Submitted:
29 September 2025
Posted:
05 October 2025
You are already at the latest version
Abstract
Fungi of the genus Parengyodontium (Ascomycota, Hypocreales, Cordycipitaceae) are emerging as promising sources of secondary metabolites with significant biotechnological potential. While traditionally understudied, species such as Parengyodontium album, Parengyodontium torokii and Parengyodontium americanum have been isolated from diverse and sometimes extreme environments—including deep-sea sediments, mangroves, and NASA clean rooms—suggesting remarkable ecological adaptability. This review presents a comprehensive synthesis of current knowledge on the chemical diversity, biological activities, and potential industrial applications of secondary metabolites produced by fungi belonging this genus. A wide variety of compounds have been identified, including polyketides (e.g., engyodontiumones, alternaphenol B2), terpenoids (e.g., cytochalasin K), alkaloids, and torrubielline derivatives. These metabolites exhibit cytotoxic, antibacterial, and antifouling properties, with promising anticancer and antimicrobial activities. In addition, recent evidence points to the genus’s role in bioremediation, particularly through the degradation of polyethylene by P. album. Despite the advances highlighted here, challenges remain in scaling production, elucidating biosynthetic pathways, and confirming in vivo efficacy. This review underscores the value of integrating chemical, genomic, and metabolomic approaches to fully unlock the biotechnological potential of Parengyodontium species. Additionnally, we broaden the perspective by comparing trends in secondary metabolites among Cordycipitaceae, highlighting lifestyle-related chemical compounds that serve as a reference for the Parengyodontium profile.
Keywords:
1. Introduction
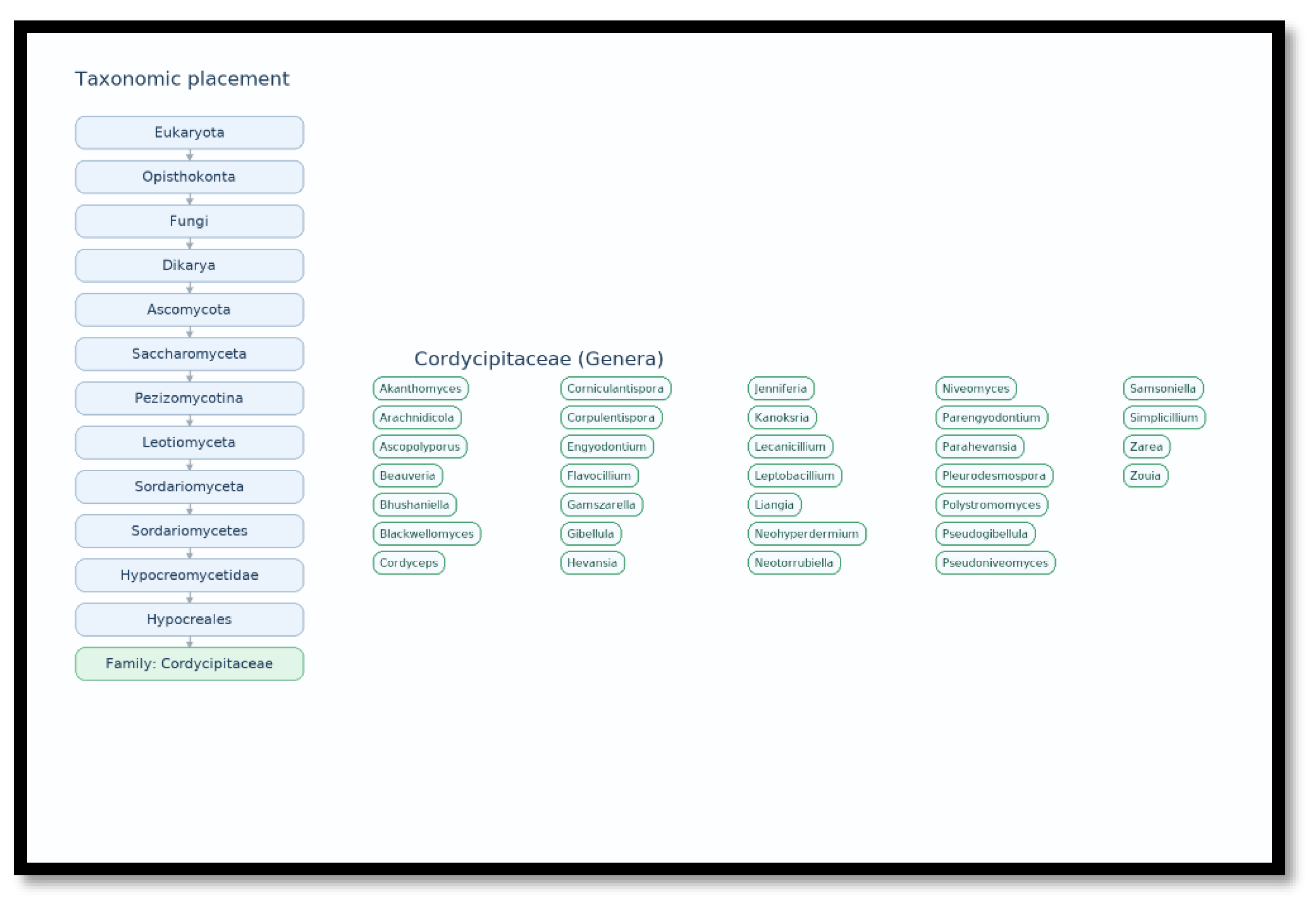
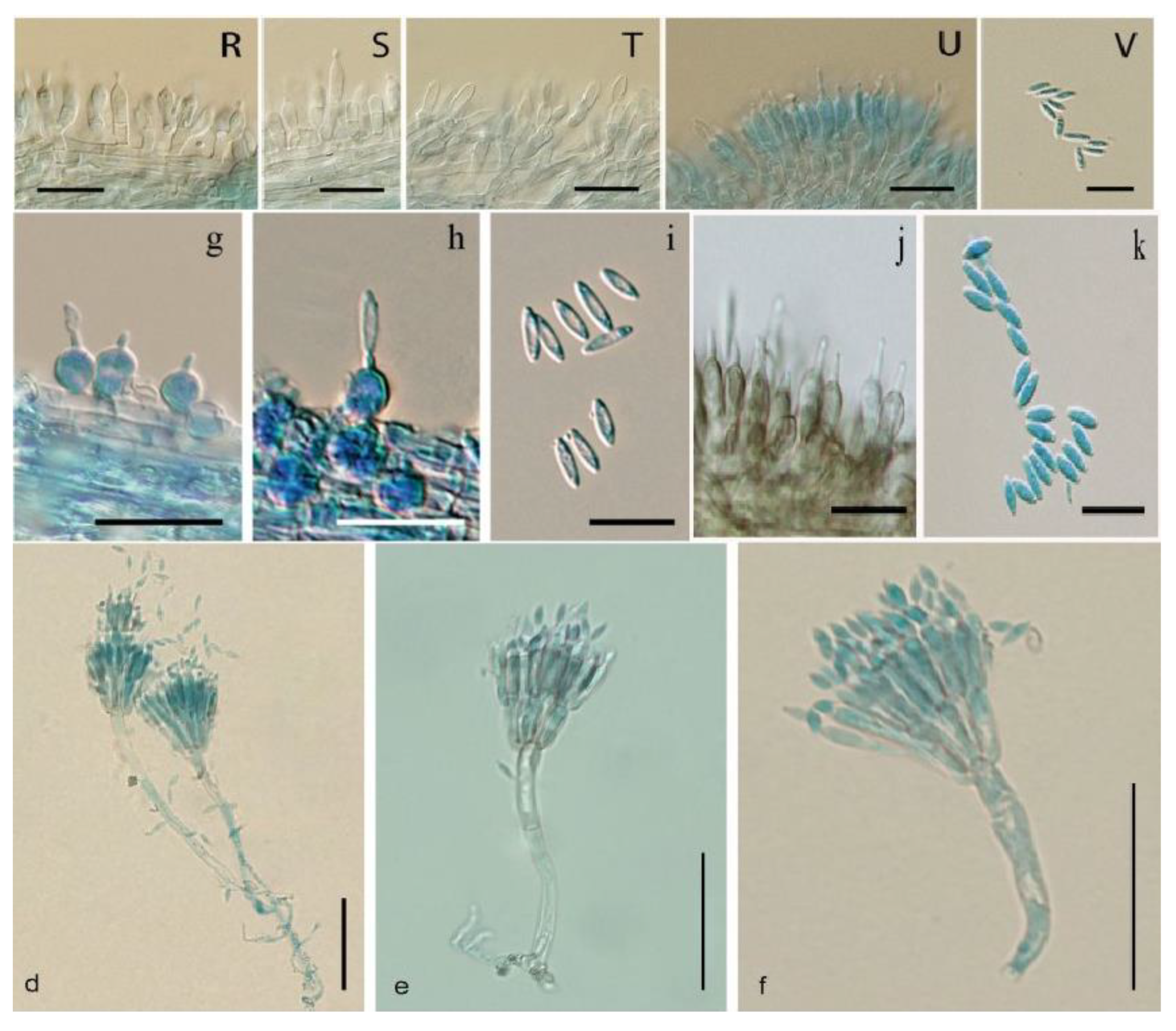
2. The Chemical Diversity of Cordycipitaceae Family: Comparison Between Entomopathogenic and Saprobic/Mycoparasitic Lineages
| Genus (representative species) | Lifestyle | Dominant metabolite classes | Flagship examples | Ecological/functional roles | References |
|---|---|---|---|---|---|
| Beauveria (e.g., B. bassiana) | Entomopathogen (insects) | Depsipeptides (bassianolide, beauvericin); Quinones (oosporein) | Bassianolide; Beauvericin; Oosporein | Virulence (membrane-active), immune modulation, cadaver defense; nematicidal/biocontrol effects | [58,59,60,61,65]#break# |
