Submitted:
15 October 2025
Posted:
16 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Nostalgia and Space
1.2. Nostalgia and Individual Differences in Spatial Ability
1.3. Nostalgia and Wellbeing
1.4. The Current Study
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Nostalgia content questionnaire
2.3.2. Proneness to Nostalgia
2.3.3. Neuroticism Scale from Big Five Inventory
2.3.4. Generalized Anxiety Disorder (GAD7)
2.3.5. Wellbeing - WHO5
2.3.6. Spatial Visualization Ability Test - Paper Folding
2.4. Statistical Approach
3. Results
3.1. Descriptive Statistics
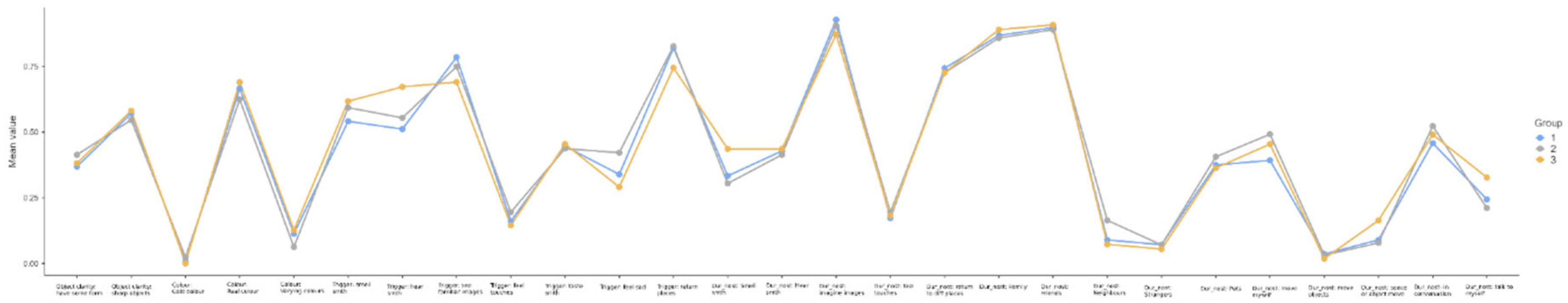
3.2. Nostalgia Content Profiles
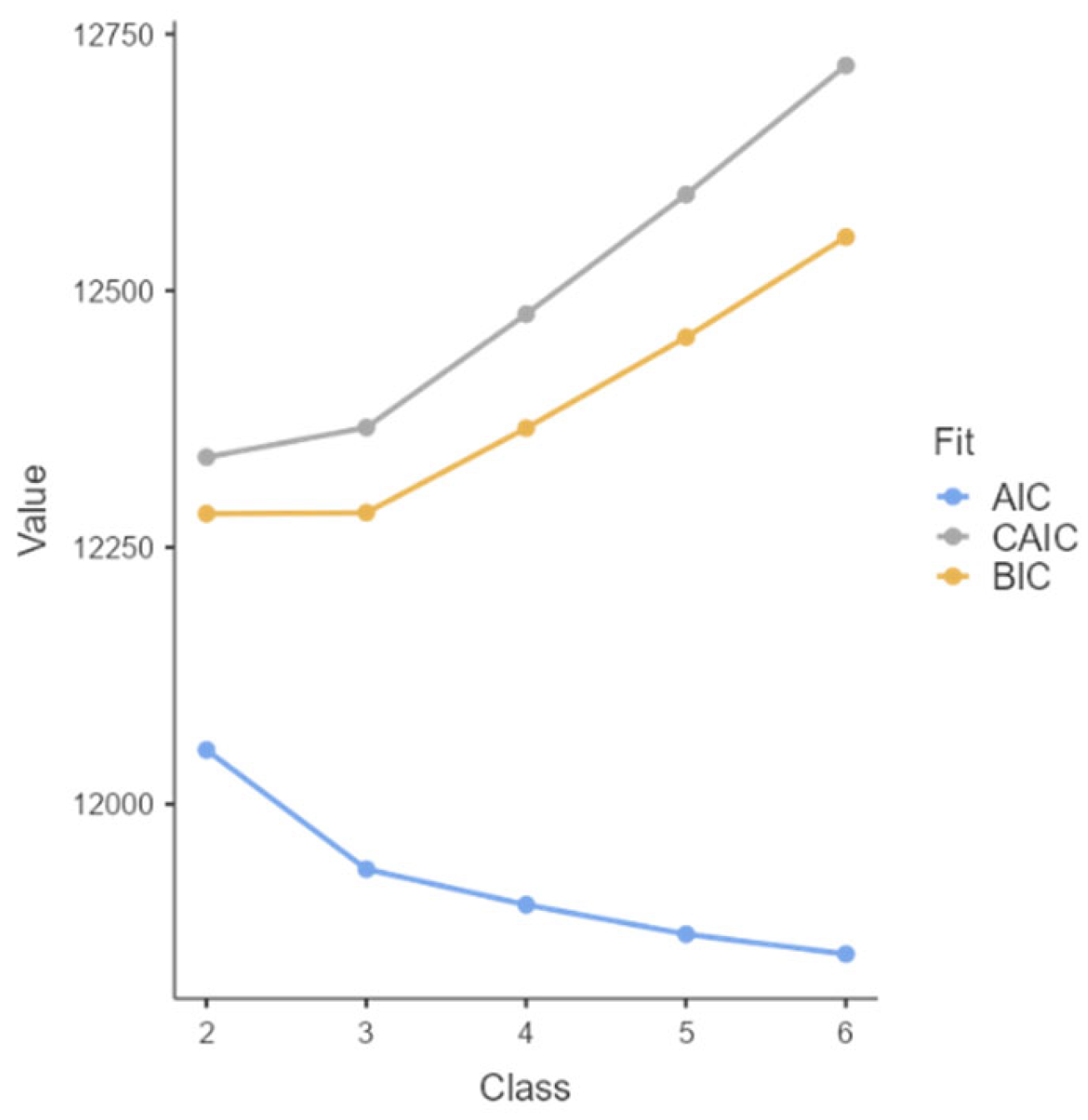
3.2.1. Latent Class Analysis
3.2.2. Profile Effects on Anxiety, Wellbeing, Nostalgia Proneness and Spatial Ability
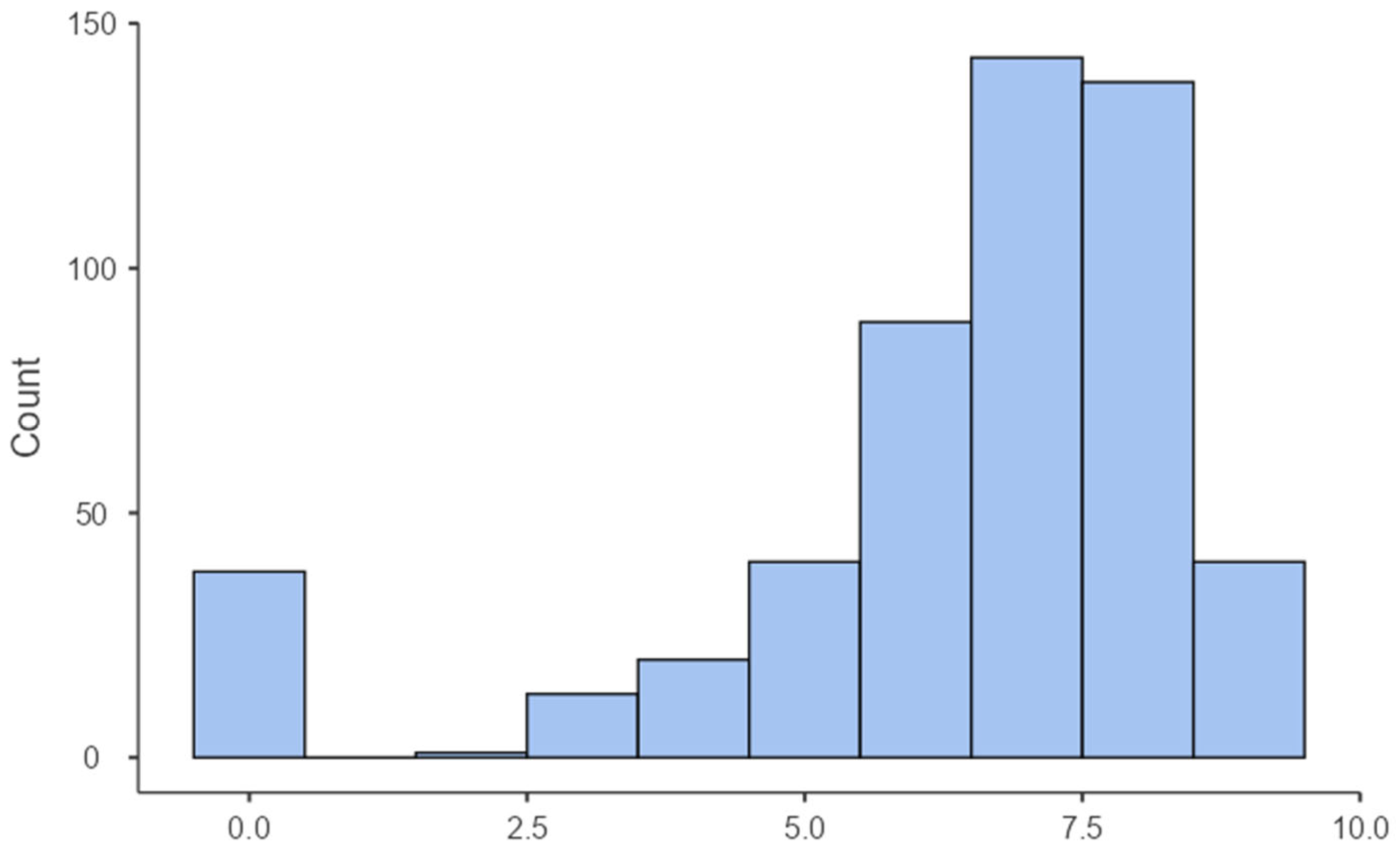
3.3. Spatial Nostalgia Score
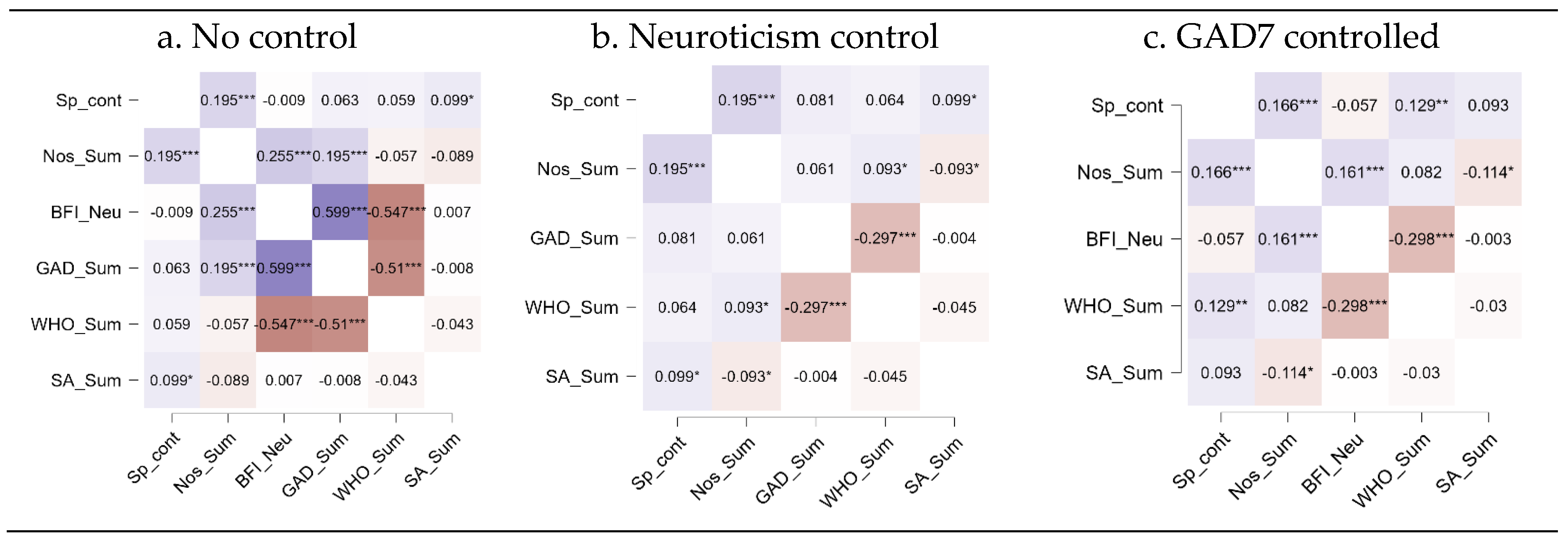
3.4. Nostalgia and Wellbeing
4. Discussion
4.1. Profiles of Nostalgia Experiences
4.2. Nostalgia and Spatial Ability
4.3. Nostalgia and Wellbeing
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrahams, S., Morris, R. G., Polkey, C. E., Jarosz, J. M., Cox, T. C. S., Graves, M., & Pickering, A. (1999). Hippocampal Involvement in Spatial and Working Memory: A Structural MRI Analysis of Patients with Unilateral Mesial Temporal Lobe Sclerosis. Brain and Cognition, 41(1), 39–65. [CrossRef]
- Agha, M. (2019). Nubia Still Exists: On the Utility of the Nostalgic Space. Humanities, 8(1), 24. [CrossRef]
- Alenina, E., Bartseva, K., Likhanov, M., Tsigeman, E., & Soldatova, E. (2023). Validation of the Russian version of the “Nostalgia Proneness Scale” questionnaire and its relationship with other psychological constructs. 386.
