Submitted:
14 October 2025
Posted:
15 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Experimental Design
3. Results
3.1. Shared Brain Networks
3.2. Brain Oscillations
3.3. Brain Structure
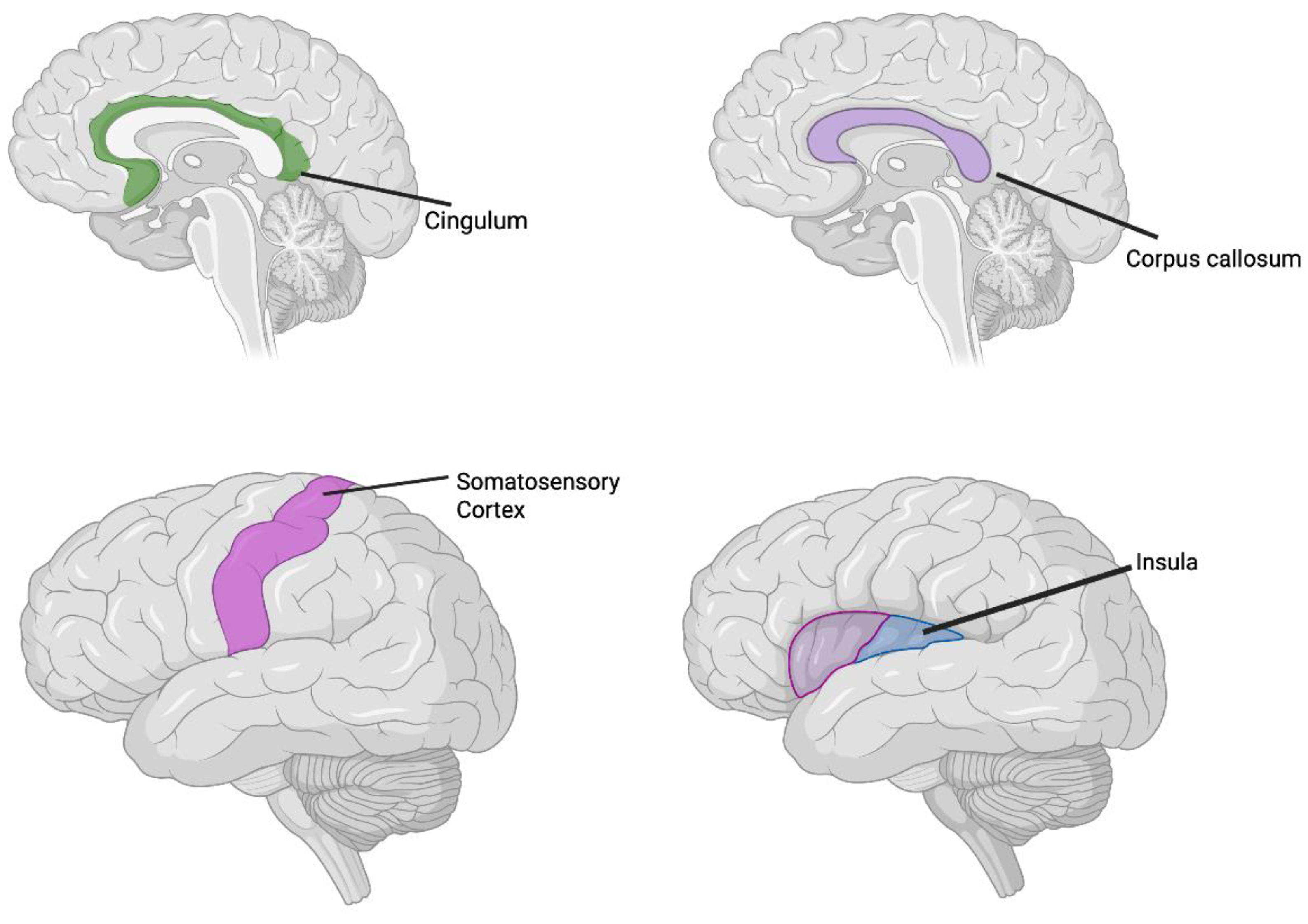
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ayres, A.J. Tactile functions: their relation to hyperactive and perceptual motor behaviour. Am J Occup Ther. 1964, 18, 83–95. [Google Scholar]
- Adra, N.; Cao, A.; Makris, N.; Valera, E.M. Sensory Modulation Disorder and its Neural Circuitry in Adults with ADHD: A Pilot Study. Brain Imaging Behav. 2021, 15, 930–940. [Google Scholar] [CrossRef]
- Albajara Sáenz, A.; Villemonteix, T.; Massat, I. Structural and functional neuroimaging in attention-deficit/hyperactivity disorder. Dev Med Child Neurol. 2019, 61, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Rubia, K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012, 51, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory. Science. 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Baddeley, A. Is working memory still working? Eur. Psychol. 2002, 7, 85–97. [Google Scholar] [CrossRef]
- Baranek, G.T.; Berkson, G. Tactile defensiveness in children with developmental disabilities: responsiveness and habituation. J Autism Dev Disord. 1994, 24, 457–471. [Google Scholar] [CrossRef]
- Bijlenga, D.; Tjon-Ka-Jie, J.Y.M.; Schuijers, F.; et al. Atypical sensory profiles as core features of adult ADHD, irrespective of autistic symptoms. Eur Psychiatry. 2017, 43, 51–57. [Google Scholar] [CrossRef]
- Björnsdotter, M.; Morrison, I.; Olausson, H. Feeling good: on the role of C fiber mediated touch in interoception. Exp Brain Res. 2010, 207, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Brotman, M.A.; Rich, B.A.; Guyer, A.E.; Lunsford, J.R.; Horsey, S.E.; Reising, M.M.; Leibenluft, E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010, 167, 61–69. [Google Scholar] [CrossRef]
- Cascio, C.J. Somatosensory processing in neurodevelopmental disorders. J. Neurodev. Disord. 2010, 2, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Fekete, T.; Siciliano, F.; et al. Neural correlates of aggression in medication-naive children with ADHD: multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 2015, 40, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, J.Y.; Lee, S.H. Bottom-up and top-down modulation of multisensory integration. Curr Opin Neurobiol. 2018, 52, 115–122. [Google Scholar] [CrossRef]
- Cortese, S. The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol. 2012, 16, 422–433. [Google Scholar] [CrossRef]
- Cubillo, A.; Halari, R.; Smith, A.; Taylor, E.; Rubia, K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012, 48, 194–215. [Google Scholar] [CrossRef]
- Cupertino, R.B.; Soheili-Nezhad, S.; Grevet, E.H.; Bandeira, C.E.; Picon, F.A.; Tavares, M.E.A.; Naaijen, J.; van Rooij, D.; Akkermans, S.; Vitola, E.S.; Zwiers, M.P.; Rovaris, D.L.; Hoekstra, P.J.; Breda, V.; Oosterlaan, J.; Hartman, C.A.; Beckmann, C.F.; Buitelaar, J.K.; Franke, B.; Bau, C.H.D.; Sprooten, E. Reduced fronto-striatal volume in attention-deficit/hyperactivity disorder in two cohorts across the lifespan. Neuroimage Clin. 2020, 28, 102403. [Google Scholar] [CrossRef]
