Submitted:
02 October 2025
Posted:
03 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Allergens from Cats
2.1. Molecular Insights of the Major Cat Allergen Fel d 1
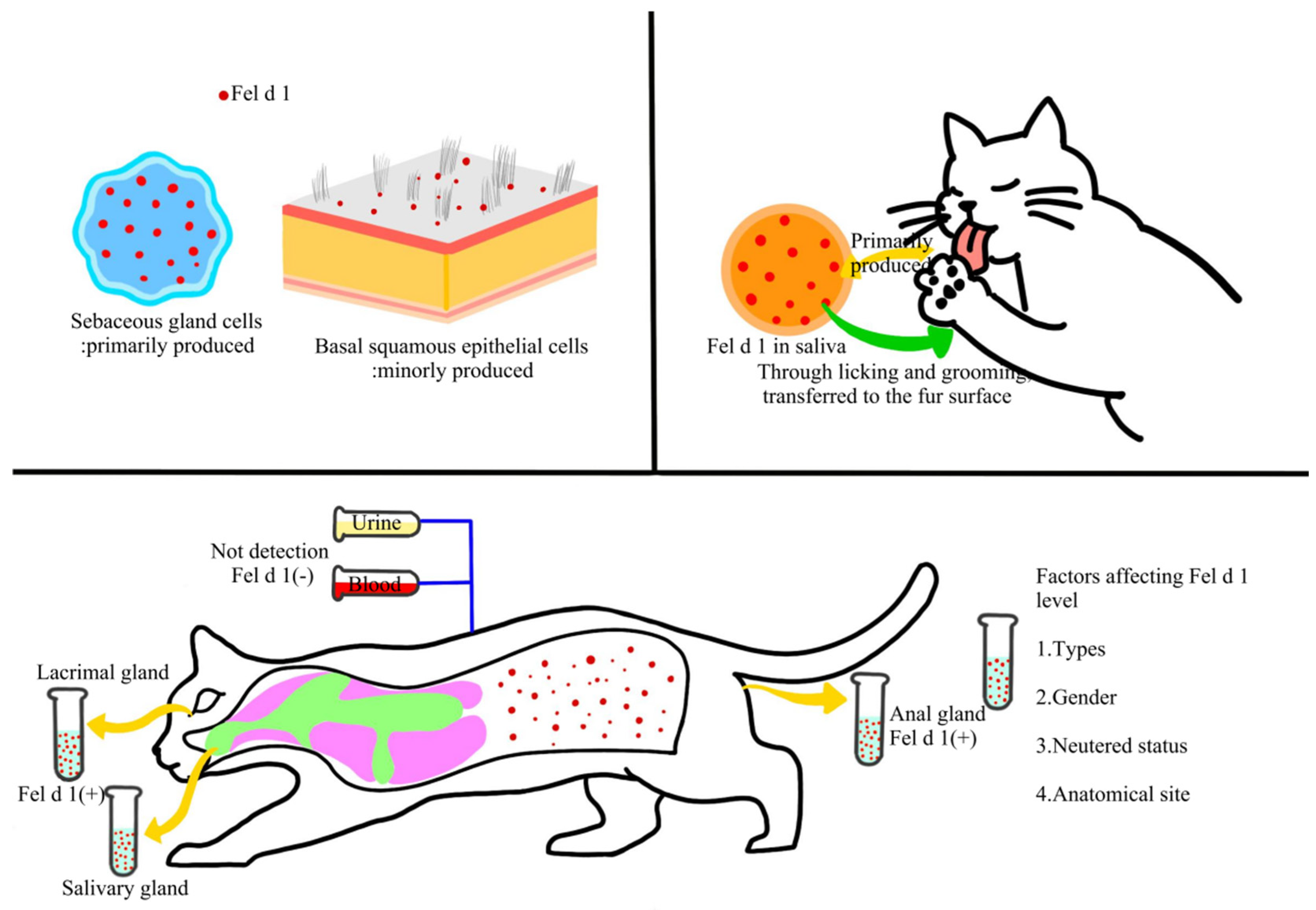
2.2. Secondary Cat Allergens and Their Cross-Reactivity Characteristics
2.3. Distribution and Transmission of Cat Allergens in Indoor Environments
2.4. Exposure Routes of Human to Cat Allergens and Sensitization Mechanisms
3. Indoor Cat Allergen Testing Methods
3.1. Immunological Assay
3.2. High-Throughput Technologies for the Multiplex Detection
3.3. Real-Time Monitoring and Emerging Immunosensing Technologies
4. Factors Influencing Indoor Cat Allergen Concentration
4.1. Cat-Related Factors
4.2. Environmental and Architectural Factors
4.3. Human Activity Factors
4.4. Spatiotemporal Variation Patterns
5. Methods for Preventing and Improving Allergies
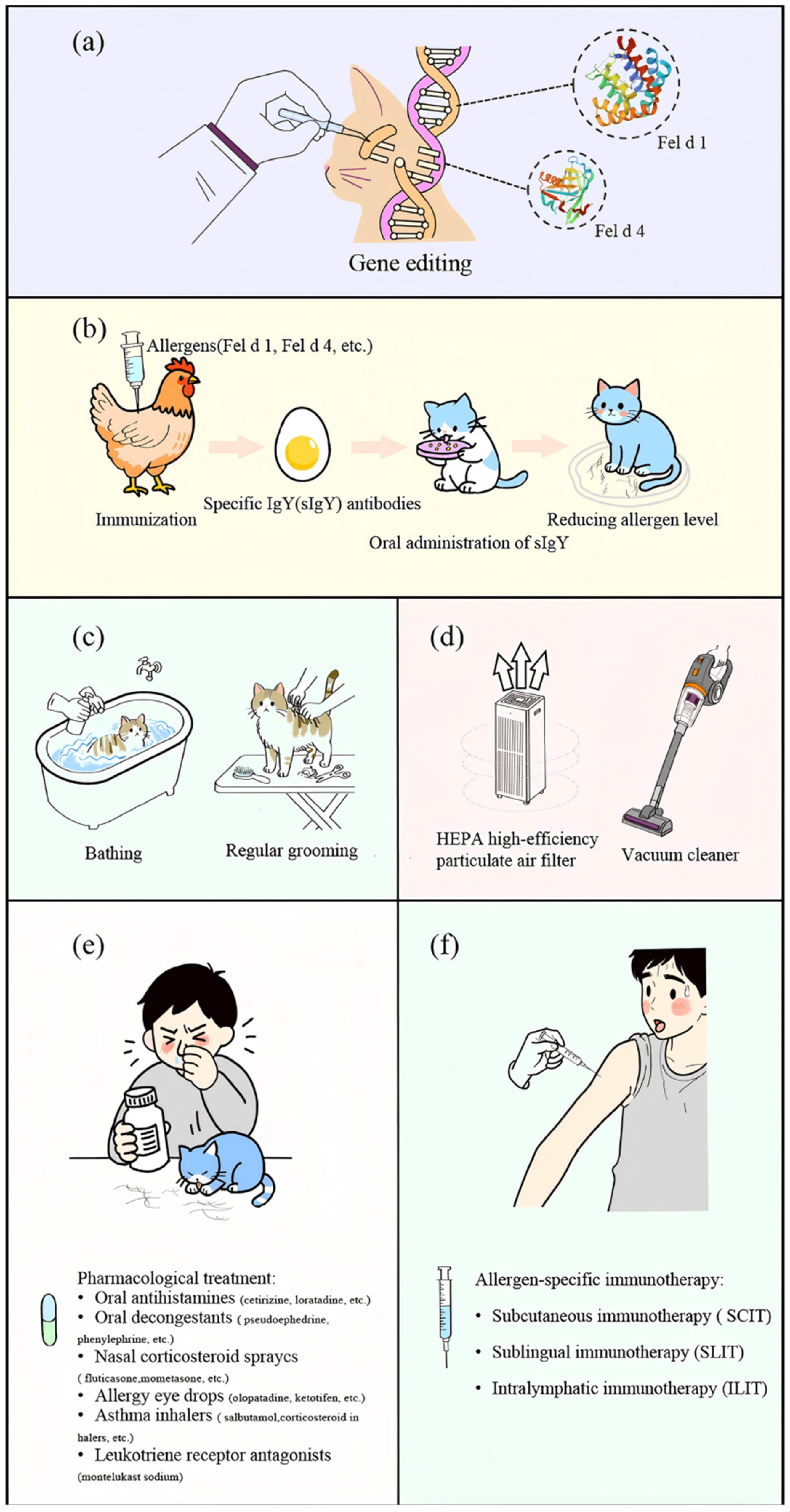
5.1. Source Control of Allergens
5.1.1. Gene Editing
5.1.2. Immunological Methods
5.1.3. Physical Methods
5.2. Environmental Interventions
5.3. Personal Protection and Medical Intervention
5.3.1. Chemical Drug Therapy
5.3.2. Immunotherapy
6. Prospects for Future Managing Indoor Cat Allergens
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Dramburg S, Hilger C, Santos A F, et al. EAACI molecular allergology user's guide 2.0. Egypt. J. Pediatr. Alle. 2023, 34, e13854.
- Holgate S T, Polosa R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008, 8, 218-230.
- Kay A B. Allergy and allergic diseases. N. Engl. J. Med. 2001, 344, 30-37.
- Asher M I, Montefort S,Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733-743.
- Park H J, Kim E, Yoon D, et al. Prevalence of self-reported allergic diseases and IgE levels: a 2010 KNHANES analysis. Allergy Asthma Immun. 2017, 9, 329.
- Edwards-Salmon S E, Padmanabhan S L, Kuruvilla M, et al. Increasing prevalence of allergic disease and its impact on current practice. Curr. Otorhinol. Rep. 2022, 10, 278-284.
- Merhej T, Zein J G. Epidemiology of asthma: prevalence and burden of disease. Adv. Exp. Med. Biol. 2023, 1426, 3-23.
- Oh J, Kim S, Kim M S, et al. Global, regional, and national burden of asthma and atopic dermatitis, 1990--2021, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Resp. Med. 2025, 13, 425-446.