| Cordyceps (e.g., C. militaris) | Entomopathogen (insects) | Nucleosides (cordycepin); Xanthones; Polysaccharides; Peptides | Cordycepin; Militarinones (rep.); Xanthones | Host manipulation, signaling/interference; broader bioactivities; production/engineering model | [66,67,68,69,70] |
| Akanthomyces | Entomopathogen (insects/arachnids) | Polyketides; Peptides (putative); Phenopicolinic-type derivatives (reported historically) | Representative polyketides/peptides (var.) | Pathogenesis and competitive interactions on arthropod hosts; genus-level idiosyncrasies | [73] |
| Lecanicillium | Entomopathogen (insects) | Polyketides; Peptides; (chemistry less mapped than Beauveria/Cordyceps) | Representative polyketides/peptides (var.) | Insect infection; potential overlaps with Akanthomyces toolkits | [74] |
| Gibellula | Entomopathogen (spiders) | Anthraquinones; Antibiofilm compounds | Pigmentosins | Antibiofilm/antimicrobial activity during host colonization and microbiome control | [75] |
| Conoideocrella | Entomopathogen (insects) | Xanthones (glycosylated); Other polyketides | Xanthone glucoside (NBRC106950) | Competitive colonization and antibiofilm/antimicrobial functions | [76] |
| Blackwellomyces | Entomopathogen (insects/arachnids) | Bioxanthracenes; Cyclodepsipeptides | Bioxanthracene derivatives; Cyclodepsipeptides | Antimicrobial/cytotoxic activities likely aiding infection and post-host defense | [77] |
| Simplicillium | Mycoparasite (on fungi) | Polyketides; NRPS/PKS-derived antimicrobials (putative) | Genomic BGC inventory (NRPS/PKS); species-level yet emerging | Antagonism of fungal pathogens (e.g., powdery mildew, coffee rust); niche competition | [78,79,80,81] |
| Parengyodontium (e.g., P. album) | Saprobe/Opportunistic | Aromatic polyketides (anthraquinones; xanthoquinodines) | Engyodontochones A–F; JBIR-99 | Antibacterial/antibiofilm, cytotoxic activities; oxidative polyethylene degradation | [42,48,49] |
3. The Chemical Diversity in Parengyodontium Genus (Focus Section)
3.1. Identified Secondary Metabolites Classified by Chemical Family
3.1.1. Polyketides
- -
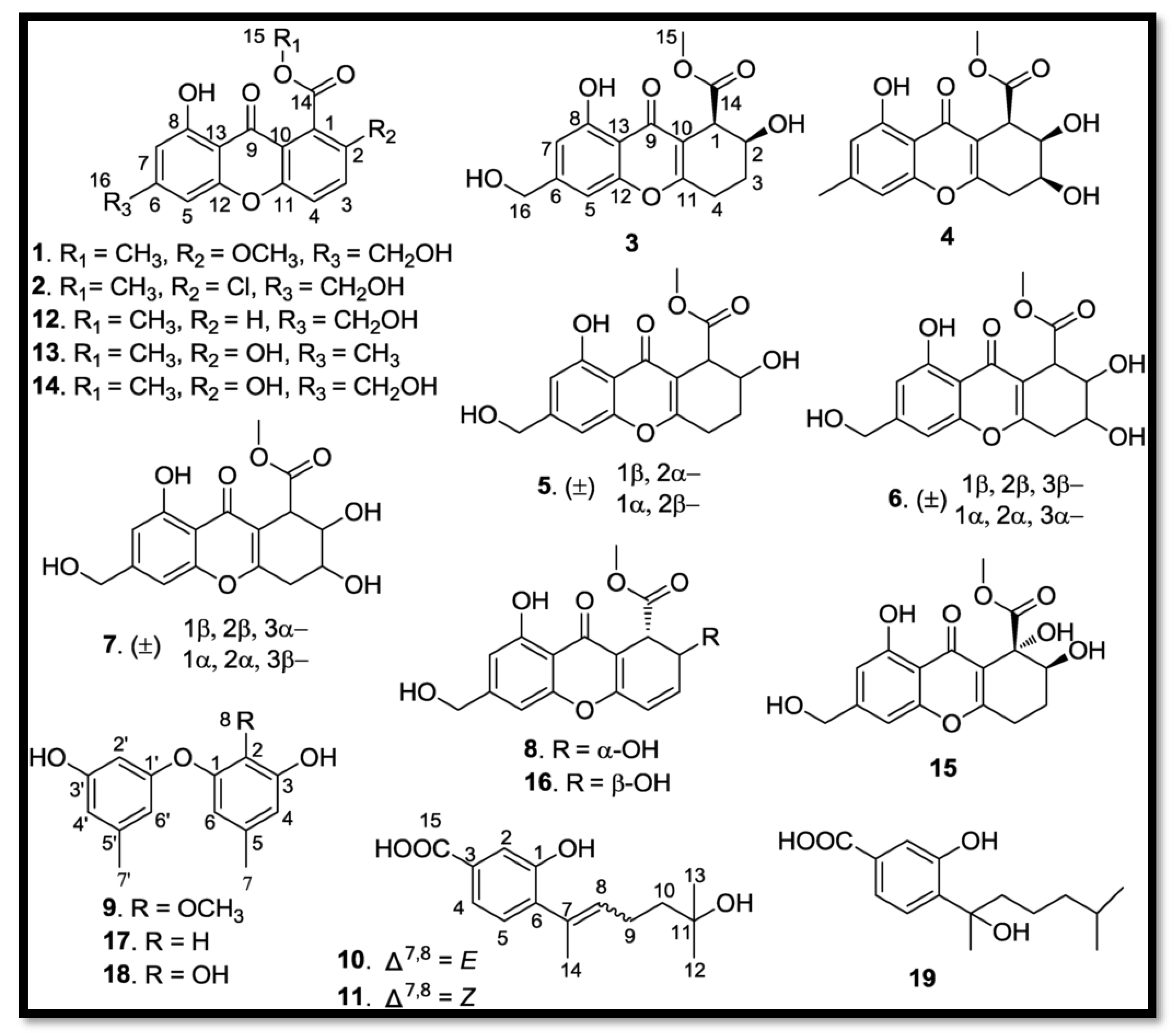
- Chromone: Engyodontium album DFFSCS021, isolated from deep marine sediments, synthesizes eight new chromones, named Engyodontiumones A-H (1-8) [82].