- Zald, D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003, 41, 88–123. [Google Scholar] [CrossRef] [PubMed]
- Dhamala, M.; Assisi, C.G.; Jirsa, V.K.; et al. Multisensory integration for timing engages different brain networks. Neuroimage. 2007, 34, 764–773. [Google Scholar] [CrossRef]
- Fast, C.D.; McGann, J.P. Amygdalar Gating of Early Sensory Processing through Interactions with Locus Coeruleus. J Neurosci. 2017, 37, 3085–3101. [Google Scholar] [CrossRef]
- Fateh, A.A.; Huang, W.; Mo, T.; Wang, X.; Luo, Y.; Yang, B.; Smahi, A.; Fang, D.; Zhang, L.; Meng, X.; Zeng, H. Abnormal Insular Dynamic Functional Connectivity and Its Relation to Social Dysfunctioning in Children With Attention Deficit/Hyperactivity Disorder. Front. Neurosci. 2022, 16, 890596. [Google Scholar] [CrossRef]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; Kuntsi, J.; Larsson, H.; Lesch, K.P.; Ramos-Quiroga, J.A.; Réthelyi, J.M.; Ribases, M.; Reif, A. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; Stauber, J.; Schaaff, N.; Koutsouleris, N.; Scheuerecker, J.; Ewers, M.; et al. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr Scand. 2010, 121, 111–118. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Sensory processing problems in children with ADHD, a systematic review. Psychiatry Investig. 2011, 8, 89–94. [Google Scholar] [CrossRef]
- Gillberg, C.; Gillberg, I.C.; Rasmussen, P.; Kadesjö, B.; Söderström, H.; Råstam, M.; Johnson, M.; Rothenberger, A.; Niklasson, L. Co-existing disorders in ADHD - implications for diagnosis and intervention. Eur. Child Adolesc. Psychiatry. 2004, 13, I80–I92. [Google Scholar] [CrossRef]
- Grandi, L.C. From Sweeping to the Caress: Similarities and Discrepancies between Human and Non-Human Primates’ Pleasant Touch. Front. Psychol. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Luo, X.; Li, B.; Chang, Q.; Sun, L.; Song, Y. Abnormal modulation of theta oscillations in children with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2020, 27, 102314. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Luo, X.S.; Wang, E.C.; Li, B.K.; Chang, Q.Y.; Sun, L.; Song, Y. Abnormal alpha modulation in response to human eye gaze predicts inattention severity in children with ADHD. Dev. Cogn. Neurosci. 2019, 38, 100671. [Google Scholar] [CrossRef]
- Güven, A.; et al. Combining functional near-infrared spectroscopy and EEG measurements for the diagnosis of attention-deficit hyperactivity disorder. Neural Comput. Appl. 2020, 32, 8367–8380. [Google Scholar] [CrossRef]
- Happel Max, F.K.; Hechavarria Julio, C.; Livia, H. Editorial: Cortical-Subcortical Loops in Sensory Processing. Frontiers in Neural Circuits. 2022, 16, 851612. [Google Scholar] [CrossRef]
- Hern, K.L.; Hynd, G.W. Clinical differentiation of the attention deficit disorder subtypes: do sensorimotor deficits characterize children with ADD/WO? Arch Clin Neuropsychol. 1992, 7, 77–83. [Google Scholar] [CrossRef]
- Herpertz, S.C.; Huebner, T.; Marx, I.; Vloet, T.D.; Fink, G.R.; Stoecker, T.; Herpertz-Dahlmann, B. Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry. 2008, 49, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.T.; Xu, J.; Wang, X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Research Reviews. 2002, 39, 181–215. [Google Scholar] [CrossRef]