- Shin Y H, Hwang J, Kwon R, et al. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: a systematic analysis for the global burden of disease study 2019. Allergy 2023, 78, 2232-2254.
- Melén E, Garcia-Aymerich J. Predicting the future global burden of asthma and atopic dermatitis: identifying strategies for prevention. Lancet Resp. Med. 2025, 13, 376-378.
- Takkouche B, González-Barcala F-J, Etminan M, et al. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy 2008, 63, 857-864.
- Chan S K, Leung D Y. Dog and cat allergies: current state of diagnostic approaches and challenges. Allergy Asthma Immun. 2018, 10, 97.
- Yin W, Xiaoli Z, Wenjin D, et al. Sensitization profiles of aeroallergens among allergic rhinitis patients in China: a 13-year multicenter retrospective study. Allergy 2023, 79, 1329-1332.
- Schoos A M, Nwaru B I, Borres M P. Component-resolved diagnostics in pet allergy: current perspectives and future directions. J. Allergy Clin. Immun. 2021, 147, 1164-1173.
- Liang H, Ouyang Z, Liu J, et al. The development trend analysis of pet industry, 2025. IEEE.
- Shen R, Shen F, Zhao Y. Pet industry analysis based on multiple linear regression and aeima models, 2025. IEEE.
- Zahradnik E, Raulf M. Animal allergens and their presence in the environment. Front. Immunol. 2014, 5, 76.
- Konradsen J R, Fujisawa T, Van Hage M, et al. Allergy to furry animals: new insights, diagnostic approaches, and challenges. Allergy Clin. Immun. 2015, 135, 616-625.
- Arbes Jr S J, Cohn R D, Yin M, et al. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: Results from the national survey of lead and allergens in housing. Ann. Allerg. Asthma Im. 2008, 101, 517.
- An W, Li T, Tian X, et al. Allergies to allergens from cats and dogs: a review and update on sources, pathogenesis, and strategies. Int. J. Mol. Sci. 2024, 25, 10520.
- Lei D K, Grammer L C. An overview of allergens. Allergy Asthma Proc. 2019, 40, 362-365.
- Chapman M D, Wood R A. The role and remediation of animal allergens in allergic diseases. Allergy Clin. Immun. 2001, 107, S414-S421.
- Nadeau K C. Allergen-specific IgG antibodies for cat allergy? Ann. Am. Thorac. Soc. 2021, 204, 1-2.
- Brożek J L, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines—2016 revision. Allergy Clin. Immun. 2017, 140, 950-958.
- Dávila I, Dom I Nguez-Ortega J, Navarro-Pulido A, et al. Consensus document on dog and cat allergy. Allergy 2018, 73, 1206-1222.
- Sahiner U M, Giovannini M, Escribese M M, et al. Mechanisms of allergen immunotherapy and potential biomarkers for clinical evaluation. J. Pers. Medi. 2023, 13, 845.
- Ohman Jr J L, Lowell F C, Bloch K J. Allergens of mammalian origin: III. Properties of a major feline allergen. J. Immunol. 1974, 113, 1668-1677.
- Klug J, Beier H M, Bernard A, et al. Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann. N. Y. Acad. Sci. 2000, 923, 348-354.
- Kaiser L, Grönlund H, Sandalova T, et al. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J. Biol. Chem. 2003, 278, 37730-37735.
- Duffort O, Carreira J E, Lombardero M. Monoclonal antibodies against Fel d I and other clinically relevant cat allergens. Immunol. Let. 1988, 17, 71-77.
- Kaiser L, Velickovic T C, Badia-Martinez D, et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J. Mol. Biol. 2007, 370, 714-727.
- Karn R C. The mouse salivary androgen-binding protein (ABP) alpha subunit closely resembles chain 1 of the cat allergen Fel dI. Biochem. Genet. 1994, 32, 271-277.
- Bonnet B, Messaoudi K, Jacomet F, et al. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Cl. Im. 2018, 14, 14.
- van Ree R, van Leeuwen W A, Bulder I, et al. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J. Allergy Clin. Imm. 1999, 104, 1223-1230.
- Cleveland III C W, Davis B W, Khatri K, et al. Genetic diversity of the major cat allergen, Fel d 1. PNAS Nexus 2024, 3, pgae447.
- Thomas W R. The advent of recombinant allergens and allergen cloning. J. Allergy Clin. Imm. 2011, 127, 855-859.
- Charpin C, Mata P, Charpin D, et al. Fel d I allergen distribution in cat fur and skin. J. Allergy Clin. Imm. 1991, 88, 77-82.
- Dabrowski A J, Van der Brempt X, Soler M, et al. Cat skin as an important source of Fel d I allergen. J. Allergy Clin. Imm. 1990, 86, 462-465.
- Bartholome K, Kissler W, Baer H, et al. Where does cat allergen 1 come from? J. Allergy Clin. Imm. 1985, 76, 503-506.
