1. Introduction
Previous studies have evidenced the catastrophic effects that helminths may inflict upon elephant host [
1,
2,
3]. These include pathological lesions, hemorrhages, tissue necrosis and even death [
2,
3,
4]. Helminth infections also suppress the host’s immunity, stagnate growth and decrease reproduction [
5].
Due to a well-developed parasite-host equilibrium, the African elephant is usually asymptomatic for gastro-intestinal (GI) parasites [
6]. Heavy worm burdens however pose a serious threat to survival. They abrade the epithelial lining of the intestines, causing damage to the rich microvasculature and gaining access to use the host’s ingested nutrients, leading to nutritional deprivation particularly in times of starvation.
Parasite infestations do not always lead to clinical symptoms. In most free-ranging populations, intestinal parasites are neglected with resultant threat to the health and ultimately, survival of especially calves and weaners. Elsheikha and Obanda [
7], hypothesized that disease is maintained at subclinical levels as a result of co-evolution between hosts and parasites also cited more recently [
6,
8]. A parasite-host equilibrium has therefore been established [
9]. In particular, helminths produce immune-evasion molecules which help maintain a balance between worm expulsions and virulence. However, clinical signs of disease may begin to show upon destabilizing of the parasite-host equilibrium. This can occur due to factors such as concurrent infection, pregnancy and lactation, or adverse weather changes.
The most common helminths in African elephants are nematodes (
Murshidia sp,
Khalilia sp, and
Quilonia sp) followed by trematodes (
Protofasciola sp, and
Fasciola sp). Intestinal coccidian infections are common, but have not been associated with any clinical symptoms [
1]. In Kenya, the nematode
Grammocephalus clathratus, the trematode Protofasciola robusta and a number of unidentified adult worms were recovered from elephant carcasses during the drought of 2009, where 38 young animals aged between 5-8 years died in the Laikipia-Samburu Ecosystem (LSE) [
3]. Eleven of the carcasses revealed pathological lesions and hemorrhages that were linked to parasitism. This suggests that helminths could play a potentially important role in regulating wildlife populations.
