1. Introduction
Endometriosis is a reproductive disorder characterized by the growth of endometrial tissue outside the uterus, affecting about 10% of women of reproductive age worldwide. Chronic pelvic pain and infertility are two primary clinical symptoms[
1] that pose a significant economic burden globally. [
2]Endometriosis is traditionally classified into three subtypes based on location: superficial endometriosis, deep infiltrating endometriosis (DIE), and ovarian endometriotic cysts or endometriomas[
3]. Ovarian endometriosis has also been identified as a precursor to ovarian clear cell and endometrioid carcinomas [
4,
5].
Unlike superficial endometriosis and ovarian endometrioma, which most likely arise from retrograde menstruation, the pathogenesis of DIE remains unclear. Recent molecular genetic studies suggest a modified paradigm for how DIE forms, where circulating epithelial progenitor or stem cells that are intended to rebuild the uterine endometrium after menstruation become excessively reactive and then malopositioned outside the uterus [
3]. These entrapped epithelium-committed progenitor cells produce new glands through clonal proliferation and recruit oligoclonal stromal cells, resulting in the formation of DIE. Once formed, the ectopic tissue is exposed to immune surveillance and reaction, leading to inflammation and fibrosis, causing chronic pain, infertility, and other gastrointestinal symptoms.
While most cases of endometriosis involve the ovaries, fallopian tubes, peritoneal surface, bladder, and bowel serosa, some cases are more widely spread throughout the body. These rare instances can appear in unusual locations and grow as nodules that may be mistaken for a neoplastic process on imaging studies. Such cases have been reported in various sites, including the groin, diaphragm, umbilicus, brain, nasal cavity, skeletal muscle, liver, pancreas, lung, kidney, and biliary tract [6-9]. Here, we use the term "tumor-like" endometriosis to describe those.
To begin characterizing the biological and pathological features of these tumor-like endometriosis cases, we aimed to identify somatic sequence mutations in cancer-driver genes within the laser-captured microdissected glandular epithelium from 14 lesions. This approach was based on evidence that mutations in KRAS, ARID1A, PIK3CA, PPP2R1A, FGFR2, and PTEN have been found in superficial endometriosis, deeply invasive endometriosis, and endometriomas [10-14]. It is uncertain whether the frequency of cancer-driver mutations differs in quantity and quality between tumor-like endometriotic lesions and conventional ones. Our results showed that the frequency of cancer-driver mutations in this cohort of tumor-like cases was comparable to those reported in the literature for conventional endometriosis. This suggests that cancer-driver mutations alone are insufficient to develop tumor-like clinical features, and that they may have other physiological functions in endometriosis beyond their canonical tumor-promoting roles.
2. Materials and Methods
2.1. Sample Selection
This retrospective case series of tumor-like endometriosis included women treated at the Johns Hopkins Medical Institution from 2007 to 2023. The institutional review board approved this study under the IRB00188126. Cases included in this report were endometriotic lesions outside the pelvis or involving lymph nodes, infiltration into the muscularis or submucosa of the gastrointestinal tracts, and cases that were clinically suspicious for neoplastic diseases. These cases were originally suspicious for neoplasms based either on clinical impression or imaging studies. H&E slides were reviewed, and the diagnosis of endometriosis was confirmed by two authors (LC and IS) following the criteria in diagnostic pathology. Cases were excluded if they had inadequate tissue samples for laser capture microdissection or any evidence of neoplastic or precancerous disease of the female reproductive tract. Clinical and demographic information was obtained through the electronic medical record, and formalin-fixed, paraffin-embedded (FFPE) tissues from qualified cases were retrieved from archival files. All cases were subsequently anonymized, and no patient health information could be retrieved or identified.
2.2. Preparation of Tissue for Whole-Exome Sequencing
We employed protocols in preparation of DNA from laser-capture microdissected epithelial cells as previously reported [
15] and the methods of whole exome sequencing, including somatic mutation callings, were detailed elsewhere [
16,
17]. Briefly, FFPE tissue blocks were sliced into 10 μm sections and mounted on PEN membrane slides (Zeiss, Germany). Slides were deparaffinized with xylene and ethanol baths and then stained with hematoxylin. Targeted tissues were dissected using a laser capture microdissection microscope (LMD7, Leica) to enrich the epithelial component from ectopic endometrial glands. Adjacent non-endometriotic tissue, such as smooth muscle, was collected as a germline control. Genomic DNA was extracted with Qiagen's QIAamp DNA FFPE Tissue Kit (Qiagen, Germantown, MD). DNA concentration was measured with a Qubit dsDNA HS Assay on a Qubit 2.0 Fluorometer (Life Technologies, CA, USA). gDNA was fragmented into 150-200 base pair pieces.
Whole-exome sequencing was performed using the Illumina NovaSeq 6000 platform and data of matched lesion/tumor and normal samples were aligned to the human reference genome (hg38) using BWA software and analyzed to identify somatic point mutations and small insertions and deletions present in the lesion/tumor but not in matched normal samples. This study defined cancer-driver genes following the guidelines and prediction algorithms, as previously reported [
18]. The functional consequence of each mutation was predicted using gene annotations with ANNOVAR 2018Apr16, using databases from SIFT, Polyphen 2HDIV prediction, and MutationTaster prediction. We focused solely on detecting DNA sequence variations in the cancer driver genes from the glandular epithelium. After identifying somatic mutations in cancer driver genes, all sequencing data were deleted in conformity to the IRB protocol.