- Brown P R, Leitermann K, Ohman Jr J L. Distribution of cat allergen 1 in cat tissues and fluids. Int. Arch. Allergy Imm. 1984, 74, 67-70.
- De Andrade A D, Birnbaum J, Magalon C, et al. Fel d I levels in cat anal glands. Clin. Exp. Allergy 1996, 26, 178-180.
- Anderson M C, Baer H, Ohman Jr J L. A comparative study of the allergens of cat urine, serum, saliva, and pelt. J. Allergy Clin Imm. 1985, 76, 563-569.
- Siebers R, Healy B, Holt S, et al. Fel d 1 levels in domestic living rooms are not related to cat color or hair length. 2001.
- Jalil-Colome J E, de Andrade A E L D, Birnbaum J E L, et al. Sex difference in Fel d 1 allergen production. J. Allergy Clin Imm. 1996, 98, 165-168.
- Zielonka T M, Charpin D, Berbis P H, et al. Effects of castration and testosterone on Fel d I production by sebaceous glands of male cats: I—immunological assessment. Revue Francaise D'allergologie et D'immunologie Clinique, 1995, 35, 359-363.
- Carayol N, Birnbaum J, Magnan A, et al. Fel d 1 production in the cat skin varies according to anatomical sites. Allergy 2000, 55, 570-573.
- Trifonova D, Curin M, Riabova K, et al. Allergenic activity of individual cat allergen molecules. Int. J. Mol. Sci. 2023, 24, 16729.
- Popescu F, Ganea C S, Panaitescu C, et al. Molecular diagnosis in cat allergy. World J. Methodol. 2021, 11, 46-60.
- Ichikawa K, Vailes L D, Pomes A, et al. Molecular cloning, expression and modelling of cat allergen, cystatin (Fel d 3), a cysteine protease inhibitor. Clin. Exp. Allergy 2001, 31, 1279-1286.
- Smith W, Butler A, Hazell L A, et al. Fel d 4, a cat lipocalin allergen. Clin. Exp. Allergy 2004, 34, 1732-1738.
- Smith W, O Neil S E, Hales B J, et al. Two newly identified cat allergens: the von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int. Arch. Allergy Imm. 2011, 156, 159-170.
- Riabova K, Karsonova A V, van Hage M, et al. Molecular allergen-specific IgE recognition profiles and cumulative specific IgE levels associated with phenotypes of cat allergy. Int. J. Mol. Sci. 2022, 23, 6984.
- Caraballo L, Valenta R, Puerta L, et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ. 2020, 13, 100118.
- Lu J, Zhu H, Yang Q, et al. Associations of protein classes with cross-reactivity and cross-sensitization in furry animal allergens: A component-resolved diagnostics study. J. Asthma Allergy 2025, 363-375.
- Saarelainen S, Rytk O Nen-Nissinen M, Rouvinen J, et al. Animal-derived lipocalin allergens exhibit immunoglobulin E cross-reactivity. Clin. Exp. Allergy 2008, 38, 374-381.
- Popescu F, Vieru M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J. Methodol. 2018, 8, 17-36.
- Hilger C, Swiontek K, Arumugam K, et al. Identification of a new major dog allergen highly cross-reactive with Fel d 4 in a population of cat-and dog-sensitized patients. J. Allergy Clin. Immun. 2012, 129, 1149-1151.
- Yamamoto K, Ishibashi O, Sugiura K, et al. Crystal structure of the dog allergen Can f 6 and structure-based implications of its cross-reactivity with the cat allergen Fel d 4. Sci. Rep. 2019, 9, 1503.
- Liu Z, Trifonova D, Tulaeva I, et al. Albumins represent highly cross-reactive animal allergens. Front. Immunol. 2023, 14, 1241518.
- Luczynska C M, Li Y, Chapman M D, et al. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Am. Rev. Respir. Dis. 1990, 141, 361-367.
- Wood R A, Laheri A N, Eggleston P A. The aerodynamic characteristics of cat allergen. Clin. Exp. Allergy 1993, 23, 733-739.
- Swanson M C, Agarwal M K, Reed C E. An immunochemical approach to indoor aeroallergen quantitation with a new volumetric air sampler: studies with mite, roach, cat, mouse, and guinea pig antigens. J. Allergy Clin. Immun. 1985, 76, 724-729.
- De Blay F, Heymann P W, Chapman M D, et al. Airborne dust mite allergens: comparison of group II allergens with group I mite allergen and cat-allergen Fel d I. J. Allergy Clin. Immun. 1991, 88, 919-926.
- Wood R A, Eggleston P A, Lind P, et al. Antigenic analysis of household dust samples1-4. Am. Rev. Respir. Dis. 1988, 137, 358-363.
- Dallongeville A, Le Cann P, Zmirou-Navier D, et al. Concentration and determinants of molds and allergens in indoor air and house dust of French dwelling. Sci. Total Environ. 2015, 536, 964-972.
- Ritz B R, Hoelscher B, Frye C, et al. Allergic sensitization owing to ‘second-hand’cat exposure in schools. Allergy 2002, 57, 357-361.
