Submitted:
28 September 2025
Posted:
29 September 2025
You are already at the latest version
Abstract
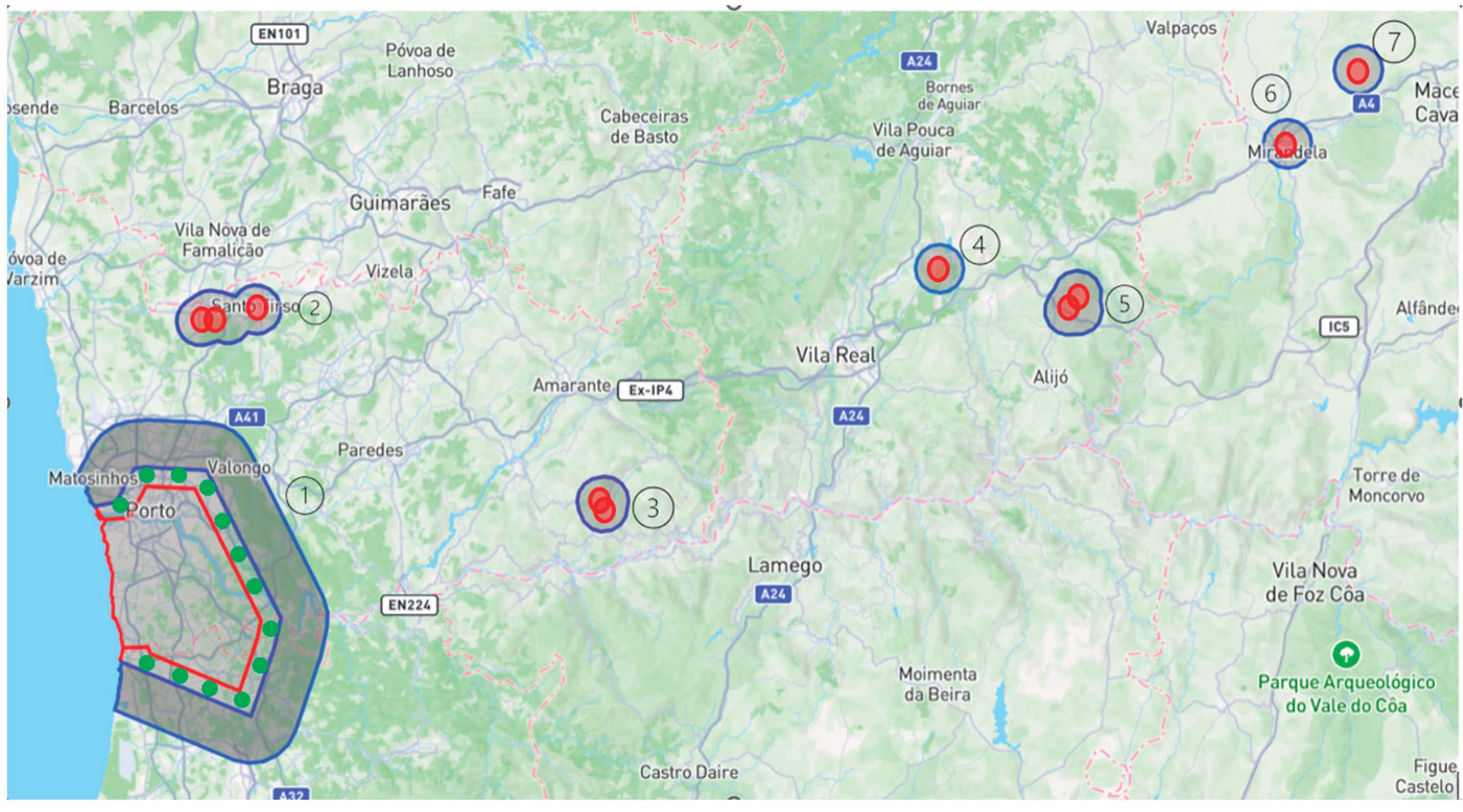
Xylella fastidiosa was first detected in Portugal in 2019 in Lavandula dentata. In response, the national plant health authorities promptly established a Demarcated Zone in the affected area and implemented a series of eradication and control measures, including the systematic removal and destruction of infected and host plants. This study analyzes the economic and operational impacts of these eradication efforts in the northern region of Portugal, with a focus on Demarcated Zones such as the Porto Metropolitan Area, Sabrosa, Alijó, Baião, Mirandela, Mirandela II, and Bougado between 2019 and June 2023. During this period, about 412,500 plants were uprooted. The majority were Pteridium aquilinum (bracken fern), with 360,324 individuals (87.3%), reflecting its wide distribution and the large area affected. Olea europaea (olive tree) was the second most common species removed, with 7,024 plants (1.7%), highlighting its economic relevance. Other notable species included Quercus robur (3,511; 0.85%), Pelargonium graveolens (3,509; 0.85%), and Rosa spp. (1,106; 0.27%). Overall, destruction costs were estimated at about €1.04 million, with replanting costs of roughly €6.81 million. In parallel, prospection activities—conducted to detect early signs of infection and monitor disease spread—generated expenses of roughly €5.94 million. While prospecting represents a significant financial investment, the results show that it is considerably more cost-effective than large-scale eradication. Prospection enables early detection and containment, preventing the widespread destruction of vegetation and minimizing disruption to agricultural