Submitted:
17 September 2025
Posted:
18 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Marine-Derived Steroids with Anti-Cancer Activity
| No | Name | Structure | Source | Anti-Cancer Activity | Reference |
| 1 | 5α-cholesta-24-en-3β,20β-diol-23-one | 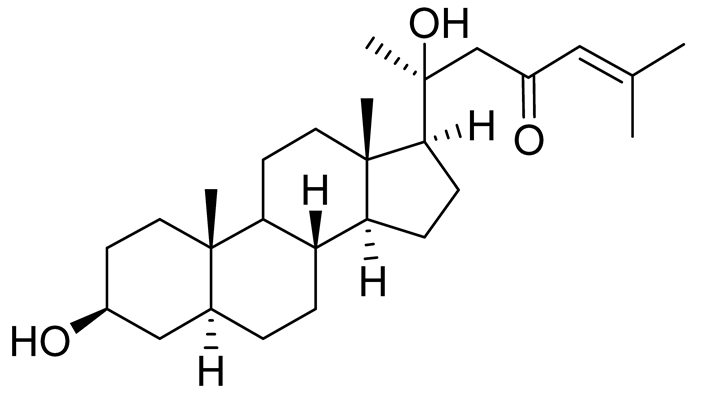 |
Crown-of-thorns starfish Acanthaster planci | Cytotoxic activity against luminal A breast cancer cells MCF-7 in MTT assay IC50 49 ± 1.6 μg/mL | [86] |
| 2 | 5α-cholesta-9(11)-en-3β,20β-diol | 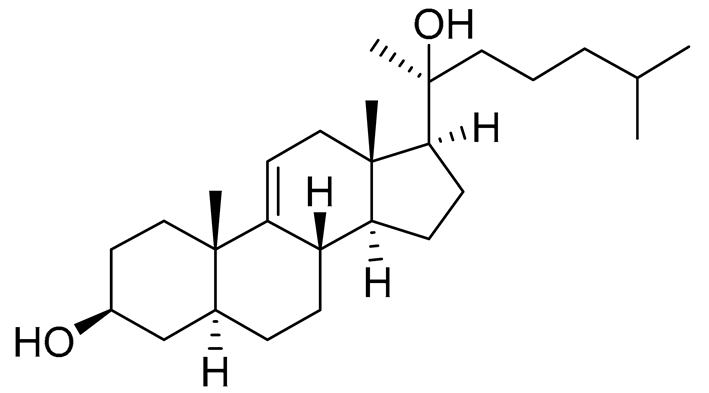 |
Crown-of-thorns starfish Acanthaster planci | Cytotoxic activity against luminal A breast cancer cells MCF-7 in MTT assay IC50 57.5 ± 1.5 μg/mL | [86] |
