1. Introduction
The Murine Double Minute 2 (MDM2) gene encodes an E3 ubiquitin ligase that serves as a principal negative regulator of the tumor suppressor p53. Under physiological conditions, MDM2 maintains low intracellular levels of p53 by mediating its ubiquitination and subsequent proteasomal degradation, thereby preventing inappropriate activation of apoptosis, cell cycle arrest, or senescence [
1]. In various malignancies, MDM2 is frequently overexpressed, leading to excessive suppression of p53 activity. The p53-MDM2 association contributes significantly to tumor initiation, progression, and therapeutic resistance [
2,
3]. Given its central oncogenic role, MDM2 has emerged as a compelling target for anticancer drug development. Innovative small-molecule inhibitors targeting the MDM2-p53 interaction are being developed to restore p53 function in tumors that harbor wild-type TP53. By disrupting this interaction, these inhibitors carry the potential to reactivate p53's tumor-suppressive capabilities, offering a promising therapeutic strategy. This manuscript provides a comprehensive analysis of MDM2 in oncology, evaluating the current landscape and prospects of MDM2-targeted therapies.
2. Materials and Methods
Information Sources and Search Strategy
A systematic search of PubMed, Embase, Cochrane, Web of Science and Scopus up until August 2025 was conducted, focusing on articles published in the last 5 years, concerning clinical trials of MDM2 inhibition as a cancer therapy. The data informationist generated a collection of search terms for each database to capture studies to be included in this review. Studies were excluded if a full text was not available. Search terms included “MDM2,” “clinical trial,“ clinical trials,” “cancer,” “oncology,”. The review was conducted in accordance with the Cochrane Handbook and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [
4]. We also searched for systematic reviews, meta-analyses, and original peer-reviewed studies, related to MDM2 biology and its therapeutic implications. Additionally, we reviewed clinical trial data from
ClinicalTrials.gov for registered and ongoing unpublished trials. Insights into clinical applications were incorporated from different profit protocols (e.g. the Boehringer Ingelheim protocol for the BI 907828 Brightline-1).
Study Screening and Data Extraction
Three authors (LDO, FC, and AP) participated in screening titles and abstracts, with each author reviewing a subset of articles. Studies that passed the initial screening underwent a full-text review conducted by two individuals to assess their eligibility. The senior author resolved any discrepancies in the screening decisions. The studies included in the full-text review underwent data extraction and were categorized based on the molecules discussed and their relationship to cancer histotypes. The extracted data were then analyzed descriptively.
3. Synthesis of Results
Twenty-seven studies addressed 22 clinical trials involving MDM2 inhibition as a cancer therapy, in the last 5 years. These studies are grouped by molecule and associated biological processes, and are reviewed below.
3.1. Biological Role of MDM2
3.1.1. p53-Mediated Oncogenic Regulation
MDM2 exerts its primary oncogenic effect through interaction with p53. It binds to the N-terminal transactivation domain of p53, suppressing its transcriptional activity and promoting its degradation via the ubiquitin-proteasome system. This interaction forms an autoregulatory loop where p53 induces MDM2 expression, which in turn downregulates p53 activity. What proved to be extremely interesting in this relation is the phosphorylation mechanism that can disrupt the interaction with p53, modifying several states, including stability, oligomerization, and subcellular localization. [
1,
5].
This tightly regulated mechanism is crucial for homeostasis, which is disrupted in tumors by MDM2 overexpression or gene amplification. In 2004, Vassilev and his team published a study revealing how potent small-molecule antagonists of MDM2 function. Their analysis of crystal structures showed that these compounds bind to the p53-binding pocket of MDM2, activating the p53 pathway in cancer cells. This leads to cell cycle arrest, apoptosis, and significant growth inhibition of human tumor xenografts in nude mice, paving the way for novel cancer therapies targeting MDM2. [
6].
3.1.2. p53-Independent Functions
It has been reported that MDM2 also possesses p53-independent oncogenic activities. In particular, it binds to other regulators such as p73, E2F1, Nbs1, DP-1, and Rb, modulating cell cycle progression, DNA repair, and apoptosis independently of p53. For example, MDM2's interaction with Nbs1 impairs homologous recombination, increasing genomic instability, interfering with DNA repair, and promoting aberrant chromosomal events that facilitate neoplastic transformation and tumour progression.
While its role as an oncogene, through not only the suppression of p53 function remains well established, emerging evidence indicates a possibility that MDM2 may act as a tumor suppressor under certain contexts. In particular, MDM2 targets Cadherins, specifically E-cadherin, which plays a critical role in the metastatic process of solid tumors of epithelial origin [
1,
7].
3.1.3. Regulation of MDM2 Activity
MDM2 is itself regulated through various post-translational modifications. ATM and Chk2-mediated phosphorylation, in response to genotoxic stress, modulate MDM2's subcellular localization and function. Moreover, the tumor suppressor ARF (p14^ARF) inhibits MDM2 by sequestering it and enhancing p53 stability during oncogenic signaling. Therefore, MDM2 inhibits p53, while ARF activates p53 by physically interacting with MDM2 to block its access to p53. The understanding of this so-called “p53-MDM2-ARF functional triangle” has been recently reviewed by Kung and collaborators. [
8,
9].
3.2. Implications in Tumor Biology of specific Cancer Histotypes
MDM2 gene amplification or protein overexpression has been reported in a range of human malignancies, including dedifferentiated liposarcoma (DDLPS), breast cancer, glioblastoma, colorectal cancer, non-small cell lung cancer (NSCLC), and urothelial carcinoma among others [
10]. In many cases, high MDM2 expression correlates with poor prognosis and resistance to chemotherapy or immunotherapy. Therefore, MDM2 has been the target of development for many synthetic small molecules, peptide- and aptamer-based therapies [
6,
11].
DDLPS in particular serves as the prototypical model of MDM2-driven cancer. MDM2 amplification is particularly common in sarcomas. Among them, liposarcoma is the most frequent histological type of soft-tissue sarcoma, including DDLPS as one of the most frequent. Nearly all DDLPS tumors harbor high-level MDM2 amplification, making it a pathognomonic biomarker and an actionable therapeutic target [
12].