- Aytürk, E., Ece, B., Göktaş, N., & Gülgöz, S. (2024). How much trait variance is captured in autobiographical memory ratings? Applied Cognitive Psychology, 38(5), e4240. [CrossRef]
- Batcho, K. I. (1995). Nostalgia: A Psychological Perspective. Perceptual and Motor Skills, 80(1), 131–143. [CrossRef]
- Batcho, K. I. (2013). Nostalgia: The bittersweet history of a psychological concept. History of Psychology, 16(3), 165–176. [CrossRef]
- Bech, P., Gudex, C., & Johansen, S. (1996). The WHO (Ten) Weil-Being Index: Validation in Diabetes. Psychotherapy and Psychosomatics, 65(4), 183–190. [CrossRef]
- Blajenkova, O., Kozhevnikov, M., & Motes, M. A. (2006). Object-spatial imagery: A new self-report imagery questionnaire. Applied Cognitive Psychology, 20(2), 239–263. [CrossRef]
- Blazhenkova, O. (2016). Vividness of Object and Spatial Imagery. Perceptual and Motor Skills, 122(2), 490–508. [CrossRef]
- Blazhenkova, O., Kotov, A., & Kotova, T. (2025). How People Estimate the Prevalence of Aphantasia and Hyperphantasia in the Population. SSRN. [CrossRef]
- Burgess, N., Maguire, E. A., & O’Keefe, J. (2002). The Human Hippocampus and Spatial and Episodic Memory. Neuron, 35(4), 625–641. [CrossRef]
- Carleton, R. N. (2016). Into the unknown: A review and synthesis of contemporary models involving uncertainty. Journal of Anxiety Disorders, 39, 30–43. [CrossRef]
- Chang, M., Hong, B., Savel, K., Du, J., Meade, M. E., Martin, C. B., & Barense, M. D. (2024). Spatial context scaffolds long-term episodic richness of weaker real-world autobiographical memories in both older and younger adults. Memory, 32(4), 431–448. [CrossRef]
- Chu, S., & Downes, J. J. (2002). Proust nose best: Odors are better cues of autobiographical memory. Memory & Cognition, 30(4), 511–518. [CrossRef]
- Dai, Y., Jiang, T., Wildschut, T., & Sedikides, C. (2024). Nostalgia Counteracts Social Anxiety and Enhances Interpersonal Competence. Social Psychological and Personality Science, 15(5), 581–591. [CrossRef]
- De Bruijn, M. J., & Bender, M. (2018). Olfactory cues are more effective than visual cues in experimentally triggering autobiographical memories. Memory, 26(4), 547–558. [CrossRef]
- Ekstrom, R. B., French, J. W., & Harmon, H. H. (1976). Manual for kit of factor-referenced cognitive tests.
- Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–353. [CrossRef]
- Fieve, J., Likhanov, M., Colé, P., & Regner, I. (2025). Different Pathways to Gender Gaps among Third-Graders: Academic Self-Esteem in Mathematics versus Complex Dynamics in Literacy. PsyArXiv. [CrossRef]
- Frankenbach, J., Wildschut, T., Juhl, J., & Sedikides, C. (2021). Does neuroticism disrupt the psychological benefits of nostalgia? A meta-analytic test. European Journal of Personality, 35(2), 249–266. [CrossRef]
- Fuentenebro De Diego, F., & Valiente Ots, C. (2014). Nostalgia: A conceptual history. History of Psychiatry, 25(4), 404–411. [CrossRef]
- Fyhn, M., Molden, S., Witter, M. P., Moser, E. I., & Moser, M.-B. (2004). Spatial Representation in the Entorhinal Cortex. Science, 305(5688), 1258–1264. [CrossRef]
- Gilboa, A. (2004). Autobiographical and episodic memory—One and the same? Neuropsychologia, 42(10), 1336–1349. [CrossRef]
- Gomez, R., & Francis, L. M. (2003). Generalised Anxiety Disorder: Relationships with Eysenck’s, Gray’s and Newman’s theories. Personality and Individual Differences, 34(1), 3–17. [CrossRef]
- Greenberg, D. L., & Knowlton, B. J. (2014). The role of visual imagery in autobiographical memory. Memory & Cognition, 42(6), 922–934. [CrossRef]
- Hafting, T., Fyhn, M., Molden, S., Moser, M.-B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436(7052), 801–806. [CrossRef]
- Hale, W. W., Klimstra, T. A., & Meeus, W. H. J. (2010). Is the Generalized Anxiety Disorder Symptom of Worry Just Another Form of Neuroticism?: A 5-Year Longitudinal Study of Adolescents From the General Population. The Journal of Clinical Psychiatry, 71(07), 942–948. [CrossRef]
- Hawes, Z., & Ansari, D. (2020). What explains the relationship between spatial and mathematical skills? A review of evidence from brain and behavior. Psychonomic Bulletin & Review, 27(3), 465–482. [CrossRef]
- Hebscher, M., Levine, B., & Gilboa, A. (2018). The precuneus and hippocampus contribute to individual differences in the unfolding of spatial representations during episodic autobiographical memory. Neuropsychologia, 110, 123–133. [CrossRef]
- Hepper, E. G., Wildschut, T., Sedikides, C., Ritchie, T. D., Yung, Y.-F., Hansen, N., Abakoumkin, G., Arikan, G., Cisek, S. Z., Demassosso, D. B., Gebauer, J. E., Gerber, J. P., González, R., Kusumi, T., Misra, G., Rusu, M., Ryan, O., Stephan, E., Vingerhoets, A. J. J., & Zhou, X. (2014). Pancultural nostalgia: Prototypical conceptions across cultures. Emotion, 14(4), 733–747. [CrossRef]
- John, O. P., Naumann, L. P., & Soto, C. J. (2008). Paradigm shift to the integrative Big Five trait taxonomy: History, measurement, and conceptual issues. Available online: https://api.semanticscholar.org/CorpusID:149343234.
