Submitted:
30 September 2025
Posted:
01 October 2025
You are already at the latest version
Abstract
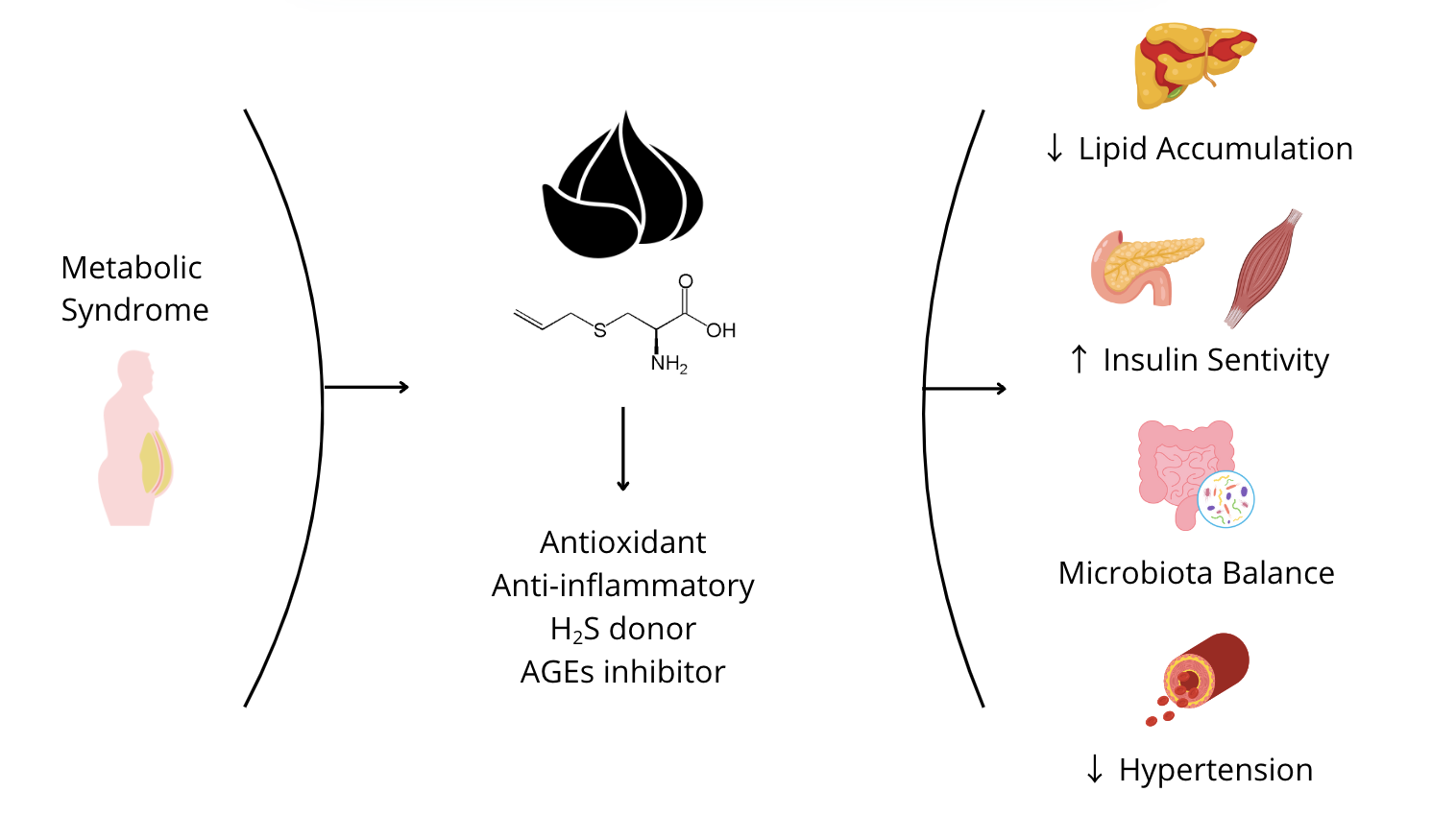
Keywords:
1. Introduction
2. Definition and Epidemiology
3. Pathophysiological Mechanisms
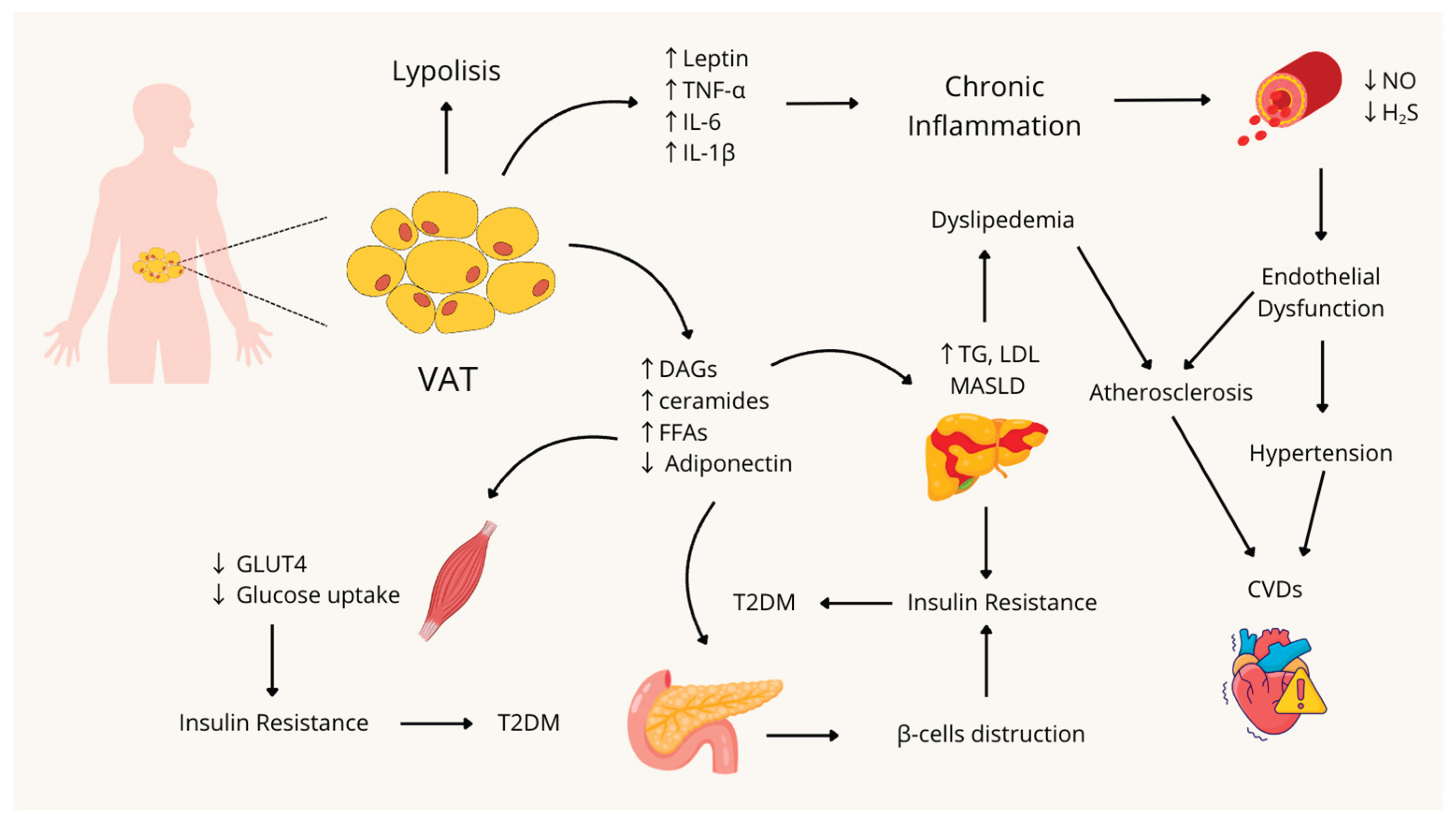
3.1. Visceral Obesity and Inflammation
3.2. Insulin Resistance
3.3. Hypertension: Focus on Endothelial Dysfunction and the Role of Gasotransmitters
3.4. The Management of Metabolic Syndrome, from Diet to Pharmacotherapy
4. S-allyl Cysteine
4.1. Origin and Derivation from Garlic Maturation, Safety, and Bioavailability
4.2. Antioxidant and Anti-Inflammatory Properties
4.3. Beneficial Effects of S-Allyl Cysteine in Insulin Resistance, Diabetes, and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): In Vivo and In Vitro Evidence
4.4. Beneficial Effects of S-Allyl Cysteine in Endothelial Dysfunction (ED)
4.5. Role of Gut Microbiota in the Development of Metabolic Syndrome and the Protective Effect of S-Allyl Cysteine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CVDs | cardiovascular disease |
| EC | endothelial cell |
| ED | endothelial dysfunction |
| eNOS | endothelial nitric oxide synthase |
| FFA | free fatty acid |
| GM | gut microbiota |
| GSH | glutathione |
| H2S | hydrogen sulfide |
| IR | insulin resistance |
| LDL | Low-Density Lipoprotein |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MedDiet | Mediterranean Diet |
| MetS | Metabolic Syndrome |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| SAC | S-allyl cysteine |
| T2DM | type II diabetes |
References
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clinics in Dermatology 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther Adv Cardiovasc Dis 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium Sativum L.). Foods 2019, 8, E246. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Ali, S.F.; Túnez, I.; Santamaría, A. On the Antioxidant, Neuroprotective and Anti-Inflammatory Properties of S-Allyl Cysteine: An Update. Neurochem Int 2015, 89, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. The Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Pigeot, I.; Ahrens, W. Epidemiology of Metabolic Syndrome. Pflugers Archiv European Journal of Physiology 2025, 477, 669–680. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Bixby, H.; Cowan, M.J.; Ali, M.K.; Taddei, C.; et al. Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4·4 Million Participants. The Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Safiri, S.; Karamzad, N.; Kaufman, J.S.; Bell, A.W.; Nejadghaderi, S.A.; Sullman, M.J.M.; Moradi-Lakeh, M.; Collins, G.; Kolahi, A.A. Prevalence, Deaths and Disability-Adjusted-Life-Years (DALYs) Due to Type 2 Diabetes and Its Attributable Risk Factors in 204 Countries and Territories, 1990-2019: Results From the Global Burden of Disease Study 2019. Frontiers in Endocrinology 2022, 13, 838027–838027. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Research and Clinical Practice 2022, 188, 109924–109924. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and Ectopic Fat, Atherosclerosis, and Cardiometabolic Disease: A Position Statement. The Lancet Diabetes and Endocrinology 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nature Reviews Endocrinology 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The Endocrine Function of Adipose Tissues in Health and Cardiometabolic Disease. Nature Reviews Endocrinology 2019 15:9 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.J.; AL-Mofarji, S.T.; Mahdi, B.M.; Ameen, R.S.; AL-Zubaidi, M.M. The Correlation between Obesity and Leptin Signaling Pathways. Cytokine 2025, 192, 156970. [Google Scholar] [CrossRef] [PubMed]