production, biodiversity, and local communities. Importantly, our findings reveal a sharp decline in confirmed cases in the initial outbreak area—the Porto Demarcated Zone—from 124 cases in 2019 to just 5 in 2023, indicating the effectiveness of the eradication and monitoring measures implemented. However, the presence of 20 active Demarcated Zones across the country as of 2023 highlights the continued risk of spread and the need for sustained vigilance. The complexity of managing Xylella fastidiosa across ecologically and logistically diverse territories justifies the high costs associated with surveillance and targeted interventions. This study reinforces the strategic value of prospection as a proactive and sustainable tool for plant health management. Effective surveillance requires the integration of advanced methodologies aligned with the phenological stages of host plants and the biological cycle of vectors. Targeting high-risk locations, optimizing sample numbers, ensuring diagnostic accuracy, and maintaining continuous training for field teams are critical for improving efficiency and reducing costs. Ultimately, the findings underscore the need to refine and adapt monitoring and eradication strategies to contain the pathogen, safeguard agricultural systems, and prevent Xylella fastidiosa from becoming endemic in Portugal.
Keywords:
1. Introduction
2. Methodology
2.1. Calculation of Prospecting Costs
2.2. Eradication Phase
- ✓
- Destruction of infected plants and others of the same species.
- ✓
- Destruction of all plants listed in Annex I and II of the EU Implementing Regulation (EU) 2020/1201.
- ✓
- Destruction of all host plants located within a 50 m radius of a confirmed infected plant, regardless of their testing status, in order to comply with eradication rules.
