3.1. Description
Systematics
Family: Pterygosomatidae Oudemans, 1910
Genus: Geckobia Mégnin, 1878
Species group
latasti sensu Fajfer [
5] (Jack’s group I [
9])
Geckobia inermis sp. nov.
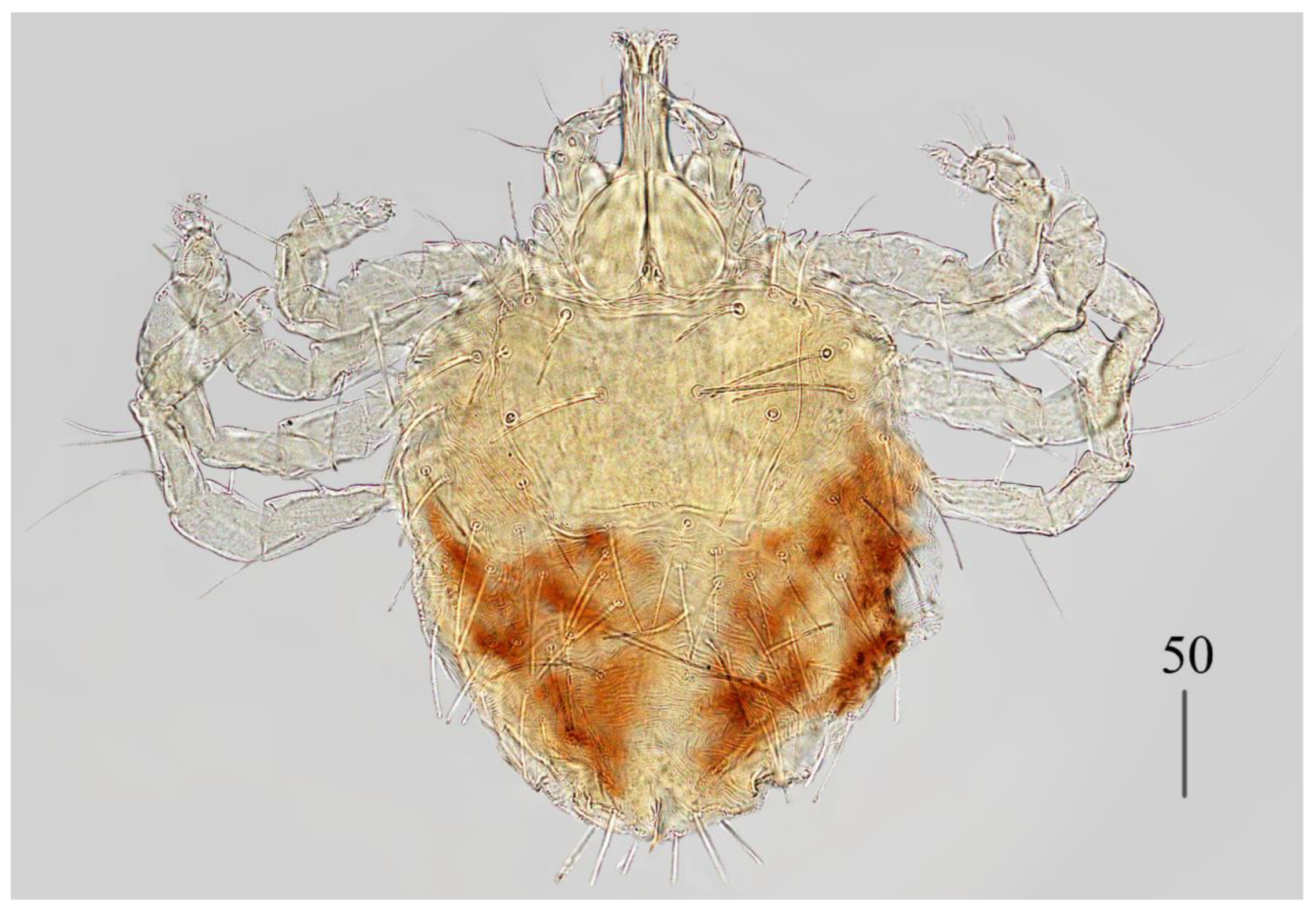
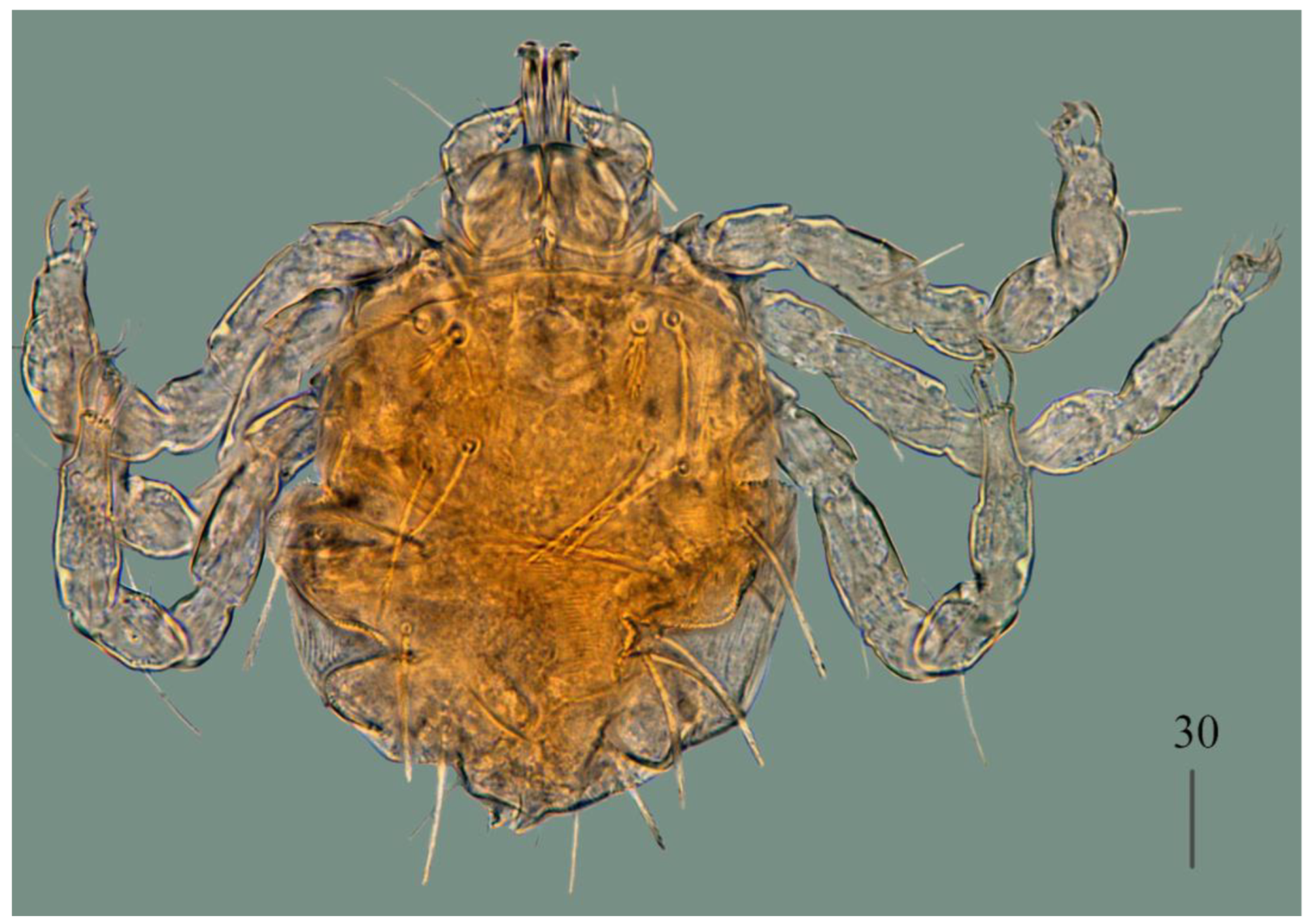
Description. Female (holotype, range for paratype) (
Figure 1 and
Figure 2).
Gnathosoma. Chelicerae 185 (220) long. Swollen, proximal part of cheliceral base 80 (110) long and slender distal part 105 (110) long. Movable cheliceral digit three-pronged, fixed cheliceral digit spinous, and approximately 15 (15) long. Palpal femur with filiform smooth or with barely discernible serration seta
dF 80 (40) long; palpal genu with filiform smooth seta
dG, 85 (80) long. Palpal tibia with three smooth setae (
dTi,
l’Ti and
l”Ti) and slender curved claw. Palpal tarsi with four smooth setae. Subcapitular seta
n filiform and smooth, 70 (70) long. Each branch of peritremes with barely visible chambers 100 (135) long. Hypostome with three-pronged apex.