- Marroquí, L.; Gonzalez, A.; Ñeco, P.; Caballero-Garrido, E.; Vieira, E.; Ripoll, C.; Nadal, A.; Quesada, I. Role of Leptin in the Pancreatic β-Cell: Effects and Signaling Pathways. J Mol Endocrinol 2012, 49, R9–17. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Hormone Molecular Biology and Clinical Investigation 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. International Journal of Molecular Sciences 2017, 18, 1321–1321. [Google Scholar] [CrossRef]
- Lee, K.W.; Shin, D. Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study. International Journal of Environmental Research and Public Health 2020, 17, 3287–3287. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Research and Clinical Practice 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediators of Inflammation 2010, 2010. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiological Reviews 2018, 98, 2133–2133. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct Target Ther 2022, 7, 216. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of Muscle Insulin Resistance and the Cross-Talk with Liver and Adipose Tissue. Physiological Reports 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.C.; Dao, X.D.; Nguyen, D.V.; Luu, D.H.; Bui, T.M.H.; Le, T.H.; Nguyen, H.T.; Le, T.N.; Hosaka, T.; Nguyen, T.T.T. Insulin Signaling and Its Application. Frontiers in Endocrinology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Maffei, P.; DeFronzo, R.A. Managing Insulin Resistance: The Forgotten Pathophysiological Component of Type 2 Diabetes. The Lancet Diabetes & Endocrinology 2024, 12, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. THE GLUCOSE FATTY-ACID CYCLE ITS ROLE IN INSULIN SENSITIVITY AND THE METABOLIC DISTURBANCES OF DIABETES MELLITUS. The Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Roden, M. Insulin Resistance and Insulin Sensitizing Agents. Metabolism 2021, 125, 154892–154892. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the Diagnosis of Insulin Resistance: Focusing on the Role of HOMA-IR and Tryglyceride/Glucose Index. Diabetes Metab Syndr 2022, 16, 102581. [Google Scholar] [CrossRef]
- González-González, J.G.; Violante-Cumpa, J.R.; Zambrano-Lucio, M.; Burciaga-Jimenez, E.; Castillo-Morales, P.L.; Garcia-Campa, M.; Solis, R.C.; González-Colmenero, A.D.; Rodríguez-Gutiérrez, R. HOMA-IR as a Predictor of Health Outcomes in Patients with Metabolic Risk Factors: A Systematic Review and Meta-Analysis. High Blood Pressure and Cardiovascular Prevention 2022, 29, 547–564. [Google Scholar] [CrossRef]