- Kolb, B., & Whishaw, I. Q. (1996). Fundamentals of human neuropsychology (4th ed). W.H. Freeman.
- Kozhevnikov, M., Kosslyn, S., & Shephard, J. (2005). Spatial versus object visualizers: A new characterization of visual cognitive style. Memory & Cognition, 33(4), 710–726. [CrossRef]
- Leunissen, J., Wildschut, T., Sedikides, C., & Routledge, C. (2021). The Hedonic Character of Nostalgia: An Integrative Data Analysis. Emotion Review, 13(2), 139–156. [CrossRef]
- Likhanov, M., Alenina, E., Bloniewski, T., Zhou, X., & Kovas, Y. (2024). Anxiety and performance in high-achieving adolescents: Associations among 8 general and specific anxiety measures and 13 school grades. [CrossRef]
- Likhanov, M., Maslennikova, E., Costantini, G., Budakova, A., Esipenko, E., Ismatullina, V., & Kovas, Y. (2022). This is the way: Network perspective on targets for spatial ability development programmes. British Journal of Educational Psychology, 92(4), 1597–1620. Scopus. [CrossRef]
- Likhanov, M. V., Ismatullina, V. I., Fenin, A. Y., Wei, W., Rimfeld, K., Maslennikova, E. P., Esipenko, E. A., Sharafeva, K. R., Feklicheva, I. V., Chipeeva, N. A., Budakova, A. V., Soldatova, E. L., Zhou, X., & Kovas, Y. V. (2018). The Factorial Structure of Spatial Abilities in Russian and Chinese Students. Psychology in Russia: State of the Art, 11(4), 96–114. [CrossRef]
- Likhanov, M. V., Tsigeman, E. S., Papageorgiou, K. A., Akmalov, A. F., Sabitov, I. A., & Kovas, Y. V. (2021). Ordinary extraordinary: Elusive group differences in personality and psychological difficulties between STEM-gifted adolescents and their peers. British Journal of Educational Psychology, 91(1), 78–100. Scopus. [CrossRef]
- Likhanov, M., Wang, F., Lyu, J., Wang, L., & Zhou, X. (2024). A special contribution from spatial ability to math word problem solving: Evidence from structural equation modelling and network analysis. Intelligence, 107, 101875. [CrossRef]
- Lohman, D. F. (1979). Spatial Ability: A Review and Reanalysis of the Correlational Literature. 204.
- Lohman, D. F. (1996). Spatial ability and g. In Human abilities: Their nature and measurement. (pp. 97–116). Lawrence Erlbaum Associates, Inc.
