Submitted:
18 October 2025
Posted:
20 October 2025
You are already at the latest version
Abstract
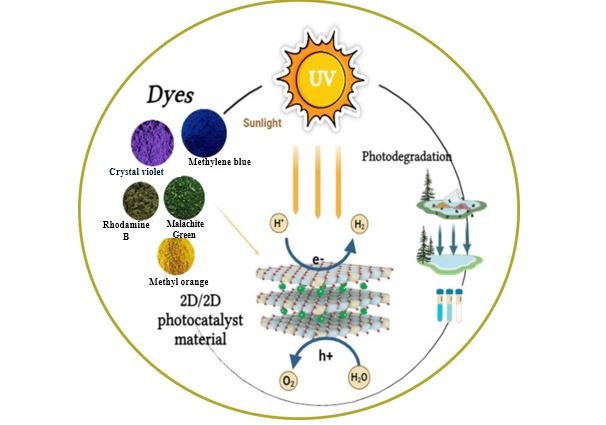
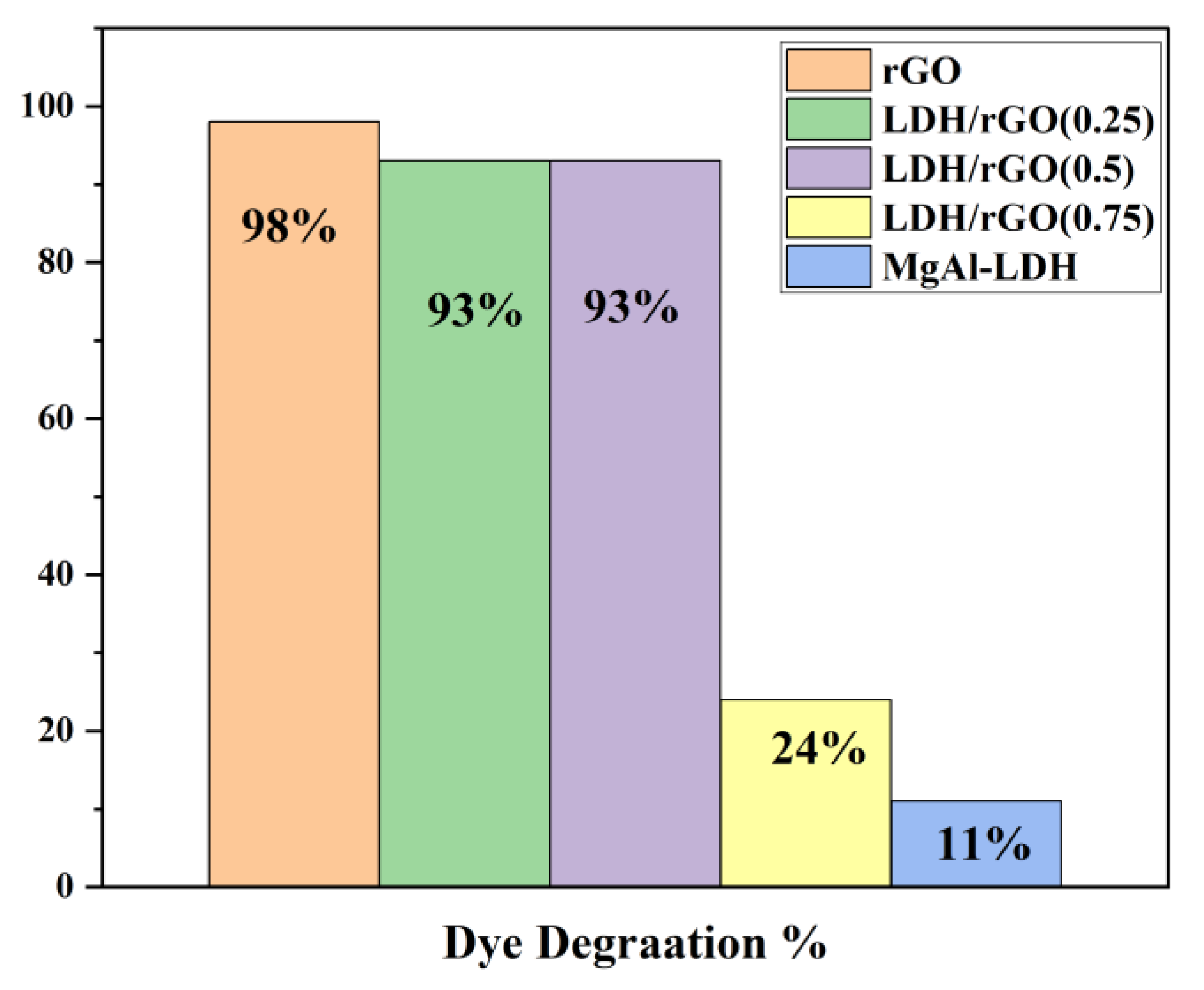
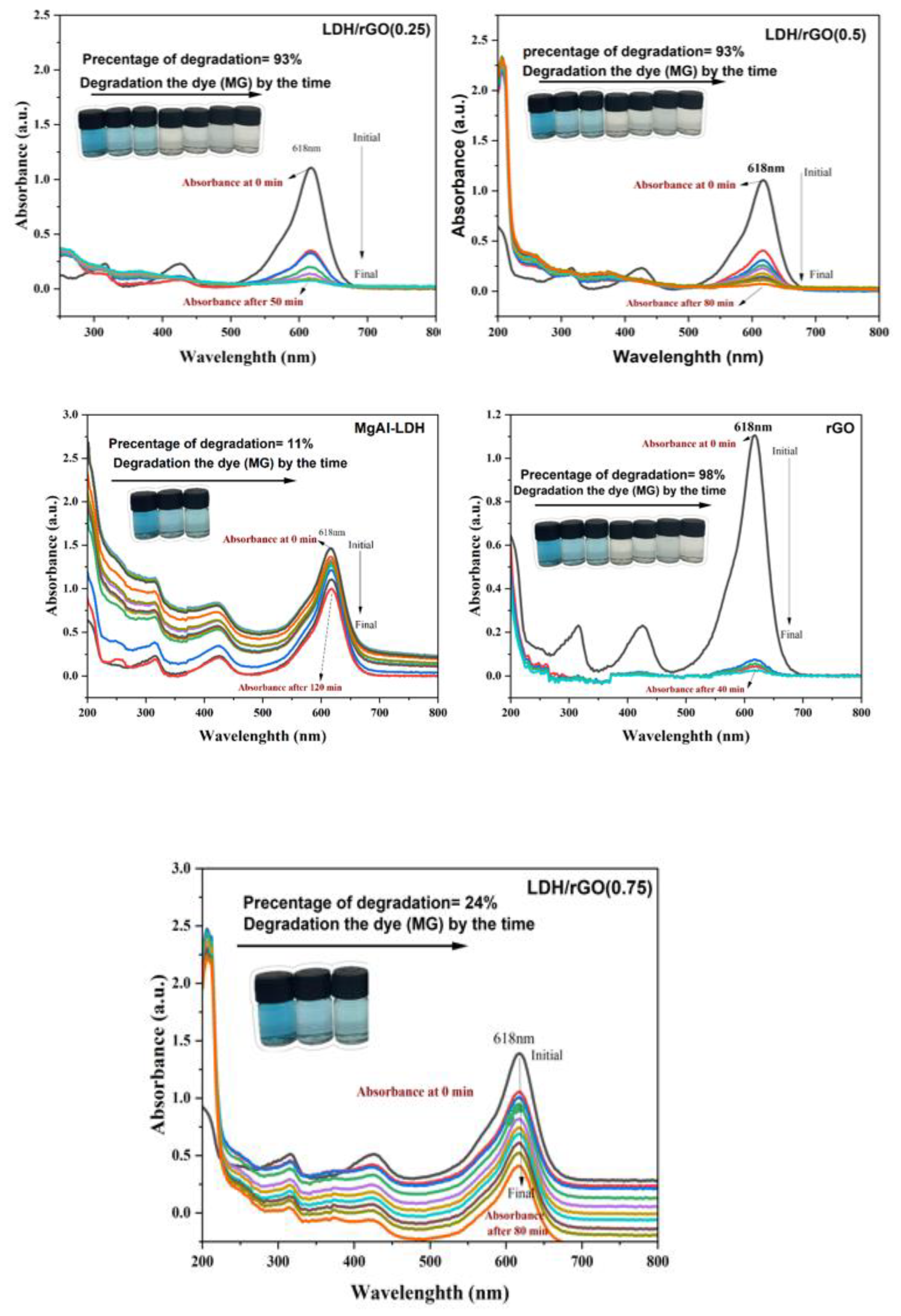
This study reports the fabrication of a novel series of photocatalysts based on reduced graphene oxide (rGO) intercalated into magnesium aluminum layered double hydroxides (MgAl-LDH), denoted as LDH/rGO(0.25), LDH/rGO(0.5), and LDH/rGO(0.75), where the numeric values represent the mass ratio of LDH to rGO. These nanocomposites were designed to address the escalating issue of organic dye pollution in wastewater. The incorporation of rGO into the LDH matrix significantly improved the electrical conductivity, surface area, and light-harvesting capacity of the hybrid material. Importantly, rGO intercalation led to a notable narrowing of the optical band gap of pristine MgAl-LDH (initially 3.5 eV) to values ranging from 2.4 to 2.9 eV, depending on the rGO content. This reduction enhanced the absorption of visible light and promoted more efficient charge carrier separation and migration. The structural, morphological, and optical properties of the synthesized composites were systematically investigated using FTIR, TEM, and UV–Vis's spectroscopy. The photocatalytic performance was evaluated under both ultraviolet (UV) and visible light irradiation, focusing exclusively on the degradation of the Malachite Green (MG) dye as a representative organic pollutant. Among the composites, LDH/rGO(0.25) exhibited the highest degradation efficiency, achieving up to 93% removal of MG within 50 minutes under optimal conditions (pH=10, dye concentration of 10 ppm, and appropriate catalyst dosage). The improvement of photocatalytic activity is assigned to the synergistic interaction between the LDH layers and rGO nanosheets, as well as the optimized band structure induced by varying rGO content. These findings demonstrate the promising potential of LDH/rGO nanohybrids particularly LDH/rGO (0.25) and LDH/rGO (0.5) as efficient, visible-light-responsive photocatalysts for sustainable wastewater treatment applications.
Keywords:
1. Introduction
2. Results and Discussion
2.1. Physicochemical and Structural Characterization
2.2. Optical Properties
2.3. Electrochemical Analysis
2.4. Photocatalytic Activity Assessment
2.4.1. Influence of Dye Structure and Charge on Degradation Behavior.
2.4.2. Role of pH in Dye Degradation
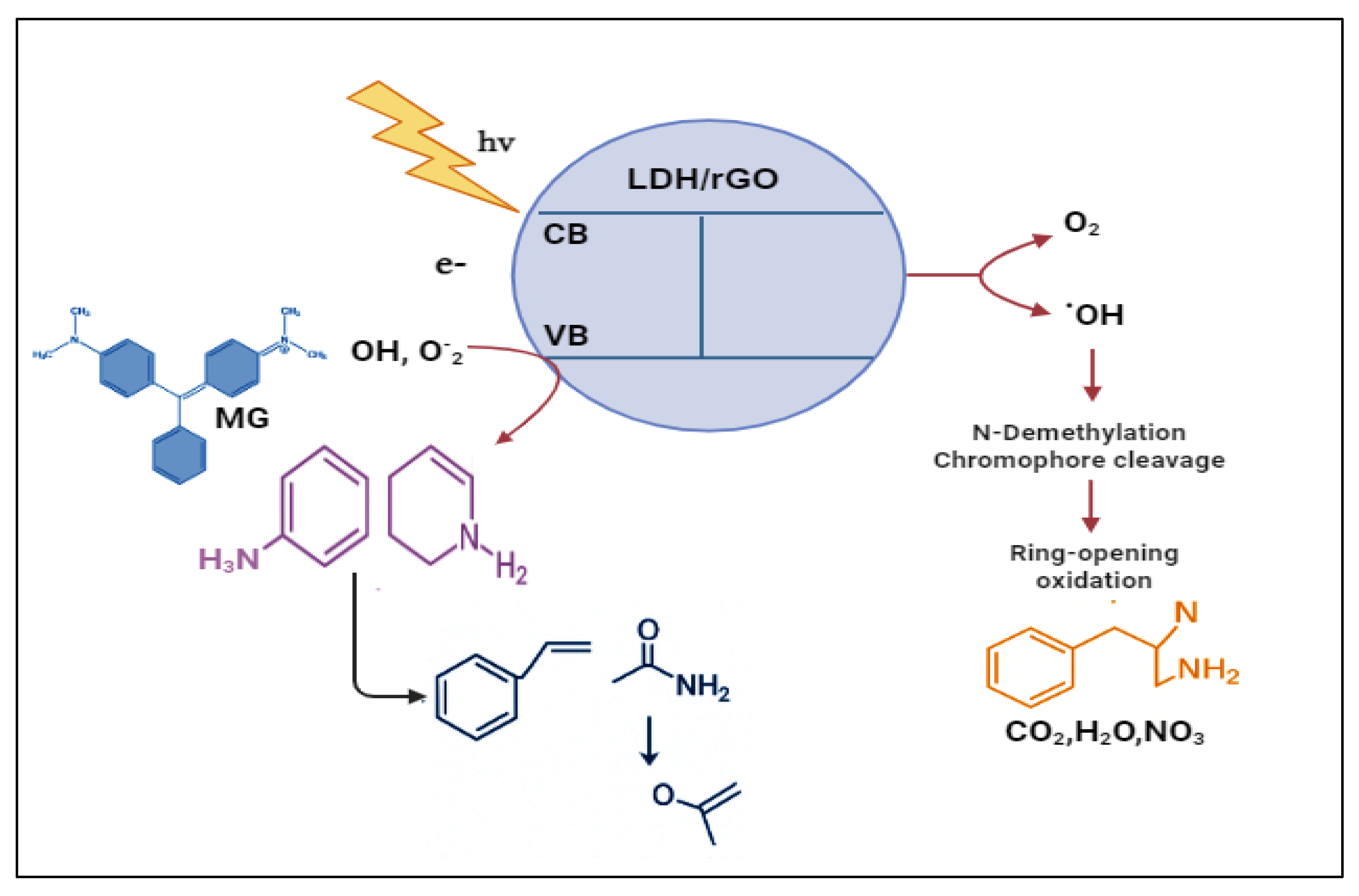
2.4.3. Proposed Mechanistic Pathways
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Mg–Al LDH and LDH/rGO Nanocomposites via Ultrasonic-Assisted Co-Precipitation and CTAB-Templated Hydrothermal Methods.
3.3. Characterization Techniques
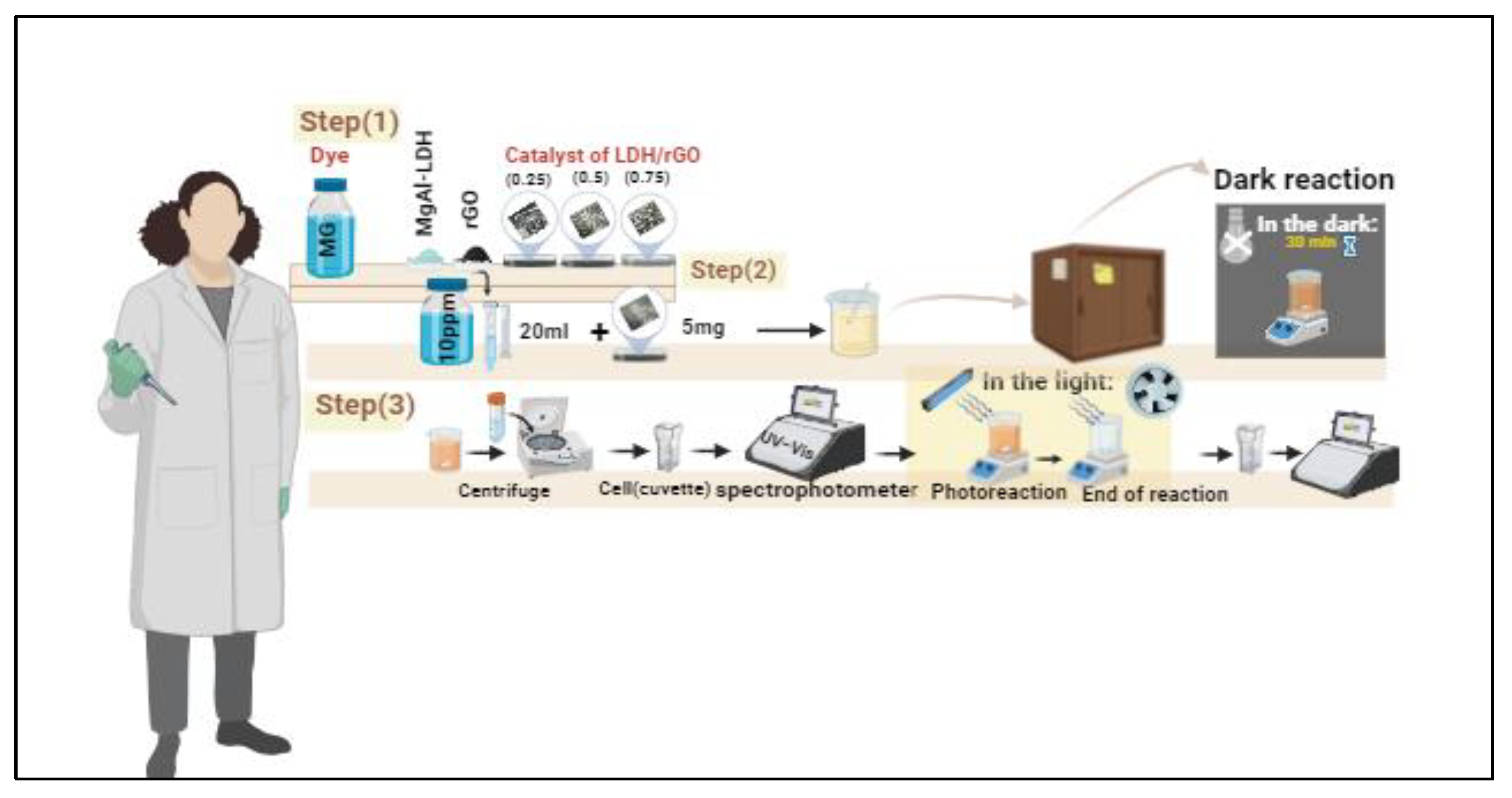
3.4. Photocatalytic Study
- Step 1: Sample Preparation
- Step 2: Adsorption–Desorption Equilibrium (Dark Reaction)
- Step 3: Photocatalytic Reaction under Visible Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad N, Savira D, Erviana D, Mohadi R, Lesbani A. A series of MgAl layer double hydroxide-based materials intercalated with Clitoria ternatea flower extract as photocatalysts in the ciprofloxacin degradation. Chem Phys Impact. 2024;8:100587. [CrossRef]
- Al-Nuaim MA, Alwasiti AA, Shnain ZY. The photocatalytic process in the treatment of polluted water. Chem Pap. 2023 Feb 1;77(2):677–701. [CrossRef]
- Gu Y, Yang Z, Zhou J, Fang Q, Tan X, Long Q. Graphene/LDHs hybrid composites synthesis and application in environmental protection. Sep Purif Technol. 2024 Jan 1;328:125042. [CrossRef]
- Gu Y, Yang Z, Zhou J, Chen Z. Application of graphene/LDH in energy storage and conversion. Sustain Mater Technol. 2023 Sept 1;37:e00695. [CrossRef]
- Raghuwanshi M, Singh A, Suryawanshi B, Jaiswal Y. Synthesis and characterization of MgAl- layered double hydroxide with graphene oxide intercalation: Application in lead removal from spent batteries effluent. Mater Today Proc. 2024 Jan 1;111:121–8. [CrossRef]
- Chaudhuri H, Yun YS. Synthesis and environmental applications of graphene oxide/layered double hydroxides and graphene oxide/MXenes: A critical review. Sep Purif Technol. 2022 Sept 15;297:121518. [CrossRef]
- Cao Y, Li G, Li X. Graphene/layered double hydroxide nanocomposite: Properties, synthesis, and applications. Chem Eng J. 2016 May 15;292:207–23. [CrossRef]
- Chen CC, Lu CS. Mechanistic Studies of the Photocatalytic Degradation of Methyl Green: An Investigation of Products of the Decomposition Processes. Environ Sci Technol. 2007 June 1;41(12):4389–96. [CrossRef]
- Khan S, Noor T, Iqbal N, Yaqoob L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega. 2024 May 21;9(20):21751–67. [CrossRef]