3.3. MDM2 Inhibitors: Mechanisms of Action and Clinical Development
Targeting the MDM2–p53 interaction is a rational strategy to reactivate p53 functions (e.g., apoptosis and cell cycle arrest) in tumors with wild-type TP53. MDM2 inhibitors bind to the p53-binding pocket on MDM2, preventing ubiquitination and proteasomal degradation of p53 [
13].
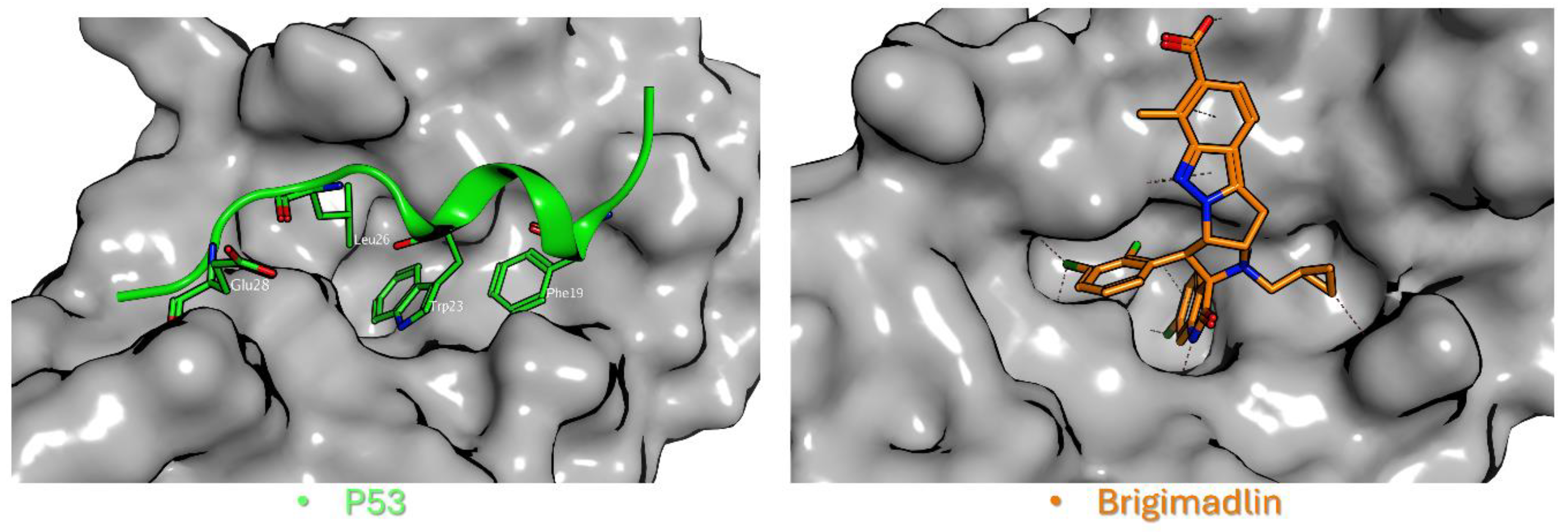
From a structural perspective, p53 adopts an α-helical conformation upon binding to MDM2, positioning three critical hydrophobic residues, Phe19, Trp23, and Leu26, into complementary pockets of the MDM2 cleft [
14] (
Figure 2, left). These pockets serve as the primary binding sites for clinical-stage MDM2 inhibitors, most of which function as competitive protein-protein interaction (PPI) inhibitors by mimicking the p53 α-helix (
Figure 2, right).
Notably, while the native p53 peptide exhibits only modest (low micromolar) affinity for MDM2 [
14], synthetic inhibitors like Nutlin-3a, SAR405838, Brigimadlin, and ALRN-6924 achieve low-nanomolar or sub-nanomolar binding affinity; a >1000-fold improvement, obtained by strategic optimization of hydrophobic contacts and additional hydrogen-bond interactions. Before making some observations on clinical progress in terms of MDM2 inhibitors, it’s important to specify that the effect of p53 activation by an MDM2 inhibitor in healthy tissue is of immense interest from a toxicology and therapeutic perspective. Several preclinical studies highlight the reduced toxicity of these molecules. Despite this, the precise mechanism for the lack of toxicity of MDM2 inhibitors to normal tissue still needs to be clarified.
Currently, clinical trials on the development of MDM2 inhibitors for cancer therapy are evaluating various compounds. A brief description of the main results addressing monotherapy is provided below.
MI-77301 (SAR405838) is a spiro-oxindole compound with high selectivity for MDM2. Preclinical data support its efficacy in liposarcoma and other p53-wild-type malignancies [
15].
Idasanutlin (RG7388) is a second-generation Nutlin with a pyrrolidine structure, for oral use with improved pharmacokinetics. A multicenter, randomized, double-blind phase III trial (MIRROS) in AML integrates Phase II safety and efficacy criteria into a Phase III study via a blinded interim analysis for futility. [
16]. In this trial the myelosuppressive effect appears to be a limitation. It remains to evaluate if dose changing or different treatment regimens could reduce neutropenia improving efficacy [
17].
BI 907828 (Brigimadlin) is an oral MDM2-p53 antagonist [
18], currently under evaluation for advanced DDLPS in the Brightline-1 Phase II/III trial (NCT05218499). Early data suggest improved tolerability and disease control [
5]. A subsequent trial (Brightline-2 Phase II/III trial -NCT03449381) is evaluating Brigimadlin as a second-line treatment for patients with advanced or metastatic cases of several cancers that often have limited treatment options and poor outcomes. These include Biliary tract cancer (BTC), Pancreatic ductal adenocarcinoma (PDAC), Lung adenocarcinoma, Bladder cancer. [
19]
Nutlin-3 belongs to the family of Nutlins, identified as cis-imidazoline inhibitors. In particular, the first small-molecule MDM2 inhibitor developed, Nutlin-3, competitively inhibits the p53-binding site of MDM2. It demonstrated robust preclinical efficacy in p53 wild-type models. Otherwise, Nutlin-3 requires functional p53 and MDM2 for its biological activity. The significant efficacy of Nutlin-3, demonstrated in several pre-clinical studies even in tumors with standard MDM2 expression levels, indicates that a broad patient population with wild-type p53 tumors may be responsive to antagonists of the p53–MDM2 pathway. [
15]
RITA (reactivation of p53 and induction of tumor cell apoptosis) binds directly to p53 and induces a conformational change that prevents MDM2 binding. In particular, RITA leads to the accumulation of p53 through an extension of its half-life. It is also reported that RITA shows p53-independent functions. The structural features identified in p53 and RITA may serve as a blueprint for the design of novel allosteric p53 activators with potential for clinical translation. Although it has a unique mechanism, poor solubility and stability have limited its clinical development until now [
20].