2.3. Price Collection Phase
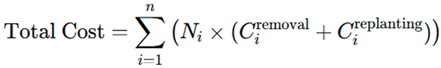
2.4. Assessing the Effectiveness of Eradication Measures
2.5. Data Collection, Organization, and Treatment Phase
3. Results
3.1. Plant Prospection Costs
3.2. Description of the Uprooted Species
3.3. Uprooting Costs by Species
3.4. Replacement Costs by Species
3.5. Assessing the Effectiveness of Eradication Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- K. Schneider, M. Mourits, W. van der Werf, and A. O. Lansink, “On consumer impact from Xylella fastidiosa subspecies pauca,” Ecological Economics, vol. 185, p. 107024, Jul. 2021. [CrossRef]
- K. Schneider et al., “Impact of Xylella fastidiosa subspecies pauca in European olives,” Proc Natl Acad Sci U S A, vol. 117, no. 17, pp. 9250–9259, Apr. 2020. [CrossRef]
- EPPO Bulletin, “PM 7/24 (4) Xylella fastidiosa,” EPPO Bulletin, vol. 49, no. 2, pp. 175–227, Aug. 2019. [CrossRef]
- N. W. Schaad, E. Postnikova, G. Lacy, M. Fatmi, and C. J. Chang, “Xylella fastidiosa subspecies: X. fastidiosa subsp piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov.,” Syst Appl Microbiol, vol. 27, no. 3, pp. 290–300, Jan. 2004. [CrossRef]
- N. W. Schaad, E. Postnikova, G. Lacy, M. Fatmi, and C. J. Chang, “Xylella fastidiosa subspecies: X. fastidiosa subsp. correction. fastidiosa correction. subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov,” Syst Appl Microbiol, vol. 27, no. 3, pp. 2004. [CrossRef]
- N. W. Schaad, E. Postnikova, G. Lacy, M. Fatmi, and C. J. Chang, “Xylella fastidiosa subspecies: X. fastidiosa subsp piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov.,” Syst Appl Microbiol, vol. 27, no. 6, p. 763, Dec. 2004. [CrossRef]
- E. L. Schuenzel, M. Scally, R. Stouthamer, and L. Nunney, “A Multigene Phylogenetic Study of Clonal Diversity and Divergence in North American Strains of the Plant Pathogen Xylella fastidiosa,” Appl Environ Microbiol, vol. 71, no. 7, p. 3832, Jul. 2005. [CrossRef]
- L. Nunney, E. L. Schuenzel, M. Scally, R. E. Bromley, and R. Stouthamerc, “Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry,” Appl Environ Microbiol, vol. 80, no. 10, pp. 3025–3033, 2014. [CrossRef]
- M. Frem et al., “The potential direct economic impact and private management costs of an invasive alien species: Xylella fastidiosa on Lebanese wine grapes,” NeoBiota 70: 43-67, vol. 70, pp. 43–67, Dec. 2021. [CrossRef]
- Alves De Souza, M. Aurélio Takita, A. Morais Do Amaral, • Helvécio, D. Coletta-Filho, and M. A. Machado, “Tree and Forestry Science and Biotechnology Citrus Responses to Xylella fastidiosa Infection, the Causal Agent of Citrus Variegated Chlorosis”, Accessed: Mar. 25, 2023. Online.. Available: www.fundecitrus.com.
- T. Cao, J. H. Connell, M. Wilhelm, and B. C. Kirkpatrick, “Influence of Inoculation Date on the Colonization of Xylella fastidiosa and the Persistence of Almond Leaf Scorch Disease Among Almond Cultivars,” https://doi.org/10.1094/PDIS-05-10-0327, vol. 95, no. 2, pp. 158–165, Jan. 2011. [CrossRef]
- D. C. Cook, R. W. Fraser, D. R. Paini, A. C. Warden, W. M. Lonsdale, and P. J. de Barro, “Biosecurity and Yield Improvement Technologies Are Strategic Complements in the Fight against Food Insecurity,” PLoS One, vol. 6, no. 10, p. e26084, Oct. 2011. [CrossRef]