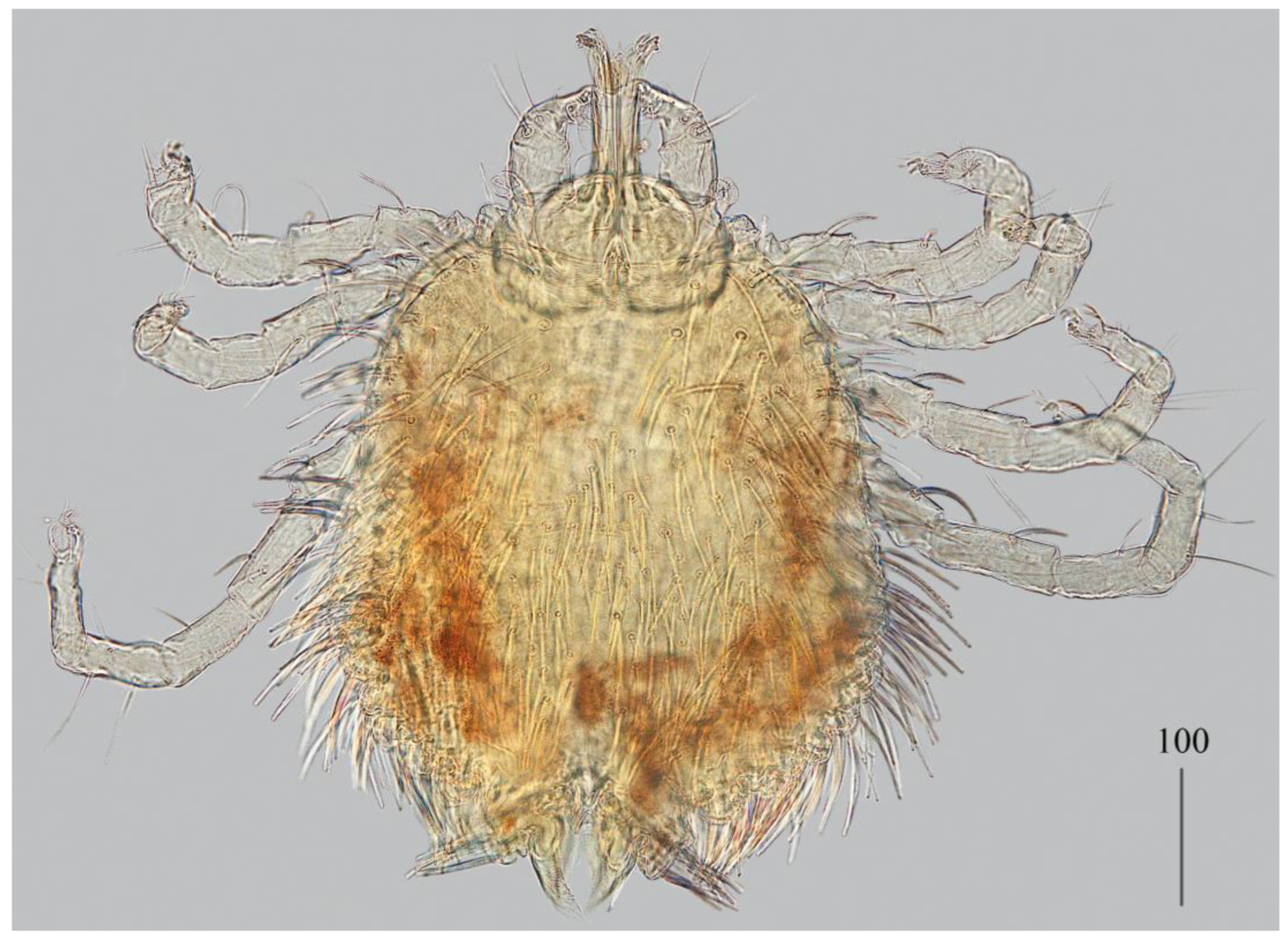
Idiosoma 400 (420) wide long and 460 (450) long. Dorsum (
Figure 1). Propodonotal shield smooth and well outlined, slightly concave in anterior and posterior part, 250 (255) wide and 85 (110) long in middle part. On propodonotal shield 13 (13) pairs of very slightly plumose thick and blunt-pointed setae, 50–70 long. One setae situate antero-laterally shorter, about 25 (30) long. Posteriorly and laterally to propodonotal shield, numerous setae resembling setae situated on propodonotal shield, 50–75 long. Eyes absent.
Venter. Anterior part with 1–2 rows of filiform smooth setae, about 30 long, below 4 rows of slightly plumose thicker and tapered setae, 35–40 long. Anteromedial part of idiosoma with plumose setae. Posterior half of idiosoma with lanceolate setae. These setae about 40 (40) long and 15 (15) wide. Most posterior peripheral setae more elongated and narrower than setae in medial part (50 long and 10 wide). Genital region. Genital setae represented by four pairs of slender blunt pointed setae g1–g4. Setae g1 and g2 about 20 long, g3 about 10 long and g4 about 30 long. Pseudanal series represented by 11 pairs of blunt-pointed smooth and flattened setae ps1–ps11, about 50 (40–55) long. Legs. Coxal setation: 1a, 1b, 2a, 2b, 3a, 3b, 3c, 3d, 4a, 4b and 4c arranged in formula: 2–2–4–3. Setae 1a, 1b, filiform and smooth, 2a and 3d serrate, 2b thick, short and plumose, 3a, 3b, 3c, 4a, 4b thick, slightly plumose, tapered and resembling those on venter. Two plumose setae present between coxal plates I and II. Leg chaetotaxy as follows: tibiae I–IV (5–5–5–5), genua I–IV (1–0–0–1), femora I–IV (3–2–2–2) and trochanters I–IV (1–1–1–1). Setae dTiI–IV, ld’TiI, ld”TiIV, v’TiI–IV, v”TiI–IV, ldGI, ld GIV, dl”FI–FIV, vFI–IV filiform and