Submitted:
18 September 2025
Posted:
18 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Wheat Grain Samples
2.2. Sample Preparation
2.3. DNA Isolation
2.4. Real-Time PCR
2.5. Statistical Analysis
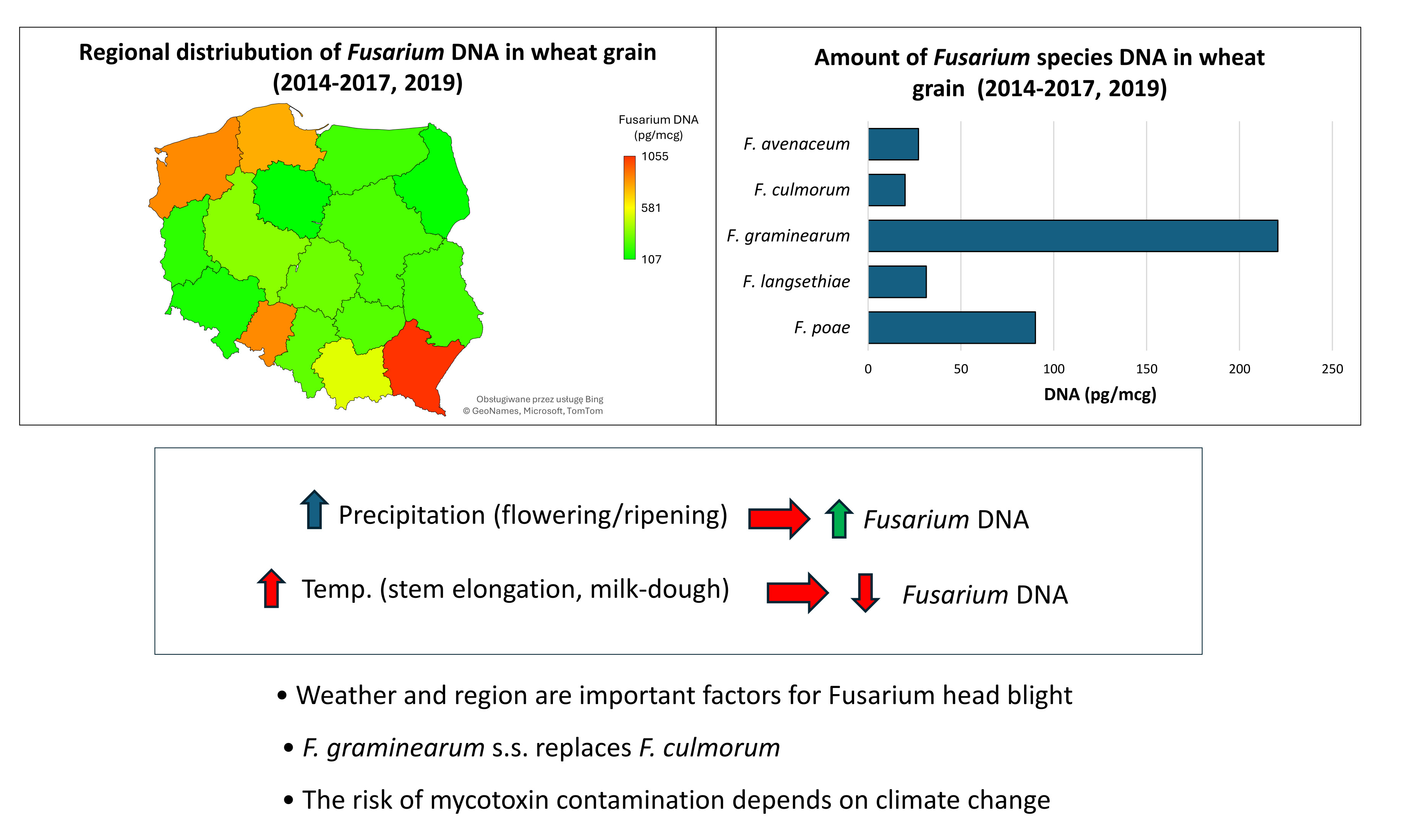
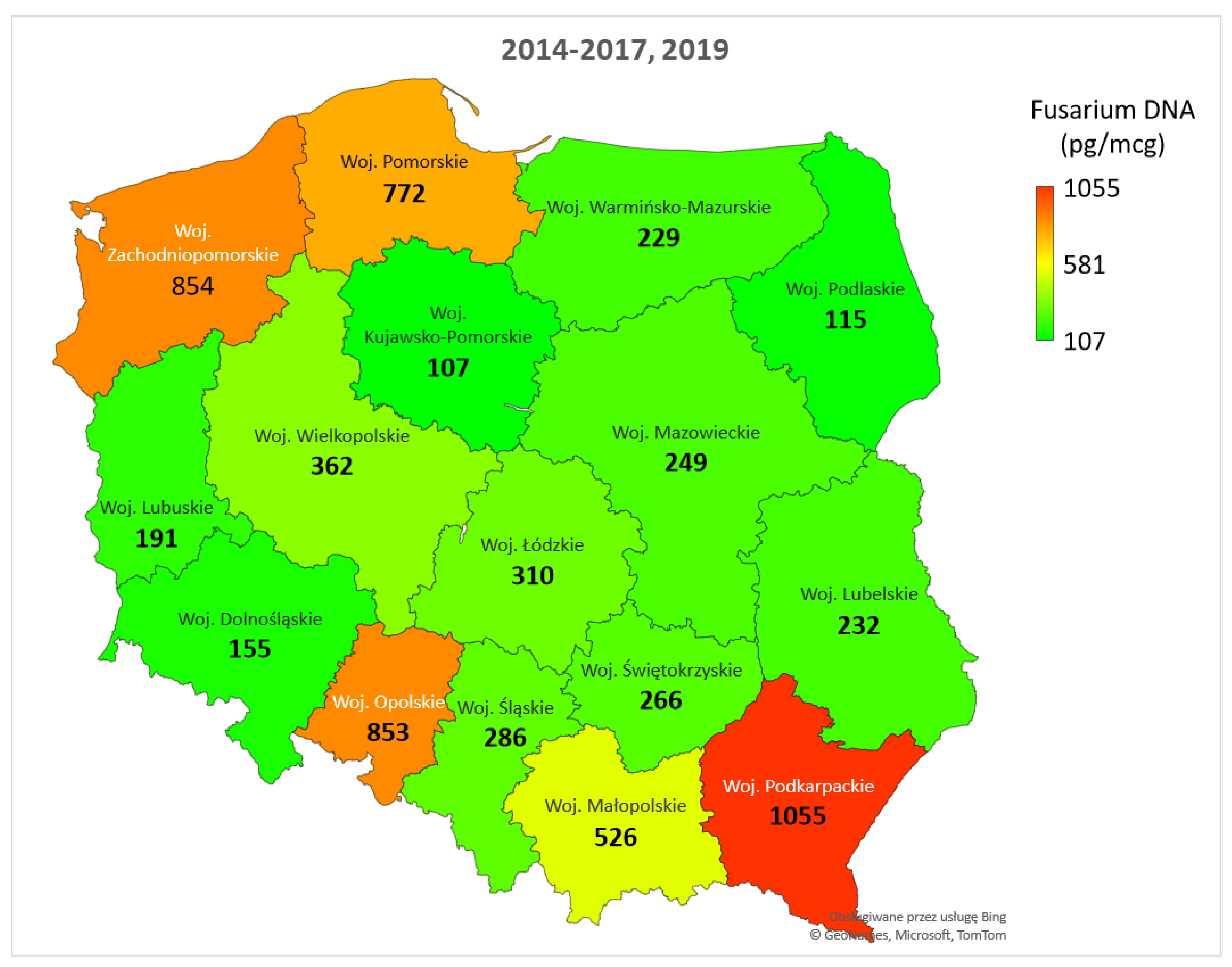
3. Results
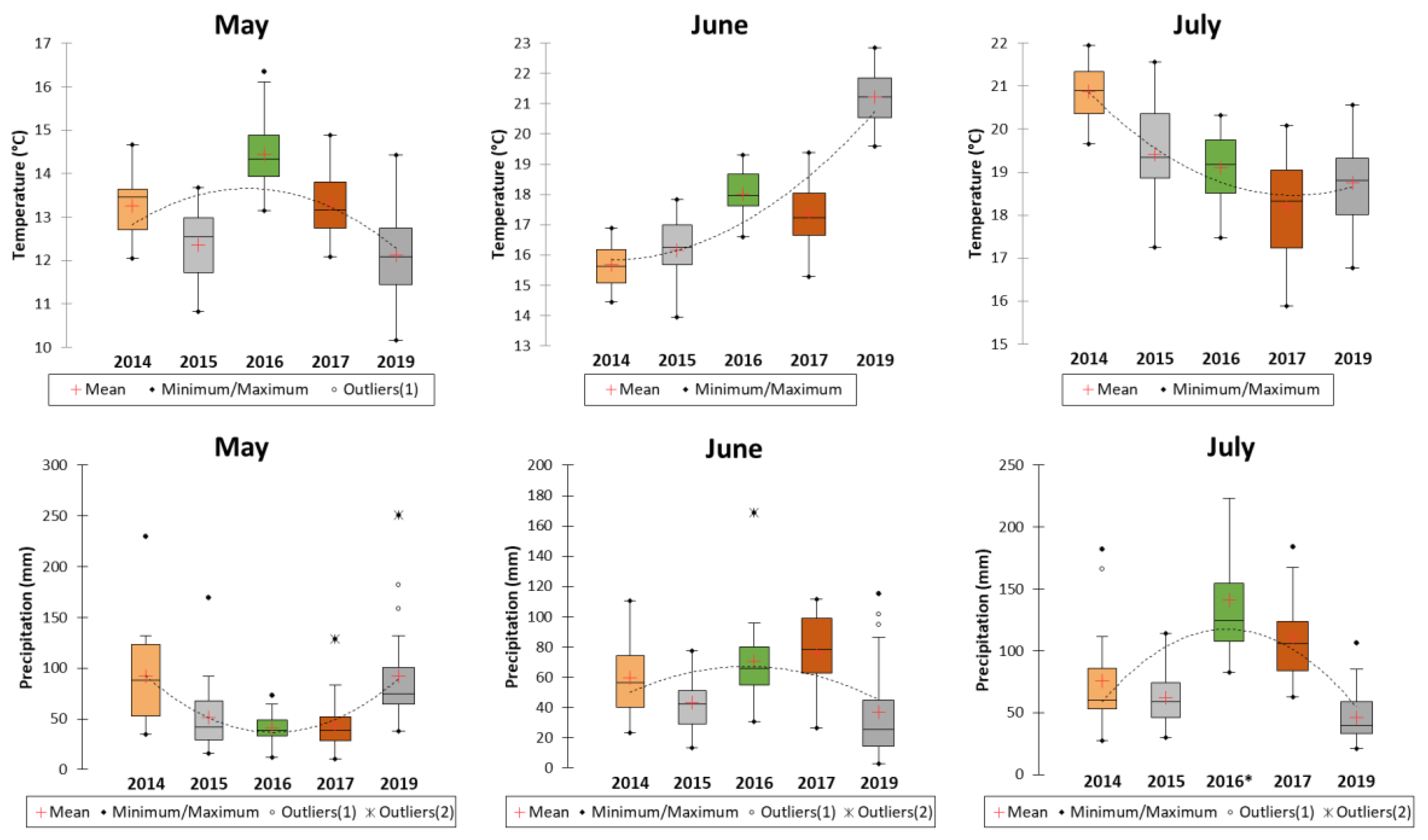
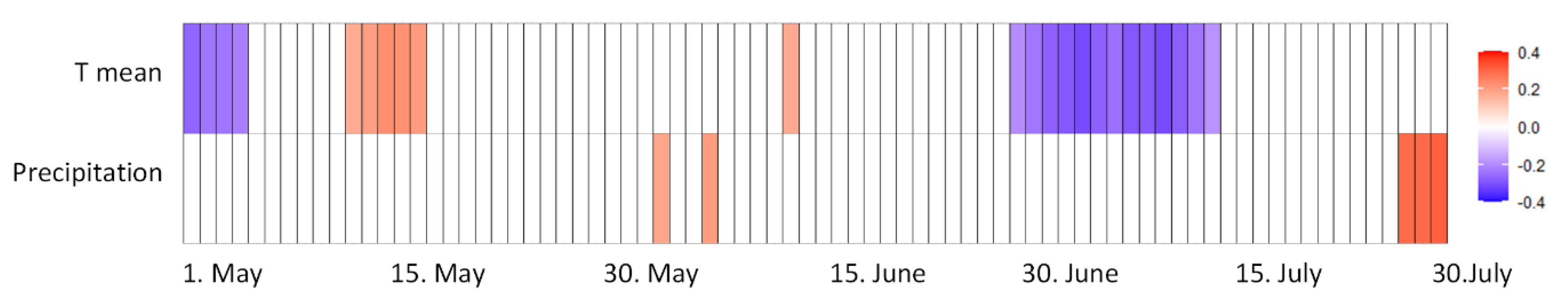
3.1. Weather Conditions
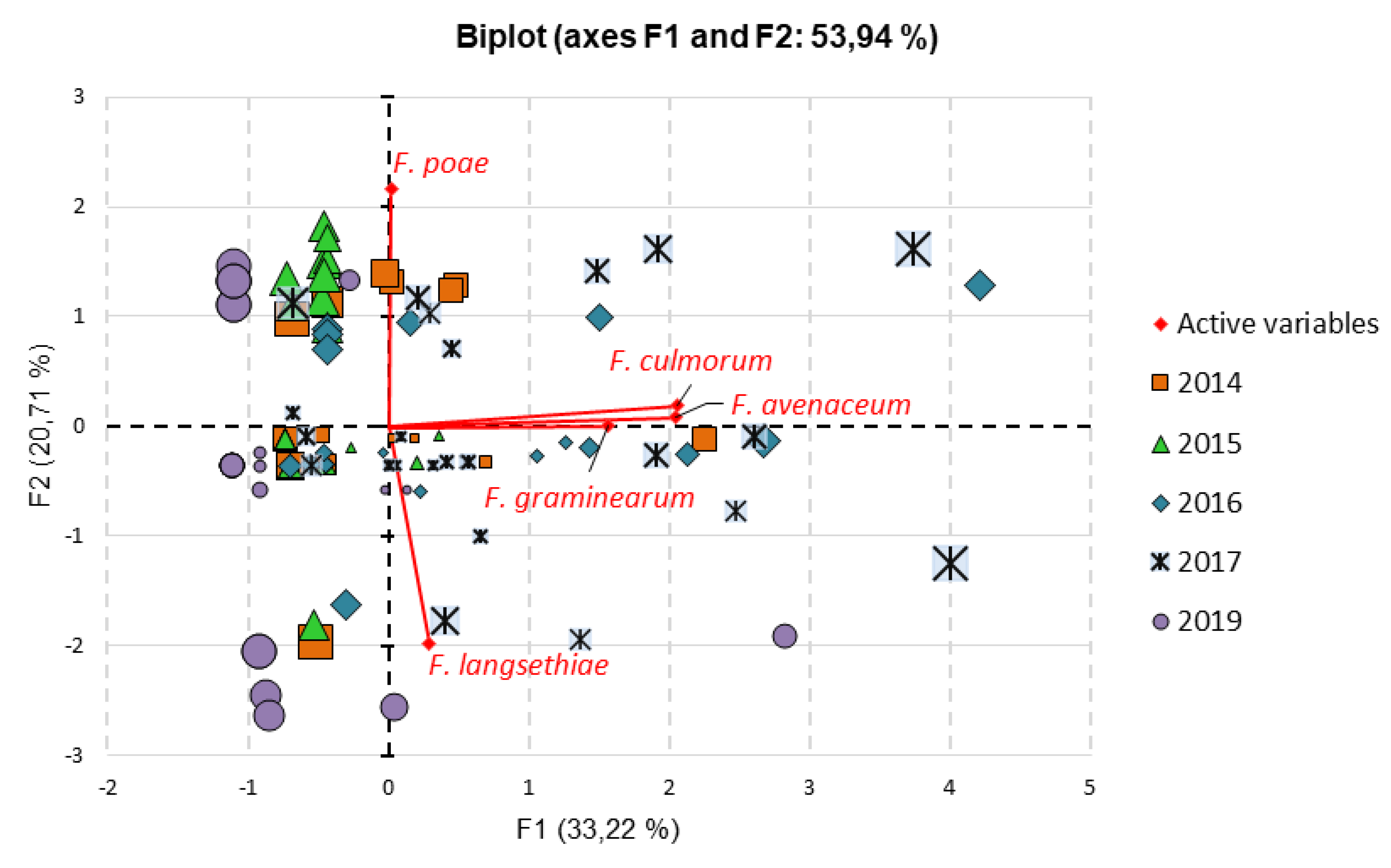
3.2. Fusarium Species
| Variables (n=111) | F. avenaceum | F. culmorum | F. graminearum s.s. | F. langsethiae |
|
F. culmorum p-value |
0.469 0.000 |
|||
|
F. graminearum s.s. p-value |
0.246 0.009 |
0.249 0.008 |
||
|
F. langsethiae p-value |
-0.012 0.899 |
0.055 0.567 |
0.067 0.488 |
|
|
F. poae p-value |
-0.050 0.599 |
0.037 0.703 |
0.031 0.745 |
-0.037 0.0698 |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium Ear Blight (Scab) in Small Grain Cereals—a Review. Plant Pathol 1995, 44, 207–238. [CrossRef]
- Bottalico, A. Fusarium Diseases of Cereals: Species Complex and Related Mycotoxin Profiles, in Europe. Journal of Plant Pathology 1998, 80, 85–103. [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur J Plant Pathol 2002, 108, 611–624. [CrossRef]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In Vitro and in Vivo Mycotoxin Production of Fusarium Species Isolated from Finnish Grains. Archives Of Phytopathology And Plant Protection 2008, 41, 545–558. [CrossRef]
- Somma, S.; Alvarez, C.; Ricci, V.; Ferracane, L.; Ritieni, A.; Logrieco, A.; Moretti, A. Trichothecene and Beauvericin Mycotoxin Production and Genetic Variability in Fusarium Poae Isolated from Wheat Kernels from Northern Italy. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2010, 27, 729–737. [CrossRef]