3.4. Combination Strategies and Novel Approaches
3.4.1. Combination Strategies
Due to the development of resistance and the occurrence of incomplete responses to monotherapy, combination therapy approaches represent a promising alternative for further investigation. These strategies aim to enhance treatment efficacy by targeting multiple pathways simultaneously. Various methods have been explored to achieve improved patient outcomes.
Chemotherapy
MDM2 inhibitors can sensitize tumor cells to genotoxic agents such as doxorubicin or cytarabine by reinforcing the DNA damage response through p53 reactivation with a synergic effect. [
21]
Over time, numerous pieces of evidence have emerged regarding the ability of a combination strategy to activate p53, reduce proliferation and increase the vulnerability of cells exposed to chemotherapy. In neuroblastoma, it has been shown that the combination of Nutlin-3a with etoposide or cisplatin significantly reduces proliferation. Furthermore, Nutlin-3a has been shown to inhibit the functioning of ABC transporters, P-glycoprotein and multidrug resistance protein 1 (MRP1; ABCC1). [
22]
Immunotherapy
Recently, Zeng and collaborators conducted a comprehensive review highlighting the crucial role of MDM2 in shaping the immune microenvironment, facilitating tumor immune evasion, and driving hyperprogression during immunotherapy.
Reactivation of p53 may enhance tumor immunogenicity, thereby increasing the efficacy of checkpoint inhibitors. Trials are ongoing for combinations with anti-PD-1/PD-L1 antibodies, especially in NSCLC and melanoma [
23]. In particular, by blocking MDM2, these drugs can help re-sensitize tumors to the effects of immunotherapy, especially in malignancies that have high levels of MDM2, as a result of overexpression or amplification. It has also been shown a crucial role in T-cell regulation for MDM2 and MDM2 inhibitors. Another interesting speculation involves Tumor Microenvironment (TME) and the mechanisms of MDM2 in various types of immune cells. In summary, combining MDM2 inhibitors with ICI therapy offers a promising strategy to enhance anti-tumor efficacy and bypass resistance, especially in cases where MDM2 monotherapy is suboptimal. Furthermore, this combined approach does not appear to heighten the toxicity associated with the MDM2 inhibitor.
Dual MDM2/MDMX Inhibitors
Stapled peptides are a novel therapeutic modality capable of disrupting protein-protein interactions like MDMX-p53. In particular, MDMX (also known as MDM4) can suppress p53 independently of MDM2. Dual inhibitors (e.g., ALRN-6924), used as chemoprotective agents, aim to overcome this compensatory resistance mechanism. A pre-clinical study explored the potential of ALRN-6924 as a combination therapy for hormone receptor-positive (ER+) breast cancer. ALRN-6924 was only effective in cancer cells with a functional, wild-type TP53 gene. When combined with chemotherapy drugs like paclitaxel and eribulin, ALRN-6924 showed a powerful synergistic effect, significantly increasing its anti-tumor activity both in cell cultures and in animal models. The strong synergy observed with ALRN-6924 and chemotherapy suggests that this combination warrants further investigation in clinical trials for patients with hormone receptor-positive breast cancer. [
24]
In a phase I clinical trial (NCT 02264613), ALRN-6924, two treatment schedules were assessed for safety, pharmacokinetics, pharmacodynamics, and antitumor efficacy in patients with solid tumors or lymphomas. ALRN-6924 resulted to be well-tolerated and exhibited promising antitumor activity. [
25]
PROTACs (Proteolysis-Targeting Chimeras)
A paradigm shift in MDM2 targeting has emerged with PROTAC-based agents such as KT-253 [
26], which combines an MDM2-binding warhead with an E3 ligase recruiter (i.e., CRBN) to induce proteasomal degradation of MDM2. These bifunctional molecules selectively degrade MDM2 through ubiquitin-mediated proteasomal pathways, reducing MDM2 concentration. Preclinical studies suggest greater efficacy with advantages in resistance settings and reduced toxicity compared to traditional inhibitors [
27].
Furthermore, next-generation compounds like KT-253 and the dual MDM2/MDMX inhibitor ALRN-6924 challenge conventional drug design through their beyond Rule of Five (bRo5) properties, including high molecular weight and extended surface engagement [
28]. Although these features complicate pharmacokinetics, they enable unprecedented specificity for MDM2's shallow interaction surface.
3.4.2. Novel Approaches. Ongoing Clinical Trials of MDM2 Inhibitors
In addition to completed studies, several ongoing clinical trials aim to define the therapeutic window and expand the applicability of MDM2 inhibitors:
Siremadlin (HDM201)
A Phase Ib/II trial (NCT05447663) is evaluating siremadlin in AML patients post-allogeneic stem cell transplant to prevent relapse. The study investigates both monotherapy and combination with donor lymphocyte infusions.[
29]
Alrizomadlin (APG-115)
A phase I trial (CTR20170975) is evaluating safety, pharmacological profiles and preliminary antitumor activity of Alrizomadlin in patients with advanced solid tumors including liposarcoma (LPS) [
30]. As a potent and selective oral antagonist, Alrizomadlin works by destabilizing the p53–MDM2 protein interaction, which in turn promotes p53 activation. The data suggest that Alrizomadlin, a new MDM2/p53 inhibitor, is a promising candidate deserving of further study.