The aim of this study was to investigate the prevalence of helminth infections and understand species diversity and intensity within two of the largest ecosystems inhabited by elephants in Kenya.
3. Results
Elephants from TENP and LSE were infected by a wide range of nematodes, mostly strongyles and a few trematodes. Overall, prevalence rates were not significantly different between TENP (95.6%) and LSE (98.5%) (χ2(1)=2.03, p>0.05) (
Table 1). Family social group in LSE exhibited prevalence rates that were significantly different from the male social group, (χ2(1) = 7.17, p<0.05) while there were no significant differences observed in the elephant social groups in TENP (χ2(1) = 0.93, p>0.05) (
Table 2). Across ages, there were no significant differences observed either; TENP (χ2(2) = 1.54, p>0.05) and LSE (χ2(2) = 1.75, p>0.05) (
Table 3). The prevalence of nematode infections was 97.1% while that of trematodes was significantly different at 32.6% (χ2(1) = 248.84, p<0.05), as shown in
Table 1. The results indicated that, in general, rates of helminth infection and the egg loads (EPG), were not statistically different between elephants in TENP and in LSE, (F(1)=0.25, p>0.05). Sex had a significant effect on mean worm burden (F(1)=5.84, p<0.05), with females exhibiting higher EPGs than males. However, EPG values within age groups, differed significantly on the basis of sex (F(1)= 4.8, p<0.05) and location (F(1)=14.04, p<0.05, CI 95%) (
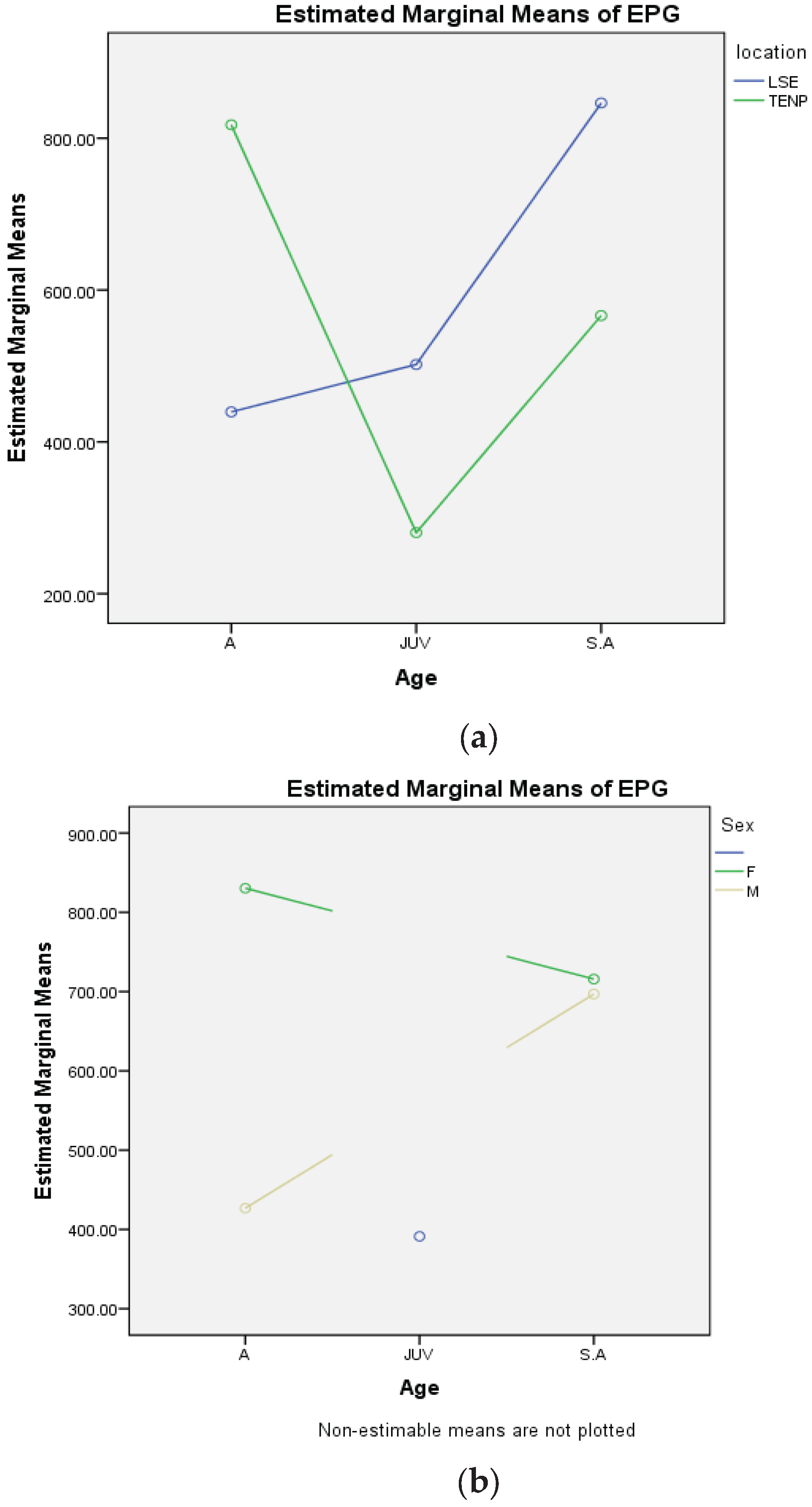
Figure 1a,b).
Figure 1.
Figure 1. (a) Plot showing the relationship between age and location against worm burden (EPG) in elephant populations from Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya. (b) Plot showing relationship between age and sex against worm burden (EPG) in elephant populations in Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
Figure 1.
Figure 1. (a) Plot showing the relationship between age and location against worm burden (EPG) in elephant populations from Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya. (b) Plot showing relationship between age and sex against worm burden (EPG) in elephant populations in Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
Table 1.
Prevalence of helminth infections in elephants from Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
Table 1.