- -
- Phenolic derivatives and benzoates: Three new phenolic derivatives were isolated from Engyodontium album DFFSCS021. These include Engyodontiumone I (10) and J (11) as well as 2-methoxyl cordyol C (9) [82]
- -
- . Other polyketides: Molecules such as sydowinine A (12), pinselin (13), sydowinine B (14), aspergillusone B (15), AGI-B4 (16), diorcinol (17), cordyol C (18), and hydroxysydonic acid (19), also classified as polyketides, have been identified in Engyodontium album [82].
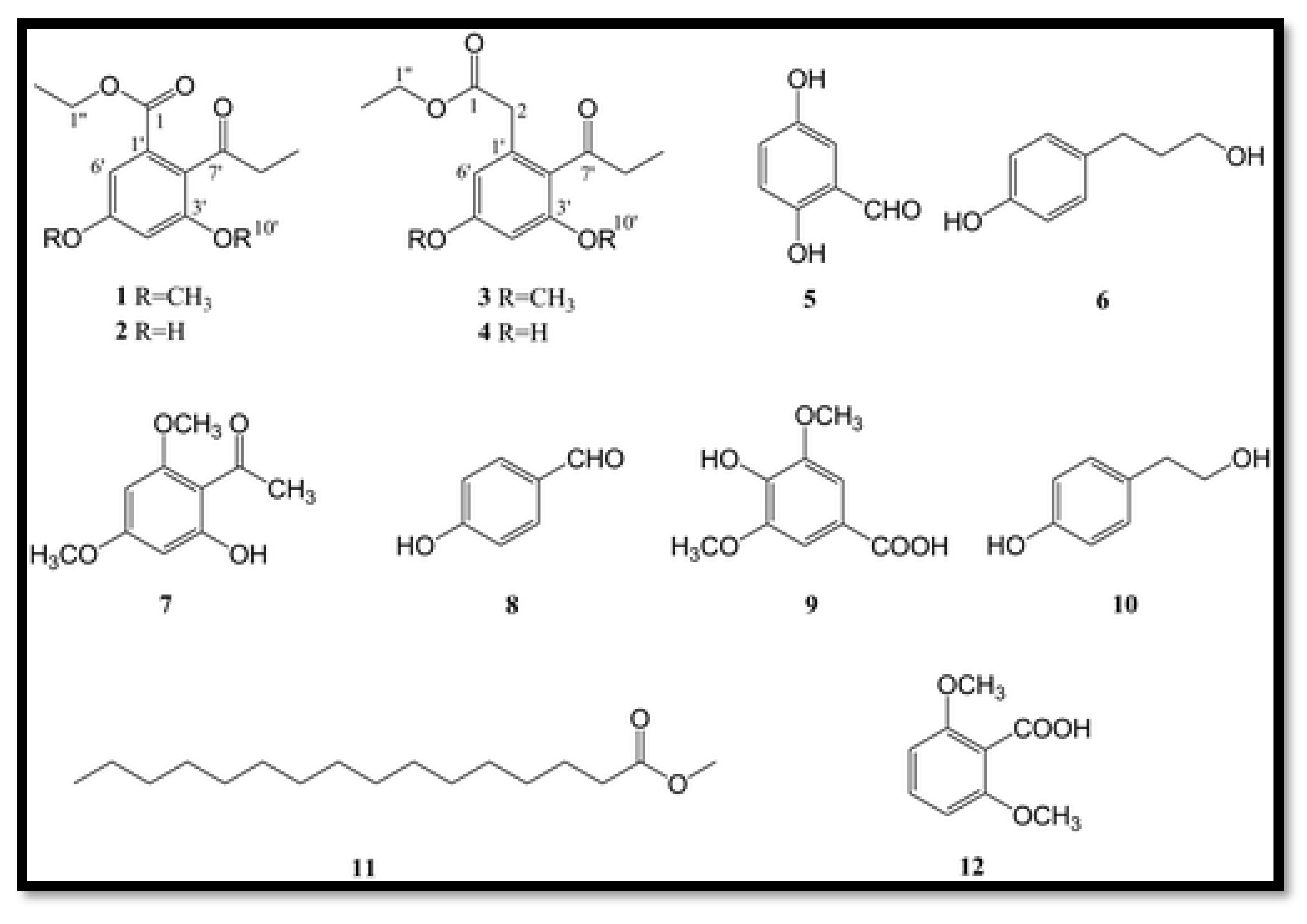
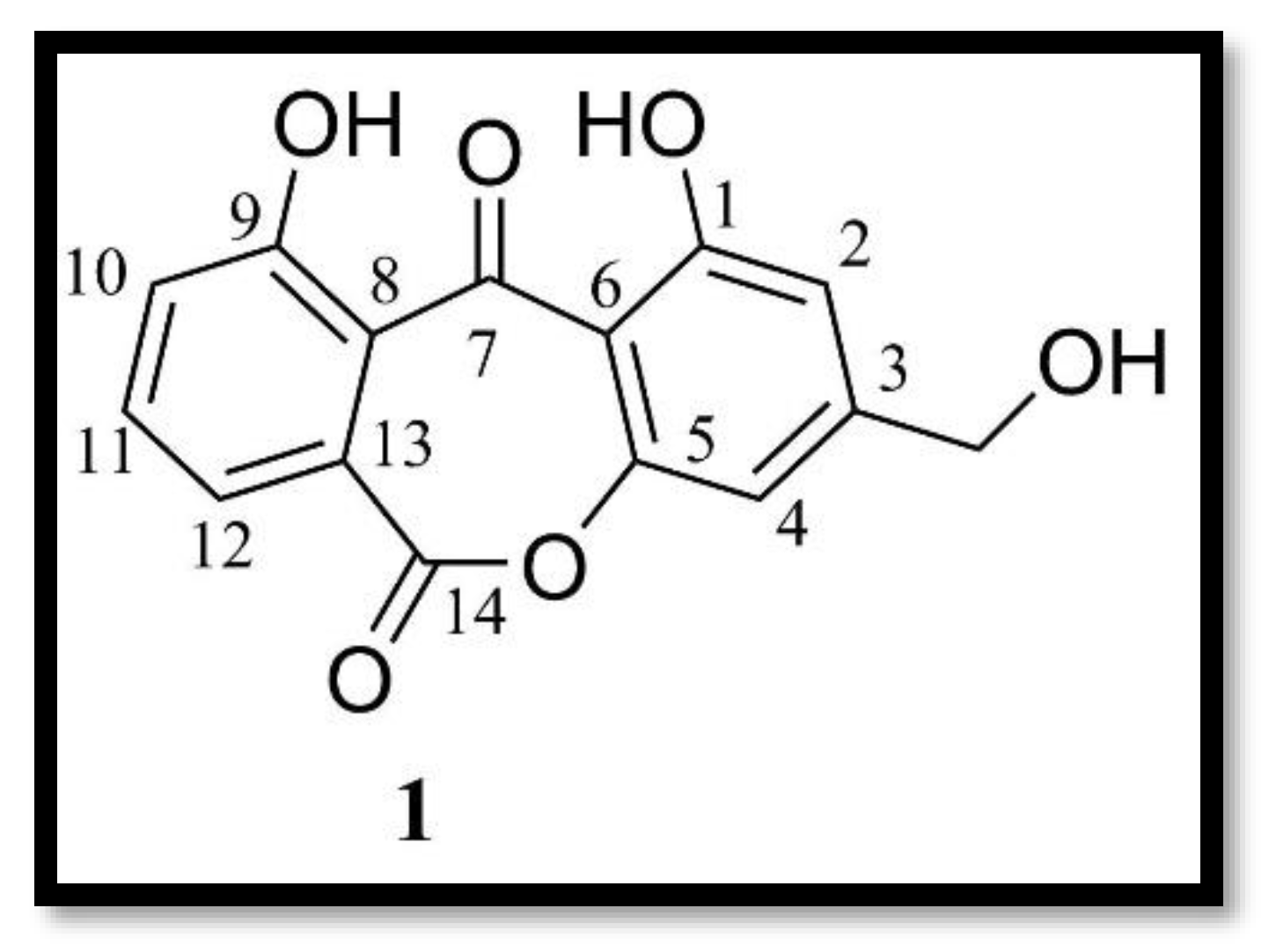
3.1.2. Terpene Compounds
- -
- Cytochalasin: Genomic analysis of Parengyodontium torokii predicted the biosynthesis of cytochalasine K, a terpenoid compound [29]. This metabolite was identified by LC-MS in a fungal extract, confirming the in silico predictions [29]. Cytochalasin compounds are known for their structural diversity and biological activities, particularly anti-cancer activities [84].
- -
- Other terpenes: Gene clusters for the production of other terpenes or related compounds such as squalestatin S1 have been identified in the species Parengyodontium torokii [29].
3.1.3. Alkaloids and Other Chemical Families
- -
- Indole alkaloids: A new indole alkaloid, 1-(4-hydroxybenzoyl)indole-3-carbaldehyde, was isolated from a strain of Engyodontium album derived from a marine sponge [50]. Alkaloids are a family of nitrogen-containing compounds known for their major pharmacological properties. They include, but are not limited to, morphine (analgesic), quinine (antimalarial), atropine (anticholinergic), etc.
