Submitted:
15 October 2025
Posted:
16 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
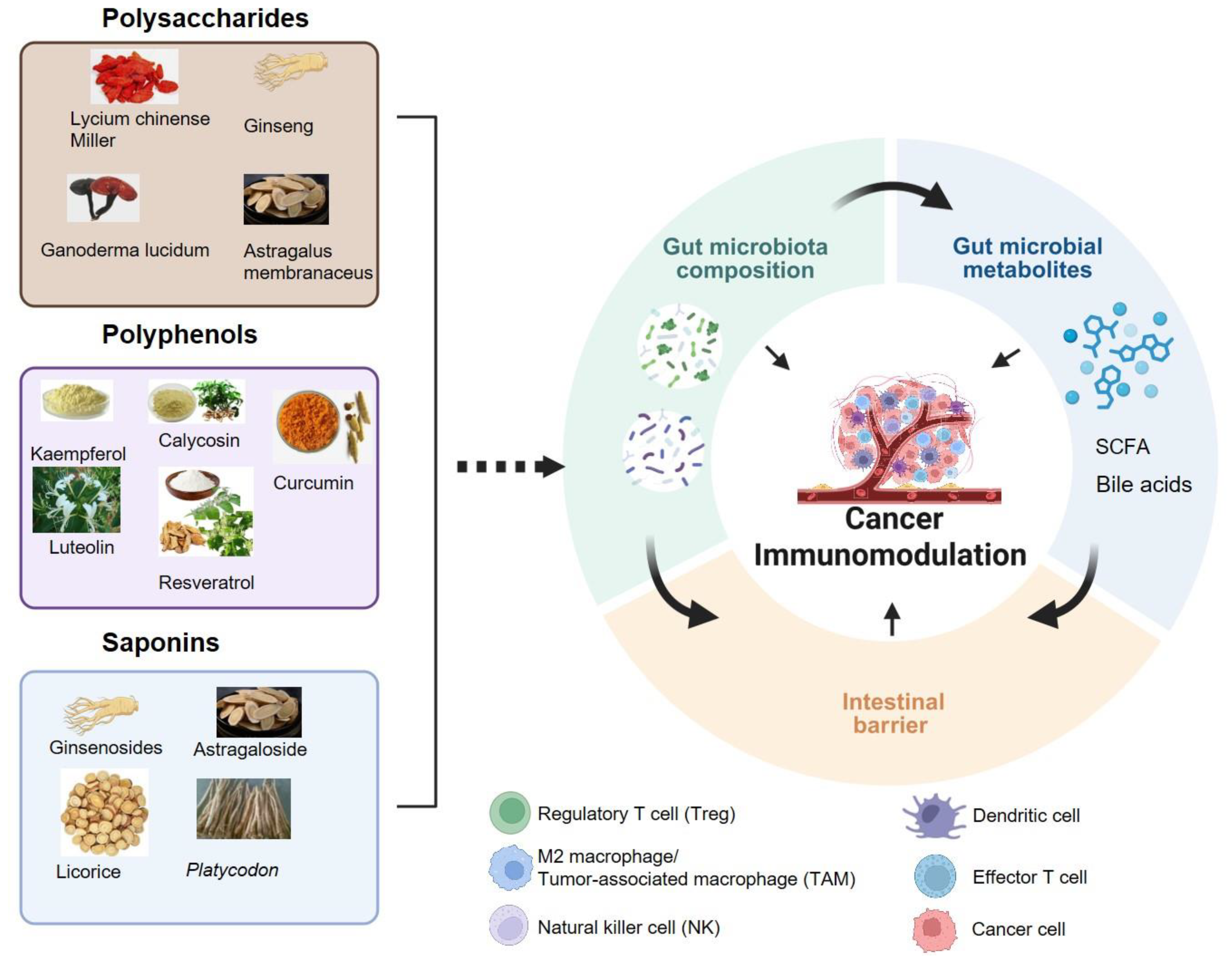
2. Bioactive Compounds from EMPs and Regulation of Gut Microbiome
2.1. Polysaccharides
2.2. Polyphenols
2.3. Saponins
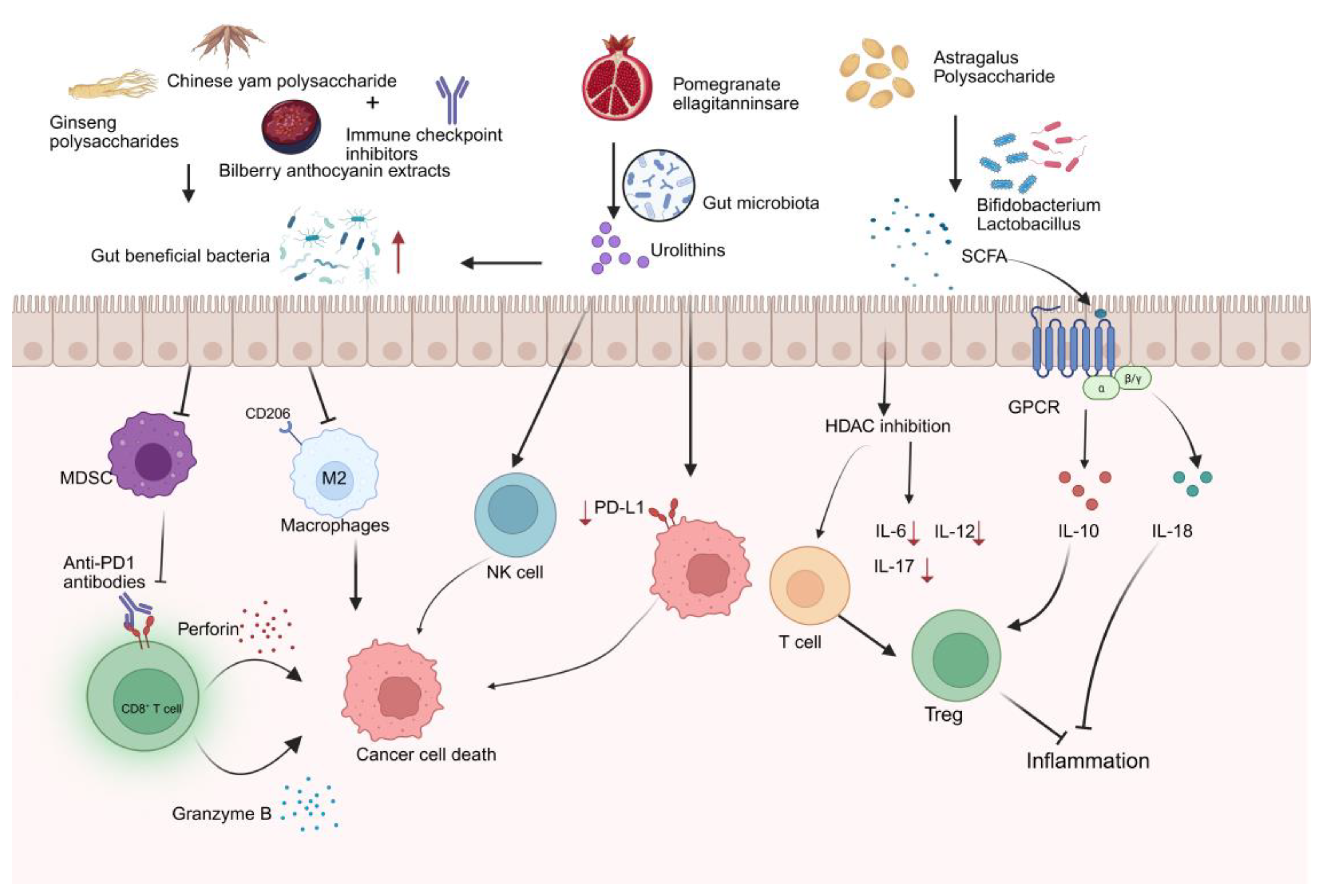
3. Synergistic Effects of the Bioactive Compounds from EMPs on Immunotherapy via Gut Microbiota Modulation
3.1. Gut Microbiota Composition Remodeling
3.2. Microbial Metabolites
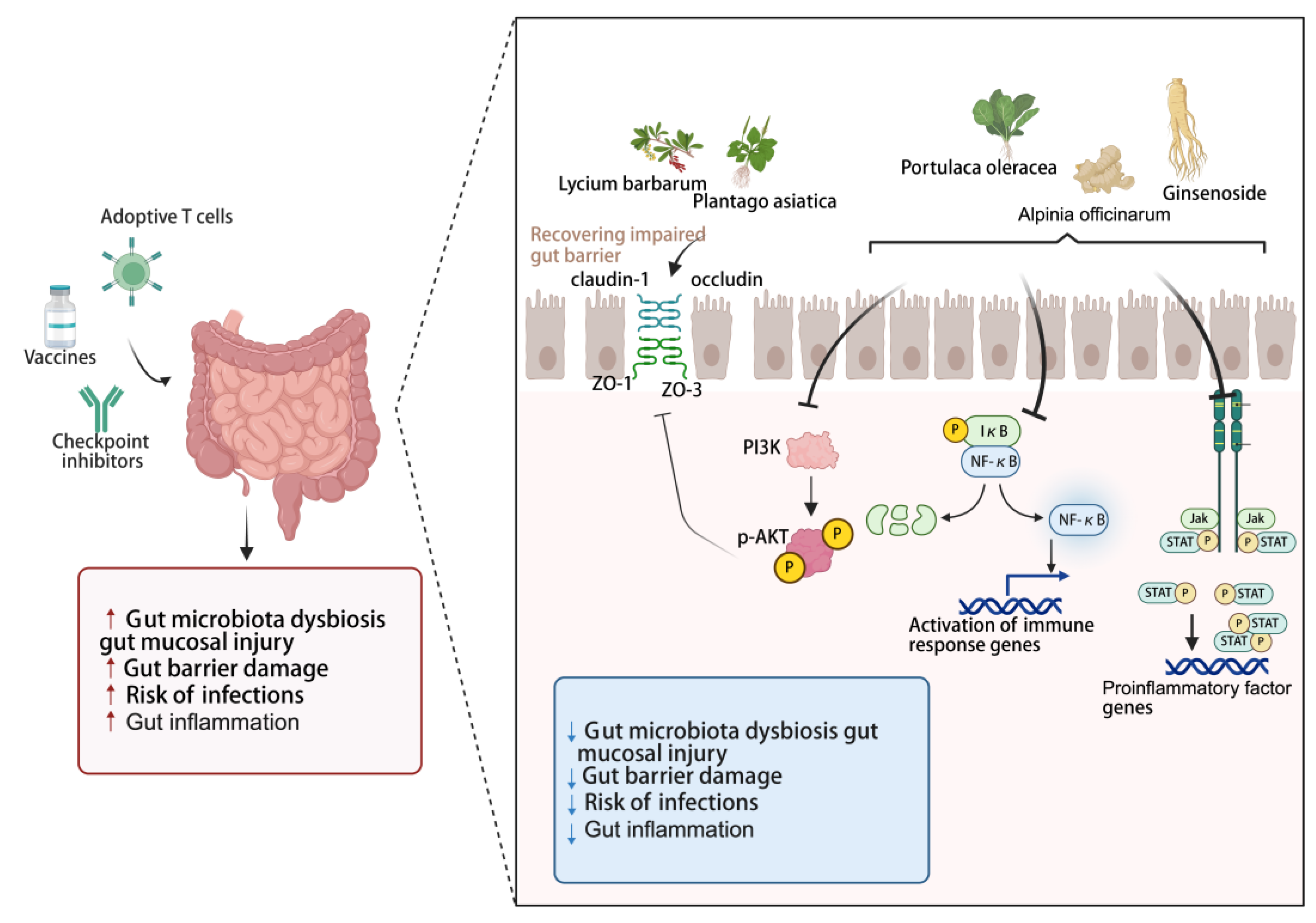
3.3. Intestinal Barrier Dysfunction and Intestinal Inflammation
4. Construct DDS Based on Components from EMPs
4.1. Advantages of Bioactive Compounds from EMPs for DDS Carriers
4.2. Intestinal-Targeting Release of the Medication
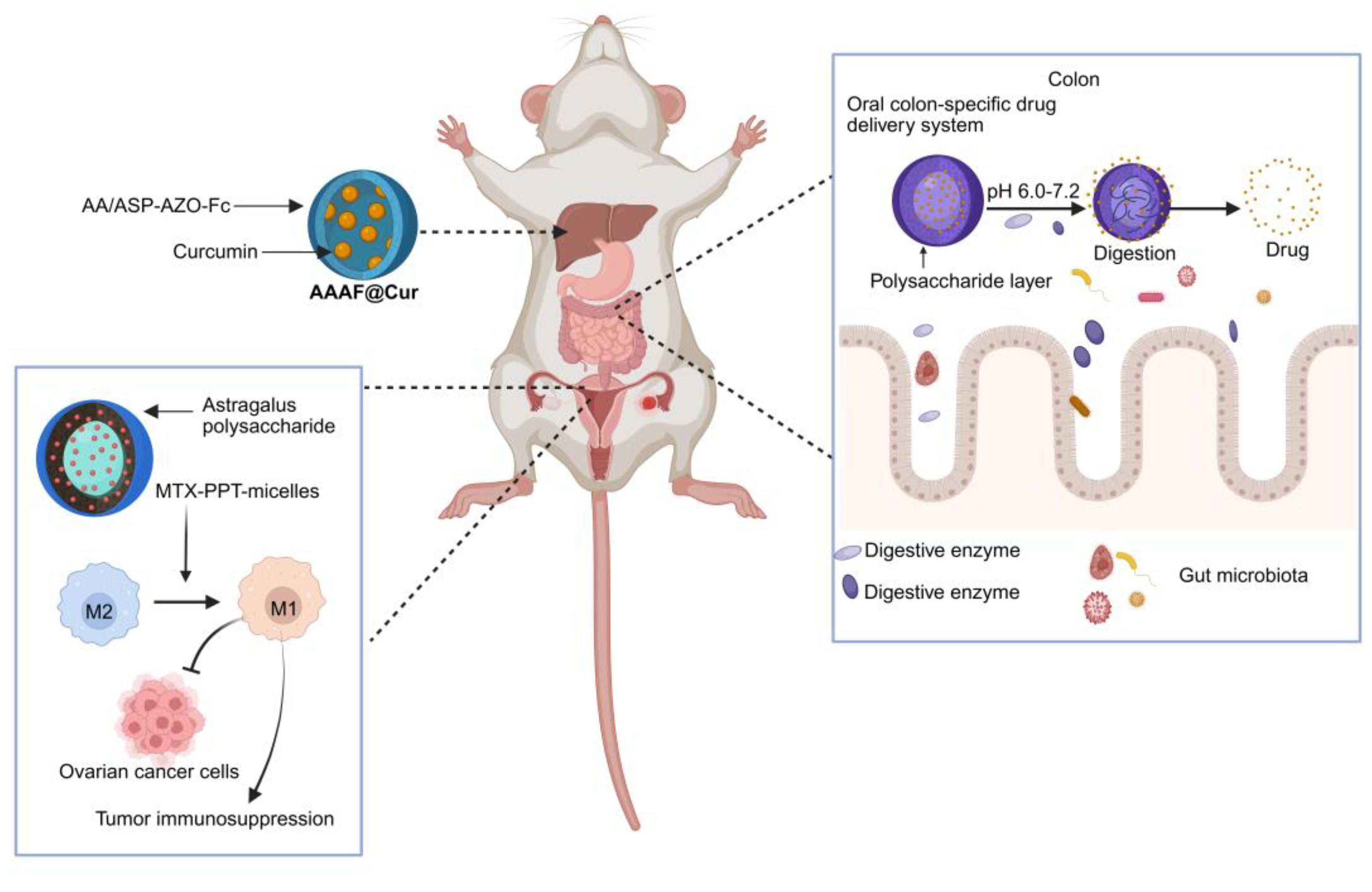
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. Cell. 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology. 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Asokan, S.; Cullin, N.; Stein-Thoeringer, C.K.; Elinav, E. CAR-T Cell Therapy and the Gut Microbiota. Cancers. 2023, 15. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Jing, N.; Wang, L.; Zhuang, H.; Jiang, G.; Liu, Z. Enhancing therapeutic effects of murine cancer vaccine by reshaping gut microbiota with Lactobacillus rhamnosus GG and jujube powder. Front. Immunol. 2023, 14, 1195075. [Google Scholar] [CrossRef]
- Tan, W.; Pan, T.; Wang, S.; Li, P.; Men, Y.; Tan, R.; Zhong, Z.; Wang, Y. Immunometabolism modulation, a new trick of edible and medicinal plants in cancer treatment. Food Chem. 2022, 376, 131860. [Google Scholar] [CrossRef]
- Xia, L.; Li, C.; Zhao, J.; Sun, Q.; Mao, X. Rebalancing immune homeostasis in combating disease: The impact of medicine food homology plants and gut microbiome. Phytomedicine. 2025, 136, 156150. [Google Scholar] [CrossRef]
- Zhou, Z.; Nan, Y.; Li, X.; Ma, P.; Du, Y.; Chen, G.; Ning, N.; Huang, S.; Gu, Q.; Li, W.; et al. Hawthorn with “homology of medicine and food”: a review of anticancer effects and mechanisms. Front. Pharmacol. 2024, 15, 1384189. [Google Scholar] [CrossRef]
- Lu, Q.; Li, R.; Yang, Y.; Zhang, Y.; Zhao, Q.; Li, J. Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chem. 2022, 368, 130610. [Google Scholar] [CrossRef]
- Wen, S.; Han, Y.; Li, Y.; Zhan, D. Therapeutic Mechanisms of Medicine Food Homology Plants in Alzheimer’s Disease: Insights from Network Pharmacology, Machine Learning, and Molecular Docking. Int. J. Mol. Sci. 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Xu, J.Y.; Zhang, H.Y.; Guo, M.; Lan, M.N.; Kong, J.; Liu, S.W.; Zheng, H.J. A medicine and food homology formula prevents cognitive deficits by inhibiting neuroinflammation and oxidative stress via activating AEA-Trpv1-Nrf2 pathway. Inflammopharmacology. 