- Vanheule, A.; De Boevre, M.; Moretti, A.; Scauflaire, J.; Munaut, F.; De Saeger, S.; Bekaert, B.; Haesaert, G.; Waalwijk, C.; Van Der Lee, T.; et al. Genetic Divergence and Chemotype Diversity in the Fusarium Head Blight Pathogen Fusarium Poae. Toxins (Basel) 2017, 9, 255. [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.-R.R. Toxigenicity and Pathogenicity of Fusarium Poae and Fusarium Avenaceum on Wheat. Eur J Plant Pathol 2008, 122, 265–276. [CrossRef]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium Head Blight of Cereals in Denmark: Species Complex and Related Mycotoxins. Phytopathology 2011, 101, 960–969. [CrossRef]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium Avenaceum – the North European Situation. Int J Food Microbiol 2007, 119, 17–24. [CrossRef]
- Yli-Mattila, T.; Paaavanen-Huhtala, P.; Parikka, P.; Hietaniemi, V.; Jestoi, M.; Rizzo, A. Real-Time PCR Detection and Quantification of Fusarium Poae as Compared to Mycotoxin Production in Grains in Finland. In the Proceedings of the 2nd International Symposium on Fusarium Head Blight; incorporating the 8th European Fusarium seminar; Canty, S.M., Boring, T., Wardwell, J., Ward, R.W., Eds.; Orlando, FL, USA. East Lansing, MI: Michigan State University., 2004; pp. 422–425.
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T.; Haikara, A.; Laaksonen, S.; Rizzo, A. Real-Time PCR Detection and Quantification of Fusarium Poae, F. Graminearum, F. Sporotrichioides and F. Langsethiae in Cereal Grains in Finland and Russia. Archives of Phytopathology and Plant Protection 2008, 41, 243–260. [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.H. Gene Genealogies Reveal Global Phylogeographic Structure and Reproductive Isolation among Lineages of Fusarium Graminearum, the Fungus Causing Wheat Scab. Proceedings of the National Academy of Sciences 2000, 97, 7905–7910. [CrossRef]
- O’Donnell, K.; Ward, T.J.; Geiser, D.M.; Kistler, H.C.; Aoki, T. Genealogical Concordance between the Mating Type Locus and Seven Other Nuclear Genes Supports Formal Recognition of Nine Phylogenetically Distinct Species within the Fusarium Graminearum Clade. Fungal Genetics and Biology 2004, 41, 600–623. [CrossRef]
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.C.; Geiser, D.M.; Suga, H.; Tóth, B.; Varga, J.; O’Donnell, K. Global Molecular Surveillance Reveals Novel Fusarium Head Blight Species and Trichothecene Toxin Diversity. Fungal Genet Biol 2007, 44, 1191–1204. [CrossRef]