Milademetan (DS-3032b)
A Phase I/II trial (NCT03634228) assesses the combination of milademetan with low-dose cytarabine, with or without venetoclax, in newly diagnosed or relapsed/refractory AML patients. Primary endpoints include tolerability and response rates. Subsequently, several pre-clinical trials supported a phase II (NCT05012397) basket study (MANTRA-2) in patients with advanced MDM2amp , TP53-wt solid tumors demonstrating a manageable safety profile and achieved responses against a variety of refractory MDM2amp , TP53 -wt solid tumors, but tumor reductions were short-lived. [
31] Another first-in-human phase I clinical trial (NCT01877382) was designed to evaluate the safety and efficacy of milademetan in patients with advanced liposarcoma, solid tumors, or lymphomas. The study concluded that intermittent dosing could reduce hematologic complications while preserving efficacy, leading to plans for a randomized Phase III trial (MANTRA). [
32]
Idasanutlin + Venetoclax
Another Phase Ib/II study (NCT03850535) investigates the dual targeting of MDM2 and BCL-2 pathways using idasanutlin and venetoclax in relapsed/refractory AML [
33]. This approach aims to enhance mitochondrial apoptosis.
These different trials (NCT05447663; NCT03634228; NCT03850535, CTR20170975, NCT05012397, NCT01877382) [
16,
17,
29,
30,
32,
33] reflect ongoing interest in exploring a potential synergistic effect combining MDM2 inhibition with other modalities to enhance therapeutic efficacy and overcome resistance, particularly in hematologic malignancies.
Table 1.
Compounds targeting MDM2 in clinical phase.
Table 1.
Compounds targeting MDM2 in clinical phase.
| Compound Name |
Clinical Phase |
Study ID |
| Nutlin-3a (RG7112) |
Phase I |
NCT00623870, NCT00559533 |
| Idasanutlin (RG7388) |
Phase III |
NCT02545283 (MIRROS), NCT02624986, NCT03566485 |
| MI-77301 (SAR405838) |
Phase I |
NCT01636479 |
| AMG-232 (Navtemadlin, KRT-232) |
Phase I – III |
NCT01723020, NCT03662126, NCT04116541 |
| DS-3032b (Milademetan) |
Phase I / II |
NCT01877382, NCT02343172, NCT05012397 |
| BI 907828 (Brigimadlin) |
Phase I – III |
NCT03449381 (Brightline-2), NCT06058793 (Brightline-4) |
| APG-115 (Alrizomadlin) |
Phase I / II |
NCT02935907, NCT03781986, NCT04785196, CTR20170975 |
| HDM201 (Siremadlin) |
Phase I / II |
NCT02143635, NCT03107780 |
| ALRN-6924 (Sulanemadlin) |
Phase I / II |
NCT02264613 |
| KT-253 |
Phase I (ongoing) |
NCT05775406 |
3.5. Clinical Implications of MDM2 Inhibition
MDM2-targeted therapy has shown encouraging potential in several tumor types—particularly those with wild-type TP53 and MDM2 overexpression or amplification. Below are key malignancies where MDM2 inhibition is being actively explored:
3.5.1. AML
In Acute Myeloid Leukemia (AML), MDM2 is highly significant due to its critical role in regulating the p53 tumor suppressor protein. In particular, in cancers with wild-type TP53 (TP53-WT), like 80% of patients with AML, targeting MDM2 may be a promising therapeutic strategy. The MIRRORS clinical trial (NCT02545283)is a randomized, placebo controlled, phase III trial to evaluate the combination between cytarabine ± idasanutlin in relapsed or refractory AML.[
16]. Another interesting strategy was evaluated testing dual MDMX/MDM2 inhibitors like ALRN-6924, recently entered phase I clinical testing. This molecule has been demonstrated to inhibit cellular proliferation by inducing cell cycle and arrest and apoptosis in cell lines and primary AML patient cells. [
34]
3.5.2. Dedifferentiated Liposarcoma (DDLPS)
DDLPS is the paradigmatic example of an MDM2-driven tumor. Over 90% of DDLPS tumors harbor amplification of the MDM2 gene, making it both a diagnostic hallmark and a prime therapeutic target [
12].
The Brightline-1 trial is a pivotal Phase II/III study evaluating brigimadlin (BI 907828) versus doxorubicin as first-line therapy in patients with advanced or metastatic DDLPS. The study includes a crossover design and incorporates quality-of-life endpoints (e.g., EORTC QLQ-C30, PGIS/PGIC) to assess both efficacy and patient experience [
12]. Preliminary data suggest that brigimadlin offers improved disease control and a favorable safety profile.
3.5.3. Urothelial Carcinoma (UC)
In muscle-invasive and metastatic UC, MDM2 overexpression has been linked to resistance to platinum-based chemotherapy, immune checkpoint inhibitors (ICIs), and immunotherapy. Molecular profiling shows that MDM2-amplified tumors exhibit lower CD8+ T-cell infiltration and decreased PD-L1 expression, contributing to an immune-cold microenvironment. These findings support trials combining MDM2 inhibitors with ICIs to reprogram the tumor immune phenotype [
35]. Therefore, it has been suggested that MDM2, beyond PD-L1, should be evaluated to predict a better response to combo/single therapies [
36].
3.5.4. Colorectal Cancer (CRC)
MDM2 amplification in CRC has been more frequently observed in primary, non-metastatic tumors than in distant metastases, suggesting a potential role in early tumorigenesis or local invasion rather than distant spread [
37]. While not yet a major therapeutic target in CRC, MDM2 may gain relevance as a predictive biomarker in select subsets with intact TP53.
3.5.5. Melanoma and Regional Chemotherapy Resistance
Although MDM2 overexpression is predominantly associated with systemic resistance mechanisms, emerging evidence suggests its potential role in predicting locoregional treatment outcomes. A study by Russano et al. (2023) analyzed MDM2 and SURVIVIN expression in 62 patients undergoing isolated limb perfusion (ILP) with TNF and melphalan for in-transit cutaneous melanoma metastases. The results showed that high MDM2 expression was independently associated with lower rates of complete clinical response and shorter disease-free survival, suggesting a role for MDM2 in mediating resistance to TNF-based regional chemotherapy. Interestingly, patients with dual expression of MDM2 and SURVIVIN had poor outcomes, whereas those with no expression of either biomarker showed the highest rates of durable response. These findings support the potential utility of MDM2 expression as a predictive biomarker even in locoregional therapeutic settings and warrant further exploration of MDM2-targeted agents to enhance ILP efficacy in melanoma [
38].
