Submitted:
27 September 2025
Posted:
28 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Anatomical Study
| Plant organ | Parameters | |
| Qualitative | Quantitative | |
| Leaf | Type of mesophyll, presence or absence of trichomes or glands, presence or absence of crystals, stomatal types, stomatal position and vascular bundle distribution. | Stomatal index and density, mesophyll thickness, cuticle thickness. |
| Stem | Type of epidermis, type of cuticle, presence or absence of trichomes, tissue organization, vascular bundle organization, and fiber classification. | Cuticle thickness, epidermal thickness, vessel diameter. |
| Root | Type of xylem, presence of exodermis and endodermis, sclerenchyma fiber bundles and vascular bundle organization. | Root diameter, periderm thickness (if present), xylem vessel diameter. |
3. Results
3.1. Leaf Anatomy
3.2. Stem Anatomy
3.3. Root Anatomy
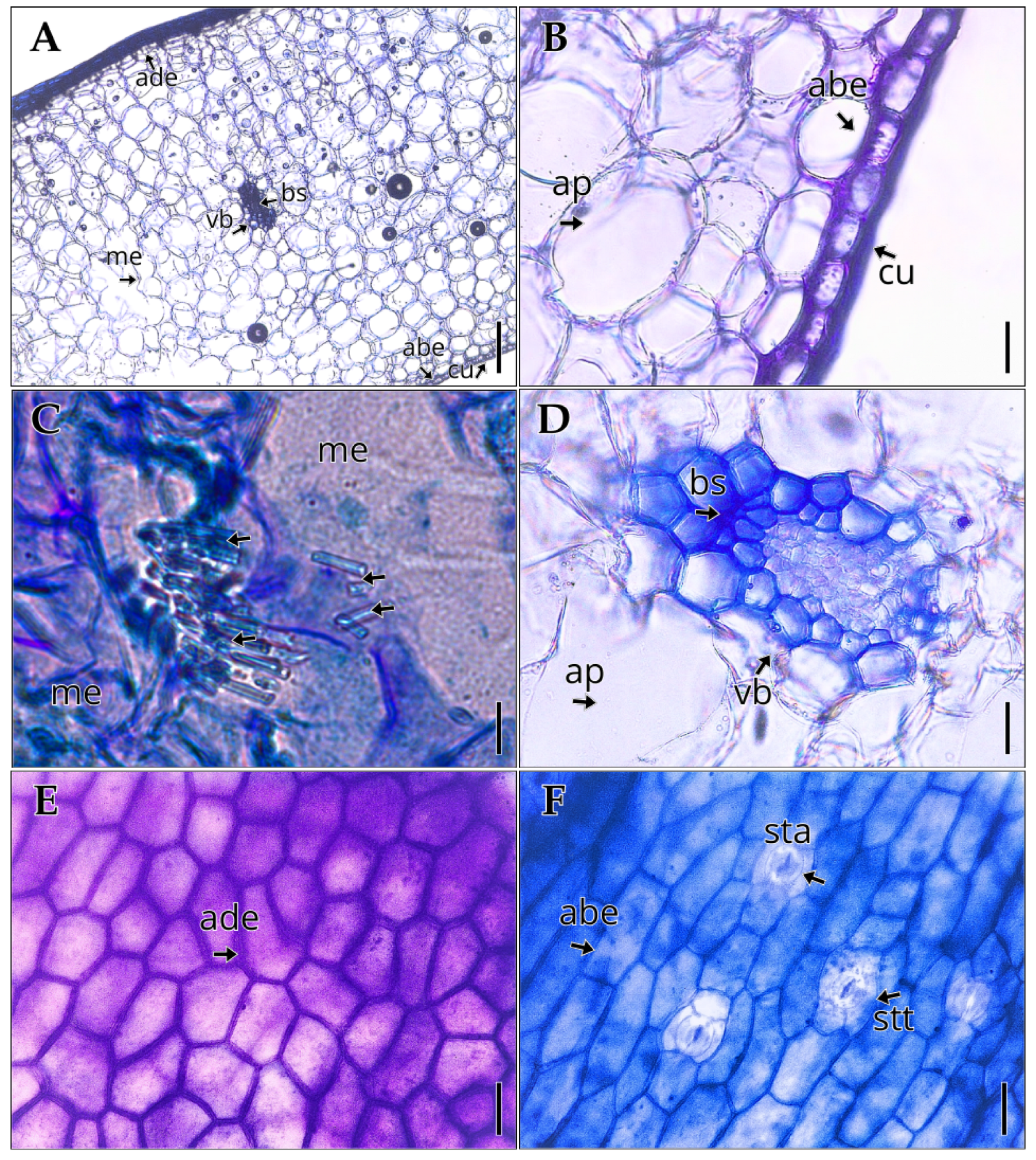
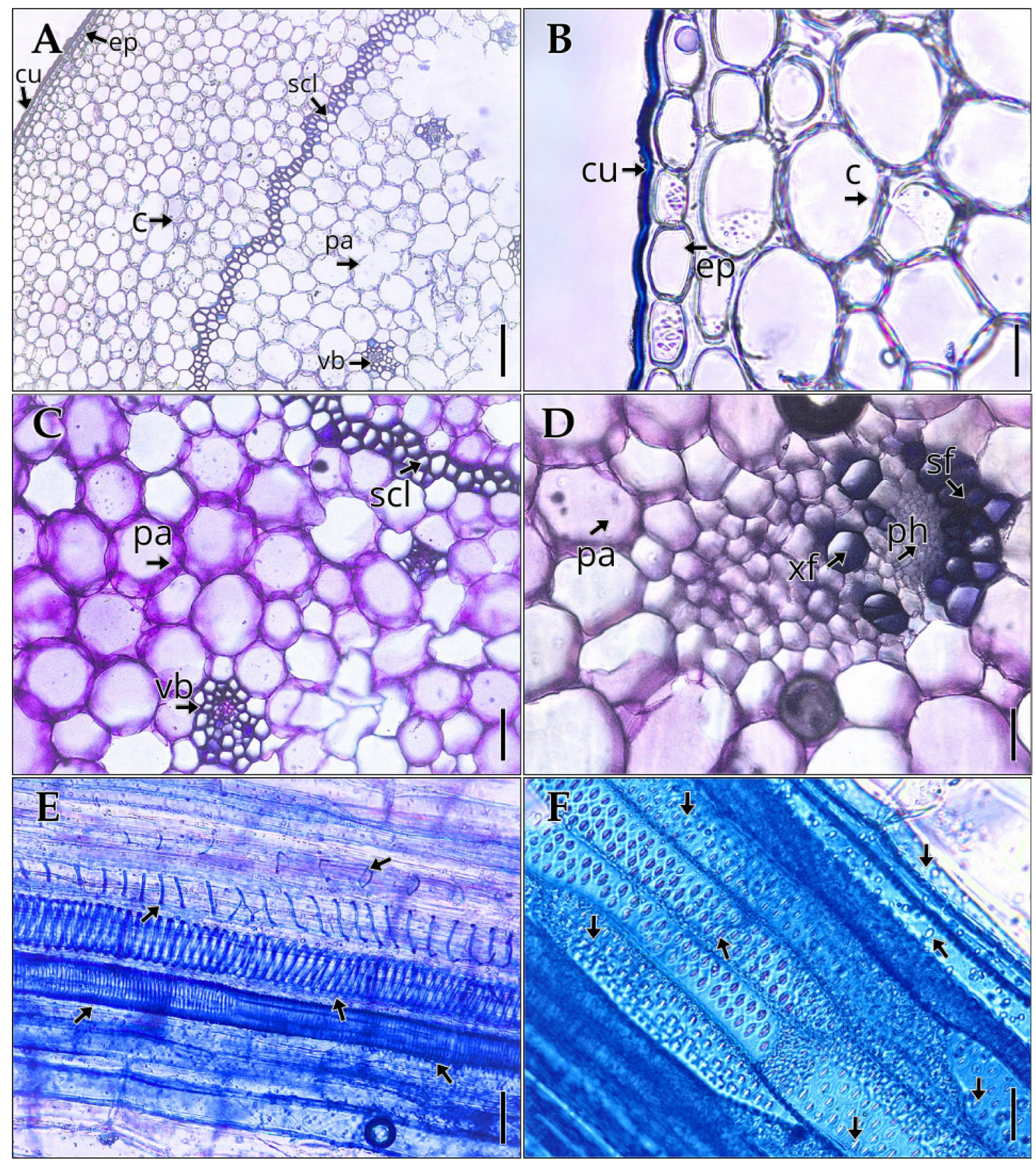
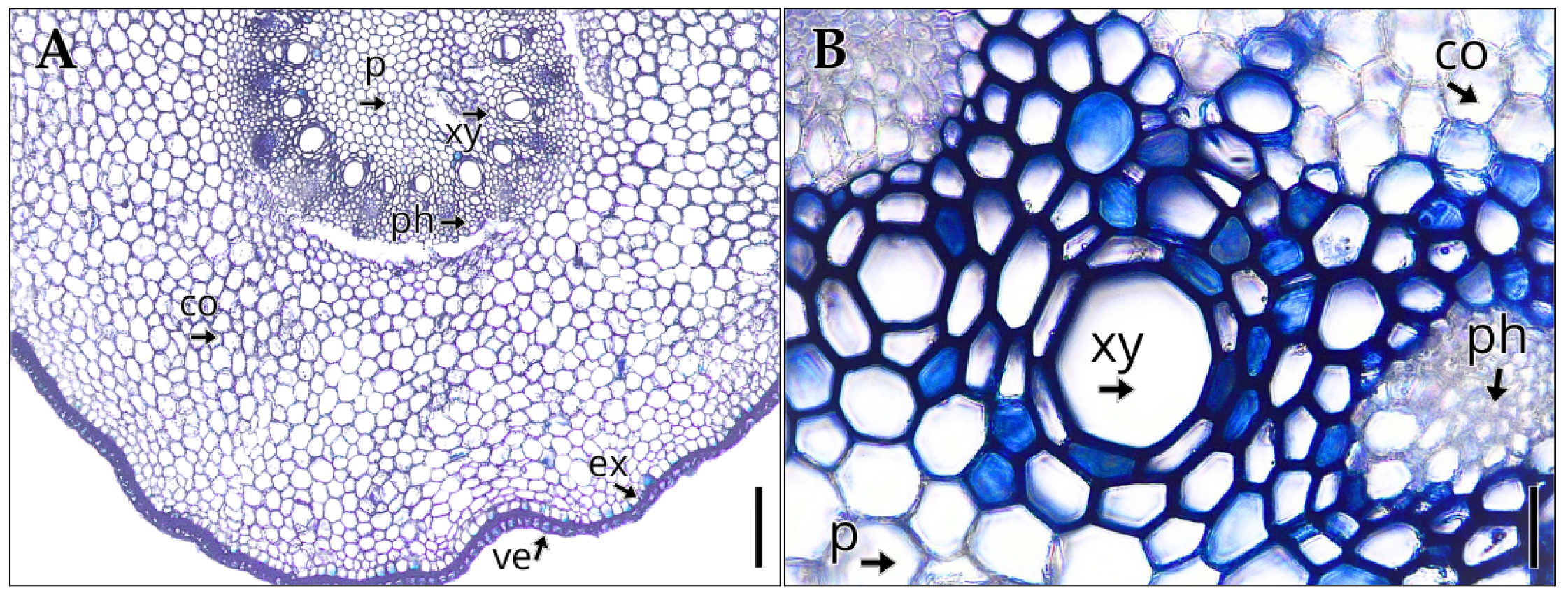
3.4. Histochemistry
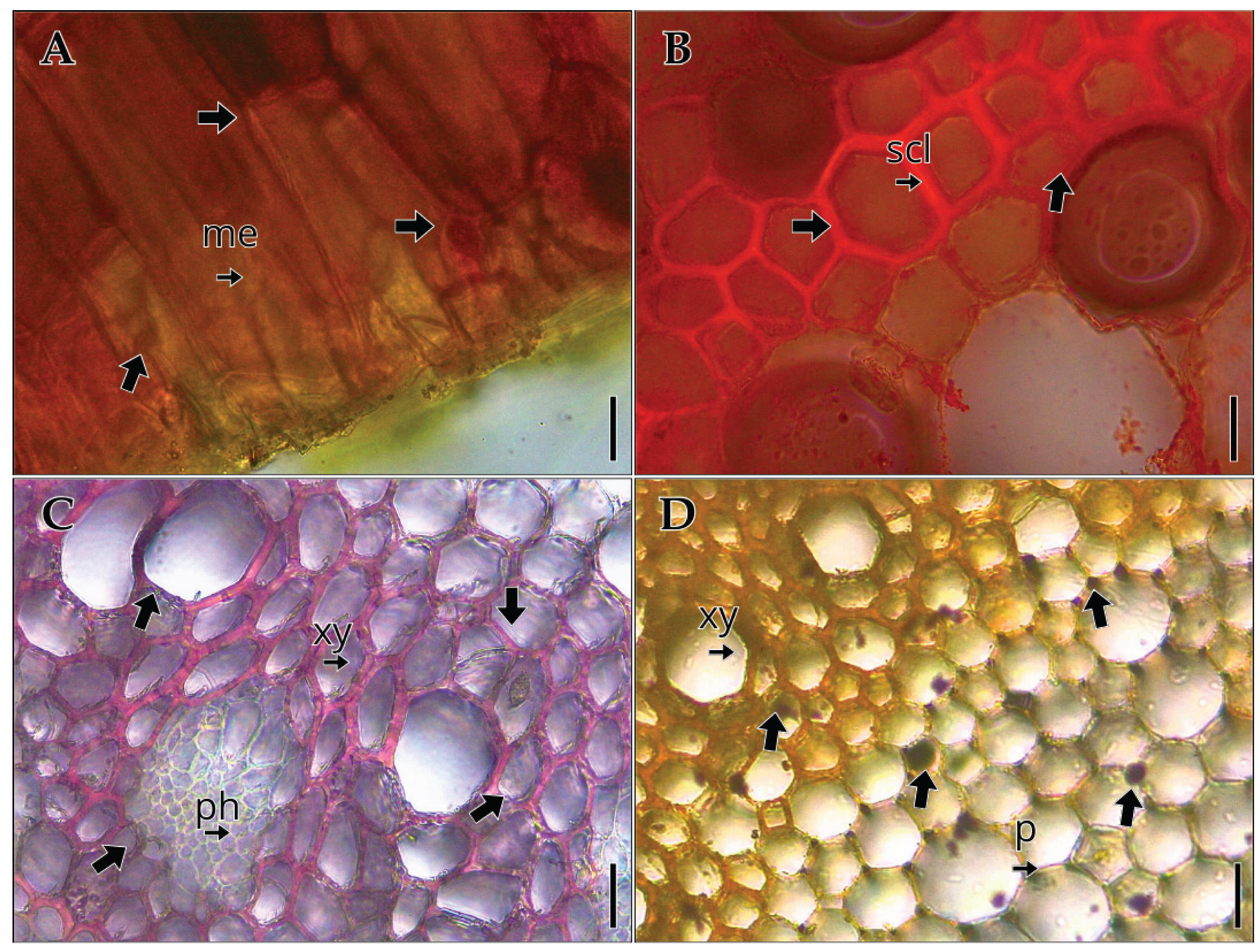
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Taylor, A.; Zotz, G.; Weigelt, P.; Cai, L.; Karger, D.N.; König, C.; Kreft, H. Vascular epiphytes contribute disproportionately to global centres of plant diversity. Glob. Ecol. Biogeogr. 2021, 30, 2584–2598. [Google Scholar] [CrossRef]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Ministerio del Ambiente. Orquídeas del Perú y herramientas para su identificación. Ministerio del Ambiente, 2017. Available online: https://www.minam.gob.pe/diversidadbiologica/wp-content/uploads/sites/131/2018/12/LIBRO-ORQUIDEAS-2017_.pdf (accessed on 15 September 2025).