- Amarasinghe, C.; Sharanowski, B.; Dilantha Fernando, W.G. Molecular Phylogenetic Relationships, Trichothecene Chemotype Diversity and Aggressiveness of Strains in a Global Collection of Fusarium Graminearum Species. Toxins (Basel) 2019, 11, 263. [CrossRef]
- Talas, F.; Parzies, H.K.; Miedaner, T. Diversity in Genetic Structure and Chemotype Composition of Fusarium Graminearum Sensu Stricto Populations Causing Wheat Head Blight in Individual Fields in Germany. Eur J Plant Pathol 2011, 131, 39–48. [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium Graminearum Species Complex and Chemotypes: A Review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2015, 32, 453–460. [CrossRef]
- Backhouse, D. Global Distribution of Fusarium Graminearum, F. Asiaticum and F. Boothii from Wheat in Relation to Climate. Eur J Plant Pathol 2014, 139, 161–173. [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium Graminearum Species Complex: A Bibliographic Analysis and Web-Accessible Database for Global Mapping of Species and Trichothecene Toxin Chemotypes. Phytopathology 2022. [CrossRef]
- Bottalico, A. Fusarium Diseases of Cereals: Species Complex and Related Mycotoxin Profiles, in Europe. Journal of Plant Pathology 1998, 80, 85–103. [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur J Plant Pathol 2002, 108, 611–624. [CrossRef]
- Sundheim, L.; Brodal, G.; Hofgaard, I.S.; Rafoss, T. Temporal Variation of Mycotoxin Producing Fungi in Norwegian Cereals. Microorganisms 2013, 1, 188–198. [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van Der Lee, T.; Brodal, G. Associations between Fusarium Species and Mycotoxins in Oats and Spring Wheat from Farmers Fields in Norway over a Six-Year Period. World Mycotoxin J 2016, 9, 365–378. [CrossRef]
- Maiorano, A.; Blandino, M.; Reyneri, A.; Vanara, F. Effects of Maize Residues on the Fusarium Spp. Infection and Deoxynivalenol (DON) Contamination of Wheat Grain. Crop Protection 2008, 27, 182–188. [CrossRef]
