1. Introduction
Hydrogels are three-dimensional networks of hydrophilic polymers capable of absorbing and retaining large amounts of water, making them one of the most versatile classes of biomaterials in biomedical science[
1]. Their high-water content, softness, and biocompatibility allow them to closely mimic the extracellular matrix (ECM) of biological tissues such as skin, cartilage, and mucosa, which has led to their widespread use in wound healing, drug delivery, tissue engineering, and biosensing applications [
2,
3]. Traditionally, bulk hydrogels have dominated the field due to their excellent swelling behavior, permeability, and responsiveness to environmental stimuli such as pH, temperature, and ionic strength [
4]. However, recent advances have shifted attention toward
hydrogel films—thin, flexible layers of hydrogel materials with thicknesses ranging from nanometers to micrometers—which offer unique advantages in biomedical contexts.[
5]
Hydrogel films represent a significant evolution in hydrogel technology [
6]. Their thin-film architecture allows for intimate contact with biological surfaces, enabling applications such as transdermal patches, implant coatings, ophthalmic devices, and bioelectronic interfaces [
7,
8,
9,
10,
11]. Compared to bulk hydrogels, hydrogel films exhibit faster response times, greater surface adaptability, and enhanced integration with biomedical devices. These properties are particularly valuable in dynamic physiological environments where responsiveness and conformability are critical [
12].
The fabrication of hydrogel films has advanced significantly, with techniques such as
solvent casting [13], spin coating [14], layer-by-layer assembly [15],
photopolymerization [16], and
3D printing [17] enabling precise control over film thickness, architecture, and functionality [
18]. These methods allow for the incorporation of
stimuli-responsive elements, bioactive molecules, and
nanomaterials, transforming hydrogel films into smart systems capable of dynamic interaction with biological environments [
19]. For example, thermo-responsive hydrogels like poly(N-isopropylacrylamide) (PNIPAm) exhibit phase transitions near body temperature, enabling on-demand drug release and real-time biosensing [
20].
Hydrogel films can be composed of
natural polymers such as chitosan, gelatin, and alginate, which offer excellent biocompatibility and biodegradability [
21,
22,
23], or
synthetic polymers like polyvinyl alcohol (PVA), polyacrylamide (PAAm), and polyethylene glycol (PEG), which provide tunable mechanical strength and chemical stability [
24,
25,
26]. Hybrid hydrogels that combine natural and synthetic components are increasingly used to balance bioactivity with durability [
27,
28]. Crosslinking mechanisms—whether physical, chemical, or enzymatic—play a crucial role in determining the mechanical and biological properties of hydrogel films. Physically crosslinked hydrogels formed via hydrogen bonding or ionic interactions are reversible and suitable for dynamic applications, while chemically crosslinked hydrogels offer permanent structures ideal for long-term implantation [
29,
30,
31].
In biomedical applications, hydrogel films have demonstrated exceptional promise [
32]. In
wound healing, they maintain a moist environment, absorb exudates, and deliver therapeutic agents, thereby accelerating tissue regeneration [
33,
34,
35,
36]. Self-healing hydrogel films that withstand mechanical stress during dressing changes further improve patient comfort and reduce secondary damage [
37,
38,
39,
40,
41].
In
drug delivery, hydrogel films serve as platforms for localized and sustained release, reducing systemic toxicity and improving therapeutic efficacy [
42,
43,
44,
45]. Their porous structure allows for high drug loading and controlled release kinetics, which can be tailored through polymer composition and crosslinking density. Stimuli-responsive hydrogel films can release drugs in response to environmental changes, such as pH shifts or temperature variations, enabling precision therapy [
46]. Commercial hydrogel products have already been developed for transdermal, ocular, and injectable drug delivery routes, demonstrating the clinical viability of hydrogel film technologies [
47].
In
tissue engineering, hydrogel films act as scaffolds that support cell growth and differentiation [
48,
49,
50]. Their ability to mimic the mechanical and biochemical properties of native tissues makes them suitable for regenerating skin, cartilage, and bone [
51,
52,
53]. Composite hydrogel films incorporating bioactive molecules like hydroxyapatite or collagen have shown improved osteogenic and angiogenic potential [
54]. Advanced fabrication techniques, such as 3D bioprinting, enable the creation of hydrogel films with complex architectures and spatial control over cell distribution, paving the way for personalized tissue constructs [
55].
Despite their promising applications, hydrogel films face several challenges. Mechanical fragility, limited scalability, and variable degradation rates can hinder clinical translation [
56,
57,
58]. Ensuring long-term biocompatibility and avoiding immune responses remain critical concerns. To address these issues, researchers are exploring
nanocomposite hydrogels, bioinspired designs, and
hybrid crosslinking strategies that enhance mechanical strength and biological performance [
59]. The integration of hydrogel films with electronic components for biosensing and soft robotics is another emerging frontier. These systems can monitor physiological signals, deliver drugs, and respond to stimuli in real time, offering new possibilities for
wearable and implantable medical devices [
60].
In summary, hydrogel films represent a transformative advancement in biomaterials science, offering a unique combination of biocompatibility, functional versatility, and engineering precision (
Scheme 1). Their ability to mimic biological tissues, respond to environmental cues, and deliver therapeutic agents positions them as key players in the future of personalized medicine, regenerative therapies, and bio-integrated technologies. As fabrication techniques and material designs continue to evolve, hydrogel films are poised to redefine the landscape of biomedical applications [
61,
62,
63].
To support this perspective, the present review provides a structured and comprehensive overview of hydrogel films, beginning with their unique physicochemical properties and fabrication strategies. We then explore their diverse biomedical applications—including wound dressings, drug delivery systems, tissue engineering scaffolds, ophthalmic devices, and implantable biosensors—highlighting both established uses and emerging innovations. Recent advances in stimuli-responsive and nanocomposite hydrogel films are discussed, followed by an analysis of current challenges such as mechanical durability, sterilization, and scalability. Finally, we present future directions for hydrogel film technologies, including personalized designs, AI-guided optimization, and clinical translation, aiming to illuminate their growing impact in next-generation biomedical solutions.
2. Materials and Composition of Hydrogel Films
2.1. Natural Biopolymers: Chitosan, Alginate, Gelatin, and Others
Natural biopolymers remain at the forefront of hydrogel film development due to inherent biocompatibility, biodegradability, and favorable biological interactions. Chitosan, derived from the deacetylation of chitin, exhibits polycationic properties that enable the formation of electrostatic complexes with negatively charged polymers, enhancing mechanical strength and biological functionality. Chitosan’s chemistry facilitates biocompatibility and antimicrobial activity, making it ideal for wound dressings and drug delivery carriers. Chitosan-based hydrogel films have been extensively studied for these properties and their applications, showing promise across antibacterial dressings and sustained drug release matrices [
64,
65,
66].
Alginate, a polysaccharide obtained from brown seaweed, is widely used in wound healing due to its gel-forming ability in the presence of divalent cations like calcium. Alginate-based hydrogels are notable for their water absorption and swelling capacity, providing moist environments and hemostatic effects, which are beneficial in wound dressings. Optimizing alginate content and crosslinking agents allows tunable properties for various biomedical needs, including enhanced mechanical integrity and controlled degradation [
67,
68].
Gelatin, denatured collagen, offers cell-interactive sequences and biodegradability, making gelatin-containing hydrogels suitable as tissue support matrices, especially in cartilage and soft tissue engineering. These hydrogels support cellular adhesion, proliferation, and differentiation, replicating natural extracellular matrix cues crucial for regenerative medicine applications [
68,
69].
2.2. Synthetic and Semi-Synthetic Polymers in Hydrogel Formulations
Synthetic polymers complement natural polymers by enhancing the mechanical strength, stability, and processability of hydrogel films. Polyethylene glycol (PEG) serves as a hydrophilic, biocompatible backbone often used in hydrogel formulations owing to its low toxicity and ability to confer swelling characteristics. Polyvinyl alcohol (PVA) is employed for its film-forming capabilities and mechanical robustness. Copolymer systems incorporating these polymers provide flexibility in tailoring physical, chemical, and biological properties to specific biomedical applications. Several studies have demonstrated PEG and PVA-based hydrogel films with controlled drug release profiles and improved mechanical parameters suited for wound dressings and transdermal delivery [
70,
71,
72].
Polyurethane copolymers have been utilized to fabricate hydrophobic films with enhanced water resistance and mechanical durability. When surface-modified with hydrophilic polymers like polyvinyl pyrrolidone via chemical reactions, these films exhibit concurrent water permeation resistance and biocompatibility, allowing their use in implantable biomedical devices that require protection from internal body fluids while maintaining compatibility [
70,
73].
Hybrid composites and functional modifications represent an advanced approach to hydrogel film design. Incorporation of materials such as humic acids or citric acid-crosslinking agents introduces antibacterial properties and enhances physicochemical behavior without compromising biodegradability. These modifications allow tuning of water absorption, thermal stability, and mechanical integrity, critical for optimized biomedical performance [
74,
75].
2.3. Composite and Multifunctional Hydrogel Films
Composite hydrogel films integrate nanoparticles, emulsions, and bioactive compounds to augment functional properties. The inclusion of Pickering emulsions stabilized by nanomaterials such as oxidized cellulose nanocrystals and silver nanoparticles has been explored to achieve self-crosslinking networks with synergistic wound healing effects, combining antimicrobial and antioxidant activities alongside mechanical reinforcement [
76]. Similarly, nanocomposite films dispersing materials like sodium montmorillonite exhibit pH-responsive drug release and enhanced mechanical strength, making them suitable for anticancer drug delivery [
77].
Natural bioactive extracts, such as mangostin from mangosteen peel and curcumin complexed with cyclodextrins, have been successfully incorporated into hydrogel films to exploit their wound healing, anti-inflammatory, and antioxidant effects. These composite systems improve therapeutic outcomes while maintaining film integrity and biocompatibility [
65,
78]
Further, composite hydrogel films have been engineered with conductive or structural enhancements using materials like carbon nanotubes and graphene, enabling applications in bioelectronics and tissue engineering. These composites demonstrate self-assembly into hierarchical fibrous structures and provide electrical conductivity without impairing biocompatibility, thereby broadening the utility of hydrogels in multifunctional biomedical devices [
79,
80]
3. Synthesis and Fabrication Techniques of Hydrogel Films
3.1. Chemical Crosslinking and Physical Gelation Methods
The synthesis of hydrogel films often employs chemical crosslinking and physical gelation techniques to establish stable polymer networks. Ionic crosslinking is widely used, particularly with biopolymers such as alginate and chitosan, where divalent cations or polyelectrolyte complexes facilitate network formation. Additionally, UV-induced polymerization enables spatial and temporal control over crosslink density, allowing the fabrication of films with tailored mechanical and swelling properties. Schiff base formation, a reaction between amino and aldehyde groups, has been utilized to create dynamic covalent crosslinks within chitosan-alginate networks, enhancing film stability and facilitating controlled degradation [
65,
81,
82].
Crosslinking agents such as glutaraldehyde, potassium persulfate, and sodium tripolyphosphate play critical roles in modulating the properties of hydrogel films. Their concentration and reaction conditions influence gelation time, mechanical strength, and swelling capacity. For example, potassium persulfate initiates free radical polymerizations, yielding biodegradable films with desirable hardness and swelling in biopolymeric systems. Crosslink density often inversely affects swelling but positively impacts mechanical robustness, reflecting the trade-offs that must be balanced during fabrication [
81,
83,
84].
3.2. Fabrication Methods of Hydrogel Films
The fabrication of hydrogel films is a critical determinant of their structural, mechanical, and functional properties. Various methods have been developed to tailor hydrogel films for specific biomedical applications, ranging from wound dressings and drug delivery systems to biosensors and tissue scaffolds. This section discusses both conventional and emerging fabrication techniques, supported by recent research.
3.2.1. Film Formation
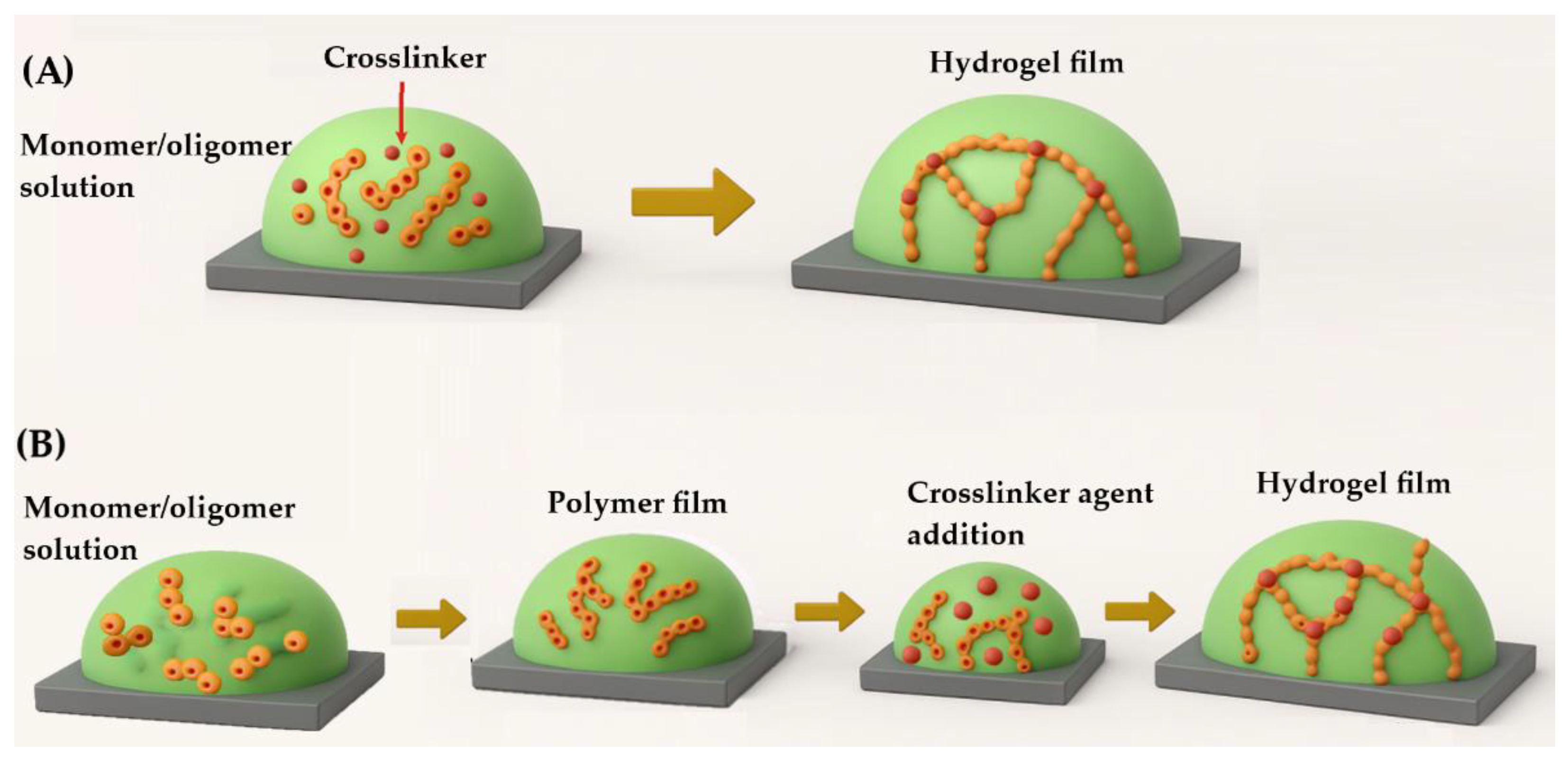
Hydrogel films can be fabricated using various techniques, which differ in their applicability depending on the material system and desired properties. These methods are generally categorized based on the timing of gelation relative to polymerization and film deposition. One key distinction is between in situ crosslinking and post-synthetic crosslinking. In the in situ approach, polymer chains are formed directly from monomers or oligomers during film deposition, with crosslinking occurring simultaneously to establish the hydrogel network [
85]. This process can be initiated by chemical agents or physical stimuli such as UV radiation
, plasma
, or thermal energy, which promote bond formation and network stabilization [
86].
In contrast, post-synthetic crosslinking involves first depositing a polymer film from a soluble precursor onto a substrate, followed by a separate crosslinking step. This secondary process may involve chemical crosslinkers or physical treatments like heat or irradiation, depending on the material and application [
87].
Figure 1 presents a schematic representation outlining the two possible methods for film preparation.
Interestingly, even when strong covalent bonds are present within the hydrogel matrix, the adhesion between the hydrogel film and the substrate is often governed by van der Waals interactions. These weak forces between polymer chains and the substrate surface allow hydrogel coatings to adhere without requiring specific surface functionalization or pre-treatment, making them compatible with a wide range of materials [
88].
Beyond conventional crosslinking methods, microwave-assisted synthesis has emerged as a promising alternative. This technique uses microwave irradiation to induce crosslinking in aqueous polymer solutions, offering advantages such as shorter reaction times
, reduced chemical waste, and higher product yields [
89,
90]. For example, Sun et al. utilized this method to fabricate carbon dot-crosslinked sodium alginate hydrogel films, while Thongsuksaengcharoen et al. applied it to prepare PVA/PVP/CA hydrogel systems
[89,91].
Microwave irradiation not only facilitates efficient crosslinking but also minimizes the need for chemical crosslinkers, potentially resulting in safer and cleaner hydrogel products. This makes it particularly attractive for biomedical applications where biocompatibility and purity are essential.
3.2.2. Preparation Methods
The selection of a suitable method for hydrogel film fabrication depends on several factors, including the chemical nature of the polymer, its adhesion behavior with the substrate, reaction kinetics, and overall cost-effectiveness of the process. Regardless of the specific route chosen, most hydrogel films are formed by applying a precursor solution onto a substrate, followed by drying or curing, which removes the solvent and promotes polymer crosslinking, resulting in a solid film.
A variety of techniques have been developed for this purpose. Below are some commonly used methods:
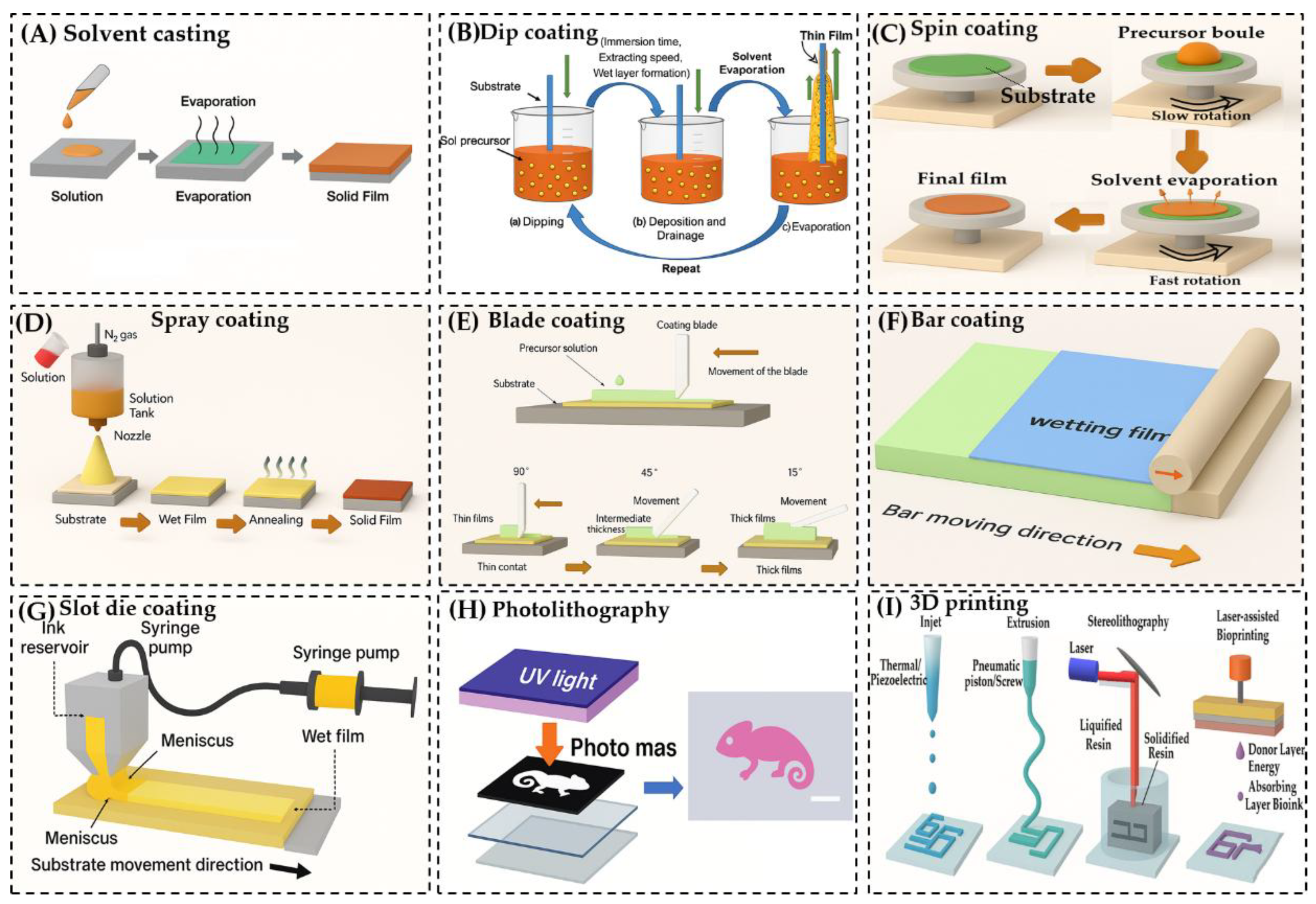
Solvent Casting [
92]: This straightforward technique involves dissolving the polymer in a solvent, casting the solution onto a substrate, and allowing the solvent to evaporate. The evaporation process leads to film formation and, in the case of hydrogels, is followed by gelation.
Dip Coating [
93]: The substrate is immersed in a polymer solution and withdrawn at a controlled rate, forming a thin film as the solution adheres to the surface.
Spin Coating [
14]: A small volume of polymer solution is dropped onto the center of a substrate, which is then rotated at high speed. Centrifugal force spreads the solution evenly, forming a uniform thin film.
Spray Coating [
94]: The polymer solution is atomized and sprayed onto the substrate, allowing for rapid and scalable film deposition.
Blade Coating [
95]: A blade is used to spread the polymer solution across the substrate, controlling film thickness through blade height and solution viscosity.