- Custovic A, Green R, Taggart S, et al. Domestic allergens in public places II: dog (Can f 1) and cockroach (Bla g 2) allergens in dust and mite, cat, dog and cockroach allergens in the air in public buildings. Clin. Exp. Allergy 1996, 26, 1246-1252.
- Partti-Pellinen K, Marttila O, M A Kinen-Kiljunen S, et al. Occurrence of dog, cat, and mite allergens in public transport vehicles. Allergy 2000, 55, 65-68.
- Enberg R N, Shamie S M, McCullough J, et al. Ubiquitous presence of cat allergen in cat-free buildings: probable dispersal from human clothing. Ann. Allergy 1993, 70, 471-474.
- De Lucca S D, O Meara T J, Tovey E R. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J. Allergy Clin. Immun. 2000, 106, 874-879.
- Karlsson A, Renstr O M A. Human hair is a potential source of cat allergen contamination of ambient air. Allergy 2005, 60, 961-964.
- Karlsson A, Renstr O M A, Hedren M, et al. Allergen avoidance does not alter airborne cat allergen levels in classrooms. Allergy, 2004, 59, 661-667.
- van Hage M, Käck U, Asarnoj A, et al. An update on the prevalence and diagnosis of cat and dog allergy--emphasizing the role of molecular allergy diagnostics. Mol. Immunol. 2023, 157, 1-7.
- Maeda Y, Akiyama K. Anaphylaxis after a cat bite. Allergol. Int. 2012, 61, 511-512.
- Leung D Y, Berdyshev E, Goleva E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immun. 2020, 145, 1485-1497.
- Reinero C R. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet. J. 2011, 190, 28-33.
- Platts-Mills T A, Woodfolk J A, Erwin E A, et al. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer Semin. Immunopathol. 2004, 25, 271-279.
- Leo G, Incorvaia C, Arasi S. Could a bite trigger the onset of cat allergy? Pediat. Aller. Immunol. 2022, 33, e13841.
- Jones M. Understanding of the molecular mechanisms of allergy. Methods Mol. Med. 2008, 138, 1-15.
- Han X, Krempski J W, Nadeau K. Advances and novel developments in mechanisms of allergic inflammation. Allergy 2020,75(12),3100-3111.
- Shamji M H, Valenta R, Jardetzky T, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021,76(12),3627-3641.
- Nwaru B I, Suzuki S, Ekerljung L, et al. Furry animal allergen component sensitization and clinical outcomes in adult asthma and rhinitis. J. Aller. Cl. Imm-Pract. 2019, 7, 1230-1238.
- Özuygur Ermis S S, Norouzi A, Borres M P, et al. Sensitization patterns to cat molecular allergens in subjects with allergic sensitization to cat dander. Clin. Transl. Allergy, 2023, 13, e12294.
- Bollinger M E, Wood R A, Chen P, et al. Measurement of cat allergen levels in the home by use of an amplified ELISA. J. Allergy Clin. Immun. 1998, 101, 124-125.
- Tasaniyananda N, Tungtrongchitr A, Seesuay W, et al. Quantification of Fel d 1 in house dust samples of cat allergic patients by using monoclonal antibody specific to a novel IgE-binding epitope. Asian Pac. J. Allergy, 2018, 36, 8-15.
- Lau S, Schulz G, Sommerfeld C, et al. Comparison of quantitative ELISA and semiquantitative dustscreen™ for determination of Der p 1, Der f 1, and Fel d 1 in domestic dust samples. Allergy, 2001,56,993-995.
- Kelly S M, Karsh J, Marcelo J, et al. Fel d 1 and Fel d 4 levels in cat fur, saliva, and urine. J. Allergy Clin. Immun. 2018, 142, 1990-1992.
- Huynh S, Menzies S, Khurana T, et al. Radial Immunodiffusion (RID) to sandwich ELISA for the quantitation of Fel d 1 and Amb a 1 in cat and short ragweed pollen allergenic extracts. J. Allergy Clin. Immun. 2012, 129, AB88.
- Rabin R L, Croote D, Chen A, et al. A human monoclonal antibody based immunoenzymetric assay to measure Fel d 1 concentrations in cat hair and pelt allergenic extracts. Frontiers in Allergy, 2024,5,1417879.
- van Ree F. Analytic aspects of the standardization of allergenic extracts. Allergy, 1997,52.
- King E M, Filep S, Smith B, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J. Immunol. Methods, 2013, 387, 89-95.
- Filep S C, Black K R, Smith B R, et al. Simultaneous quantification of specific food allergen proteins using a fluorescent multiplex array. Food Chem. 2022, 389, 132986.
- Earle C D, King E M, Tsay A, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J. Allergy Clin Immun. 2007, 119, 428-433.
- Earle C D, King E M, Tsay A, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry, 2009,55,4611-4622.
- Scherer E, Valot B, Vacheyrou M, et al. Assessment of pets (cats and dogs) in homes using electrostatic dust collectors and QPCR: new tools to evaluate exposure and risk of allergies. Int. J. Environ. Heal. R. 2016, 26, 589-599.