- Obst, A.; Lepschy-von Gleissenthall, J.; Beck, R. On the Etiology of Fusarium Head Blight of Wheat in South Germany – Preceding Crops, Weather Conditions for Inoculum Production and Head Infection, Proneness of the Crop to Infection and Mycotoxin Production. Cereal Res Commun 1997, 25, 699–703. [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected Shifts in Fusarium Species’ Composition on Cereal Grain in Northern Europe Due to Climatic Change. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012, 29, 1543–1555. [CrossRef]
- Miller, J.D. Mycotoxins in Small Grains and Maize: Old Problems, New Challenges. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2008, 25, 219–230. [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium Culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol Plant Pathol 2013, 14, 323–341. [CrossRef]
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu Rev Phytopathol 2009, 47, 83–103. [CrossRef]
- Stępień, Ł.; Chełkowski, J. Fusarium Head Blight of Wheat: Pathogenic Species and Their Mycotoxins. World Mycotoxin J 2010, 3, 107–119. [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Żelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins (Basel) 2018, 10, 325. [CrossRef]
- Kuzdraliński, A.; Nowak, M.; Szczerba, H.; Dudziak, K.; Muszyńska, M.; Leśniowska-Nowak, J. The Composition of Fusarium Species in Wheat Husks and Grains in South-Eastern Poland. J Integr Agric 2017, 16, 1530–1536. [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Agnieszka, J.; Abrowska, D.˛; Radwí Nska, J. Prevalence of Fusarium Fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [CrossRef]
- Iwaniuk, P.; Konecki, R.; Snarska, K.; Łozowicka, B. Quantitative Evaluation of Fusarium Species and Crop Quality Traits in Wheat Varieties of Northeastern Poland. J Plant Prot Res 2018, 58, 413–419. [CrossRef]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium Species Infecting Wheat Heads in Poland. Cent Eur J Biol 2014, 9, 163–172. [CrossRef]