- Cirino, G.; Vellecco, V.; Bucci, M. Nitric Oxide and Hydrogen Sulfide: The Gasotransmitter Paradigm of the Vascular System. British J Pharmacology 2017, 174, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Endothelial Dysfunction in Metabolic Syndrome: Prevalence, Pathogenesis and Management. Nutrition, Metabolism and Cardiovascular Diseases 2010, 20, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Shruthi, N.R.; Banerjee, A.; Jothimani, G.; Duttaroy, A.K.; Pathak, S. Endothelial Dysfunction, Platelet Hyperactivity, Hypertension, and the Metabolic Syndrome: Molecular Insights and Combating Strategies. Front. Nutr. 2023, 10, 1221438. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascular Pharmacology 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Hsieh, H.-J.; Liu, C.-A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-Induced Endothelial Mechanotransduction: The Interplay between Reactive Oxygen Species (ROS) and Nitric Oxide (NO) and the Pathophysiological Implications. J Biomed Sci 2014, 21, 3. [Google Scholar] [CrossRef]
- Marchesi, C.; Ebrahimian, T.; Angulo, O.; Paradis, P.; Schiffrin, E.L. Endothelial Nitric Oxide Synthase Uncoupling and Perivascular Adipose Oxidative Stress and Inflammation Contribute to Vascular Dysfunction in a Rodent Model of Metabolic Syndrome. Hypertension 2009, 54, 1384–1392. [Google Scholar] [CrossRef]
- Smimmo, M.; Casale, V.; Casillo, G.M.; Mitidieri, E.; d’Emmanuele Di Villa Bianca, R.; Bello, I.; Schettino, A.; Montanaro, R.; Brancaleone, V.; Indolfi, C.; et al. Hydrogen Sulfide Dysfunction in Metabolic Syndrome-Associated Vascular Complications Involves cGMP Regulation through Soluble Guanylyl Cyclase Persulfidation. Biomedicine & Pharmacotherapy 2024, 174, 116466. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Hüttl, M.; Markova, I.; Berenyiova, A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules 2021, 11, 108. [Google Scholar] [CrossRef]
- Birulina, Yu.G.; Ivanov, V.V.; Buyko, E.E.; Gabitova, I.O.; Kovalev, I.V.; Nosarev, A.V.; Smagliy, L.V.; Gusakova, S.V. Role of H2S in Regulation of Vascular Tone in Metabolic Disorders. Bull Exp Biol Med 2021, 171, 431–434. [Google Scholar] [CrossRef]
- Pott, A.; Hiane, P.; Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. 2023. [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Adry, E.M.˛ High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. 2021. [CrossRef]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western Dietary Pattern Is Prospectively Associated with Cardio-Metabolic Traits and Incidence of the Metabolic Syndrome. British Journal of Nutrition 2018, 119, 1168–1176. [Google Scholar] [CrossRef]
- Seral-Cortes, M.; Larruy-García, A.; De Miguel-Etayo, P.; Labayen, I.; Moreno, L.A. Mediterranean Diet and Genetic Determinants of Obesity and Metabolic Syndrome in European Children and Adolescents. Genes 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Giacco, A.; Cioffi, F.; Silvestri, E. Mediterranean Diet and Metabolic Syndrome. Nutrients 2025, 17, 2364–2364. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic Syndrome, Mediterranean Diet, and Polyphenols: Evidence and Perspectives. Journal of Cellular Physiology 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and Metabolic Syndrome: A Narrative Review. Internal and Emergency Medicine 2023, 18, 1007–1017. [Google Scholar] [CrossRef]
- Dayi, T.; Ozgoren, M. Effects of the Mediterranean Diet on the Components of Metabolic Syndrome. Journal of Preventive Medicine and Hygiene 2022, 63, E56–E56. [Google Scholar] [CrossRef]