- -
- Torrubiellin derivatives: A strain of P.album isolated from the leaves of Avicennia marina (in mangroves) produces new torrubiellin derivatives, named parengyomarin A (1) and B (2), in addition to the already known torrubiellin B (3) . Other compounds such as emodin and emodic acid have also been identified in extracts of this fungus [44].
- -
- Other compounds: Metabolomic analysis of P.torokii identified several other molecules, including cyclic peptides such as cyclo(L-Leu-L-Pro) and (3β,22E)-cyclo(L-Pro-L-Leu), fatty acids (6,9-octadecadienoic acid), and compounds such as cephalochromin and betulinan [29]. In silico predictions have also suggested the presence of equistetin, cephalosporin C, EQ-4, curcupallide-B, pyranonigrin E and dimethylcoprogen [29].
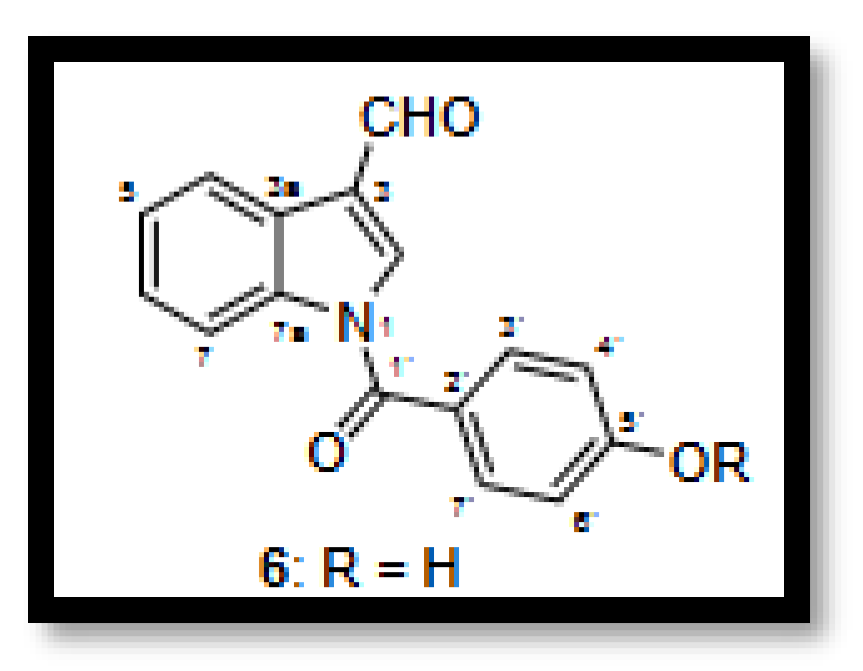
4. Potential appLications of Secondary Metabolites Produced by Parengyodontium spp.
- -
- Anticancer activities: Polyketides, such as Engyodontiumones, have shown selective cytotoxicity against the human histiocytic lymphoma cell line U937, with IC50 values of 4.9 and 8.8 µM for compounds 8 and 16, respectively [82]. Cytochalasin K, identified in P. torokii, has been shown to influence the final stages of mitosis and have a marked synergistic effect on cancer cells [29]. Cytotoxic polyketides (Xanthoquinodin JBIR-99) have been isolated from Engyodontium album [48]. Alternaphenol B2 from P. album showed selective inhibitory activity against mutant isocitrate dehydrogenase R132H (IDH1m), a relevant target for cancer treatment, with an IC50 of 41.9 µM [46].