2.3. Statistical Analysis
Fisher's exact test was used for all categorical variables. Analysis of variance was used for all continuous variables. A p-value of 0.05 was considered statistically significant.
3. Results
Table 1 summarizes the overall clinical and demographic data for all 14 cases in this cohort. The mean age at the time of surgery to remove tumor-like endometriosis was 39.6 years, and the mean BMI was 29.1. As expected, most cases (85.7%) presented as stage IV disease, and 57.1% of patients had a history of previous surgery for endometriosis. Uterine leiomyoma or adenomyosis was found in every patient. Two-thirds of the patients were nulliparous.
Table 2 lists the clinical features, treatment histories, and somatic mutation statuses for individual cases. Cases 1 to 5 showed distant involvement, manifesting as an umbilical nodule, a groin mass, a large lesion encroaching the aorta, lymph node enlargement, and an omental mass. Four cases (cases 11-14) presented with appendiceal endometriosis together with other concurrent endometriotic lesions: three of the four cases were found to have concomitant ovarian endometriosis. Gastrointestinal endometriosis was found in six cases, showing deep infiltration of endometriosis into the muscularis and submucosa of the bowel wall. We highlighted individual cases as follows.
Case 1 involves a 45-year-old woman who was evaluated for surgical removal of a solitary subcutaneous nodule at the umbilicus. Her symptoms started 15 years ago, with the lesion gradually becoming increasingly swollen, painful, and bloody during menstruation. The clinical impressions included skin appendage neoplasia and endometriosis. She underwent excision of her umbilical nodule to diagnose endometriosis, which was also found to affect the anterior cul-de-sac, uterosacral ligaments, and ovarian tissue. A primary umbilical hernia complicated her postoperative recovery. She was not found to have any cancer driver mutations in this umbilical endometriosis.
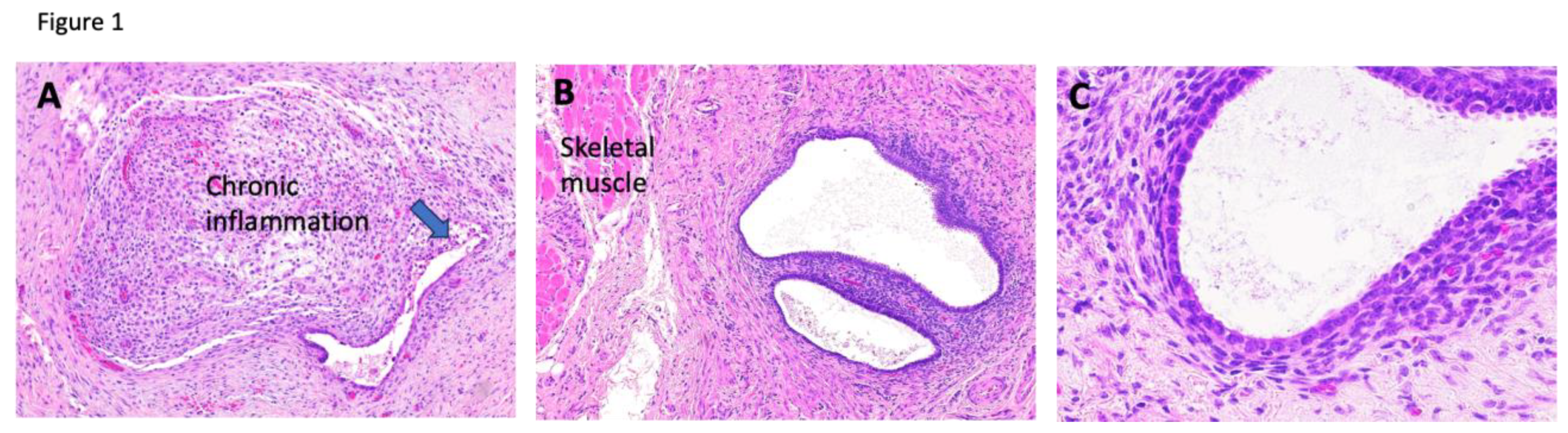
Case 2 involves a 35-year-old woman with a past medical history of infertility and prolactinoma who was initially evaluated for a right inguinal mass suspected to be a desmoid tumor. The mass had been present for 10 years, and she reported that it became prominent and tender during menstruation. MRI detected a right inguinal mass in the subcutaneous fat alongside a left para-ovarian cyst. The pathology report after excision revealed florid endometriosis with focal chronic inflammation (
Figure 1). She had no history of cesarean section or other gynecologic surgeries. Her inguinal endometriosis harbors a
KRAS mutation.