- Sofi, F.; Martini, D.; Angelino, D.; Cairella, G.; Campanozzi, A.; Danesi, F.; Dinu, M.; Erba, D.; Iacoviello, L.; Pellegrini, N.; et al. Mediterranean Diet: Why a New Pyramid? An Updated Representation of the Traditional Mediterranean Diet by the Italian Society of Human Nutrition (SINU). Nutrition, Metabolism and Cardiovascular Diseases 2025, 35, 103919–103919. [Google Scholar] [CrossRef]
- Bruna-Mejias, A.; San Martin, J.; Arciniegas-Diaz, D.; Meneses-Caroca, T.; Salamanca-Cerda, A.; Beas-Gambi, A.; Paola-Loaiza-Giraldo, J.; Ortiz-Ahumada, C.; Nova-Baeza, P.; Oyanedel-Amaro, G.; et al. Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences 2025, 26, 5887–5887. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652–1652. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Corella, D.; Martínez-González, M.Á.; Babio, N.; Martínez, J.A.; Forga, L.; Alonso-Gómez, Á.M.; Wärnberg, J.; Vioque, J.; Romaguera, D.; et al. Comparison of an Energy-Reduced Mediterranean Diet and Physical Activity Versus an Ad Libitum Mediterranean Diet in the Prevention of Type 2 Diabetes : A Secondary Analysis of a Randomized Controlled Trial. Annals of internal medicine 2025. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, L. Research Advances in the Therapy of Metabolic Syndrome. Frontiers in Pharmacology 2024, 15, 1364881–1364881. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Yudhistira, B.; Punthi, F.; Lin, J.-A.; Sulaimana, A.S.; Chang, C.-K.; Hsieh, C.-W. S-Allyl Cysteine in Garlic (Allium Sativum): Formation, Biofunction, and Resistance to Food Processing for Value-Added Product Development. Compr Rev Food Sci Food Saf 2022, 21, 2665–2687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, M.; Wang, C.; Zhou, H.; Fan, L.; Huang, X. Thermolysis Kinetics and Thermal Degradation Compounds of Alliin. Food Chemistry 2017, 223, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Ohtani, M. Pharmacokinetics of Sulfur-Containing Compounds in Aged Garlic Extract: S-Allylcysteine, S-1-Propenylcysteine, S-Methylcysteine, S-Allylmercaptocysteine and Others (Review). Exp Ther Med 2025, 29, 102. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, Chemical, and Biological Properties of S-Allylcysteine, an Amino Acid Derived from Garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef]
- Basu, C.; Sur, R. S-Allyl Cysteine Alleviates Hydrogen Peroxide Induced Oxidative Injury and Apoptosis through Upregulation of Akt/Nrf-2/HO-1 Signaling Pathway in HepG2 Cells. Biomed Res Int 2018, 2018, 3169431. [Google Scholar] [CrossRef]
- Chen, P.; Hu, M.; Liu, F.; Yu, H.; Chen, C. S-Allyl-l-Cysteine (SAC) Protects Hepatocytes from Alcohol-Induced Apoptosis. FEBS open bio 2019, 9, 1327–1336. [Google Scholar] [CrossRef]
- gianni S-Allyl Cysteine Protects Retinal Pigment Epithelium Cells from Hydroquinone-Induced Apoptosis through Mitigating Cellular Response to Oxidative Stress. European Review 2020.
- Geddo, F.; Querio, G.; Asteggiano, A.; Antoniotti, S.; Porcu, A.; Occhipinti, A.; Medana, C.; Gallo, M.P. Improving Endothelial Health with Food-Derived H2S Donors: An in Vitro Study with S-Allyl Cysteine and with a Black-Garlic Extract Enriched in Sulfur-Containing Compounds. Food Funct 2023, 14, 4163–4172. [Google Scholar] [CrossRef]
