Submitted:
17 September 2025
Posted:
24 September 2025
You are already at the latest version
Abstract
Keywords:
Introduction:
Discussion:
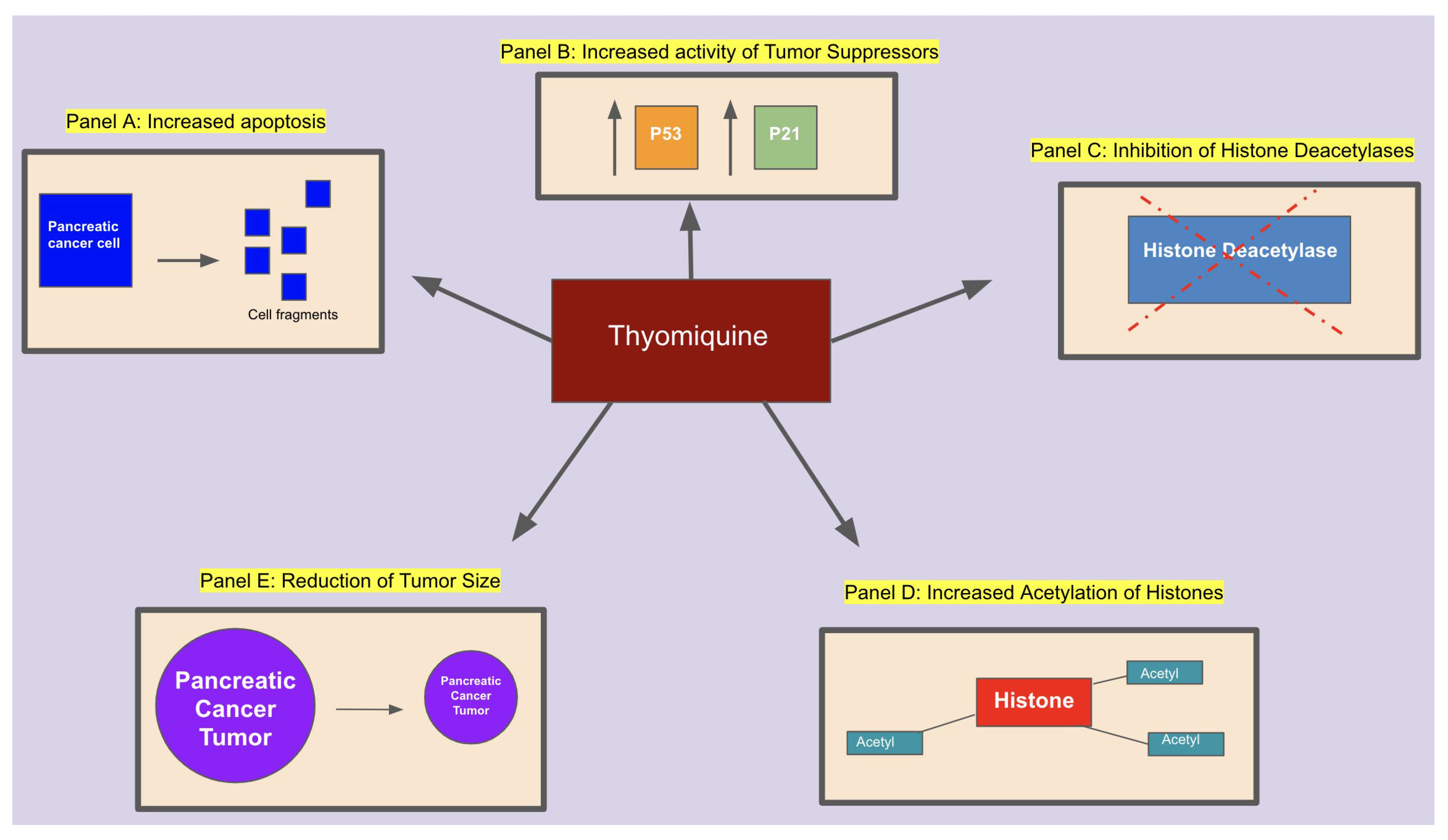
Conclusion:
References
- American Cancer Society. (2020). Cancer.Org. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
- American Institute for Cancer Research. (2018). Analysing Research on Cancer Prevention and Survival. In wcrf.org. https://www.wcrf.org/sites/default/files/The-cancer-process.pdf.
- Chakraborty, S., & Singh, S. (2013). Surgical resection improves survival in pancreatic cancer patients without vascular invasion- a population based study. Annals of Gastroenterology, 26(4), 346–352. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959484/.
- Chehl, N., Chipitsyna, G., Gong, Q., Yeo, C. J., & Arafat, H. A. (2009). Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB, 11(5), 373–381. [CrossRef]
- Clinisciences. (2020). LMK 235 [1418033-25-6] Clinisciences. Clinisciences.Com. https://www.clinisciences.com/autres-produits-186/lmk-235-1418033-25-6-182003944.html.
