules 2023, 13, 1262. [CrossRef]
- Obeagu, E. I., Igwe, M. C., & Obeagu, G. U. (2024). Oxidative stress's impact on red blood cells: Unveiling implications for health and disease. Medicine, 103(9), e37360. [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 5, 84. [CrossRef]
- Hans, M., Malik, P. K., Hans, V. M., Chug, A., & Kumar, M. (2023). Serum levels of various vitamins in periodontal health and disease- a cross-sectional study. Journal of oral biology and craniofacial research, 13(4), 471–475. [CrossRef]
- Wu, D.; Lin, Z.; Zhang, S.; Cao, F.; Liang, D.; Zhou, X. Decreased Hemoglobin Concentration and Iron Metabolism Disorder in Periodontitis: Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 1620. [CrossRef]
- Anumolu, V. N., Srikanth, A., & Paidi, K. (2016). Evaluation of the relation between anemia and periodontitis by estimation of blood parameters: A cross-sectional study. Journal of Indian Society of Periodontology, 20(3), 265–272. [CrossRef]
- Agarwal, N., Kumar, V. S., & Gujjari, S. A. (2009). Effect of periodontal therapy on hemoglobin and erythrocyte levels in chronic generalized periodontitis patients: An interventional study. Journal of Indian Society of Periodontology, 13(1), 6–11. [CrossRef]
| Covariate |
No Periodontal Disease N=2930 |
Periodontal Disease N=1739 |
Total N=4669 |
P-Value |
|
Sex male female |
1221 (29.1%) 1709 (38.5%) |
995 (18.5 %) 744 (13.8%) |
2216 (47.6%) 2453 (100%) |
<0.001 |
|
Age 30-34 35-49 50-64 65+ |
354 (8.1%) 982 (23.4%) 817 (20.76%) 777 (15.3%) |
147 (2.8%) 485 (9.9%) 619 (11.85%) 488 (7.7 %) |
501 (52.3%) 1467 (10.9%) 1436 (33.3%) 1265 (23%) |
<0.001 |
|
Race Non-Hispanic White Non-Hispanic Black Hispanic Non-Hispanic Asian Other |
1402 (48.4%) 539 (6.5%) 574 (7.8%) 341 (3.3%) 74 (64.35%) |
638 (19.5%) 409 (4.5%) 445 (5.5 %) 206 (2 %) 41 (0.85%) |
2040 (67.9%) 948 (11%) 1019 (13.3%) 547 (5.3%) 115 (2.3%) |
<0.001 |
|
Education < High School High School/GED College or More |
563 (8.7%) 593 (12.7%) 1772 (46.1%) |
479 (6.7 %) 454 (8.6%) 804 (16.9%) |
1042 (15.4%) 1047 (21.4%) 2576 (63%) |
<0.001 |
|
Smoking No Yes |
1716 (39.6%) 1214 (28%) |
824 (14.8 %) 915 (17.5%) |
2540 (54.4%) 2129 (45.5%) |
<0.001 |
|
Family Income Ratio to FPL <138% 138%-399% >400% |
826 (12.6%) 1012 (23.5) 1092 (31.4) |
646 (9.3%) 646 (13.2%) 447 (9.8) |
1472 (21.9%) 1658 (36.7%) 1539 (41.2) |
<0.001 |
|
Any disease No Yes |
1626 (37.5%) 1294 (30%) |
942 (17.1%) 793 (15.2 %) |
2568 (54.6%) 2087 (45.3%) |
0.356 |
| Variable (Total N) | No Periodontitis (n, Mean ± SD) | Periodontitis (n, Mean ± SD) | P-Value |
|
White Blood Cells (10³ cells/µL) (N = 4504) |
2,817, 7.18 ± 2.21 | 1,687, 7.49 ± 2.53 | <0.001 |
| Serum Albumin (g/dL) (N=4449) | 2,776, 4.23 ± 0.33 | 1,673, 4.20 ± 0.32 | 0.0067 |
|
MCHC (g/dL) (N = 4,504) |
2,817, 33.71 ± 0.95 | 1,687, 33.84 ± 1.37 | 0.0003 |
| Composite | ||||
| Covariate | Odds Ratio | Confidence Interval | p Value | |
| Lower | Upper | |||
| White blood cells | 1.075 | 1.044 | 1.107 | <0.001 |
| Serum albumin | 0.760 | 0.615 | 0.939 | 0.011 |
| MCHC | 1.138 | 1.062 | 1.219 | <0.001 |
| Sex (reference: Male) | 0.551 | 0.481 | 0.631 | <0.001 |
| Education (reference: <high school) | ||||
| High school/GED | 1.006 | 0.832 | 1.217 | 0.947 |
| Some college or more | 0.734 | 0.616 | 0.874 | 0.001 |
| Poverty (reference: <138 FPL) | ||||
| 138%-399% | 0.883 | 0.754 | 1.035 | 0.127 |
| >400% | 0.608 | 0.511 | 0.724 | <0.001 |
| Race (reference: Non-Hispanic White) | ||||
| Non-Hispanic Black | 1.927 | 1.600 | 2.321 | <0.001 |
| Hispanic | 1.689 | 1.416 | 2.014 | <0.001 |
| Non-Hispanic Asian | 1.811 | 1.455 | 2.253 | <0.001 |
| Other | 1.338 | 0.909 | 2.118 | 0.128 |
| Smoking (reference: No Smoking) | 1.311 | 1.146 | 1.500 | <0.01 |
| Any Disease (reference: No Disease) | 0.906 | 0.781 | 1.050 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