- Góral, T.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D. Species of the Genus Fusarium and Fusarium Toxins in the Grain of Winter and Spring Wheat in Poland. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 2021, 296, 25–42. [CrossRef]
- Postupolski, J.; Starski, A.; Ledzion, E.; Kurpińska-Jaworska, J.; Szczęsna, M. Assessment of Changes in the Occurrence of Fusarium Toxin and Ochratoxin A in Poland Related to Extreme Weather Phenomena. Rocz Panstw Zakl Hig 2019, 70, 127–135. [CrossRef]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Yoshinari, T.; Waskiewicz, A.; Szymczyk, K. Contamination of Wheat Cultivated in Various Regions of Poland during 2017 and 2018 Agricultural Seasons with Selected Trichothecenes and Their Modified Forms. Toxins (Basel) 2019, 11, 88. [CrossRef]
- Kowalska, G.; Kowalski, R. Occurrence of Mycotoxins in Selected Agricultural and Commercial Products Available in Eastern Poland. Open Chem 2021, 19, 653–664. [CrossRef]
- Marzec-Schmidt, K.; Börjesson, T.; Suproniene, S.; Jędryczka, M.; Janavičienė, S.; Góral, T.; Karlsson, I.; Kochiieru, Y.; Ochodzki, P.; Mankevičienė, A.; et al. Modelling the Effects of Weather Conditions on Cereal Grain Contamination with Deoxynivalenol in the Baltic Sea Region. Toxins (Basel) 2021, 13, 737. [CrossRef]
- Gourdain, E.; Piraux, F.; Barrier-Guillot, B. A Model Combining Agronomic and Weather Factors to Predict Occurrence of Deoxynivalenol in Durum Wheat Kernels. World Mycotoxin J 2011, 4, 129–139. [CrossRef]
- Aleksandrowicz, E. Factors Influencing the Occurrence of Fusarium Mycotoxins in the Grain of Winter Wheat. Polish Journal of Agronomy 2020, 103–112. [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10. [CrossRef]
- Góral, T. Nowe Dopuszczalne Limity Zawartości Toksyn Fuzaryjnych (Deoksyniwalenol, Toksyny T-2/HT-2) w Ziarnie Zbóż i Produktach Zbożowych. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 2024, 45–47. [CrossRef]