Bar Coating [
96]: Similar to blade coating, but uses a cylindrical bar wrapped with wire to distribute the solution evenly across the substrate.
Slot Die Coating [
97]: The polymer solution is dispensed through a narrow slit (die) directly onto the moving substrate, allowing for precise control over film thickness and uniformity.
Photolithography [
98]: A photosensitive polymer is exposed to UV light through a patterned mask, enabling the creation of microstructured hydrogel films with high spatial resolution.
3D Printing [
99]: Hydrogel structures are built layer-by-layer using techniques such as extrusion-based printing or stereolithography (SLA), which uses light to polymerize photosensitive resins with high precision.
These methods can be applied in both in situ crosslinking and post-synthetic crosslinking workflows, depending on the material system and desired film properties. Each technique offers unique advantages, but not all are universally compatible with every polymer type.
Solvent Casting Method
Solvent casting is one of the most accessible and widely used techniques for fabricating hydrogel films. The process begins by dissolving a polymer—along with any necessary additives—into a suitable solvent to form a homogeneous solution. This solution is then applied to a substrate, either by pouring or spreading, and left to dry under controlled conditions. As the solvent evaporates, the polymer chains align and consolidate into a solid film. In hydrogel systems, this is typically followed by a gelation step, which stabilizes the network structure [
Figure 2A][
100]. This method is favored for its simplicity, low cost, and minimal equipment requirements, making it suitable for both laboratory-scale and industrial applications. It also offers flexibility in terms of polymer selection and additive incorporation. The thickness and uniformity of the resulting film can be finely tuned by adjusting the solution concentration, volume, and drying conditions. However, solvent casting does have limitations. The use of organic solvents can pose toxicity risks, and residual solvent may remain trapped within the film matrix, potentially compromising biocompatibility and purity—especially in biomedical applications. Additionally, films produced via this method often exhibit lower mechanical strength compared to those fabricated using more advanced techniques.
Despite these drawbacks, solvent casting remains a valuable method for producing hydrogel films, particularly when ease of fabrication and material versatility are prioritized.
Dip Coating Method
Dip coating is a widely used and straightforward technique for producing thin hydrogel films. The process involves immersing a substrate into a container filled with
a polymer precursor solution, then withdrawing it at a controlled speed. After removal, the coated substrate is typically dried either by air drying, heating, or baking to solidify the film [
Figure 2B]. [
85,
94]. This method is popular due to its simplicity
, scalability, and potential for automation, making it suitable for both laboratory and industrial applications. The thickness of the resulting film is influenced by several factors, including the viscosity of the solution and the withdrawal speed. Generally, a faster withdrawal rate results in a thicker coating, while slower rates produce thinner films.
As the solvent evaporates, the liquid film on the substrate begins to thin and form a wedge-like profile, ending in a distinct drying line. When the speed of the drying front matches the withdrawal speed, the system reaches a steady-state condition. In hydrogel systems, gelation typically occurs in the upper region of the film, where polymer concentration and crosslinking agent levels are highest due to solvent loss and molecular accumulation.
This method allows for uniform coating on complex geometries and is compatible with various polymers and crosslinking strategies. A schematic illustration of the dip coating process is typically used to visualize the dynamics of film formation and drying.
Spin Coating Method
Spin coating is a widely adopted and efficient technique for producing uniform and thin films on flat substrates. The process involves placing a liquid precursor solution—typically containing polymers or hydrogel-forming agents—onto the center of a substrate. The substrate is then rotated at high speeds, typically ranging from 500 to 4000 rpm, which spreads the solution outward due to centrifugal force [
101] [
Figure 2C]. The solution can be applied either while the substrate is stationary or during slow rotation. Once dispensed, the substrate is rapidly accelerated to the desired spin speed. As the substrate spins, the liquid film thins out and begins to dry, forming a solid layer. The final film thickness is influenced by several key parameters:
Spin speed: Higher speeds generally produce thinner films.
Viscosity of the solution: More viscous solutions tend to yield thicker coatings.
Solvent evaporation rate: Faster evaporation can lead to quicker solidification and thinner films.
Volume of the applied solution: Larger volumes may result in thicker layers.
This method is particularly useful for creating hydrogel films with controlled thickness and surface uniformity. It is compatible with both in situ and post-synthetic crosslinking approaches, depending on the formulation and desired properties. A schematic diagram is often used to illustrate the spin coating process and its stages.
Spray Coating and Blade Coating Methods
Spray coating is a versatile technique used to fabricate hydrogel films by applying a fine mist of polymer precursor solution onto a substrate [
102]. This method is particularly effective for producing thin, uniform, and potentially porous coatings, and it can be adapted to substrates with complex geometries, making it suitable for a wide range of biomedical and industrial applications [
Figure 2D]. The process involves atomizing the precursor solution using a pressurized gas stream, which disperses the liquid into fine droplets through a spray nozzle or gun. These droplets are deposited onto the substrate in a controlled manner. As the solvent evaporates, a thin layer of polymer remains, which can then be crosslinked—either chemically or physically—to form a stable hydrogel network. Parameters such as gas pressure, nozzle design, and spray duration can be adjusted to control the thickness, surface roughness, and porosity of the final film. Depending on the formulation and process settings, the resulting hydrogel film may be porous or non-porous, offering flexibility for different functional requirements. Spray coating is valued for its scalability, cost-efficiency, and adaptability, making it suitable for both small-scale research and large-scale production. The ability to fine-tune coating parameters also allows for precise control over film characteristics.
Blade Coating and Bar Coating Methods
Blade coating, also known as doctor blade coating, is a straightforward and efficient technique for fabricating hydrogel films [
103]. In this method, a blade is moved across the surface of a substrate to evenly distribute a polymer solution. A small gap between the blade and the substrate determines the wet film thickness, which is influenced by factors such as the viscosity of the solution, coating speed, and blade geometry [
Figure 2E]. After the solution is spread, it undergoes drying, resulting in a solid hydrogel film. The angle of the blade plays a crucial role in controlling the final film thickness and uniformity:
A 90° blade angle generates high shear, ideal for producing thin and uniform coatings.
A 45° angle offers a balance between removing excess material and retaining some on the surface, yielding medium-thickness films.
Angles between 15° and 30° reduce shear pressure, allowing for thicker coatings, which are beneficial when working with high-viscosity formulations.
Blade coating is compatible with a wide range of polymer viscosities and can be applied to both rigid and flexible substrates. It is also scalable, making it suitable for industrial production of hydrogel films. However, it may not be ideal for producing films thinner than several microns, and the reproducibility of film thickness can be a challenge. Compared to other methods, blade-coated films may exhibit less uniformity, depending on the process parameters and material properties.
Bar Coating Methods
Bar coating is a film deposition technique closely related to blade coating, where a cylindrical rod, typically wrapped with wire, is used to spread a polymer solution across a substrate [
95,
103]. As the rod moves over the surface, the gap between the wire and the substrate determines how much solution is deposited, directly influencing the film thickness. This gap can be adjusted to control the coating outcome, and other parameters such as rod pressure, solution viscosity, and deposition rate can be optimized for better performance. Bar coating is appreciated for its simplicity, low cost, and scalability, especially for large-area coatings. However, it is generally limited to producing films with thicknesses above 10 microns, and the process tends to be slower due to reliance on capillary filling. Additionally, it is not suitable for creating gradient patterns or highly precise structures [
Figure 2F].
Slot Die Coating Methods
Slot die coating is a precision technique used to apply thin films by metering a liquid solution through a narrow slit onto a moving substrate [
97,
104]. The solution flows through a specially designed coating head, forming a controlled meniscus or liquid curtain that ensures uniform deposition. The wet film thickness is determined by the volume of solution dispensed, while other parameters such as flow rate, substrate speed, coating width, and solution viscosity can be fine-tuned to achieve consistent and stable coatings [
Figure 2G]. This method supports both high- and low-viscosity solutions, making it versatile for various materials, including polymers and hydrogels. Slot die coating is highly scalable and suitable for high-speed production, although it requires complex equipment and careful optimization of multiple variables. A schematic diagram typically illustrates the flow dynamics and coating head configuration.
Photolithography Methods
Photolithography is a modern technique widely used in microfabrication and nanotechnology to create patterned hydrogel films [
105]. It involves applying a photosensitive polymer layer—often prepared via spin coating—onto a substrate. When exposed to electromagnetic radiation (e.g., UV light or X-rays), the polymer undergoes chemical changes, such as crosslinking, in the illuminated regions [
Figure 2H]. By using masks, selective exposure can be achieved, allowing for the creation of complex surface patterns. Additionally, by layering polymers with different optical properties, vertical patterning is possible. This method enables high-resolution structuring of hydrogel films, making it ideal for applications in biosensors, microfluidics, and tissue engineering. A schematic typically includes the substrate, light source, mask, and movement axes.
3D Printing Techniques
Three-dimensional (3D) printing enables the rapid fabrication of hydrogel films and structures, allowing for swift design iterations and customization without the need for expensive molds. This technique supports the creation of intricate geometries and complex architectures that are often unachievable through conventional manufacturing methods. A key advantage of 3D printing is its material efficiency—it deposits only the required amount of material, thereby minimizing waste compared to subtractive manufacturing approaches. However, its scalability remains limited, making it more suitable for prototyping or small-batch production rather than mass manufacturing. A schematic overview of the principles of 3D printing applied to hydrogel film fabrication is illustrated in [
Figure 2I] [
106].
Selecting the best method is challenging, as it depends on the film’s application, material properties, cost factors, and often simply on the equipment available to the researchers or organization.
Table 1 shows a comparison of the main characteristics of these methods.
4. Unique Properties of Hydrogel Films
Hydrogel films possess a distinctive combination of physicochemical and biological properties that make them highly suitable for biomedical applications. Their thin-film format, high water content, permeability control, and tissue-conforming behavior enable them to function effectively in wound healing, drug delivery, tissue engineering, and biosensing.
4.1. Thin-Film Architecture and Flexibility
Hydrogel thin films, typically fabricated with micrometer-scale thicknesses, have emerged as a versatile platform for biomedical applications due to their ability to conform intimately to soft, irregular biological surfaces. This thin-film architecture not only enhances tissue contact and drug absorption but also improves mechanical adaptability, making it ideal for applications requiring minimal invasiveness and high biocompatibility.
The flexibility of hydrogel films is largely attributed to their high water content and polymeric network structure, which mimics the extracellular matrix (ECM). These properties allow them to maintain softness
, stretchability
, and permeability, essential for dynamic biological environments. For instance, Gong et al. emphasized the importance of double-network hydrogels in achieving both toughness and flexibility, which has inspired the design of thin-film variants for wearable and implantable devices [
110].
Recent advancements have focused on optimizing the mechanical strength of thin hydrogel films without compromising their flexibility. Nguyen et al. introduced a novel thin-film hydrogel (TFH) with a thickness of approximately 100 µm and 60% water content, synthesized via hydrophobic benzene–benzene interactions [
111]
. These TFHs exhibited remarkable mechanical properties, including a tensile strength of 2.35 MPa and a Young’s modulus of 4.7 MPa
, along with excellent environmental stability
, making them suitable for use as biological membranes and scaffolds
. Moreover, thin-film hydrogels have been explored for ocular applications, such as corneal patches, where transparency, oxygen permeability, and conformability are critical. For example, Maulvi et al. (2016) developed a hydrogel-based contact lens for sustained drug delivery to the eye, demonstrating the potential of thin-film hydrogels in ophthalmology [
112].
In transdermal drug delivery, thin hydrogel films serve as adhesive patches that can deliver therapeutic agents through the skin in a controlled manner. Their moisture-retaining and non-irritating nature makes them superior to traditional patches. Studies by Ngo et al. have shown that incorporating nanocarriers into hydrogel films can further enhance drug loading and release profiles [
113].
Additionally, implant coatings made from hydrogel films can reduce foreign body responses and improve integration with host tissues. Their ability to be functionalized with bioactive molecules or antimicrobial agents adds another layer of utility in regenerative medicine and infection control.
In summary, the thin-film architecture of hydrogels offers a unique combination of mechanical compliance, biocompatibility, and functional versatility, making them indispensable in next-generation biomedical devices and therapies.
4.2. High Water Content
Hydrogels are composed of three-dimensional, hydrophilic polymer networks capable of absorbing and retaining substantial amounts of water—often exceeding 90% by weight. This high water content closely mimics the extracellular matrix (ECM) of natural tissues, creating a moist, nutrient-rich, and mechanically compliant environment that supports cellular functions and tissue regeneration.
The water-rich nature of hydrogels plays a pivotal role in their biocompatibility. As highlighted by researchers, the porous and hydrated structure of hydrogels facilitates oxygen and nutrient diffusion, promotes cell adhesion and proliferation, and minimizes mechanical mismatch with soft tissues. These characteristics make hydrogels particularly suitable for applications in wound healing
, tissue engineering
, and implantable devices [
114]. Moreover, the low interfacial tension between hydrogel surfaces and biological tissues reduces the risk of inflammation and immune rejection. Studies have shown that hydrogels can modulate the foreign body response by minimizing fibrotic encapsulation, especially when engineered with bioinert or bioactive surface chemistries [
115],[
116]. Wei et al. further emphasized that the elastic moduli of many hydrogels are comparable to those of soft biological tissues (ranging from a few kPa to hundreds of kPa), which is critical for maintaining mechanical harmony with the host environment [
117]. This mechanical compatibility is essential in applications such as drug delivery systems, where the hydrogel must deform with tissue movement, and in biological electrodes, where soft interfaces reduce tissue damage and improve signal fidelity. In addition, hydrogels can be functionalized with peptides, growth factors, or nanoparticles to enhance their bioactivity and targeted therapeutic performance. For example, Britton et al. developed a hydrogel with embedded exosomes for enhanced skin regeneration, demonstrating how water-rich matrices can serve as both structural and biochemical scaffolds [
118].
Therefore, the high water content of hydrogels is not merely a structural feature but a biomimetic advantage that underpins their biocompatibility, mechanical tunability, and therapeutic potential across a wide range of biomedical applications.
4..3. Permeability and Diffusion Control
Hydrogel films are uniquely suited for biomedical applications due to their selective permeability and diffusion-regulating capabilities. Their hydrated polymer networks allow for the controlled transport of gases, nutrients, and therapeutic agents, which is essential for applications such as sustained drug release, biosensing, wound healing, and tissue regeneration.
The diffusion behavior in hydrogels is governed by several factors, including polymer composition, crosslinking density, pore size, and hydration level. These parameters can be finely tuned to achieve precise control over molecular transport, enabling the design of hydrogels that respond to specific physiological or environmental stimuli. Lavrentev et al. conducted a comprehensive review of diffusion-limited processes in hydrogels, emphasizing their role in drug encapsulation, nutrient delivery, and stimuli-responsive systems [
119]. Their findings highlighted how denser crosslinking reduces pore size and slows diffusion, while loosely crosslinked networks allow for faster transport. This tunability is critical for designing time-controlled drug delivery systems and biosensors that require consistent analyte exchange.
In a complementary study, Kanduč et al. used molecular dynamics simulations to demonstrate that molecular shape, size, and chemistry significantly influence hydrogel permeability [
120]. Their work revealed that collapsed hydrogel states, often induced by environmental triggers (e.g., pH, temperature, ionic strength), exhibit high selectivity by restricting the passage of larger or hydrophobic molecules. This property can be exploited to create smart hydrogels that release drugs only under specific conditions, enhancing therapeutic precision and minimizing side effects.
Furthermore, stimuli-responsive hydrogels—also known as “intelligent” or “smart” hydrogels—have been developed to dynamically alter their permeability in response to external cues. For example, thermo-responsive hydrogels based on poly(N-isopropylacrylamide) (PNIPAM) undergo a volume phase transition near body temperature, enabling on-demand drug release [
121]. Similarly, pH-sensitive hydrogels have been used in gastrointestinal drug delivery, where they remain stable in the acidic stomach but swell and release their payload in the more neutral intestines [
122].
In tissue engineering, oxygen and nutrient diffusion through hydrogel scaffolds is vital for maintaining cell viability and tissue integration. Hydrogels with gradient permeability or multi-layered structures have been engineered to mimic natural tissue interfaces, such as the skin or cornea, where different layers require distinct transport properties [
123].
Hence, the permeability and diffusion control of hydrogel films are a cornerstone of their functionality in biomedical applications. Through careful design of their network architecture and responsive behavior, hydrogels can be tailored to meet the complex demands of controlled release, biosensing, and regenerative medicine.
4.4. Surface Adhesion and Conformability to Tissues
One of the most compelling features of hydrogel films is their ability to adhere to moist biological surfaces without the need for external adhesives. This intrinsic adhesion, combined with their softness and flexibility, allows hydrogel films to conform intimately to irregular tissue geometries, which is critical for enhancing therapeutic efficacy, sensor accuracy, and mechanical integration in biomedical applications.
Hydrogels achieve this conformability through their low elastic modulus
, hydrated polymeric structure
, and interfacial compatibility with biological tissues. These properties enable them to form tight, non-irritating interfaces with soft organs such as the brain, heart, lungs, and skin. The ability to maintain stable contact even under dynamic physiological conditions makes hydrogel films ideal for implantable devices
, wound dressings
, and bioelectronic interfaces
. A notable example is the work by Chen et al.
, who developed a gelatin-based metamaterial hydrogel film with a tunable elastic modulus ranging from 20 to 420 kPa and a Poisson’s ratio from −0.25 to 0.52 [
124]. These tunable mechanical properties allowed the hydrogel to match the mechanical behavior of ultra-soft tissues, such as the myocardium and pulmonary tissue. The films were successfully used to monitor cardiac deformation and respiratory signals, demonstrating their potential in implantable bioelectronics and real-time physiological monitoring
.
In parallel
, Bovone et al. reviewed advanced strategies for engineering hydrogel adhesion through chemical junction design, including covalent bonding, supramolecular interactions, and dynamic reversible bonds [
125]
. These approaches significantly enhance adhesion strength and durability
, especially in wet and mechanically active environments
. For instance, catechol-functionalized hydrogels, inspired by mussel adhesive proteins, have shown strong and reversible adhesion to wet tissues, making them promising candidates for surgical glues
, biosensors
, and wearable electronics
[126]. Furthermore
, bioinspired adhesion mechanisms—such as those mimicking gecko feet or octopus suckers—have been integrated into hydrogel designs to improve reusability
, directional adhesion, and detachment control. These innovations are particularly valuable in soft robotics
, flexible electronics
, and dynamic tissue interfaces [
127,
128].
Therefore, the surface adhesion and conformability of hydrogel films are key enablers of their success in non-invasive, implantable, and wearable biomedical technologies. By tailoring their mechanical properties and interfacial chemistry, hydrogel films can be engineered to achieve stable, biocompatible, and functional integration with a wide range of biological tissues.
4.5. Biocompatibility of Hydrogel Films for Biomedical Applications
Hydrogel films are extensively used in biomedical applications due to their outstanding biocompatibility, which arises from their high water content, soft mechanical properties, and tunable chemical composition. These features allow hydrogels to mimic the extracellular matrix (ECM), promoting cell adhesion, proliferation, and tissue integration [
129]. Structurally, hydrogels are three-dimensional polymeric networks capable of absorbing over 90% of their weight in water, facilitating nutrient diffusion and reducing mechanical mismatch with soft tissues, thereby minimizing inflammation. Both natural (e.g., gelatin, alginate, hyaluronic acid) and synthetic polymers (e.g., PEG, PVA, polyacrylamide) are commonly used to fabricate biocompatible hydrogel films [
108,
130].
Polymer selection, crosslinking methods, and surface functionalization are critical in enhancing hydrogel-tissue interactions. Hydrogels crosslinked via dynamic covalent bonds or supramolecular interactions show improved tissue adhesion and mechanical resilience while maintaining cytocompatibility [
31,
131,
132,
133]. In vitro studies using cell lines such as L929 fibroblasts and HeLa cells consistently report low cytotoxicity, while in vivo applications demonstrate minimal immune response and favorable tissue remodeling, especially when bioactive or anti-inflammatory agents are incorporated [
134].
Hydrogels are tailored for specific biomedical applications. In drug delivery, they enable localized and sustained release of therapeutics, reducing systemic toxicity. For instance, PAM/CNT nanocomposite hydrogel films exhibit excellent cytocompatibility and hemocompatibility, making them effective carriers for doxorubicin in cancer therapy [
44]. In tissue engineering, hydrogels act as scaffolds that support cell infiltration, angiogenesis, and matrix deposition, with tunable biodegradability and mechanical properties suited to regenerating tissues [
33,
135,
136]. For implantable devices, hydrogel coatings reduce foreign body responses and enhance biointegration, as demonstrated by gelatin-based metamaterial hydrogels compatible with soft organs like the heart and lungs [
137].
Recent advances focus on multifunctional hydrogels that combine biocompatibility with antibacterial, anti-inflammatory, or immunomodulatory properties [
138,
139,
140]. Phenylboronic acid-modified chitosan hydrogels, for example, offer both cytocompatibility and antibacterial activity, making them ideal for wound healing [
141]. Additionally, smart hydrogels responsive to environmental stimuli (e.g., pH, temperature, enzymes) are being developed for precision medicine, enabling adaptive tissue interaction and on-demand therapeutic delivery [
142,
143].
4.6. Biodegradability of Hydrogel Films for Biomedical Applications
Biodegradability is a critical property of hydrogel films used in biomedical applications such as tissue engineering, drug delivery, and wound healing [
2,
4,
144,
145]. Biodegradable hydrogels are designed to safely degrade within the body through enzymatic, hydrolytic, or stimuli-responsive mechanisms, eliminating the need for surgical removal and reducing long-term complications [
1]. These materials align with tissue regeneration timelines or drug release schedules, improving therapeutic outcomes and patient compliance [
146].
Hydrogel degradation occurs via hydrolysis (common in synthetic polymers like PLA, PGA, and PEG-based copolymers), enzymatic breakdown (typical in natural polymers such as gelatin, chitosan, alginate, and hyaluronic acid), or stimuli-responsive mechanisms triggered by pH, temperature, enzymes, light, or oxidative stress [
147,
148,
149,
150,
151]. Design strategies for tunable biodegradability include adjusting crosslinking density, blending fast-degrading natural polymers with stable synthetic ones, and incorporating stimuli-responsive elements for spatiotemporal control over degradation and drug release [
148,
149].