- Zotz, G.; Weigelt, P.; Kessler, M.; Kreft, H.; Taylor, A. EpiList 1.0: A global checklist of vascular epiphytes. Ecology 2021, 102, e03326. [Google Scholar] [CrossRef]
- Gastelbondo, M.; Nicholls, U.; Chen, S.; Chambers, A.; Wu, X. First gynogenesis of Vanilla planifolia for haploid production and ploidy verification protocol. Plants 2024, 13, 1733. [Google Scholar] [CrossRef]
- Karremans, A.P.; Chinchilla, I.F.; Rojas-Alvarado, G. Studies on Costa Rican Vanilloideae: The return of Cleistes rosea and a reaffirmation of Epistephium ellipticum. Lankesteriana 2023, 23, 613–622. [Google Scholar] [CrossRef]
- Azofeifa-Bolaños, J.B.; Paniagua-Vásquez, A.; García-García, J.A. Importancia y desafíos de la conservación de Vanilla spp. (Orchidaceae) en Costa Rica. Agron. Mesoam. 2014, 25, 189–202. Available online: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1659-13212014000100019 (accessed on 15 September 2025). [CrossRef]
- Leyva, V.E.; Lopez, J.M.; Zevallos-Ventura, A.; Cabrera, R.; Cañari-Chumpitaz, C.; Toubiana, D.; Maruenda, H. NMR-based leaf metabolic profiling of Vanilla planifolia and three endemic Vanilla species from the Peruvian Amazon. Food Chem. 2021, 358, 129365. [Google Scholar] [CrossRef]
- Rocha-Flores, R.G.; Herrera-Cabrera, B.E.; Velasco-Velasco, J.; Salazar-Rojas, V.M.; Delgado-Alvarado, A.; Mendoza-Castillo, M.C. Determinación preliminar de componentes de rendimiento para el cultivo de vainilla (Vanilla planifolia Jacks. ex Andrews) en la región Totonacapan, México. Agro Prod. 2018, 11, 9–14. [Google Scholar]
- Bouétard, A.; Lefuvre, P.; Gigant, R.; Bory, S.; Pignal, M.; Besse, P.; Grisoni, M. Evidence of transoceanic dispersion of the genus Vanilla based on plastid DNA phylogenetic analysis. Mol. Phylogenet. Evol. 2010, 55, 621–630. [Google Scholar] [PubMed]
- Rodríguez-Deméneghi, M.V.; Ramírez-Mosqueda, M.A.; Armas-Silva, A.A.; Aguilar-Rivera, N.; Gheno-Heredia, Y.A. Biofábricas de vainilla (Vanilla planifolia) en México como oportunidad de desarrollo agrario. Cuad. Biodivers. 2022, 63, 49–54. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.; Zarco-González, M.; Gómez Ticerán, D.; Endara-Agramont, A.; Monroy-Vilchis, O. Effects of human disturbance on above-ground carbon stocks in north-west Amazonian Mauritia flexuosa peat swamp forests. Mires and Peat 2023, 29, 1–19. [Google Scholar]
- Humboldt, A. Essai politique sur le royaume de la Nouvelle-Espagne, 2 vols.; F. Schoell: Paris, France, 1811; p. 1–[vol. 2 end]. [Google Scholar]
- Humboldt, A.; Bonpland, A.; Kunth, C. Nova Genera et Species Plantarum, Quarto ed.; Librariae Graeco-Latino-Germaniae: Paris, France, 1815; Volume 1, pp. 1–377. [Google Scholar]
- Kraenzlin, F. Plantae novae andinae imprimis weberbauerianae I. Bot. Jahrb. Syst. 1906, 37, 373–398. [Google Scholar]
- Schlechter, R. Die Orchideenfloren der sudamerikanischen Kordillerenstaaten, IV. Perú. Repert. Spec. Nov. Regni Veg., Beihefte 1921, 9, 80–400. [Google Scholar]
- Schweinfurth, C. Orchids of Perú. Fieldiana Bot. 1958, 30, 1–260. [Google Scholar]
- Damián-Parizaca, L. Taxonomía del género Vanilla Plum. ex Mill. (Orchidaceae: Vanilleae) en el Perú. Bachelor’s Thesis, Universidad Nacional Mayor de San Marcos, Lima, Perú, 2020. [Google Scholar]