- Huang, X. pei; Shi, Z. hua; Ming, G. feng; Xu, D. miao; Cheng, S. qiao S-Allyl-L-Cysteine (SAC) Inhibits Copper-Induced Apoptosis and Cuproptosis to Alleviate Cardiomyocyte Injury. Biochemical and biophysical research communications 2024, 730. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Bentke, A.; Lasota, M.; Wróbel, M. Effect of S-Allyl –L-Cysteine on MCF-7 Cell Line 3-Mercaptopyruvate Sulfurtransferase/Sulfane Sulfur System, Viability and Apoptosis. Int J Mol Sci 2020, 21, 1090. [Google Scholar] [CrossRef]
- Reyes-Soto, C.Y.; Ramírez-Carreto, R.J.; Ortíz-Alegría, L.B.; Silva-Palacios, A.; Zazueta, C.; Galván-Arzate, S.; Karasu, Ç.; Túnez, I.; Tinkov, A.A.; Aschner, M.; et al. S-Allyl-Cysteine Triggers Cytotoxic Events in Rat Glioblastoma RG2 and C6 Cells and Improves the Effect of Temozolomide through the Regulation of Oxidative Responses. Discov Oncol 2024, 15, 272. [Google Scholar] [CrossRef]
- Orozco-Morales, M.; Hernández-Pedro, N.Y.; Barrios-Bernal, P.; Arrieta, O.; Ruiz-Godoy, L.M.; Aschner, M.; Santamaría, A.; Colín-González, A.L. S-Allylcysteine Induces Cytotoxic Effects in Two Human Lung Cancer Cell Lines via Induction of Oxidative Damage, Downregulation of Nrf2 and NF-κB, and Apoptosis. Anticancer Drugs 2021, 32, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Su, D.; Zhu, L.; Zhang, S.; Ma, S.; Wu, K.; Yuan, Q.; Lin, N. S-Allylcysteine Suppresses Ovarian Cancer Cell Proliferation by DNA Methylation through DNMT1. J Ovarian Res 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.-N.; Kang, M.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Byun, S.-S. Anticancer Effect of S-Allyl-L-Cysteine via Induction of Apoptosis in Human Bladder Cancer Cells. Oncol Lett 2018, 15, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.P.; Guo, D.Y.; Cheng, Q.; Geng, W.; Ling, C.C.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Lo, C.M.; Poon, R.T.P.; et al. A Garlic Derivative, S-Allylcysteine (SAC), Suppresses Proliferation and Metastasis of Hepatocellular Carcinoma. PLoS One 2012, 7, e31655. [Google Scholar] [CrossRef]
- Neufeld, B.H.; Tapia, J.B.; Lutzke, A.; Reynolds, M.M. Small Molecule Interferences in Resazurin and MTT-Based Metabolic Assays in the Absence of Cells. Anal. Chem. 2018, 90, 6867–6876. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. Harnessing Gasotransmitters to Combat Age-Related Oxidative Stress in Smooth Muscle and Endothelial Cells. Pharmaceuticals (Basel) 2025, 18, 344. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P.; Kumar, G.P.S.; Rajarajan, T. Antidiabetic Properties of S-Allyl Cysteine, a Garlic Component on Streptozotocin-Induced Diabetes in Rats. Journal of Applied Biomedicine 2009, 7, 151–159. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P. Beneficial Effect of S-Allylcysteine (SAC) on Blood Glucose and Pancreatic Antioxidant System in Streptozotocin Diabetic Rats. Plant Foods Hum Nutr 2010, 65, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P.; Begum, M.S. Effect of S-Allylcysteine, a Sulphur Containing Amino Acid on Iron Metabolism in Streptozotocin Induced Diabetic Rats. Journal of Trace Elements in Medicine and Biology 2013, 27, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P. S-Allylcysteine Improves Streptozotocin-Induced Alterations of Blood Glucose, Liver Cytochrome P450 2E1, Plasma Antioxidant System, and Adipocytes Hormones in Diabetic Rats. Int J Endocrinol Metab 2013, 11, e10927. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Minamiyama, Y.; Kodai, S.; Shinkawa, H.; Tsukioka, T.; Okada, S.; Azuma, H.; Kubo, S. S-Allyl Cysteine Improves Nonalcoholic Fatty Liver Disease in Type 2 Diabetes Otsuka Long-Evans Tokushima Fatty Rats via Regulation of Hepatic Lipogenesis and Glucose Metabolism. J Clin Biochem Nutr 2013, 53, 94–101. [Google Scholar] [CrossRef]