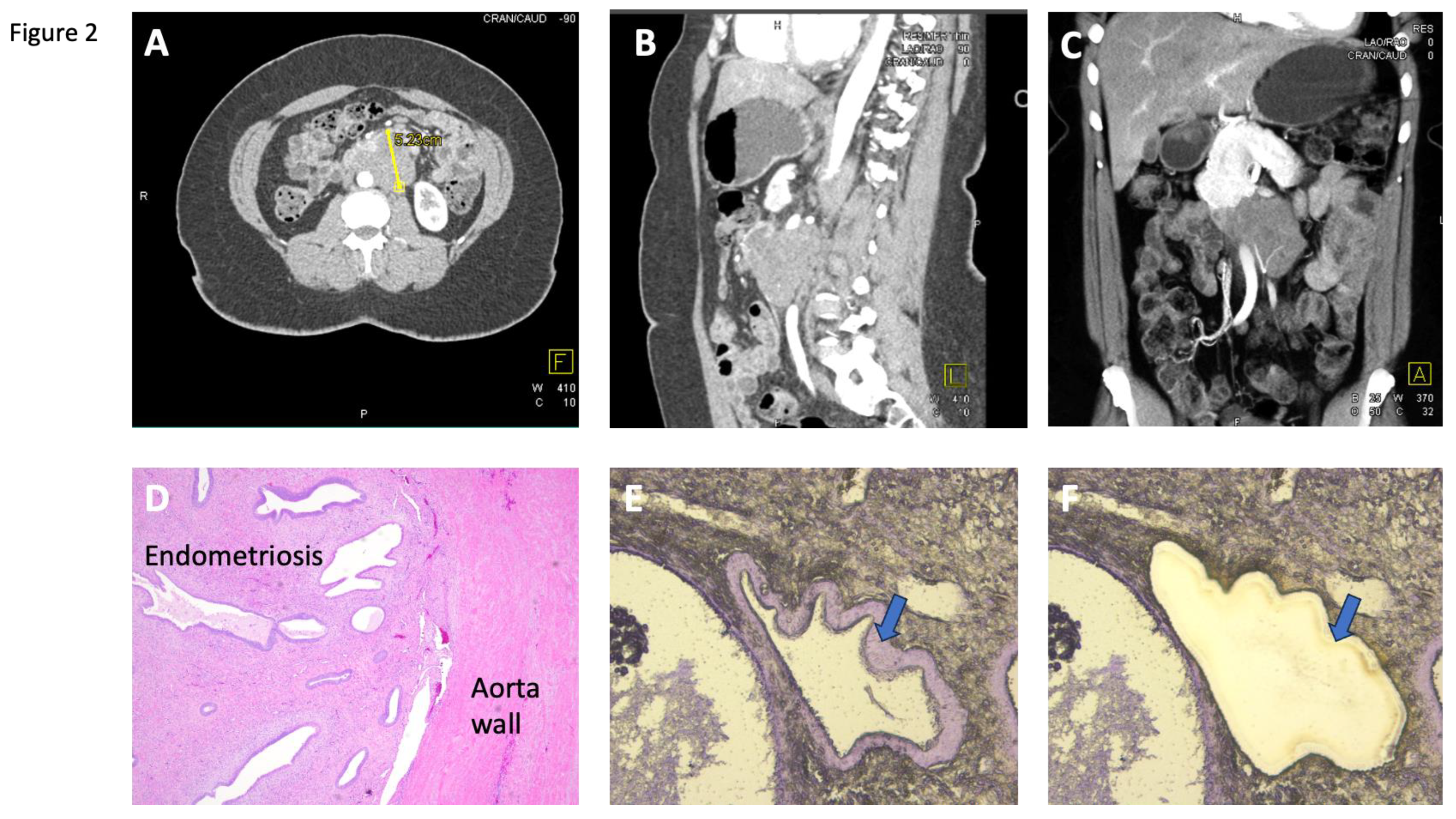
Case 3, a 49-year-old woman, had a history of endometriosis for which she underwent hysterectomy and bilateral oophorectomy. Nine years later, she developed abdominal pain and bowel obstruction. Her abdominal CT scan showed a slowly enlarging retroperitoneal mass involving the abdominal aorta, initially suspected to be an abdominal aneurysm or a retroperitoneal soft tissue tumor (
Figure 2). Subsequently, she underwent exploratory laparotomy with en bloc resection of the large retroperitoneal mass which was surprisingly identified on pathologic analysis as an endometriotic mass. She was found to have mutations in
CTNNB1 and
ARID1A in this retroperitoneal endometriosis involving the aorta.