- Lopis, D., Le Pape, T., Manetta, C., & Conty, L. (2021). Sensory Cueing of Autobiographical Memories in Normal Aging and Alzheimer’s Disease: A Comparison Between Visual, Auditory, and Olfactory Information. Journal of Alzheimer’s Disease, 80(3), 1169–1183. [CrossRef]
- Luo, Y. L. L., Welker, K. M., Way, B., DeWall, N., Bushman, B. J., Wildschut, T., & Sedikides, C. (2019). 5-HTTLPR polymorphism is associated with nostalgia proneness: The role of neuroticism. Social Neuroscience, 14(2), 183–190. [CrossRef]
- Maguire, E. A., Burgess, N., Donnett, J. G., Frackowiak, R. S. J., Frith, C. D., & O’Keefe, J. (1998). Knowing Where and Getting There: A Human Navigation Network. Science, 280(5365), 921–924. [CrossRef]
- Maguire, E. A., Burke, T., Phillips, J., & Staunton, H. (1996). Topographical disorientation following unilateral temporal lobe lesions in humans. Neuropsychologia, 34(10), 993–1001. [CrossRef]
- Maguire, E. A., Intraub, H., & Mullally, S. L. (2016). Scenes, Spaces, and Memory Traces: What Does the Hippocampus Do? The Neuroscientist, 22(5), 432–439. [CrossRef]
- Maguire, E. A., & Mummery, C. J. (1999). Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus, 9(1), 54–61. [CrossRef]
- Maguire, E. A., Mummery, C. J., & Büchel, C. (2000). Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus, 10(4), 475–482. [CrossRef]
- Marks, D. F. (1973). VISUAL IMAGERY DIFFERENCES IN THE RECALL OF PICTURES. British Journal of Psychology, 64(1), 17–24. [CrossRef]
- Mazard, A., Tzourio-Mazoyer, N., Crivello, F., Mazoyer, B., & Mellet, E. (2004). A PET meta-analysis of object and spatial mental imagery. European Journal of Cognitive Psychology, 16(5), 673–695. [CrossRef]
- McGill, R., Tukey, J. W., & Larsen, W. A. (1978). Variations of Box Plots. The American Statistician, 32(1), 12. [CrossRef]
- Mishkevich, A., Shchebetenko, S., Perm State University, HSE University, Kalugin, A., Perm State Humanitarian Pedagogical University, Soto, C. J., Colby College, John, O. P., & University of California, Berkeley. (2022). The Short and Extra-Short Forms of the Russian Version of the Big Five Inventory-2: BFI-2-S AND BFI-2-XS. Psikhologicheskii Zhurnal, 43(1), 95–108. [CrossRef]
- Monzel, M., Leelaarporn, P., Lutz, T., Schultz, J., Brunheim, S., Reuter, M., & McCormick, C. (2024). Hippocampal-occipital connectivity reflects autobiographical memory deficits in aphantasia. eLife, 13, RP94916. [CrossRef]
- Moscovitch, M., Nadel, L., Winocur, G., Gilboa, A., & Rosenbaum, R. S. (2006). The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology, 16(2), 179–190. [CrossRef]
- Oba, K., Noriuchi, M., Atomi, T., Moriguchi, Y., & Kikuchi, Y. (2016). Memory and reward systems coproduce ‘nostalgic’ experiences in the brain. Social Cognitive and Affective Neuroscience, 11(7), 1069–1077. [CrossRef]
- O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Clarendon Press ; Oxford University Press.
- Palombo, D. J., Sheldon, S., & Levine, B. (2018). Individual Differences in Autobiographical Memory. Trends in Cognitive Sciences, 22(7), 583–597. [CrossRef]
- Papageorgiou, K. A., Likhanov, M., Li, J., Alenina, E., Tsigeman, E., Bartseva, K., Kovas, Y., & Luo, Y. L. L. (2025). Light in the Dark: Cross-Sectional and Longitudinal Investigation of the Network of Dark Triad and Big Five Personality Traits, Resilience and Anxiety. PsyArXiv. [CrossRef]
- Reid, C. A., Green, J. D., Wildschut, T., & Sedikides, C. (2015). Scent-evoked nostalgia. Memory, 23(2), 157–166. [CrossRef]
- Rimfeld, K., Shakeshaft, N. G., Malanchini, M., Rodic, M., Selzam, S., Schofield, K., Dale, P. S., Kovas, Y., & Plomin, R. (2017). Phenotypic and genetic evidence for a unifactorial structure of spatial abilities. Proceedings of the National Academy of Sciences, 114(10), 2777–2782. [CrossRef]
- Robin, J. (2018). Spatial scaffold effects in event memory and imagination. WIREs Cognitive Science, 9(4), e1462. [CrossRef]
- Robin, J., Buchsbaum, B. R., & Moscovitch, M. (2018). The Primacy of Spatial Context in the Neural Representation of Events. The Journal of Neuroscience, 38(11), 2755–2765. [CrossRef]
- Robin, J., Garzon, L., & Moscovitch, M. (2019). Spontaneous memory retrieval varies based on familiarity with a spatial context. Cognition, 190, 81–92. [CrossRef]
- Routledge, C., Arndt, J., Sedikides, C., & Wildschut, T. (2008). A blast from the past: The terror management function of nostalgia. Journal of Experimental Social Psychology, 44(1), 132–140. [CrossRef]