- Naidu, P.B.; Sathibabu Uddandrao, V.V.; Naik, R.R.; Pothani, S.; Munipally, P.K.; Meriga, B.; Begum, M.S.; Varatharaju, C.; Pandiyan, R.; Saravanan, G. Effects of S-Allylcysteine on Biomarkers of the Polyol Pathway in Rats with Type 2 Diabetes. Canadian Journal of Diabetes 2016, 40, 442–448. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, S.H.; Cho, Y.J.; Pan, J.H.; Cho, H.T.; Kim, J.H.; Bong, H.; Lee, Y.; Chang, M.H.; Jeong, Y.J.; et al. Preparation of S-Allylcysteine-Enriched Black Garlic Juice and Its Antidiabetic Effects in Streptozotocin-Induced Insulin-Deficient Mice. J. Agric. Food Chem. 2017, 65, 358–363. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pischetsrieder, M.; Ahmed, N. Aged Garlic Extract and S-Allyl Cysteine Prevent Formation of Advanced Glycation Endproducts. European Journal of Pharmacology 2007, 561, 32–38. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Saini, S.; Ghosh, S.; Roy, P.; Ali, N.; Parvez, M.K.; Al-Dosari, M.S.; Mishra, A.K.; Singh, L.R. Organosulfurs, S-Allyl Cysteine and N-Acetyl Cysteine Sequester Di-Carbonyls and Reduces Carbonyl Stress in HT22 Cells. Sci Rep 2023, 13, 13071. [Google Scholar] [CrossRef]
- Kavitha, S.A.; Zainab, S.; Muthyalaiah, Y.S.; John, C.M.; Arockiasamy, S. Mechanism and Implications of Advanced Glycation End Products (AGE) and Its Receptor RAGE Axis as Crucial Mediators Linking Inflammation and Obesity. Mol Biol Rep 2025, 52, 1–19. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Kim, H.G.; Choi, J.H.; Do, M.T.; Chung, Y.C.; Jeong, T.C.; Jeong, H.G. S-Allyl Cysteine Attenuates Free Fatty Acid-Induced Lipogenesis in Human HepG2 Cells through Activation of the AMP-Activated Protein Kinase-Dependent Pathway. The Journal of Nutritional Biochemistry 2013, 24, 1469–1478. [Google Scholar] [CrossRef]
- Sharif, A.; Majimbi, M.; Mamo, J.; Lam, V.; Nesbit, M.; Takechi, R. Differential Effects of S-Allyl Cysteine and Cannabidiol on Enterocytic and Plasma Amyloid-β in Db/Db Diabetic Mice. Sci Rep 2025, 15, 20448. [Google Scholar] [CrossRef]
- Sakayanathan, P.; Loganathan, C.; Thayumanavan, P. Astaxanthin-S-Allyl Cysteine Ester Protects Pancreatic β-Cell From Glucolipotoxicity by Suppressing Oxidative Stress, Endoplasmic Reticulum Stress and mTOR Pathway Dysregulation. Journal of Biochemical and Molecular Toxicology 2024, 38, e70058. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of Hydrogen Sulfide in Endothelial Dysfunction: Pathophysiology and Therapeutic Approaches. J Adv Res 2021, 27, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front Pharmacol 2020, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Lau, B.H.S. Garlic Compounds Protect Vascular Endothelial Cells from Oxidized Low Density Lipoprotein-Induced Injury. Journal of Pharmacy and Pharmacology 1997, 49, 908–911. [Google Scholar] [CrossRef]