Recent innovations include multi-layered hydrogel films with sequential degradation behavior, enabling phase-specific drug delivery. For example, a double-layer hydrogel system with curcumin-loaded chitosan nanoparticles and pirfenidone-encapsulated gelatin microspheres demonstrated synchronized degradation and drug release aligned with wound healing stages, promoting scar-free regeneration in vivo [
152].
In drug delivery, biodegradable hydrogels act as temporary depots, releasing therapeutic agents over time and degrading into non-toxic byproducts, thereby enhancing bioavailability and minimizing side effects [
153]. In tissue engineering, they serve as scaffolds that support cell infiltration, angiogenesis, and ECM deposition, then degrade to leave behind newly formed tissue [
154]. In wound healing, biodegradable films maintain a moist environment, protect against infection, and degrade in synchrony with tissue repair, eliminating the need for dressing removal [
152,
155].
However, safety concerns remain. Partial degradation can produce toxic monomers or oligomers [
156]. For instance, while PEG is generally safe, its monomer ethylene glycol is nephrotoxic and neurotoxic [
157]. Short-chain degradation products may accumulate in organs or cross the blood-brain barrier, posing risks [
158]. Therefore, evaluating the biocompatibility of degradation byproducts is essential, especially for long-term or implantable applications.
In summary, the biodegradability of hydrogel films is a key enabler of their success in biomedical applications. Through rational material design, stimuli-responsive engineering, and toxicity-aware formulation, hydrogel films can be tailored to degrade in harmony with therapeutic needs while ensuring safety, efficacy, and patient comfort [
159,
160]
In contemporary biomedical practice, hydrogel films serve a multifaceted role across a spectrum of applications encompassing wound dressings, sustained drug delivery systems, and scaffolds for tissue engineering. Their water absorption capabilities and biocompatibility allow hydrogel films to create optimal microenvironments for cell growth and regeneration [
65]. Moreover, hydrogels facilitate the controlled release of therapeutic agents, enhancing treatment efficacy while reducing systemic side effects [
66]. The versatility of these systems extends to complex wound care, including chronic and burn wounds, where traditional dressings often fall short. Compared to conventional materials, hydrogel films possess distinct advantages such as the ability to maintain moisture balance, conform to irregular wound surfaces, and provide enhanced patient comfort [
161]. Innovations in polymer design and surface modification have further improved the integration of hydrogel films with biological tissues, minimizing foreign body reactions and promoting healing outcomes [
73]. Looking ahead, the continual development of hydrogel films is anticipated to align with personalized medicine trends, incorporating smart, responsive materials that adapt dynamically to changes in the wound or tissue milieu, thereby offering tailored therapeutic interventions [
162,
163].
4.7. Wound Dressings: Moisture Retention, Antimicrobial, Anti-Inflammatory Incorporation, and Smart Monitoring
Hydrogel films have emerged as versatile platforms in wound care due to their ability to maintain a moist healing environment, deliver therapeutic agents, support tissue regeneration, and enable real-time monitoring. Their hydrophilic nature, biocompatibility, and tunable mechanical properties make them ideal for treating both acute and chronic wounds, including burns, diabetic ulcers, and pressure sores [
164,
165,
166].
Maintaining optimal hydration at the wound site is critical for epithelialization, autolytic debridement, and minimizing scarring. Hydrogel films mimic the extracellular matrix (ECM), facilitating cell migration, proliferation, and angiogenesis [
108,
137,
167]. Commercial products such as Healoderm and Intrasite Gel exemplify clinically successful hydrogel dressings that promote granulation and re-epithelialization while reducing pain and infection risk [
168,
169,
170].
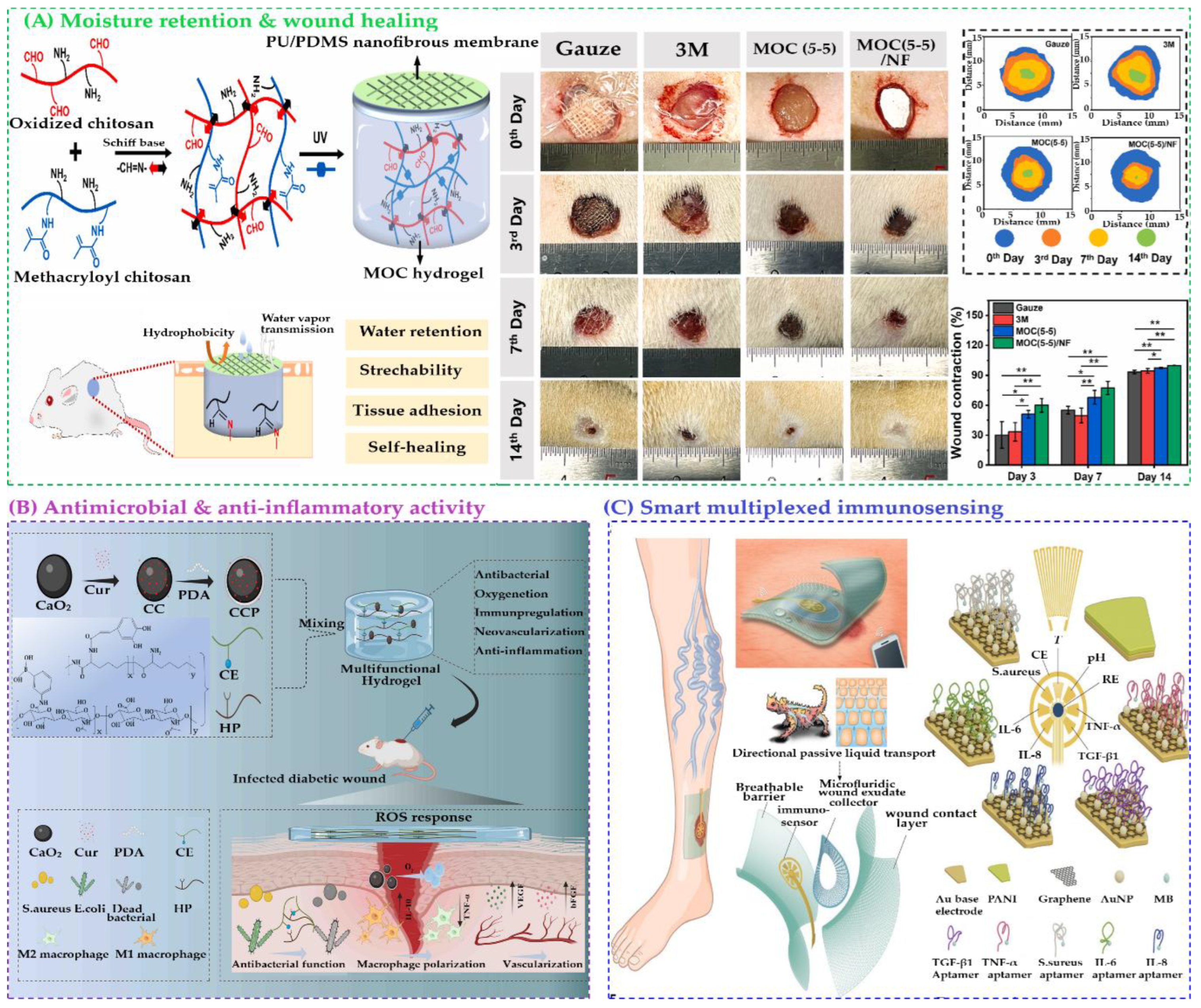
Recent innovations have focused on hydrogel/nanofibrous membrane composites, which combine the moisture-retaining properties of hydrogels with the mechanical strength of nanofibers. Li et al. developed a bilayered PU/PDMS nanofibrous membrane with a self-healing chitosan-based hydrogel, showing enhanced stretchability and water retention (
Figure 3A) [
171]. Cheng et al. introduced a ZIF-8-encapsulated alginate hydrogel/polylactic acid nanofiber (CAH/PLANF) composite with photodynamic antibacterial properties and extracellular matrix (ECM) -like architecture, accelerating healing in infected wounds [
172]. Ruan et al. further reviewed nanohybrid hydrogels integrating antibacterial agents, antioxidants, and stimuli-responsive drug delivery systems [
173].
To address infection and inflammation—two major impediments to wound healing—hydrogel films have been engineered to incorporate antimicrobial agents, anti-inflammatory drugs, and natural bioactives. Ullah et al. developed a collagen-based hydrogel with Sr/Fe-substituted hydroxyapatite nanoparticles, ciprofloxacin, and dexamethasone, demonstrating antibacterial, anti-inflammatory, and osteogenic effects [
174]. Pratinthong et al. modified CMC/PVA hydrogel films with citric acid and glutaraldehyde to enhance the anti-inflammatory efficacy of triamcinolone acetonide [
175]. Xi et al. incorporated Fructus Ligustri Lucidi polysaccharide into PVA/pectin hydrogels, resulting in enhanced antibacterial activity, collagen deposition, and reduced inflammation [
176].
Natural extracts have also been widely explored. Chuysinuan et al. formulated CMC/silk sericin hydrogel films with turmeric extract, showing strong antibacterial, antioxidant, and anti-inflammatory effects [
177]. Fan et al. designed a multifunctional curcumin-loaded PVA/chitosan/sodium alginate hydrogel with potent antimicrobial, antioxidative, and angiogenic properties, promoting macrophage polarization and collagen synthesis in diabetic wound models [
170]. Ahmady et al. embedded thymol-loaded alginate microparticles into chitosan-gelatin films, achieving controlled drug release, broad-spectrum antibacterial activity, and enhanced epithelialization in vivo [
178].
Figure 3.
Schematic representation of the synthesis and applications of (A) a polyurethane/polydimethylsiloxane (PU/PDMS) nanofibrous (NF) membrane composite and methacrylated chitosan/oxidized chitosan (MOC (5–5))/NF hydrogel composites for wound healing. Reproduced with permission from [
171], Elsevier; (B) HP-CE@CCP hydrogel dressings, highlighting their antimicrobial and anti-inflammatory mechanisms in accelerating the healing of infected diabetic wounds. Reproduced with permission from [
179], American Chemical Society; (C) Flexible microfluidic multiplexed immunosensing platform for point-of-care, in situ profiling of wound microenvironment, inflammation, and infection through multiplexed biomarker detection. Reproduced with permission from [
180], American Association for the Advancement of Science.
Figure 3.
Schematic representation of the synthesis and applications of (A) a polyurethane/polydimethylsiloxane (PU/PDMS) nanofibrous (NF) membrane composite and methacrylated chitosan/oxidized chitosan (MOC (5–5))/NF hydrogel composites for wound healing. Reproduced with permission from [
171], Elsevier; (B) HP-CE@CCP hydrogel dressings, highlighting their antimicrobial and anti-inflammatory mechanisms in accelerating the healing of infected diabetic wounds. Reproduced with permission from [
179], American Chemical Society; (C) Flexible microfluidic multiplexed immunosensing platform for point-of-care, in situ profiling of wound microenvironment, inflammation, and infection through multiplexed biomarker detection. Reproduced with permission from [
180], American Association for the Advancement of Science.
Cadinoiu et al. created chitosan/PVA biocomposite films with silver nanoparticles and ibuprofen, showing synergistic antimicrobial and anti-inflammatory effects, validated through in vitro and in vivo studies [
181]. Hashempur et al. developed a chitosan xerogel film with Nigella sativa extract using deep eutectic solvents, demonstrating strong antioxidant and antimicrobial activity against multiple pathogens [
182].
To address chronic infected wounds, which are often exacerbated by persistent inflammation and reactive oxygen species (ROS), Tang et al. developed an ROS-responsive injectable hydrogel composed of ε-polylysine grafted with caffeic acid (EPL-CA) and hyaluronic acid grafted with phenylboronic acid (HP)
. The hydrogel was loaded with CaO
2@Cur-PDA (CCP) nanoparticles
, combining calcium peroxide (CaO
2)
, curcumin (Cur)
, and polydopamine (PDA) (
Figure 3B) [
179]. Upon exposure to the wound microenvironment, the hydrogel gradually dissociates, enabling sequential release of therapeutic agents. Initially, caffeic acid-grafted ε-polylysine (CE) provides antibacterial and antioxidant effects, while hyaluronic acid (HA) mimics the extracellular matrix. Subsequently, CCP decomposes, releasing Cur, which promotes angiogenesis. This multi-phase release strategy aligns with the dynamic stages of wound healing, demonstrating effective bacterial clearance, ROS scavenging, and tissue regeneration in vivo. Boateng et al. formulated Polyox/carrageenan films loaded with streptomycin and diclofenac, achieving sustained drug release, high fluid absorption, and synergistic antibacterial and anti-inflammatory effects [
183].
In addition to therapeutic functionalities, smart hydrogel films have been developed for real-time wound monitoring, particularly pH-responsive systems [
184]. These dressings detect changes in wound pH—a key biomarker for infection and healing progression—and respond accordingly. Chronic wounds often exhibit elevated pH levels (above 7.0), indicating bacterial colonization and inflammation, while healing wounds maintain an acidic environment (pH 4.0–6.0) [
185].
Wound pH is a reliable indicator of infection, with elevated levels (7.5–9.0) often signaling bacterial colonization and impaired healing. Gamerith et al. developed a silane-anchored bromocresol purple sensor that changes color from yellow to blue with increasing pH, enhancing visual contrast for infection detection [
186]. Eskilson et al. improved spatial resolution using bacterial nanocellulose dressings embedded with mesoporous silica nanoparticles carrying pH-sensitive dyes, maintaining optimal wound dressing properties [
187]. Electrochemical sensing offers real-time quantification. Rahimi et al. created a flexible pH sensor using laser-scribed ITO electrodes functionalized with polyaniline, achieving −55 mV/pH sensitivity across pH 4–10 and enabling wireless smartphone readout via NFC [
188].
Hydrogel films have emerged as a promising platform for smart, multiplexed immunosensing in chronic wound care due to their biocompatibility, flexibility, and ability to interface directly with biological tissues. Chronic wounds, often caused by disrupted healing mechanisms, require continuous monitoring of multiple biomarkers to guide personalized treatment. Traditional diagnostic methods are limited in scope and accessibility, prompting the development of integrated biosensing systems. To address the complexity of chronic wounds, Gao et al. developed a graphene-based microfluidic immunosensor array that simultaneously detects tumor necrosis factor–α (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), and transforming growth factor–β1 (TGF-β1)],
S. aureus, pH, and temperature. Clinical trials showed strong correlation between these biomarkers and delayed healing over five weeks (
Figure 3C) [
180]. A notable advancement is the creation of a flexible, hydrogel-based microfluidic platform capable of simultaneously detecting inflammatory cytokines (TNF-α, IL-6, IL-8, TGF-β1), microbial burden (
Staphylococcus aureus), and physicochemical parameters (pH and temperature). This system, exemplified by the VeCare device, incorporates microdrop functionalization, novel aptamer sequences, and wireless electronics for real-time, smartphone-based data readout. The hydrogel film serves as both a sensing matrix and a wound interface, enabling in situ, point-of-care diagnostics. Clinical validation in animal models and human wound exudates demonstrates its potential to transform chronic wound management by enabling timely, personalized interventions and improving healing outcomes.
Han et al. categorized pH-responsive hydrogel mechanisms into morphological changes via dynamic bonds (e.g., Schiff base, catechol–Fe coordination), swelling behavior through protonation/deprotonation, degradation control using ester or imine bonds, and drug release modulation based on ion concentration and drug-polymer affinity [
189]. These systems integrate optical indicators and electrochemical sensors, enabling color change or electrical signal generation in response to pH fluctuations. Representative studies include Mariani et al.’s textile-based smart bandage with a semiconducting polymer/IrOx pH sensor [
190], and Kaewpradub et al.’s fully-printed wearable bandage-based electrochemical sensor with pH correction [
191]. Du et al. developed dual drug-loaded GelMA/HA-CHO hydrogels with pH-responsive release of gentamicin and lysozyme [
192], while Zhao et al. designed a multilayer hydrogel film (K-E-AGB) for diabetic wounds with glucose-responsive EGF release and early anti-inflammatory action [
193]. Moeinipour et al. created a stimuli-responsive hydrogel film based on hydrogen-bonded organic frameworks (HOFs) for temperature and pH-triggered drug release [
194].
These smart systems not only monitor the wound microenvironment but also actively respond to pathological changes, offering a personalized and proactive approach to wound care. Future directions include multi-parameter sensing (e.g., combining pH with temperature and bacterial load) [
195], personalized therapy through responsive drug release [
185], AI-guided analytics, and 3D bioprinting for customizable hydrogel architectures.
4.8. Hydrogel Films as Cell Culture
Hydrogel films have gained prominence as dynamic platforms for cell culture due to their tunable physicochemical properties, biocompatibility, and ability to mimic the native extracellular matrix. Recent innovations in hydrogel design have focused on enhancing cellular interactions, spatial organization, and mechanical stability to support both mono- and co-culture systems.
One notable approach involves photocrosslinkable dextran-based hydrogel films, which utilize benzophenone-functionalized carboxymethyl dextran (BP-CMD) to form stable networks upon UV irradiation. These films can be reinforced with silica nanoparticles and gelatin microparticles, offering improved mechanical integrity and porosity. Importantly, the covalent immobilization of bioactive molecules such as BMP-2 enables targeted stimulation of cell growth. This system has demonstrated excellent support for both osteoblast and endothelial cell proliferation, making it highly suitable for bone tissue engineering and vascularization studies [
196].
Complementing this, surface-patterned hydrogel films present a versatile scaffold for 2D and 3D co-culture. By integrating magnetic silica rods on the hydrogel surface, these films allow spatial separation and simultaneous culture of different cell types—one embedded within the hydrogel matrix and another adhered to the patterned surface. This dual-architecture mimics tissue interfaces and facilitates the study of cell-cell interactions in a controlled microenvironment. The fabrication process is simple and scalable, offering potential for applications in organ-on-a-chip systems and multi-layered tissue constructs.
Together, these hydrogel film technologies represent a significant advancement in cell culture engineering. Their modularity, bioactivity, and spatial control capabilities make them promising tools for regenerative medicine, tissue modeling, and drug screening platforms [
197].
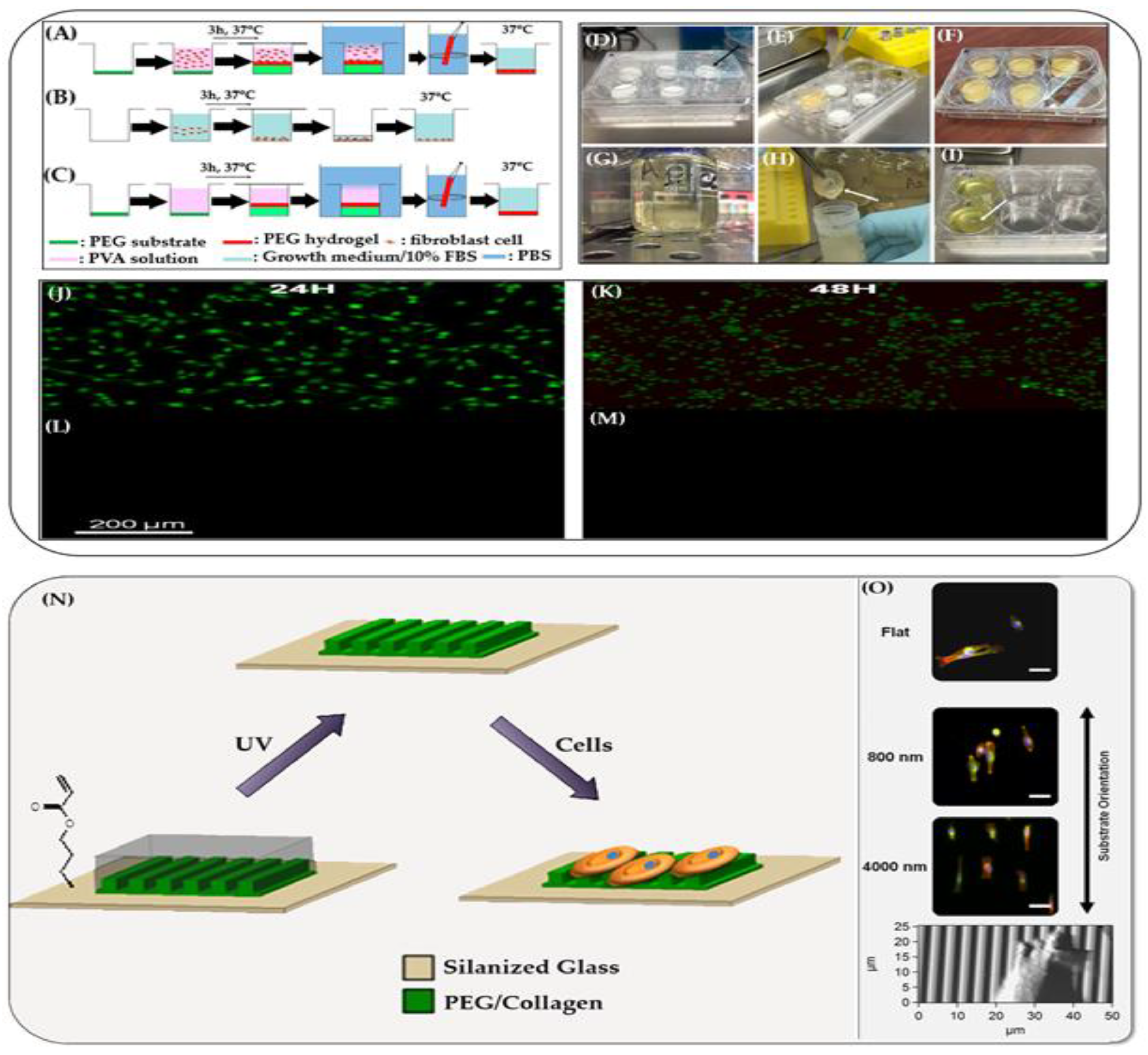
Hydrogels have a physical structure similar to the natural extracellular matrix, making them excellent materials for cell culture. A novel method was demonstrated by Moreau et al. for encapsulating cells in freestanding poly(vinyl alcohol) (PVA) hydrogel films
, formed spontaneously through swelling-induced gelation
. Mouse fibroblasts (NIH 3T3) were suspended in a PVA solution containing growth medium and fetal bovine serum, then poured into wells with dry, un–cross-linked PEG substrates. As the PEG swelled and dissolved, it triggered the formation of freestanding PVA hydrogel membranes (
Figure 4, A-M) [
198].