- Routledge, C., Arndt, J., Wildschut, T., Sedikides, C., Hart, C. M., Juhl, J., Vingerhoets, A. J. J. M., & Schlotz, W. (2011). The past makes the present meaningful: Nostalgia as an existential resource. Journal of Personality and Social Psychology, 101(3), 638–652. [CrossRef]
- Rubin, D. C. (2006). The Basic-Systems Model of Episodic Memory. Perspectives on Psychological Science, 1(4), 277–311. [CrossRef]
- Rubin, D. C. (2021). Properties of autobiographical memories are reliable and stable individual differences. Cognition, 210, 104583. [CrossRef]
- Rubin, D. C., Schrauf, R. W., & Greenberg, D. L. (2003). Belief and recollection of autobiographical memories. Memory & Cognition, 31, 887–901. [CrossRef]
- Sargolini, F., Fyhn, M., Hafting, T., McNaughton, B. L., Witter, M. P., Moser, M.-B., & Moser, E. I. (2006). Conjunctive Representation of Position, Direction, and Velocity in Entorhinal Cortex. Science, 312(5774), 758–762. [CrossRef]
- Schlintl, C., Zorjan, S., & Schienle, A. (2023). Olfactory imagery as a retrieval method for autobiographical memories. Psychological Research, 87(3), 862–871. [CrossRef]
- Sedikides, C., & Wildschut, T. (2019). The sociality of personal and collective nostalgia. European Review of Social Psychology, 30(1), 123–173. [CrossRef]
- Sedikides, C., & Wildschut, T. (2022). Nostalgia across cultures. Journal of Pacific Rim Psychology, 16, 18344909221091649. [CrossRef]
- Seehusen, J., Cordaro, F., Wildschut, T., Sedikides, C., Routledge, C., Blackhart, G. C., Epstude, K., & Vingerhoets, A. J. J. M. (2013). Individual differences in nostalgia proneness: The integrating role of the need to belong. Personality and Individual Differences, 55(8), 904–908. [CrossRef]
- Setton, R., Lockrow, A. W., Turner, G. R., & Spreng, R. N. (2022). Troubled past: A critical psychometric assessment of the self-report Survey of Autobiographical Memory (SAM). Behavior Research Methods, 54(1), 261–286. [CrossRef]
- Sinha, P., Calfee, C. S., & Delucchi, K. L. (2021). Practitioner’s Guide to Latent Class Analysis: Methodological Considerations and Common Pitfalls. Critical Care Medicine, 49(1), e63–e79. [CrossRef]
- Slominski, T., Odeleye, O. O., Wainman, J. W., Walsh, L. L., Nylund-Gibson, K., & Ing, M. (2024). Calling for Equity-focused Quantitative Methodology in Discipline-based Education Research: An Introduction to Latent Class Analysis. CBE—Life Sciences Education, 23(4), es11. [CrossRef]
- Spiers, H. J., Burgess, N., Hartley, T., Vargha-Khadem, F., & O’Keefe, J. (2001). Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus, 11(6), 715–725. [CrossRef]
- Spitzer, R. L., Kroenke, K., Williams, J. B. W., & Löwe, B. (2006). A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092. [CrossRef]
- Stella, F., Cerasti, E., Si, B., Jezek, K., & Treves, A. (2012). Self-organization of multiple spatial and context memories in the hippocampus. Neuroscience & Biobehavioral Reviews, 36(7), 1609–1625. [CrossRef]
- Tsao, A., Sugar, J., Lu, L., Wang, C., Knierim, J. J., Moser, M.-B., & Moser, E. I. (2018). Integrating time from experience in the lateral entorhinal cortex. Nature, 561(7721), 57–62. [CrossRef]
- Tullett, A. M., Wildschut, T., Sedikides, C., & Inzlicht, M. (2015). Right-frontal cortical asymmetry predicts increased proneness to nostalgia. Psychophysiology, 52(8), 990–996. [CrossRef]
- Tulving, E. (1983). Elements of episodic memory. Clarendon Press ; Oxford University Press.
- Uttal, D. H., Meadow, N. G., Tipton, E., Hand, L. L., Alden, A. R., Warren, C., & Newcombe, N. S. (2013). The malleability of spatial skills: A meta-analysis of training studies. Psychological Bulletin, 139(2), 352–402. [CrossRef]
- Van Tilburg, W. A. P., Sedikides, C., Wildschut, T., & Vingerhoets, A. J. J. M. (2019). How nostalgia infuses life with meaning: From social connectedness to self-continuity. European Journal of Social Psychology, 49(3), 521–532. [CrossRef]
- Vargha-Khadem, F., Gadian, D. G., Watkins, K. E., Connelly, A., Van Paesschen, W., & Mishkin, M. (1997). Differential Effects of Early Hippocampal Pathology on Episodic and Semantic Memory. Science, 277(5324), 376–380. [CrossRef]
- Verplanken, B. (2012). When bittersweet turns sour: Adverse effects of nostalgia on habitual worriers. European Journal of Social Psychology, 42(3), 285–289. [CrossRef]
- Viviani, G., Visalli, A., Finos, L., Vallesi, A., & Ambrosini, E. (2023). A comparison between different variants of the spatial Stroop task: The influence of analytic flexibility on Stroop effect estimates and reliability. Behavior Research Methods, 56(2), 934–951. [CrossRef]