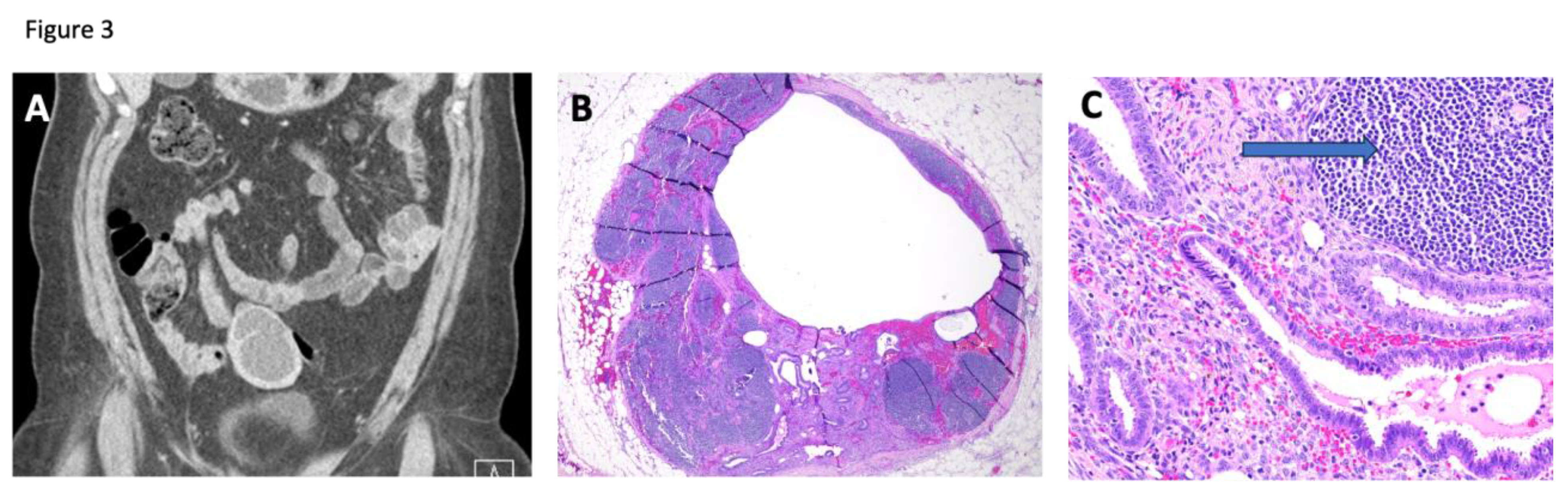
Case 4, a 49-year-old woman, developed a right-sided ovarian mass and left ureteral obstruction requiring percutaneous nephrostomy. Her abdomen and pelvis CT scans showed a right ovarian lesion with suspicious features concerning for neoplastic disease. Pathologic analysis revealed a right endometriotic ovarian cyst and evidence of endometriosis within an enlarged and engorged left pelvic sidewall lymph node and a portion of the sigmoid colon. These samples also demonstrated evidence of adenomyosis. The imaging and pathology of the involved lymph node are shown in
Figure 3. Because lymph node endometriosis is less common, we examined its epithelium and found no cancer driver mutations.
Case 5 involves a 28-year-old woman with a history of cervical atresia, infertility, severe endometriosis with dysmenorrhea, and known homozygous MTHFR germline mutations. She developed extensive adhesions among the pelvic organs, including the uterus, rectum, sigmoid colon, ovaries, fallopian tubes, and pelvic sidewalls. During hysterectomy and bilateral salpingo-oophorectomy, an omental lesion was discovered. Her pathology report indicated omental endometriosis with no cancer driver mutations.
Case 6, a 39-year-old woman, presented with one week of abdominal pain and vomiting. The clinical impression included possible small bowel obstruction, cholecystitis, gastritis, abdominal aortic aneurysm, or gastroenteritis. She underwent an exploratory laparotomy and the pathology report revealed extensive endometriosis involving all layers of the wall of the sigmoid colon. Evidence of endometriosis was found in a pelvic lymph node, on the mesentery, and within the abdominal wall, the colonic serosa, muscularis, and submucosa. The sigmoid colon endometriosis lesion was found to have somatic mutations in CHD4, MYD88, and STAG1.
Case 7 was a 17-year-old female with a history of constipation. On exam, she had a well-defined, non-tender firmness in the right lower quadrant that was rapidly enlarging. Pathology showed bilateral endometriotic cysts, and endometriosis in the rectum (serosa, muscularis, and submucosa) and omentum. Her omental endometriosis was not found to have any cancer driver mutations.
Case 8 involved a 39-year-old presenting with a bowel obstruction. Ultrasound and MRI revealed a large, complex cystic mass, with differential diagnoses including a sizable endometrioma and ovarian carcinoma. She underwent an exploratory laparotomy with bilateral oophorectomy, ovarian cystectomy, extensive ureterolysis, ileocecal resection, and reanastomosis. Her symptoms resolved following surgery. The pathology report confirmed endometriotic cysts on both sides with transmural involvement of the rectosigmoid colon and ileocecum by extensive endometriosis. The endometriosis in her rectosigmoid had mutations in KRAS and ARID1A.
Case 9 involved a 51-year-old presenting with abnormal findings on a barium enema and an inability to pass sigmoidoscopy. The pathology report showed transmural endometriosis of the sigmoid colon, leading to bowel obstruction. No cancer driver mutations were found in her sigmoid endometriosis.
Case 10 involved a 46-year-old who presented with complications following a robotic hysterectomy performed at an outside hospital due to endometriosis. Segment resection of her colon revealed focal endometriosis involving the muscularis propria and pericolonic fibroadipose tissue. Her pain improved after surgery. Her colonic endometriosis was found to have a KRAS mutation.
Case 11 presented at age 34 with nausea, vomiting, and diarrhea associated with menses. Abdominal and pelvic CT scans showed a dilated appendix in addition to a right adnexal cyst. The pathology report on her appendectomy specimen revealed extensive endometriosis involving the muscularis propria and adipose tissue of the appendix. Nucleotide sequencing fer endometriosis lesion did not detect any cancer driver mutations.