2024, 32, 3745–3759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, C.; Zhou, W.; Li, Y.; Xie, Y.; Hu, H.; Wang, Z. Polygonati Rhizoma with the homology of medicine and food: A review of ethnopharmacology, botany, phytochemistry, pharmacology and applications. J. Ethnopharmacol. 2023, 309, 116296. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L. - A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef]
- Liu, C.; He, P.; Qiao, R.; Yang, X.; Ding, C.; He, F. Mechanistic study of Lonicerae Japonicae Flos (Caprifoliaceae) in non-small cell lung cancer prevention and treatment through integrative pharmacology, multi-machine learning, artificial intelligence, and in vitro experiments. J. Ethnopharmacol. 2025, 348, 119832. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, W.; Wei, J.; Ou, Y.; Xiao, X.; Jia, Y. Synergist for antitumor therapy: Astragalus polysaccharides acting on immune microenvironment. Discover oncology. 2023, 14, 179. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.Y. Ginseng-derived compounds as potential anticancer agents targeting cancer stem cells. J. Ginseng Res. 2024, 48, 266–275. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 27, 118231. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Z.W.; Sheng, H.P.; He, L.J.; Fan, X.W.; He, Z.X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Devel. Ther. 2015, 9, 33–78. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules. 2019, 9. [Google Scholar] [CrossRef]
- Guo, H.; Gibson, S.A.; Ting, J.P.Y. Gut microbiota, NLR proteins, and intestinal homeostasis. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obes Control Ther. Open Access. 2017, 4. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Z.; Gong, G.; Huang, W.; Huang, L.; Song, S.; Zhu, B. Effects of Lycium barbarum Polysaccharides on Immunity and Metabolic Syndrome Associated with the Modulation of Gut Microbiota: A Review. Foods. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, Z.; Chen, H.; Liu, W.; Gu, F.; Li, J. Extraction and Identification of Polysaccharide from Lentinus edodes and Its Effect on Immunosuppression and Intestinal Barrier Injury Induced by Cyclophosphamide. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, W.; Hong, Y.; Wei, W.; Zheng, N.; He, X.; Bao, Y.; Gao, X.; Huang, W.; Sheng, L.; et al. Astragalus polysaccharides attenuate chemotherapy-induced immune injury by modulating gut microbiota and polyunsaturated fatty acid metabolism. Phytomedicine. 2024, 128, 155492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Xu, W.; Bai, H.; Geng, Z.; Yu, Z.; Li, H.; Liu, T. Potential mechanisms underlying inhibition of xenograft lung cancer models by kaempferol: modulation of gut microbiota in activating immune cell function. J. Cancer. 2024, 15, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar] [CrossRef]
