Submitted:
09 October 2025
Posted:
10 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Collection and Preparation
2.2.1. Main Experiment
2.2.2. Preliminary Experiment for Evaluation of the Effects of Salivette
2.3. VOC Extraction and Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.3.1. VOC Extraction
2.3.2. GC/MS Analysis
2.4. Data Analysis
3. Results
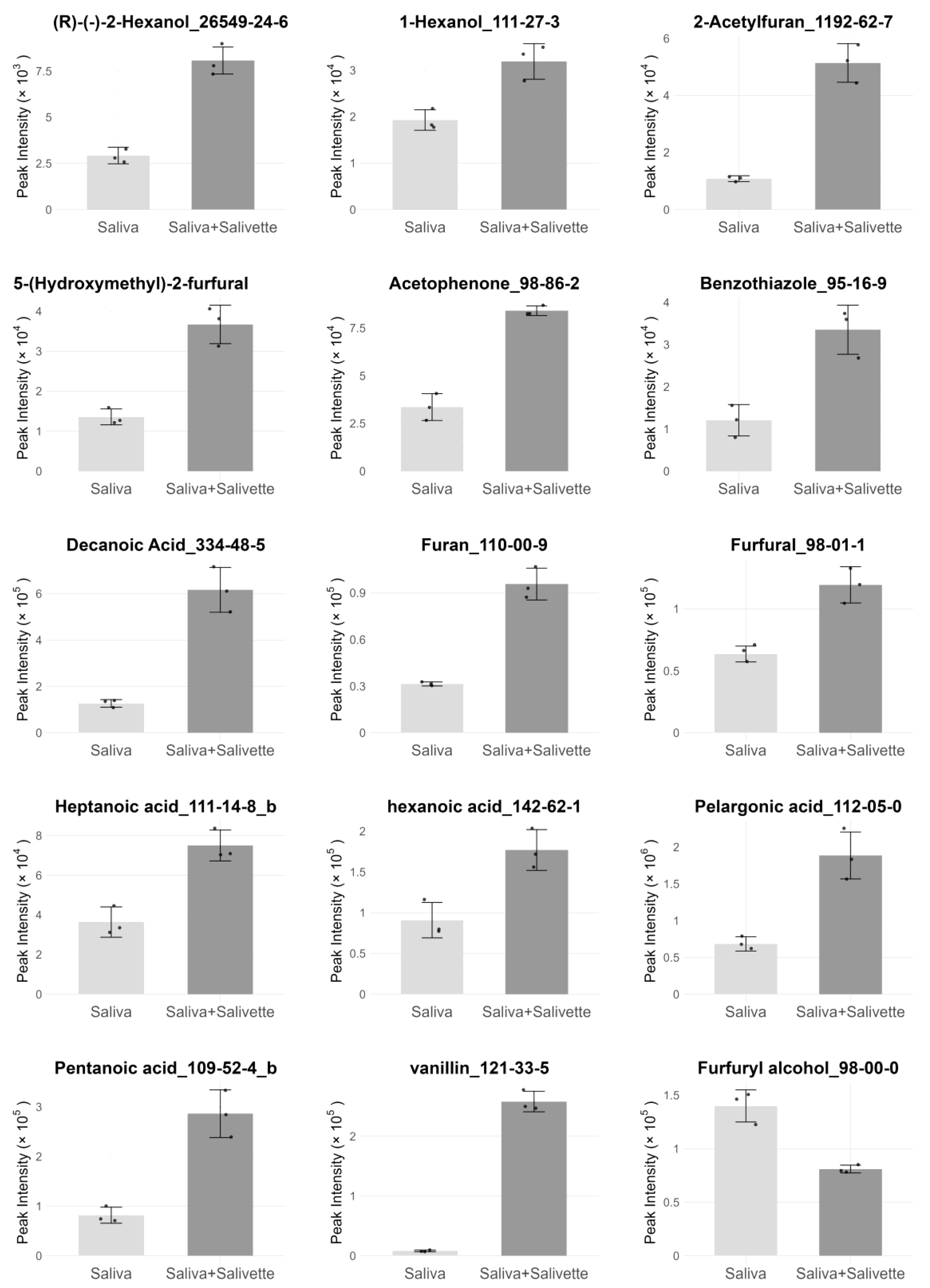
3.1. Evaluation of the Effects of Salivette on Whole Saliva VOC Profiles
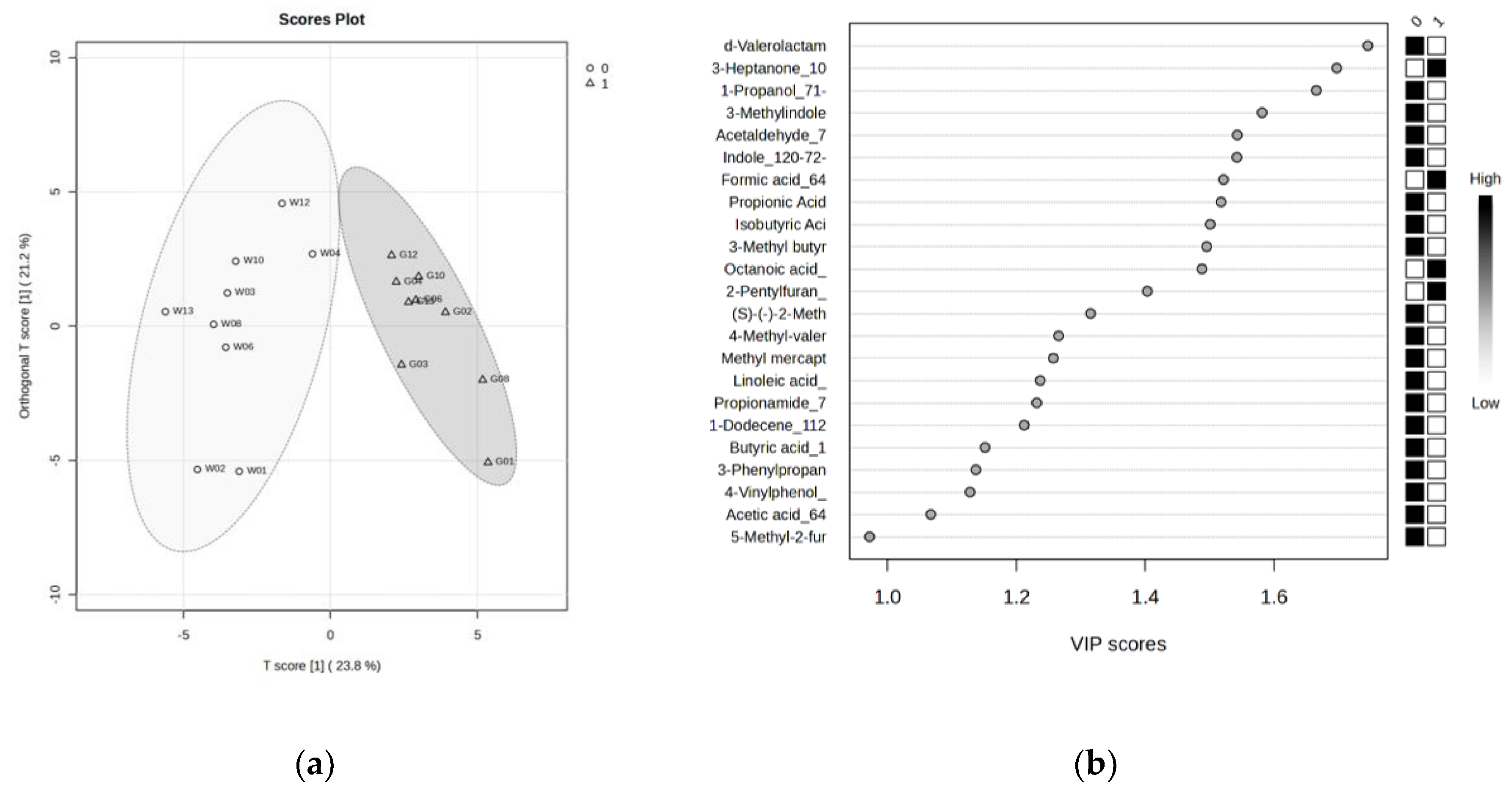
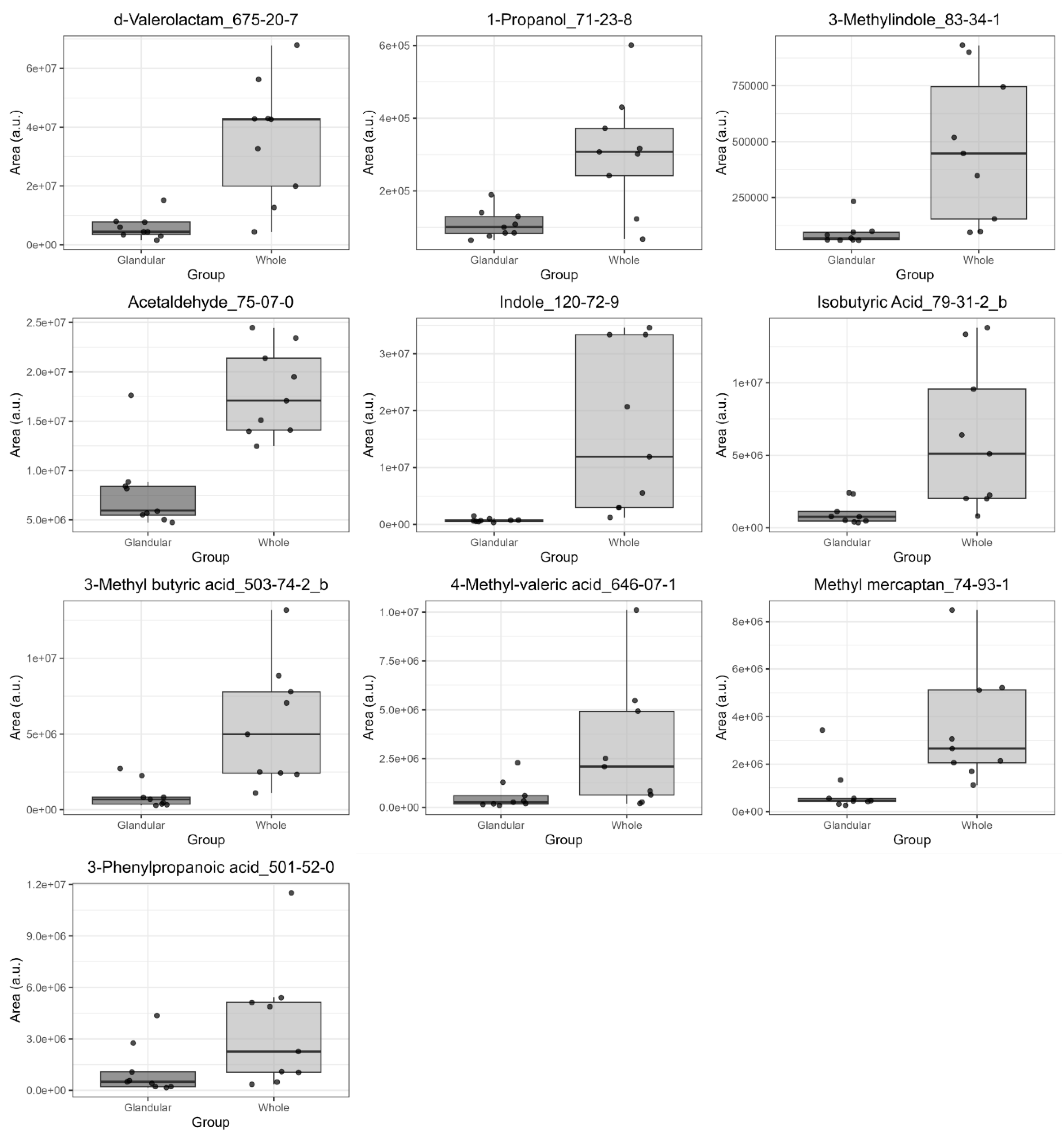
3.2. Comparison of VOC Profiles in Whole and Glandular Saliva
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| GC | Gas chromatography |
| MS | Mass spectrometry |
| RI | Retention index |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| VSCs | Volatile sulfur compounds |
| VIP | Variable importance for prediction |
References
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The Human Volatilome: Volatile Organic Compounds (VOCs) in Exhaled Breath, Skin Emanations, Urine, Feces and Saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- AL-Kateb, H.; de Lacy Costello, B.; Ratcliffe, N. An Investigation of Volatile Organic Compounds from the Saliva of Healthy Individuals Using Headspace-Trap/GC-MS. J. Breath Res. 2013, 7, 036004. [Google Scholar] [CrossRef]
- Arulvasan, W.; Chou, H.; Greenwood, J.; Ball, M.L.; Birch, O.; Coplowe, S.; Gordon, P.; Ratiu, A.; Lam, E.; Hatch, A.; et al. High-Quality Identification of Volatile Organic Compounds (VOCs) Originating from Breath. Metabolomics 2024, 20, 102. [Google Scholar] [CrossRef]
- Monedeiro, F.; dos Reis, R.B.; Peria, F.M.; Sares, C.T.G.; De Martinis, B.S. Investigation of Sweat VOC Profiles in Assessment of Cancer Biomarkers Using HS-GC-MS. J. Breath Res. 2020, 14, 026009. [Google Scholar] [CrossRef]