- R. D. Zenni, F. Essl, E. García-Berthou, and S. M. McDermott, “The economic costs of biological invasions around the world,” NeoBiota 67: 1-9, vol. 67, pp. 1–9, Jul. 2021. [CrossRef]
- R. P. P. Almeida, L. De La Fuente, R. Koebnik, J. R. S. Lopes, S. Parnell, and H. Scherm, “Addressing the New Global Threat of Xylella fastidiosa,” Phytopathology, vol. 109, no. 2, pp. 172–174, Feb. 2019. [CrossRef]
- T. Loureiro et al., “Xylella fastidiosa: A Glimpse of the Portuguese Situation,” Microbiology Research 2023, Vol. 14, Pages 1568-1588, vol. 14, no. 4, pp. 1568–1588, Oct. 2023. [CrossRef]
- M. Saponari et al., “Infectivity and transmission of Xylellua fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy,” J Econ Entomol, vol. 107, no. 4, pp. 1316–1319, 2014. [CrossRef]
- S. Avosani, C. Tattoni, V. Mazzoni, and M. Ciolli, “Occupancy and detection of agricultural threats: The case of Philaenus spumarius, European vector of Xylella fastidiosa,” Agric Ecosyst Environ, vol. 324, p. 107707, Feb. 2022. [CrossRef]
- G. Pérez-Donoso, J. J. Lenhof, K. Pinney, and J. M. Labavitch, “Vessel embolism and tyloses in early stages of Pierce’s disease,” Aust J Grape Wine Res, vol. 22, no. 1, pp. 81–86, Feb. 2016. [CrossRef]
- Ingel et al., “Xylella fastidiosa causes transcriptional shifts that precede tylose formation and starch depletion in xylem,” Mol Plant Pathol, vol. 22, no. 2, pp. 175–188, Feb. 2021. [CrossRef]
- M. De Benedictis et al., “Vessel occlusion in three cultivars of Olea europaea naturally exposed to Xylella fastidiosa in open field,” Journal of Phytopathology, vol. 165, no. 9, pp. 589–594, Sep. 2017. [CrossRef]
- V. Montilon et al., “Xylella fastidiosa subsp. pauca ST53 exploits pit membranes of susceptible olive cultivars to spread systemically in the xylem,” Plant Pathol, vol. 72, no. 1, pp. 144–153, Jan. 2023. [CrossRef]
- P. S. Pereira, “Xylella fastidiosa - a new menace for Portuguese agriculture and forestry.,” Revista de Ciências Agrárias (Portugal), vol. 38, no. 2, pp. 149–154, 2015.
- DGAV, Plano de Contingência Xylella fastidiosa e seus vetores. 2022.
- D. Gibin, L. Pasinato, and A. Delbianco, “Update of the Xylella spp. host plant database – systematic literature search up to 31 December 2022,” EFSA Journal, vol. 21, no. 6, p. e08061, Jun. 2023. [CrossRef]
- C. Baccari, E. Antonova, and S. Lindow, “Biological control of Pierce’s disease of grape by an endophytic bacterium,” Phytopathology, vol. 109, no. 2, pp. 248–256, Feb. 2019. 2019. [CrossRef]
- T. Loureiro, L. Serra, Â. Martins, I. Cortez, and P. Poeta, “Xylella fastidiosa Dispersion on Vegetal Hosts in Demarcated Zones in the North Region of Portugal,” Microbiology Research 2024, Vol. 15, Pages 1050-1072, vol. 15, no. 3, pp. 1050–1072, Jun. 2024. [CrossRef]
- V. Cavalieri et al., “Update of the Xylella spp. host plant database – Systematic literature search up to 31 December 2024,” EFSA Journal, vol. 23, no. 7, Jul. 2025. [CrossRef]
- Paula, A. Cruz De Carvalho, A. Cruz De Carvalho Dn: C=pt, Title=subdiretora Geral, G. De Alimentação E Veterinária, P. De Almeida, and C. De Carvalho, “PLANO DE AÇÃO PARA ERRADICAÇÃO DE Xylella fastidiosa e controlo dos seus vetores ZONA DEMARCADA DA ÁREA METROPOLITANA DO PORTO Atualizado em fevereiro de 2022 Aprovado,” 2022.