- Jasper, H.; Penfield, W. Electrocorticograms in man: Effect of voluntary movement upon the electrical activity of the precentral gyrus. Archiv Für Psychiatrie Und Nervenkrankheiten. 1949, 183, 163–174. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.E.; Chidekel, D. Sensory integration, sensory processing, and sensory modulation disorders: putative functional neuroanatomic underpinnings. Cerebellum. 2011, 10, 770–792. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D. ADHD and Sensory Processing Disorders: Placing the Diagnostic Issues in Context. Applied Neuropsychology: Child. 2012, 1, 137–144. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef]
- Lenartowicz, A.; Lu, S.; Rodriguez, C.; Lau, E.P.; Walshaw, P.D.; McCracken, J.T.; Cohen, M.S.; Loo, S.K. Alpha desynchronization and fronto-parietal connectivity during spatial working memory encoding deficits in ADHD: A simultaneous EEG-fMRI study. Neuroimage Clin. 2016, 11, 210–223. [Google Scholar] [CrossRef]
- Lopez-Larson, M.P.; King, J.B.; Terry, J.; McGlade, E.C.; Yurgelun-Todd, D. Reduced insular volume in attention deficit hyperactivity disorder. Psychiatry Res. 2012, 204, 32–39. [Google Scholar] [CrossRef]
- Jurek, L.; Duchier, A.; Gauld, C.; Hénaault, L.; Giroudon, C.; Fourneret, P.; Cortese, S.; Nourredine, M. Sensory Processing in Individuals With Attention-Deficit/Hyperactivity Disorder Compared With Control Populations: A Systematic Review and Meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry. [CrossRef]
- Luman, M.; Oosterlaan, J.; Sergeant, J. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clin. Psychol. Rev. 2005, 25, 183–213. [Google Scholar] [CrossRef]
- MacAluso, E.; Noppeney, U.; Talsma, D.; et al. The curious incident of attention in multisensory integration: bottom-up vs. top-down. Multisens Res. 2016, 29, 557–583. [Google Scholar] [CrossRef]
- Marsh, A.A.; Finger, E.C.; Mitchell, D.G.V.; Reid, M.E.; Sims, C.; Kosson, D.S.; Blair, R.J.R. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008, 165, 712–720. [Google Scholar] [CrossRef]
- Marshall, A.C.; Gentsch-Ebrahimzadeh, A.; Schütz-Bosbach, S. From the inside out: Interoceptive feedback facilitates the integration of visceral signals for efficient sensory processing. NeuroImage. 2022, 251, 119011. [Google Scholar] [CrossRef]
- Mason, D.J.; Humphreys, G.W.; Kent, L. Insights into the control of attentional set in ADHD using the attentional blink paradigm. J Child Psychol Psychiatry. 2005, 46, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Mufson, E.J. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology. 1982, 212, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Nielsen, D.M.; Schoen, S.A.; Brett-Green, B.A. Perspectives on sensory processing disorder: a call for translational research. Front Integr Neurosci. 2009, 3, 22. [Google Scholar] [CrossRef]
- Morrison, I.; Björnsdotter, M.; Olausson, H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci. 2011, 31, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Mesulam, M.M.; Pandya, D.N. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981, 6, 1231–1248. [Google Scholar] [CrossRef]