smooth, vTrI-IV , dl’FI serrate. Setation of tarsi I: 14 setae (ft, tc’, tc”, p’, p”, a’, a”, it’, it”, u’, u”, vs’, vs” and pl’) and solenidion w1; tarsi II: 10 setae (tc’, tc”, p’, p”, a’, a”, u’, u”, vs’ and vs”) and w1; tarsi III and IV with 10 setae each (tc’, tc”, p’, p”, a’, a”, u’, u”, vs’ and vs”). Solenidion w1 (about 25 long) longer than seta ft (about 5 long). Setae tc’, tc”, it’ and it” of leg I represented by euphatidia; tc’ and tc” of legs II–IV, u’, u”, vs’, vs”, a’, a” and pl’ of legs I–IV filiform.
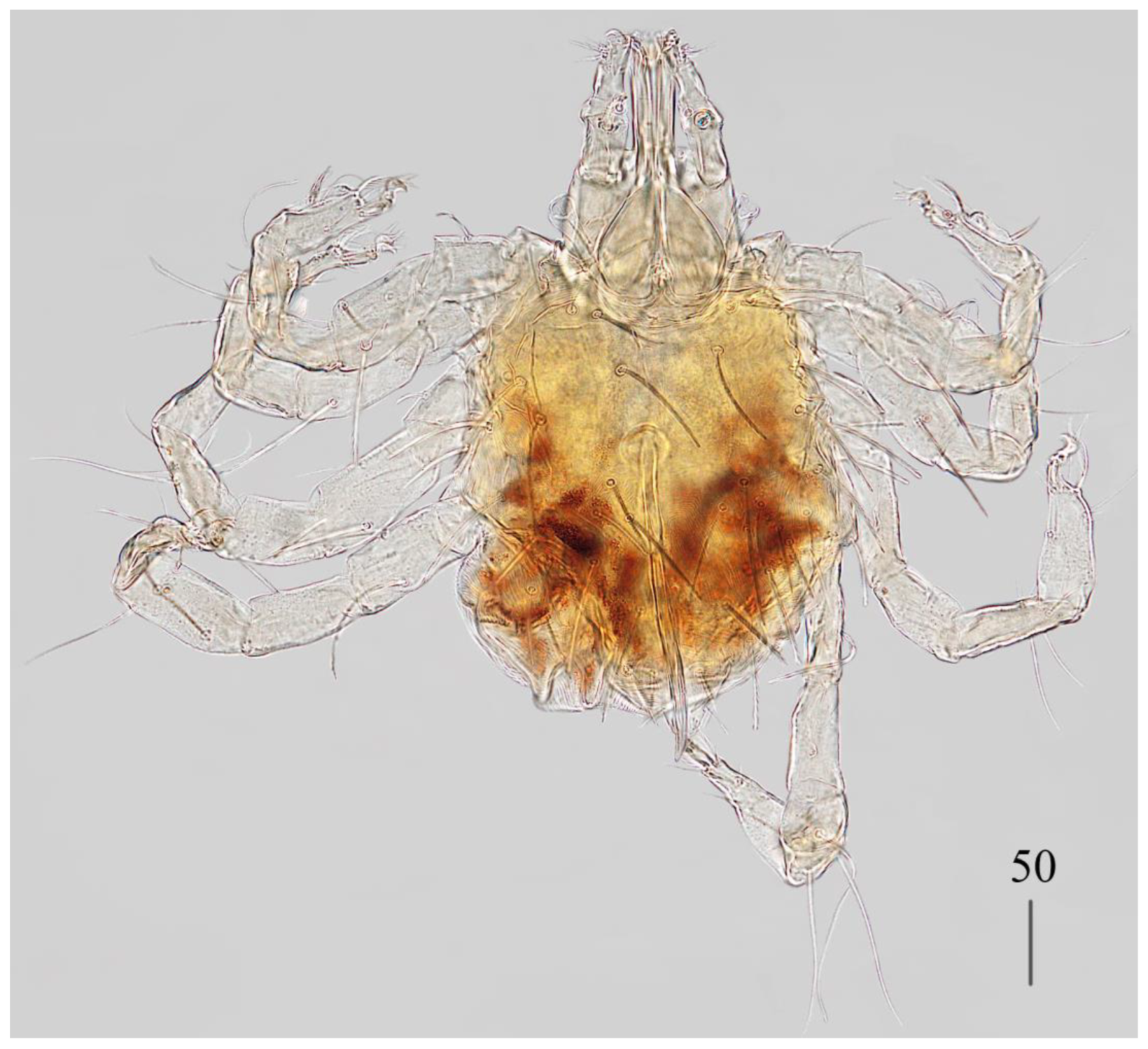
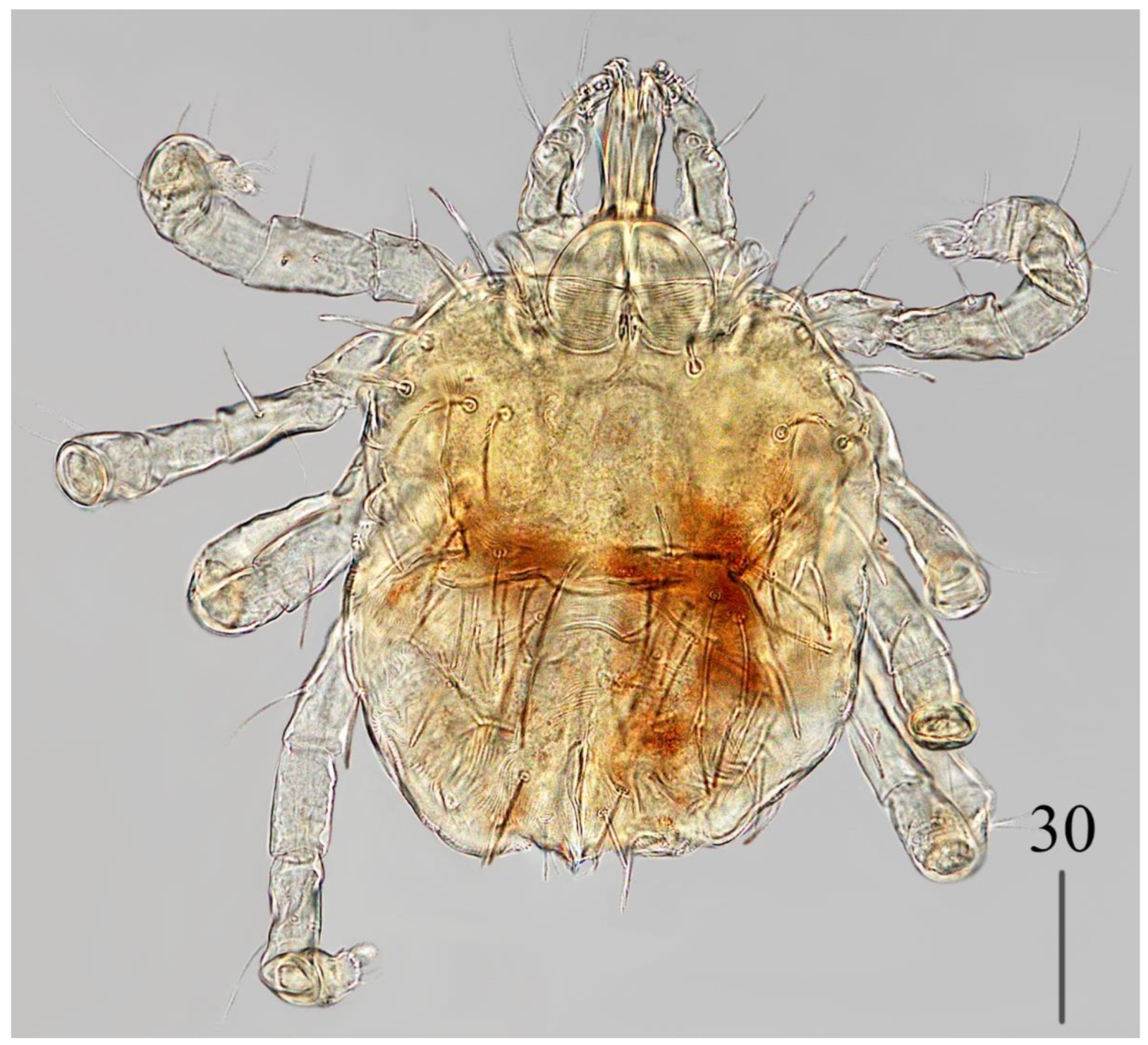
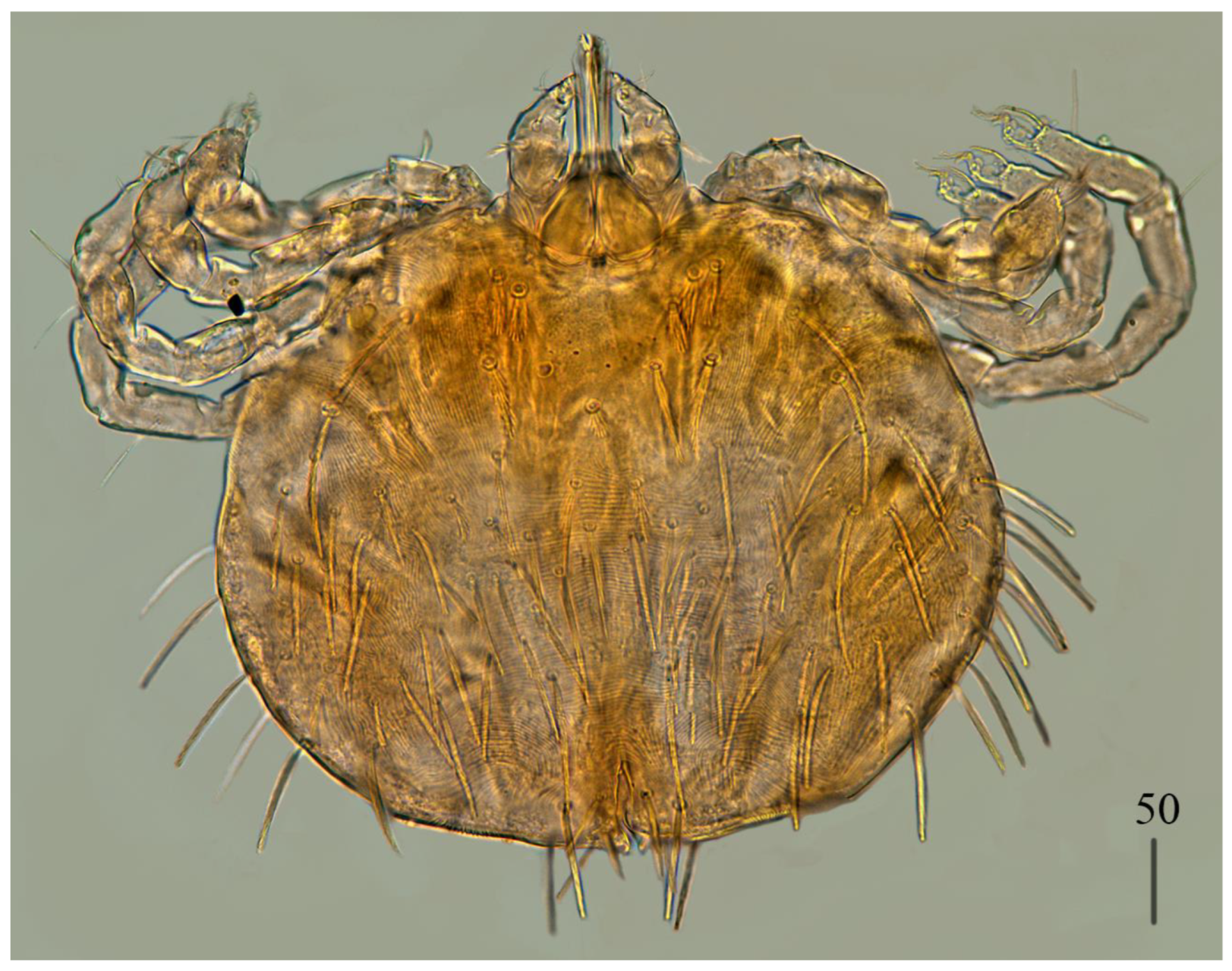
Male (
Figure 3).
Gnathosoma as in female. Chelicerae about 145 long; slender cheliceral part 80 long, swollen basal part 65 long. Fixed cheliceral digit about 10 long. Setae
dF thick, serrate, about 20 long; setae
dG filiform, smooth, about 50 long. Subcapitular seta
n 35–40 long. Each branch of peritremes about 100 long. Hypostome with ornamented apex.
Idiosoma 215 (200–255) wide, 265 (260–295) long. Dorsum with propodonotal shield 80 long, 155 wide, accompanied by ocular plate on lateral margins. On propodonotal shield 7 pairs of serrate setae: 4 pairs situated antero-laterally (30–60 long), 1 pair medially (60 long), 2 pairs postero-laterally (including 1 on ocular plate), and 3 pairs of longer setae 35–40 long. Medial and posterior part with about 22 pairs of serrate setae, 30–50 long. Aedeagus 185 long. Genital cone with 2 filiform setae 35 and 10 long situated dorsally, and one filiform seta situated ventrally, 25 long. Venter with 5 pairs of setae (about 45 long) situated medially.
Legs. Coxae in formula: 2–2–2–2. Setae
1a,
1b,
2a,
2b filiform and smooth; setae
3a,
3b,
4a and
4b serrate. Setae of tibiae–trochanters I–IV as in female.