3.5.6. Glioblastoma
The strategy of inhibiting the interaction between MDM2 and p53 is a promising new approach to fighting brain cancer, especially in the treatment of glioblastoma (GBM). Preclinical studies in cell cultures and animal models have shown encouraging results, suggesting that MDM2 inhibitors may be effective for a specific group of GBM patients who have a wild-type (non-mutated) TP53 gene. [
39] In addition, the idea is that combining MDM2 inhibitors with standard treatments like radiation and chemotherapy, or with other targeted molecular therapies, could enhance the overall effect and lead to a more significant therapeutic response also in GBM.
Despite these promising findings, many MDM2 inhibitors are still in the early stages of clinical trials for GBM. Their efficacy, both alone and in combination, has not yet been definitively confirmed. [
40]
3.5.7. Breast Cancer
MDM2 role in breast cancer is complex and involves both p53-dependent and p53-independent mechanisms. MDM2 levels can be elevated in up to 40% of estrogen receptor-positive breast cancers, often due to gene amplification or overexpression.[
41]. Breast cancer cells with estrogen and progesterone receptors showed MDM2 overexpression in wild-type TP53. MDM2 treatment is specific to breast cancer patients with wild-type TP53, because of the modified binding of TP53 to MDM2. In patients with breast cancer in the advanced stages of metastasis, SMAD family member 3 (SMAD3) has been implicated in inducing MDM2 transcription via its second promoter. MDM2 expression may provide a more accurate prognostic indicator in breast cancer patients than solely evaluating the p53 status. As discussed in the previous cases a combination therapy approach appears to be the most promising therapeutic alternative also for breast cancer. [
41,
42]
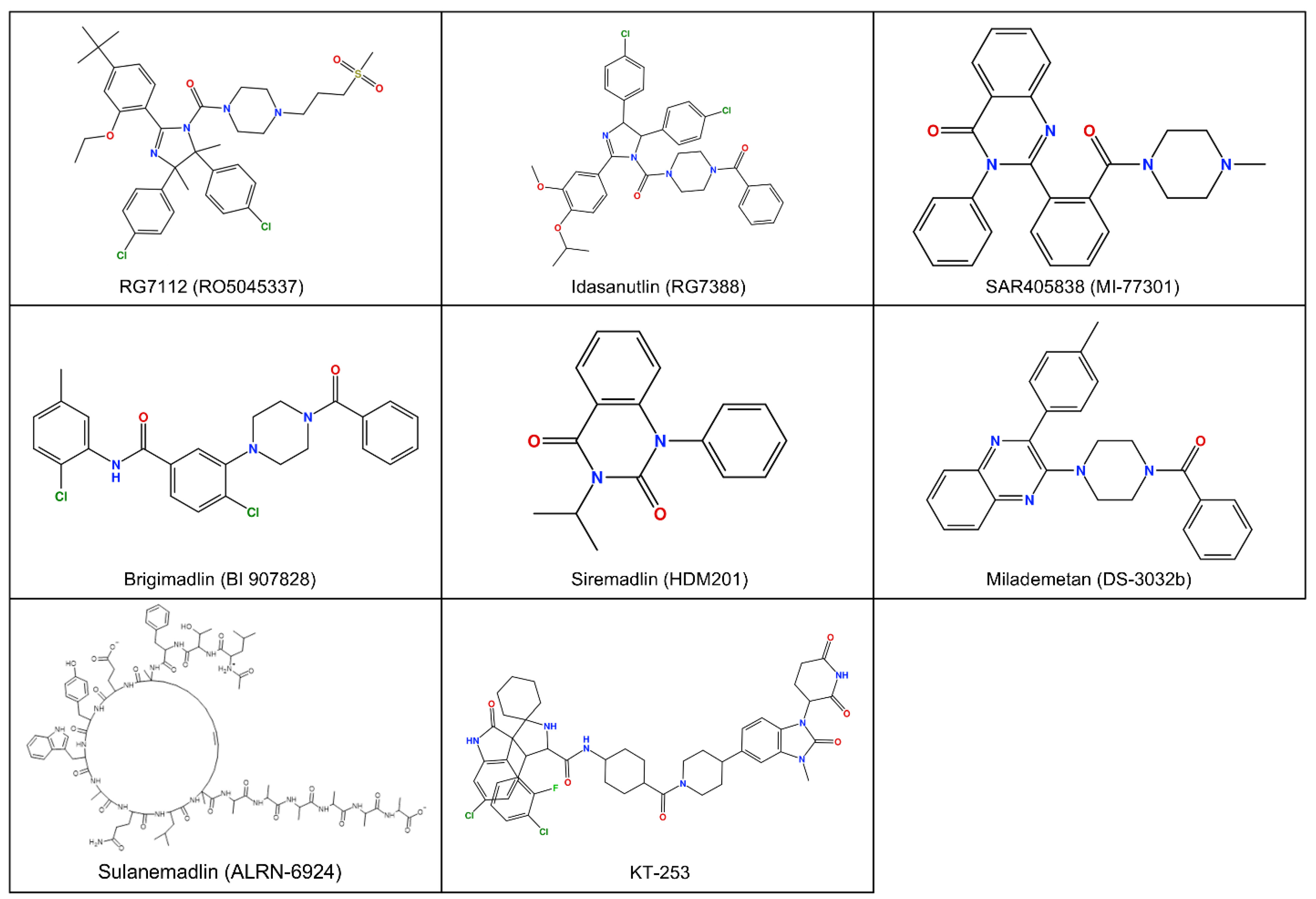
Figure 1.
Chemical 2D structure of compounds targeting MDM2 in clinical phase
Figure 1.
Chemical 2D structure of compounds targeting MDM2 in clinical phase
Figure 2.
Structural basis of p53-MDM2 protein-protein interaction inhibition. Left: The interaction between p53 (green) and MDM2 (gray) highlights the native binding mechanism (PDB ID: 1YCR) [
11]. The p53 α-helix inserts into the MDM2 cleft, with three critical hydrophobic residues (Phe19, Trp23, and Leu26) and one polar Glu28 residue anchoring the peptide. Right: Brigimadlin (orange), a clinical-stage MDM2 inhibitor, binds competitively to the same hydrophobic cleft on MDM2 (gray), sterically blocking p53 association. The compound mimics the helical p53 interface, exploiting hydrophobic contacts (e.g., Trp23 pocket) to disrupt MDM2’s negative regulation of p53 (PDB ID: 8PWC) [
25].