- M. Godefroid, A. Cruaud, J.-C. Streito, J.-Y. Rasplus, and J.-P. Rossi, “Climate change and the potential distribution of Xylella fastidiosa in Europe,” bioRxiv, p. 289876, Mar. 2018. [CrossRef]
- C. Chen, C. H. Bock, and P. M. Brannen, “Novel Primers and Sampling for PCR Detection of Xylella fastidiosa in Peach,” Phytopathology, vol. 109, no. 2, pp. 307–317, Feb. 2019. [CrossRef]
- M. Román-Écija et al., “Two Xylella fastidiosa subsp. multiplex strains isolated from almond in Spain differ in plasmid content and virulence traits,” Phytopathology, Dec. 2022. [CrossRef]
- Cavalieri Vincenzo et al., “TRANSMISSION OF THE CODIRO STRAIN OF XYLELLA FASTIDIOSA BY DIFFERENT INSECT SPECIES,” XI European Congress of Entomology, 2018. [CrossRef]
- “Portal do INE.” Accessed: Mar. 18, 2024. Online.. Available: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000709&xlang=pt&contexto=bd&selTab=tab2.
- GPP, “Newsletter Azeite e Azeitona,” 2022, Accessed: Mar. 18, 2024. Online.. Available: www.gpp.pt/index.php/sima/sistema-de-informacao-de-mercados-agricolas-sima-.
- R. Scholten, L. Martinez Sanchez, A. Hornero, J. A. Navas-Cortes, P. J. Zarco-Tejada, and P. S. A. Beck, “Monitoring the impact of Xylella on Apulia’s olive orchards using Sentinel-2 satellite data and aerial photographs Second European conference on Xylella fastidiosa,” 2019.
- K. P. Tumber, J. M. Alston, and K. B. Fuller, “Pierce’s disease costs California $104 million per year,” Calif Agric (Berkeley), vol. 68, no. 1–2, pp. 20–29, 2014. [CrossRef]
- B. Sánchez, O. E. Mosbach-Schulz Rodríguez Cerezo, B. J. Hurle, and S. I. Embodas, “Estimating the economic, social and environmental impacts of EU priority pests: a joint EFSA and JRC project with a focus on Xylella fastidiosa”.
- G. Cardone et al., “Potential socio-economic impact of xylella fastidiosa in the near east and north africa (Nena): Risk of introduction and spread, risk perception and socio-economic effects,” New Medit, vol. 20, no. 2, pp. 27–52, 2021. [CrossRef]
- Luvisi, F. Nicolì, and L. De Bellis, “Sustainable Management of Plant Quarantine Pests: The Case of Olive Quick Decline Syndrome,” Sustainability 2017, Vol. 9, Page 659, vol. 9, no. 4, p. 659, Apr. 2017. [CrossRef]
- “Árvores de Fruto, Portal do INE.” Accessed: Mar. 18, 2024. Online.. Available: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000709&xlang=pt&contexto=bd&selTab=tab2.
- M. Nielsen, K. Everett, V. Marroni, G. Greer, and S. Bulman, “Risks to New Zealand’s primary industries from Xylella”, Accessed: Mar. 13, 2024. Online.. Available: https://pubmlst.org/X.
- “IVV // Produção.” Accessed: Mar. 18, 2024. Online.. Available: https://www.ivv.gov.pt/np4/163.
- T. de Figueiredo, A. Martins, Z. Hernández, C. Carlos, and F. Fonseca, “Proteção do solo em viticultura de montanha: manual técnico para a região do Douro,” Proteção do Solo em Viticultura de Montanha Manual Técnico para a Região do Douro, p. 25, 2015, Accessed: Mar. 18, 2024. Online.. Available: https://bibliotecadigital.ipb.pt/handle/10198/14009.
- M. E. Grebus and J. M. Henry, “Evaluation of Pruning as a Method to Reduce Damage by Oleander Leaf Scorch”.