- Norman, L.J.; Carlisi, C.; Lukito, S.; et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016, 73, 815–825. [Google Scholar] [CrossRef]
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.G.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, Å.B.; Bushnell, M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002, 5, 900–904. [Google Scholar] [CrossRef]
- Dubljević, O.; Pavković, Ž.; Srbovan, M.; Potrebić, M.; Stanojlović, M.; Pešić, V. Attention-deficit/hyperactivity disorder-related psychomotor activity and altered neuronal activity in the medial prefrontal cortex and striatum in the A53T mouse model of Parkinson's disease and other synucleinopathies: Findings from an “endophenotype” approach. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2025, 137, 111273. [Google Scholar] [CrossRef]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Parush, S.; Sohmer, H.; Steinberg, A.; Kaitz, M. Somatosensory function in boys with ADHD and tactile defensiveness. Physiol Behav. 2007, 90, 553–558. [Google Scholar] [CrossRef]
- Passarello, N.; Tarantino, V.; Chirico, A.; Menghini, D.; Costanzo, F.; Sorrentino, P.; Fucà, E.; Gigliotta, O.; Alivernini, F.; Oliveri, M.; Lucidi, F.; Vicari, S.; Mandolesi, L.; Turriziani, P. Sensory Processing Disorders in Children and Adolescents: Taking Stock of Assessment and Novel Therapeutic Tools. Brain Sci. 2022, 12, 1478. [Google Scholar] [CrossRef]
- Perlov, E.; Philipsen, A.; van Elst, L.T.; Ebert, D.; Henning, J.; Maier, S.; et al. Hippocampus and amygdala morphology in adults with attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2008, 33, 509–515. [Google Scholar] [CrossRef]
- Pevzner, A.; Izadi, A.; Lee, D.J.; Shahlaie, K.; Gurkoff, G.G. Making waves in the brain: what are oscillations, and why modulating them makes sense for brain injury. Front Syst Neurosci. 2016, 10, 30. [Google Scholar] [CrossRef]
- Piccardi, E.S.; Begum Ali, J.; Jones, E.J.H.; et al. Behavioural and neural markers of tactile sensory processing in infants at elevated likelihood of autism spectrum disorder and/or attention deficit hyperactivity disorder. J Neurodevelop Disord. 2021, 13, 1. [Google Scholar] [CrossRef]
- Plessen, K.J.; Bansal, R.; Zhu, H.; Whiteman, R.; Amat, J.; Quackenbush, G.A.; et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006, 63, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.; De Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Rach, S.; Diederich, A.; Colonius, H. On quantifying multisensory interaction effects in reaction time and detection rate. Psychol Res. 2011, 75, 77–94. [Google Scholar] [CrossRef]
- Schneider, M.L.; Moore, C.F.; Ahlers, E.O.; Barnhart, T.E.; Christian, B.T.; DeJesus, O.T.; Converse, A.K. PET Measures of Dl, D2, and DAT Binding Are Associated with Heightened Tactile Responsivity in Rhesus Macaques: Implications for Sensory Processing Disorder. Front Integr Neurosci. 2019, 13, 29. [Google Scholar] [CrossRef]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef]
- Schulze, M.; Aslan, B.; Stöcker, T.; Stirnberg, R.; Lux, S.; Philipsen, A. Disentangling early versus late audiovisual integration in adult ADHD: a combined behavioural and resting-state connectivity study. J Psychiatry Neurosci. 2021, 46, E528–E537. [Google Scholar] [CrossRef]