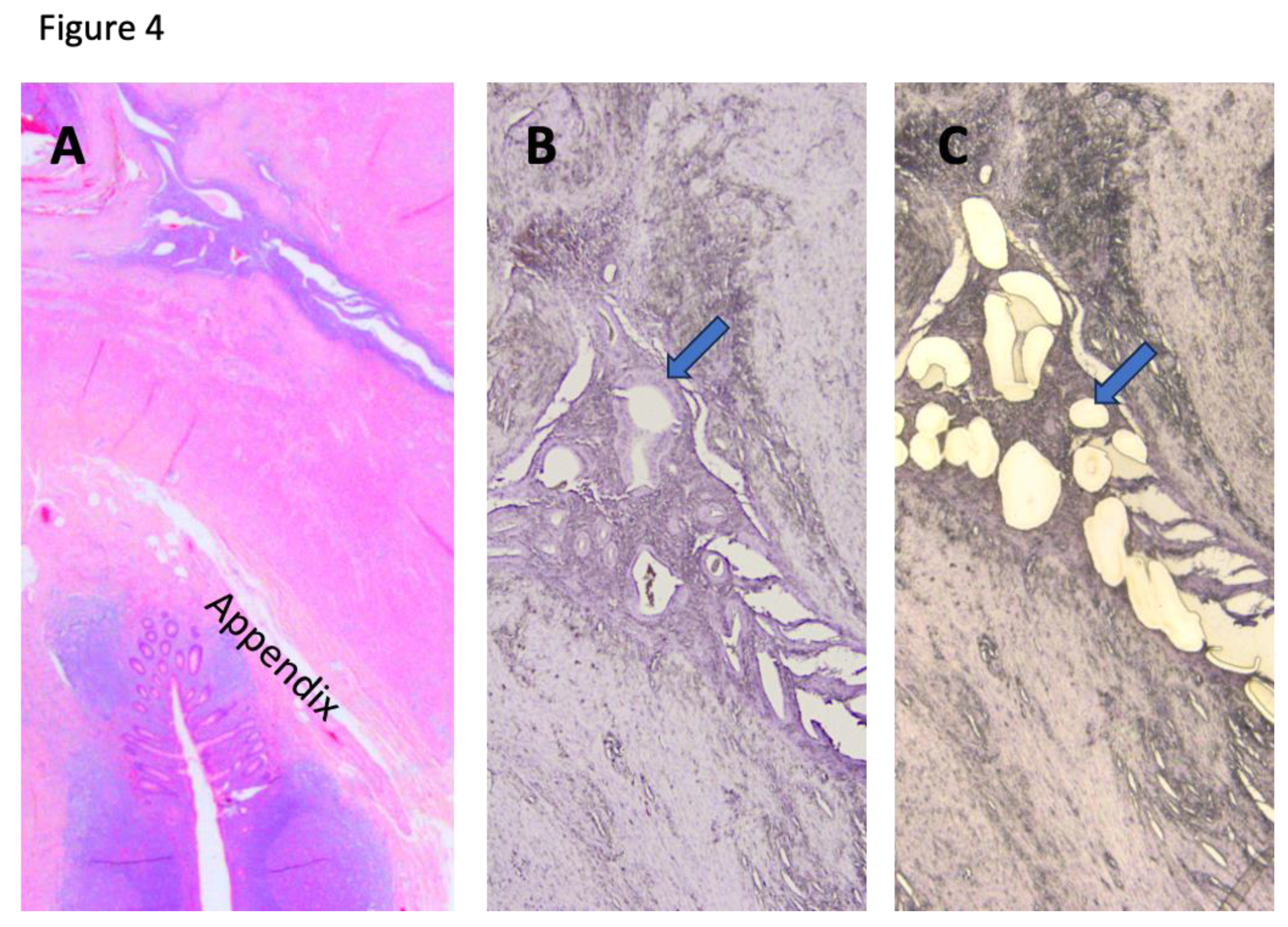
Case 12 presented at age 41 with dyschezia during menses. She had undergone a diagnostic laparoscopy to reveal an appendiceal mass, suspicious for appendiceal neoplasm. Her pathology findings show endometriosis of the appendix with extensive involvement of peri-appendiceal soft tissue, forming fibrosis and accounting for the tumor-like lesion (
Figure 4). Besides, endometriosis also involved the serosal surface of the uterus, and the right fallopian tube. Her pain improved after surgical removal of the appendix, uterus, and fallopian tubes. Her appendiceal endometriosis showed
KRAS and
PIK3CA mutations.
Case 13 involved a 31-year-old woman who presented with chronic pelvic pain and cyclic small bowel obstructions. She underwent an ileocolic resection that revealed endometriosis affecting the ileal serosa, causing stricture formation, serosal adhesions, and adhesion of the appendix. Her endometriosis did not contain any cancer driver mutations. Case 14 involved a 55-year-old woman who was found to have endometriosis incidentally during surgery for metastatic neuroendocrine cancer. She had multiple foci of endometriosis involving the serosa of the ileum and both the wall and serosa of the appendix. Her appendiceal endometriosis did not demonstrate evidence of any cancer driver mutations.