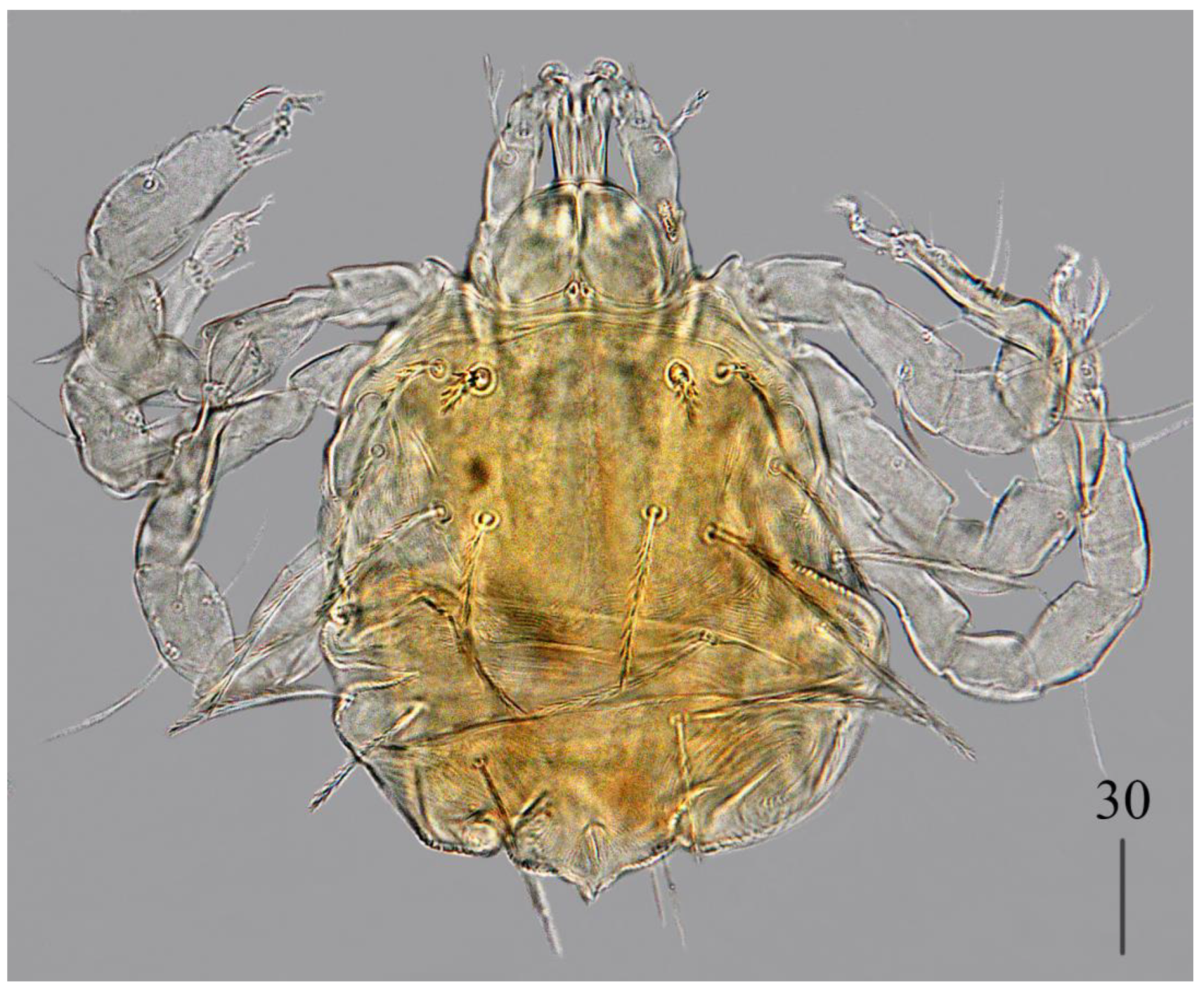
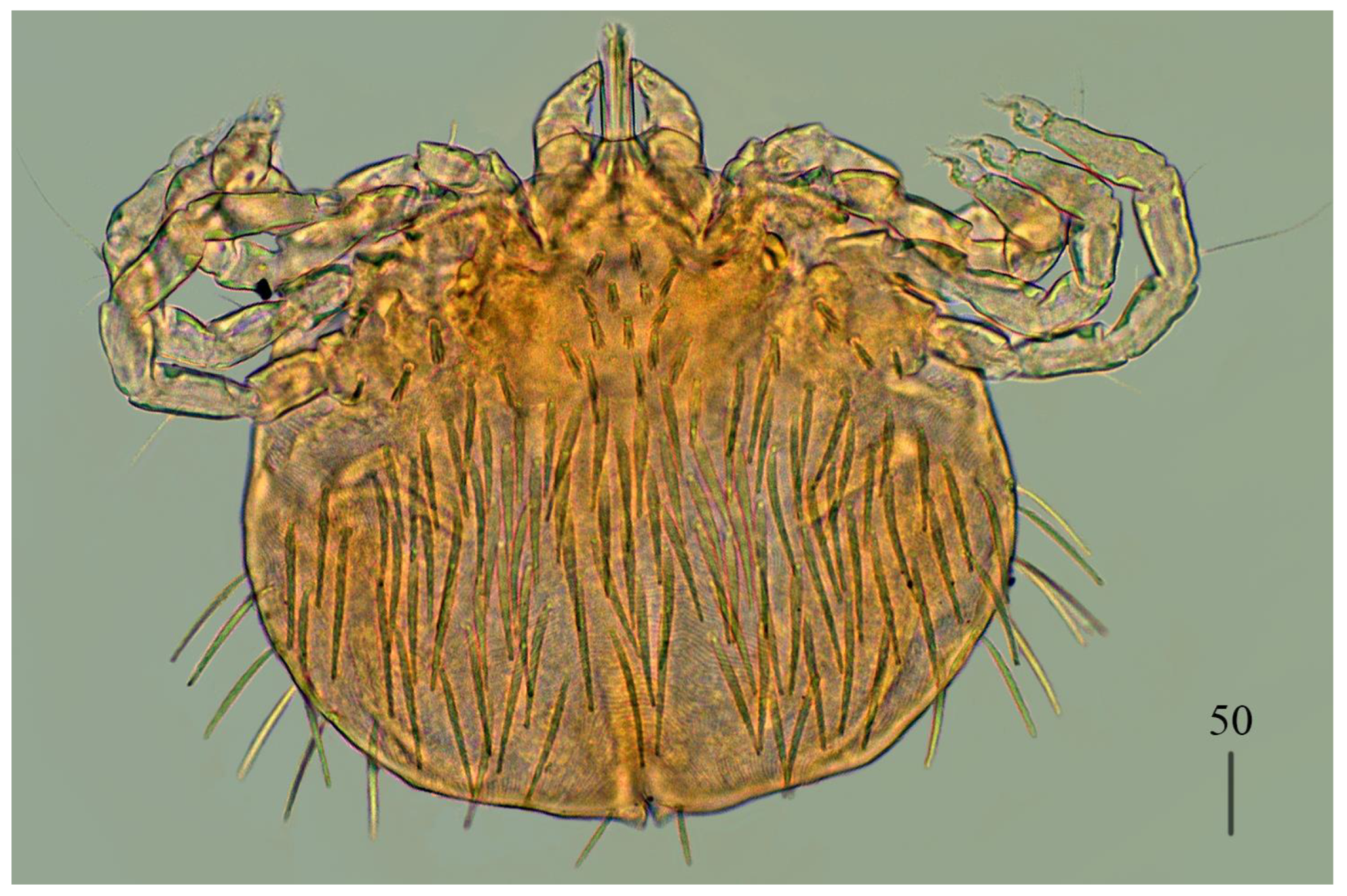
Deutonymph (
Figure 4).
Gnathosoma as in female. Chelicerae 110–130 long; swollen cheliceral part and slender distal part about 60 long. Fixed cheliceral digit 10 long. Setae
dF slightly serrate, 60 long; setae
dG filiform, smooth, 50 long. Subcapitular setae
n about 45 long. Each branch of peritremes about 90 long.
Idiosoma 250–275 long and 175–234 wide. Dorsum. Propodonotal shield about 90 long and 140 wide, with 5–6 pairs of slightly serrate setae: 3 pairs situated antero-laterally (35–60 long), 1 pair medially (55–60 long), 1 pair medio-laterally (about 60 long). Laterally to propodonotal shield an eye on oval ocular plate (20 wide and 25 long) with associated serrate seta (60 long). About 25 pairs of serrate setae (35–60 long) situated in lateral and medial part of idiosoma. Venter with 12–13 shorter serrate setae (15–25 long) in antero-medial part, and 25 longer setae (35–65 long) in medial part. Coxae in formula: 2–2–2–2. Setae
1a,
1b filiform; setae
2a,
2b,
3a,
3b,
4a,
4b serrate.
Legs. Setae of trochanters-tarsi I–IV as in female. Genital area with 2 pseudanal setae
ps1 and
ps2 with barely visible serration and fine-pointed setae
g1–g3. Setae
ps1 30–35 long, setae
ps2 25 long; setae
g1–g3 about 15 long.
Protonymph (
Figure 5).
Gnathosoma as in female. Swollen cheliceral part 35 long, slender distal part 30 long. Setae
dF slightly serrate and 55 long, setae
dG smooth and 55–60 long. Each branch of peritremes 65 long.
Idiosoma 210–220 long and 190–200 wide. Dorsum with densely serrate setae. Propodonotal shield 125 wide and 95 long with 4 setae present on shield: 2 short setae situated anteriorly (about 25 long) and 2 longer setae situated medially (60 long). Laterally to propodonotal shield eye on ocular plate present with associated serrated seta (65 long). In medial part numerous setae, 35–45 long, present. Venter with tapered setae 35–40 long. In anterior part about 6 short setae (20–25 long), in medial part numerous longer setae (30–40 long). Genital setae
g1 with barely discernible serration,
g2–g3 smooth, setae
g1–
g3 15–20 long. Pseudanal setae
ps1 and
ps2 slightly serrate and 30–35 long.
Legs as in female.
Larva (
Figure 6).
Gnathosoma as in female. Chelicerae about 55–60 long; slender cheliceral part 30–40 long, swollen distal part about 30–35 long. Setae
dF 25 long, setae
dG 30 long. Peritremes about 30 long. Subcapitular setae
n absent.
Idiosoma 125–230 long and 115–220 wide. Dorsum. Propodonotal shield 65 long, 80 wide; bearing one pair of short densely serrate setae, 15 long, and three pairs of longer serrate setae, 35 long. Laterally to propodonotal shield eyes on oval ocular plates (15 wide, 25 long) with one associated serrate seta (35 long) present. Posteriorly to propodonotal shield six pairs of serrate setae, about 35–45 long. Venter devoid of any setation. Genital area with three filiform genital setae
g1–g3, about 10 long, and two slightly serrate pseudanal setae
ps1–ps2 20 long. Coxae in formula: 2–0–1. Setae
1a,
1b filiform;
3a short, densely serrate. Setation of trochanters-tarsi I–III as in female and typical for pterygosomatid larva (Figure 5 and Table 2 in [
30]).