- Monedeiro, F.; Monedeiro-Milanowski, M.; Zmysłowski, H.; De Martinis, B.S.; Buszewski, B. Evaluation of Salivary VOC Profile Composition Directed towards Oral Cancer and Oral Lesion Assessment. Clin Oral Invest 2021, 25, 4415–4430. [Google Scholar] [CrossRef]
- Topkas, E.; Keith, P.; Dimeski, G.; Cooper-White, J.; Punyadeera, C. Evaluation of Saliva Collection Devices for the Analysis of Proteins. Clin. Chim. Acta 2012, 413, 1066–1070. [Google Scholar] [CrossRef]
- Bosman, P.; Pichon, V.; Acevedo, A.C.; Chardin, H.; Combes, A. Development of Analytical Methods to Study the Salivary Metabolome: Impact of the Sampling. Anal Bioanal Chem 2022, 414, 6899–6909. [Google Scholar] [CrossRef]
- Meleti, M.; Quartieri, E.; Antonelli, R.; Pezzi, M.E.; Ghezzi, B.; Viani, M.V.; Setti, G.; Casali, E.; Ferrari, E.; Ciociola, T.; et al. Metabolic Profiles of Whole, Parotid and Submandibular/Sublingual Saliva. Metabolites 2020, 10, 318. [Google Scholar] [CrossRef]
- Gardner, A.; Parkes, H.G.; So, P.-W.; Carpenter, G.H. Determining Bacterial and Host Contributions to the Human Salivary Metabolome. J. Oral Microbiol. 2019, 11, 1617014. [Google Scholar] [CrossRef]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. The Proteomes of Human Parotid and Submandibular/Sublingual Gland Salivas Collected as the Ductal Secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef]
- Monedeiro, F.; Milanowski, M.; Ratiu, I.-A.; Zmysłowski, H.; Ligor, T.; Buszewski, B. VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules 2019, 24, 2977. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Shalygin, S.P.; Postnova, T.V.; Kosenok, V.K. Identification of Salivary Volatile Organic Compounds as Potential Markers of Stomach and Colorectal Cancer: A Pilot Study. J. Oral Biosci. 2020, 62, 212–221. [Google Scholar] [CrossRef]