This process minimized the need for direct handling and maintained aseptic conditions. The resulting films exhibited a thickness gradient (2 mm at edges to 0.5 mm at center). Epifluorescence imaging showed efficient cell encapsulation, with viability reaching ~70% for cells located more than 1 mm from the PEG interface after 24–48 hours. Cells near the interface experienced reduced survival, likely due to hypertonic stress during swelling. This hydrogel environment offers a gentle, supportive, biocompatible and scalable approach for the in vitro cell culture applications.
Hydrogel films designed to replicate the topography and mechanical compliance of the basement membrane offer a biologically relevant substrate for cell culture. These films aim to simulate the native microenvironment that cells experience in vivo, which is critical for maintaining physiological cell behavior.
Garland et al. developed a substrate with nano- to microscale surface features and tissue-like softness, closely resembling the basement membrane. This biomimetic design supports cell adhesion, spreading, and differentiation, particularly for epithelial and stem cells (
Figure 4, N,O) [
199]. The substrate’s compliance and topographical cues were shown to influence cellular mechanotransduction, guiding cell morphology and function more effectively than conventional rigid culture surfaces. Such hydrogel-based substrates provide a more accurate platform for in vitro modeling of tissue behavior, drug testing, and regenerative medicine, bridging the gap between traditional cell culture systems and the complexity of living tissues
4.9. Drug Delivery Systems via Hydrogel Films
Hydrogel films have garnered substantial interest in drug delivery applications due to their unique combination of biocompatibility
, high water content
, and tunable physicochemical properties
. These films, composed of three-dimensional hydrophilic polymer networks, can encapsulate a wide range of therapeutic agents—including small molecules, proteins, peptides, and nanoparticles—making them highly adaptable for various administration routes [
200].
One of the key advantages of hydrogel films is their ability to provide controlled and sustained drug release, which enhances therapeutic efficacy while minimizing systemic side effects. Hydrogel films offer precise control over drug release kinetics, maintaining therapeutic levels while minimizing dosing frequency and side effects [
200]. Their ability to localize drug delivery reduces systemic toxicity, making them ideal for applications such as wound care and localized chemotherapy [
201]. These systems also address challenges like burst release and premature drug degradation, ensuring a stable therapeutic window [
108].
The adjustable crosslinking and mesh size of hydrogels enable sustained release that is tailored to drug properties and clinical requirements [
202]. For example, ciprofloxacin-loaded hydrogels demonstrated prolonged antibacterial activity and controlled release over 10 hours, following first-order kinetics [
203]. Additionally, effective osteochondral repair requires simultaneous regeneration of both cartilage and subchondral bone, which is often limited by single-agent delivery systems. To address this, Kang et al. developed a supramolecular hydrogel film capable of co-delivering two distinct therapeutic agents: kartogenin@polydopamine (KGN@PDA
) nanoparticles for cartilage regeneration and miRNA@calcium phosphate (miRNA@CaP) nanoparticles for bone repair (
Figure 5A) [
204]. These agents were in situ deposited onto a patterned UPy-modified gelatin hydrogel via metal ion coordination, enabling spatially organized and targeted delivery. The hydrogel system supports controlled release of both KGN and miR-26a, promoting chondrogenic and osteogenic differentiation through the JNK/RUNX1 and GSK-3β/β-catenin pathways, respectively. In vivo, the cylindrical hydrogel plug mimicking the Haversian canal structure facilitated integrated regeneration of cartilage and bone, demonstrating enhanced tissue formation and functional restoration.
Transdermal hydrogel patches exploit controlled permeability to deliver drugs systemically while bypassing gastrointestinal degradation and first-pass metabolism, enhancing bioavailability and patient adherence [
206].
Abedini et al. developed dual-anionic hydrogel films using alginate and quince seed gum to deliver curcumin transdermally [
207]. To improve compatibility with the hydrophilic matrix, curcumin was modified with stearic acid, enabling uniform dispersion and sustained release over 48 hours. This system enhanced wound healing markers, demonstrating the potential of combining natural polymers and surface modification for effective transdermal delivery of hydrophobic drugs.
Mucosal films for oral and ocular drug delivery benefit from the adhesive, hydrating, and sustained release properties of hydrogel films, improving therapeutic outcomes in sensitive tissue environments [
200]. The flexibility and conformability of such films greatly increase patient compliance, especially in chronic conditions requiring long-term treatment [
108]. For example, to enhance sublingual delivery of antifungal agents, researchers developed multilayered mucoadhesive hydrogel films incorporating nystatin [
208]. The system combined Ocimum basilicum seed mucilage, thiolated alginate, and dopamine-modified hyaluronic acid, with a polydopamine (PDA) coating to improve adhesion and drug retention. This layered structure enabled controlled and sustained release of nystatin, improving mucosal permeability and therapeutic efficacy. The study highlights the potential of multifunctional hydrogel films for localized delivery of bioactive agents in oral applications.
Özakar et al. developed fast-dissolving hydrogel-based oral thin films incorporating pregabalin and methylcobalamin for improved management of neuropathic pain (
Figure 5B) [
205]. Designed for patients with swallowing difficulties, the films enable rapid disintegration and targeted delivery. The dual-drug system ensures simultaneous release of both agents, enhancing therapeutic efficacy while maintaining biocompatibility and ease of administration. This approach highlights the potential of oral thin films for delivering multiple active compounds in a patient-friendly format.
Hydrogel films have emerged as a promising platform for multi-drug delivery due to their high water content, biocompatibility, and tunable network structures. These properties enable controlled release profiles and spatial separation of therapeutic agents, which are critical for complex treatment regimens such as wound healing, cancer therapy, and immunosuppression.
Recent studies have explored diverse strategies for multi-drug incorporation. Yoon et al. reviewed hydrogel–nanoparticle composites that enable dual-drug delivery through physical embedding, covalent integration, and layer-by-layer assembly [
209]. These designs allow for programmable, multi-phase release, enhancing therapeutic synergy and reducing side effects. Similarly, Manghnani et al. demonstrated how Michael addition-based PEG hydrogels can be chemically tuned to control the release of micro-crystalline fenofibrate [
210]. By altering the crosslinking chemistry, the release duration was modulated from 4 hours to 10 days, offering precise temporal control over drug availability.
Zhang et al. designed a biodegradable double-layer hydrogel film for scar-free wound healing, enabling multi-drug loading and sequential release kinetics (
Figure 5C) [
152]. The lower layer contained curcumin-loaded chitosan nanoparticles for early anti-inflammatory action, while the upper layer housed pirfenidone-encapsulated gelatin microspheres for delayed anti-fibrotic effects. The distinct degradation rates and mechanical properties of each layer facilitated phase-specific drug release, aligning with the wound healing stages. This controlled, time-resolved delivery strategy accelerated tissue regeneration and minimized scarring, demonstrating the hydrogel’s potential for multi-phase therapeutic regulation.
Hu et al. provided a comprehensive review of hydrogel drug delivery systems, emphasizing the role of polymer-drug interactions, crosslink density, and external stimuli (e.g., pH, temperature) in shaping release kinetics [
202]. Their work highlights how hydrogels can be engineered to respond to physiological conditions, enabling site-specific and sustained drug release across various tissues.
Despite these advances, challenges remain in achieving reproducible multi-drug loading, precise spatial control, and predictive modeling of in vivo release behavior. Future research is expected to focus on integrating real-time monitoring systems and developing smart hydrogels capable of adaptive release in response to dynamic biological environments.
4.10. Tissue Engineering
Hydrogel films have garnered significant attention within tissue engineering due to their ability to closely mimic the native extracellular matrix (ECM), which is crucial for directing cellular behavior and tissue regeneration. The ECM is inherently hydrated and exhibits a porous, soft, and viscoelastic nature, providing both physical support and biochemical signaling to resident cells. Hydrogel films reproduce this soft, hydrated environment through their three-dimensional polymeric networks, offering a microenvironment conducive to promoting cell adhesion, proliferation, and differentiation. Notably, they ensure sufficient nutrient and gas exchange, necessary for cell viability and function, by virtue of their porous structure [
64,
65].
In contrast to traditional scaffolds made from rigid or synthetic materials, hydrogel films provide enhanced mechanical compliance, which better recapitulates the physiological stiffness of soft tissues, thereby minimizing foreign body reactions and fibrosis risks post-implantation. Their intrinsic biocompatibility allows for seamless integration with host tissues, enabling more effective tissue regeneration. These advantages not only aid in improving cell-matrix interactions but also facilitate the dynamic remodeling characteristic of natural tissues, thus reinforcing the utility of hydrogel films in regenerative medicine [
69].
Hydrogel films have become indispensable in tissue engineering due to their ability to mimic the extracellular matrix (ECM), support cellular functions, and serve as barrier layers or scaffolds for tissue regeneration. Their high water content, biocompatibility, and tunable mechanical properties make them ideal for both soft and hard tissue applications, including skin, bone, cartilage, and mucosal tissues
Barrier-forming hydrogel films are designed to protect damaged tissues
, prevent infection, and regulate biochemical exchange at wound or implant interfaces. These films act as bioadhesive interfaces, promoting tissue integration while preventing microbial infiltration and fluid loss. Recent innovations include Janus hydrogels, which feature asymmetric surfaces—one side optimized for adhesion and integration, the other for anti-fouling and protection [
124]. These structures mimic natural biological barriers and have shown promise in skin and mucosal wound repair
, hemostasis
, and post-surgical sealing
[211]. Biocompatible hydrogel films also demonstrated promising applications in tissue adhesives and sealants as a potential alternative or adjunct to sutures or staples in various clinical indications [
212,
213,
214,
215]. However, traditional adhesive hydrogels often struggle to stick to wet tissues and cannot prevent unwanted tissue adhesion after surgery.
To solve this problem, Cui et al. developed a Janus hydrogel
—a film with two different surfaces: one sticky and one non-sticky (
Figure 6A) [
216]. This was achieved by dipping one side of a negatively charged hydrogel into a solution containing positively charged oligosaccharides. The dipping process created a gradient of electrostatic interactions, resulting in two distinct surfaces. The lightly treated side became highly adhesive, even underwater, due to increased hydrophobicity and better water drainage. It could strongly bond to wet tissues like pig skin, stomach, and intestine. In contrast, the heavily treated side became non-adhesive because the chemical groups responsible for sticking were neutralized. This design allowed the hydrogel to seal internal wounds while preventing external tissue from sticking, which is important for avoiding complications after surgery. In animal tests, the Janus hydrogel successfully repaired stomach perforations in rabbits and degraded naturally over 14 days. Its ability to heal itself in water and its safe interaction with tissues make it a promising material for future medical applications in internal tissue repair and anti-adhesion barriers.
Hydrogels are widely used in wound healing due to their ability to maintain a moist environment and adhere well to tissues, which helps stop bleeding and promote healing. However, traditional hydrogels with uniform structure can cause unwanted tissue adhesion, leading to secondary injuries. To overcome this, Fang et al. developed a Janus hydrogel with two distinct sides using a one-pot fabrication method. This hydrogel, called JPs@PAA-PU, is made from polyacrylic acid (PAA) and polyurushiol (PU), stabilized by special Janus particles. It has a water–oil layered structure without a separate adhesive layer, giving it unique physical and chemical properties (Figure B-F) [
217]. The PAA side strongly adheres to tissues, red blood cells, and platelets, while the PU side supports blood clotting and acts as a physical barrier. This dual function allows the hydrogel to stop bleeding faster (as quickly as 32 seconds) and reduce blood loss compared to conventional materials. It also shows strong antibacterial activity against common bacteria like E. coli and Staphylococcus aureus, and has been proven safe for clinical use. The hydrogel’s asymmetric toughness—with higher strength on the adhesive side—further supports its role in targeted tissue interaction. Overall, this Janus hydrogel offers a promising solution for rapid hemostasis, infection control, and prevention of tissue adhesion in wound care and surgical applications.
Hydrogel barriers are also being explored for implant coatings, where they reduce immune response and enhance biocompatibility. For example, nanocellulose-based hydrogels have demonstrated excellent mechanical strength and bioactivity, making them suitable for barrier applications in bone and vascular implants
[218].
Hydrogel films act as structural scaffolds that provide mechanical support for cell growth and tissue formation. These scaffolds can be engineered from natural polymers (e.g., collagen, gelatin, alginate, chitosan) or synthetic polymers (e.g., PEG, PVA), often in hybrid combinations to balance biological activity and mechanical resilience [
154,
219]. Their mechanical properties can be finely tuned through polymer selection, crosslinking density, and incorporation of reinforcing composite materials to closely approximate native tissue stiffness [
72]. These adjustments ensure that scaffolds can sustain physiological loads and accommodate cellular remodeling without premature failure.
Nanofillers such as cellulose nanocrystals and silver nanoparticles impart enhanced mechanical strength and toughness while simultaneously contributing antimicrobial properties that protect against infection during tissue regeneration [
76,
220]. Additionally, design strategies incorporating anisotropic and hierarchical architectures emulate native tissue organization, improving functional outcomes by directing cell alignment and extracellular matrix deposition [
80]. In advanced wound care, hydrogel films serve not only as drug carriers but also as structural scaffolds that support tissue regeneration. A recent study introduced a self-crosslinked chitosan (CS) hydrogel film reinforced with oxidized cellulose nanocrystal–silver nanoparticles (CNC-AgNPs), which stabilized a Pickering emulsion (PE) for delivering quercetin (Qu) [
76]. This design enabled the formation of a robust interpenetrated network through Schiff base bonding between aldehyde groups of CNC-AgNPs and amino groups of CS, enhancing the film’s mechanical integrity. The hydrogel demonstrated excellent biocompatibility
, non-hemolytic behavior
, and promoted cell migration and collagen synthesis
, crucial for full-thickness wound healing. In vivo studies confirmed accelerated wound closure and tissue regeneration, positioning this multifunctional hydrogel as a promising scaffold for clinical applications in skin repair.
Biomaterial-based scaffolds play a critical role in enhancing cell survival and functional maturation in skeletal muscle regeneration. Nanocomposite fibrous hydrogel films incorporating graphene have been shown to support this process by providing anisotropic, bioactive scaffolds that mimic the native muscle extracellular matrix. Patel et al. developed hierarchically aligned fibrous hydrogel films using a microfluidic self-assembly technique that combines graphene with natural polysaccharides. In this context, when C2C12 myoblasts were cultured on these films, they initially formed aggregates and later differentiated into myotubes under myogenic conditions (
Figure 7A-E) [
80]. At lower graphene concentrations (0.01% and 0.05%), cells tended to form dense, rounded aggregates with positive expression of myosin heavy chain (MHC), indicating early myogenic activity. In contrast, films with 0.1% graphene promoted the formation of elongated, multinucleated myotubes aligned along the fiber direction, suggesting enhanced and directional myogenesis. This transition from aggregation to alignment is attributed to the synergistic effects of graphene-induced nanoroughness, increased electrical conductivity, and optimal surface wettability. These properties collectively enhance cell–matrix interactions, promote cell spreading and fusion, and support the structural and functional maturation of muscle tissue. Such nanocomposite hydrogels offer a promising platform for skeletal muscle regeneration by combining mechanical reinforcement with bioinstructive cues.
Recent studies have emphasized the importance of porosity
, degradation rate, and mechanotransduction in scaffold design. For instance, gelatin methacryloyl (GelMA) granular hydrogel scaffolds with macropores have shown enhanced macrophage-mediated healing and reduced inflammation in full-thickness skin wounds [
6]. Similarly, injectable hydrogel scaffolds have been developed for cartilage regeneration, offering minimally invasive delivery and controlled release of bioactive agents [
222,
223].
In bone tissue engineering, hydrogel scaffolds are being used to deliver osteogenic cells and growth factors, promote vascularization, and support mineralization. Studies have shown that hydrogel-based systems can effectively mimic the bone microenvironment and facilitate osteoblast differentiation, especially when combined with bioactive ceramics or nanoparticles [
224].
Hydrogels have emerged as a promising class of biomaterials in bone tissue engineering (BTE) due to their ability to mimic the extracellular matrix and facilitate osteogenic drug delivery (Figure F)[
221]. Their polymeric networks, particularly those derived from synthetic and natural biomacromolecules, offer inherent biocompatibility and tunable biofunctionality. Recent developments have emphasized hydrogels fabricated from biomacromolecules to enhance tissue integration and reduce inflammatory responses, eliminating the need for surgical removal in case of failure. These materials can be engineered for specific geometries suitable for implantation or injection, with controlled degradation rates, porosity, and drug release profiles achieved through tailored cross-linking strategies. Importantly, hydrogels provide structural support while creating a conducive microenvironment for bone regeneration. They enable osteoblast adhesion both on the surface and within the porous matrix, promoting cell proliferation, differentiation, and maturation—key processes in effective bone healing.
4.11. Ophthalmic Applications
Hydrogel films have revolutionized ophthalmic applications due to their exceptional biocompatibility
, optical transparency, and moisture-retention capabilities
, which are critical for maintaining ocular surface integrity and comfort. Their ability to conform to the complex anatomy of the eye and respond to physiological stimuli makes them ideal for a wide range of therapeutic and diagnostic uses
, including contact lenses
, corneal patches
, and ocular drug delivery systems [
225].
Hydrogel films have become foundational materials in ophthalmology, particularly in the development of soft contact lenses and corneal patches, due to their biocompatibility
, optical clarity
, moisture retention
, and oxygen permeability. These properties allow hydrogel films to mimic the natural hydration and mechanical environment of the cornea, making them ideal for both vision correction and therapeutic applications
. Hydrogels such as poly(2-hydroxyethyl methacrylate) (pHEMA)
, polyvinyl alcohol (PVA)
, and silicone-based hydrogels are widely used in soft contact lenses. Their hydrophilic nature enables high water content, which enhances comfort and reduces friction with the ocular surface [
226]. Recent advances include copolymer hydrogel systems that improve drug loading capacity and release kinetics, enabling contact lenses to serve as ocular drug delivery platforms [
227].
Wei et al. developed smart contact lenses featuring a highly porous, gas-permeable, and optically transparent design using a metal-coated nanofiber mesh (metalc-NM) (
Figure 8 A-C) [
228]. Their fabrication process involved sputtering a thin layer of gold (~110 nm) onto electrospun polyacrylonitrile (PAN) nanofibers, which were then integrated with commercial hydrogel-based disposable contact lenses to form a composite structure. To enhance adhesion between the metal layer and the substrate, an electrochemical deposition of poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) was applied. The resulting smart lenses demonstrated excellent gas permeability, hydration, wettability, optical clarity, and mechanical durability, making them suitable for advanced wearable biomedical applications.
Innovative designs such as microfluidic hydrogel-embedded contact lenses have demonstrated pH-responsive drug release, allowing for on-demand delivery of therapeutics in response to ocular conditions like inflammation [
231]. These lenses also offer potential for real-time diagnostics, such as intraocular pressure monitoring and tear fluid analysis [
232]
Hydrogel films are also being developed as corneal patches
for wound healing
, post-surgical repair, and tissue regeneration. Their ability to adhere to the corneal surface without sutures makes them attractive for minimally invasive treatments
. For example
, adhesive hydrogels composed of oxidized guar gum and carboxymethyl chitosan have shown self-healing
, injectable
, and tissue-adhesive properties, promoting corneal regeneration in rabbit models [
233].
GelPatch, composed of gelatin methacryloyl (GelMA) and glycidyl methacrylated hyaluronic acid (HAGM), is a photocrosslinkable adhesive hydrogel film engineered for ocular tissue sealing (
Figure 8 D-L) [
229]. Designed to address laceration-type injuries, it exhibits high burst pressure, minimal swelling, and strong adhesion to both scleral and subconjunctival tissues. These properties enable rapid and effective wound closure without sutures, positioning GelPatch as a promising candidate for sutureless ocular repair.
Another promising material, GelCORE, a light-crosslinkable bioadhesive hydrogel, has demonstrated superior tissue adhesion
, transparency
, and stromal regeneration compared to commercial adhesives [
213]. These hydrogel patches can be tailored to match the geometry of corneal defects and support epithelial and stromal healing
.
Hydrogel films can be functionalized with stem cells
, extracellular vesicles, and bioactive molecules to enhance their regenerative capabilities. For instance, MSC-exosome-loaded hydrogels have been shown to promote collagen deposition
, reduce inflammation, and accelerate healing in corneal injuries [
233]. Additionally, heparin-functionalized hydrogels have been designed to sequester inflammatory cytokines and prevent fibrosis, offering a pathway to scarless corneal repair
[234]. The integration of 3D bioprinting
, stimuli-responsive polymers
, and bioelectronic interfaces is expected to further advance hydrogel film technologies in ophthalmology. These innovations aim to create multifunctional platforms that combine drug delivery, diagnostics, and regenerative therapy in a single device.
Hydrogel films in contact lenses and corneal patches are not only improving patient comfort and compliance, but also redefining the possibilities for non-invasive ocular treatment and vision restoration.
4.12. Ocular Drug Delivery Films
Hydrogel films have emerged as
a transformative solution for ocular drug delivery, addressing the limitations of conventional eye drops and ointments, which suffer from low bioavailability
, rapid clearance
, and poor patient compliance
. Their high water content
, biocompatibility, and mucoadhesive properties make them ideal for sustained, localized, and non-invasive drug release directly at the ocular surface [
235].
The eye’s unique anatomy—including the corneal epithelium
, blinking reflex
, and nasolacrimal drainage system—poses significant barriers to drug absorption. Less than 5% of topically administered drugs typically reach intraocular tissues [
236]. Hydrogel films overcome these challenges by forming a protective, drug-loaded matrix that adheres to the ocular surface and releases medication over extended periods [
235].
Hydrogel films for ocular drug delivery are fabricated using natural polymers (e.g., hyaluronic acid, chitosan, alginate) and synthetic polymers (e.g., polyvinyl alcohol, polyacrylamide, PEG derivatives). These materials can be engineered to respond to physiological stimuli such as pH
, temperature, or ionic strength
, enabling in situ gelation and on-demand drug release [
235,
237].
Tighsazzadeh et al. explore the development of hydrogel films composed of matrix hyaluronic acid (HA) and bilayer poly-hydroxyethyl methacrylate (pHEMA)-HA as innovative platforms for ocular drug delivery (
Figure 8 M-P) [
230]. These films combine the biocompatibility and hydration properties of HA with the mechanical stability and permeability control of pHEMA, forming a layered structure suitable for sustained drug release. The bilayer design allows for modulated diffusion, enhancing therapeutic retention on the ocular surface while maintaining optical transparency and comfort. Such hydrogel films offer a promising alternative to conventional eye drops by improving bioavailability, residence time, and patient compliance in treating ocular conditions.