- Góral, T.; Walentyn-Góral, D. Zróżnicowanie Podatności Odmian Pszenicy Ozimej i Jarej Na Fuzariozę Kłosów Badanych w Latach 2009–2016. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 2018, 284, 3–11. [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-Time PCR for Quantification of Eleven Individual Fusarium Species in Cereals. J Microbiol Methods 2009, 76, 234–240. [CrossRef]
- Skelsey, P.; Newton, A.C. Future Environmental and Geographic Risks of Fusarium Head Blight of Wheat in Scotland. Eur J Plant Pathol 2015, 142, 133–147. [CrossRef]
- Zhang, X.; Halder, J.; White, R.P.; Hughes, D.J.; Ye, Z.; Wang, C.; Xu, R.; Gan, B.; Fitt, B.D.L. Climate Change Increases Risk of Fusarium Ear Blight on Wheat in Central China. Annals of Applied Biology 2014, 164, 384–395. [CrossRef]
- Madgwick, J.W.; West, J.S.; White, R.P.; Semenov, M.A.; Townsend, J.A.; Turner, J.A.; Fitt, B.D.L. Impacts of Climate Change on Wheat Anthesis and Fusarium Ear Blight in the UK. Eur J Plant Pathol 2011, 130, 117–131. [CrossRef]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, E.R.T.; Fernando, W.G.D. Weather-Based Models for Forecasting Fusarium Head Blight Risks in Wheat and Barley: A Review. Plant Pathol 2024, 73, 492–505. [CrossRef]
- Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of Post-Anthesis Rainfall, Fungicide and Harvesting Time on the Concentration of Deoxynivalenol and Zearalenone in Wheat. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2015, 32, 2075–2085. [CrossRef]
- Cowger, C.; Patton-Ozkurt, J.; Brown-Guedira, G.; Perugini, L. Post-Anthesis Moisture Increased Fusarium Head Blight and Deoxynivalenol Levels in North Carolina Winter Wheat. Phytopathology 2009, 99, 320–327. [CrossRef]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U. Trichothecene Genotypes Analysis of Fusarium Isolates from Di-, Tetra- And Hexaploid Wheat. Agronomy 2019, 9, 698. [CrossRef]
- Birzele, B.; Meier, A.; Hindorf, H.; Krämer, J.; Dehne, H.W. Epidemiology of Fusarium Infection and Deoxynivalenol Content in Winter Wheat in the Rhineland, Germany. Eur J Plant Pathol 2002, 108, 667–673. [CrossRef]