- Che, Y.; Li, L.; Kong, M.; Geng, Y.; Wang, D.; Li, B.; Deng, L.; Chen, G.; Wang, J. Dietary supplementation of Astragalus flavonoids regulates intestinal immunology and the gut microbiota to improve growth performance and intestinal health in weaned piglets. Front. Immunol. 2024, 15, 1459342. [Google Scholar] [CrossRef]
- Hirsch, D.; Hardt, J.; Sauer, C.; Heselmeyer-Hadded, K.; Witt, S.H.; Kienle, P.; Ried, T.; Gaiser, T. Molecular characterization of ulcerative colitis-associated colorectal carcinomas. Mod. Pathol. 2021, 34, 1153–1166. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Erdogan Orhan, I.; Rizwan, M.; Atif, M.; et al. Corrigendum to “Luteolin, a flavonoid, as an anticancer agent: A review” [Biomed. Pharmacother. 112 (2019) 108612]. Biomed. Pharmacother. 2019, 116, 109084. [Google Scholar] [CrossRef]
- Liu, F.; Guo, C.; Liu, X.; Gu, Z.; Zou, W.; Tang, X.; Tang, J. Luteolin in Inflammatory Bowel Disease and Colorectal Cancer: A Disease Continuum Perspective. Curr. Issues Mol. Biol. 2025, 47. [Google Scholar] [CrossRef]
- Tang, L.; Gao, J.; Li, X.; Cao, X.; Zhou, B. Molecular Mechanisms of Luteolin Against Atopic Dermatitis Based on Network Pharmacology and in vivo Experimental Validation. Drug Des. Devel. Ther. 2022, 16, 4205–4221. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Luo, H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Vieth, M. Different levels of healing in inflammatory bowel diseases: mucosal, histological, transmural, barrier and complete healing. Gut. 2023, 72, 2164–2183. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Zhao, D.Y.; Du, P.L.; Wang, X.T.; Yang, Q.; Cai, Y.R. Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. Inflamm. Res. 2021, 70, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Luo, B.; An, Q.; Lei, J.; Tan, D.; Liu, X.; Li, H.; Zhao, Y.; Qin, J.; Zhang, C.; Zhang, Y.; et al. Resveratrol amplifies the anti-tumor effect of α-PD-1 by altering the intestinal microbiome and PGD2 content. Gut Microbes. 2025, 17, 2447821. [Google Scholar] [CrossRef]
- Zhou, H.; Zhuang, Y.; Liang, Y.; Chen, H.; Qiu, W.; Xu, H.; Zhou, H. Curcumin exerts anti-tumor activity in colorectal cancer via gut microbiota-mediated CD8(+) T Cell tumor infiltration and ferroptosis. Food Funct. 2025, 16, 3671–3693. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients. 2021, 13. [Google Scholar] [CrossRef]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, F.; Hou, L.; Wan, C. Anti-Inflammatory and Immunostimulatory Activities of Astragalosides. Am. J. Chin. Med. 2017, 45, 1157–1167. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Zhang, Z.; Song, Z.; Xu, J.; Zhang, M.; Gong, M. Overview of Panax ginseng and its active ingredients protective mechanism on cardiovascular diseases. J. Ethnopharmacol. 2024, 334, 118506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Tang, Y.; Keep, R.F.; Ma, X.; Xiang, J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014, 21, 1189–1195. [Google Scholar] [CrossRef]