4. Discussion
This study reported clinical and molecular genetic findings of "tumor-like endometriosis" to clarify the biological nature of these unusual lesions, which appear in uncommon anatomical locations, including lymph nodes and intestinal endometriosis, mimicking neoplastic diseases. The findings from this study are expected to have several clinical and biological implications.
First, we observed a significant delay in diagnosing endometriosis in these women. Before confirming endometriosis through pathology, various clinical diagnoses were considered, including ovarian carcinoma, gastrointestinal neoplasia, desmoid tumor, abdominal aortic aneurysm, gastroenteritis, cannabinoid hyperemesis, pregnancy, viral infection, appendicitis, pancreatitis, gastric ulcer, cholecystitis, and gastritis. These differential diagnoses were clinically reasonable, but some patients disclosed to healthcare providers experiencing worsening pain symptoms during menstruation. Therefore, taking a detailed history and being aware of the possibility of extensive or tumor-like endometriosis are essential in primary care. For instance, the woman in case 2 was regularly treated for suspected cannabis hyperemesis syndrome or functional abdominal discomfort despite histopathologic evidence of endometriosis. The treatments she received did not target the underlying cause of her symptoms. In hindsight, her cannabis use was probably related to the need for additional pain relief when her endometriosis was untreated.
Second, we did not identify any specific age group associated with increasing tumor-like endometriosis, as their ages at the time of clinical presentations ranged from 17 to 51 years old, indicating that tumor-like endometriosis can occur at any age. Patients in this cohort experienced varying levels of treatment success with medical management. Progesterone and NSAIDs managed pain in some cases but not others. In four patients, different treatments alleviated particular symptoms but not all. One patient saw improvement in gastrointestinal symptoms but not pain after systemic progestin treatment. Pain continued despite surgical intervention surgery in three patients. Future prospective studies should aim to systematically evaluate medical and surgical therapy options and their effects on patient symptoms. Our findings also underscore the importance of a multimodal intervention to endometriosis, including progesterone, NSAIDs, and surgical options. It's essential to explain to patients that specific treatments may target some symptoms more effectively than others, and that treatment plans should be individualized for their constellation of symptoms and the locations of endometriotic lesions.
Third, the presence of endometriosis in unusual anatomical locations is of great interest. The umbilical and groin endometriosis lesions (case 1 and case 2) represent classic examples of extrapelvic endometriosis. These lesions are considered uncommon, but they have been documented in a significant number of cases. [
7]. The involvement of the aorta, reported as case 3 here, is probably the second known case in the literature [
19]. For individuals with endometriosis in rare anatomic locations, increased knowledge and clinical vigilance about the disease, along with a multidisciplinary approach, are recommended to ensure timely diagnosis and improve patient outcomes. Additionally, among the 14 cases, two showed lymph node endometriosis, resulting in nodal enlargement. Although uncommon, lymph node endometriosis has been documented in the literature. Most of these lesions involve the mesentery and pelvic lymph nodes, but the para-aortic obturator node has also been reported [
20]. The presence of endometriosis in lymph nodes confirms that endometriosis may spread via lymphatic pathways rather than only through local direct dissemination. The occurrence of appendiceal endometriosis in four cases supports the idea that these lesions are not uncommon. One study involving patients undergoing laparoscopic endometriosis surgery at a tertiary referral center estimated the prevalence of appendiceal endometriosis at 2.8%, with a higher risk observed in women with ovarian and bladder endometriosis [
21].
Fourth, our results emphasize that several somatic cancer-driver mutations are of great interest in the study of endometriosis because these mutations and the pathways they affect may play a role in the disease's development. The original study that identified cancer driver mutations in endometriosis found that 26% of deep-infiltrating endometriotic lesions contained cancer-causing mutations in the endometriotic epithelium, including
KRAS,
PIK3CA,
ARID1A, and
PPP2R1A, and proposed a clonal origin for endometriosis [
10]. Uterine endometrioid carcinomas and endometriosis-related ovarian malignancies typically exhibit these gene mutations [
22]. Moreover, those mutations are also detected in the precursor lesions of uterine endomtroid carcinomas [
16], supporting that (ovarian) endometriosis predisposes to ovarian endometrioid or clear cell carcinomas.
Most importantly, our data showed that the mutation frequency of cancer-driver genes in our cohort was comparable to that of other endometriosis cohorts, including superficial, deeply infiltrating, and endometriomas [10-14]. In light of tumor-promoting functions of somatic mutations in cancer-associated genes [
23], the finding thereof may be surprising. This is because endometriosis contains non-neoplastic tissues, minimal proliferation, and is histologically indistinguishable from eutopic endometrium. It is unclear whether these or other cancer driver mutations are involved in the development of tumor-like endometriosis in some cases within this cohort. Several explanations for why tumor-like endometriosis lesions do not show a higher frequency of cancer-driver mutations include the following. The combination of mutations in cancer-driver genes may not be ideal for tumor formation. For instance, concurrent inactivation of the tumor suppressors ARID1A and PTEN is necessary to increase proliferation in endometrioid intraepithelial neoplasia, the immediate precursor lesion of the endometrium. [
24], and induce endometrioid carcinoma in a mouse model [
25], likely through activating the MAPK signaling via DUSP4 downregulation [
26]. However, we did not observe co-mutation of
ARID1A and
PTEN in this cohort.