Recent advances include microfluidic hydrogel films embedded in contact lenses, which release drugs in response to ocular pH changes. For example, pH-responsive hydrogel microcavities have been shown to accelerate drug release under acidic conditions associated with inflammation [
232].
The integration of nanoparticles into hydrogel films has further enhanced their drug delivery capabilities. These hydrogel–nanoparticle composites allow for dual-drug loading
, targeted delivery, and programmable release kinetics. A 2024 review by Arabpour et al. highlighted the use of nanosuspensions and nanoemulsions within hydrogel matrices to treat conditions such as glaucoma
, dry eye disease
, and retinal disorders [
238]. These systems improve drug stability and enable delivery of large molecules and biologics to both anterior and posterior segments of the eye, overcoming anatomical barriers such as the blood-retinal barrier [
239].
Hydrogel films are being developed for a wide range of ophthalmic conditions:
Dry eye syndrome: Films loaded with lubricants and anti-inflammatory agents [
235].
Glaucoma: Sustained release of prostaglandin analogs to reduce intraocular pressure [
238].
Post-surgical care: Antibiotic-loaded films to prevent infection and promote healing [
240].
Retinal diseases: Intravitreal hydrogel implants for long-term drug delivery [
239].
Commercial formulations are beginning to emerge. For instance, CsA-PG ophthalmic gel, a cyclosporine-loaded hydrogel, is currently in Phase III clinical trials for treating moderate to severe dry eye disease, showing promising results in reducing inflammation and improving tear production [
241].
The future of ocular drug delivery films lies in the development of multifunctional, stimuli-responsive systems that combine therapeutics, diagnostics, and regenerative capabilities. Promising directions include:
Despite their promise, most hydrogel-based ocular drug delivery systems remain in preclinical stages
, and further clinical validation is needed to ensure safety, efficacy, and scalability [
235].
4.13. Implant Coatings and Biosensors
Hydrogel films have gained significant attention in biomedical engineering as implant coatings and biosensing interfaces, owing to their biocompatibility
, mechanical tunability, and functional versatility [
242]. Their ability to mimic the extracellular matrix (ECM), retain moisture, and respond to physiological stimuli makes them ideal for enhancing implant integration
, reducing immune response, and enabling real-time diagnostics [
243].
Hydrogel films are increasingly used as surface coatings for medical implants, including orthopedic devices, neural interfaces, and cardiovascular implants. Their soft, hydrated nature reduces mechanical mismatch between the implant and surrounding tissue, thereby minimizing foreign body reactions (FBR) and fibrotic encapsulation [
244].
Recent studies have shown that hydrogels with elastic moduli below 1 kPa can suppress activation of mechanosensitive cells such as astrocytes and glia, promoting long-term biointegration in neural implants [
244]. These coatings can also be engineered to release anti-inflammatory agents
, antibiotics, or growth factors, enhancing tissue healing and preventing infection. Advanced hydrogel coatings incorporate conductive polymers (e.g., PEDOT, polypyrrole) or nanomaterials (e.g., carbon nanotubes, graphene) to create electrically conductive hydrogels, which facilitate charge transfer in bioelectronic implants while maintaining mechanical compliance [
244]. Such coatings are particularly valuable in neural stimulation
, cardiac pacing, and biosignal recording. Moreover, antibacterial hydrogel coatings have been developed to combat implant-associated infections
, especially in orthopedic applications. These coatings utilize strategies such as contact killing
, biofilm disruption
, and drug delivery to prevent bacterial colonization and enhance implant longevity [
245].
Hydrogel films have emerged as promising coatings for titanium implants, addressing key challenges such as bacterial infection and poor soft tissue integration. In a recent study, an enzymatically-degradable hydrogel was engineered to cover titanium surfaces, offering a self-adaptive response to infection (
Figure 9A) [
246]. Under normal conditions, the hydrogel promotes fibroblast viability and soft tissue compatibility
. Upon bacterial invasion, the hydrogel degrades, exposing an underlying ZnO nanostructure that activates antibacterial effects. This dual-function system enhances implant safety and healing by combining infection control with tissue regeneration, demonstrating the potential of smart hydrogel coatings in biomedical implants.
4.14. Hydrogel Films Integrated Biosensors
Hydrogel films are also being integrated into biosensors for diagnostics
, wearable health monitoring, and implantable bioelectronics
. Their porous structure
, high water content, and biomolecule immobilization capacity make them ideal for detecting analytes such as glucose
, lactate
, urea, and pathogens
[250].
Hydrogel films are ideal for implantable biosensors due to their high biocompatibility, flexibility, and tissue-like mechanical properties. A recent study Gao et al. introduced a microfiber composite hydrogel (MF-CH), integrating electrospun polyurethane (PU) microfibers into a poly(vinyl alcohol) (PVA) matrix (
Figure 9B-F) [
247]. This design achieved an ultrasoft, ultrathin (<5 μm), and mechanically robust structure, mimicking the extracellular matrix (ECM). The MF-CH exhibited high tensile strength (~6 MPa), tunable modulus (5 kPa–tens of MPa), and excellent anti-tearing properties. Enhanced with glycerol and ionic salts, the hydrogel demonstrated improved ionic conductivity and dehydration resistance, ensuring stable performance in physiological environments. Its application in electromyography (EMG) sensing confirmed its potential as a high-fidelity, long-term bioelectronic interface. These findings position MF-CHs as promising candidates for next-generation implantable biosensors, offering seamless tissue integration and reliable signal acquisition.
Hydrogel-based biosensors can be functionalized with enzymes
, antibodies
, or aptamers, enabling high specificity and sensitivity
. These sensors are used in applications ranging from point-of-care diagnostics to continuous monitoring of chronic diseases
. Recent innovations include electrochemical hydrogel biosensors embedded in wearable devices, capable of real-time monitoring of physiological parameters such as sweat composition
, temperature, and strain [
251]. Conductive hydrogels with nanocomposite fillers have enabled the development of flexible, stretchable, and self-healing biosensors, suitable for electronic skin and prosthetics [
252].
The future of hydrogel films in implant coatings and biosensors lies in the development of multifunctional, smart materials that combine therapeutic, diagnostic, and regenerative capabilities. Promising directions include:
Self-healing hydrogel coatings for long-term implant durability
Stimuli-responsive biosensors for dynamic health monitoring
3D-printed hydrogel interfaces for personalized implant design
Bioelectronic hydrogel platforms for integrated sensing and stimulation
These innovations are paving the way for next-generation biomedical devices that are minimally invasive, highly adaptive, and clinically effective.
4.15. Anti-fouling and Biointegration
Hydrogel films are increasingly recognized for their dual role in preventing biofouling and promoting biointegration in biomedical devices and implants. Their hydrated, soft, and tunable surface chemistry allows them to mimic the extracellular matrix (ECM), reduce immune responses, and resist nonspecific protein adsorption and microbial colonization—two critical factors in long-term implant success.
Biofouling begins with the adsorption of proteins and biomolecules onto implant surfaces, which can lead to bacterial colonization
, biofilm formation
, and chronic inflammation [
253]. Hydrogel films, particularly those based on zwitterionic polymers such as poly(sulfobetaine methacrylate) (pSBMA), have shown excellent resistance to protein and cell adhesion due to their strong hydration layers. These coatings can be further enhanced by incorporating cationic bactericidal polymers, creating dual-functional surfaces that both repel and kill bacteria like
E. coli and
S. aureus [
254].
Zwitterionic hydrogel films have gained attention for implantable applications due to their exceptional antifouling properties and tunable mechanical performance. By photografting zwitterionic polymers onto substrates like PDMS, researchers achieved up to a 20-fold reduction in fibrinogen adsorption and significantly decreased macrophage (30-fold) and fibroblast (10-fold) adhesion, minimizing foreign body response (
Figure 9G-I) [
248].Cross-linking with PEGDMA modulates swelling, compressive modulus, and lubricity, with optimal densities balancing mechanical integrity and biological inertness. Notably, these films exhibit a lower coefficient of friction, enhancing suitability for insertional implants. Overall, zwitterionic hydrogels offer a robust platform for bioinert, durable, and lubricious implant coatings.
Zwitterionic hydrogels (ZIHs) have also shown exceptional promise in antibiofouling applications for implantable devices due to their superhydrophilicity and charge neutrality. In a recent study, a sulfobetaine-based ZIH was covalently bonded to a polyurethane-based dural patch (NP
®), forming an interpenetrating network (NP
®@AZ) reinforced by hydrogen bonds [
255]. This coating significantly reduced protein and bacterial adhesion, as confirmed by in vitro and in vivo tests, without relying on antibiotics. The ZIH’s lubricity also minimized post-implantation adhesion to brain tissue and bone flaps.
Advanced formulations, such as PNIPAAm–co–PMPC hydrogels, combine thermo-responsiveness with antifouling behavior, enabling temperature-triggered drug release while maintaining resistance to biofilm formation [
256]. These smart hydrogels are particularly useful in wound care and implantable devices where infection risk is high. Hydrogel coatings based on polyethylene glycol (PEG) also remain a gold standard for antifouling due to their well-established safety profile and ability to minimize thrombosis and foreign body reactions [
257]. However, newer materials are being developed to overcome PEG’s limitations in mechanical durability and long-term stability.
Beyond resisting fouling, hydrogel films can be engineered to promote biointegration by facilitating cell adhesion
, tissue ingrowth
, and immune modulation. For example
, Janus hydrogels—with asymmetric surface designs—have been developed to provide one side optimized for tissue adhesion and the other for anti-fouling and anti-wear properties [
258]. These biomimetic interfaces support robust integration with soft tissues while minimizing postoperative complications.
Hydrogel-based biointerfaces also play a key role in human–machine integration, such as in neural implants and wearable electronics. Their mechanical compliance
, electrical conductivity, and biocompatibility enable seamless interfacing with biological tissues, reducing foreign body responses and improving signal fidelity [
10,
242]. Recent studies have also explored immunomodulatory hydrogels, which can actively regulate the local immune environment to promote healing and reduce inflammation. These hydrogels are particularly promising for applications in tissue regeneration
, wound healing
, and implantable biosensors [
259].
4.16. Responsive Films for Diagnostics
Hydrogel films are increasingly being developed as stimuli-responsive diagnostic platforms, offering real-time, non-invasive, and highly sensitive detection of physiological and pathological biomarkers. Their soft, hydrated, and tunable polymeric networks allow for dynamic interaction with biological environments, making them ideal for biosensing, point-of-care diagnostics, and wearable health monitoring.
Responsive hydrogel films are engineered to react to external stimuli such as pH
, temperature
, ionic strength
, light
, and electric fields
, or internal biological cues like enzyme activity
, metabolite concentration
, and biomolecular interactions [
260]. These responses typically manifest as changes in swelling behavior
, optical properties
, electrical conductivity
, or mechanical deformation, which can be transduced into measurable signals. For example, DNA-functionalized hydrogels have been used to detect progesterone, mRNA, and other biomarkers through fluorescence resonance energy transfer (FRET) and electrochemical sensing [
260]. These systems offer high specificity and sensitivity, and can be tailored for multiplexed detection.
Recent advancements in biosensing technologies have increasingly focused on the detection of microRNAs (miRNAs) due to their critical role in cancer diagnostics. Among these, surface-enhanced Raman scattering (SERS) platforms have emerged as powerful tools for sensitive and multiplexed detection. Si et al. introduced a novel SERS sensor array that utilizes a dynamic “ON/OFF” Raman signal modulation strategy tailored to miRNA responsiveness (Figure J) [
249]. This system comprises nine distinct sensing units, enabling simultaneous identification of multiple cancer-related miRNAs within a single biological sample.
The fabrication process involved the synthesis of DNA-based hydrogels, which serve as a scaffold for the integration of AuAg nanoparticles—functioning as SERS signal tags. To enhance specificity and catalytic efficiency, multi-component nucleic acid enzymes (MNAzymes) were incorporated into the array. These enzymes facilitate target recognition and signal amplification, thereby improving the overall sensitivity of the platform. The design reflects a strategic convergence of nanomaterials and molecular biology, offering a promising route for non-invasive cancer diagnostics and real-time biomarker monitoring.
Responsive hydrogel films have been applied in various diagnostic contexts:
Hydrogel films are also being integrated into wearable biosensors for monitoring sweat composition, temperature, and strain. These devices leverage the mechanical compliance and biocompatibility of hydrogels to interface seamlessly with skin and tissues [
263]. For instance, electrically responsive hydrogel biosensor arrays have been developed for non-invasive vascular mapping, outperforming traditional imaging techniques in locating perforating arteries [
264].
The future of responsive hydrogel films in diagnostics lies in the development of multifunctional, adaptive systems that combine:
Real-time sensing and feedback
Wireless data transmission
Integration with therapeutic platforms
AI-guided signal interpretation
Emerging technologies such as 4D bioprinting, molecular imprinting, and bioelectronic interfaces are expected to further enhance the diagnostic capabilities of hydrogel films, bringing them closer to clinical translation and personalized medicine.
5. Recent Advances in Hydrogel Films for Biomedical Applications
Hydrogel films have evolved from simple water-retaining matrices into sophisticated, multifunctional platforms capable of responding to environmental stimuli, integrating nanomaterials, and interfacing with electronics. These innovations have significantly expanded their utility across precision medicine, diagnostics, regenerative therapies, and bioelectronics.
5.1. Stimuli-Responsive Hydrogel Films
Stimuli-responsive hydrogel films are engineered to undergo physical or chemical changes in response to specific triggers such as pH, temperature, light, enzymes, or reactive oxygen species (ROS). These smart materials enable on-demand drug release, dynamic tissue interaction, and real-time diagnostics, making them highly valuable in precision medicine, biosensing, and targeted therapy.
Enzyme-responsive systems have shown promise in site-specific drug delivery and diagnostic imaging. For example, hydrogels composed of chitosan, hyaluronic acid, PEGDA, and GelMA degrade selectively in the presence of MMP-2 and hyaluronidase, releasing doxorubicin at tumor sites while sparing healthy cells. These systems also incorporate fluorescent dyes and superparamagnetic iron oxide nanoparticles (SPIONs) for dual optical and MRI-based diagnostics, demonstrating their theranostic potential [
265].
Multi-stimuli-responsive hydrogels react to combinations of triggers such as pH, temperature, light, and magnetic fields, offering precise control over therapeutic actions. These systems are being applied in cancer therapy, wound healing, and biosensing [
266,
267]. For instance, hydrogels that respond to acidic pH and elevated temperatures—common features of tumor microenvironments—can release chemotherapeutics only at diseased sites, reducing systemic toxicity [
266]. Similarly, light-responsive hydrogels allow spatiotemporal control of drug release or activation of therapeutic agents using external light sources [
148].
In diagnostics, these hydrogels convert environmental changes into optical, electrochemical, or mechanical signals. They can detect biomarkers such as glucose, lactate, or inflammatory enzymes, and are being integrated into wearable devices and implantable sensors [
268].
5.2. Nanocomposite and Hybrid Hydrogel Films
Nanocomposite and hybrid hydrogel films represent a significant leap forward in the design of multifunctional biomaterials. By integrating nanostructures and polymeric diversity, these systems overcome limitations of conventional hydrogels—such as poor mechanical strength and limited bioactivity—while enabling targeted therapy, tissue regeneration, and biosensing.
Nanocomposite hydrogels incorporate nanoparticles—including carbon nanotubes (CNTs), graphene, metal oxides, and polymeric nanostructures—into hydrogel matrices to enhance mechanical strength, electrical conductivity, and biological functionality. These materials exhibit sustained therapeutic activity, reduced dosing frequency, and improved cellular interactions [
54]. For example, PAM/CNT nanocomposite hydrogel films have demonstrated excellent biocompatibility, structural stability, and sustained doxorubicin release at acidic pH, effectively inhibiting breast cancer cell proliferation [
44].
Hybrid hydrogels combine natural and synthetic polymers or integrate nano/microstructures to create materials with enhanced mechanical properties, controlled degradation, and tailored drug release profiles. Techniques such as click chemistry, 3D printing, and photopatterning enable precise control over structure and function [
27,
269].
Applications include cancer therapy, wound healing, tissue engineering, and biosensing [
252], with future directions pointing toward electrically conductive hydrogels for neural interfaces, 4D-printed hydrogels, and AI-guided design [
270].
5.3. 3D and 4D Printing of Hydrogel Films
Advances in 3D bioprinting and emerging 4D printing technologies have revolutionized hydrogel film fabrication. These printed hydrogel films are now used in drug delivery systems, tissue scaffolds, implantable devices, and soft robotics. 3D bioprinting enables the layer-by-layer deposition of hydrogel materials with spatial control over composition, porosity, and mechanical properties. Hydrogels based on GelMA, alginate, and PEG derivatives are commonly used due to their biocompatibility and crosslinking versatility. Applications include cartilage repair, bone regeneration, and vascular tissue engineering [
271].
4D printing introduces time-dependent responsiveness, allowing hydrogel films to change shape or function in response to stimuli. These systems are being explored for drug delivery capsules, responsive wound dressings, and bioactuators [
271].
Fabrication techniques include extrusion-based bioprinting, inkjet and laser-assisted printing, photopolymerization, and click chemistry-based modular assembly.
5.4. Biofunctionalization and Smart Materials
Biofunctionalized hydrogel films actively interact with biological systems through embedded bioactive molecules, peptides, or responsive moieties. These intelligent hydrogels respond to ROS, glucose, temperature, pH, and other stimuli, enabling site-specific drug delivery and real-time diagnostics [
271]. Fabrication strategies such as surface functionalization, click-based orthogonal chemistry, and self-assembly allow fine-tuning of hydrogel behavior. Applications include artificial skin, smart drug delivery systems, and implantable medical devices.
Future directions include integration with bioelectronics, AI-guided hydrogel design, and engineered living materials (ELMs) for autonomous regeneration.
5.5. Integration with Wearable and Flexible Electronics
Hydrogel films are increasingly integrated into wearable and flexible electronic systems for health monitoring, diagnostics, and human–machine interfaces. Their softness, stretchability, and biocompatibility make them ideal for skin-like sensors and implantable devices. Hydrogels maintain ionic conductivity and mechanical compliance, enabling detection of physiological signals such as strain, temperature, and biochemical markers. Transparent hydrogel-based electronics support real-time biosensing and interactive displays [
272]. Advanced formulations include PEDOT:PSS/PVA organohydrogels for strain sensing and alginate–gelatin hydrogels for multimodal sensing. Applications span sweat sensors, neural interfaces, cardiac diagnostics, and interactive prosthetics [
273,
274,
275]. Future innovations include self-healing hydrogel electronics, wireless systems using energy-harvesting hydrogels, AI-integrated biosensors, and 3D-printed hydrogel circuits.
6. Challenges and Limitations of Hydrogel Films for Biomedical Applications
Despite their promising potential, hydrogel films face several critical challenges that hinder their widespread clinical and commercial adoption. These limitations span mechanical, chemical, regulatory, and economic domains, requiring multidisciplinary efforts to overcome.
6.1. Mechanical Durability and Tear Resistance
Hydrogel films often suffer from low mechanical strength, brittleness, and poor tear resistance, especially under dynamic physiological conditions. Their soft, hydrated nature, while beneficial for biocompatibility, makes them vulnerable to mechanical failure. Recent reviews highlight that conventional hydrogels exhibit fracture energies far below those of biological tissues, limiting their use in load-bearing applications [
276]. Strategies such as double
-network hydrogels, fiber-reinforced composites, and supramolecular crosslinking have been explored to enhance toughness and elasticity [
277]. For example, hydrogels mimicking tendon-like structures have achieved fracture energies up to 30 kJ/m
2, approaching natural tissue performance [
278]. However, integrating these enhancements into thin-film formats without compromising flexibility and transparency remains a technical bottleneck.
6.2. Sterilization and Storage Stability
Sterilization is essential for biomedical use, but hydrogel films are highly sensitive to conventional sterilization methods. Techniques like autoclaving, gamma irradiation, and ethylene oxide treatment can alter polymer networks, degrade bioactive components, and compromise mechanical integrity. A comparative study found that gamma irradiation, while effective, often causes chain scission and crosslinking changes, affecting swelling behavior and drug release profiles. Supercritical CO
2 and cold plasma sterilization are emerging alternatives that preserve hydrogel properties but require specialized equipment and validation [
279].
Storage stability is another concern. Hydrogels may lose responsiveness or degrade over time due to water loss, microbial contamination, or chemical instability [
280,
281]. Maintaining sterility and functionality during long-term storage remains a challenge, especially for stimuli-responsive and biofunctionalized films [
282].
6.3. Regulatory and Clinical Translation
Hydrogel films face complex regulatory hurdles due to their hybrid nature—often combining device-like structure with drug-like functionality. In the U.S., the FDA classifies hydrogels as either medical devices or combination products, depending on their primary mode of action [
283]. The European MDR (Medical Device Regulation) imposes stricter requirements, including biocompatibility testing, degradation profiling, and risk-based classification [
283]. Only around 100 hydrogel-based products have received clinical approval globally, reflecting the difficulty of navigating regulatory pathways [
284]. Clinical translation is further slowed by the need for extensive preclinical testing, long approval timelines (7–12 years), and high development costs. Many promising hydrogel technologies remain confined to academic labs due to lack of funding and commercialization support [
284].
6.4. Cost and Scalability of Production
Scaling hydrogel film production from lab to industry presents significant technical and economic challenges. Traditional batch processes struggle with consistency, while mixing, casting, and drying operations require precise control to maintain product quality [
285]. Drying methods like freeze-drying and supercritical drying are effective but energy-intensive and expensive, limiting their feasibility for large-scale manufacturing [
286]. In-line monitoring technologies for real-time quality control are still underdeveloped, increasing waste and production costs. The average production cost of hydrogel films remains 30–40% higher than traditional alternatives, creating barriers in price-sensitive markets [
287]. Automation, modular manufacturing systems, and sustainable formulations are needed to reduce costs and improve scalability.
Hydrogel films hold immense promise for biomedical applications, but their mechanical fragility, sterilization sensitivity, regulatory complexity, and production costs pose significant challenges. Addressing these limitations requires innovations in material science, process engineering, and regulatory strategy. Collaborative efforts between academia, industry, and regulatory bodies will be essential to unlock the full potential of hydrogel films in clinical practice.
7. Future Perspectives of Hydrogel Films for Biomedical Applications
Hydrogel films are poised to play a transformative role in next-generation biomedical technologies. As research advances, the focus is shifting toward personalized, intelligent, sustainable, and clinically translatable hydrogel systems. This section explores emerging directions that are expected to shape the future of hydrogel film development and application.