- Baik, I.H.; Kim, K.H.; Lee, K.A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules. 2021, 26. [Google Scholar] [CrossRef]
- Khan, M.I.; Karima, G.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, T.; Li, J. Research progress on the antitumor effects of astragaloside IV. Eur. J. Pharmacol. 2023, 938, 175449. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yoo, D.S.; Cha, M.R.; Choi, C.W.; Kim, Y.S.; Choi, S.U.; Lee, K.R.; Ryu, S.Y. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J. Nat. Prod. 2010, 73, 1863–1867. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Li, Y.; Fang, B.; Zhu, X.; Xu, B.; Zhang, J.; Wang, M.; Fang, J. New insight into 20(S)-ginsenoside Rh2 against T-cell acute lymphoblastic leukemia associated with the gut microbiota and the immune system. Eur. J. Med. Chem. 2020, 203, 112582. [Google Scholar] [CrossRef]
- Yousuf, S.; Liu, H.; Yingshu, Z.; Zahid, D.; Ghayas, H.; Li, M.; Ding, Y.; Li, W. Ginsenoside Rg1 modulates intestinal microbiota and supports re-generation of immune cells in dexamethasone-treated mice. Acta Microbiol. Immunol. Hung. 2022, 69, 259–269. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Liu, Y.; Deng, J.; Fei, Q.; Duan, Z.; Zhu, C.; Fan, D. Ginsenoside Rk3 modulates gut microbiota and regulates immune response of group 3 innate lymphoid cells to against colorectal tumorigenesis. J. Pharm. Anal. 2024, 14, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Ai, Y.; Ni, J.; Zhang, Y.; Chen, J.; Wang, T.; Ma, J.; Zheng, J.; Li, R.; Ma, X.; et al. Astragaloside IV: A Promising Drug to Prevent the Transition from Colitis to Colorectal Cancer. Am. J. Chin. Med. 2025, 53, 1065–1091. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology. 2023, 164, 198–213. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, W.; Liang, Z.; Wang, J.; Zeng, Z.; Kołat, D.; Li, X.; Zhou, D.; Xu, X.; Zhao, L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 33. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer. 2022, 22, 703–722. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Marcos-Kovandzic, L.; Avagliano, M.; Ben Khelil, M.; Srikanthan, J.; Abdallah, R.; Petrocelli, V.; Rengassamy, J.; Alfaro, A.; Bied, M.; Fidelle, M.; et al. Gut microbiota modulation through Akkermansia spp. supplementation increases CAR-T cell potency. 2025. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer. 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018, 32, 317–326. [Google Scholar] [CrossRef]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO open. 2017, 2, e000213. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009, 114, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X.; et al. Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe. 2023, 31, 418–432.e418. [Google Scholar] [CrossRef]