- Soini, H.A.; Klouckova, I.; Wiesler, D.; Oberzaucher, E.; Grammer, K.; Dixon, S.J.; Xu, Y.; Brereton, R.G.; Penn, D.J.; Novotny, M.V. Analysis of Volatile Organic Compounds in Human Saliva by a Static Sorptive Extraction Method and Gas Chromatography-Mass Spectrometry. J. Chem. Ecol. 2010, 36, 1035–1042. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Tian, X.; Zhang, J.; Zhang, Z.; Shi, J.; Xu, J.; Ren, X. Determination of Volatile Profiles inside Apple Fruit Storage Facilities Using MonotrapTM Monolithic Silica Adsorbent and GC–MS. Hortic. Plant J. 2021, 7, 267–274. [Google Scholar] [CrossRef]
- Mori, A.; Taniguchi, M.; Kuboniwa, M.; Amano, A.; Fukusaki, E. Profiling Volatile Compounds from Culture Supernatants of Periodontal Bacteria Using Gas Chromatography/Mass Spectrometry/Olfactometry Analysis with a Monolithic Silica Gel Adsorption Device. J. Biosci. Bioeng. 2022, 134, 77–83. [Google Scholar] [CrossRef]
- Yee, K.L.; Jansen, L.E.; Lajoie, C.A.; Penner, M.H.; Morse, L.; Kelly, C.J. Furfural and 5-Hydroxymethyl-Furfural Degradation Using Recombinant Manganese Peroxidase. Enzym. Microb. Technol. 2018, 108, 59–65. [Google Scholar] [CrossRef]
- Łojewski, T.; Sawoszczuk, T.; Łagan, J.M.; Zięba, K.; Barański, A.; Łojewska, J. Furfural as a Marker of Cellulose Degradation. A Quantitative Approach. Appl. Phys. A 2010, 100, 873–884. [Google Scholar] [CrossRef]
- Roosen, M.; Van Laere, T.; Decottignies, V.; Morel, L.; Schnitzler, J.-L.; Schneider, J.; Schlummer, M.; Lase, I.S.; Dumoulin, A.; De Meester, S. Tracing the Origin of VOCs in Post-Consumer Plastic Film Bales. Chemosphere 2023, 324, 138281. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Compounds Responsible for Off-Odors in Several Samples Composed by Polypropylene, Polyethylene, Paper and Cardboard Used as Food Packaging Materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef]