- Visible Light-Driven Photocatalytic Degradation of Methylene Blue Dye Using a Highly Efficient Mg–Al LDH@g-C3N4@Ag3PO4 Nanocomposite | ACS Omega [Internet]. [cited 2025 July 14]. Available from: https://pubs.acs.org/doi/full/10.1021/acsomega.3c07326.
- Djeda R, Mailhot G, Prevot V. Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering. 2020 June;4(2):39. [CrossRef]
- Laipan M, Yu J, Zhu R, Zhu J, Smith AT, He H, et al. Functionalized layered double hydroxides for innovative applications. Mater Horiz. 2020 Mar 9;7(3):715–45. [CrossRef]
- Manuda KRJ, Tillekaratne A, Jayasundara DR. In situ real-time assessment of wavelength dependent degradation of methyl orange on rGO-TiO2 photocatalyst. iScience. 2025 May;28(5):112304.
- Farhan A, Khalid A, Maqsood N, Iftekhar S, Sharif HMA, Qi F, et al. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci Total Environ. 2024 Feb 20;912:169160. [CrossRef]
- Hanifah Y, Mohadi R, Mardiyanto M, Lesbani A. Photocatalytic Degradation of Malachite Green by NiAl-LDH Intercalated Polyoxometalate Compound. Bull Chem React Eng Catal. 2022 Sept 30;17(3):627–37. [CrossRef]
- Rajoba SJ, Sartale SD, Jadhav LD. Investigating functional groups in GO and r-GO through spectroscopic tools and effect on optical properties. Optik. 2018 Dec;175:312–8. [CrossRef]
- Li B, Zhao Y, Zhang S, Gao W, Wei M. Visible-Light-Responsive Photocatalysts toward Water Oxidation Based on NiTi-Layered Double Hydroxide/Reduced Graphene Oxide Composite Materials. ACS Appl Mater Interfaces. 2013 Oct 23;5(20):10233–9. [CrossRef]
- Nayak S, Parida K. Recent Progress in LDH@Graphene and Analogous Heterostructures for Highly Active and Stable Photocatalytic and Photoelectrochemical Water Splitting. Chem – Asian J. 2021;16(16):2211–48. [CrossRef]
- Ma X, Liu T, Liu E, Zhang Y. Preparation and performance of Cd-MgAl-LDHs@RGO in high efficiency electrocatalytic reduction of CO2 to CO. Mol Catal. 2023 Jan 15;535:112876. [CrossRef]
- Nayak S, Parida KM. Deciphering Z-scheme Charge Transfer Dynamics in Heterostructure NiFe-LDH/N-rGO/g-C3N4 Nanocomposite for Photocatalytic Pollutant Removal and Water Splitting Reactions. Sci Rep [Internet]. 2019 Feb 21 [cited 2025 July 8];9(1). Available from: https://www.nature.com/articles/s41598-019-39009-4.
- Zhang L. Photocatalysts with adsorption property for dye-contaminated water purification [Internet] [PhD Thesis]. The University of Queensland; 2017 [cited 2025 July 8]. Available from: http://espace.library.uq.edu.au/view/UQ:656461.