- Wildschut, T., & Sedikides, C. (2024). Psychology and Nostalgia. In T. Becker & D. Trigg, The Routledge Handbook of Nostalgia (1st ed., pp. 54–69). Routledge. [CrossRef]
- Wildschut, T., Sedikides, C., & Alowidy, D. (2019). Hanin: Nostalgia among Syrian refugees. European Journal of Social Psychology, 49(7), 1368–1384. [CrossRef]
- Wildschut, T., Sedikides, C., Arndt, J., & Routledge, C. (2006). Nostalgia: Content, triggers, functions. Journal of Personality and Social Psychology, 91(5), 975–993. [CrossRef]
- Wilson, J. L. (2015). Here and Now, There and Then: Nostalgia as a Time and Space Phenomenon: Here Now, There and Then. Symbolic Interaction, 38(4), 478–492. [CrossRef]
- Yang, Z., Izuma, K., & Cai, H. (2023). Nostalgia in the brain. Current Opinion in Psychology, 49, 101523. [CrossRef]
- Yang, Z., Wildschut, T., Izuma, K., Gu, R., Luo, Y. L. L., Cai, H., & Sedikides, C. (2022). Patterns of brain activity associated with nostalgia: A social-cognitive neuroscience perspective. Social Cognitive and Affective Neuroscience, 17(12), 1131–1144. [CrossRef]
| `№ | Question | Response Options | Coding in SNS* |
|---|---|---|---|
| Nost 2 | The last time I indulged in nostalgic memories | a – never / a long time ago | |
| b – a month ago | |||
| c – a week ago | |||
| d – a couple of days ago | |||
| e – today | |||
| Nost_3 | The objects in my nostalgic memories usually... | a – Cannot be clearly described |
0 1 2 |
| b – Have some form and shape | |||
| c – Have a clear form and shape | |||
| Nost_4 | The surrounding environment in my nostalgic memories is usually... | a – Black and white | |
| b – Colored If you selected this option, the question Nost4_2 will be presented |
|||
| Nost_4_2 | The colors in my nostalgic memories are... | a – Warm colors | |
| b – Cold colors | |||
| c – As they were in reality | |||
| d – Vary from time to time | |||
| Nost_5 | I usually experience nostalgic memories when... (choose all that apply) | a – I smell something from the past | |
| b – I hear sounds that remind me of the past | |||
| c – I see familiar images | 1 | ||
| d – I feel special touches from the past | |||
| e – I taste something that triggers memories | |||
| f – I feel sad | |||
| g – I return to places tied to memories | 1 | ||
| Nost_6 | Most often during nostalgic memories, I... (choose all that apply) | a – Smell something (e.g., baked goods) | |
| b – Hear something (e.g., a voice) | |||
| c – Imagine only visual images | 1 | ||
| d – Feel touch (e.g., a person, breeze) | |||
| e – Other (please specify) | |||
| Nost_7 | In my nostalgic memories, I return... | a – Mostly to the same place | 1 |
| b – Mostly to different places | 2 | ||
| Nost_8 | In my nostalgic memories, I am usually... | a – Alone | |
| b – With someone else | |||
| Nost_8_2 | For those who selected ‘with someone else’ in Nost_8 The people usually present in my nostalgic memories are... (choose all that apply) |
a – Family members | |
| b – Friends | |||
| c – Neighbors | |||
| d – Stranger | |||
| e – Pets | |||
| f – Other (please specify) | |||
| Nost_9 | In my nostalgic memories, I... | a – Move around dynamically (e.g., inside a house) | 1 |
| b – Move objects in space | 1 | ||
| c – Mostly observe passively without moving | |||
| d – The space or objects move around me | 1 | ||
| Nost_10 | I most often engage in nostalgic memories... | a – Alone | |
| b – In conversation with someone | |||
| Nost_11 | In my nostalgic memories, I usually... | a – Speak words (out loud or to myself) | |
| b – Visualize something without speaking | 1/0 | ||
| Note: *the formula for calculating Spatial Nostalgia Score (SNS) was as follows: Nost_3 +Nost5c+ Nost7 + Nost_9a+Nost_9b+Nost_9d+ Nost_11b; questions and response options included in the SNS are in bold | |||
| BFI: Neuroticism | GAD7 | Nostalgia proneness | WHO5 | Spatial ability | |||||||
| Valid | 473 | 440 | 486 | 474 | 445 | ||||||
| Mean | 2.811 | 7.314 | 29.893 | 13.751 | 7.265 | ||||||
| Std. Deviation | 0.889 | 4.936 | 9.338 | 4.845 | 3.459 | ||||||
| Skewness | 0.120 | 0.980 | -0.454 | -0.178 | 0.280 | ||||||
| Kurtosis | -0.749 | 0.417 | -0.315 | -0.562 | -0.911 | ||||||
| Minimum | 1.000 | 1.000 | 1.920 | 2.000 | 1.000 | ||||||
| Maximum | 5.000 | 21.817 | 48.000 | 25.000 | 15.000 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