Differential diagnosis. This new species is most similar to
Geckobia bochkovi Fajfer, 2023, described from
Ptyodactylus guttatus Heyden (Phyllodactylidae) in Israel [
19]. Both species share the presence of a well-defined propodonotal shield, slightly serrate dorsal setae, lanceolate ventral setae, and a comparable leg chaetotaxy. However, the new species differs from
G. bochkovi in several key morphological traits: the propodonotal shield lacks anterior and posterior concavity; the dorsal setae are uniform in size; the anterior part of the ventral surface bears 1–2 rows of filiform smooth setae; seta
2a is serrate; coxal setae
3c and
3d are present; pseudanal series are represented by 11 pairs of blunt-pointed, smooth, flattened setae
ps1–ps11 and eyes are absent. In contrast, in
G. bochkovi, the propodonotal shield is concave both anteriorly and posteriorly; the dorsal setae are fine-pointed and slightly increase in length posteriorly; coxal seta
2a is smooth and filiform; coxal setae
3c and
3d are absent; setae
ps1–
ps12 are slightly lanceolate with minute serration and tapered at tips ,and eyes are present.
Type material. Holotype female and 1 female paratype, 2 males, 2 nymphs, 4 larvae from Ptyodactylus puiseuxi Boutan, 1893 (tympanum) (HUJ no. 18522), Israel: Northern District: Golan: Nahal, 6 May 1987, coll. Wered Werner.
Type material deposition. Holotype female, male, deutonymph and protonymph, 2 larvae in the HUJ (HUJINV-Acari_Pte00003.1–7), female paratype and 2 larvae in the CSWU (CSWU–Pte0019.1–3).
Etymology. This species names is derived from the name inermis which is a Latin adjective meaning “unarmed” or “without spines”, referring to the blunt-pointed, smooth pseudanal setae of this species.
Geckobia squameum Bertrand, Finkelman and Paperna, 2000
Geckobia squameum Bertrand, Finkelman and Paperna, 2000: 294, Figures 42−47
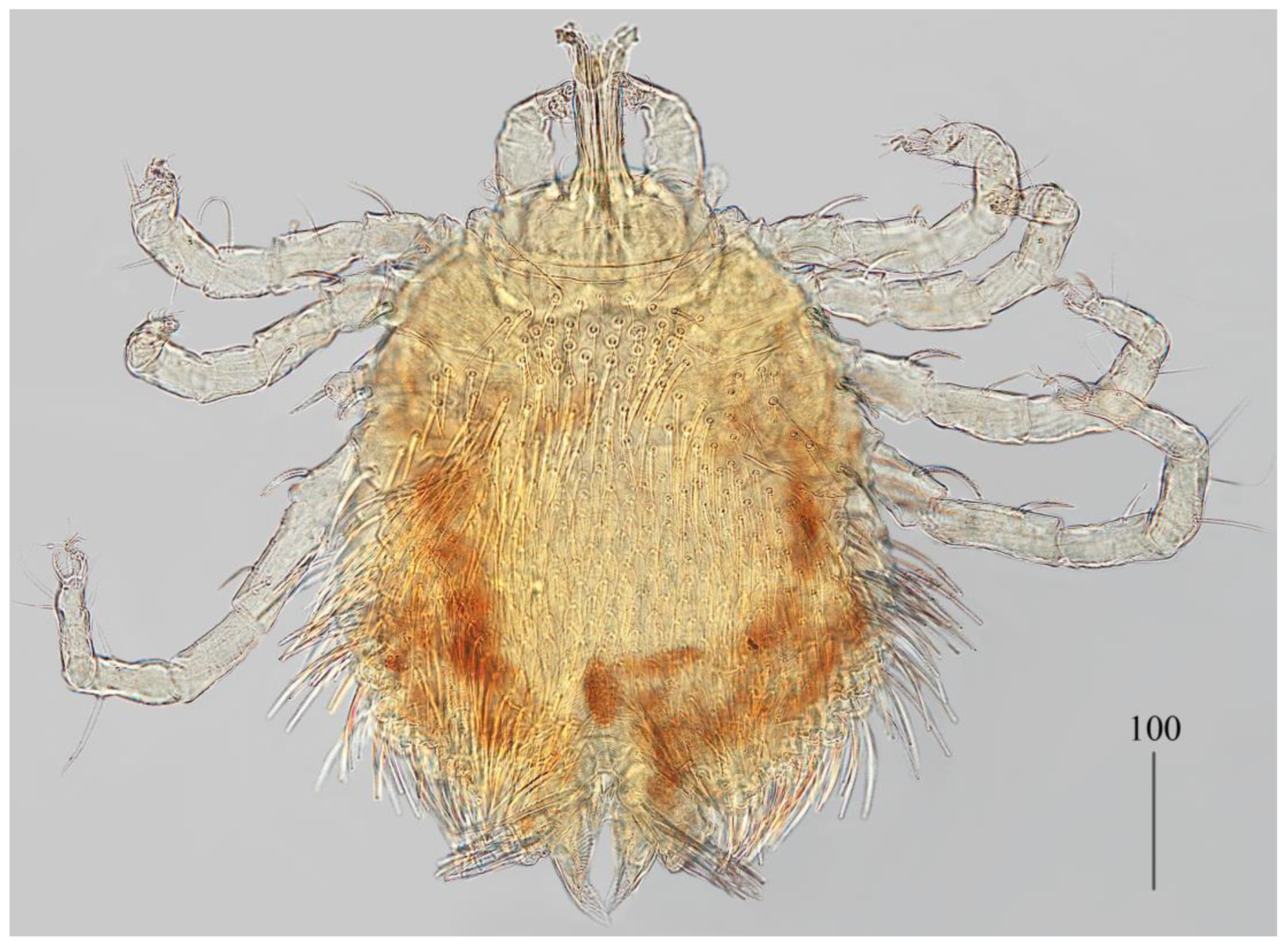
Male (
Figure 7).
Gnathosoma as in female. Chelicerae 70 long; slender cheliceral part about 40 long, swollen basal part 30 long. Fixed cheliceral digit about 5 long. Setae
dF thick, serrate, about 15 long; setae
dG filiform, smooth, about 35 long. Subcapitular seta
n 35 long. Each branch of peritremes about 45 long. Hypostome with ornamented apex.
Idiosoma 175-190 long, 175–195 wide. Dorsum with propodonotal shield 85 long and 175 wide, accompanied by ocular plate on lateral margins. On propodonotal shield 5 pairs of serrate setae: 2 pairs situated antero-laterally (one seta shorter about 25 long, second setae about 50 long, 1 pair medially (65 long), 1 pairs postero-laterally, about 60 long, and one pair 60 long , near ocular plate. Medial and posterior part with about 23 pairs of serrate setae, 40–60 long. Aedeagus 110 long. Genital cone with one pair of setae about 25 long. Genital cone situated dorsally. Venter with 10 pairs of very slightly serrate setae (20–30 long) situated antero-medially and about 30 pairs of setae situated in posterior half of idiosoma.
Legs. Coxae in formula: 2–2–2–2. Setae
1a,
1b,
2a,
2b filiform and smooth; setae
3a,
3b,
4a and
4b serrate. Setae of tibiae–trochanters I–IV as in female.
Nymph chrysalis. Gnathosoma with visible peritremes. Idiosoma almost circular(290 wide and 280 long in one specimen, 205 long and 230 wide in second) with fully formed male inside. Dorsal and ventral side of idiosoma devoid of any setation, only coxae visible.
Non-type material. Two males from Ptyodactylus guttatus Heyden, 1827 from Israel, coll. unknown; 6 males and 2 nymph chrysalis from same host, Israel, Northern District: 1 km South West of Tubas, 01.02.1986, coll. Yaacov Pesach.
Material deposition. Eight males and 2 nymph chrysalis in the CSWU (CSWU-Pte0017.1–10).
Geckobia bochkovi Fajfer, 2023
Geckobia bochkovi Fajfer, 2023: 252, Figures 1−3
Imagochrysalis. Gnathosoma barely discernible, inserted at ventral surface of idiosoma. Peritremes with barely discernible chambers. Idiosoma 420–545 long and 375–490 wide. Only coxae I and II visible. Inside fully formed female visible.
Larva (
Figure 8).
Gnathosoma as in female. Chelicerae about 60 long; slender cheliceral part 30 long, swollen part about 30 long. Setae
dF filiform and slightly serrate, 25 long; setae
dG filiform and smooth, about 35 long. Peritremes about 35 long. Subcapitular setae
n absent.
Idiosoma 130 long and 155 wide. Dorsum. Propodonotal shield 60 long and 75 wide; well-outlined, bearing four pairs of densely serrate setae: 2 pairs situated antero-laterally (one pair longer, 40 long, one pair shorter, 20 long), and 2 pairs situated posteriorly on the shield, both about 45 long. Laterally to propodonotal shield eye on ocular plate and one seta present (40 long). In posterior half of idiosoma five pairs of posterior setae 40–50 long present. Venter devoid of any setation. Genital area with 3 filiform genital setae
g1–g3, 5–10 long, and two slightly serrate pseudanal setae
ps1–ps2 15–20 and 20–25 long. Coxae in formula: 2–0–1. Setae
1a,
1b filiform;
3a short, densely serrate. Setation of trochanters-tarsi I–IV as in female, except for presence in several specimens of
dGI; setation of legs I–III typical for larva (Figure 5 and Table 2 in [
30]).
Non-type material. One imago chrysalis from Ptyodactylus guttatus Heyden (HUJ no. 2915)(tympanum) from Israel, Haifa district, Coastal Plain: Atlit, April 1955, coll. Michael Warburg; 1 imago chrysalis from same host species (HUJ no. 2916) ( tympanum) and locality, April 1955, coll. Michael Warburg; 1 larva with from same host (HUJ no 2798) (tympanum), Israel, Haifa district: Mount Carmel above Nesher, 15.02.1955, coll. Yehudah L. Werner.
Material deposition. Two imagochrysalis, 1 larva in CSWU (CSWU-Pte0018.3–6).