- Waalwijk, C.; Van Der Heide, R.; De Vries, I.; Van Der Lee, T.; Schoen, C.; Costrel-de Corainville, G.; Häuser-Hahn, I.; Kastelein, P.; Köhl, J.; Lonnet, P.; et al. Quantitative Detection of Fusarium Species in Wheat Using TaqMan. Eur J Plant Pathol 2004, 110, 481–494. [CrossRef]
- Isebaert, S.; De Saeger, S.; Devreese, R.; Verhoeven, R.; Maene, P.; Heremans, B.; Haesaert, G. Mycotoxin-Producing Fusarium Species Occurring in Winter Wheat in Belgium (Flanders) during 2002-2005. Journal of Phytopathology 2009, 157, 108–116. [CrossRef]
- Giraud, F.; Pasquali, M.; El Jarroudi, M.; Vrancken, C.; Brochot, C.; Cocco, E.; Hoffmann, L.; Delfosse, P.; Bohn, T. Fusarium Head Blight and Associated Mycotoxin Occurrence on Winter Wheat in Luxembourg in 2007/2008. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2010, 27, 825–835. [CrossRef]
- Chandelier, A.; Nimal, C.; André, F.; Planchon, V.; Oger, R. Fusarium Species and DON Contamination Associated with Head Blight in Winter Wheat over a 7-Year Period (2003–2009) in Belgium. Eur J Plant Pathol 2011, 130, 403–414. [CrossRef]
- Talas, F.; Parzies, H.K.; Miedaner, T. Diversity in Genetic Structure and Chemotype Composition of Fusarium Graminearum Sensu Stricto Populations Causing Wheat Head Blight in Individual Fields in Germany. Eur J Plant Pathol 2011, 131, 39–48. [CrossRef]
- Laszlo, E.; Varga, B.; Veisz, O. Composition of Fusarium Species Causing Natural Spike Infection in Wheat. Acta Agronomica Hungarica 2011, 59, 255–260. [CrossRef]
- van der Fels-Klerx, H.J.; de Rijk, T.C.; Booij, C.J.H.; Goedhart, P.W.; Boers, E.A.M.; Zhao, C.; Waalwijk, C.; Mol, H.G.J.; van der Lee, T.A.J. Occurrence of Fusarium Head Blight Species and Fusarium Mycotoxins in Winter Wheat in the Netherlands in 2009. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012, 29, 1716–1726. [CrossRef]
- Goliński, P.; Perkowski, J.; Kostecki, M.; Grabarkiewicz-Szczȩsna, J.; Chełkowski, J. Fusarium Species and Fusarium Toxins in Wheat in Poland - a Comparison with Neighbour Countries. Sydowia 1996, 48, 12–22.