- Mureseanu M, Cioatera N, Carja G. Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water. Nanomaterials. 2023 Jan;13(6):981. [CrossRef]
- Wang HW, Bringans C, Hickey AJR, Windsor JA, Kilmartin PA, Phillips ARJ. Cyclic Voltammetry in Biological Samples: A Systematic Review of Methods and Techniques Applicable to Clinical Settings. Signals. 2021 Mar;2(1):138–58. [CrossRef]
- Amara UE, Majeed A, Al-Rawi SS, Iqbal MA, Shahzadi A, Zakaria M, et al. Harnessing Nanotechnology for Sunlight-Driven Detoxification: Advanced Photocatalytic Strategies for Malachite Green Degradation. Comments Inorg Chem. 2025 May 31;1–45. [CrossRef]
- Yadav P, Manori S, Shukla RK. Contact electro catalysis driven degradation of malachite green dye by RGO/ZnO nanohybrid. Solid State Commun. 2024 Oct 1;389:115578. [CrossRef]
- Hanifah Y, Mohadi R, Mardiyanto, Lesbani A. Polyoxometalate Intercalated MgAl-Layered Double Hydroxide for Degradation of Malachite Green. Ecol Eng Environ Technol [Internet]. 2023 [cited 2025 Oct 12];Vol. 24, iss. 2. Available from: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-1e8ce9dc-3303-4e27-a6fe-29b8070cbc56.