Figure 2.
Structural basis of p53-MDM2 protein-protein interaction inhibition. Left: The interaction between p53 (green) and MDM2 (gray) highlights the native binding mechanism (PDB ID: 1YCR) [
11]. The p53 α-helix inserts into the MDM2 cleft, with three critical hydrophobic residues (Phe19, Trp23, and Leu26) and one polar Glu28 residue anchoring the peptide. Right: Brigimadlin (orange), a clinical-stage MDM2 inhibitor, binds competitively to the same hydrophobic cleft on MDM2 (gray), sterically blocking p53 association. The compound mimics the helical p53 interface, exploiting hydrophobic contacts (e.g., Trp23 pocket) to disrupt MDM2’s negative regulation of p53 (PDB ID: 8PWC) [
25].
3.6. Future Perspectives
Several avenues are being investigated to enhance the clinical effectiveness of MDM2 inhibitors.
Biomarker Stratification: TP53 mutational status remains the primary criterion for patient selection. However, additional markers (such as MDMX co-expression, p14^ARF levels, and immune profiles) may refine patient stratification, thus optimizing the identification of those most likely to benefit from treatment [
7,
25].
Overcoming Resistance: The emergence of resistance to MDM2 inhibitors is a major limit to their long-term effectiveness. The most common causes include acquisitive mutations in the TP53 gene that compromise its function, overexpression of MDMX, compensatory for MDM2 inhibition, and activation of alternative pro-oncogenic signalling pathways such as PI3K/AKT and MAPK. For this reason, combination therapy strategies aimed at bypassing or overcoming these resistance mechanisms are currently being studied. These approaches include the use of dual inhibitors that act simultaneously on MDM2 and MDMX, combination with immunotherapies based on immune checkpoint blockade (e.g., anti-PD-1/PD-L1, anti-CTLA-4), and the use of PROTACs for the targeted degradation of MDM2, potentially overcoming the limitations of traditional inhibitors. [
25,
26,
43].
Toxicity Management: Hematologic toxicities, including thrombocytopenia and neutropenia, are common effects of MDM2 inhibitors. This often limits the dose that can be administered and compromises the treatment compliace. Optimizing dosing schedules, including intermittent or fractionated administration and using supportive care interventions (e.g., GCSFs, transfusions) are crucial to sustaining the therapeutic index [
16].
Novel Delivery Platforms: Nanocarrier formulations and tumor-selective delivery mechanisms would allow overcoming the limitations associated with systemic distribution, enhancing issue-specific activity and reducing off-target toxic effects. These new strategies are under evaluation in different preclinical settings.
Expanding to New Indications:
Beyond DDLPS and AML, MDM2 inhibitors are being investigated in breast cancer, NSCLC, and gliomas with wild-type TP53 [
26,
44,
45].
4. Conclusions
MDM2 is a critical negative regulator of p53 and functions as an oncogenic driver in a wide array of human cancers. Its overexpression or amplification contributes to tumorigenesis via both p53-dependent and independent mechanisms. The therapeutic strategy of disrupting the MDM2-p53 interaction has led to the development of multiple targeted agents, some of which have shown encouraging results in preclinical and early clinical trials, particularly in tumors with wild-type TP53, such as dedifferentiated liposarcoma and certain hematologic malignancies. However, the clinical utility of MDM2 inhibitors is tempered by challenges such as acquired resistance (e.g., TP53 mutations), MDMX co-overexpression, and hematologic toxicity.
Rational combination strategies, including association with chemotherapy, immune checkpoint inhibitors, and dual degraders or PROTACs, may improve the efficacy and durability of response to MDM2 inhibitors.
Ongoing trials will further clarify the role of MDM2 inhibition across solid and hematologic malignancies. In summary, MDM2 represents both a robust biomarker and a therapeutically actionable target for future precision oncology.
Author Contributions
Conceptualization, F.R., L.D.O. and S.M.1,3 ; methodology, F.R. and M.R..; software, M.S.; investigation, F.R., M.S., L.D.O. and F.C. ; data curation, D.B., P.D.F., V.P. and A.P.; writing—original draft preparation, F.R., M.S. and S.M.2; writing—review and editing, L.D.O., F.C. and M.R.; supervision, M.R. and S.M.1,3; funding acquisition, S.M.1,3 All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was funded by “Current Research” funds from the Italian Ministry of Health to cover publication costs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
Biliary tract cancer (BTC), Colorectal Cancer (CRC), Differentiated Liposarcoma (DDLPS), Immune Checkpoint Inhibitors (ICIs), Isolated Limb Perfusion (ILP), Murine Double Minute 2 (MDM2), Non-Small Cell Lung Cancer (NSCLC), Pancreatic ductal adenocarcinoma (PDAC), Protein-Protein Interaction (PPI), Proteolysis-Targeting Chimeras (PROTACs), Reactivation of p53 and Induction of Tumor cell Apoptosis (RITA), Urothelial Carcinoma (UC).
References
- Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013 Feb;13(2):83-96. [CrossRef]
- Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998 Aug 1;26(15):3453-9.
- Kato S, Ross JS, Gay L, Dayyani F, Roszik J, Subbiah V, Kurzrock R. Analysis of MDM2 Amplification: Next-Generation Sequencing of Patients With Diverse Malignancies. JCO Precis Oncol. 2018;2018:PO.17.0023. [CrossRef]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Oct;62(10):e1-34. [CrossRef] [PubMed]
- Schöffski P, Lahmar M, Lucarelli A, Maki RG. Brightline-1: phase II/III trial of the MDM2-p53 antagonist BI 907828 versus doxorubicin in patients with advanced DDLPS. Future Oncol. 2023 Mar;19(9):621-629.
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004 Feb 6;303(5659):844-8. [CrossRef]
- Manfredi JJ. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010 Aug 1;24(15):1580-9. Erratum in: Genes Dev. 2010 Sep 15;24(18):2105.
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998 Sep 1;17(17):5001-14.