- Bustin S A, Benes V, Garson J A, et al. Unbiased quantitative proteomics reveals a crucial role of the allergen context for the activation of human dendritic cells. Sci. Rep-UK. 2017, 7, 16638.
- Krutz N L, Kimber I, Winget J, et al. Identification and semi-quantification of protein allergens in complex mixtures using proteomic and AllerCatPro 2.0 bioinformatic analyses: a proof-of-concept investigation. J. Immunotoxicol. 2024, 21, 2305452.
- López-Pedrouso M, Lorenzo J M, Dios Alché J D, et al. Advanced proteomic and bioinformatic tools for predictive analysis of allergens in novel foods. Biology, 2023, 12, 714.
- Wéry N, Galès A, Brunet Y. Bioaerosol sources. Microbiology of Aerosols, 2017,115-135.
- Haig C W, Mackay W G, Walker J T, et al. Bioaerosol sampling: sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242.
- Kabir E, Azzouz A, Raza N, et al. Recent advances in monitoring, sampling, and sensing techniques for bioaerosols in the atmosphere. ACS sensors, 2020, 5, 1254-1267.
- Morris D R, Fatisson J, Olsson A L, et al. Real-time monitoring of airborne cat allergen using a QCM-based immunosensor. Sensor. Actuat. B-Chem. 2014, 190, 851-857.
- Toma K, Horibe M, Kishikawa C, et al. Rapid and repetitive immunoassay with a surface acoustic wave device for monitoring of dust mite allergens. Sensor. Actuat. B-Chem. 2017, 248, 924-929.
- Hossenbaccus L, Walker T, Ellis A K. Technical validation of controlled exposure to cat dander in the specialized particulate control environmental exposure unit (SPaC-EEU). Allergy Asthma Cl. Im. 2025, 21, 6.
- Salimifard P, Rim D, Freihaut J D. Evaluation of low-cost optical particle counters for monitoring individual indoor aerosol sources. Aerosol Sci. Tech. 2020, 54, 217-231.
- Fong A, Fong B. Indoor air quality control for asthma patients using smart home technology, 2011. IEEE.
- Bastien B C, Gardner C, Satyaraj E. Influence of time and phenotype on salivary Fel d1 in domestic shorthair cats. J. Feline Med. Surg. 2019, 21, 867-874.
- Bienboire-Frosini C E C, Lebrun R E G, Vervloet D, et al. Distribution of core fragments from the major cat allergen Fel d 1 is maintained among the main anatomical sites of production. Int. Arch. Allergy Imm 2010, 152, 197-206.
- Peterson E L, Ownby D R, Johnson C C. The relationship of housing and household characteristics to the indoor concentrations of Der f 1, Der p 1, and Fel d 1 measured in dust and air samples. Ann. Allergy Asthma Im. 2003, 90, 564-571.
- Neal J S, Arlian L G, Morgan M S. Relationship among house-dust mites, Der 1, Fel d 1, and Can f 1 on clothing and automobile seats with respect to densities in houses. Ann. Allergy Asthma Im. 2002, 88, 410-415.
- Montoya L D, Hildemann L M. Evolution of the mass distribution of resuspended cat allergen (Fel d 1) indoors following a disturbance. Atmos. Environ. 2001, 35, 859-866.
- Corsi R L, Siegel J A, Chiang C. Particle resuspension during the use of vacuum cleaners on residential carpet. J. Occup. Environ. Hyg. 2008, 5, 232-238.
- De Blay F, Spirlet F, Gries P, et al. Effects of various vacuum cleaners on the airborne content of major cat allergen (Fel d 1). Allergy, 1998, 53, 411-414.
- Hegarty J M, Rouhbakhsh S, Warner J A, et al. A comparison of the effect of conventional and filter vacuum cleaners on airborne house dust mite allergen. Resp. Med. 1995, 89, 279-284.
- Chew G L, Higgins K M, Gold D R, et al. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy, 1999, 54, 1058-1066.
- Chew G L, Saha S. Impacts of climate change on indoor allergens. Impacts of climate change on allergens and allergic diseases. Cambridge University Press Cambridge, 2016,119.
- Egmar A, Almqvist C, Emenius G, et al. Deposition of cat (Fel d 1), dog (Can f 1), and horse allergen over time in public environments--a model of dispersion. Allergy, 1998, 53, 957-961.
- Lee S R, Lee K, Song S, et al. Generation and cloning of CH2 knockout cats by CRISPR-Cas9 system: Hypoallergenic cats. Authorea Preprints, 2023.
- Brackett N F, Davis B W, Adli M, et al. Evolutionary biology and gene editing of cat allergen, Fel d 1. CRISPR J. 2022, 5, 213-223.
- Brackett N, Riedy J, Adli M, et al. CRISPR gene editing of the major cat allergen, Fel d 1. Allergy, 2020,75,59-60.
- Lee S R, Lee K, Song S, et al. Generation of Fel d 1 chain 2 genome-edited cats by CRISPR-Cas9 system. Scientific Rep-UK. 2024, 14, 4987.
- Zhu D, Kepley C L, Zhang K, et al. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat. Med. 2005, 11, 446-449.