- Dastjerdi, M., Mehdiabady, E., Iranpour, F., & Bahramian, H. (2016). Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. International Journal of Preventive Medicine, 7(1), 66. [CrossRef]
- Dirican, A., Erten, C., Atmaca, H., Bozkurt, E., Kucukzeybek, Y., Varol, U., Oktay Tarhan, M., Karaca, B., & Uslu, R. (2014). Enhanced cytotoxicity and apoptosis by thymoquinone in combination with zoledronic acid in hormone- and drug-resistant prostate cancer cell lines. Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology, 19(4), 1055–1061. https://www.ncbi.nlm.nih.gov/pubmed/25536616.
- Diyabalanage, H. V. K., Granda, M. L., & Hooker, J. M. (2013). Combination therapy: histone deacetylase inhibitors and platinum-based chemotherapeutics for cancer. Cancer Letters, 329(1), 1–8. [CrossRef]
- DNA fragmentation and Apoptosis - Flow Cytometry Core Facility. (2020). Qmul.Ac.Uk. http://www.icms.qmul.ac.uk/flowcytometry/uses/apoptosis/dnafragmentation/.
- Dzoyem, J. P., McGaw, L. J., Kuete, V., & Bakowsky, U. (2017). Anti-inflammatory and Anti-nociceptive Activities of African Medicinal Spices and Vegetables. Medicinal Spices and Vegetables from Africa, 239–270. [CrossRef]
- Garner, E., & Raj, K. (2008). Protective mechanisms of p53-p21-pRb proteins against DNA damage-induced cell death. Cell Cycle, 7(3), 277–282. [CrossRef]
- Harvard Health Publishing. (2009, April). The Whipple procedure - Harvard Health. Harvard Health; Harvard Health. https://www.health.harvard.edu/newsletter_article/The_Whipple_procedure.
- He, X., Li, Y., Su, T., Lai, S., Wu, W., Chen, L., Si, J., & Sun, L. (2018). The impact of a history of cancer on pancreatic ductal adenocarcinoma survival. United European Gastroenterology Journal, 6(6), 888–894. [CrossRef]
- Herrero, A., Rojas, E., Misiewicz-Krzeminska, I., Krzeminski, P., & Gutiérrez, N. (2016). Molecular Mechanisms of p53 Deregulation in Cancer: An Overview in Multiple Myeloma. International Journal of Molecular Sciences, 17(12), 2003. [CrossRef]
- Hirshberg Foundation. (2015). Prognosis. pancreatic.org. http://pancreatic.org/pancreatic-cancer/about-the-pancreas/prognosis/.
- Isaji, S., Mizuno, S., Windsor, J. A., Bassi, C., Fernández-Del Castillo, C., Hackert, T., Hayasaki, A., Katz, M. H. G., Kim, S.-W., Kishiwada, M., Kitagawa, H., Michalski, C. W., & Wolfgang, C. L. (2018). International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology : Official Journal of the International Association of Pancreatology (IAP)... [et Al.], 18(1), 2–11. [CrossRef]
- Jang, J.-Y., Han, Y., Lee, H., Kim, S.-W., Kwon, W., Lee, K.-H., Oh, D.-Y., Chie, E. K., Lee, J. M., Heo, J. S., Park, J. O., Lim, D. H., Kim, S. H., Park, S. J., Lee, W. J., Koh, Y. H., Park, J. S., Yoon, D. S., Lee, I. J., & Choi, S. H. (2018). Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Annals of Surgery, 268(2), 215–222. [CrossRef]
- Javed, A. A., Wright, M. J., Siddique, A., Blair, A. B., Ding, D., Burkhart, R. A., Makary, M., Cameron, J. L., Narang, A., Herman, J., Zheng, L., Laheru, D., Weiss, M. J., Wolfgang, C., & He, J. (2018). Outcome of Patients with Borderline Resectable Pancreatic Cancer in the Contemporary Era of Neoadjuvant Chemotherapy. Journal of Gastrointestinal Surgery, 23(1), 112–121. [CrossRef]
- Kat Arney. (2019). Discovering the p53 cancer protein. Cancer Research UK - Science Blog. https://scienceblog.cancerresearchuk.org/2009/10/04/high-impact-science-p53/.
- Kimball, J. (2014, March 8). Apoptosis. Biology-Pages.Info. http://www.biology-pages.info/A/Apoptosis.html.
- Lane, A., & Chabner, B. (2009). Histone Deacetylase Inhibitors in Cancer Therapy | Journal of Clinical Oncology. Journal of Clinical Oncology. https://ascopubs.org/doi/full/10.1200/JCO.2009.22.1291.