- Kung CP, Weber JD. It's Getting Complicated-A Fresh Look at p53-MDM2-ARF Triangle in Tumorigenesis and Cancer Therapy. Front Cell Dev Biol. 2022 Jan 26;10:818744.
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80-3. [CrossRef]
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009 Dec;9(12):862-73.
- Ray-Coquard I, Blay JY, Italiano A, Le Cesne A, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, Geho D, Middleton SA, Vassilev LT, Nichols GL, Bui BN. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012 Nov;13(11):1133-40.
- Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008 Sep 1;14(17):5318-24.
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996 Nov 8;274(5289):948-53.
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1888-93.
- Montesinos P, Beckermann BM, Catalani O, Esteve J, Gamel K, Konopleva MY, Martinelli G, Monnet A, Papayannidis C, Park A, Récher C, Rodríguez-Veiga R, Röllig C, Vey N, Wei AH, Yoon SS, Fenaux P. MIRROS: a randomized, placebo-controlled, Phase III trial of cytarabine ± idasanutlin in relapsed or refractory acute myeloid leukemia. Future Oncol. 2020 May;16(13):807-815.
- Konopleva MY, Röllig C, Cavenagh J, Deeren D, Girshova L, Krauter J, Martinelli G, Montesinos P, Schäfer JA, Ottmann O, Petrini M, Pigneux A, Rambaldi A, Recher C, Rodriguez-Veiga R, Taussig D, Vey N, Yoon SS, Ott M, Muehlbauer S, Beckermann BM, Catalani O, Genevray M, Mundt K, Jamois C, Fenaux P, Wei AH. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022 Jul 26;6(14):4147-4156.
- LoRusso P, Yamamoto N, Patel MR, Laurie SA, Bauer TM, Geng J, Davenport T, Teufel M, Li J, Lahmar M, Gounder MM. The MDM2-p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced or Metastatic Solid Tumors: Results of a Phase Ia, First-in-Human, Dose-Escalation Study. Cancer Discov. 2023 Aug 4;13(8):1802-1813.
- Yoo C, Lamarca A, Choi HJ, Vogel A, Pishvaian MJ, Goyal L, Ueno M, Märten A, Teufel M, Geng L, Morizane C. Brightline-2: a phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024;20(16):1069-1077. [CrossRef]
- Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004 Dec;10(12):1321-8.
- Alaseem AM. Advancements in MDM2 inhibition: Clinical and pre-clinical investigations of combination therapeutic regimens. Saudi Pharm J. 2023 Oct;31(10):101790.
- Chen L, Zhao Y, Halliday GC, Berry P, Rousseau RF, Middleton SA, Nichols GL, Del Bello F, Piergentili A, Newell DR, Lunec J, Tweddle DA. Structurally diverse MDM2-p53 antagonists act as modulators of MDR-1 function in neuroblastoma. Br J Cancer. 2014 Aug 12;111(4):716-25. [CrossRef]
- Zeng Q, Zeng S, Dai X, Ding Y, Huang C, Ruan R, Xiong J, Tang X, Deng J. MDM2 inhibitors in cancer immunotherapy: Current status and perspective. Genes Dis. 2024 Mar 28;11(6):101279.
- Pairawan S, Zhao M, Yuca E, Annis A, Evans K, Sutton D, Carvajal L, Ren JG, Santiago S, Guerlavais V, Akcakanat A, Tapia C, Yang F, Bose PSC, Zheng X, Dumbrava EI, Aivado M, Meric-Bernstam F. First in class dual MDM2/MDMX inhibitor ALRN-6924 enhances antitumor efficacy of chemotherapy in TP53 wild-type hormone receptor-positive breast cancer models. Breast Cancer Res. 2021 Mar 4;23(1):29.
- Saleh MN, Patel MR, Bauer TM, Goel S, Falchook GS, Shapiro GI, Chung KY, Infante JR, Conry RM, Rabinowits G, Hong DS, Wang JS, Steidl U, Naik G, Guerlavais V, Vukovic V, Annis DA, Aivado M, Meric-Bernstam F. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin Cancer Res. 2021 Oct 1;27(19):5236-5247. Erratum in: Clin Cancer Res. 2022 Jan 15;28(2):429. [CrossRef]
- Churcher I. Protac-induced protein degradation in drug discovery: Breaking the Rules or Just Making New Ones? J Med Chem. 2018 Jan 25;61(2):444-452.
- Chutake YK, Mayo MF, Dumont N, Filiatrault J, Breitkopf SB, Cho P, Chen D, Dixit VS, Proctor WR, Kuhn EW, Bollinger Martinez S, McDonald AA, Qi J, Hu KN, Karnik R, Growney JD, Sharma K, Schalm SS, Gollerkeri AM, Mainolfi N, Williams JA, Weiss MM. KT-253, a Novel MDM2 Degrader and p53 Stabilizer, Has Superior Potency and Efficacy than MDM2 Small-Molecule Inhibitors. Mol Cancer Ther. 2025 Apr 2;24(4):497-510.
- Guerlavais V, Sawyer TK, Carvajal L, Chang YS, Graves B, Ren JG, Sutton D, Olson KA, Packman K, Darlak K, Elkin C, Feyfant E, Kesavan K, Gangurde P, Vassilev LT, Nash HM, Vukovic V, Aivado M, Annis DA. Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized α-Helical Peptide in Clinical Development. J Med Chem. 2023 Jul 27;66(14):9401-9417.
- Stein EM, DeAngelo DJ, Chromik J, Chatterjee M, Bauer S, Lin CC, Suarez C, de Vos F, Steeghs N, Cassier PA, Tai D, Kiladjian JJ, Yamamoto N, Mous R, Esteve J, Minami H, Ferretti S, Guerreiro N, Meille C, Radhakrishnan R, Pereira B, Mariconti L, Halilovic E, Fabre C, Carpio C. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin Cancer Res. 2022 Mar 1;28(5):870-881.
- Zhang X, Wen X, Peng R, Pan Q, Weng D, Ma Y, Zhang Y, Yang J, Men L, Wang H, Liang E, Wang C, Yang D, Zhang L, Zhai Y. A first-in-human phase I study of a novel MDM2/p53 inhibitor alrizomadlin in advanced solid tumors. ESMO Open. 2024 Aug;9(8):103636.