7.1. Personalized and Patient-Specific Hydrogel Films
The integration of hydrogel films into precision medicine is gaining momentum. Personalized hydrogel systems are being designed to match individual patient needs, including tissue-specific mechanical properties, drug release profiles, and biological compatibility. Recent developments in 3D bioprinting have enabled the fabrication of patient-specific hydrogel constructs for organ models, wound dressings, and tissue scaffolds [
288]. These systems can be tailored using patient-derived cells and biomaterials, allowing for customized therapeutic interventions. Moreover, biomaterials-based hydrogels are being explored for personalized drug delivery, where the hydrogel matrix is optimized for the patient’s metabolic profile and disease state [
289]. This approach enhances therapeutic efficacy and minimizes adverse effects.
7.2. AI-Guided Design and Optimization
Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing hydrogel design by enabling predictive modeling, property optimization, and automated formulation discovery. AI-driven platforms can analyze complex datasets to identify optimal polymer compositions, crosslinking strategies, and drug release kinetics [
290]. For example, Bayesian optimization and neural networks have been used to fine-tune polyacrylamide/alginate hydrogels for flexible electronics and biomedical sensors [
291]. These computational tools accelerate development timelines, reduce experimental costs, and facilitate the creation of multi-functional hydrogel films with enhanced performance [
292].
7.3. Sustainable and Biodegradable Materials
Sustainability is becoming a core criterion in hydrogel film development. Researchers are increasingly turning to bio-based and biodegradable polymers such as bacterial cellulose, recombinant collagen, silk fibroin, and xanthan gum [
293]. These materials are produced via biotechnological methods like microbial fermentation and genetic engineering, reducing reliance on petrochemical sources and minimizing environmental impact. Sustainable hydrogels are being used in wound healing, tissue regeneration, and drug delivery, aligning with the One Health paradigm that integrates human, animal, and environmental health [
293]. Natural polymer-based hydrogels also offer biodegradability, reducing long-term waste and improving safety in implantable applications [
294].
7.4. Clinical Trials and Commercialization Pathways
Hydrogel films are transitioning from laboratory research to clinical and commercial use. Several hydrogel-based products have received FDA and EMA approval, while others are undergoing active clinical trials for applications in drug delivery, wound care, and tissue engineering [
7]. Challenges in clinical translation include regulatory classification (device vs. drug), long approval timelines (7–12 years), and manufacturing scalability. However, recent reviews highlight successful commercialization of hydrogel products for ocular, transdermal, and injectable applications [
295]. The global hydrogel market is projected to grow significantly, driven by innovations in stimuli-responsive systems, wearable diagnostics, and bioprinted implants. Strategic partnerships between academia, industry, and regulatory bodies are essential to accelerate commercialization and ensure safety and efficacy [
283].
The future of hydrogel films lies in their ability to adapt to individual patient needs, integrate intelligent design frameworks, and align with sustainability goals. Advances in AI, bioprinting, and biomaterials science are converging to create hydrogel systems that are not only functional but also ethical and environmentally responsible. As clinical trials expand and commercialization pathways mature, hydrogel films are expected to become central to personalized and regenerative healthcare.
8. Conclusion
Hydrogel films have emerged as a transformative class of biomaterials, offering a unique combination of high water content, biocompatibility, tunable mechanical properties, and responsiveness to environmental stimuli. These characteristics have enabled their integration into a wide array of biomedical applications, including wound healing, drug delivery, tissue engineering, ophthalmology, and biosensing.
Over the past decade, remarkable advancements have been made in the design, fabrication, and functionalization of hydrogel films. Innovations such as the incorporation of antimicrobial agents, growth factors, nanoparticles, and smart polymers have significantly enhanced their therapeutic and diagnostic potential. Stimuli-responsive systems, nanocomposites, and biofunctionalized hydrogels have shown promising outcomes in both preclinical and clinical settings. However, challenges remain—particularly in improving mechanical durability, ensuring effective sterilization, navigating regulatory pathways, and scaling up production for widespread clinical use.
Despite these hurdles, hydrogel films have already demonstrated substantial biomedical impact by improving patient outcomes, minimizing treatment invasiveness, and enabling real-time physiological monitoring. Their adaptability to diverse anatomical sites and therapeutic needs positions them as ideal candidates for personalized medicine, especially in areas such as chronic wound care, targeted drug delivery, and regenerative therapies.
Looking forward, the future of hydrogel films lies at the intersection of materials science, biotechnology, and digital health. Key directions for future research and development include the creation of personalized hydrogel systems tailored to individual patient profiles, the application of AI-driven design for performance optimization, the use of sustainable and biodegradable materials, and the establishment of robust clinical and commercialization pathways. Achieving these goals will require interdisciplinary collaboration among scientists, engineers, clinicians, and regulatory experts to fully unlock the potential of hydrogel films and usher in a new era of advanced biomedical solutions.
Author Contributions
S.C.S. & H.S.: Conceptualization, literature search, validation, data analysis, original draft preparation, review, and editing. W.K and H.J.: review and editing. H.J.: Resources, project administration, funding acquisition, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Konkuk University, Glocal Campus, South Korea.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors gratefully acknowledge the financial support from Konkuk University, Gocal Campus, South Korea.
Conflicts of Interest
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author(s) used Copilot to improve the language and readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the publication content.
References
- Gajurel, B.; Tamang, K.B.; Das, D.; Adhikari, R. Advances in synthetic strategies and applications of polymeric hydrogels. Polymer Engineering & Science 2025, 65, 2803–2840. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogosanu, G.D.; Bita, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers (Basel) 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, W.S.; Jeong, J.O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10. [Google Scholar] [CrossRef]
- Ubaldini, A.; Calistri, S. Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields. Applied Sciences 2025, 15, 9579. [Google Scholar] [CrossRef]
- Le, H.H.; Tran, V.T.; Mredha, M.T.I.; Na, J.Y.; Seon, J.-K.; Jeon, I. Thin-film hydrogels with superior stiffness, strength, and stretchability. Extreme Mechanics Letters 2020, 37, 100720. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J.; Zo, S.M.; Kummara, M.R.; Kim, C.M.; Kumar, A.; Bae, H.J.; Sood, A.; Han, S.S. Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery. Gels 2025, 11, 49. [Google Scholar] [CrossRef]
- Raeisi, A.; Farjadian, F. Commercial hydrogel product for drug delivery based on route of administration. Front Chem 2024, 12, 1336717. [Google Scholar] [CrossRef]
- Labie, H.; Blanzat, M. Hydrogels for dermal and transdermal drug delivery. Biomaterials Science 2023, 11, 4073–4093. [Google Scholar] [CrossRef]
- Alavi, S.E.; Panah, N.; Page, F.; Gholami, M.; Dastfal, A.; Ajay Sharma, L.; Ebrahimi, H. Hydrogel-based therapeutic coatings for dental implants. European Polymer Journal 2022, 181, 111652. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nature Reviews Materials 2022, 7. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. Hydrogels for Translucent Wearable Electronics: Innovations in Materials, Integration, and Applications. Gels 2025, 11. [Google Scholar] [CrossRef]
- Khan, S.; Maryam, L.; Gulzar, A.; Mansoor, M.A.; Iqbal, M. smart and active hydrogels in biotechnology—synthetic techniques and applications. Journal of Materials Science 2024, 59, 16449–16471. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, J.; Gao, D.; Du, Y.; Zhang, Y.; Lai, Y. Stimuli-responsive biomedical polymeric films for tissue regeneration. Microstructures 2025, 5, 2025055. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Tian, Y.; Zhang, X.N.; Du, M.; Song, Y.; Wu, Z.L.; Zheng, Q. Spin-coating-assisted fabrication of ultrathin physical hydrogel films with high toughness and fast response. Soft Matter 2018, 14, 5888–5897. [Google Scholar] [CrossRef] [PubMed]
- Yola, A.M.; Campbell, J.; Volodkin, D. Microfluidics meets layer-by-layer assembly for the build-up of polymeric scaffolds. Applied Surface Science Advances 2021, 5, 100091. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Applied Polymer Materials 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Uysal, B.; Madduma-Bandarage, U.S.K.; Jayasinghe, H.G.; Madihally, S. 3D-Printed Hydrogels from Natural Polymers for Biomedical Applications: Conventional Fabrication Methods, Current Developments, Advantages, and Challenges. Gels 2025, 11, 192. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Huang, W.; Lei, Y. Injectable smart stimuli-responsive hydrogels: pioneering advancements in biomedical applications. Biomaterials Science 2024, 12, 8–56. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Tang, L.; Shi, D.; Lam, E.; Bae, J. 3D shape morphing of stimuli-responsive composite hydrogels. Materials Chemistry Frontiers 2023, 7, 5989–6034. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.; Vermonden, T. Stimuli-responsive hydrogels: the dynamic smart biomaterials of tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Angaria, N.; Saini, S.; Hussain, M.S.; Sharma, S.; Singh, G.; Khurana, N.; Kumar, R. Natural polymer-based hydrogels: versatile biomaterials for biomedical applications. International Journal of Polymeric Materials and Polymeric Biomaterials 2024, 73, 1550–1568. [Google Scholar] [CrossRef]
- Wang, C.; Yokota, T.; Someya, T. Natural Biopolymer-Based Biocompatible Conductors for Stretchable Bioelectronics. Chemical Reviews 2021, 121, 2109–2146. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. Journal of Biomaterials Science, Polymer Edition 2022, 33, 1595–1622. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Dalvi, Y.B.; Lochana, P.; Achinthya, S.; Somani, B.O.; Karnaver, P.; Thomas, N.G.; Rupesh, S.; Varghese, N.; VP, J. Physiochemical and Biomedical Properties of Hydrogels: From Fundamentals to Applications. Hydrogels and Nanogels-Applications in Medicine 2024. [Google Scholar]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. International Journal of Polymer Science 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Kumar, A.C.; Erothu, H. Synthetic Polymer Hydrogels. In Biomedical Applications of Polymeric Materials and Composites; 2016; pp. 141–162.
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. International Journal of Pharmaceutics 2025, 672, 125323–125323. [Google Scholar] [CrossRef]
- Sevinc Ozdemir, N.; Kenar, H. Properties and Preparation Techniques of Hydrogels. In Hydrogels and Bioinks in Tissue Engineering; Springer: 2025; pp. 57–74.
- Yammine, P.; El Safadi, A.; Kassab, R.; El-Nakat, H.; Obeid, P.J.; Nasr, Z.; Tannous, T.; Sari-Chmayssem, N.; Mansour, A.; Chmayssem, A. Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry 2025, 7, 61. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Physical crosslinking of hydrogels: The potential of dynamic and reversible bonds in burn care. Coordination Chemistry Reviews 2025, 542, 216868. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol Pharm 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioactive Materials 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Yousaf, M.T.; Ullah, S.; Yousaf, H.; Li, Y.; Jin, H.; Shen, J.; Xu, H.-T. Advancements in hydrogels: A comprehensive review of natural, synthetic, and hybrid innovations for wound healing. International Journal of Biological Macromolecules 2025, 327, 147270. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Luo, S.; Huang, W.; Lu, X.; Kankala, R.K.; Wang, S.; Xu, P.; Chen, A. ECM-inspired stem cell secretome sustained releasing composite nanofibrous membranes for accelerated wound healing. Mater Today Bio 2025, 34, 102141. [Google Scholar] [CrossRef] [PubMed]
- Sawadkar, P.; Lali, F.; Garcia-Gareta, E.; Garrido, B.G.; Chaudhry, A.; Matharu, P.; Kyriakidis, C.; Greco, K. Innovative hydrogels in cutaneous wound healing: current status and future perspectives. Frontiers in Bioengineering and Biotechnology 2025, 13, 1454903. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Kang, L.; Li, Z.M.; Tseng, S.L.; Wang, L.Q.; Li, T.H.; Li, Z.J.; Huang, J.Z.; Yu, N.Z.; Long, X. Biological scaffold as potential platforms for stem cells: Current development and applications in wound healing. World J Stem Cells 2024, 16, 334–352. [Google Scholar] [CrossRef]
- Bogadi, S.; Malayandi, R.; Vasanth Raj, P.; Suresh Kumar, A.; Parvathaneni, M.; Kumar Kundu, M.; Rabiul Islam, M.; Khan, F.S.; Tagde, P.; Kumar Mondal, T.; et al. Silk fibroin and sericin: Multifunctional formulations for treating diabetic wound healing. European Polymer Journal 2024, 220, 113465. [Google Scholar] [CrossRef]
- Nifontova, G.; Safaryan, S.; Khristidis, Y.; Smirnova, O.; Vosough, M.; Shpichka, A.; Timashev, P. Advancing wound healing by hydrogel-based dressings loaded with cell-conditioned medium: a systematic review. Stem cell research & therapy 2024, 15, 371. [Google Scholar]
- Qutub, M.; Tatode, A.; Taksande, J.; Premchandani, T.; Umekar, M.; Hussain, U.M.; Biyani, D.; Mane, D. Stimuli-responsive supramolecular hydrogels for paclitaxel delivery: Progress and prospects. Aspects of Molecular Medicine 2025, 5, 100062. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sargazi, S.; Baino, F. Recent advances in hydrogel-based drug delivery systems for enhanced cancer therapy: A review. Materials Today Communications 2025, 48, 113615. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A.; Sillanpaa, M.; Ghasemzadeh, H.; Mohammadi, E. Biocompatible porous PAM/CNT nanocomposite hydrogel films for sustained drug delivery and cancer therapy. Scientific Reports 2025, 15, 22387. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Amin Yavari, S. Liposome-integrated hydrogel hybrids: Promising platforms for cancer therapy and tissue regeneration. Journal of Controlled Release 2024, 368, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current advances in stimuli-responsive hydrogels as smart drug delivery carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Nasseri, R.; Bouzari, N.; Huang, J.; Golzar, H.; Jankhani, S.; Tang, X.; Mekonnen, T.H.; Aghakhani, A.; Shahsavan, H. Programmable nanocomposites of cellulose nanocrystals and zwitterionic hydrogels for soft robotics. Nature Communications 2023, 14, 6108. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Spicer, C. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polymer Chemistry 2019, 11. [Google Scholar] [CrossRef]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Sanchez-Schmitz, G.; Ramos-Payan, R. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Frontiers in Materials 2021, Volume 8 - 2021. [CrossRef]
- Zhang, B.; Zhang, M.; Jiang, C.; Yan, W.; Pan, Y.; Meng, F. Engineered polysaccharide scaffolds for cartilage regeneration: Mechanisms, functionalization, and clinical prospects. Colloids and Surfaces B: Biointerfaces 2026, 257, 115134. [Google Scholar] [CrossRef]
- Lu, J.; Gao, Y.; Cao, C.; Wang, H.; Ruan, Y.; Qin, K.; Liu, H.; Wang, Y.; Yang, P.; Liu, Y.; et al. 3D bioprinted scaffolds for osteochondral regeneration: advancements and applications. Materials Today Bio 2025, 32, 101834. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, D.; Li, G.; Liu, J.; Chen, X.; Su, J. Engineering bone/cartilage organoids: strategy, progress, and application. Bone Res 2024, 12, 66. [Google Scholar] [CrossRef]
- Baishya, G.; Parasar, B.; Limboo, M.; Kumar, R.; Dutta, A.; Hussain, A.; Phukan, M.M.; Saikia, D. Advancements in nanocomposite hydrogels: A comprehensive review of biomedical applications. Discover Materials 2024, 4, 40. [Google Scholar] [CrossRef]
- Gong, W.; Kim, J.; Kim, C.; Chang, H.; Ahn, Y.; Schaffer, D.V.; Baek, J. Hydrogel Fabrication Techniques for Advanced Artificial Sensory Systems. International Journal of Extreme Manufacturing 2025. [Google Scholar] [CrossRef]
- Gorantla, A.; Hall, J.T.V.E.; Troidle, A.; Janjic, J.M. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines 2024, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M.; et al. Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Frontiers in Bioengineering and Biotechnology 2022, Volume 10 - 2022. [CrossRef]
- Han, I.K.; Chung, T.; Han, J.; Kim, Y.S. Nanocomposite hydrogel actuators hybridized with various dimensional nanomaterials for stimuli responsiveness enhancement. Nano Convergence 2019, 6, 18. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel actuators and sensors for biomedical soft robots: brief overview with impending challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Lee, H.K.; Yang, Y.J.; Koirala, G.R.; Oh, S.; Kim, T.-i. From lab to wearables: Innovations in multifunctional hydrogel chemistry for next-generation bioelectronic devices. Biomaterials 2024, 310, 122632. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Fan, M.-H.; Pi, J.-K.; Zou, C.-Y.; Jiang, Y.-L.; Li, Q.-J.; Zhang, X.-Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.-Q. Hydrogel-exosome system in tissue engineering: A promising therapeutic strategy. Bioactive Materials 2024, 38, 1–30. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis. Applied Sciences 2019, 9, 5235. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: an update on potential biomedical and pharmaceutical applications. Marine drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, B.; Mignon, A.; Declercq, H.; Van Vlierberghe, S.; Dubruel, P. Development of gelatin-alginate hydrogels for burn wound treatment. Macromolecular bioscience 2019, 19, 1900123. [Google Scholar] [CrossRef]
- Salamon, A.; Van Vlierberghe, S.; Van Nieuwenhove, I.; Baudisch, F.; Graulus, G.-J.; Benecke, V.; Alberti, K.; Neumann, H.-G.; Rychly, J.; Martins, J.C. Gelatin-based hydrogels promote chondrogenic differentiation of human adipose tissue-derived mesenchymal stem cells in vitro. Materials 2014, 7, 1342–1359. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.T.; Madeira, A.B.; Pereira, P.; Branco, R.; Morais, P.V.; Coelho, J.F.; Fonseca, A.C.; Serra, A.C. ROS-degradable PEG-based wound dressing films with drug release and antibacterial properties. European Polymer Journal 2022, 177, 111447. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and evaluation of chitosan/PVA based hydrogel films loaded with honey for wound healing application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Mahardian, A. Biocompatible hydrogel film of polyethylene oxide-polyethylene glycol dimetacrylate for wound dressing application. In Proceedings of the IOP Conference Series: Materials Science and Engineering; 2018; p. 012076. [Google Scholar]
- Jeong, H.I.; An, D.H.; Lim, J.W.; Oh, T.; Lee, H.; Park, S.-M.; Jeong, J.H.; Chung, J.W. Hydrogel surface-modified polyurethane copolymer film with water permeation resistance and biocompatibility for implantable biomedical devices. Micromachines 2021, 12, 447. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Lebedeva, K.; Cherkashina, A.; Lebedev, V.; Tsereniuk, O.; Krygina, N. Study of hybrid modification with humic acids of environmentally safe biodegradable hydrogel films based on hydroxypropyl methylcellulose. C 2022, 8, 71. [Google Scholar] [CrossRef]
- Mali, K.; Dhawale, S.; Dias, R.; Dhane, N.; Ghorpade, V. Citric Acid Crosslinked Carboxymethyl Cellulose-based Composite Hydrogel Films for Drug Delivery. Indian Journal of Pharmaceutical Sciences 2018, 80. [Google Scholar] [CrossRef]
- Sharma, G.; George Joy, J.; Sharma, A.R.; Kim, J.-C. Accelerated full-thickness skin wound tissue regeneration by self-crosslinked chitosan hydrogel films reinforced by oxidized CNC-AgNPs stabilized Pickering emulsion for quercetin delivery. Journal of Nanobiotechnology 2024, 22, 323. [Google Scholar] [CrossRef]
- Kouser, R.; Vashist, A.; Zafaryab, M.; Rizvi, M.A.; Ahmad, S. Na-montmorillonite-dispersed sustainable polymer nanocomposite hydrogel films for anticancer drug delivery. ACS omega 2018, 3, 15809–15820. [Google Scholar] [CrossRef]
- Motoyama, K.; Higashi, T.; Okajima, M.K.; Kaneko, T.; Arima, H. Potential use of sacran hydrogels as wound dressing material. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan 2018, 138, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Huang, Y.; Wang, Y.; Xu, H.; Xing, M.; Zhong, W. Mussel-inspired dopamine and carbon nanotube leading to a biocompatible self-rolling conductive hydrogel film. Materials 2017, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Xue, Y.; Hartley, R.; Sant, V.; Eles, J.R.; Cui, X.T.; Stolz, D.B.; Sant, S. Hierarchically aligned fibrous hydrogel films through microfluidic self-assembly of graphene and polysaccharides. Biotechnology and bioengineering 2018, 115, 2654–2667. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Scientific Reports 2021, 11, 21255. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Dodou, K. Effect of drug loading method and drug physicochemical properties on the material and drug release properties of poly (ethylene oxide) hydrogels for transdermal delivery. Polymers 2017, 9, 286. [Google Scholar] [CrossRef]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and characterization of low methoxyl pectin/gelatin/carboxymethyl cellulose absorbent hydrogel film for wound dressing applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef]
- Aman, J.; Shahi, N.C.; Lohani, U.C.; Balodhi, D.; Singh, R.; Kumar, N.; Bhat, M.I.; Kumar, A.P. [Retracted] Process Optimization for Development of Guar Gum-Based Biodegradable Hydrogel Film Using Response Surface Methodology. Bioinorganic Chemistry and Applications 2022, 2022, 9180000. [Google Scholar] [CrossRef]
- Park, K.M.; Park, K.D. In situ cross-linkable hydrogels as a dynamic matrix for tissue regenerative medicine. Tissue Engineering and Regenerative Medicine 2018, 15, 547–557. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.-W. A Comprehensive review of radiation-induced hydrogels: Synthesis, properties, and multidimensional applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Wolfel, A.; Romero, M.R.; Igarzabal, C.I.A. Post-synthesis modification of hydrogels. Total and partial rupture of crosslinks: Formation of aldehyde groups and re-crosslinking of cleaved hydrogels. Polymer 2017, 116, 251–260. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. European Polymer Journal 2022, 173, 111277. [Google Scholar] [CrossRef]
- Cook, J.P.; Goodall, G.W.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Microwave-assisted hydrogel synthesis: a new method for crosslinking polymers in aqueous solutions. Macromolecular rapid communications 2012, 33, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Lutton, R.E.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-assisted preparation of hydrogel-forming microneedle arrays for transdermal drug delivery applications. Macromolecular materials and engineering 2015, 300, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Thongsuksaengcharoen, S.; Samosorn, S.; Songsrirote, K. A facile synthesis of self-catalytic hydrogel films and their application as a wound dressing material coupled with natural active compounds. Acs Omega 2020, 5, 25973–25983. [Google Scholar] [CrossRef]
- Pargaonkar, S.S.; Ghorpade, V.S.; Mali, K.K.; Dias, R.J.; Havaldar, V.D.; Kadam, V.J.; Pargaonkar, M.S.S. Hydrogel films of citric acid cross-linked hydroxypropyl methylcellulose/methylcellulose for hydrophilic drug delivery. Indian J. Pharm. Educ. Res 2023, 57, 718–727. [Google Scholar] [CrossRef]
- Brinker, C.; Hurd, A.; Schunk, P.; Frye, G.; Ashley, C. Review of sol-gel thin film formation. Journal of Non-Crystalline Solids 1992, 147, 424–436. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Li, S.; Zhang, X.; Miao, Z.; Zeng, Z.; Ruan, D.; Shen, Y.; Yuan, H.; Zhao, Y.; et al. Tough and Functional Hydrogel Coating by Electrostatic Spraying. Small 2025, 21, 2408780. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.J.; Xi, S.; Zhang, Y.; Rao, P.; You, X.; Qu, S. Lignin-Based Ultrathin Hydrogel Coatings with Strong Substrate Adhesion Enabled by Hydrophobic Association. Advanced Functional Materials 2025, 35, 2413464. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, J.; Qiu, X.; Liu, H.; Liu, X.; Yao, P.; Huang, J.; Cui, X.; Liang, X.; Huang, C. Towards Large-Scale Fabrication of Self-Healable Functional Hydrogel Coatings for Anti-Fog/Frost Surfaces and Flexible Sensors. Advanced Materials Technologies 2021, 6, 2001267. [Google Scholar] [CrossRef]