- Lane, D. P., & Crawford, L. V. (1979). T antigen is bound to a host protein in SY40-transformed cells. Nature, 278(5701), 261–263. [CrossRef]
- Li, Y., Fan, J., & Ju, D. (2019). Neurotoxicity concern about the brain targeting delivery systems. Brain Targeted Drug Delivery System, 377–408. [CrossRef]
- Liou, Y., Chen, P., Chu, S., Kao, S., Chang, Y., Hsieh, Y., & Chang, H. (2019). Thymoquinone suppresses the proliferation of renal cell carcinoma cells via reactive oxygen species-induced apoptosis and reduces cell stemness. Environmental Toxicology, 34(11), 1208–1220. [CrossRef]
- Morton, J. P., Timpson, P., Karim, S. A., & Sansom, O. J. (2009, December). Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. ResearchGate; National Academy of Sciences. https://www.researchgate.net/publication/40696085_Mutant_p53_drives_metastasis_and_overcomes_growth_arrestsenescence_in_pancreatic_cancer.
- Murphy, P. J. M., Galigniana, M. D., Morishima, Y., Harrell, J. M., Kwok, R. P. S., Ljungman, M., & Pratt, W. B. (2004). Pifithrin-α Inhibits p53 Signaling after Interaction of the Tumor Suppressor Protein with hsp90 and Its Nuclear Translocation. Journal of Biological Chemistry, 279(29), 30195–30201. [CrossRef]
- Ozturk, S., Alp, E., Saglam, A. Y., Konac, E., & Menevse, E. (2017). The effects of thymoquinone and genistein treatment on telomerase activity, apoptosis, angiogenesis, and survival in thyroid cancer cell lines. Journal of Cancer Research and Therapeutics, 0(0), 0. [CrossRef]
- Pancreatic Cancer - Statistics. (2020, January). Cancer.Net. https://www.cancer.net/cancer-types/pancreatic-cancer/statistics.
- Relles, D., Chipitsyna, G. I., Gong, Q., Yeo, C. J., & Arafat, H. A. (2016). Thymoquinone Promotes Pancreatic Cancer Cell Death and Reduction of Tumor Size through Combined Inhibition of Histone Deacetylation and Induction of Histone Acetylation. Advances in Preventive Medicine, 2016, 1–9. [CrossRef]
- Ropero, S., & Esteller, M. (2007). The role of histone deacetylases (HDACs) in human cancer. Molecular Oncology, 1(1), 19–25. [CrossRef]
- Samarghandian, S., Azimi-Nezhad, M., & Farkhondeh, T. (2019). Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. Journal of Cellular Physiology, 234(7), 10421–10431. [CrossRef]
- Torres, M. P., Ponnusamy, M. P., Chakraborty, S., Smith, L. M., Das, S., Arafat, H. A., & Batra, S. K. (2010). Effects of Thymoquinone in the Expression of Mucin 4 in Pancreatic Cancer Cells: Implications for the Development of Novel Cancer Therapies. Molecular Cancer Therapeutics, 9(5), 1419–1431. [CrossRef]
- Treatment Before Surgery. (2017). Cancertodaymag.Org. https://www.cancertodaymag.org/Pages/Fall2017/Treatment-Before-Surgery-Chemotherapy.aspx.
- Valentini, A., Gravina, P., Federici, G., & Bernardini, S. (2007). Valproic acid induces apoptosis, p16INK4A upregulation and sensitization to chemotherapy in human melanoma cells. Cancer Biology & Therapy, 6(2), 185–191. [CrossRef]
- Wang, X., Simpson, E. R., & Brown, K. A. (2015). p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Research, 75(23), 5001–5007. [CrossRef]
- Yoon, C. Y., Park, M. J., Lee, J. S., Lee, S. C., Oh, J. J., Park, H., Chung, C. W., Abdullajanov, M. M., Jeong, S. J., Hong, S. K., Byun, S. S., Lee, E. S., & Lee, S. E. (2011). The histone deacetylase inhibitor trichostatin A synergistically resensitizes a cisplatin resistant human bladder cancer cell line. The Journal of Urology, 185(3), 1102–1111. [CrossRef]
- Zhang, J., & Zhong, Q. (2014). Histone deacetylase inhibitors and cell death. Cellular and Molecular Life Sciences, 71(20), 3885–3901. [CrossRef]
- Zhang, L., Bai, Y., & Yang, Y. (2016). Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncology Letters, 12(4), 2840–2845. [CrossRef]
- Zhang, M., Du, H., Huang, Z., Zhang, P., Yue, Y., Wang, W., Liu, W., Zeng, J., Ma, J., Chen, G., Wang, X., & Fan, J. (2018). Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chemico-Biological Interactions, 292, 65–75. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