- Ide, N.; Lau, B.H.S. S -Allylcysteine Attenuates Oxidative Stress in Endothelial Cells. Drug Development and Industrial Pharmacy 1999, 25, 619–624. [Google Scholar] [CrossRef]
- Ide, N.; Lau, B.H.S. Garlic Compounds Minimize Intracellular Oxidative Stress and Inhibit Nuclear Factor-κB Activation. The Journal of Nutrition 2001, 131, 1020S–1026S. [Google Scholar] [CrossRef]
- Lau, B.H. Suppression of LDL Oxidation by Garlic Compounds Is a Possible Mechanism of Cardiovascular Health Benefit. The Journal of Nutrition 2006, 136, 765S–768S. [Google Scholar] [CrossRef]
- Kim, K.-M.; Chun, S.-B.; Koo, M.-S.; Choi, W.-J.; Kim, T.-W.; Kwon, Y.-G.; Chung, H.-T.; Billiar, T.R.; Kim, Y.-M. Differential Regulation of NO Availability from Macrophages and Endothelial Cells by the Garlic Component S-Allyl Cysteine. Free Radical Biology and Medicine 2001, 30, 747–756. [Google Scholar] [CrossRef]
- Ho, S.E.; Ide, N.; Lau, B.H.S. S-Allyl Cysteine Reduces Oxidant Load in Cells Involved in the Atherogenic Process. Phytomedicine 2001, 8, 39–46. [Google Scholar] [CrossRef]
- Syu, J.-N.; Yang, M.-D.; Tsai, S.-Y.; Chiang, E.-P.I.; Chiu, S.-C.; Chao, C.-Y.; Rodriguez, R.L.; Tang, F.-Y. S-Allylcysteine Improves Blood Flow Recovery and Prevents Ischemic Injury by Augmenting Neovasculogenesis. Cell Transplant 2017, 26, 1636–1647. [Google Scholar] [CrossRef]
- Wen, Y.-D.; Wang, H.; Kho, S.-H.; Rinkiko, S.; Sheng, X.; Shen, H.-M.; Zhu, Y.-Z. Hydrogen Sulfide Protects HUVECs against Hydrogen Peroxide Induced Mitochondrial Dysfunction and Oxidative Stress. PLoS ONE 2013, 8, e53147. [Google Scholar] [CrossRef]
- Bentke-Imiolek, A.; Szlęzak, D.; Zarzycka, M.; Wróbel, M.; Bronowicka-Adamska, P. S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines. Biomolecules 2024, 14, 188. [Google Scholar] [CrossRef]
- Brahmanaidu, P.; Uddandrao, V.V.S.; Sasikumar, V.; Naik, R.R.; Pothani, S.; Begum, M.S.; Rajeshkumar, M.P.; Varatharaju, C.; Meriga, B.; Rameshreddy, P.; et al. Reversal of Endothelial Dysfunction in Aorta of Streptozotocin-Nicotinamide-Induced Type-2 Diabetic Rats by S-Allylcysteine. Mol Cell Biochem 2017, 432, 25–32. [Google Scholar] [CrossRef]
- Sasidharan Pillai, S.; Gagnon, C.A.; Foster, C.; Ashraf, A.P. Exploring the Gut Microbiota: Key Insights Into Its Role in Obesity, Metabolic Syndrome, and Type 2 Diabetes. The Journal of Clinical Endocrinology and Metabolism 2024, 109, 2709–2709. [Google Scholar] [CrossRef]
- Nie, P.; Hu, L.; Feng, X.; Xu, H. Gut Microbiota Disorders and Metabolic Syndrome: Tales of a Crosstalk Process. Nutrition Reviews 2025, 83, 908–924. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging and Disease 2022, 13, 1106–1106. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome Connections with Host Metabolism and Habitual Diet from 1,098 Deeply Phenotyped Individuals. Nature Medicine 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.M.; Luque, E.; Aguilar-Luque, M.; Feijóo, M.; Caballero-Villarraso, J.; Torres, L.A.; Ramirez, V.; García-Maceira, F.I.; Agüera, E.; Santamaria, A.; et al. Dose-Dependent S-Allyl Cysteine Ameliorates Multiple Sclerosis Disease-Related Pathology by Reducing Oxidative Stress and Biomarkers of Dysbiosis in Experimental Autoimmune Encephalomyelitis. European Journal of Pharmacology 2017, 815, 266–273. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Frontiers in Nutrition 2018, 5, 122–122. [Google Scholar] [CrossRef] [PubMed]