Remarks. Northern Israeli specimens require host verification through DNA barcoding (see Discussion).
Species group diversipilis (sensu Jack[
9]
)
Geckobia parva sp. nov.
Female (holotype, range for paratypes) (
Figure 9 and
Figure 10).
Gnathosoma. Chelicerae 95 (75–85) long; swollen proximal part of cheliceral base 45 (35–40) long, slender distal part 50 (40–50) long. Movable cheliceral digit three-pronged; fixed cheliceral digit spinous, approximately 5 (5) long. Palpal femur with serrate seta
dF 25 (20–25) long; palpal genu with filiform smooth seta
dG 30 (25–35) long. Palpal tibia with 3 smooth setae (
dTi,
l’Ti,
l”Ti) and slender curved claw. Palpal tarsi with 3 smooth setae. Subcapitular seta
n filiform, smooth, 25 (25–30) long. Each branch of peritremes with barely visible chambers, 55 (55) long. Hypostome with three-pronged apex.
Idiosoma 275 (290–380) wide and 210 (240–340) long. Dorsum. Propodonotal shield well outlined, with minute punctuation in medial part, very slightly concave anteriorly and posteriorly, 95 (100–105) wide in anterior part, 70 (80–85) long in middle part; bearing six pairs of very slightly plumose, thick, blunt-pointed setae, 20–40 long; one seta situated medially shorter, about 10 (10–15) long. Posteriorly and laterally to propodonotal shield numerous setae (about 38 pairs), less serrate than those on shield, 20–40 long. Eyes present laterally to propodonotal shield on small plate with one serrate seta 40 long. Venter. Anterio-medial part with 4 rows (14 setae, 10–15 long) of plumose antero-median short setae (11–13 setae in paratypes); below, in posterior half of idiosoma, several rows of slightly serrate, thicker tapered setae (96 in holotype, 70– 86 in paratypes), 40–55 long.
Genital region. Genital setae represented by four pairs of slender, slightly serrate setae
g1–g4 situated dorsally;
g1,
g2 about 20 (25) long,
g3–g4 about 10 long. Pseudanal series represented by 3 pairs of blunt-pointed, flattened, slightly serrate setae
ps1–ps3, about 40, 35 and 25 long, respectively;
ps1, and
ps2 situated dorsally,
ps3 ventrally.
Legs. Coxal setation: 1a, 1b, 2a, 2b, 3a, 3b, 3c, 3d, 4a, 4b, 4c arranged in formula: 2–2–4–3. Setae 1a, 1b filiform, smooth; 2a, 2b, 3a, 3b, 4a, 4b thick, plumose. One short plumose seta present between coxal plates II and antero-median setae. Leg chaetotaxy of tibiae I–IV (5–5–5–5), genua I–IV (0–0–0–1), femora I–IV (3–2–2–2), trochanters I–IV (1–1–1–1). Setae vTrI–IV and dFI serrate; ldFII–IV, vFII–IV, l’TII–IV, dTI–IV, vdTI–IV long and smooth; vFI filiform with barely discernible serration. Setation of tarsi I: 14 setae (ft, tc’, tc”, p’, p”, a’, a”, it’, it”, u’, u”, vs’, vs”, pl’) and solenidion w1; tarsi II: 10 setae (tc’, tc”, p’, p”, a’, a”, u’, u”, vs’, vs”) and w1; tarsi III, IV: 10 setae each (tc’, tc”, p’, p”, a’, a”, u’, u”, vs’, vs”). Solenidion w1 (about 25 long) longer than seta ft (about 5 long). Setae tc’, tc”, it’ and it” of leg I represented by eupathidia; tc’, tc” of legs II–IV, u’, u”, vs’, vs”, a’, a” and pl’ of legs I–IV filiform.
Imagochrysalis. Gnathosoma barely discernible, inserted at ventral surface of idiosoma. Peritremes with barely discernible chambers. Idiosoma 300 long, 300 wide. Only coxae I and II visible.
Larva.
Gnathosoma as in female. Chelicerae about 55 long; slender cheliceral part 25 long, swollen basal part about 30 long. Setae
dF serrate, 30 long; setae
dG 40 long. Peritremes about 35 long. Subcapitular setae
n absent.
Idiosoma almost rounded, 150–265 long and 170–275 wide. Dorsum. Propodonotal shield 65–70 long and 50–55 wide; well-outlined, bearing four pairs of densely serrate setae: 2 pairs situated antero-laterally (1 pair longer, 45 long, 1 pair shorter, 20 long), and 2 pairs situated posteriorly on the shield, both about 45 long. Laterally to propodonotal shield eye on ocular plate present and accompanied by 1 seta. In posterior half of idiosoma five pairs of posterior setae 40–50 long present.
Venter devoid of any setation. Genital area with 3 filiform genital setae
g1–g3, about 5–10 long, and 2 slightly serrate pseudanal setae
ps1–ps2 15–20 and 20–25 long. Coxae in formula: 2–0–1. Setae
1a,
1b filiform;
3a short, densely serrate. Setation of trochanters–tarsi I–IV as in female, except for presence of
dGI in several specimens; setation of legs I–III typical for pterygosomatidlarva (Figure 5 and Table 2 in [
30]).
Differential diagnosis.
G parva n. sp. is most similar to
Geckobia bochkovi Fajfer, 2023 from
Ptyodactylus guttatus (Heyden) from Israel [
19]. In females of both species, the propodonotal shield is well outlined and slightly concave in anterior and posterior part of idiosoma, palp seta
dG and subcapitular setae
n are filiform and smooth, eyes are present laterally to propodonotal shield, in the medial and posterior part of the idiosomal venter lanceolate setae are present, coxal setae
1a and
1b are filiform, whereas setae
2b and
3c are thick and densely serrate, and four pairs of genital setae are present. In this new species, the idiosoma is much smaller (275–380 wide and 210–340 long) and propodonotal shiels bears six pairs of setae, leg seta
lGI is absent, coxal setae
3d is present, pseudanal series is represented by three pairs of setae
ps. In
G. bochkovi the idiosoma is much bigger (560–650 wide and 520–650 long), the propodonotal shield bears 14 pairs of setae, leg seta
lGI is present and coxal seta
3d is absent, the pseudanal series is represented by 12 pairs of setae
ps.
Type material. Female holotype, 1 imagochrysalis and 9 larvae from Ptyodactylus puiseuxi Boutan, (HUJ no. 18259) (tympanum), Jordan: Wadi Khalid, 16.01.1987, coll. Yaacov Pesach; 1 female from same host (HUJ no. 18258) (tympanum), Israel, Northern district, Golan: Qazbiya, 24.05.1987, coll. Yehudah L. Werner; 3 females, 3 imagochrysalis and 16 larvae from same host (HUJ n. 18672), Israel, Northern District, Golan: foot of Rekhes Bashanit, 24.05.1987, coll. Yehudah L. Werner.
Type material deposition. Holotype female, 2 imagochrysalis, 10 larvae in the HUJ (HUJINV-Acari_Pte00004.1–13), 3 female paratypes, 1 imagochrysalis and 14 larvae in the CSWU (CSWU-Pte0020.1-18).
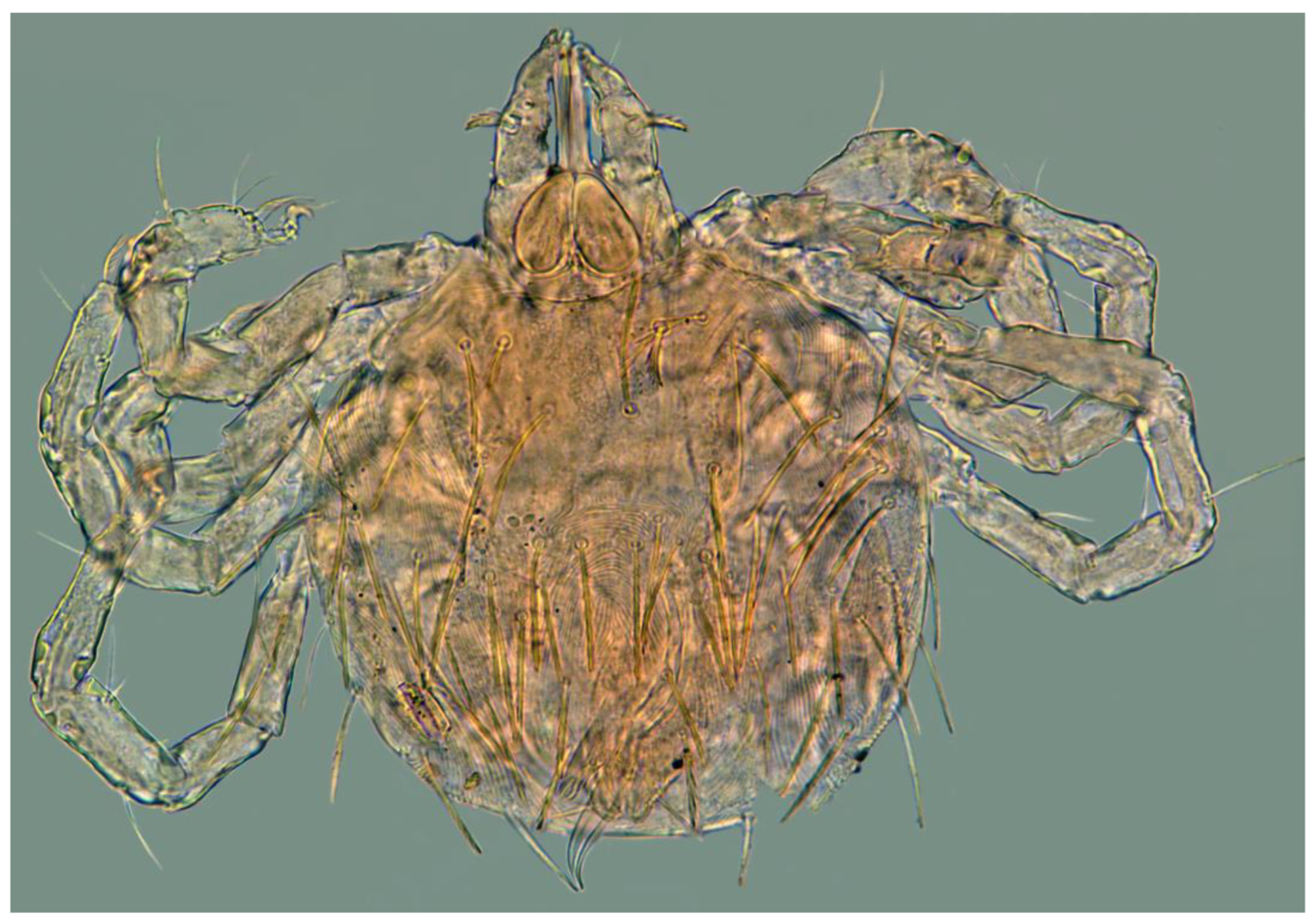
Figure 11.