- T. de Figueiredo, A. Martins, Z. Hernández, C. Carlos, and F. Fonseca, “Proteção do solo em viticultura de montanha: manual técnico para a região do Douro,” Proteção do Solo em Viticultura de Montanha Manual Técnico para a Região do Douro, p. 25, 2015, Accessed: Mar. 18, 2024. Online.. Available: https://bibliotecadigital.ipb.pt/handle/10198/14009. [CrossRef]
- T. de Figueiredo, A. Martins, Z. Hernández, C. Carlos, and F. Fonseca, “Proteção do solo em viticultura de montanha: manual técnico para a região do Douro,” Proteção do Solo em Viticultura de Montanha Manual Técnico para a Região do Douro, p. 25, 2015, Accessed: Mar. 18, 2024. Online.. Available: https://bibliotecadigital.ipb.pt/handle/10198/14009.
- B. Gould and J. H. Lashomb, “Bacterial Leaf Scorch of Shade Trees.,” APSnet Feature Articles, 2005. [CrossRef]
- M. Ali, W. van der Werf, and A. Oude Lansink, “Assessment of the environmental impacts of Xylella fastidiosa subsp. pauca in Puglia,” Crop Protection, vol. 142, p. 105519, Apr. 2021. [CrossRef]
- Bragard et al., “Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory,” EFSA Journal, vol. 17, no. 5, p. e05665, May 2019. [CrossRef]
- T. Semeraro et al., “Changes in Olive Urban Forests Infected by Xylella fastidiosa: Impact on Microclimate and Social Health,” International Journal of Environmental Research and Public Health 2019, Vol. 16, Page 2642, vol. 16, no. 15, p. 2642, Jul. 2019. [CrossRef]
- F. Sanna, N. Mori, G. Santoiemma, D. D’Ascenzo, M. A. Scotillo, and L. Marini, “Ground cover management in olive groves reduces populations of philaenus spumarius (Hemiptera: Aphrophoridae), Vector of Xylella fastidiosa,” J Econ Entomol, vol. 114, no. 4, pp. 1716–1721, Aug. 2021. [CrossRef]
- M. G. Berman, J. Jonides, and S. Kaplan, “The Cognitive Benefits of Interacting With Nature,” https://doi.org/10.1111/j.1467-9280.2008.02225.x, vol. 19, no. 12, pp. 1207–1212, Dec. 2008. [CrossRef]
- M. P. White, I. Alcock, B. W. Wheeler, and M. H. Depledge, “Would You Be Happier Living in a Greener Urban Area? A Fixed-Effects Analysis of Panel Data,” https://doi.org/10.1177/0956797612464659, vol. 24, no. 6, pp. 920–928, Apr. 2013. [CrossRef]
- G. N. Bratman, J. P. Hamilton, K. S. Hahn, G. C. Daily, and J. J. Gross, “Nature experience reduces rumination and subgenual prefrontal cortex activation,” Proc Natl Acad Sci U S A, vol. 112, no. 28, pp. 8567–8572, Jul. 2015. [CrossRef]
- T. Loureiro et al., “Histological analysis of Xylella fastidiosa infection in Quercus pyrenaica in Northern Portugal,” AIMS Agriculture and Food 2024 2:607, vol. 9, no. 2, pp. 607–627, 2024. [CrossRef]

| Xylella fastidiosa subs fastidosa | |
| Acacia dealbata | 1 |
| Genista tridentata | 1 |
| Lavandula stoechas | 1 |
| Citrus × aurantium var. paradisi | 1 |
| Citrus × limon | 1 |
| Xylella fastidiosa subs. multiplex st7 | |
| Acacia longifolia | 2 |
| Acacia melanoxylon | 1 |
| Adenocarpus lainzii | 2 |