Prevalence of helminth infections in elephants from Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
| Category |
Variables |
TENP |
LSE |
Total |
| |
Total Count (N) |
137 |
136 |
273 |
| Sedimentation |
Number infected (n) |
131 |
134 |
265 |
| Prevalence (%) |
95.6 |
98.5 |
97.1 |
| Trematode infection |
Number infected (n) |
54 |
35 |
89 |
| Prevalence (%) |
39.4 |
25.7 |
32.6 |
Table 2.
Prevalence of helminth infections based on elephant social groups in the Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
Table 2.
Prevalence of helminth infections based on elephant social groups in the Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
| Category |
Variables |
TENP |
LSE |
Total |
| Male social group |
Count (N) |
65 |
30 |
95 |
| Sedimentation |
Number infected (n) |
61 |
28 |
89 |
| Prevalence (%) |
93.9 |
93.3 |
93.7 |
| Trematode infections |
Number infected (n) |
31 |
5 |
36 |
| Prevalence (%) |
47.7 |
16.7 |
37.9 |
| Family social group |
Count (N) |
72 |
106 |
178 |
| Sedimentation |
Number infected (n) |
70 |
106 |
176 |
| Prevalence (%) |
97.2 |
100 |
98.9 |
| Trematode infections |
Number infected (n) |
23 |
30 |
53 |
| Prevalence (%) |
31.9 |
28.3 |
29.8 |
Table 3.
Prevalence of helminth infections based on elephant age groups in the Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
Table 3.
Prevalence of helminth infections based on elephant age groups in the Tsavo East National Park and Laikipia-Samburu ecosystem, Kenya.
| Category |
Variables |
TENP |
LSE |
Total |
| Adult |
Count (N) |
87 |
73 |
160 |
| Sedimentation |
Number infected (n) |
83 |
71 |
154 |
| Prevalence (%) |
95.4 |
97.3 |
96.3 |
| Trematode infections |
Number infected (n) |
34 |
18 |
52 |
| Prevalence (%) |
39.1 |
24.7 |
32.5 |
| Sub-adult |
Count (N) |
30 |
38 |
68 |
| Sedimentation |
Number infected (n) |
28 |
38 |
66 |
| Prevalence (%) |
93.3 |
100 |
97.1 |
| Trematode infections |
Number infected (n) |
10 |
10 |
20 |
| Prevalence (%) |
33.3 |
26.3 |
29.4 |
| Juvenile |
Count (N) |
20 |
25 |
45 |
| Sedimentation |
Number infected (n) |
20 |
25 |
45 |
| Prevalence (%) |
100 |
100 |
100 |
| Trematode infections |
Number infected (n) |
10 |
7 |
17 |
| Prevalence (%) |
50 |
28 |
37.8 |
There is no significant difference in overall helminth prevalence rate observed between adults, sub-adults and juveniles in both TENP (χ2(2) = 1.54, p= 0.462) and LSE (χ2(2) = 1.75, p=0.416). Trematode prevalence based on age also shows no significant differences in both TENP (χ2(2) = 1.4q, p= 0.495) and LSE (χ2(2)= 0.12, p= 0.943) (
Table 3).