- Wang H, Zhong Q, Lin J. Egg yolk antibody for passive immunization: status, challenges, and prospects. J. Agr. Food Chem. 2023, 71, 5053-5061.
- Zhang X, Calvert R A, Sutton B J, et al. IgY: a key isotype in antibody evolution. Biol. Rev. 2017, 92, 2144-2156.
- Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. T. 2012, 3, 163-182.
- Satyaraj E, Li Q, Sun P, et al. Anti-Fel d1 immunoglobulin Y antibody-containing egg ingredient lowers allergen levels in cat saliva. J. Feline Med. Surg. 2019, 21, 875-881.
- Satyaraj E, Wedner H J, Bousquet J. Keep the cat, change the care pathway: a transformational approach to managing Fel d 1, the major cat allergen. Allergy, 2019, 74, 5-17.
- Matulka R A, Thompson L, Corley D. Multi-level safety studies of anti Fel d 1 IgY ingredient in cat food. Front. Vet. Sci. 2020, 6, 477.
- Hedrick E D, Matulka R A, Conboy-Schmidt L, et al. Evaluation of anti-Fel d 1 IgY ingredient for pet food on growth performance in kittens. Front. Vet. Sci. 2024, 11, 1355390.
- Satyaraj E, Gardner C, Filipi I, et al. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immun. Inflamm. Dis. 2019, 7, 68-73.
- Thoms F, Jennings G T, Maudrich M, et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J. Allergy Clin. Immun. 2019, 144, 193-203.
- Thoms F, Haas S, Erhart A, et al. Immunization of cats against Fel d 1 results in reduced allergic symptoms of owners. Viruses, 2020, 12, 288.
- Bergmann K, Graessel A, Raab J, et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients—first evaluation in a standardized allergen exposure chamber. Allergo Journal International, 2021,30(4),141-149.
- Bergmann K, Raab J, Krause L, et al. Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo Journal International, 2022, 31, 161-171.
- Jensen-Jarolim E, Jensen S A, Bergmann K. Allergy to the cat—from diagnosis to management. Allergo Journal International, 2023,32(5),130-137.
- Bergmann K, Raab J, Graessel A, et al. The holo beta-lactoglobulin lozenge reduces symptoms in cat allergy—evaluation in an allergen exposure chamber and by titrated nasal allergen challenge. Clin. Transl. Allergy. 2023, 13, e12274.
- Wood R A, Chapman M D, Adkinson Jr N F, et al. The effect of cat removal on allergen content in household-dust samples. J. Allergy Clin. Immun. 1989, 83, 730-734.
- Matsui E, Kagey-Sobotka A, Chichester K, et al. Allergic potency of recombinant Fel d 1 is reduced by low concentrations of chlorine bleach. J. Allergy Clin. Immun. 2003, 111, 396-401.
- Avner D B, Perzanowski M S, Platts-Mills T A, et al. Evaluation of different techniques for washing cats: quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J. Allergy Clin. Immun. 1997, 100, 307-312.
- Nageotte C, Park M, Havstad S, et al. Duration of airborne Fel d 1 reduction after cat washing. J. Allergy Clin. Immun. 2006, 118, 521-522.
- Maya-Manzano J E M I, Pusch G, Ebner Von Eschenbach C, et al. Effect of air filtration on house dust mite, cat and dog allergens and particulate matter in homes. Clin. Transl. Allergy, 2022, 12, e12137.
- Gherasim A, de Blay F E D E. Does air filtration work for cat allergen exposure? Curr. Allergy Asthm. R. 2020, 20, 18.
- Devadoss D, Surbaugh K, Manevski M, et al. Indoor-air purification by photoelectrochemical oxidation mitigates allergic airway responses to aerosolized cat dander in a murine model. Sci. Rep-UK, 2023, 13, 10980.
- Golightly L K, Greos L S. Second-generation antihistamines: actions and efficacy in the management of allergic disorders. Drugs, 2005, 65, 341-384.
- Linton S, Hossenbaccus L, Ellis A K. Evidence-based use of antihistamines for treatment of allergic conditions. Ann. Allergy Asthma Im. 2023, 131, 412-420.
- Desjardins P J, Berlin R G. Efficacy of phenylephrine. Brit. J. Clin. Pharmaco. 2007, 64, 555.
- Krause H F. Antihistamines and decongestants. Otolaryngolog. Head Neck , 1992, 107, 835-840.
- Paggiaro P L, Dahle R, Bakran I, et al. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. The Lancet, 1998, 351, 773-780.
- Penagos M, Compalati E, Tarantini F, et al. Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta-analysis of randomized, double-blind, placebo-controlled, clinical trials. Allergy, 2008, 63, 1280-1291.
- Hoang M P, Chitsuthipakorn W, Seresirikachorn K, et al. As-needed intranasal corticosteroid spray for allergic rhinitis: a systematic review and meta-analysis. Rhinology, 2022, 60, 242-251.
- Abelson M B. A review of olopatadine for the treatment of ocular allergy. Expert Opin Pharmaco. 2004, 5, 1979-1994.