- Zong, G.; Deng, R.; Pan, Y.; Liu, M.; Zhu, H.; Tao, R.; Shan, Y.; Wei, Z.; Lu, Y. Ginseng polysaccharides ameliorate colorectal tumorigenesis through Lachnospiraceae-mediated immune modulation. Int. J. Biol. Macromol. 2025, 307, 142015. [Google Scholar] [CrossRef]
- Zhang, G.; Pan, J.; Xu, X.; Nie, S.; Lu, L.; Jing, Y.; Yang, F.; Ji, G.; Xu, H. Chinese yam polysaccharide enhances anti-PD-1 immunotherapy in colorectal cancer through alterations in the gut microbiota and metabolites. Int. J. Biol. Macromol. 2025, 310, 143323. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, G.; Jing, N.; Liu, X.; Li, Q.; Liang, W.; Liu, Z. Bilberry anthocyanin extracts enhance anti-PD-L1 efficiency by modulating gut microbiota. Food Funct. 2020, 11, 3180–3190. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nature reviews. Immunology. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv. Sci. 2023, 10, e2207366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, L.; Ma, Q.; Zhang, X.; Chen, M.; Liu, F.; Zhang, T.; Jia, W.; Zhu, L.; Qi, W.; et al. Astragalus Polysaccharide Modulates the Gut Microbiota and Metabolites of Patients with Type 2 Diabetes in an In Vitro Fermentation Model. Nutrients. 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, J.; Zheng, Y.; Li, M.; Xu, X.; Chen, H.; Rui, W. Structural changes of polysaccharides from Astragulus after honey processing and their bioactivities on human gut microbiota. J. Sci. Food Agric. 2023, 103, 7241–7250. [Google Scholar] [CrossRef]
- Yue, B.; Zong, G.; Tao, R.; Wei, Z.; Lu, Y. Crosstalk between traditional Chinese medicine-derived polysaccharides and the gut microbiota: A new perspective to understand traditional Chinese medicine. Phytother. Res. 2022, 36, 4125–4138. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United U.S.A. 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 2010, 285, 27601–27608. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Chen, Q.; Song, H.; Wang, Z.; Xing, W.; Jin, S.; Song, X.; Yang, H.; Zhao, W. The Gut Microbiota Metabolite Urolithin B Prevents Colorectal Carcinogenesis by Remodeling Microbiota and PD-L1/HLA-B. Oxid. Med. Cell. Longev. 2023, 2023, 6480848. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Lo, B.C.; Kryczek, I.; Yu, J.; Vatan, L.; Caruso, R.; Matsumoto, M.; Sato, Y.; Shaw, M.H.; Inohara, N.; Xie, Y.; et al. Microbiota-dependent activation of CD4(+) T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science. 2024, 383, 62–70. [Google Scholar] [CrossRef]