3.1. Intestinal Parasites in Elephants from TENP and LSE Areas
3.1.1. Egg Morphology and Morphometry
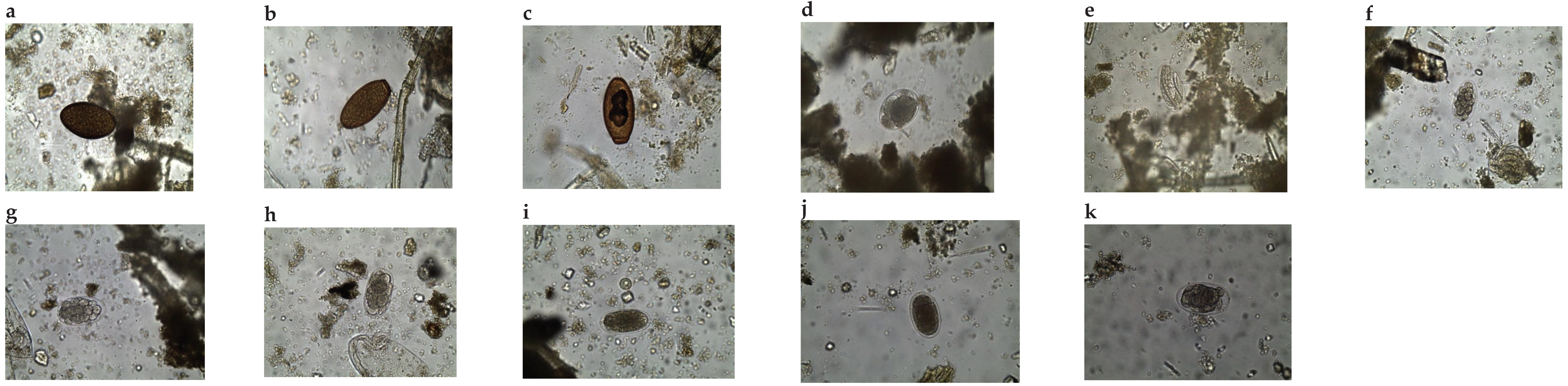
A total of 273 elephants were sampled for this analysis, 137 (50.2%) from TENP and 136 from LSE (49.8%). Populations from the sampled areas are infected by nematodes whose eggs were of a typical strongyle-type morphology, as described by [
1]. Some nematode genera identified from eggs and their subsequent morphometry are shown in
Figure 2a–k. They included those of
Quilonia (80-90*40-55μm),
Murshidia (70-75*35-50μm),
Grammocephalus (65-75*40-50μm), and
Khalilia (80-92*44-60μm). Trematode species identified had similar lengths to those of
Protofasciola (84-104*56-64μm,) and
Fasciola. The eggs of
Fasciola hepatica were easily distinguishable from those of the other trematodes due to the absence of an operculum, which is distinct in
Protofasciola robusta and
Brumptia bicaudata species [
9].
3.1.2. Adult Worm Identification
No adult worms were recovered from the TENP samples. However, from the LSE samples, 29 worms were recovered from 8 out of the 136 samples collected. Of the worms recovered, 26 (89.7%) were identified up to genus level. Using keys and descriptions provided by Anderson et al., [
16], Monnig, [
17] and Van Der Westhuysen, [
18], infections from
Quilonia sp (
Figure 3 a–f) and
Murshidia sp (
Figure 3 images g-u) were identified. The morphology of the anterior and posterior regions of the worms were found most useful for identification.
3.2. Comparison of Helminth Occurrence in TENP and LSE
Comparison of Prevalence
A total of 273 individual elephants were examined for intestinal helminths. Out of these, 137 (50.2%) individuals were from TENP and 136 (49.8%) individuals from LSE. Based on social grouping, individuals sampled from male social groups were 95 (34.8%) while those samples from family social groups were 178 (65.2%). Adult elephants sampled in this study were 160 (58.6%) of which 87 individuals were from TENP and 73 from LSE. Sub adults were 68 (24.9%), with 30 individuals from TENP and 38 from LSE. Lastly, the total number of juveniles sampled were 45 (16.5%) with 20 individuals from TENP and 25 from LSE. Prevalence rate established from floatation method was 93% while that obtained from sedimentation was 97.1%, this difference proved to be statistically significant (χ2(1)= 4.72, p<0.05) (
Table 1).
There was no significant difference in prevalence rates based on location (χ2 (1) = 2.03, p>0.05). The prevalence of trematodes (32.6%) differed from that of nematodes (97.1%) (χ2(1) = 248.84, p<0.001). Elephants from TENP (39.4%) had a trematode prevalence rate that differed from that observed from the population in LSE (25.7%) (χ2(1) =5.81, p<0.05) (
Table 1).
There was no significant difference in observed prevalence rates in the male and family social groups of the elephant populations of TENP (χ2 (1)= 0.93, p=0.335). In LSE however the family social group recorded a prevalence rate (100%) that differed from that of the male social group (93.3%) (χ2(1) = 4.715, p= 0.007). There was no significant difference in trematode prevalence based on social groups from both locations: TENP, (χ2(1) = 3.55, p= 0.06) and LSE, (χ2(1) = 1.66, p= 0.198) (
Table 2).