- Kulik, T.; Jestoi, M. Quantification of Fusarium Poae DNA and Associated Mycotoxins in Asymptomatically Contaminated Wheat. Int J Food Microbiol 2009, 130, 233–237. [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M. A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and Association of Pathogenic Fungi Causing Fusarium Ear Blight in Wheat in Four European Countries. Eur J Plant Pathol 2005, 112, 143–154. [CrossRef]
- Sakalauskas, S.; Stumbriene, K.; Suproniene, S.; Svegzda, P. Changes in Fusarium Link Species Composition From Lithuanian Wheat Grain in Years 2005-2007 to 2011-2013. Proceedings of the Latvia University of Agriculture 2014, 32, 45–50. [CrossRef]
- Audenaert, K.; van Broeck, R.; van Bekaert, B.; de Witte, F.; Heremans, B.; Messens, K.; Höfte, M.; Haesaert, G.; Broeck, R.; Bekaert, B.; et al. Fusarium Head Blight (FHB) in Flanders: Population Diversity, Inter-Species Associations and DON Contamination in Commercial Winter Wheat Varieties. Eur J Plant Pathol 2009, 125, 445–458. [CrossRef]
- Hörberg, H.M. Patterns of Splash Dispersed Conidia of Fusarium Poae and Fusarium Culmorum. Eur J Plant Pathol 2002, 108, 73–80. [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected Shifts in Fusarium Species’ Composition on Cereal Grain in Northern Europe Due to Climatic Change. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012, 29, 1543–1555. [CrossRef]

| Target | Primer name | Sequence (5’-3’) |
|---|---|---|
| F. graminearum | Fgram F | CCATTCCCTGGGCGCT |
| species complex | Fgram R | CCTATTGACAGGTGGTTAGTGACTGG |
| F. culmorum | Fcul F | CACCGTCATTGGTATGTTGTCACT |
| Fcul R | CGGGAGCGTCTGATAGTCG | |
| F. langsethiae | Flang F | CAAGTCGACCACTGTGAGTACCTCT |
| Flang R | TGTCAAAGCATGTCAGTAAAGATGAC | |
| F. avenaceum | Fave F | TATGTTGTCACTGTCTCACACCACC |
| Fave R | AGAGGGATGTTAGCATGATGAAG | |
| F. poae | Fpoae F | ACCGAATCTCAACTCCGCTTT |
| Fpoae R | GTCTGTCAAGCATGTTAGCACAAGT | |
| EF1α | Hor1F | TCTCTGGGTTTGAGGGTGAC |
| Hor2R | GGCCCTTGTACCAGTCAAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