- Zhou, Y.; Medik, Y.B.; Patel, B.; Zamler, D.B.; Chen, S.; Chapman, T.; Schneider, S.; Park, E.M.; Babcock, R.L.; Chrisikos, T.T.; et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Batten, M.; Reijers, I.L.M.; Read, M.; Silva, I.P.; Versluis, J.M.; Ribeiro, R.; Angelatos, A.S.; Tan, J.; et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 2022, 28, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cance. 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue barriers. 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Qi, P.; Yin, L.; Wei, R.; Yang, S.; Liu, Z.; Huang, P.; Yu, Q.; Xiong, S.; Wang, M.; Deng, Y.; et al. Chronic stress synergizes with Listeria monocytogenes to promote intestinal adenomagenesis via myeloid-derived suppressor cells. Front. Immunol. 2025, 16. [Google Scholar] [CrossRef]
- Jin-quan, Z.; Jian-song, W.; Hui, Z. Immunosuppression Mechanism of MDSCs and Regulatory T Cells in Inflammation and Tumor Microenvironment. J. Int. Oncol. 2013. [Google Scholar]
- Gill, G.S.; Kharb, S.; Goyal, G.; Das, P.; Kurdia, K.C.; Dhar, R.; Karmakar, S. Immune checkpoint inhibitors and immunosuppressive tumor microenvironment: current challenges and strategies to overcome resistance. Immunopharmacol. Immunotoxicol. 2025, 47, 485–507. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective Effects of Natural Polysaccharides on Intestinal Barrier Injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers. 2018, 6, e1425085. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Li, W.; Gao, M.; Han, T. Lycium barbarum polysaccharides ameliorate intestinal barrier dysfunction and inflammation through the MLCK-MLC signaling pathway in Caco-2 cells. Food Funct. 2020, 11, 3741–3748. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Chen, X.; Yang, Z.; Cao, Y. Effects of the polysaccharide SPS-3-1 purified from Spirulina on barrier integrity and proliferation of Caco-2 cells. Int. J. Biol. Macromol. 2020, 163, 279–287. [Google Scholar] [CrossRef]
- Dougan, M.; Pietropaolo, M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J. Clin. Invest. 2020, 130, 51–61. [Google Scholar] [CrossRef]
- Dougan, M. Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front. Immunol. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, F.; Li, Y.; Fan, N.; Zhao, K.; Zhang, A.; Kang, J.; Lin, Y.; Xue, X.; Jiang, X. Blockade of PI3K/AKT signaling pathway by Astragaloside IV attenuates ulcerative colitis via improving the intestinal epithelial barrier. J. Transl. Med. 2024, 22, 406. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, W.; Zhang, R.; Wang, X.; Zhang, L. Improving curcumin bioavailability: Targeted delivery of curcumin and loading systems in intestinal inflammation. Food Res. Int. 2024, 196, 115079. [Google Scholar] [CrossRef]