| Artemisia arborescens | 2 |
| Asparagus acutifolius | 1 |
| Athyrium filix-femina | 1 |
| Berberis thunbergii | 1 |
| Calluna vulgaris | 1 |
| Cistus inflatus | 3 |
| Cistus monspeliensis | 2 |
| Cistus salviifolius | 1 |
| Cytisus scoparius | 3 |
| Dodonaea viscosa | 2 |
| Echium plantagineum | 1 |
| Elaeagnus × submacrophylla | 1 |
| Erica cinerea | 1 |
| Erigeron canadensis | 1 |
| Erodium moschatum | 1 |
| Euryops chrysanthemoides | 2 |
| Frangula alnus | 1 |
| Gazania rigens | 2 |
| Genista tridentata | 1 |
| Hebe sp. | 3 |
| Helichrysum italicum | 2 |
| Hibiscus syriacus | 1 |
| Hypericum androsaemum | 1 |
| Hypericum perforatum | 1 |
| Ilex aquifolium | 1 |
| Laurus nobilis | 1 |
| Lavandula angustifolia | 2 |
| Lavandula dentata | 6 |
| Lavandula stoechas | 1 |
| Lonicera periclymenum | 1 |
| Magnolia grandiflora | 3 |
| Magnolia × soulangeana | 1 |
| Medicago sativa | 5 |
| Metrosideros excelsa | 2 |
| Myrtus communis | 2 |
| Nerium oleander | 3 |
| Olea europaea | 5 |
| Pelargonium graveolens | 1 |
| Plantago lanceolata | 1 |
| Prunus cerasifera | 1 |
| Prunus laurocerasus | 1 |
| Prunus persica | 1 |
| Pteridium aquilinum | 1 |
| Quercus robur | 2 |
| Species | Price for Prospection (€) |
| Asparagus acutifolius | 7 151,1 € |
| BrassicaL. | 9 621,5 € |
| Citrus | 27,303.2 € |
| Citrus limon | 10,011.2 € |
| Citrus sinensis | 11,831.4 € |
| Dodonea viscosa | 11,831.8 € |
| Eugenia myrtifoliaSims | 6,631.0 € |
| Euryops chrysanthemoides | 12,741.9 € |
| Ficus caricaL. | 11,051.3 € |
| Hebe | 9 751,5 € |
| Hedera helixL. | 20,933.2 € |
| Ilex AquifoliumL. | 15,017.3 € |
| Laurus nobilisL. | 25,028.8 € |
| Lavandula angustifóliaL. | 6,891.0 € |
| Lavandula dentataL. | 20,608.1 € |
| Lonicera japonicaThunb. | 8,451.3 € |
| Metrosideros excelsea | 19,828.0 € |
| Nerium oleanderL. | 21,583.3 € |
| Olea europaeaL. | 54,736.3 € |
| Unknown | 480,228.1 € |
| Pelargonium graveolens | 27,889.2 € |
| Prunus domesticaL. | 9,036 € |
| Prunus dulcis | 10,726.2 € |
| Prunus laurocerasus | 16,836.9 € |
| Prunus lusitanica | 6,695.8 € |
| Prunus persica | 7,215.8 € |
| Prunus sp. | 11,246.3 € |
| Pteridium aquilinum | 10,206.6 € |
| Quercus roburL. | 10,011.2 € |
| Quercus sp. | 16,316.9 € |
| Quercus suberL. | 19,762.3 € |
| Rosa spp. | 22,818.5 € |
| Rubus | 10,921.7 € |
| Strelitzia reginaeAiton | 7,801.2 € |
| Veronica sp. | 6,956.1 € |
| Vitis vinifera | 11,898.3 € |
| Total | 997,570.2 € |
| Species | Number of Plants |
| Asparagus acutifolius | 175 |
| Citrus limon | 21 |
| Citrus sinensis | 14 |
| Dodonea viscosa | 1,943 |
| Euryops chrysanthemoides | 245 |
| Ficus carica | 17 |
| Hebesp. | 2,178 |
| Hedera helix | 840 |
| Ilex aquifolium | 197 |
| Laurus nobilis | 402 |
| Lavandula angustifólia | 148 |
| Lavandula dentata | 1,152 |
| Lonicera japonica | 496 |
| Metrosideros excelsea | 447 |
| Nerium oleander | 239 |
| Olea europaea | 7,024 |
| Pelargonium graveolens | 3,509 |
| Pelargoniumsp. | 37 |