3.3. Comparison of Worm Burden
Generalized Linear Modeling at 95% confidence level was used using IBM SPSS Statistics 20, to determine the effect of age sex and location on the worm burden. Based on this model, it was possible to determine the effect of location, age and sex, on the mean worm burden observed. Results obtained from the General Linear Model showed that age alone (F(1)=0.789, p= 0.375 CI 95%) and location alone (F(1)=0.247, p= 0.620, CI 95%) had no significant effect on mean worm burdens observed. Sex had a significant effect on mean worm burden (F(1)= 5.842, p= 0.016, CI 95%), with females exhibiting higher EPGs than males.
Interaction between age and location (F(1)=14.043, p= 0.000219, CI 95%) and age and sex (F(1)=4.858, p= 0.028, CI 95%) did have significant effect on mean EPGs observed as shown in the plots in
Figure 1a,b.
Based on location alone, though the difference between them was not statistically significant, adult elephants in TENP exhibited highest EPG means as indicated in
Table 4, followed by sub-adults and Juveniles recording the least mean EPG. In LSE, on the other hand, adult elephants recorded the least mean EPG, followed by juveniles and sub-adults recording the highest mean EPG as shown in
Table 4. Adults in TENP are more likely to experience higher intensity of worm burdens as compared to adult elephants in LSE. However Juvenile and sub-adult elephants in LSE are more likely to harbor more helminths than those in TENP.
Infection intensity in adult females was higher compared to adult males while sub-adult males and females seem to have similar levels of infection intensity. Mean EPG in adult females is higher than that of sub- adults while adult males have lower egg burdens as compared to sub-adults. It proved difficult to sex elephant calves while collecting samples in the field, hence the missing comparison of sex and age with regard to this group (
Figure 1b).
4. Discussion
Our study revealed that African elephants exhibit high prevalence of helminth infections with varied patterns between elephant populations in the TENP and LSE. It should be known that elephants’ habitat loss and fragmentation have led to elephants’ splitting to many sub-populations as a result of movement restriction. King’ori et al [
11], have suggested that these elephant sub-populations are likely to suffer different rates of parasite infestation, and in our study, we reported intestinal parasites’ prevalence of 95.6% and 98.5% in elephants from TENP and LSE respectively. Other workers have also reported high prevalence rates in Kenya including 87.5% by Elsheikha et al., [
19], and 97.5% by King’ori et al., [
11]. These studies established that irrespective of age, social group or location, African elephants are highly susceptible to helminth, especially the nematodes.
In our study, more nematode infection (97.1%) than trematode (32.6%) was observed. Similarly, King’ori et al., [
11] in Kenya, and Baines et al., [
1] in the Okavango-Delta, have reported more nematodes (97.5% and 73%) than trematodes (39.1% and 26%) respectively. The complex life cycle of trematode may have accounted for the lower prevalence rates, which include the necessity of an intermediate host (aquatic snails), whose presence is largely determined by the presence of a permanent water source. Where these special conditions are not met, trematode infection may be suboptimal compared to nematodes, which undergo a more direct lifecycle [
19]. However, during the seasons with abundance of water, elephants do enjoy lengthy periods of time in the water bodies to cool their body temperatures on hot days. Such behavior may serve as risk factor and increase trematode infection.
Apart from the differences in the life cycles of nematodes and trematodes, the abundance and distribution of aquatic snails within the ecosystem serve as risk factors for trematode prevalence. Factors which support aquatic snail distribution include: physico-chemical water quality (water temperature, dissolved oxygen, ions and salts in the water, depth, availability of food), and predation among others, all affect snail species distribution, with resultant effect on trematode prevalence [
20]. Although we determined that elephants from TENP recorded higher trematode prevalence than those from LSE, King’ori et al., [
11] have observed different result where elephants from LSE recorded higher trematode prevalence than those from TENP. A full scale malacological survey of wildlife habitats in Kenya covering all seasons, would therefore be useful in shedding light on trematode distribution and prevalence in animals in the wild. We hypothesized that location rather than social group influence trematode prevalence.