- -
- Antibacterial activities: Several metabolites exhibited antibacterial properties. Compounds 8, 15, and 16 from Engyodontium album showed moderate antibacterial activity against Escherichia coli and Bacillus subtilis [82]. A phenylacetate derivative (compound 3) from Engyodontium album exhibited inhibitory activity against methicillin-resistant Staphylococcus aureus (MRSA) and Vibrio vulnificus, with MICs of 7.8 and 15.6 µg/mL, respectively [52]. Torrubielline derivatives have also demonstrated antibacterial activities [44]. Fungal mycelium extracts possess antimicrobial properties, with superior efficacy against Gram-positive bacteria [47].
- -
- Antilaryngeal activities: Compound 15, a polyketide from Engyodontium album DFFSCS021, showed potent antilaryngeal activity against the establishment of barnacle larvae (Balanus amphitrite) [82]. This property suggests potential for the development of biofoulants.
- -
- Enzymes and other applications: Genomic analyses of P. torokii have revealed the presence of enzyme families such as GH33 glycosyl hydrolases (sialidases) and GT20 and GT34 glycosyltransferases. These enzymes may have biotechnological applications, particularly for the modification of glycoconjugates or the biosynthesis of disaccharides and oligosaccharides [29]. In addition, the genus Parengyodontium is of interest in bioremediation, as evidenced by P. album, which is capable of biodegrading certain synthetic plastics such as polyethylene [42]. This result suggests the presence of enzymes such as laccases, oxidases, and peroxidases [42]. Laccases are multi-copper oxidases widely found in fungi, plants and bacteria. Fungal laccases are particularly valued because they oxidize a wide range of phenolic and non-phenolic substrates (often with redox mediators) while reducing O2 to H2O, enabling applications ranging from lignin modification to green synthesis and pollutant removal [85,86]. Peroxydases, including lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP), and dye-decolorizing peroxidases (DyPs) are heme enzymes that use H2O2 to attack lignin and recalcitrant aromatic compounds. Recent work highlights engineered VPs, MnP-mediated oxidation via Mn3+ chelates, and DyP diversity across fungi for lignin/dye transformation [87,88,89]. Oxidases generate H2O2 from O2. An example is glucose oxidase, which oxidizes β-D-glucose to D-glucono-δ-lactone and H2O2 and remains central in biosensors and food applications [90,91,92]. In white-rot systems, aryl-alcohol oxidase supplies H2O2 to lignilolytic peroxidases and can also act as a quinone reductase, enhancing the degradation of lignin by peroxidases [93,94].
| Anticancer | Antibacterial | Antilaryngeal | Enzymes & Bioremediation |
|---|---|---|---|
| Polyketides (Engyodontiumones): selective cytotoxicity (U937) [82] Cytochalasin K in P. torokii: impacts late mitosis; synergy on cancer cells [29] Cytotoxic polyketides: Xanthoquinodin JBIR-99 [48] Alternaphenol B2 (P. album): IDH1 R132H inhibitor [46] |
E.album compounds 8, 15, 16: moderate activity vs E. coli & B. subtilis [82] Phenylacetate derivative: active vs MRSA & V. vulnificus [52] Torrubielline derivatives [44] Fungal mycelial extracts [47] |
Compound 15 (E. album DFFSCS021): anti-settlement of Balanus Amphitrite (Potential for biofouling control) [82] |
P. torokii genomics: GH33 sialidases; GT20/GT34 glycosyltransferases [29] P. album biodegrades UV-pretreated polyethylene [42] |
5. Discussion: Perspectives, Limitations and Futures Directions
6. Conclusions
Funding
Conflicts of Interest
References
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 4.6–01. [Google Scholar] [CrossRef]
- Atli, B.; Ozcakir, B.; Isik, B.; Mursaliyeva, V.; Mammadov, R. Secondary Metabolites in Fungi. Nat. Prod. Biotechnol. 2022, 2. [Google Scholar] [CrossRef]
- Demain, A.L.; Fang, A. The Natural Functions of Secondary Metabolites. In History of Modern Biotechnology I; Fiechter, A., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2000; Vol. 69, pp. 1–39; ISBN 978-3-540-67793-2. [Google Scholar]
- Avalos, J.; Limón, M.C. Fungal Secondary Metabolism. Encyclopedia 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.-L. Metabolite Induction via Microorganism Co-Culture: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary Metabolites of Fungi from Marine Habitats. Nat. Prod. Rep. 2011, 28, 290. [Google Scholar] [CrossRef] [PubMed]
- Kempken, F. Marine Fungi: A Treasure Trove of Novel Natural Products and for Biological Discovery. PLOS Pathog. 2023, 19, e1011624. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic Fungi: A Reservoir of Antibacterials. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of Fungal Secondary Metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.-H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A Phylogenetically-Based Nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-B.; Wang, Y.; Fan, Q.; Duan, D.-E.; Zhang, G.-D.; Dai, R.-Q.; Dai, Y.-D.; Zeng, W.-B.; Chen, Z.-H.; Li, D.-D.; et al. Multigene Phylogeny of the Family Cordycipitaceae (Hypocreales): New Taxa and the New Systematic Position of the Chinese Cordycipitoid Fungus Paecilomyces Hepiali. Fungal Divers. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Khonsanit, A.; Thanakitpipattana, D.; Mongkolsamrit, S.; Kobmoo, N.; Phosrithong, N.; Samson, R.A.; Crous, P.W.; Luangsa-ard, J.J. A Phylogenetic Assessment of Akanthomyces Sensu Lato in Cordycipitaceae ( Hypocreales, Sordariomycetes ): Introduction of New Genera, and the Resurrection of Lecanicillium. Fungal Syst. Evol. 2024, 14, 271–306. [Google Scholar] [CrossRef]