- Long, D.; Mao, C.; Zhang, W.; Zhu, Y.; Xu, Y. Natural products for the treatment of ulcerative colitis: focus on the JAK/STAT pathway. Front. Immunol. 2025, 16, 1538302. [Google Scholar] [CrossRef]
- Jia, X.; Huang, Y.; Liu, G.; Li, Z.; Tan, Q.; Zhong, S. The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers. Nutrients. 2024, 16. [Google Scholar] [CrossRef]
- Li, Z.; Chu, T.; Sun, X.; Zhuang, S.; Hou, D.; Zhang, Z.; Sun, J.; Liu, Y.; Li, J.; Bian, Y. Polyphenols-rich Portulaca oleracea L. (purslane) alleviates ulcerative colitis through restiring the intestinal barrier, gut microbiota and metabolites. Food Chem. 2025, 468, 142391. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, W.; Leslie, F.; Yang, J.; Guo, M.; Sun, M.; Zhang, G.; Zhang, Q.; Wang, F. Nano-formulated delivery of active ingredients from traditional Chinese herbal medicines for cancer immunotherapy. Acta Pharm. Sin. B. 2024, 14, 1525–1541. [Google Scholar] [CrossRef]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B. 2022, 12, 378–393. [Google Scholar] [CrossRef]
- Man, S.; Liu, W.; Bi, J.; Bai, J.; Wu, Q.; Hu, B.; Hu, J.; Ma, L. Smart Mesoporous Silica Nanoparticles Loading Curcumin Inhibit Liver Cancer. J. Agric. Food Chem. 2024, 72, 25743–25754. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Chen, Z.; Wang, M.; Wen, B.; Deng, X. Polysaccharide-modified liposomes and their application in cancer research. Chem. Biol. Drug Des. 2023, 101, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Y.; Sun, Q.; Zhang, Z.; Zhao, M.; Peng, C.; Shi, S. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J. Nanobiotechnology. 2021, 19, 322. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano. 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef]
- Lynam, S.; Lugade, A.A.; Odunsi, K. Immunotherapy for Gynecologic Cancer: Current Applications and Future Directions. Clin. Obstet. Gynecol. 2020, 63, 48–63. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.X.; Wang, J.H.; Guo, R.B.; Zhang, L.; Kong, L.; Yu, Y.; Zang, J.; Liu, Y.; Li, X.T. Immunomodulatory and anti-ovarian cancer effects of novel astragalus polysaccharide micelles loaded with podophyllotoxin. Int. J. Biol. Macromol. 2025, 290, 138960. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science. 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Mastrobattista, E.; Schiffelers, R.M. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013, 10, 1399–1410. [Google Scholar] [CrossRef]