- Pemble, O.J.; Bardosova, M.; Povey, I.M.; Pemble, M.E. A Slot-Die Technique for the Preparation of Continuous, High-Area, Chitosan-Based Thin Films. Polymers 2021, 13, 1566. [Google Scholar] [CrossRef]
- Li, C.Y.; Hao, X.P.; Wu, Z.L.; Zheng, Q. Photolithographically Patterned Hydrogels with Programmed Deformations. Chemistry – An Asian Journal 2019, 14, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C. Recent advances in 3D printing hydrogel for topical drug delivery. MedComm – Biomaterials and Applications 2022, 1. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian Journal of Pharmaceutical Sciences 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Bauer, M.; Duerkop, A.; Baeumner, A.J. Critical review of polymer and hydrogel deposition methods for optical and electrochemical bioanalytical sensors correlated to the sensor’s applicability in real samples. Analytical and Bioanalytical Chemistry 2023, 415, 83–95. [Google Scholar] [CrossRef]
- Li, Y.; Ni, C.; Cao, R.; Jiang, Y.; Xia, L.; Ren, H.; Chen, Y.; Xie, T.; Zhao, Q. Sprayable porous hydrogel coating for efficient and sustainable evaporative cooling. Matter 2024, 7, 4270–4280. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Jeong, T.-J.; Yu, X.; Harris, T.A.L. Scaled Production of Functionally Gradient Thin Films Using Slot Die Coating on a Roll-to-Roll System. ACS Applied Materials & Interfaces 2024, 16, 9264–9274. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Ke, L.-Y.; Wei, S.-Y.; Poddar, M.S.; Liu, C.-H. Optofluidic thin-film lithography for photocrosslinking hydrogel-based microarchitectures and the assembling of modular cell-embedded microarchitectures. Sensors and Actuators B: Chemical 2022, 352, 131048. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K. 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Rao Bhaviripudi, V.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. 2024. [CrossRef]
- Dong, M.; Jiao, D.; Zheng, Q.; Wu, Z.L. Recent progress in fabrications and applications of functional hydrogel films. Journal of Polymer Science 2023, 61, 1026–1039. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels … A review. Saudi Pharmaceutical Journal 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Tran, T.V.; Trai, V.K. Facile fabrication route of stretchable thin-film hydrogels with high strength. MRS Advances 2024, 9, 1672–1677. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug delivery 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Ngo, H.V.; Tran, P.H.L.; Lee, B.-J.; Tran, T.T.D. Development of film-forming gel containing nanoparticles for transdermal drug delivery. Nanotechnology 2019, 30, 415102. [Google Scholar] [CrossRef]
- Sun, W.; Wu, W.; Dong, X.; Yu, G. Frontier and hot topics in the application of hydrogel in the biomedical field: a bibliometric analysis based on CiteSpace. Journal of Biological Engineering 2024, 18, 40. [Google Scholar] [CrossRef]
- Asadikorayem, M.; Weber, P.; Surman, F.; Puiggalí-Jou, A.; Zenobi-Wong, M. Foreign body immune response to zwitterionic and hyaluronic acid granular hydrogels made with mechanical fragmentation. Advanced Healthcare Materials 2025, 14, 2402890. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Willerth, S.M.; de la Torre, L.G.; Han, S.W. Trends in hydrogel-based encapsulation technologies for advanced cell therapies applied to limb ischemia. Materials Today Bio 2022, 13, 100221. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, J. Recent Progress in Hydrogel Synthesis and Biomedical Applications. Gels 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Britton, D.; Almanzar, D.; Xiao, Y.; Shih, H.-W.; Legocki, J.; Rabbani, P.; Montclare, J.K. Exosome Loaded Protein Hydrogel for Enhanced Gelation Kinetics and Wound Healing. ACS Applied Bio Materials 2024, 7, 5992–6000. [Google Scholar] [CrossRef] [PubMed]
- Lavrentev, F.V.; Shilovskikh, V.V.; Alabusheva, V.S.; Yurova, V.Y.; Nikitina, A.A.; Ulasevich, S.A.; Skorb, E.V. Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules 2023, 28, 5931. [Google Scholar] [CrossRef]
- Kanduč, M.; Kim, W.K.; Roa, R.; Dzubiella, J. How the shape and chemistry of molecular penetrants control responsive hydrogel permeability. ACS nano 2020, 15, 614–624. [Google Scholar] [CrossRef]
- Narayana, S.; Gowda, B.J.; Hani, U.; Ahmed, M.G.; Asiri, Z.A.; Paul, K. Smart poly (N-isopropylacrylamide)-based hydrogels: a tour D’horizon of biomedical applications. Gels 2025, 11, 207. [Google Scholar] [CrossRef]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Frontiers in Bioengineering and Biotechnology 2023, 11, 1270364. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Global Cardiology Science and Practice 2013, 2013, 38. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Hu, Z.; Lu, W.; Li, Z.; Gao, N.; Liu, N.; Li, Y.; He, J.; Gao, Q. Gelatin-based metamaterial hydrogel films with high conformality for ultra-soft tissue monitoring. Nano-Micro Letters 2024, 16, 34. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomaterials Science & Engineering 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Sun, K.; Seong, M.; Hwang, I.; Jang, H.; Park, S.; Choi, G.; Lee, S.-H.; Kim, J.; Jeong, H.E. Applications of bioinspired reversible dry and wet adhesives: A review. Frontiers in Mechanical Engineering 2021, 7, 668262. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Nie, L.; Muñoz-Camargo, C.; Ganguly, S.; Bahsis, L.; Cruz, J.C.; Mohammadinejad, R.; Nicosia, A.; Reyes, L.H.; Wang, X. Editorial: Biocompatible hydrogels: properties, synthesis and applications in biomedicine. Frontiers in Chemistry 2024, Volume 12 - 2024. [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ren, P.; Yang, L.; Wei, D.; Liang, M.; Xu, L.; Zhang, T.; Hu, W.; Zhang, Z.; Zhang, Q. Alginate/polyacrylamide host-guest supramolecular hydrogels with enhanced adhesion. International Journal of Biological Macromolecules 2023, 242, 124885. [Google Scholar] [CrossRef]
- Ollier, R.C.; Webber, M.J. Mechanoresponsive Hydrogels Emerging from Dynamic and Non-Covalent Interactions. Advanced Materials 2025. [Google Scholar] [CrossRef]
- Feliciano, A.J.; van Blitterswijk, C.; Moroni, L.; Baker, M.B. Realizing tissue integration with supramolecular hydrogels. Acta Biomaterialia 2021, 124, 1–14. [Google Scholar] [CrossRef]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses : iv. Viral multiplication in a stable strain of human malignant epithelial cells (strain hela) derived from an epidermoid carcinoma of the cervix. Journal of Experimental Medicine 1953, 97, 695–710. [Google Scholar] [CrossRef]
- Yi, B.; Wang, X.; Yu, J.; Diao, J.; Wang, G.; Li, S.; Bo, J.; Zhang, X.; Zhang, C.; Guimarães, C.F.; et al. Biomimetic hydrogel micro-/nanofibers for in situ soft tissue repair and regeneration. Bioactive Materials 2026, 55, 485–502. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioactive Materials 2022, 9, 198–220. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Hu, Z.; Lu, W.; Li, Z.; Gao, N.; Liu, N.; Li, Y.; He, J.; Gao, Q.; et al. Gelatin-Based Metamaterial Hydrogel Films with High Conformality for Ultra-Soft Tissue Monitoring. Nano-Micro Letters 2023, 16, 34. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Materials Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lin, C.; Hu, H.; Zhao, Y.; Liao, J.; Al-Smadi, F.; Mi, B.; Hu, Y.; Liu, G. Recent advances and challenges in hydrogel-based delivery of immunomodulatory strategies for diabetic wound healing. Theranostics 2026, 16, 516–544. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Kang, H.W. Recent progress in multifunctional theranostic hydrogels: the cornerstone of next-generation wound care technologies. Biomater. Sci. 2025, 13, 4358–4389. [Google Scholar] [CrossRef]
- Han, Y.; Cao, J.; Li, M.; Ding, P.; Yang, Y.; Okoro, O.V.; Sun, Y.; Jiang, G.; Shavandi, A.; Nie, L. Fabrication and characteristics of multifunctional hydrogel dressings using dopamine modified hyaluronic acid and phenylboronic acid modified chitosan. Frontiers in Chemistry 2024, Volume 12 - 2024. [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17. [Google Scholar] [CrossRef]
- Naranđa, J.; Bračič, M.; Maver, U.; Trojner, T. Recent Advancements in Smart Hydrogel-Based Materials in Cartilage Tissue Engineering. Materials 2025, 18, 2576. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, C.; and Zou, H. Current Status and Prospects of Biomaterials (Review). Journal of Jinan University (Natural Science Edition) 2000, 21, 122–125. [Google Scholar]
- Chiellini, E.; Solaro, R. Multifunctional bioerodible/biodegradable polymeric materials. Macromolecular Symposia 1995, 98, 803–824. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. International Journal of Molecular Sciences 2022, 23, 4421. [Google Scholar] [CrossRef]
- Ding, K.; Liao, M.; Wang, Y.; Lu, J.R. Advances in Composite Stimuli-Responsive Hydrogels for Wound Healing: Mechanisms and Applications. Gels 2025, 11, 420. [Google Scholar] [CrossRef]
- Zhang, X.; Zu, Q.; Deng, C.; Gao, X.; Liu, H.; Jin, Y.; Yang, X.; Wang, E. Biodegradable Double-Layer Hydrogels with Sequential Drug Release for Multi-Phase Collaborative Regulation in Scar-Free Wound Healing. Journal of Functional Biomaterials 2025, 16, 164. [Google Scholar] [CrossRef]
- Hu, B.; Gao, J.; Lu, Y.; Wang, Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.-C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels 2025, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.M.; Hasan, I.T.; Jwaid, M.M.; Dawood, A.H.; Jawad, M.A. Enhanced Drug Delivery and Wound Healing with Novel Hydrogel Nanocomposite. Engineering Proceedings 2023, 59, 219. [Google Scholar]
- Mukhopadhyay, P.; Eid, N.; Abdelmegeed, M.A.; Sen, A. Interplay of Oxidative Stress, Inflammation, and Autophagy: Their Role in Tissue Injury of the Heart, Liver, and Kidney. Oxidative Medicine and Cellular Longevity 2018, 2018, 2090813. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.M.; Bakaic, E.; Stewart, S.A.; Hoare, T.; Adronov, A. Properties of Poly(ethylene glycol) Hydrogels Cross-Linked via Strain-Promoted Alkyne–Azide Cycloaddition (SPAAC). Biomacromolecules 2016, 17, 1093–1100. [Google Scholar] [CrossRef]
- Liew, K.-F.; Hanapi, N.A.; Chan, K.-L.; Yusof, S.R.; Lee, C.-Y. Assessment of the Blood-Brain Barrier Permeability of Potential Neuroprotective Aurones in Parallel Artificial Membrane Permeability Assay and Porcine Brain Endothelial Cell Models. Journal of Pharmaceutical Sciences 2017, 106, 502–510. [Google Scholar] [CrossRef]
- Stidl, R.; Denne, M.; Goldstine, J.; Kadish, B.; Korakas, K.I.; Turecek, P.L. Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety. Pharmaceuticals 2018, 11, 75. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, X.; Huang, F.; Chen, X. Anti-PEG Antibodies and Their Biological Impact on PEGylated Drugs: Challenges and Strategies for Optimization. Pharmaceutics 2025, 17, 1074. [Google Scholar] [CrossRef]
- Muktar, M.Z.; Bakar, M.A.A.; Amin, K.A.M.; Che Rose, L.; Wan Ismail, W.I.; Razali, M.H.; Abd Razak, S.I.; in het Panhuis, M. Gellan gum hydrogels filled edible oil microemulsion for biomedical materials: Phase diagram, mechanical behavior, and in vivo studies. Polymers 2021, 13, 3281. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Jang, K.-I.; Chung, H.U.; Xu, S.; Lee, C.H.; Luan, H.; Jeong, J.; Cheng, H.; Kim, G.-T.; Han, S.Y.; Lee, J.W. Soft network composite materials with deterministic and bio-inspired designs. Nature communications 2015, 6, 6566. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. Journal of Composites Science 2025, 9, 133. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Y.; Xu, K. Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels 2025, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microscopy research and technique 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clinical and Translational Medicine 2022, 12, e1094. [Google Scholar] [CrossRef]
- Hasan, N.; Jiafu, C.; Mustopa, A.Z.; Himawan, A.; Umami, R.N.; Ullah, M.; Wathoni, N.; Yoo, J.-W. Recent advancements of nitric oxide-releasing hydrogels for wound dressing applications. Journal of Pharmaceutical Investigation 2023, 53, 781–801. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. International journal of biological macromolecules 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Li, M.; Dong, Y.; Wang, M.; Lu, X.; Li, X.; Yu, J.; Ding, B. Hydrogel/nanofibrous membrane composites with enhanced water retention, stretchability and self-healing capability for wound healing. Composites Part B: Engineering 2023, 257, 110672. [Google Scholar] [CrossRef]
- Cheng, H.; Newton, M.A.A.; Rajib, M.; Zhang, Q.; Gao, W.; Lu, Z.; Zheng, Y.; Dai, Z.; Zhu, J. A ZIF-8-encapsulated interpenetrated hydrogel/nanofiber composite patch for chronic wound treatment. Journal of Materials Chemistry B 2024, 12, 2042–2053. [Google Scholar] [CrossRef]
- Ruan, L.; Pan, C.; Ran, X.; Wen, Y.; Lang, R.; Peng, M.; Cao, J.; Yang, J. Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection. Gels 2024, 10, 219. [Google Scholar] [CrossRef]
- Ullah, I.; Hussain, Z.; Ullah, S.; Zahra, Q.u.a.; Zhang, Y.; Mehmood, S.; Liu, X.; Kamya, E.; Waseem Ghani, M.; Mansoorianfar, M.; et al. An osteogenic, antibacterial, and anti-inflammatory nanocomposite hydrogel platform to accelerate bone reconstruction. Journal of Materials Chemistry B 2023, 11, 5830–5845. [Google Scholar] [CrossRef]
- Pratinthong, K.; Punyodom, W.; Jantrawut, P.; Jantanasakulwong, K.; Tongdeesoontorn, W.; Sriyai, M.; Panyathip, R.; Thanakkasaranee, S.; Worajittiphon, P.; Tanadchangsaeng, N.; et al. Modification of a Carboxymethyl Cellulose/Poly(vinyl alcohol) Hydrogel Film with Citric Acid and Glutaraldehyde Crosslink Agents to Enhance the Anti-Inflammatory Effectiveness of Triamcinolone Acetonide in Wound Healing. Polymers 2024, 16, 1798. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Hu, L.; Chen, X.; Zuo, L.; Bai, X.; Du, W.; Xu, N. Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing. Molecules 2024, 29, 1423. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric Herb Extract-Incorporated Biopolymer Dressings with Beneficial Antibacterial, Antioxidant and Anti-Inflammatory Properties for Wound Healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, A.R.; Razmjooee, K.; Saber-Samandari, S.; Toghraie, D. Fabrication of chitosan-gelatin films incorporated with thymol-loaded alginate microparticles for controlled drug delivery, antibacterial activity and wound healing: in-vitro and in-vivo studies. International journal of biological macromolecules 2022, 223, 567–582. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, R.; Yi, M.; Ge, Z.; Wang, D.; Wang, G.; Deng, X. Multifunctional Hydrogel Enhances Inflammatory Control, Antimicrobial Activity, and Oxygenation to Promote Healing in Infectious Wounds. Biomacromolecules 2024, 25, 2423–2437. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Lim, S.B.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Science Advances 2021, 7, eabg9614. [Google Scholar]
- Cadinoiu, A.N.; Rata, D.M.; Daraba, O.M.; Ichim, D.L.; Popescu, I.; Solcan, C.; Solcan, G. Silver Nanoparticles Biocomposite Films with Antimicrobial Activity: In Vitro and In Vivo Tests. International Journal of Molecular Sciences 2022, 23, 10671. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Sabili, A.; Karami, F.; Zomorodian, K.; Shenavari, S.; Vaez, A.; Sahraeian, K.; Zareshahrabadi, Z. Synthesize, antioxidant and antimicrobial properties of a chitosan xerogel film with Nigella Sativa extract. Scientific Reports 2025, 15, 24635. [Google Scholar] [CrossRef]
- Boateng, J.; Mani, J.; Kianfar, F. Improving drug loading of mucosal solvent cast films using a combination of hydrophilic polymers with amoxicillin and paracetamol as model drugs. BioMed Research International 2013, 2013, 198137. [Google Scholar] [CrossRef]
- Xin, H.; Maruf, D.A.A.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Materials 2025, 8, 1339–1356. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.Y.; Yeo, J.C.C.; Soo, X.Y.D.; Thitsartarn, W.; Liu, S.; Tan, B.H.; Suwardi, A.; Li, Z.; Zhu, Q. Responsive hydrogel dressings for intelligent wound management. BMEMat 2023, 1, e12021. [Google Scholar] [CrossRef]
- Gamerith, C.; Luschnig, D.; Ortner, A.; Pietrzik, N.; Guse, J.-H.; Burnet, M.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E. pH-responsive materials for optical monitoring of wound status. Sensors and Actuators B: Chemical 2019, 301, 126966. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R. Nanocellulose composite wound dressings for real-time pH wound monitoring. Materials Today Bio 2023, 19, 100574. [Google Scholar]
- Rahimi, R.; Brener, U.; Chittiboyina, S.; Soleimani, T.; Detwiler, D.A.; Lelièvre, S.A.; Ziaie, B. Laser-enabled fabrication of flexible and transparent pH sensor with near-field communication for in-situ monitoring of wound infection. Sensors and Actuators B: Chemical 2018, 267, 198–207. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive wound dressings: advances and prospects. Nanoscale Horizons 2023, 8, 422–440. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time pH Monitoring. ACS Sensors 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Kaewpradub, K.; Veenuttranon, K.; Jantapaso, H.; Mittraparp-arthorn, P.; Jeerapan, I. A Fully-Printed Wearable Bandage-Based Electrochemical Sensor with pH Correction for Wound Infection Monitoring. Nano-Micro Letters 2024, 17, 71. [Google Scholar] [CrossRef]
- Du, M.; Jin, J.; Zhou, F.; Chen, J.; Jiang, W. Dual drug-loaded hydrogels with pH-responsive and antibacterial activity for skin wound dressing. Colloids and Surfaces B: Biointerfaces 2023, 222, 113063. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L.; Lin, G.; Tong, M.; Xie, Y.; Pan, H.; Shangguan, J.; Yao, Q.; Xu, S.; Xu, H. Skin-adaptive film dressing with smart-release of growth factors accelerated diabetic wound healing. International journal of biological macromolecules 2022, 222, 2729–2743. [Google Scholar] [CrossRef]
- Moeinipour, A.; Afkhami, A.; Madrakian, T. Stimuli-responsive polymeric film based on hydrogen-bonded organic framework designing as a smart wound dressing. Iranian Polymer Journal 2025, 34, 1387–1397. [Google Scholar] [CrossRef]
- Li, X.; Xue, X.; Xie, P. Smart dressings and their applications in chronic wound management. Cell Biochemistry and Biophysics 2024, 82, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Brunsen, A.; Ritz, U.; Mateescu, A.; Hoefer, I.; Frank, P.; Menges, B.; Hofmann, A.; Rommens, P.; Knoll, W.; Jonas, U. Photocrosslinkable dextran hydrogel films as substrates for osteoblast and endothelial cell growth. Journal of Materials Chemistry 2012, 22, 19590–19604. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, Y.; Yang, S.; Wang, Q.; Liang, F.; Qu, X.; Hu, Z. Surface patterned hydrogel film as a flexible scaffold for 2D and 3D cell co-culture. RSC Advances 2016, 6, 61185–61189. [Google Scholar] [CrossRef]
- Moreau, D.; Chauvet, C.; Etienne, F.; Rannou, F.P.; Corté, L. Hydrogel films and coatings by swelling-induced gelation. Proceedings of the National Academy of Sciences 2016, 113, 13295–13300. [Google Scholar] [CrossRef]
- Garland, S.P.; McKee, C.T.; Chang, Y.-R.; Raghunathan, V.K.; Russell, P.; Murphy, C.J. A cell culture substrate with biologically relevant size-scale topography and compliance of the basement membrane. Langmuir 2014, 30, 2101–2108. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-D.; Karmakar, M.; Cho, H.-J. Injectable hydrogel systems for local cancer therapy. Journal of Pharmaceutical Investigation 2024, 54, 555–591. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, C.; Xiong, Y.; Ma, S.; Sun, C.; Xu, W. A review of recent advances in drug loading, mathematical modeling and applications of hydrogel drug delivery systems. Journal of Materials Science 2024, 59, 15077–15116. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Taha, G.M. Investigation and kinetics of hydrogel scaffold with sustained release ciprofloxacin hydrochloride. Polymer Bulletin 2024, 81, 17393–17411. [Google Scholar] [CrossRef]
- Kang, J.; Li, Y.; Qin, Y.; Huang, Z.; Wu, Y.; Sun, L.; Wang, C.; Wang, W.; Feng, G.; Qi, Y. In Situ Deposition of Drug and Gene Nanoparticles on a Patterned Supramolecular Hydrogel to Construct a Directionally Osteochondral Plug. Nano-Micro Letters 2023, 16, 18. [Google Scholar] [CrossRef]
- Özakar, E.; Sevinç-Özakar, R.; Yılmaz, B. Preparation, characterization, and evaluation of cytotoxicity of fast dissolving hydrogel based oral thin films containing pregabalin and methylcobalamin. Gels 2023, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Ahmed, K.A.; Shirsand, S.B.; Raikar, P.K.; Hiraskar, A. Transdermal Patches: Design, Evaluation, and Potential Applications in Modern Therapeutics. Biomedical Materials & Devices 2025, 1–19. [Google Scholar] [CrossRef]
- Abedini, A.A.; Pircheraghi, G.; Kaviani, A.; Hosseini, S. Exploration of curcumin-incorporated dual anionic alginate-quince seed gum films for transdermal drug delivery. International Journal of Biological Macromolecules 2023, 248, 125798. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Kamali, B.; Nabid, M.R. Multilayered mucoadhesive hydrogel films based on Ocimum basilicum seed mucilage/thiolated alginate/dopamine-modified hyaluronic acid and PDA coating for sublingual administration of nystatin. International Journal of Biological Macromolecules 2022, 203, 93–104. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, J.M.; Jo, M.J.; Kang, S.J.; Yoo, M.K.; Park, S.Y.; Bong, S.; Park, C.-S.; Park, C.-W.; Kim, J.-S. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels 2025, 11, 520. [Google Scholar] [CrossRef]
- Manghnani, P.N.; Nelson, A.Z.; Wong, K.; Lee, Y.W.; Khan, S.A.; Doyle, P.S. From burst to controlled release: Using hydrogel crosslinking chemistry to tune release of micro-crystalline active pharmaceutical ingredients. RSC Pharmaceutics 2025, 2, 94–101. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Luo, J.; Cui, Y.; Chen, J.; Zeng, B.; Deng, Z.; Shao, L. “Double-sided protector” Janus hydrogels for skin and mucosal wound repair: applications, mechanisms, and prospects. Journal of Nanobiotechnology 2025, 23, 387. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.; Pereira, M.J.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the translational barriers of tissue adhesives. Nature Reviews Materials 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Science advances 2019, 5, eaav1281. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Bai, A.; Cai, H.; Bai, Y.; Jiang, W.; Yang, H.; Wang, X.; Yang, L.; Sun, N. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nature biomedical engineering 2019, 3, 632–643. [Google Scholar] [CrossRef]
- Stapleton, L.M.; Steele, A.N.; Wang, H.; Lopez Hernandez, H.; Yu, A.C.; Paulsen, M.J.; Smith, A.A.; Roth, G.A.; Thakore, A.D.; Lucian, H.J. Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier. Nature biomedical engineering 2019, 3, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, T.; Chen, X.; Liu, Y.; Li, Y.; Xu, Z.; Fan, C.; Liu, W. A janus hydrogel wet adhesive for internal tissue repair and anti-postoperative adhesion. Advanced Functional Materials 2020, 30, 2005689. [Google Scholar] [CrossRef]
- Fang, Y.; Zheng, Y.; Chi, C.; Jiang, S.; Qin, W.; Zhang, Y.; Liu, H.; Chen, Q. PAA-PU Janus Hydrogels Stabilized by Janus Particles and its Interfacial Performance During Hemostatic Processing. Advanced Healthcare Materials 2024, 13, 2303802. [Google Scholar] [CrossRef] [PubMed]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. Journal of Materials Chemistry B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Prakashini, R.S.; Thangam, T.; Hemamalani, A.U.; Parthasarathy, K. Role of Natural Polymers in Hydrogel-Based Scaffolds for Tissue Engineering Applications. Regenerative Engineering and Translational Medicine 2025. [Google Scholar] [CrossRef]
- Ragab, A.; El-Badry, N.; Tamer, N.; Naas, A.; Hamdy, A.; Tawakey, S.H.; Hassan, A.H.; Salim, A.I. Biodegradable chitosan/PVA-based hydrogel incorporating green synthesized silver nanoparticles for wound healing applications. BMC chemistry 2025, 19, 1–16. [Google Scholar] [CrossRef]
- Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regenerative biomaterials 2019, 6, 129–140. [Google Scholar] [CrossRef]
- Li, C.-S.; Xu, Y.; Li, J.; Qin, S.-H.; Huang, S.-W.; Chen, X.-M.; Luo, Y.; Gao, C.-T.; Xiao, J.-H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. BioMedical Engineering OnLine 2025, 24, 13. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidi, Y. Hydrogel-based scaffolds for bone and cartilage tissue engineering and regeneration. Reactive and Functional Polymers 2022, 177, 105313. [Google Scholar] [CrossRef]
- Hajirasouliha, E.; Zandi, M.; Hashemi Tabatabaei, M.; Zarrinbakhsh, P. Ocular contact lenses: smart materials for biomedical applications. Polymer Bulletin 2024, 81, 7791–7832. [Google Scholar] [CrossRef]
- Adrus, N.; bin Mohd Farizal, M.A.; Jamaluddin, J.; bin Syaiful Azim, F.S.; Mizi, F.M.; Nanda Kumar, S.; Govindasamy, J.J. Hydrogel as a Foundational Material for Contact Lens. In Contact Lenses: Research, Industry, and User Perspectives, Adrus, N., bin Mohd Farizal, M.A., Jamaluddin, J., bin Syaiful Azim, F.S., Mizi, F.M., Nanda Kumar, S., Govindasamy, J.J., Eds.; Springer Nature Singapore: Singapore, 2025; pp. 13–42.