- Pattanaik R, Pradhan D, Kamal R, Dash SK. Facile synthesis of Sr-Bi4Ti3O12: A promising photocatalyst for enhanced degradation of malachite green dye under solar irradiation. Mater. 2025 July 1;8:100847. [CrossRef]
- Balderas-León I, Silva-Jara JM, López-Álvarez MÁ, Ortega-Gudiño P, Barrera-Rodríguez A, Neri-Cortés C. Degradation of Malachite Green Dye by Solar Irradiation Assisted by TiO2 Biogenic Nanoparticles Using Vaccinium corymbosum Extract. Sustainability. 2024 Jan;16(17):7638. [CrossRef]
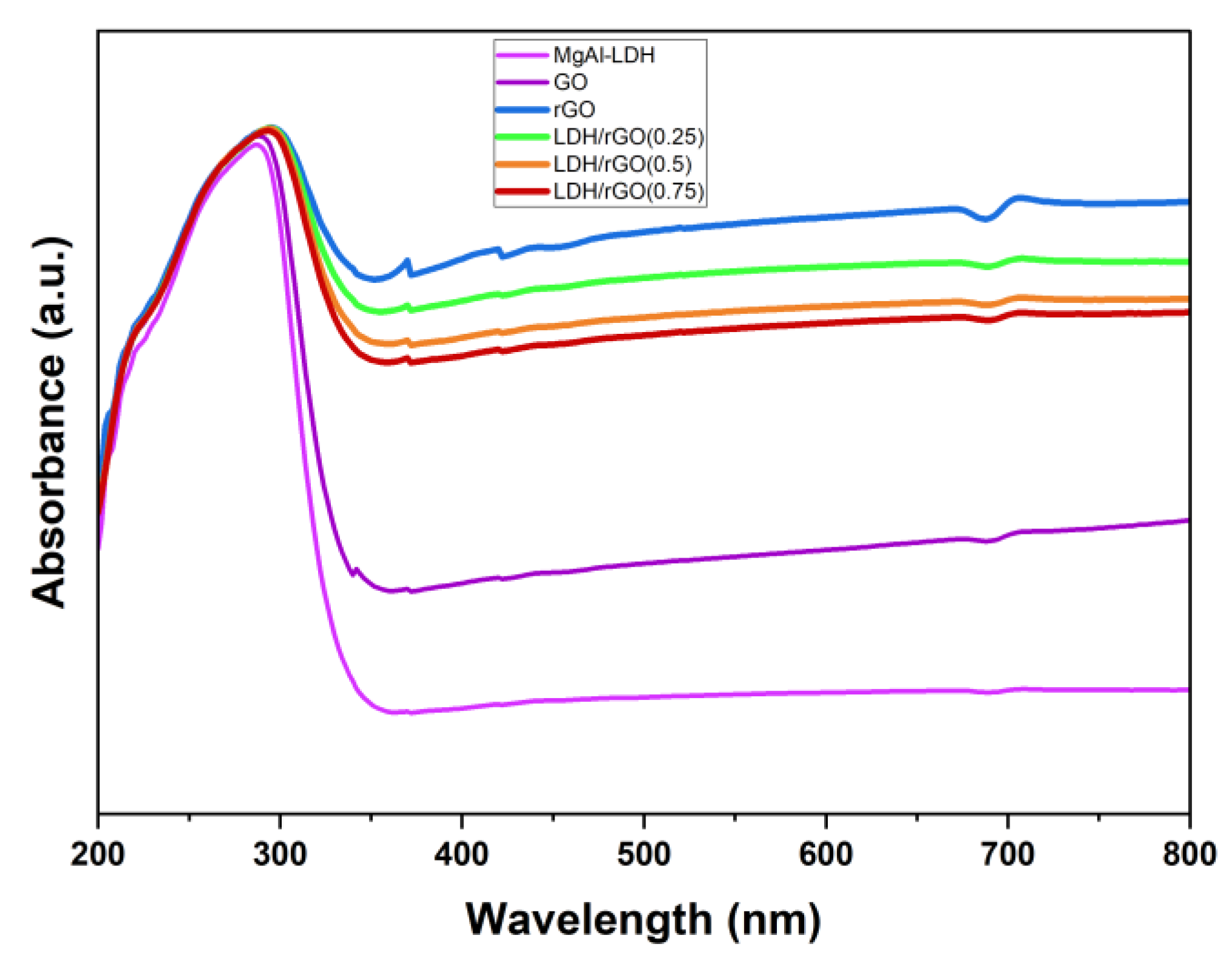
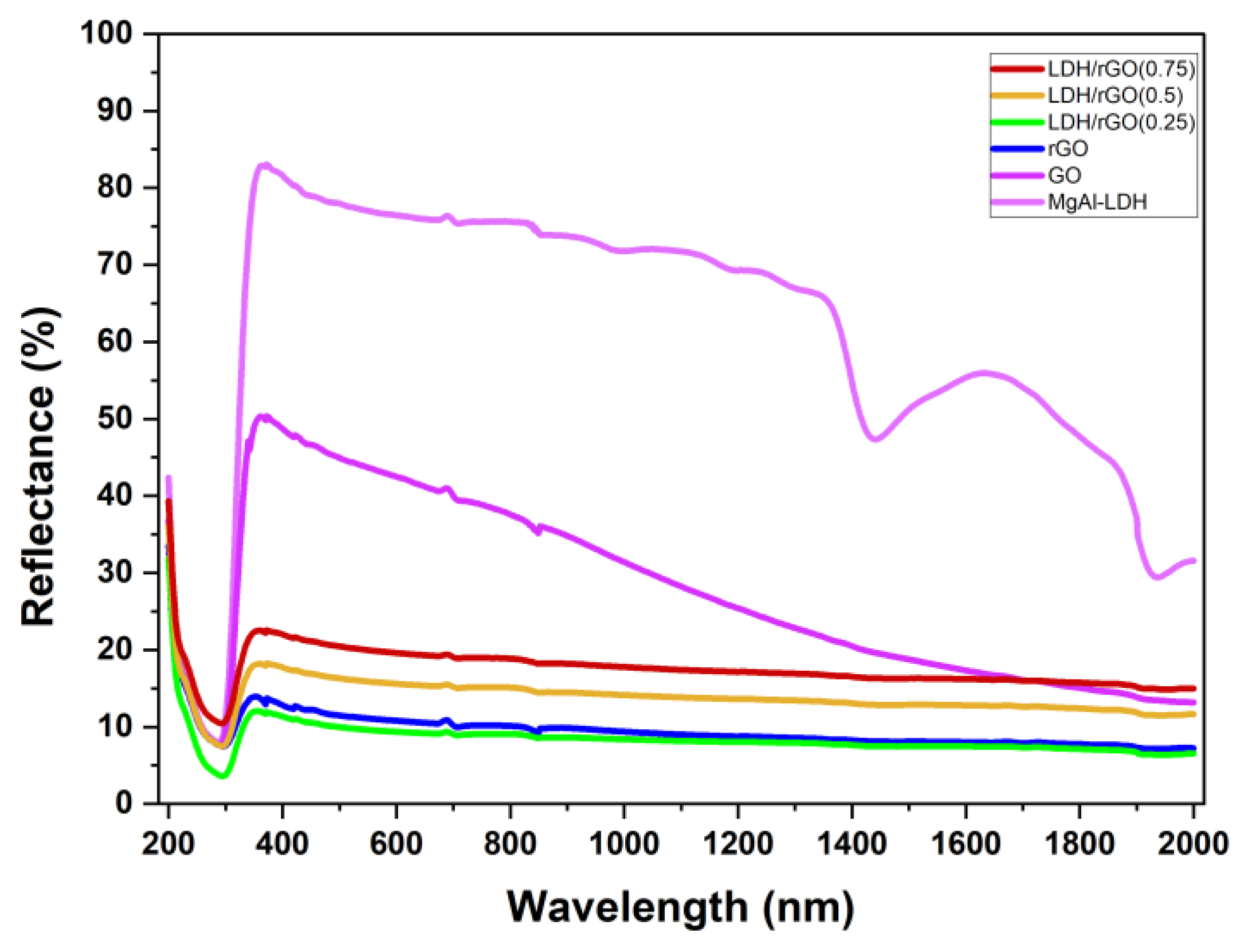
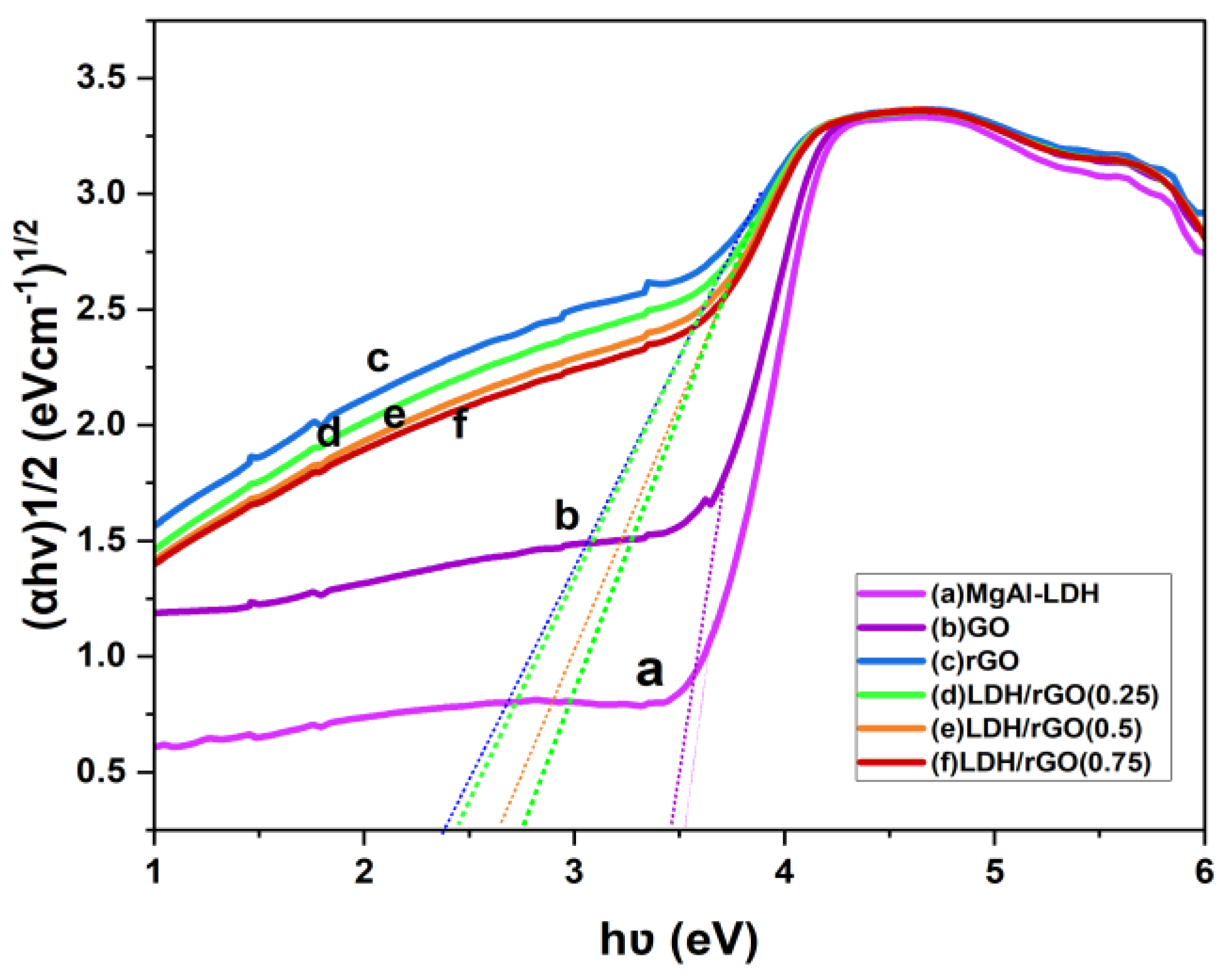