Geckobia parva sp. nov. , larva in dorsal view.
Figure 11.
Geckobia parva sp. nov. , larva in dorsal view.
3.2. Ecological Analyses
A total of 1,135
Ptyodactylus specimens were examined from museum collections. Among these, 37 hosts (3.26%, 95% CI: 2.31-4.47%) were infected with five
Geckobia species with species-specific prevalence patterns (host-level, 95% CI) as shown in
Table 1. A total of 264 individual mites were collected. Intensity averaged 7.14 ± 8.46 with a median of 4.0 (IQR 1.0-10.0), while abundance was 0.233. Five
Geckobia species were identified.
Co-infections with multiple Geckobia species occurred in 5 of 37 infected hosts (13.5%). The most frequent combinations were G. bochkovi + G. synthesys (3 hosts), G. bochkovi + G. squameum (1 host), and G. inermis + G. squameum (1 host). Single-species infections predominated (32 hosts, 86.5%).
Host species analysis across all 1135 examined specimens revealed differential infection patterns (see host species-specific prevalence in
Table 2).
Ptyodactylus guttatus, the dominant species (904 hosts, 79.6%), showed 27 infections, 2.99% prevalence (95% CI: 1.88–4.10%) with a mean intensity of 5.44 ± 6.41 mites per infected host.
Ptyodactylus puiseuxi (177 hosts) exhibited 7 infections, 3.95% prevalence (95% CI: 1.08–6.83%) with higher mean intensity 14.29 ± 12.59.
Ptyodactylus hasselquistii showed 2 infections out of 41 hosts (4.88% prevalence, 95% CI: 0.00–11.47%) and a mean intensity of 1.50 ± 0.71. Unidentified to species
Ptyodactylus sp. exhibited zero infections (0/12 hosts, 0.00% prevalence [95% CI: 0.00–0.00%]). Among infected hosts, mean intensity differed markedly between
P. puiseuxi and
P. guttatus (Hedges’ g = 1.12, 95% CI 0.24–1.99; large effect).
Exact binomial tests revealed significant deviations from 1:1 sex ratios in three species (
G. squameum,
G. synthesys,
G. parva) before multiple comparison correction. After Holm correction for multiple comparisons, only
G. squameum showed significant female bias (31♀:9♂, p = 0.0034, Cohen's h = 0.73 [large effect], 95% CI: 0.31-1.15, power = 0.94) whereas
G. synthesys (16♀:5♂, raw p = 0.0266, corrected p = 0.1064) and
G. parva (6♀:0♂, raw p = 0.0312, corrected p = 0.0938) showed trends toward female bias but were not significant after correction (
Table 3).
G. bochkovi (21♀:16♂, p = 1.0000) and
G. inermis (2♀:2♂, p = 1.0000) showed balanced sex ratios.
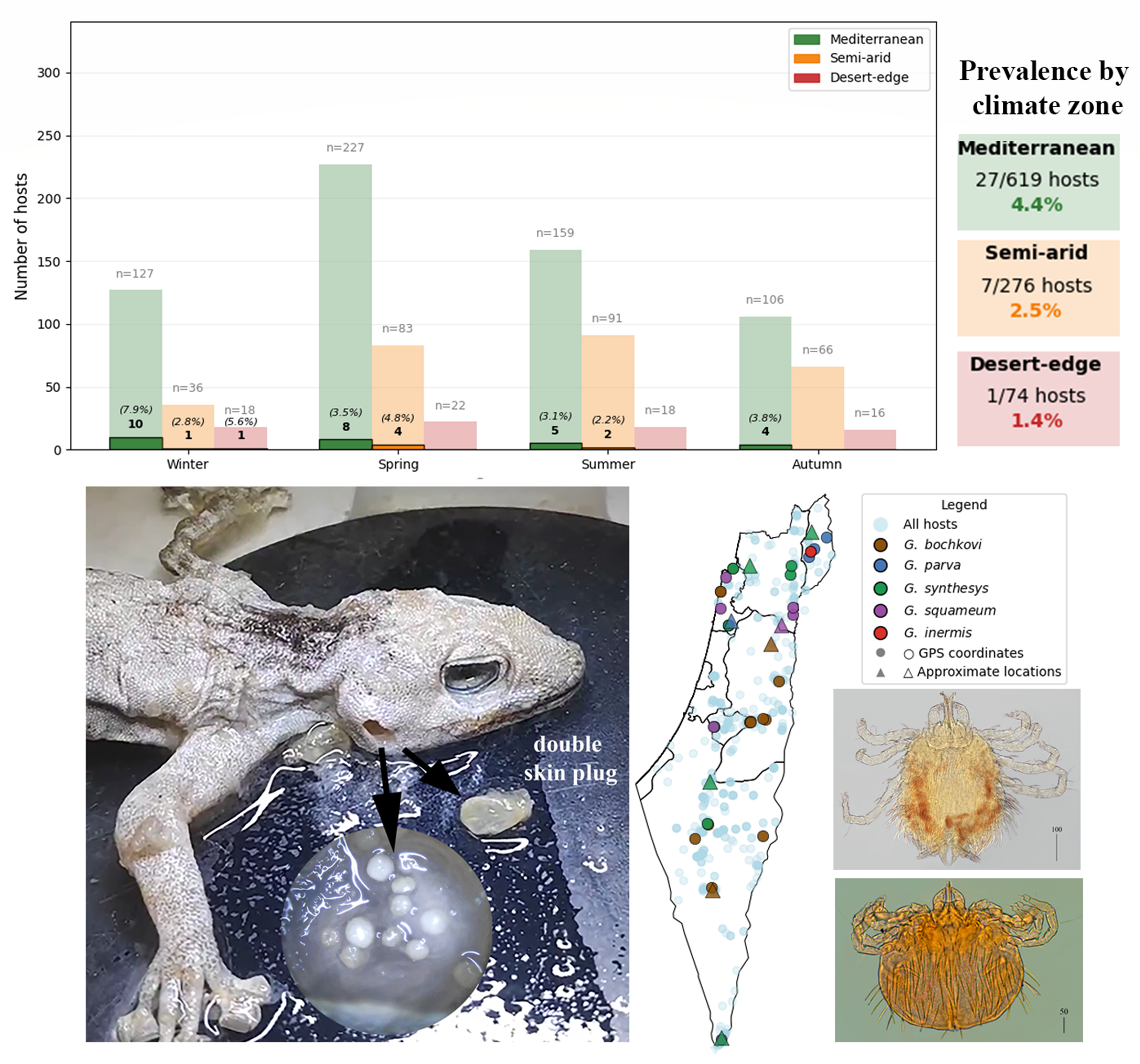
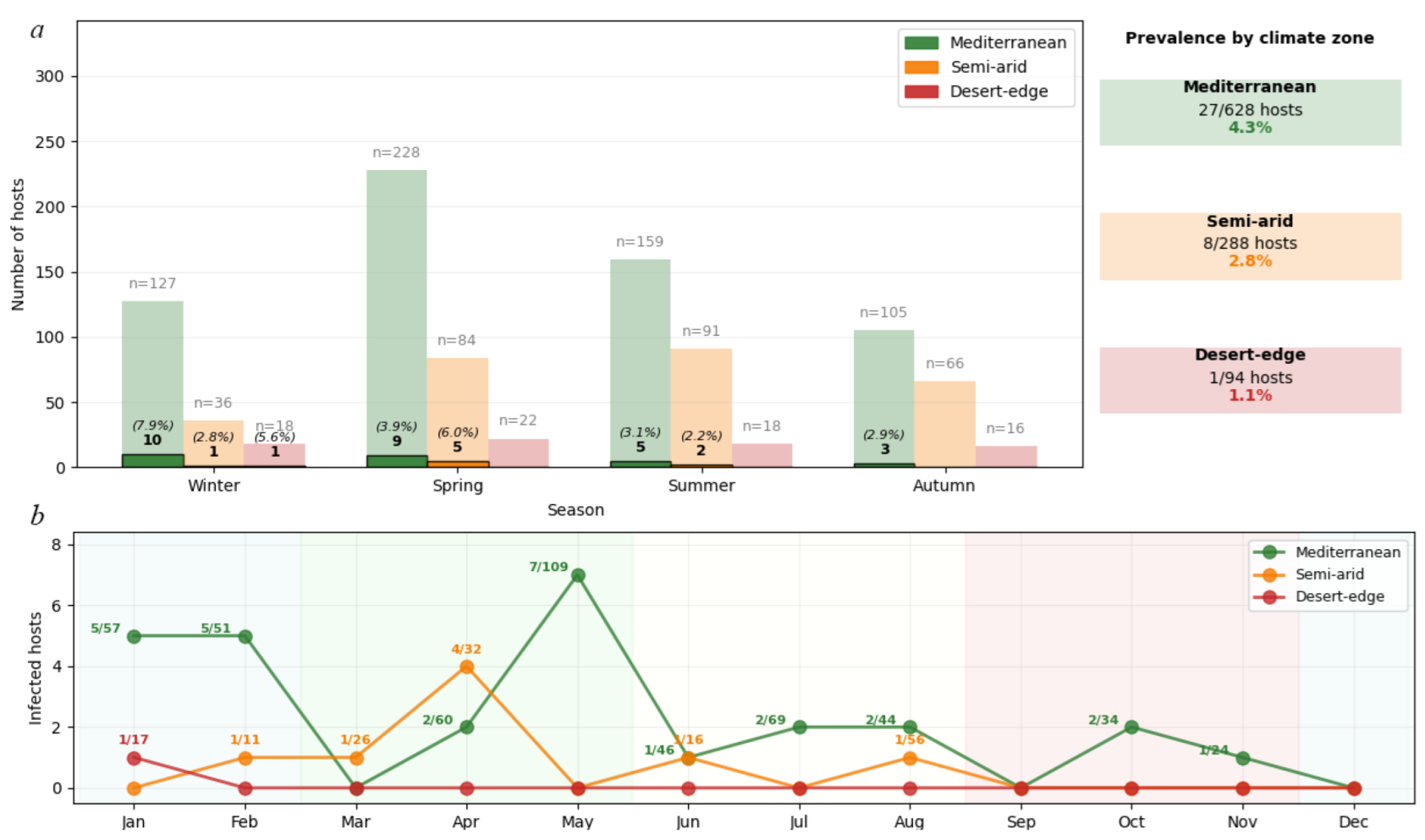
Environmental filtering showed a systematic prevalence decline: Mediterranean 4.3% (27/628 hosts), semi-arid 2.8% (8/288), desert-edge 1.1% (1/94). Due to low expected values in the desert-edge category, Fisher's exact test confirmed significant association between climate zone and infection status (p = 0.029). The climate effect (Cramér's V = 0.078, 95% CI: 0.045-0.112) exceeded host species effect (Cramér's V = 0.015, 95% CI: 0.001-0.034) by 5.2-fold (95% CI: 2.3-11.7), confirming climate as the primary structuring force (χ² = 8.74, df = 3, p = 0.033, 1-β = 0.60). The systematic prevalence decline across the climate gradient supports the role of climate in shaping mite distributions (Cramér's V = 0.078 vs 0.015 for host species). Pairwise risk ratios (RR) for prevalence across climate zones were: Mediterranean vs desert-edge RR = 4.04 (95% CI 0.56–29.4), Mediterranean vs semi-arid RR = 1.55 (0.71–3.36), and semi-arid vs desert-edge RR = 2.61 (0.33–20.6); overall association strength Cramér’s V = 0.078 (95% CI 0.045–0.112).