| 3 | Dendrodoristerol | 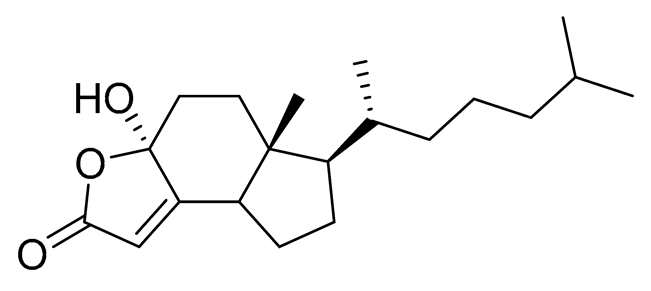 |
Sea slug Dendrodoris fumata |
Cytotoxic activity against hepatocellular carcinoma cells HepG2, prostate cancer cells LNCaP, breast cancer cells MCF-7, lung adenocarcinoma cells SK-LU-1, epidermal carcinoma cells KB, leukemia cells HL-60 in SRB assay IC50 21.63 ± 2.22, 22.22 ± 1.81, 24.53 ± 2.47, 41.19 ± 3.25, 25.34 ± 3.81, and 21.59 ± 1.38 μM | [111] |
| 4 | (25S)-5α-cholestane-3β,5,6β,15α,16β,26-hexaol | 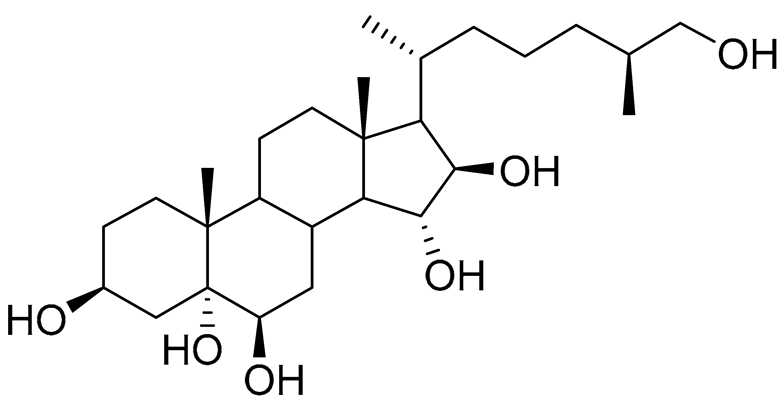 |
Mud star Ctenodiscus crispatus | Shows cytotoxic activity against hepatocellular carcinoma cells HepG2 in MTT assay | [88] |