- Bu, J.; Wei, D.-P.; Liu, Z.-H.; Yang, Y.; Liu, Z.-L.; Kang, J.-C.; Peng, X.-C.; Xie, S.-W.; Zhang, H.-G.; He, Z.-J.; et al. Molecular Phylogeny and Morphology Reveal Four Novel Species in Cordycipitaceae in China. MycoKeys 2025, 116, 91–124. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Noisripoom, W.; Tasanathai, K.; Kobmoo, N.; Thanakitpipattana, D.; Khonsanit, A.; Petcharad, B.; Sakolrak, B.; Himaman, W. Comprehensive Treatise of Hevansia and Three New Genera Jenniferia, Parahevansia and Polystromomyces on Spiders in Cordycipitaceae from Thailand. MycoKeys 2022, 91, 113–149. [Google Scholar] [CrossRef]
- Kobmoo, N.; Tasanathai, K.; Araújo, J.P.M.; Noisripoom, W.; Thanakitpipattana, D.; Mongkolsamrit, S.; Himaman, W.; Houbraken, J.; Luangsa-Ard, J.J. New Mycoparasitic Species in the Genera Niveomyces and Pseudoniveomyces Gen. Nov. ( Hypocreales : Cordycipitaceae ), with Sporothrix-like Asexual Morphs, from Thailand. Fungal Syst. Evol. 2023, 12, 91–110. [Google Scholar] [CrossRef]
- Chen, W.-H.; Li, D.; Shu, H.-L.; Liang, J.-D.; Zhao, J.-H.; Tian, W.-Y.; Han, Y.-F. Four New Araneogenous Species and a New Genus in Hypocreales (Clavicipitaceae, Cordycipitaceae) from the Karst Region of China. MycoKeys 2025, 112, 335–359. [Google Scholar] [CrossRef]
- Dong, Q.-Y.; Wang, Y.; Wang, Z.-Q.; Tang, D.-X.; Zhao, Z.-Y.; Wu, H.-J.; Yu, H. Morphology and Phylogeny Reveal Five Novel Species in the Genus Cordyceps (Cordycipitaceae, Hypocreales) From Yunnan, China. Front. Microbiol. 2022, 13, 846909. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Dong, Q.; Fan, Q.; Dao, V.-M.; Yu, H. Morphological and Phylogenetic Characterization Reveals Five New Species of Samsoniella (Cordycipitaceae, Hypocreales). J. Fungi 2022, 8, 747. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.-C.; Wu, L.-X.; Wang, Y.; Xu, A.; Lin, W.-F. Blackwellomyces Kaihuaensis and Metarhizium Putuoense (Hypocreales), Two New Entomogenous Fungi from Subtropical Forests in Zhejiang Province, Eastern China. Forests 2023, 14, 2333. [Google Scholar] [CrossRef]
- Sung, G.-H.; Hywel-Jones, N.L.; Sung, J.-M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic Classification of Cordyceps and the Clavicipitaceous Fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Aini, A.N.; Mongkolsamrit, S.; Wijanarka, W.; Thanakitpipattana, D.; Luangsa-ard, J.J.; Budiharjo, A. Diversity of Akanthomyces on Moths (Lepidoptera) in Thailand. MycoKeys 2020, 71, 1–22. [Google Scholar] [CrossRef]
- Chen, M.-J.; Wang, T.; Lin, Y.; Huang, B. Morphological and Molecular Analyses Reveal Two New Species of Gibellula from China. MycoKeys 2022, 90, 53–69. [Google Scholar] [CrossRef]
- Mendes-Pereira, T.; De Araújo, J.P.M.; Kloss, T.G.; Costa-Rezende, D.H.; De Carvalho, D.S.; Góes-Neto, A. Disentangling the Taxonomy, Systematics, and Life History of the Spider-Parasitic Fungus Gibellula (Cordycipitaceae, Hypocreales). J. Fungi 2023, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Q.; Wang, D.; Zou, W.-Q.; Tang, D.-X.; Hongthong, P.; Yu, H. Species Diversity and Virulence Potential of the Beauveria Bassiana Complex and Beauveria Scarabaeidicola Complex. Front. Microbiol. 2022, 13, 841604. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Pell, J.K. Fungal Entomopathogens: New Insights on Their Ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Humber, R.A. Identification of Entomopathogenic Fungi. In Manual of Techniques in Invertebrate Pathology; Elsevier, 2012; pp. 151–187 ISBN 978-0-12-386899-2.
- Parker, C.W.; Teixeira, M. de M.; Singh, N.K.; Raja, H.A.; Cank, K.B.; Spigolon, G.; Oberlies, N.H.; Barker, B.M.; Stajich, J.E.; Mason, C.E.; et al. Genomic Characterization of Parengyodontium Torokii Sp. Nov., a Biofilm-Forming Fungus Isolated from Mars 2020 Assembly Facility. J. Fungi Basel Switz. 2022, 8, 66. [Google Scholar] [CrossRef]
- Sung, G.-H.; Spatafora, J.W.; Zare, R.; Hodge, K.T.; Gams, W. A Revision of Verticillium Sect. Prostrata. II. Phylogenetic Analyses of SSU and LSU Nuclear rDNA Sequences from Anamorphs and Teleomorphs of the Clavicipitaceae. Nova Hedwig. 2001, 72, 311–328. [Google Scholar] [CrossRef]
- Wei, D.-P.; Wanasinghe, D.N.; Hyde, K.D.; Mortimer, P.E.; Xu, J.; Xiao, Y.-P.; Bhunjun, C.S.; To-anun, C. The Genus Simplicillium. MycoKeys 2019, 60, 69–92. [Google Scholar] [CrossRef]
- Thanakitpipattana, D.; Mongkolsamrit, S.; Khonsanit, A.; Himaman, W.; Luangsa-ard, J.J.; Pornputtapong, N. Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus Spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand. J. Fungi 2022, 8, 516. [Google Scholar] [CrossRef]
- Tsang, C.-C.; Chan, J.F.W.; Pong, W.-M.; Chen, J.H.K.; Ngan, A.H.Y.; Cheung, M.; Lai, C.K.C.; Tsang, D.N.C.; Lau, S.K.P.; Woo, P.C.Y. Cutaneous Hyalohyphomycosis Due to Parengyodontium Album Gen. et Comb. Nov. Med. Mycol. 2016, 54, 699–713. [Google Scholar] [CrossRef]
- Leplat, J.; François, A.; Bousta, F. Diversity of Parengyodontium Spp. Strains Isolated from the Cultural Heritage Environment: Phylogenetic Diversity, Phenotypical Diversity, and Occurrence. Mycologia 2022, 114, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Belfiori, B.; Rubini, A.; Riccioni, C. Diversity of Endophytic and Pathogenic Fungi of Saffron (Crocus Sativus) Plants from Cultivation Sites in Italy. Diversity 2021, 13, 535. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.-Y.; You, X.-L.; Li, Y.-H. Diversity of Endophytic Fungi from Roots of Panax Ginseng and Their Saponin Yield Capacities. SpringerPlus 2013, 2, 107. [Google Scholar] [CrossRef]