Seasonal Phenological Plasticity
Geckobia mites exhibited distinct seasonal activity patterns (
Figure 12) that varied systematically across climate zones. Mediterranean populations showed pronounced winter activity peaks, with highest infection rates in Winter (10/127 hosts, 7.9%) followed by Spring (9/228 hosts, 3.9%), Summer (5/159 hosts, 3.1%), and Autumn (3/105 hosts, 2.9%). Peak reproductive activity occurred during the mild, humid Mediterranean winter when temperature-moisture conditions optimize mite survival and inter-host transmission.
In contrast, semi-arid populations demonstrated spring-shifted phenology, with maximum activity in Spring (5/84 hosts, 6.0%) compared to Winter (1/36 hosts, 2.8%) and Summer (2/91 hosts, 2.2%). No infections were detected in semi-arid autumn samples (0/66 hosts, 0.0%). This temporal shift reflects adaptation to the brief post-winter period before extreme summer desiccation, when conditions briefly achieve suitable humidity levels for mite’s survival and reproduction.
Desert-edge populations showed minimal and sporadic activity, with infections detected only in winter (1/18 hosts, 5.6%), while spring (0/22 hosts), summer (0/18 hosts), and autumn (0/16 hosts) showed complete absence despite adequate sampling effort. This pattern indicates that climatic conditions rarely reach thresholds suitable for sustained mite reproduction in hyperarid environments.
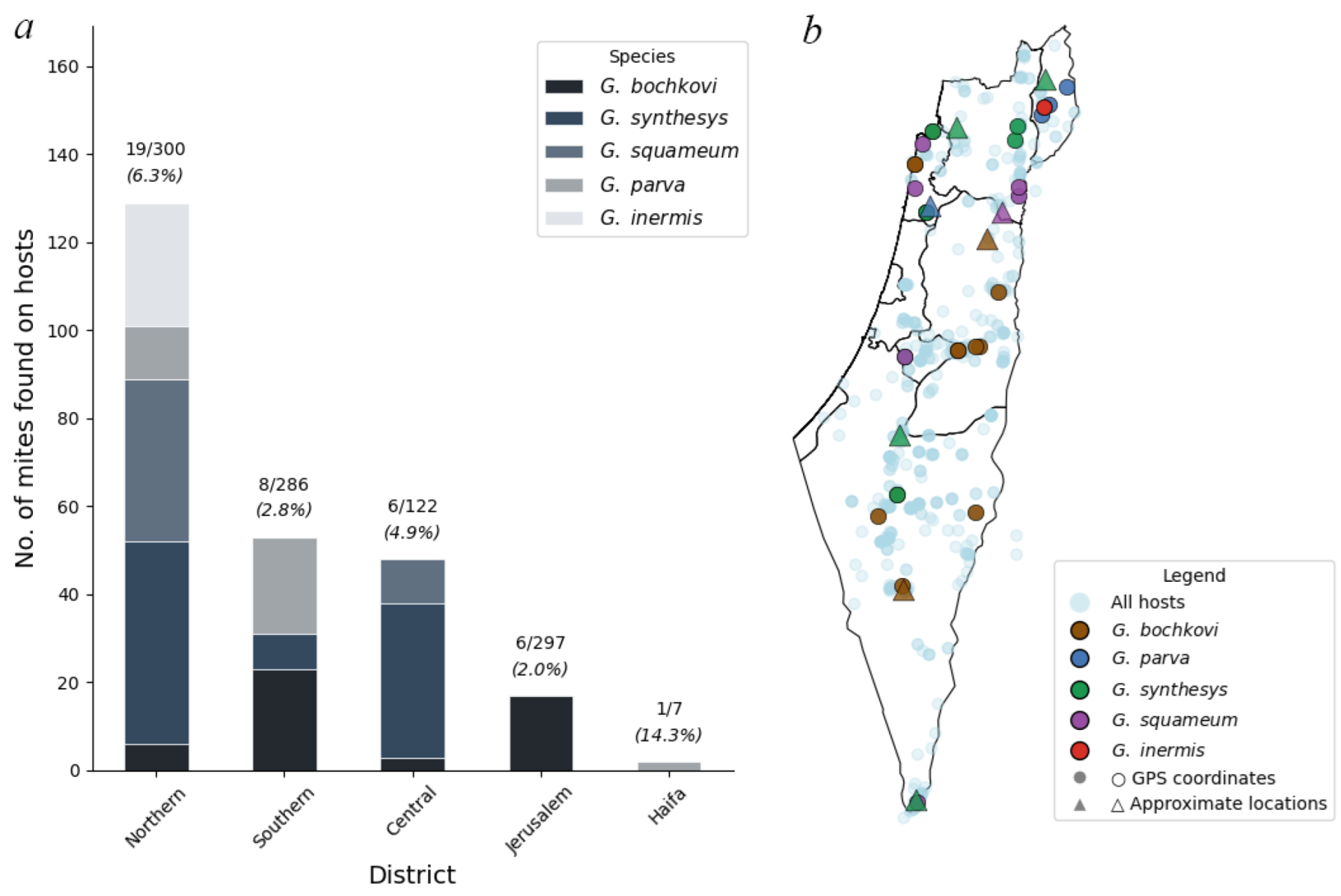
Analysis of mite distribution across Israeli administrative districts (
Figure 13) revealed significant geographic heterogeneity in both prevalence and species composition. Among hosts with district assignments, infections were recorded from all five main administrative districts, with the highest prevalence in Northern District (19/300 hosts, 6.3%) followed by Central District (6/122 hosts, 4.9%), Southern District (8/286 hosts, 2.8%), Jerusalem District (6/297 hosts, 2.0%), and Haifa District (1/7 hosts, 14.3%). The small sample size in Haifa District (n=7) limits interpretation.
Geckobia species showed distinct district-level distribution patterns.
G. bochkovi demonstrated the broadest geographic range, occurring in four districts with highest abundance in Southern District (23 individuals from 5 hosts, mean intensity = 4.6) (
Figure 13).
G. synthesys showed preference for northern and central regions, with highest concentrations in Central District (35 individuals from 4 hosts, mean intensity = 8.8) and Northern District (46 individuals from 5 hosts, mean intensity = 9.2).
G. squameum was restricted primarily to Northern District (37 individuals from 7 hosts, mean intensity = 5.3) with limited occurrence in Central District.
G. parva occurred mainly in Northern and Southern Districts, while
G. inermis was recorded only from Northern District (28 individuals from 1 host).
Mite abundance (total individuals per host examined) varied considerably across districts: Northern District (0.430), Central District (0.393), Haifa District (0.286), Southern District (0.185), and Jerusalem District (0.057). The 7.5-fold difference in abundance between Northern and Jerusalem Districts suggests strong regional environmental or ecological factors influencing mite establishment and population growth.
Co-infections with multiple Geckobia species were most frequent in Northern District, where 5 of 16 infected hosts (31.3%) harbored more than one species. Analysis of realized versus potential geographic ranges revealed incomplete exploitation of host distributions. While Mediterranean and semi-arid zones showed consistent mite occurrence across the sampled range, desert-edge zones exhibited patchy and sporadic infections despite substantial host availability. This pattern indicates that climate sets hard boundaries on mite distribution, creating "climate refugia" where mites persist only under locally favorable conditions.