- Smirni, D.; Smirni, P.; Carotenuto, M.; Parisi, L.; Quatrosi, G.; Roccella, M. Noli Me Tangere: Social Touch, Tactile Defensiveness, and Communication in Neurodevelopmental Disorders. Brain Sci. 2019, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.L.; Wiedholz, L.M.; Bassett, D.S.; et al. A validated network of effective amygdala connectivity. Neuroimage. 2007, 36, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.C.; Haney-Caron, E. Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. J Psychiatry Neurosci. 2012, 37, 389–398. [Google Scholar] [CrossRef] [PubMed]
- ter Huurne, N.; Lozano-Soldevilla, D.; Onnink, M.; Kan, C.; Buitelaar, J.; Jensen, O. Diminished modulation of preparatory sensorimotor mu rhythm predicts attention-deficit/hyperactivity disorder severity. Psychological Medicine. 2017, 47, 1947–1956. [Google Scholar] [CrossRef]
- ter Huurne, N.; Onnink, M.; Kan, C.; Franke, B.; Buitelaar, J.; Jensen, O. Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013, 74, 227–233. [Google Scholar] [CrossRef]
- Turner, B.H.; Herkenham, M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol. 1991, 313, 295–325. [Google Scholar] [CrossRef]
- Vassalli, A.; Dellepiane, J.M.; Emmenegger, Y.; Jimenez, S.; Vandi, S.; Plazzi, G.; Franken, P.; Tafti, M. Electroencephalogram paroxysmal θ characterizes cataplexy in mice and children. Brain. 2013, 136, 1592–1608. [Google Scholar] [CrossRef]
- Vassalli, A.; Franken, P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc Natl Acad Sci U S A. 2017, 114, E5464–E5473. [Google Scholar] [CrossRef]
- Villemonteix, T.; De Brito, S.A.; Kavec, M.; Baleriaux, D.; Metens, T.; Slama, H.; et al. Grey matter volumes in treatment naive vs. chronically treated children with attention deficit/hyperactivity disorder: A combined approach. Eur Neuropsychopharmacol. 2015, 25, 1118–1127. [Google Scholar] [CrossRef]
- Vollebregt, M.A.; Zumer, J.M.; ter Huurne, N.; Buitelaar, J.K.; Jensen, O. Posterior alpha oscillations reflect attentional problems in boys with attention deficit hyperactivity disorder. Clin. Neurophysiol. 2016, 127, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Völter, C.; Thomas, J.P.; Maetzler, W.; Guthoff, R.; Grunwald, M.; Hummel, T. Sensory Dysfunction in Old Age. Dtsch Arztebl Int. 2021, 118, 512–520. [Google Scholar] [CrossRef]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Wing, L. The handicaps of autistic children: A comparative study. Journal of Child Psychology and Psychiatry. 1969, 10, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Owen, J.P.; Pojman, N.J.; Thieu, T.; Bukshpun, P.; Wakahiro, M.L.; Berman, J.I.; Roberts, T.P.; Nagarajan, S.S.; Sherr, E.H. White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PLoS One. 2015, 10, e0123656. [Google Scholar] [CrossRef] [PubMed]
- Yochman, A.; Parush, S.; Ornoy, A. Responses of preschool children with and without ADHD to sensory events in daily life. Am J Occup Ther. 2004, 58, 294–302. [Google Scholar] [CrossRef]
- Zaher, A.; Leonards, J.; Reif, A.; et al. Functional connectivity of the nucleus accumbens predicts clinical course in medication adherent and non-adherent adult ADHD. Sci Rep. 2025, 15, 19663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