The next interpretation is that mutations in these genes may have functions beyond carcinogenesis. For example, studies show that increased KRAS signaling pathway activity, whether through genetic or epigenetic processes, but not activating mutations, may facilitate the survival of ectopic endometrium and contribute to progesterone resistance. Activation of the KRAS pathway in mouse models was associated with endometriosis-like lesions on the peritoneum and ovaries [
27], and endometriosis lesions originating from mice with
Kras activating mutations exhibit prolonged survival compared to those in wild-type mice [
28]. A separate study found that KRAS pathway activation caused abnormal overexpression of
SIRT1, which co-localizes with BCL6, thereby promoting progesterone resistance through the inactivation of the
GLI1 promoter [
29]. In a retrospective longitudinal study, mutations in
KRAS were associated with higher disease severity and surgical difficulty [
14].
Another possible explanation is that these cancer-driver mutations may serve as clonal markers associated with their development, with less biological significance. It has been reported that individual endometrial glands and microdissected tissues from normal uterine endometrium share a similar set of somatic cancer-associated mutations as those found in endometriosis [
11,
30]. From this perspective, normal endometrial glands undergo clonal expansion carrying specific mutations in epithelial cells, which can stay
in situ, exit the uterine cavity through retrograde menstruation, or spread potential endometrial progenitors via circulation, leading to endometriosis [
3,
31]. From this perspective, cancer-associated mutations are considered indolent and occur alongside the growth of endometriotic lesions that harbor mutations.
Lastly, we identified somatic mutations in genes that have not been previously reported in endometriosis. Our data show that
KRAS,
ARID1A,
PIK3CA,
CTNNB1, and
MYD88 are known somatic mutations that have been reported in endometriosis, while
CHD4 and
STAG1 are newly identified in the current study. The SNF2/RAD54 helicase family includes the transcriptional repressor
CHD4. Mutations in these genes have been observed in endometrial carcinomas and their precancerous lesions [
16,
32]. The
CHD4 R975H mutation is linked to endometrial cancer cell stemness and M2-like polarization in tumor-associated macrophages [
32]. It is prevalent in the endometrium. On the other hand,
STAG1 maintains telomere cohesion and controls mitotic chromosome segregation as a member of the SCC3 family.
ARID1A, which has inactivating mutations in endometriotic lesions, upregulates
STAG1, promoting genomic stability by increasing telomere cohesion [
33].
ARID1A or
STAG1 mutations may cause telomere cohesion problems of which effects on the pathogenesis of endometriosis warrant further studies.
Despite the new insights gained from this study, several limitations are also recognized . The relatively small sample size may limit the statistical power needed for a correlative study between somatic mutation status and clinical parameters. Because tumor-like endometriosis is relatively rare, a future collaborative effort is necessary to clarify its associations. Additionally, all included patients were undergoing surgical intervention at a single center, which could introduce selection bias. Moreover, the DNA quality is known to be affected by the age of tissue blocks, and the quantity is also limited by laser capture microdissection. Although this did not impact the identification of cancer driver mutations, it may lead to underdetection of mutations that were not enriched in the epithelium of endometriosis.
5. Conclusions
In summary, this study highlights the importance of educating all providers about endometriosis, including atypical presentations, to ensure it remains on the differential diagnosis for various painful and gastrointestinal symptoms [
7]. Our data also demonstrate that tumor-like endometriosis did not enrich the cancer-driver mutations, warranting further investigation into the possible roles of these mutations in the biology of endometriosis.
Competing interests statement and acknowledgements
The authors have no conflicts of interest to disclose. IS is supported, in part, by the Richard W. TeLinde Endowment, and JS is supported, in part, by the Howard and Georgeanna Jones Endowment at the Johns Hopkins University.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Johns Hopkins eIRB (IRB00188126) on 1 October 2019.