- Milanowski, M.; Pomastowski, P.; Ligor, T.; Buszewski, B. Saliva – Volatile Biomarkers and Profiles. Crit. Rev. Anal. Chem. 2017, 47, 251–266. [Google Scholar] [CrossRef]
- Codipilly, D.; Kleinberg, I. Generation of Indole/Skatole during Malodor Formation in the Salivary Sediment Model System and Initial Examination of the Oral Bacteria Involved. J. Breath Res. 2008, 2, 017017. [Google Scholar] [CrossRef]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines 2023, 11, 2749. [Google Scholar] [CrossRef]
- Van den Velde, S.; van Steenberghe, D.; Van hee, P.; Quirynen, M. Detection of Odorous Compounds in Breath. J Dent Res 2009, 88, 285–289. [Google Scholar] [CrossRef]
- Gardner, A.; Carpenter, G.; So, P.-W. Salivary Metabolomics: From Diagnostic Biomarker Discovery to Investigating Biological Function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef]
- Malkar, A.; Devenport, N.A.; Martin, H.J.; Patel, P.; Turner, M.A.; Watson, P.; Maughan, R.J.; Reid, H.J.; Sharp, B.L.; Thomas, C.L.P.; et al. Metabolic Profiling of Human Saliva before and after Induced Physiological Stress by Ultra-High Performance Liquid Chromatography–Ion Mobility–Mass Spectrometry. Metabolomics 2013, 9, 1192–1201. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M.; Nie, Y.; Wang, C.; Sun, F.; Jiang, W.; Hu, W.; Wu, X. Integrated Analysis of the Salivary Microbiome and Metabolome in Chronic and Aggressive Periodontitis: A Pilot Study. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Khalid, T.Y.; Saad, S.; Greenman, J.; de Lacy Costello, B.; Probert, C.S.J.; Ratcliffe, N.M. Volatiles from Oral Anaerobes Confounding Breath Biomarker Discovery. J. Breath Res. 2013, 7, 017114. [Google Scholar] [CrossRef]