- Householder, E.; Janovec, J.; Balarezo, A.; Maceda, H.; Wells, J.; Valega, R. Diversity, natural history, and conservation of Vanilla (Orchidaceae) in Amazonian wetlands of Madre de Dios, Perú. J. Bot. Res. Inst. Tex. 2010, 4, 227–243. [Google Scholar]
- Janovec, J.; Householder, J.E.; Tobler, M. Evaluación de los actuales impactos y amenazas inminentes en aguajales y cochas de Madre de Dios; World Wildlife Fund (WWF): Lima, Perú, 2013; pp. 1–244. [Google Scholar]
- Soto, M.A.; Cribb, P. A new infrageneric classification and synopsis of the genus Vanilla Plum. ex Mill. (Orchidaceae: Vanillinae). Lankesteriana 2010, 9, 355–398. [Google Scholar]
- Herrera, X. Aprovechamiento de la fibra de coco y la hojarasca para la propagación de vainilla (Vanilla pompona subsp. grandiflora (Lindl.) y Vanilla odorata C. (Presl)) con fines de mitigación ambiental y conservación de la biodiversidad. Bachelor’s Thesis, Universidad Peruana Unión, Lima, Perú, 2019. Available online: https://repositorio.upeu.edu.pe/items/f4c465d5-6907-4fe6-adec-c15ddebe593 (accessed on 15 September 2025).
- Raffi, A.; Abdullah; Yunus, N. -S.M.; Go, R. Preliminary foliar anatomical assessment of four Vanilla species (Orchidaceae) from Perak, Malaysia. Malays. Appl. Biol. 2019, 42, 807–812. Available online: https://openurl.ebsco.com/EPDB%3Agcd%3A13%3A33252973/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A136874117&crl=c&link_origin=scholar.google.com.pe (accessed on 15 September 2025).
- Martínez-Quezada, D.M.; Sandoval-Zapotitla, E.; Solís-De la Cruz, J.; Velázquez-Vázquez, D.E.; Herrera-Cabrera, E. Caracterización anatómica y análisis de variación de epidermis foliar y caulinar entre dos genotipos de Vanilla planifolia Jacks. ex Andrews. Agro Prod. 2018, 9, 1. Available online: https://revista-agroproductividad.org/index.php/agroproductividad/article/view/704 (accessed on 15 September 2025).
- Odoux, E.; Brillouet, J.-M. Anatomy, histochemistry and biochemistry of glucovanillin, oleoresin and mucilage accumulation sites in green mature vanilla pod (Vanilla planifolia; Orchidaceae): A comprehensive and critical reexamination. Fruits 2009, 64, 221–241. [Google Scholar] [CrossRef]
- Stern, W.L.; Judd, W.S. Comparative vegetative anatomy and systematics of Vanilla (Orchidaceae). Bot. J. Linn. Soc. 1999, 131, 353–382. [Google Scholar] [CrossRef]
- Flores Jiménez, Á.; López, D.R.; García, D.J.; Arenas, O.R.; Rivera, A.; Lara, M.H.; Silva, A.P. Diversidad de Vanilla spp. en México: Distribución geográfica y perfiles bioclimáticos. Rev. Mex. Cienc. For. 2017, 8, 62–86. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.M.; Cabrera Mestanza, D.; Macedo Bedoya, J.; Santos Linares, V.; Salinas Inga, A. Propagación vegetativa de Vanilla pompona subsp. grandiflora (Orchidaceae) en territorios inundables del Valle del Alto Mayo, Perú. Acta Bot. Mex. 2024, 131. [Google Scholar] [CrossRef]
- Ahmad, H.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Jilani, M.I. Vanilla. In Elsevier EBooks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 657–669. [Google Scholar] [CrossRef]
- Nakata, P. A. (2003). Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Ribeiro, J.; Paula-Souza, J.; da Silva, C. Morfoanatomia de órgãos vegetativos de duas espécies de Cattleya (Orchidaceae) nativas do Brasil. Rodriguésia 2020, 71. [Google Scholar] [CrossRef]