| Prunus domestica | 21 |
| Prunus dulcis | 25 |
| Prunus laurocerasus | 154 |
| Prunus persica | 50 |
| Pteridium aquilinum | 360,324 |
| Quercus robur | 3,511 |
| Quercus suber | 236 |
| Rosaspp. | 1,106 |
| Rubussp. | 43 |
| Spontaneous herbaceous plants | 27,636 |
| Strelitzia reginae | 85 |
| Veronicasp. | 242 |
| Vitis vinifera | 7 |
| Total | 412,524 |
| Species | Price for Destruction (€) |
| Asparagus acutifolius | 700 |
| Citrus limon | 525 |
| Citrus sinensis | 350 |
| Dodonea viscosa | 7,772 |
| Euryops chrysanthemoides | 735 |
| Ficus carica | 425 |
| Hebesp. | 8,712 |
| Hedera helix | 1,260 |
| Ilex aquifolium | 6,895 |
| Laurus nobilis | 4,422 |
| Lavandula angustifolia | 592 |
| Lavandula dentata | 4,608 |
| Lonicera japonica | 1,984 |
| Metrosideros excelsea | 6,705 |
| Nerium oleander | 2,151 |
| Olea europaea | 245,840 |
| Pelargonium graveolens | 7,018 |
| Pelargoniumsp. | 74 |
| Prunus domestica | 588 |
| Prunus dulcis | 725 |
| Prunus laurocerasus | 1,848 |
| Prunus persica | 1,250 |
| Pteridium aquilinum | 540,486 |
| Quercus robur | 140,440 |
| Quercus suber | 9,440 |
| Rosaspp. | 4,424 |
| Rubussp. | 258 |
| Spontaneous herbaceous plants | 41,454 |
| Strelitzia reginae | 1,530 |
| Veronica sp. | 363 |
| Vitis vinifera | 77 |
| Total | 1,043,651 |
| Species | Price for Replacement |
| Asparagus acutifolius | 2,625 |
| Citrus limon | 840 |
| Citrus sinensis | 560 |
| Dodonea viscosa | 21,373 |
| Euryops chrysanthemoides | 1,715 |
| Ficus carica | 680 |
| Hebe | 17,424 |
| Hedera helix | 2,310 |
| Ilex Aquifolium | 16,745 |
| Laurus nobilis | 15,276 |
| Lavandula angustifólia | 1,332 |
| Lavandula dentata | 10,368 |
| Lonicera japonica | 5,952 |
| Metrosideros excelsea | 20,115 |
| Nerium oleander | 6,453 |
| Olea europaea | 526,800 |
| Pelargonium graveolens | 11,404.25 |
| Pelargonium sp. | 129.5 |
| Prunus domestica | 1,260 |
| Prunus dulcis | 1,375 |
| Prunus laurocerasus | 3,850 |
| Prunus persica | 2,750 |
| Pteridium aquilinum | 5,765,184 |
| Quercus robur | 333,545 |
| Quercus suber | 21,240 |
| Rosa spp. | 9,954 |
| Rubus | 645 |
| Spontaneous herbaceous plants | 0 |
| Strelitzia reginae Aiton | 2,720 |
| Veronica sp. | 968 |
| Vitis vinifera | 98 |
| Total | 6,805,690.75 |
| 2019 | 2020 | 2021 | 2022 | 2023 | ||||||
| P | S | P | S | P | S | P | S | P | S | |
| Porto ZD | 124 | 4977 | 51 | 2914 | 31 | 3115 | 82 | 3287 | 5 | 951 |
| Baião ZD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 45 |
| Bougado ZD | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 74 | 1 | 50 |
| Alijó Zd | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 52 | 0 | 222 |
| Sabrosa ZD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 110 |
| Mirandela ZD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 149 | 0 | 338 |
| MirandelaI II ZD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 141 | 0 | 716 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