- Moein Moghimi, S.; Hamad, I.; Bünger, R.; Andresen, T.L.; Jørgensen, K.; Hunter, A.C.; Baranji, L.; Rosivall, L.; Szebeni, J. Activation of the human complement system by cholesterol-rich and PEGylated liposomes-modulation of cholesterol-rich liposome-mediated complement activation by elevated serum LDL and HDL levels. J. Liposome Res. 2006, 16, 167–174. [Google Scholar] [CrossRef]
- Szebeni, J.; Baranyi, L.; Savay, S.; Bodo, M.; Morse, D.S.; Basta, M.; Stahl, G.L.; Bünger, R.; Alving, C.R. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1319–1328. [Google Scholar] [CrossRef]
- Su, Y.; Wang, L.; Liang, K.; Liu, M.; Liu, X.; Song, Y.; Deng, Y. The accelerated blood clearance phenomenon of PEGylated nanoemulsion upon cross administration with nanoemulsions modified with polyglycerin. Asian J. Pharm. Sci. 2018, 13, 44–53. [Google Scholar] [CrossRef]
- Verhoef, J.J.; Anchordoquy, T.J. Questioning the Use of PEGylation for Drug Delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Baskaran, R.; Maeng, H.J.; Yoo, B.K. Ginsenoside improves physicochemical properties and bioavailability of curcumin-loaded nanostructured lipid carrier. Arch. Pharm. Res. 2017, 40, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nano-Micro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Fox, C.B.; Kim, J.; Le, L.V.; Nemeth, C.L.; Chirra, H.D.; Desai, T.A. Micro/nanofabricated platforms for oral drug delivery. J. Control. Release. 2015, 219, 431–444. [Google Scholar] [CrossRef]
- Laffleur, F.; Mayer, A.H. Oral nanoparticulate drug delivery systems for the treatment of intestinal bowel disease and colorectal cancer. Expert Opin. Drug Deliv. 2023, 20, 1595–1607. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.; Khan, M.A. Strategies of targeting oral drug delivery systems to the colon and their potential use for the treatment of colorectal cancer. Pharm. Dev. Technol. 2012, 17, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef]
- Gong, T.; Liu, X.; Wang, X.; Lu, Y.; Wang, X. Applications of polysaccharides in enzyme-triggered oral colon-specific drug delivery systems: A review. Int. J. Biol. Macromol. 2024, 275, 133623. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, C.; Li, X.; McClements, D.J.; Sang, S.; Jiao, A.; Jin, Z. Polysaccharide-based nano-delivery systems for encapsulation, delivery, and pH-responsive release of bioactive ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 187–201. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, N.; Li, B.; Zu, M.; Ma, Y.; Xu, H.; Zhu, Z.; Reis, R.L.; Kundu, S.C.; Xiao, B. Natural lipid nanoparticles extracted from Morus nigra L. leaves for targeted treatment of hepatocellular carcinoma via the oral route. J. Nanobiotechnology. 2024, 22, 4. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 2019, 176, 998–1013.e1016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