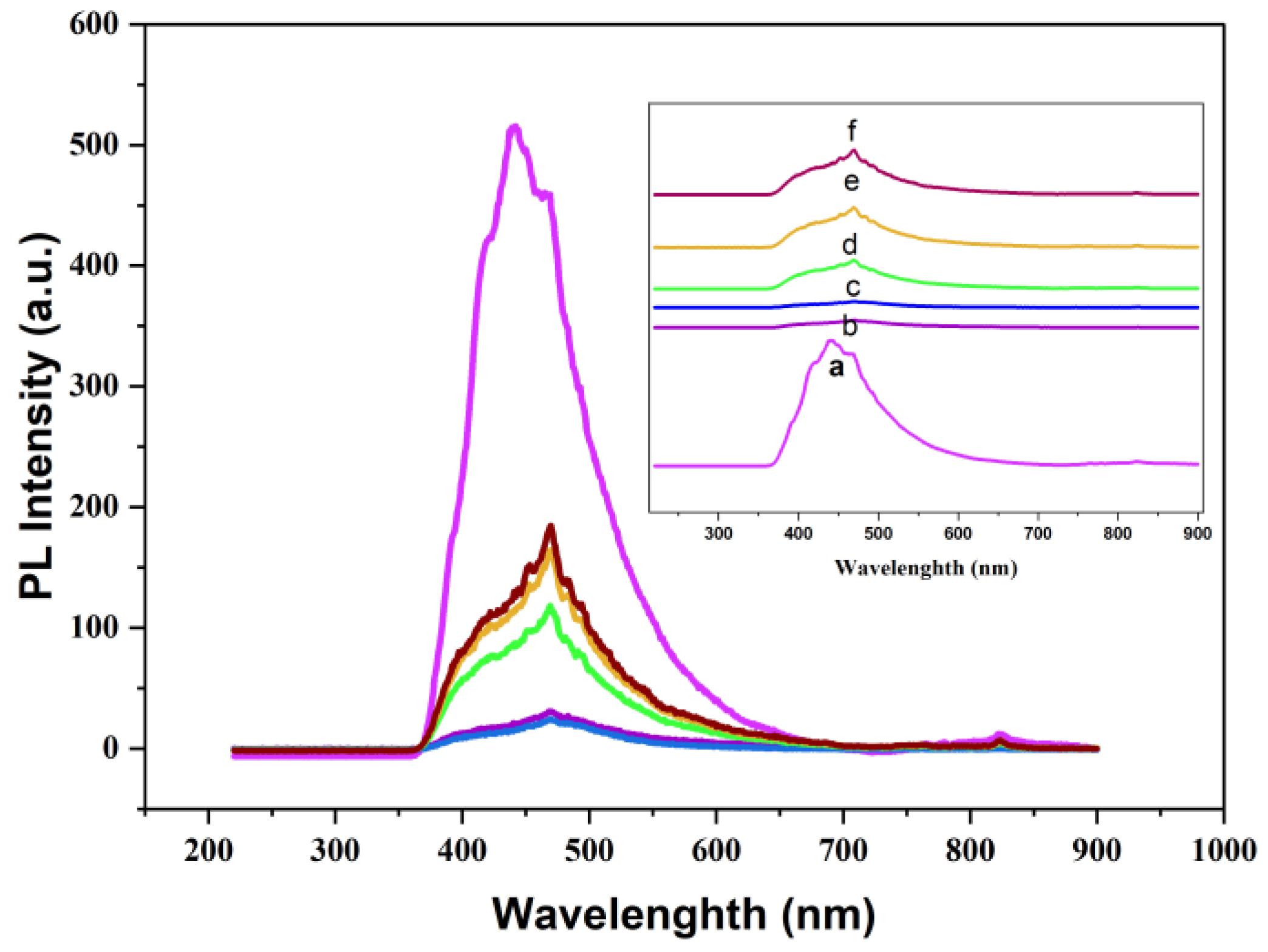
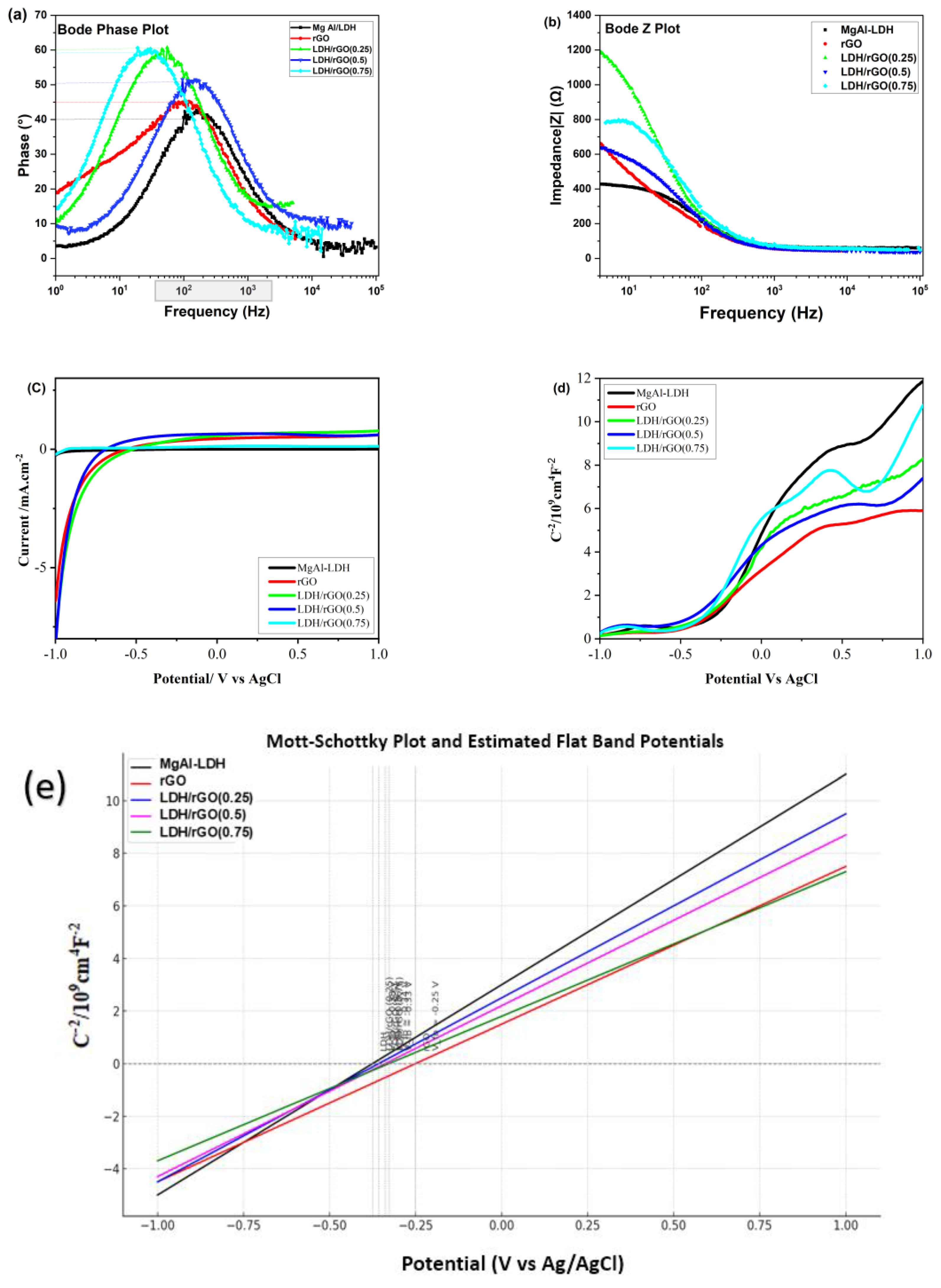
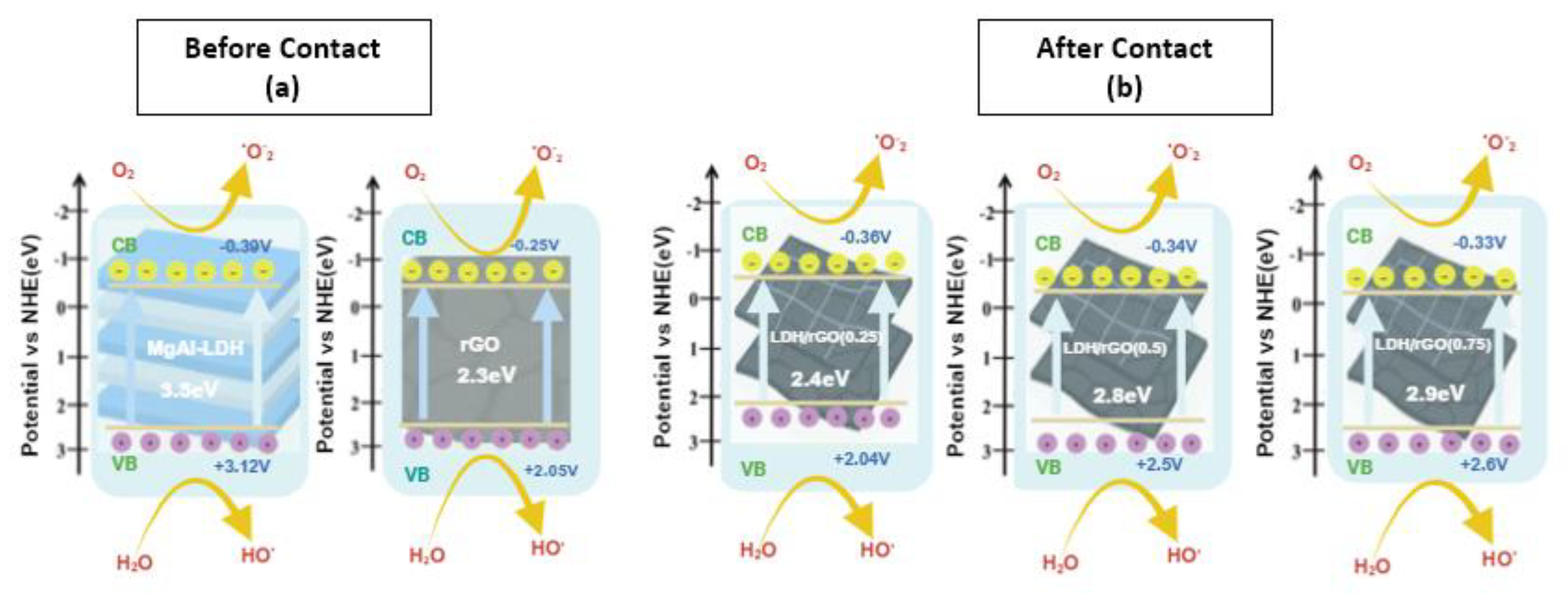
| N# | Samples | Band Gap, Eg [eV] for the Prepared Samples |
|---|---|---|
| a | MgAl-LDH | 3.5eV |
| b | GO | 3.5eV |
| c | rGO | 2.3eV |
| d | LDH/rGO[0.25] | 2.4eV |
| e | LDH/rGO[0.5] | 2.8eV |
| f | LDH/rGO[0.75] | 2.9eV |
| Dye | Optimal pH | Mechanistic Effect | Ref |
|---|---|---|---|
| MG | 10 (alkaline) | Favors ·OH generation, dye deprotonation, better rGO interaction. | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