References
- Bulun, S.E. Endometriosis. N Engl J Med 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Wilbur, M.A.; Shih, I.M.; Segars, J.H.; Fader, A.N. Cancer Implications for Patients with Endometriosis. Semin Reprod Med 2017, 35, 110–116. [Google Scholar] [CrossRef]
- Chui, M.H.; Wang, T.L.; Shih, I.M. Endometriosis: benign, malignant, or something in between? Oncotarget 2017, 8, 78263–78264. [Google Scholar] [CrossRef]
- Victory, R.; Diamond, M.P.; Johns, D.A. Villar's nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol 2007, 14, 23–32. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrao, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J Minim Invasive Gynecol 2020, 27, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Dalkalitsis, A.; Salta, S.; Tsakiridis, I.; Dagklis, T.; Kalogiannidis, I.; Mamopoulos, A.; Daniilidis, A.; Athanasiadis, A.; Navrozoglou, I.; Paschopoulos, M.; et al. Inguinal endometriosis: A systematic review. Taiwan J Obstet Gynecol 2022, 61, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Ishihara, S. Omental Endometriosis. N Engl J Med 2021, 384, e21. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N Engl J Med 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Li, L.; Antero, M.F.; Zhang, M.; Chu, T.; Seckin, T.; Ayhan, A.; Pisanic, T.; Wang, T.L.; Cope, L.; Segars, J.; et al. Mutation and methylation profiles of ectopic and eutopic endometrial tissues. J Pathol 2021, 255, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Verhoef, L.; Aguirre-Hernandez, R.; Nazeran, T.M.; Tessier-Cloutier, B.; Praetorius, T.; Orr, N.L.; Noga, H.; Lum, A.; Khattra, J.; et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum Reprod 2019, 34, 69–78. [Google Scholar] [CrossRef]
- Orr, N.L.; Albert, A.; Liu, Y.D.; Lum, A.; Hong, J.; Ionescu, C.L.; Senz, J.; Nazeran, T.M.; Lee, A.F.; Noga, H.; et al. KRAS mutations and endometriosis burden of disease. J Pathol Clin Res 2023, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Noe, M.; Ayhan, A.; Wang, T.L.; Shih, I.M. Independent development of endometrial epithelium and stroma within the same endometriosis. J Pathol 2018, 245, 265–269. [Google Scholar] [CrossRef]
- Li, L.; Yue, P.; Song, Q.; Yen, T.T.; Asaka, S.; Wang, T.L.; Beavis, A.L.; Fader, A.N.; Jiao, Y.; Yuan, G.; et al. Genome-wide mutation analysis in precancerous lesions of endometrial carcinoma. J Pathol 2021, 253, 119–128. [Google Scholar] [CrossRef]
- Wu, R.C.; Wang, P.; Lin, S.F.; Zhang, M.; Song, Q.; Chu, T.; Wang, B.G.; Kurman, R.J.; Vang, R.; Kinzler, K.; et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol 2019, 248, 41–50. [Google Scholar] [CrossRef]
- Tokheim, C.J.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; Karchin, R. Evaluating the evaluation of cancer driver genes. Proc Natl Acad Sci U S A 2016, 113, 14330–14335. [Google Scholar] [CrossRef]
- Notzold, A.; Moubayed, P.; Sievers, H.H. Endometriosis in the thoracic aorta. N Engl J Med 1998, 339, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Beavis, A.L.; Matsuo, K.; Grubbs, B.H.; Srivastava, S.A.; Truong, C.M.; Moffitt, M.N.; Maliglig, A.M.; Lin, Y.G. Endometriosis in para-aortic lymph nodes during pregnancy: case report and review of literature. Fertil Steril 2011, 95, 2429–e2429. [Google Scholar] [CrossRef]
- Centini, G.; Ginetti, A.; Colombi, I.; Cannoni, A.; Giorgi, M.; Ferreira, H.; Fedele, F.; Pacifici, M.; Martire, F.G.; Zupi, E.; et al. Endometriosis of the appendix: prevalence, associated lesions, and proposal of pathogenetic hypotheses. A retrospective cohort study with prospectively collected data. Arch Gynecol Obstet 2024, 310, 1669–1675. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Mao T-L. ; Rahmanto, Y.S.; Zeppernick, F.; Ogawa, H.; Wu, R.C.; Wang, T.L.; Shih, I.M. Increased proliferation in atypical hyperplasia/endometrioid intraepithelial neoplasia of the endometrium with concurrent inactivation of ARID1A and PTEN tumour suppressors. J Patho Clin Res 2015, 1, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Suryo Rahmanto, Y.; Shen, W.; Shi, X.; Chen, X.; Yu, Y.; Yu, Z.C.; Miyamoto, T.; Lee, M.H.; Singh, V.; Asaka, R.; et al. Inactivation of Arid1a in the endometrium is associated with endometrioid tumorigenesis through transcriptional reprogramming. Nat Commun 2020, 11, 2717. [Google Scholar] [CrossRef]
- Mandal, J.; Yu, Z.C.; Shih, I.M.; Wang, T.L. ARID1A loss activates MAPK signaling via DUSP4 downregulation. J Biomed Sci 2023, 30, 94. [Google Scholar] [CrossRef]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 2005, 11, 63–70. [Google Scholar] [CrossRef]
- Cheng, C.W.; Licence, D.; Cook, E.; Luo, F.; Arends, M.J.; Smith, S.K.; Print, C.G.; Charnock-Jones, D.S. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J Pathol 2011, 224, 261–269. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci Rep 2017, 7, 6765. [Google Scholar] [CrossRef]
- Pandya, D.; Tomita, S.; Rhenals, M.P.; Swierczek, S.; Reid, K.; Camacho-Vanegas, O.; Camacho, C.; Engelman, K.; Polukort, S.; RoseFigura, J.; et al. Mutations in cancer-relevant genes are ubiquitous in histologically normal endometrial tissue. Gynecol Oncol 2024, 185, 194–201. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis and ovulatory menstruation: beyond the Sampson principle. J Clin Invest 2025, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, F.; Tong, Y.; Shi, D.; Zhang, J. CHD4 R975H mutant activates tumorigenic pathways and promotes stemness and M2-like macrophage polarization in endometrial cancer. Sci Rep 2024, 14, 18617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, J.; Rong, L.; Wu, S.; Deng, Z.; Fatkhutdinov, N.; Zundell, J.; Fukumoto, T.; Liu, Q.; Kossenkov, A.; et al. ARID1A promotes genomic stability through protecting telomere cohesion. Nat Commun 2019, 10, 4067. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).