| Main Effect | Experimental Model |
SAC Administration |
References |
| Decrease in blood glucose and increase in plasma insulin levels Increase in plasma and pancreatic antioxidant enzyme activity Increase in plasma concentrations of ferritin, bilirubin, and iron, and a decrease in transferrin level Increase in leptin and adiponectin levels Increase in liver P450 2E1 activity |
Streptozotocin (STZ)-induced diabetic adult Wistar strain albino male rats |
150 mg/kg body weight for 45 days | [72,73,74,75] |
| Decrease in blood glucose, insulin, and hemoglobinA1c levels Decrease in LDL cholesterol and triglyceride levels Increase in mRNA and protein expression of PPARα and γ Increase in protein involved in lipid and glucose metabolism regulation |
Otsuka Long-Evans Tokushima Fatty rats |
0.45% dietary mixture for 13 weeks |
[76] |
|
Decrease in blood glucose and increase in plasma insulin levels Decrease in lipid peroxidation products and increase in the nonenzymatic antioxidant levels |
STZ and nicotinamide-induced diabetic male Wistar rats |
150 mg/kg body weight for 45 days |
[77] |
| Decrease in blood glucose levels Decrease in visceral fat depots Increase in pancreatic insulin content and suppression of β-cell apoptosis |
Streptozotocin (STZ)-induced diabetic male C57BL/6J mice |
SAC-enriched black garlic juice (200 mg/kg) for 31 days | [78] |
| Inhibition of metal-catalyzed protein fragmentation and AGE formation Decrease in carboxymethyllysine levels |
In vitro biochemical assays | 0-84 mg/ml of aged garlic extract for 7/21/35 days | [79] |
| Inhibition of protein glycation and oxidative modifications | HT22 cells | 0.25–1 mM | [80] |
| Inhibition of FFA-induced hepatocyte injury Decrease in FFA-induced lipid accumulation levels Decrease in protein expression levels of SREBP-1c and FAS |
HepG2 cells | 0.5–10 mM | [82] |
| Decrease in plasma amyloid-β levels | Male db/db and db/ + C57BLK/6 J mice |
0.04% w/w | [83] |
| Decrease in oxidative stress levels Decrease in DNA fragmentation levels Increase in gene expression levels in correlation with insulin secretion |
Mus musculus pancreatic β-cell line |
5-15 μg/ml of AST-SAC | [84] |
| Main Effect | Experimental Model |
SAC Administration |
References |
| Decrease in LDL oxidation levels Increase in GSH levels and decrease in peroxide release Inhibition of NFkB |
Bovine pulmonary artery ECs and HUVECs |
1-20 mM | [87,88,89,90] |
| Increase in NO production Decrease in hydroxyl radical and superoxide anion levels |
HUVECs | 20-80 µM | [91] |
| Inhibition of LDL oxidation, hydrogen peroxide production, and NFkB activation | HUVECs | 0.1-10 mM | [92] |
| Activation of AKT/eNOS signaling cascades Stimulation of neovasculogenesis |
Human endothelial progenitor cells (EPCs)/Neovasculogenesis xenograft model mice |
10-150 µM/ 0.2 or 2 mg/kg |
[93] |
| Increase in NO production Increase GSH, SOD, and CAT levels Restoration of the normal vascular structure in aorta rings |
STZ and nicotinamide-induced diabetic male Wistar rats |
150 mg/kg for 45 days | [96] |
| Increase in H2S production Decrease in ROS levels Increase in eNOS phosphorylation and NO production |
Bovine aortic endothelial cells (BAE-1) | 100 µM | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