- van den Velde, S.; Quirynen, M.; Van hee, P.; van Steenberghe, D. Halitosis Associated Volatiles in Breath of Healthy Subjects. J. Chromatogr. B 2007, 853, 54–61. [Google Scholar] [CrossRef]
- Homann, N.; Tillonen, J.; Meurman, J.H.; Rintamäki, H.; Lindqvist, C.; Rautio, M.; Jousimies-Somer, H.; Salaspuro, M. Increased Salivary Acetaldehyde Levels in Heavy Drinkers and Smokers: A Microbiological Approach to Oral Cavity Cancer. Carcinogenesis 2000, 21, 663–668. [Google Scholar] [CrossRef]
| ID | Age | Sex |
|---|---|---|
| 01 | 72 | M |
| 02 | 44 | F |
| 03 | 48 | F |
| 04 | 30 | M |
| 06 | 49 | F |
| 08 | 22 | M |
| 10 | 22 | M |
| 12 | 24 | F |
| 13 | 23 | F |
| 17 | 27 | F |
| Compound | OPLS-DA VIP |
Wilcoxon signed-rank test (q < 0.05) |
Salivette blank evaluation* |
|---|---|---|---|
| δ-Valerolactam | 1.75 | 0.028 | No |
| 3-Heptanone | 1.70 | 0.016 | Yes |
| 1-Propanol | 1.67 | 0.016 | No |
| Skatole | 1.58 | 0.016 | No |
| Acetaldehyde | 1.54 | 0.016 | No |
| Indole | 1.54 | 0.016 | No |
| Formic acid | 1.52 | 0.016 | ** |
| Propionic acid | 1.52 | 0.035 | Yes |
| Isobutyric acid | 1.50 | 0.016 | No |
| Isovaleric acid | 1.50 | 0.016 | No |
| Octanoic acid | 1.49 | 0.016 | Yes |
| 2-Pentylfuran | 1.40 | 0.016 | ** |
| 2-Methyl-1-butanol | 1.32 | 0.086 | Yes |
| 4-Methylvaleric acid | 1.27 | 0.035 | No |
| Methyl mercaptan | 1.26 | 0.035 | No |
| Linoleic acid | 1.24 | 0.016 | ** |
| Propionamide | 1.23 | 0.128 | No |
| 1-Dodecene | 1.21 | 0.016 | ** |
| Butyric acid | 1.15 | 0.289 | Yes |
| 3-Phenylpropionic acid | 1.14 | 0.028 | No |
| 4-Vinylphenol | 1.13 | 0.065 | ** |
| Acetic acid | 1.07 | 0.065 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