- Muthukumar, T.; Shenbagam, M. Vegetative anatomical adaptations of Epidendrum radicans (Epidendroideae, Orchidaceae) to epiphytic conditions of growth. Mod. Phytomorphol. 2017, 11, 117–130. [Google Scholar] [CrossRef]
- Barreda-Castillo, J.M.; Monribot-Villanueva, J.L.; Velázquez-Rosas, N.; Bayman, P.; Guerrero-Analco, J.A.; Menchaca-García, R.A. Morphological and physio-chemical responses to PEG-induced water stress in Vanilla planifolia and V. pompona hybrids. Int. J. Mol. Sci. 2023, 24, 4690. [Google Scholar] [CrossRef] [PubMed]
- Saoncella, A.L.; Marteline, M.A.; de Moraes, C.P. Anatomia dos órgãos vegetativos de Cattleya violacea (Kunth) Rolfe (Orchidaceae). Iheringia, Sér. Bot. 2017, 72, 114–126. [Google Scholar] [CrossRef]
- de Lima, J.F.; Moreira, A.S.F.P. Structural plasticity in roots of the hemiepiphyte Vanilla phaeantha Rchb.f. (Orchidaceae): A relationship between environment and function. Sci. Nat. 2022, 109, 46. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.F.; Coelho de Oliveira, D.; Coelho Kuster, V.; Franco Pinheiro Moreira, A.S. Aerial and terrestrial root habits influence the composition of the cell walls of Vanilla phaeantha (Orchidaceae). Protoplasma 2024, 262, 87–98. [Google Scholar] [CrossRef]
- Deseo, N.B.; de Guzman, C.C.; Nuevo, P.A.; Torreta, N.K. Morpho-Anatomy of Adventitious Roots of Vanilla (Vanilla planifolia Jacks. ex Andrews) during Attachment to Support Post. Thailand Nat. Hist. Mus. J. 2020, 14, 31–36. [Google Scholar]
- Botomanga, A.; Jeannoda, V.H.; Fuzzati, N.; Ramarosandratana, A.V. Morpho-anatomical responses of leafless Vanilla spp. roots to drought and habitat degradation. Flora 2024, 317, 152562. [Google Scholar] [CrossRef]
- Ravier, A.; Chalut, P.; Belarbi, S.; Santerre, C.; Vallet, N.; Nhouchi, Z. Impact of the post-harvest period on the chemical and sensorial properties of Vanilla planifolia and V. pompona. Molecules 2024, 29, 839. [Google Scholar] [CrossRef] [PubMed]
- Hickey, L.J. Classification of the architecture of dicotyledonous leaves. Am. J. Bot. 1973, 60, 17–33. [Google Scholar] [CrossRef]
- Ash, A.; Ellis, B.; Hickey, L.J.; Johnson, K.; Wilf, P.; Wing, S.L. Manual of Leaf Architecture: Morphological Description and Categorization of Dicotyledonous and Net-Veined Monocotyledonous Angiosperms; Smithsonian Institution: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- D’Ambrogio de Argüeso, A. Manual de Técnicas en Histología Vegetal; Hemisferio Sur S.A.: Buenos Aires, Argentina, 1986. [Google Scholar]
- Lock, O. Investigación Fitoquímica: Métodos en el Estudio de Productos Naturales, 2ª ed.; Pontificia Universidad Católica del Perú: Lima, Perú, 1994. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Evans, W.C. Trease y Evans Farmacognosia, 13th ed.; Interamericana-McGraw-Hill: Madrid, Spain, 1991. [Google Scholar]


| Quantitative parameter | Measurement |
| Mesophyll thickness (µm) | 1144.88 ± 54.17 |
| Stomatal density (stomata/mm²) | 4.5 ± 0.85 |
| Stomatal index (%) | 7.92 ± 1.32 |
| Leaf cuticle thickness (µm) | 4.50 ± 0.37 |
| Stem epidermis thickness (µm) | 34.09 ± 5.92 |
| Stem cuticle thickness (µm) | 5.26 ± 1.90 |
| Stem xylem vessel diameter (µm) | 35.52 ± 4.27 |
| Root diameter (µm) | 1482.55 ± 10.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