- Grant S M, Goa K L, Fitton A, et al. Ketotifen: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs, 1990, 40, 412-448.
- Barnes P J, Pedersen S O R, Busse W W. Efficacy and safety of inhaled corticosteroids: new developments. Am. J. Crit. Care .1998, 157, S1-S53.
- Marques L, Vale N. Salbutamol in the management of asthma: A review. Int. J. Mol. Sci. 2022, 23, 14207.
- Nayak A, Langdon R B. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs, 2007, 67, 887-901.
- Noonan M J, Chervinsky P, Brandon M, et al. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Eu. Respir. J. 1998, 11, 1232-1239.
- Lipworth B J. Leukotriene-receptor antagonists. The Lancet, 1999, 353, 57-62.
- Montuschi P, Peters-Golden M L. Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy, 2010, 40, 1732-1741.
- Arshad S H. An update on allergen immunotherapy. Clin. Med. Res. 2016, 16, 584-587.
- Adlany Y K, V S O V S I C L, Senti G, et al. Quality of life in allergic rhinitis patients treated with intralymphatic immunotherapy (ILIT): A 19-year follow-up. J. Allergy Clin. Immun. 2023, 2, 43-50.
- Orengo J M, Radin A R, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9,1 421.
- Yuan W, Hu S, Li M, et al. Efficacy and safety of omalizumab in Chinese patients with anti-histamine refractory chronic spontaneous urticaria. Dermatol. Ther. 2022, 35, e15303.
- Trifonova D, Curin M, Focke-Tejkl M, et al. Recombinant hypoallergenic cat allergy vaccines. Allergy 2025, 80, 2622-2635.
- De Filippo M, Votto M, Caminiti L, et al. Safety of allergen-specific immunotherapy in children. Pediat. Allerg. Imm-UK. 2022, 33, 27-30.
- Remes S T, Castro-Rodriguez J A, Holberg C J, et al. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J. Allergy Clin. Immun. 2001, 108, 509-515.
- Celed O N J C, Litonjua A A, Ryan L, et al. Exposure to cat allergen, maternal history of asthma, and wheezing in first 5 years of life. The Lancet, 2002, 360, 781-782.
- Dias R, Torkamani A. Artificial intelligence in clinical and genomic diagnostics. Genome Med., 2019, 11, 70.
- MacMath D, Chen M, Khoury P. Artificial intelligence: exploring the future of innovation in allergy immunology. Curr. Allergy Asthm. R. 2023, 23, 351-362.
- Alanazi H H. Role of artificial intelligence in advancing immunology. Immunol. Res. 2025, 73, 76.
- Indolfi C, Klain A, Dinardo G, et al. Artificial intelligence in the transition of allergy: A valuable tool from childhood to adulthood. Front. Med-PRC. 2024, 11, 1469161.
| Countries | Methods | Total | Cats | Dogs |
| China | Blood testing | 16,664 | 15.47% | 10.50% |
| Russia | Blood testing | 513 | 24.10% | 21.40% |
| South Korea | Skin prick test | 7504 | 20.60% | 15.20% |
| Germany | Blood testing | 356 | 34.80% | 31.70% |
| Japan | Blood testing | 12,205,097 | 18.20% | 18.90% |
| America | Blood testing | 478 | 54.40% | 64.70% |
| Canada | Skin prick test | 623 | 53.10% | 17.30% |
| Qatar | Skin prick test | 473 | 6.18% | 0.50% |
| Lebanon | Skin prick test | 919 | 29.90% | 21.90% |
| Thailand | Skin prick test | 1516 | 12.90% | 10.00% |
| Nepal | Skin prick test | 170 | 15.30% | 14.10% |
| Mexico | Skin prick test | 761 | 26.70% | 33.90% |
| Methods | Technical Features | Application Scenarios | Sensitivity | References |
| Signal amplification ELISA | Employing catalytic reported deposition (CARD) technology for the cascade amplification of enzyme-substrate reactions | Ultra-low concentration air samples | 156 pg Fel d 1 / mL | [84] |
| Double-point sandwich ELISA | MAbC48 targets conserved IgE epitopes, avoiding interference from Fel d 1 polymorphisms | Indoor dust samples | 390 pg Fel d 1 / mL | [85] |
| Immunodot assay (Dustscreen™) | Semi-quantitative, 4h results, capable of multiple allergen testing | Rapid screening for allergens in public areas | 100 pg Fel d 1/mL | [86] |
| Double antibody sandwich ELISA | Quantified using manufacturer standard curves, between plates CV<10% | For detecting allergen content in clinical or research settings | 80,000 pg Fel d 1 | [87] |
| scFv-Sandwich ELISA | The 96-well high-throughput format replaces traditional radial diffusion, eliminating the need for subjective interpretation | Specific for the potency verification of commercially available standardized allergen extracts | 500 pg Fel d 1/mL | [88] |
| Human IgG4-tweezed sandwich ELISA | Using high-affinity humanized monoclonal antibodies, between plates CV<9% | Suitable for manufacturers calibration of cat allergen concentrations | 250 pg Fel d 1/mL | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