- Lucero, M.E.; Barrow, J.R.; Osuna, P.; Reyes, I. Plant–Fungal Interactions in Arid and Semi-Arid Ecosystems: Large-Scale Impacts from Microscale Processes. J. Arid Environ. 2006, 65, 276–284. [Google Scholar] [CrossRef]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Kachuei, R.; Emami, M.; Naeimi, B.; Diba, K. Isolation of Keratinophilic Fungi from Soil in Isfahan Province, Iran. J. Mycol. Médicale 2012, 22, 8–13. [Google Scholar] [CrossRef]
- Pindi, P.K. Diversity of Fungi at Various Depths of Marine Water. Res. Biotechnol. 2012, 3. [Google Scholar]
- Lefkowitz, R.J. Identification of Adenylate Cyclase-Coupled Beta-Adrenergic Receptors with Radiolabeled Beta-Adrenergic Antagonists. Biochem. Pharmacol. 1975, 24, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Vielfaure, H.; Polerecky, L.; Kienhuis, M.V.M.; Van Der Meer, M.T.J.; Pflüger, T.; Egger, M.; Niemann, H. Biodegradation of Polyethylene by the Marine Fungus Parengyodontium Album. Sci. Total Environ. 2024, 934, 172819. [Google Scholar] [CrossRef]
- Banchi, E.; Manna, V.; Muggia, L.; Celussi, M. Marine Fungal Diversity and Dynamics in the Gulf of Trieste (Northern Adriatic Sea). Microb. Ecol. 2024, 87, 78. [Google Scholar] [CrossRef]
- Liu, S.; Mao, Y.; Lu, H.; Zhao, Y.; Bilal, M.; Proksch, P.; Hu, P. Two New Torrubiellin Derivatives from the Mangrove Endophytic Fungus Parengyodontium Album. Phytochem. Lett. 2021, 46, 149–152. [Google Scholar] [CrossRef]
- Teixeira, M.D.M.; Muszewska, A.; Travis, J.; Moreno, L.F.; Ahmed, S.; Roe, C.; Mead, H.; Steczkiewicz, K.; Lemmer, D.; De Hoog, S.; et al. Genomic Characterization of Parengyodontium Americanum Sp. Nov. Fungal Genet. Biol. 2020, 138, 103351. [Google Scholar] [CrossRef]
- Li, X.; Shen, W.; Li, G.; Song, Y.; Lu, X.; Wong, N.-K.; Yan, Y. Alternaphenol B2, a New IDH1 Inhibitor from the Coral-Derived Fungus Parengyodontium Album SCSIO SX7W11. Nat. Prod. Res. 2023, 1–7. [Google Scholar] [CrossRef]
- Wong Chin, J.M.; Puchooa, D.; Bahorun, T.; Jeewon, R. Antimicrobial Properties of Marine Fungi from Sponges and Brown Algae of Mauritius. Mycology 2021, 12, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Eugenio, G.D.; Rebollar-Ramos, D.; González, M.D.C.; Raja, H.; Mata, R.; Carcache De Blanco, E.J. Apoptotic Activity of Xanthoquinodin JBIR-99, from Parengyodontium Album MEXU 30054, in PC-3 Human Prostate Cancer Cells. Chem. Biol. Interact. 2019, 311, 108798. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiese, J.; Wenzel-Storjohann, A.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Engyodontochones, Antibiotic Polyketides from the Marine Fungus Engyodontium Album Strain LF069. Chem. – Eur. J. 2016, 22, 7452–7462. [Google Scholar] [CrossRef]
- Meng, L.-H.; Chen, H.-Q.; Form, I.; Konuklugil, B.; Proksch, P.; Wang, B.-G. New Chromone, Isocoumarin, and Indole Alkaloid Derivatives from Three Sponge-Derived Fungal Strains. Nat. Prod. Commun. 2016, 11, 1293–1296. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhang, L.; Liu, Z.; Liu, W.; Xu, H.; Zhang, Q.; Zhang, H.; Yan, Y.; Liu, Z.; et al. Structures and Absolute Configurations of Phomalones from the Coral-Associated Fungus Parengyodontium Album Sp. SCSIO 40430. Org. Biomol. Chem. 2021, 19, 6030–6037. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Luo, Z.; Wang, W.; Chen, J. Two New Benzoate Derivatives and One New Phenylacetate Derivative from a Marine-Derived Fungus Engyodontium Album. Nat. Prod. Res. 2017, 31, 758–764. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-ard, J.J. Disentangling Cryptic Species with Isaria-like Morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Tasanathai, K.; Khonsanit, A.; Thanakitpipattana, D.; Himaman, W.; Kobmoo, N.; Luangsa-ard, J.J. Molecular Phylogeny and Morphology Reveal Cryptic Species in Blackwellomyces and Cordyceps (Cordycipitaceae) from Thailand. Mycol. Prog. 2020, 19, 957–983. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, W.; Feng, M.-G. Secondary Metabolites from Hypocrealean Entomopathogenic Fungi. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef] [PubMed]
- Berestetskiy, A.O.; Hu, X. The Chemical Ecology of Fungi That Attack Insects. Microorganisms 2021, 9, 1379. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, M.-G.; Keller, N.P. Genomics-Driven Discovery of Natural Products in Filamentous Fungi. Nat. Prod. Rep. 2020, 37, 1164–1180. [Google Scholar] [CrossRef]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Espinosa-Artiles, P.; Gunatilaka, A.A.L.; Stock, S.P.; Molnár, I. Biosynthesis of the Cyclooligomer Depsipeptide Bassianolide, an Insecticidal Virulence Factor of Beauveria Bassiana. Fungal Genet. Biol. 2009, 46, 353–364. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, X.; Keyhani, N.O.; Tang, G.; Pei, Y.; Zhang, W.; Pei, Y.; Xiao, Y.; Fang, W.; Wang, C. Regulatory Cascade and Biological Activity of Beauveria Bassiana Oosporein That Limits Bacterial Growth after Host Death. Proc. Natl. Acad. Sci. USA 2017, 114, E1578–E1586. [Google Scholar] [CrossRef]
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Oosporein Is Required for Fungal Virulence by Evading Host Immunity to Facilitate Multiplication in Insects. Proc. Natl. Acad. Sci. USA 2015, 112, E109–E118. [Google Scholar] [CrossRef]
- Wang, H.; Fang, W.; Wang, C. The Toxins of Beauveria Bassiana and the Strategies for Enhancing Their Virulence to Insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Raghunandan, B.L.; Dave, A.; Baria, P.R.; Manjari Applications of Bioactive Compounds from Fungal Entomopathogens. In Entomopathogenic Fungi; Deshmukh, S.K., Sridhar, K.R., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 453–478 ISBN 978-981-97-5990-3.