- Dumbrava EE, Stinchcombe TE, Gounder M, Cote GM, Hanna GJ, Sumrall B, Wise-Draper TM, Kanaan M, Duffy S, Sumey C, Cobb P, Forbes A, Beckmann A, Schadt E, Ku N, Tirunagaru VG, Singh K, Pei X, Xu F, Doebele RC, Chen CT. Milademetan in advanced solid tumors with MDM2 amplification and wild-type TP53: pre-clinical and phase 2 clinical trial results. Clin Cancer Res. 2025 Aug 11. [CrossRef]
- Zhang X, Wen X, Peng R, Pan Q, Weng D, Ma Y, Zhang Y, Yang J, Men L, Wang H, Liang E, Wang C, Yang D, Zhang L, Zhai Y. A first-in-human phase I study of a novel MDM2/p53 inhibitor alrizomadlin in advanced solid tumors. ESMO Open. 2024 Aug;9(8):103636.
- Daver NG, Dail M, Garcia JS, Jonas BA, Yee KWL, Kelly KR, Vey N, Assouline S, Roboz GJ, Paolini S, Pollyea DA, Tafuri A, Brandwein JM, Pigneux A, Powell BL, Fenaux P, Olin RL, Visani G, Martinelli G, Onishi M, Wang J, Huang W, Green C, Ott MG, Hong WJ, Konopleva MY, Andreeff M. Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood. 2023 Mar 16;141(11):1265-1276.
- Carvajal LA, Neriah DB, Senecal A, Benard L, Thiruthuvanathan V, Yatsenko T, Narayanagari SR, Wheat JC, Todorova TI, Mitchell K, Kenworthy C, Guerlavais V, Annis DA, Bartholdy B, Will B, Anampa JD, Mantzaris I, Aivado M, Singer RH, Coleman RA, Verma A, Steidl U. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci Transl Med. 2018 Apr 11;10(436):eaao3003.
- Wu H, Jin H, Cai Y. Unveiling therapeutic prospects: targeting MDM-2 in non-muscle invasive bladder cancer. J Biomol Struct Dyn. 2024 Mar 18:1-10.
- Brunelli M, Tafuri A, Cima L, Cerruto MA, Milella M, Zivi A, Buti S, Bersanelli M, Fornarini G, Vellone VG, Rebuzzi SE, Procopio G, Verzoni E, Bracarda S, Sabbatini R, Baldessari C, Eccher A, Passalacqua R, Perrucci B, Giganti MO, Donini M, Panni S, Tucci M, Prati V, Ortega C, Caliò A, Alongi F, Munari E, Pappagallo G, Iacovelli R, Mosca A, Porta C, Martignoni G, Antonelli A. MDM2 gene amplification as selection tool for innovative targeted approaches in PD-L1 positive or negative muscle-invasive urothelial bladder carcinoma. J Clin Pathol. 2022 Jan;75(1):39-44.
- Forslund A, Zeng Z, Qin LX, Rosenberg S, Ndubuisi M, Pincas H, Gerald W, Notterman DA, Barany F, Paty PB. MDM2 gene amplification is correlated to tumor progression but not to the presence of SNP309 or TP53 mutational status in primary colorectal cancers. Mol Cancer Res. 2008 Feb;6(2):205-11. [CrossRef]
- Russano F, Del Fiore P, Cassalia F, Benna C, Dall'Olmo L, Rastrelli M, Mocellin S. Do Tumor SURVIVIN and MDM2 Expression Levels Correlate with Treatment Response and Clinical Outcome in Isolated Limb Perfusion for In-Transit Cutaneous Melanoma Metastases? J Pers Med. 2023 Nov 28;13(12):1657.
- Pellot Ortiz KI, Rechberger JS, Nonnenbroich LF, Daniels DJ, Sarkaria JN. MDM2 Inhibition in the Treatment of Glioblastoma: From Concept to Clinical Investigation. Biomedicines. 2023 Jul 2;11(7):1879.
- Vaubel RA, Zhang W, Oh JH, Mladek AC, Pasa TI, Gantchev JK, Waller KL, Baquer G, Stopka SA, Regan MS, Hossain MA, Decker PA, Kosel ML, Gupta SK, Jain S, Sarkaria PP, Hu Z, Ott LL, Carlson BL, Bakken KK, Talele S, Zhang W, Ligon KL, Lee EQ, Eckel Passow JE, Burgenske DM, Agar NYR, Elmquist WF, Sarkaria JN. Preclinical Modeling of Navtemadlin Pharmacokinetics, Pharmacodynamics, and Efficacy in IDH-Wild-type Glioblastoma. Clin Cancer Res. 2025 Sep 2;31(17):3771-3786.
- Di Grazia G, Conti C, Nucera S, Stella S, Massimino M, Giuliano M, Schettini F, Vigneri P, Martorana F. Bridging the Gap: the role of MDM2 inhibition in overcoming treatment resistance in breast cancer. Crit Rev Oncol Hematol. 2025 Oct;214:104834.
- Yousuf A, Khan NU. Targeting MDM2-p53 interaction for breast cancer therapy. Oncol Res. 2025 Mar 19;33(4):851-861.
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002 Mar;12(1):9-18.
- Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barrière C, Stuckey JA, Meagher JL, Bai L, Liu L, Hoffman-Luca CG, Lu J, Shangary S, Yu S, Bernard D, Aguilar A, Dos-Santos O, Besret L, Guerif S, Pannier P, Gorge-Bernat D, Debussche L. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014 Oct 15;74(20):5855-65. [CrossRef]
- Gollner A, Rudolph D, Weyer-Czernilofsky U, Baumgartinger R, Jung P, Weinstabl H, Ramharter J, Grempler R, Quant J, Rinnenthal J, Pérez Pitarch A, Golubovic B, Gerlach D, Bader G, Wetzel K, Otto S, Mandl C, Boehmelt G, McConnell DB, Kraut N, Sini P. Discovery and Characterization of Brigimadlin, a Novel and Highly Potent MDM2-p53 Antagonist Suitable for Intermittent Dose Schedules. Mol Cancer Ther. 2024 Dec 3;23(12):1689-1702.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).