- Li, Z.; Cheng, H.; Ke, L.; Liu, M.; Wang, C.; Jun Loh, X.; Li, Z.; Wu, Y. Recent advances in new copolymer hydrogel-formed contact lenses for ophthalmic drug delivery. ChemNanoMat 2021, 7, 564–579. [Google Scholar] [CrossRef]
- Wei, S.; Yin, R.; Tang, T.; Wu, Y.; Liu, Y.; Wang, P.; Wang, K.; Mei, M.; Zou, R.; Duan, X. Gas-permeable, irritation-free, transparent hydrogel contact lens devices with metal-coated nanofiber mesh for eye interfacing. ACS nano 2019, 13, 7920–7929. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Chen, X.; Yung, A.; Naderi, A.; Ghovvati, M.; Liu, Y.; Farzad, A.; Mostafavi, A.; Dana, R.; Annabi, N. Development and optimization of an ocular hydrogel adhesive patch using definitive screening design (DSD). Biomaterials Science 2023, 11, 1318–1334. [Google Scholar] [CrossRef] [PubMed]
- Tighsazzadeh, M.; Boateng, J. Matrix hyaluronic acid and bilayer poly-hydroxyethyl methacrylate-hyaluronic acid films as potential ocular drug delivery platforms. International Journal of Biological Macromolecules 2024, 260, 129496. [Google Scholar] [CrossRef] [PubMed]
- Manjeri, A.; George, S.D. Hydrogel-embedded polydimethylsiloxane contact lens for ocular drug delivery. ACS Applied Bio Materials 2024, 7, 7324–7331. [Google Scholar] [CrossRef]
- Kulbay, M.; Wu, K.Y.; Truong, D.; Tran, S.D. Smart molecules in ophthalmology: Hydrogels as responsive systems for ophthalmic applications. Smart molecules 2024, 2, e20230021. [Google Scholar] [CrossRef]
- Wei, R.; Wang, Y.; Feng, Z.; Liu, R.; Liu, C.; Hu, X.; Liu, Y.; Kong, B.; Zhou, X.; Li, M. Self-healing adhesive oxidized guar gum hydrogel loaded with mesenchymal stem cell exosomes for corneal wound healing. Journal of Nanobiotechnology 2025, 23, 321. [Google Scholar] [CrossRef]
- Safi, S.Z.; Fazil, S.; Saeed, L.; Shah, H.; Arshad, M.; Alobaid, H.M.; Rehman, F.; Sharif, F.; Selvaraj, C.; Orakzai, A.H. Chitosan-and heparin-based advanced hydrogels: their chemistry, structure and biomedical applications. Chemical Papers 2024, 78, 9287–9309. [Google Scholar] [CrossRef]
- Truong, D.; Wu, K.Y.; Nguyen, L.; Tran, S.D. Advancements in hydrogel technology for ocular drug delivery. Exploration of BioMat-X 2024, 1, 331–352. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Frontiers in Bioengineering and Biotechnology 2020, Volume 8 - 2020. [CrossRef]
- Chattopadhyay, D.; Orasugh, J.T.; Adhikari, A.; Ray, S.S. Stimuli-Responsive Hydrogels for Ophthalmic Drug Delivery; Elsevier: 2024.
- Arabpour, Z.; Salehi, M.; An, S.; Moghtader, A.; Anwar, K.N.; Baharnoori, S.M.; Shah, R.J.; Abedi, F.; Djalilian, A.R. Exploring Hydrogel Nanoparticle Systems for Enhanced Ocular Drug Delivery. Gels 2024, 10, 589. [Google Scholar] [CrossRef]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Jarchizadeh, R. Hydrogel-based formulations for drug delivery to the anterior segment of the eye. Journal of Drug Delivery Science and Technology 2023, 81, 104250. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, Q.; Liu, Q.; Han, Y.; Liu, Z. Advances in adhesive hydrogels applied for ophthalmology: An overview focused on the treatment. Theranostics 2025, 15, 915. [Google Scholar] [CrossRef] [PubMed]
- A Phase 3, Multicenter, Randomized, Double-Masked, Active-Controlled Study to Evaluate the Efficacy and Safety of Treatment With CsA-PG Ophthalmic Gel in Dry Eye Patients. 2025.
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and their role in biosensing applications. Advanced healthcare materials 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Sagdic, K.; Fernández-Lavado, E.; Mariello, M.; Akouissi, O.; Lacour, S.P. Hydrogels and conductive hydrogels for implantable bioelectronics. MRS Bulletin 2023, 48, 495–505. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Yin, C.; Dai, S.; Fan, X.; Jiang, Y.; Liu, X.; Fang, J.; Yi, B.; Zhou, Q.; et al. Recent Progress in antibacterial hydrogel coatings for targeting biofilm to prevent orthopedic implant-associated infections. Frontiers in Microbiology 2023, Volume 14 - 2023. [CrossRef]
- Leng, J.; He, Y.; Yuan, Z.; Tao, B.; Li, K.; Lin, C.; Xu, K.; Chen, M.; Dai, L.; Li, X.; et al. Enzymatically-degradable hydrogel coatings on titanium for bacterial infection inhibition and enhanced soft tissue compatibility via a self-adaptive strategy. Bioactive Materials 2021, 6, 4670–4685. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, F.; Li, Y.; Li, L.; Liu, M.; Wang, S.; Wang, Y.; Li, T.; Liu, L.; Feng, S.; et al. Biological Tissue-Inspired Ultrasoft, Ultrathin, and Mechanically Enhanced Microfiber Composite Hydrogel for Flexible Bioelectronics. Nano-Micro Letters 2023, 15, 139. [Google Scholar] [CrossRef]
- Jensen, M.J.; Peel, A.; Horne, R.; Chamberlain, J.; Xu, L.; Hansen, M.R.; Guymon, C.A. Antifouling and Mechanical Properties of Photografted Zwitterionic Hydrogel Thin-Film Coatings Depend on the Cross-Link Density. ACS Biomaterials Science & Engineering 2021, 7, 4494–4502. [Google Scholar] [CrossRef]
- Si, Y.; Xu, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Target microRNA-responsive DNA hydrogel-based surface-enhanced Raman scattering sensor arrays for microRNA-marked cancer screening. Analytical chemistry 2020, 92, 2649–2655. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Saeidi, M.; Chenani, H.; Orouji, M.; Adel Rastkhiz, M.; Bolghanabadi, N.; Vakili, S.; Mohamadnia, Z.; Hatamie, A.; Simchi, A. Electrochemical Wearable Biosensors and Bioelectronic Devices Based on Hydrogels: Mechanical Properties and Electrochemical Behavior. Biosensors 2023, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Afshari, R.; Jain, S.; Zheng, Y.; Lin, M.-H.; Zenkar, S.; Yin, J.; Chen, J.; Peppas, N.A.; Annabi, N. Advances in conducting nanocomposite hydrogels for wearable biomonitoring. Chemical Society Reviews 2025, 54, 2595–2652. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Chien, J.C.; Axpe, E.; Blankemeier, L.; Baker, S.W.; Swaminathan, S.; Piunova, V.A.; Zubarev, D.Y.; Maikawa, C.L.; Grosskopf, A.K. Combinatorial polyacrylamide hydrogels for preventing biofouling on implantable biosensors. Advanced Materials 2022, 34, 2109764. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Jang, S.Y.; Jung, Y.; Lee, K.; Yoo, J. Development of Substrate-Independent Antifouling and Bactericidal Surfaces Using Visible Light Cross-Linked Hydrogel Coatings for Biomedical Applications. Advanced Healthcare Materials 2025, 14, 2402565. [Google Scholar] [CrossRef]
- Rong, H.; Sun, S.; Lu, M.; Zhang, Y.; Liu, L.; Guo, Z.; Zhang, Z.; Ye, Z.; Zhang, J.; Chen, B. Super-hydrophilic and super-lubricating Zwitterionic hydrogel coatings coupled with polyurethane to reduce postoperative dura mater adhesions and infections. Acta Biomaterialia 2025, 192, 206–217. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, S.; Altenried, S.; Zuber, F.; Chen, Q.; Ren, Q. Advanced antifouling and antibacterial hydrogels enabled by controlled thermo-responses of a biocompatible polymer composite. Biomaterials Science 2022, 10, 6146–6159. [Google Scholar] [CrossRef]
- Wancura, M.; Nkansah, A.; Robinson, A.; Toubbeh, S.; Talanker, M.; Jones, S.; Cosgriff-Hernandez, E. PEG-Based Hydrogel Coatings: Design Tools for Biomedical Applications. Annals of Biomedical Engineering 2024, 52, 1804–1815. [Google Scholar] [CrossRef]
- Pan, M.; Shui, T.; Zhao, Z.; Xiang, L.; Yan, B.; Gu, N.; Zeng, H. Engineered Janus hydrogels: biomimetic surface engineering and biomedical applications. National Science Review 2024, 11, nwae316. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Y.; Ju, Y.; Yang, P.; Shen, N.; Yang, A.; Wu, R.; Fang, B.; Liu, L. Immunomodulatory hydrogels for tissue repair and regeneration. APL Materials 2024, 12. [Google Scholar] [CrossRef]
- Quazi, M.Z.; Hwang, J.; Song, Y.; Park, N. Hydrogel-Based Biosensors for Effective Therapeutics. Gels 2023, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Lucío, M.I.; Cubells-Gómez, A.; Maquieira, Á.; Bañuls, M.-J. Hydrogel-based holographic sensors and biosensors: past, present, and future. Analytical and Bioanalytical Chemistry 2022, 414, 993–1014. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gerber, A.; Santa, C.; Aktug, G.; Hengerer, B.; Clark, H.A.; Jonas, U.; Dostalek, J.; Sergelen, K. Molecularly responsive aptamer-functionalized hydrogel for continuous plasmonic biomonitoring. Journal of the American Chemical Society 2025, 147, 11485–11500. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Y.; Yu, R.; Feng, Y.; Han, Z.; Liu, C.; Zhu, P.; Lu, L.; Mao, Y. Recent Progress of Biomaterial-Based Hydrogels for Wearable and Implantable Bioelectronics. Gels 2025, 11, 442. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, Y.; Pang, B.; Guo, W.; Liu, S.; Zheng, Q.; Zhou, S.; Tian, J.; Liu, W.; Xie, B. A reusable hydrogel biosensor array with electrically responsive hydrogel interfaces for noninvasive locating of perforating arteries. Science Advances 2025, 11, eadw6166. [Google Scholar] [CrossRef]
- Zahid, S.; Ali, R.; Kousar, S.; Yousafzai, M.A.Z.; Muhammad, R.K.; Iqbal, K.; Mahmood, W.; Hasan, T.; Khan, M. ENZYME-RESPONSIVE HYDROGELS FOR TARGETED THERAPEUTIC DELIVERY AND DIAGNOSTIC APPLICATIONS.
- Solanki, R.; Bhatia, D. Stimulus-Responsive Hydrogels for Targeted Cancer Therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New Journal of Chemistry 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Toews, P.M.; Velraj, A.; Bates, J.S. Stimuli-responsive hydrogels, their mass transfer, intermolecular interactions, and applications in biomedical devices. Journal of Materials Science: Materials in Engineering 2025, 20, 66. [Google Scholar] [CrossRef]
- Sojdeh, S.; Panjipour, A.; Yaghmour, A.; Arabpour, Z.; Djalilian, A.R. Click Chemistry-Based Hydrogels for Tissue Engineering. Gels 2025, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, Q.; Xie, T.; Wu, J. DLP 3D printing of electrically conductive hybrid hydrogels via polymerization-induced phase separation and subsequent in situ assembly of polypyrrole. Journal of Materials Chemistry A 2024, 12, 5348–5356. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent hydrogels and their biomedical applications. Materials Advances 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. International Journal of Molecular Sciences 2022, 23, 3665. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Z.; Li, K.; Ma, C.; Zhou, W.; Lin, T.; Xu, J.; Liu, X. Self-Healable PEDOT:PSS-PVA Nanocomposite Hydrogel Strain Sensor for Human Motion Monitoring. Nanomaterials 2023, 13, 2465. [Google Scholar] [CrossRef]
- Ahmad Ruzaidi, D.A.; Maurya, M.R.; Yempally, S.; Abdul Gafoor, S.; Geetha, M.; Che Roslan, N.; Cabibihan, J.-J.; Kumar Sadasivuni, K.; Mahat, M.M. Revealing the improved sensitivity of PEDOT:PSS/PVA thin films through secondary doping and their strain sensors application. RSC Advances 2023, 13, 8202–8219. [Google Scholar] [CrossRef]
- Jurin, F.E.; Buron, C.C.; Frau, E.; del Rossi, S.; Schintke, S. The Electrical and Mechanical Characteristics of Conductive PVA/PEDOT:PSS Hydrogel Foams for Soft Strain Sensors. Sensors 2024, 24, 570. [Google Scholar]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Rahman Khan, M.M. Progress in hydrogel toughening: addressing structural and crosslinking challenges for biomedical applications. Discover Materials 2025, 5, 5. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. Journal of polymer science 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Li, X.; Gong, J.P. Design principles for strong and tough hydrogels. Nature Reviews Materials 2024, 9, 380–398. [Google Scholar] [CrossRef]
- Heinemann, C.; Buchner, F.; Lee, P.S.; Bernhardt, A.; Kruppke, B.; Wiesmann, H.-P.; Hintze, V. Effects of Gamma Irradiation and Supercritical Carbon Dioxide Sterilization on Methacrylated Gelatin/Hyaluronan Hydrogels. Journal of Functional Biomaterials 2023, 14, 317. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; García-Carvajal, Z.Y. Stimuli-Responsive Hydrogels in Drug Delivery. In Functional Biomaterials: Drug Delivery and Biomedical Applications, Jana, S., Jana, S., Eds.; Springer Singapore: Singapore, 2022; pp. 75–103. [Google Scholar]
- Khonakdar, H.; Ehsani, M.; Naderi, G.; Shokrolahi, F.; Khonakdar, H.A. Recent Advances in Injectable Stimuli-Responsive Hydrogels for Biomedical Applications. Journal of Polymers and the Environment 2025, 33, 3512–3554. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, P.; Ji, Q.; Yan, C.; Gong, A. Stimuli-responsive hydrogel for disease therapy. Polymer Bulletin 2024, 81, 1981–2000. [Google Scholar] [CrossRef]
- Fam, S.B. Regulations for Hydrogels and Bioinks in Clinical Trials. In Hydrogels and Bioinks in Tissue Engineering, Hasirci, N., Hasirci, V., Eds.; Springer Nature Switzerland: Cham, 2025; pp. 359–367.
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the clinic: An update. Bioengineering & Translational Medicine 2024, 9, e10680. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of polymer hydrogel composites and their applications. Journal of Polymers and the Environment 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Huang, C.; Xu, L.; Huang, S.; Qu, M.; Xu, Z.; Zhang, D.; Guo, B.; Ji, C. Optimization of 3D Printing Process for Hydrogel-Based Thermal Management Patches in Engineering Machinery. In Proceedings of the The 9th International Conference on Advances in Construction Machinery and Vehicle Engineering, Singapore, 2026//, 2026; pp. 235–247.
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal transduction and targeted therapy 2021, 6, 426. [Google Scholar] [CrossRef]
- Huang, X.; Fu, D.; Zha, X.; Ling, T.; Huang, J. High-precision 3D printing of hydrogel: Material innovations, process breakthroughs, and translational applications in regenerative medicine. APL Materials 2025, 13. [Google Scholar] [CrossRef]
- Musuc, A.M.; Chelu, M. Biomaterials-Based Hydrogels for Therapeutic Applications. In Biomaterials in Microencapsulation, Sharma, A., Ed.; IntechOpen: London, 2024.
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, X.; Wang, S.; Chen, Z.; Pan, P.; Huang, Q. Integrating machine learning for the optimization of polyacrylamide/alginate hydrogel. Regenerative Biomaterials 2024, 11, rbae109. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI energized hydrogel design, optimization and application in biomedicine. Materials Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Yazdanpanah, S.; Petillo, O.; Conte, R.; Sepe, F.; Peluso, G.; Calarco, A. Sustainable Hydrogels for Medical Applications: Biotechnological Innovations Supporting One Health. Gels 2025, 11, 559. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in natural polymer-based hydrogels: synthesis, applications, and future directions in biomedical and environmental fields. Discover Polymers 2025, 2, 6–6. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Kang, J.; Noh, I. Status for Commercialization of FDA-approved Hydrogels and Their Intellectual Properties. 2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).