Elephants’ grazing habits encourage re-infection with nematodes. Grazing areas and the general savannah are characterized by scattered faecal material from elephants and other wild animal species that are found in these areas. In the dry season, most adult elephants preferred to feed on shrubs and trees, due to unavailability of grass. Where grass was found, it was scanty, dry and very close to the ground. Elephants would use their trunk to collect grass, and use their feet to remove dirt and soil from the grass before ingesting. Such feeding habits in contaminated areas will lead to inadvertent ingestion of helminth ova, creating conducive conditions for infection and re-infection, a situation that may account for the high nematode prevalence in elephant populations.
The presence of
Protofasciola robusta and
Fasciola hepatica can be attributed to the presence of marsh, swamps, streams and even possible watering holes that provide suitable environments for the intermediate snail hosts. Since elephants feed in marshy areas, with the possibilities of ingesting metacercariae, with consequent infection with these parasites. P robusta has been isolated from the duodenum and distal entrance of the bile ducts and small intestines of elephants [
2,
3], and the parasite has been associated with hemorrhage, intestinal tissue damage and calf fatality [
3]. Fasciola hepatica adults have been found occupying the elephant’s bile ducts and can lead to anorexia, constipation, jaundice, anemia and ultimately death. Fowler and Mikota, [
9] have explain that chronic infection could lead to obstruction of bile ducts, elevation of intrahepatic blood pressure, hypoproteinemia, hemorrhage and death.
We detected the eggs of elephant hook worm, Grammocephalus clathratus in this study. The adults of this worm are found in the liver and bile ducts of the host and causes hemorrhages and lesions in the liver and bile ducts [
3]. Heavy infestation in the bile ducts by these three species can occlude the bile ducts and cause eventual death. Similarly,
Murshidia and
Quilonia species, whose pathology has not been well defined, were occasionally found in the large intestines and sometimes in the small intestines [
2]. While healthy elephants are asymptomatic to helminth infections, starvation, and nutrient deprivation by helminths can cause pathological lesions on the intestinal mucosa [
3], and in aggravated helminthosis, elephants’ survival fitness may be greatly compromised.
In this study, we utilized opportunistic non-invasive methods to obtain adult worms that were excreted together with faeces. Because helminth species from same host are subject to extreme variations [
18], uncertainties in species classification may exist. We utilized multiple methods to classify the
Murshidia and
Quilonia spp up to the genus level [
16,
17,
18]. These two species have earlier been found highly concentrated in the caecum and colon of elephant hosts by Condy, [
2]. The possible pathological effects of these two species in elephants have been discussed earlier [
15,
21,
22]. Their eggs have also been identified by King’ori et al., [
11] from Kenyan elephants across various populations.
We recorded higher mean EPGs in family social groups than in the male social group, an indication that the social structure in elephant populations does have an effect on intensity of helminth infections [
11]. Family groups tend to associate in larger herds as compared to bachelor herds and lone bulls. This association in large herds creates an environment of re-infection as they tend to feed for long in the same areas and they defecate in these areas as they feed as explained earlier. In addition, infection intensity in the social groups is a factor of the foraging dynamics found in elephant social groups. Family herds rarely move far from water sources in dry seasons. This is because family herds consist of calves and sub-adults who may not move as fast as adults and are at higher risks of predation and mortality due to exhaustion. Lone bulls and bachelor herds on the other are not held back and can therefore travel further distances, in search of water and even better food in times of drought, with less risk [
23]. Thus, in the dry season, when family herds are utilizing dwindled and diminishing resources, the adult bulls are able to acquire better feed and water by traveling further. This therefore means that family herds will undergo nutritive and hydric stress more, leading to increase in intensity of helminth infections due to lowered immunity and thus record higher EPGs as compared to their male counterparts, as observed in this study. Mean EPGs recorded reveal similar patterns of infection intensity, whereby female elephant hosts are more parasitized than male hosts [
1,
5,
11,
18]. However, some studies that have looked at the effect of sex on helminth infections in elephants confirmed opposite results [
22]. In our case, mean EPGs recorded in female elephants (707) and male elephants (556), was an indication of relatively moderate levels of infection intensity by helminth parasites.