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Stock, S.P.; Molnár, I. Biosynthesis of the Cyclooligomer Depsipeptide Beauvericin, a Virulence Factor of the Entomopathogenic Fungus Beauveria Bassiana. Chem. Biol. 2008, 15, 898–907. [Google Scholar] [CrossRef]
- Safavi, S.A. In Vitro and In Vivo Induction, and Characterization of Beauvericin Isolated from Beauveria Bassiana and Its Bioassay on Galleria Mellonella Larvae. mdrsjrns 2013, 15, 1–10. [Google Scholar]
- Sánchez-Gómez, T.; González-Llamazares, Á.; Sánchez-Hernández, M.P.; et al. Nematicidal Effect of Beauveria Species and the Mycotoxin Beauvericin against Bursaphelenchus Xylophilus. Front. For. Glob. Change 2023, 6, 1229456. [Google Scholar] [CrossRef]
- Qu, S.-L.; Li, S.-S.; Li, D.; Zhao, P.-J. Metabolites and Their Bioactivities from the Genus Cordyceps. Microorganisms 2022, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Suparmin, A.; Yoon, J.-H.; Lee, T.-S. New Insights into Cordycepin Biosynthesis and Regulation in Cordyceps Militaris. PLOS ONE 2017, 12, e0187052. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Zhang, Y.; Wang, C. Advances in the Chemical Biology and Biosynthesis of Compounds from Cordyceps: The Case of Cordyceps Militaris. Front. Microbiol. 2024, 15, 1386855. [Google Scholar] [CrossRef]
- Li, P.; Xu, X.; Zhang, Y. Cordyceps Militaris: A Promising Model Organism for Cordycepin Biosynthesis and Applications. Front. Chem. Eng. 2024, 6, 1446454. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Song, J. Current Progress Regarding Cordyceps Militaris as a Macromycete Medicine. Appl. Sci. 2024, 14, 4610. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhang, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, C. Genome Sequence of the Insect Pathogenic Fungus Cordyceps Militaris, a Valued Traditional Chinese Medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.-Q.; Luo, R.; Souvanhnachit, S.; Thanarut, C.; Dao, V.M.; Yu, H. Species Diversity and Major Host/Substrate Associations of the Genus Akanthomyces (Hypocreales, Cordycipitaceae). MycoKeys 2024, 101, 113–141. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Zhi, J.-R.; Qu, J.-J.; Zou, X. Estimated Divergence Times of Lecanicillium in the Family Cordycipitaceae Provide Insights into the Attribution of Lecanicillium. Front. Microbiol. 2022, 13, 859886. [Google Scholar] [CrossRef]
- Helaly, S.E.; Kuephadungphan, W.; Phainuphong, P.; Ibrahim, M.A.A.; Tasanathai, K.; Mongkolsamrit, S.; Luangsa-ard, J.J.; Phongpaichit, S.; Rukachaisirikul, V.; Stadler, M. Pigmentosins from Gibellula Sp. as Antibiofilm Agents and a New Glycosylated Asperfuran from Cordyceps Javanica. Beilstein J. Org. Chem. 2019, 15, 2968–2981. [Google Scholar] [CrossRef]
- Yoneyama, T.; Iguchi, M.; Yoshii, K.; Elshamy, A.I.; Ban, S.; Noji, M.; Umeyama, A. Xanthone Glucoside from an Insect Pathogenic Fungus Conoideocrella Luteorostrata NBRC106950. Nat. Prod. Res. 2021, 36, 6349–6353. [Google Scholar] [CrossRef]
- Phutthacharoen, K.; Llanos-López, N.A.; Toshe, R.; Noisripoom, W.; Khonsanit, A.; Luangsa-ard, J.J.; Hyde, K.D.; Ebada, S.S.; Stadler, M. Bioactive Bioxanthracene and Cyclodepsipeptides from the Entomopathogenic Fungus Blackwellomyces Roseostromatus BCC56290. Antibiotics 2024, 13, 585. [Google Scholar] [CrossRef]
- Wei, D.; Wanasinghe, D.N.; Hyde, K.D.; Mortimer, P.E.; Xu, J.-C.; Xiao, Y.-P.; Bhunjun, C.S.; To-anun, C. The Genus Simplicillium. MycoKeys 2019, 60, 69–92. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, L.; Ren, X.-X.; Zhao, J.-H.; Han, F.-Y.; Liang, Z.-Q. Taxonomic and Phylogenetic Characterizations Reveal Four New Species of Simplicillium (Cordycipitaceae, Hypocreales) from China. Sci. Rep. 2021, 11, 14893. [Google Scholar] [CrossRef]
- Zhu, M.; Yin, F.; Luo, Q.; et al. Deciphering the Genome of Simplicillium Aogashimaense to Understand Its Mycoparasitic Behavior against Wheat Powdery Mildew. Phytopathol. Res. 2022, 4, 21. [Google Scholar] [CrossRef]
- Baró Robaina, Y.; González Marrero, I.; Lorenzo Nicao, M.E.; et al. First Description of Simplicillium Lanosoniveum, a Potential Antagonist of the Coffee Leaf Rust from Cuba. Appl. Microbiol. 2024, 4, 275–283. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic Polyketides from the Deep-Sea-Derived Fungus Engyodontium Album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef]
- A Plethora of Polyketides: Structures, Biological Activities, and Enzymes. ACS Symposium Series; American Chemical Society: Washington, DC, 2007; pp. 2–14; ISBN 978-0-8412-3978-4. [Google Scholar]
- Formánek, B.; Dupommier, D.; Volfová, T.; Rimpelová, S.; Škarková, A.; Herciková, J.; Rösel, D.; Brábek, J.; Perlíková, P. Synthesis and Migrastatic Activity of Cytochalasin Analogues Lacking a Macrocyclic Moiety. RSC Med. Chem. 2024, 15, 322–343. [Google Scholar] [CrossRef]
- Aza, P.; Camarero, S. Fungal Laccases: Fundamentals, Engineering and Classification Update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef]
- Brugnari, T.; Braga, D.M.; Dos Santos, C.S.A.; Torres, B.H.C.; Modkovski, T.A.; Haminiuk, C.W.I.; Maciel, G.M. Laccases as Green and Versatile Biocatalysts: From Lab to Enzyme Market—an Overview. Bioresour. Bioprocess. 2021, 8, 131. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, P.K. Biotechnological Applications of Manganese Peroxidases for Sustainable Management. Front. Environ. Sci. 2022, 10, 875157. [Google Scholar] [CrossRef]
- Adamo, M.; Comtet-Marre, S.; Büttner, E.; Kellner, H.; Luis, P.; Vallon, L.; Prego, R.; Hofrichter, M.; Girlanda, M.; Peyret, P.; et al. Fungal Dye-Decolorizing Peroxidase Diversity: Roles in Either Intra- or Extracellular Processes. Appl. Microbiol. Biotechnol. 2022, 106, 2993–3007. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Mindel, V.; Garcia-Ruiz, E.; Weinstein, J.J.; Alcalde, M.; Fleishman, S.J. Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences. J. Am. Chem. Soc. 2022, 144, 3564–3571. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- Guoqiang, G.; Liang, Q.; Yani, Z.; Pengyun, W.; Fanzhuo, K.; Yuyang, Z.; Zhiyuan, L.; Xing, N.; Xue, Z.; Qiongya, L.; et al. Recent Advances in Glucose Monitoring Utilizing Oxidase Electrochemical Biosensors Integrating Carbon-Based Nanomaterials and Smart Enzyme Design. Front. Chem. 2025, 13, 1591302. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Qi, Y.; Hu, Y.; Ni, Z.; Li, C. Update Application of Enzyme in Food Processing, Preservation, and Detection. Food Bioeng. 2024, 3, 380–394. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Koschorreck, K. Pecularities and Applications of Aryl-Alcohol Oxidases from Fungi. Appl. Microbiol. Biotechnol. 2021, 105, 4111–4126. [Google Scholar] [CrossRef]
- Ferreira, P.; Carro, J.; Balcells, B.; Martínez, A.T.; Serrano, A. Expanding the Physiological Role of Aryl-Alcohol Flavooxidases as Quinone Reductases. Appl. Environ. Microbiol. 2023, 89, e01844–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).