Overall, the patterns observed in the study showed that elephants from TENP have a higher mean EPG (623.06 ± 653.798) compared to elephants from LSE (589.34 ± 589.237) (p> 0.05); and adults in TENP had significantly higher EPGs as compared to adults in LSE. Perhaps, the habitat range and resource distribution in LSE and TENP played a role in this observation. TENP is a gazetted and fenced national park, covering approximately 12,000 Km
2. The park has one main source of permanent water, Galana River, with seasonal sources including rivers Tiva and Voi, Aruba dam, scattered ponds, swamps and watering holes [
24]. In the dry seasons, elephant home ranges in TENP shrink considerably as water resources become scarce. The elephants retreat to areas along Galana, Voi and Tiva rivers, to increase their chances of survival in the dry season [
24]. The reduction in home range therefore increases chances of heavy parasite infestation especially for family social groups, due to foraging in the same grasslands over a prolonged period of time. LSE, on the other hand, covers a much larger area of 33,817Km
2 [
25]. The ecosystem has a wide range of habitats, associated with climatic gradients within the region: hot and dry lowlands in the north, cool wet highlands to the south, interrupted with rugged mountains and open landscapes. The ecosystem allows for mostly free movement of elephants in between the different land uses due to the wildlife corridors maintained in these areas [
25]. Elephant populations in LSE, therefore, have access to a wider range of habitat as compared to those in TENP. During the dry season, elephants in LSE expand their home range in search for water and food, as was observed in Samburu National Reserve during the conducting of this study. Elephant data from Save the Elephants Foundation, included elephant families that were residents of the reserve, migratory herds, and newcomers. This could explain the differences in mean egg burden observed between the two locations. We suggest that elephants in TENP experience more stress in dry periods due to reduction of habitat range and water resources as compared to those in LSE, and therefore experience higher mean egg burden.
Using egg measurements alone for identification of species present presents a few challenges. It has been noted that egg measurements for a single species varies greatly across different elephant populations [
11]. To overcome this downfall, it is important, where possible, to study larval stages and adult worm morphology to determine species present. The most assured way however for species determination is through molecular characterization [
15].
Whether host species variation exist was not studied in this work, but this knowledge is needed as part of the elephant ecologic system and will be insightful for the parasite-host equilibrium. The patterns observed including sex, age and location may affect mean worm burdens in elephant populations, but more studies are needed to understand the Kenyan ecosystems’ elephants worm burdens and establish ‘high/low worm burden’. This is useful for routine surveillance and health monitoring in wildlife (elephant) health. Ecosystem fragmentation increases pressure on animals (population, movement and resources), which may even be more dire during drought, and these are worsened by high helminth prevalence, especially in calves.
Author Contributions
Conceptualization, S.K.M., E.M.M., and W.W.M.; methodology, S.K.M., E.M.M., W.W.M. and F.O.F.; software, S.K.M., W.W.M. and F.O.F.; validation, S.K.M., E.M.M., W.W.M. and F.O.F.; formal analysis, S.K.M., E.M.M., W.W.M. and F.O.F.; investigation, S.K.M., E.M.M., and W.W.M.; resources, S.K.M., E.M.M., W.W.M. and F.O.F.; data curation, S.K.M., and F.O.F.; writing—original draft preparation, S.K.M., E.M.M., and F.O.F.; writing—review and editing, S.K.M., E.M.M., and W.W.M. and F.O.F.; visualization, S.K.M., E.M.M., W.W.M. and F.O.F.; supervision, E.M.M., and W.W.M.; project administration, S.K.M., E.M.M., and W.W.M.; funding acquisition, S.K.M., and F.O.F. All authors have read and agreed to the published version of the manuscript.