| 5 | (3E)-cholest-4-en-3,6-dione-3-oxime | 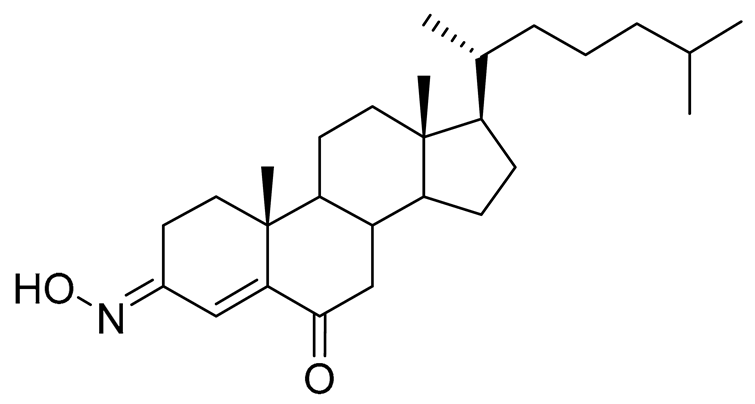 |
Sea sponge Cinachyrella australiensis | Cytotoxic activity against hepatocellular carcinoma cells HepG2 in MTT assay IC50 2.91 mg/mL | [89] |
| 6 | Gracilosulphate A |
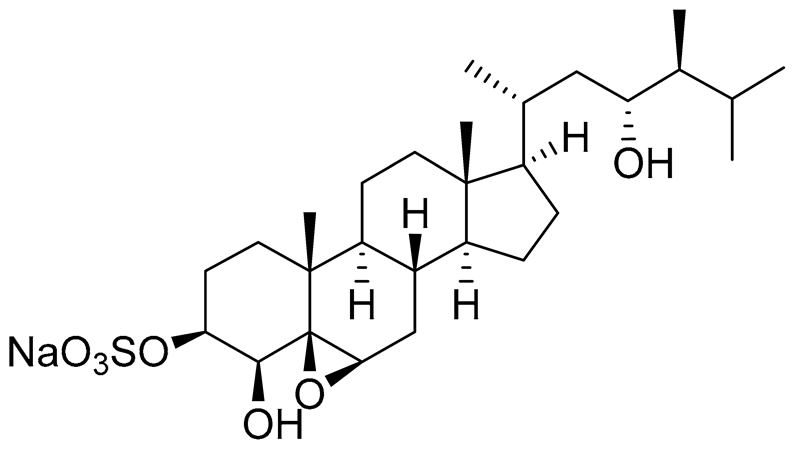 |
Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 64.4 ± 14.9 μM | [90] |
| 7 | Gracilosulphate B |
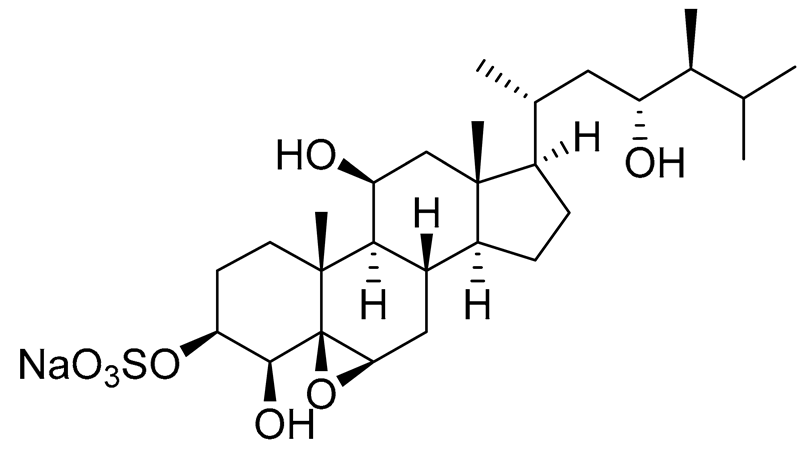 |
Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 8 | Gracilosulphate D |
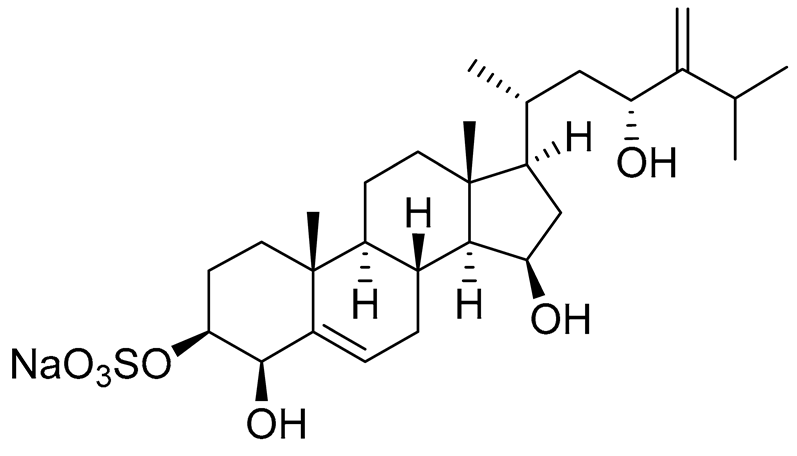 |
Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 9 | Gracilosulphate F | 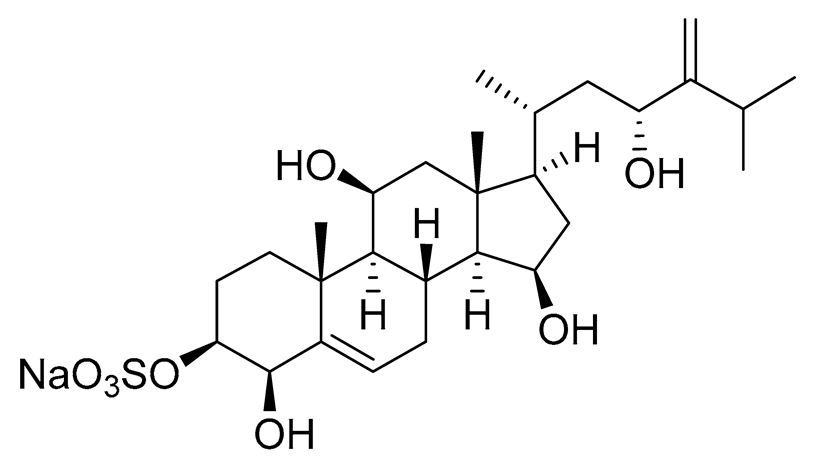 |
Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 10 | Gracilosulphate G |
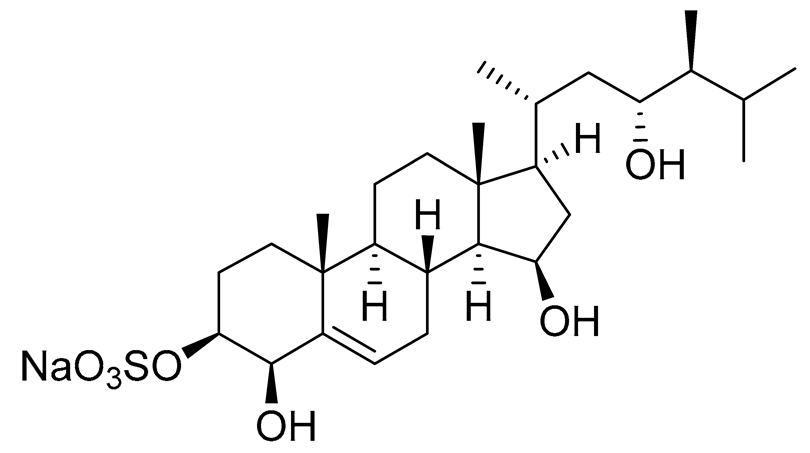 |
Sea sponge Haliclona gracilis | Cytotoxic activity against prostate cancer cell line 22Rv1 in MTT assay IC50 > 100 μM | [90] |
| 11 | β-sitosterol-3-O-(3Z)-pentacosenoate | 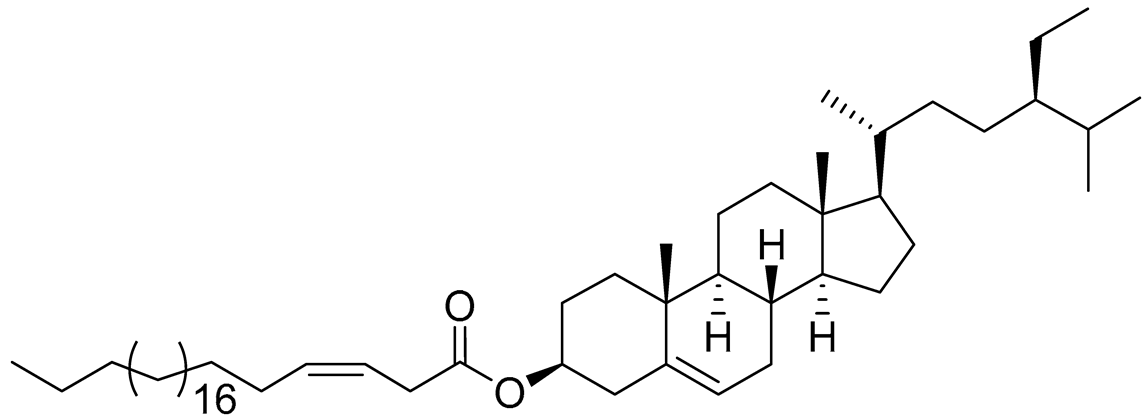 |
Sea sponge Echinoclathria gibbosa | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 64 μM | [91] |
| 12 | 5α-pregna-3β-acetoxy-12β,16β-diol-20-one | 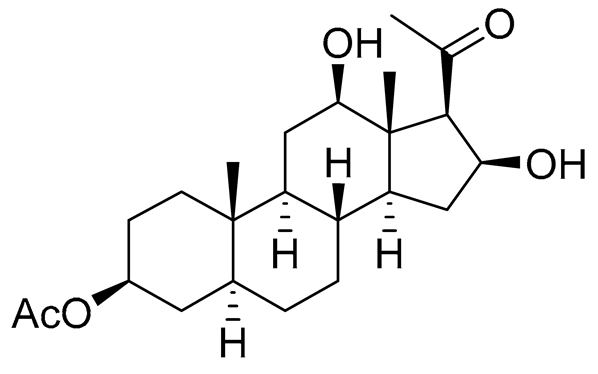 |
Sea sponge Echinoclathria gibbosa | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 > 100 μM | [91] |
| 13 | 3α,12α,16α-trihydroxy-24ξ-ethylcholest-25-ene | 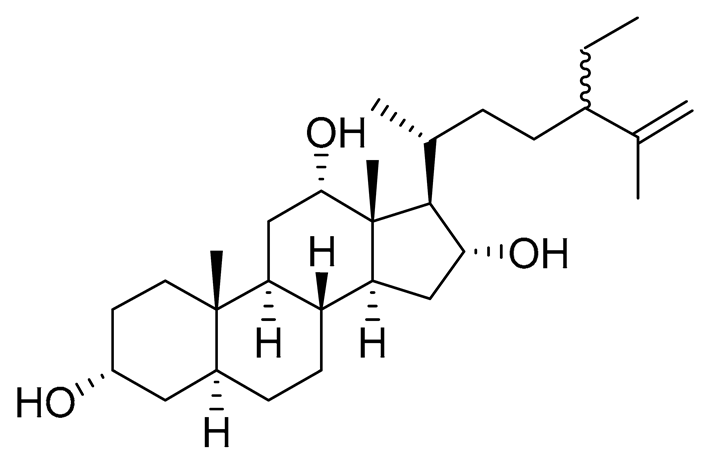 |
Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 13 ± 1 μM | [92] |
| 14 | 3α,12α,16α-trihydroxy-24R-methylcholest-22E-ene | 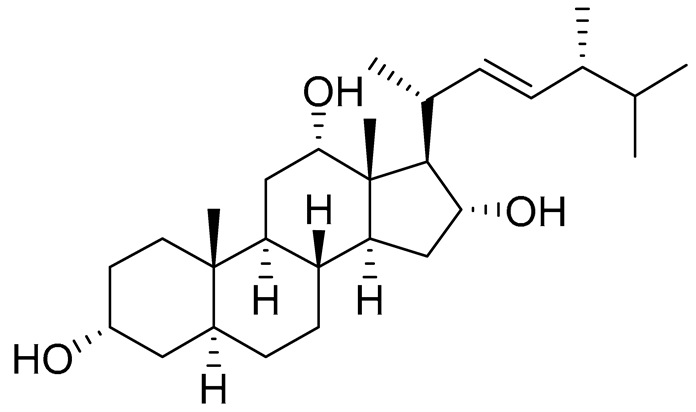 |
Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 27 ± 1 μM | [92] |
| 15 | 3α,12α,16α-trihydroxy-24-methylcholest-24(28)-ene | 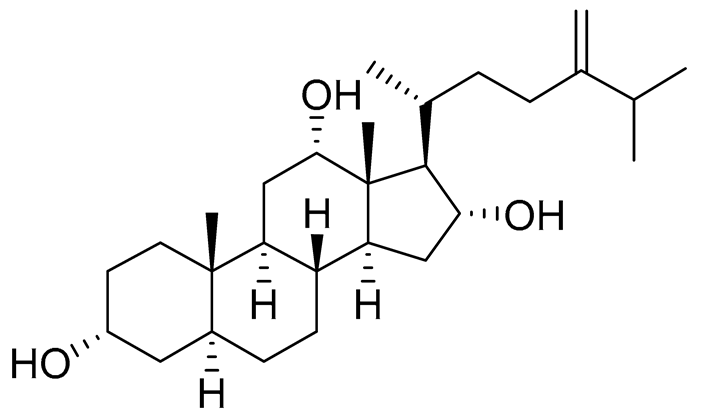 |
Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 27 ± 1 μM | [92] |
| 16 | 3α,12α,16α-trihydroxycholestane | 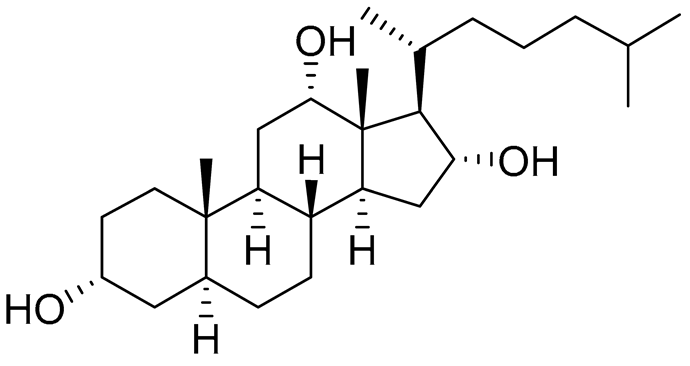 |
Sea sponge Psammoclema | Cytotoxic activity against prostate cancer cells DU-145 in MTT assay GI50 6.7 ± 0.2 μM | [92] |
| 17 | Archasteroside A | 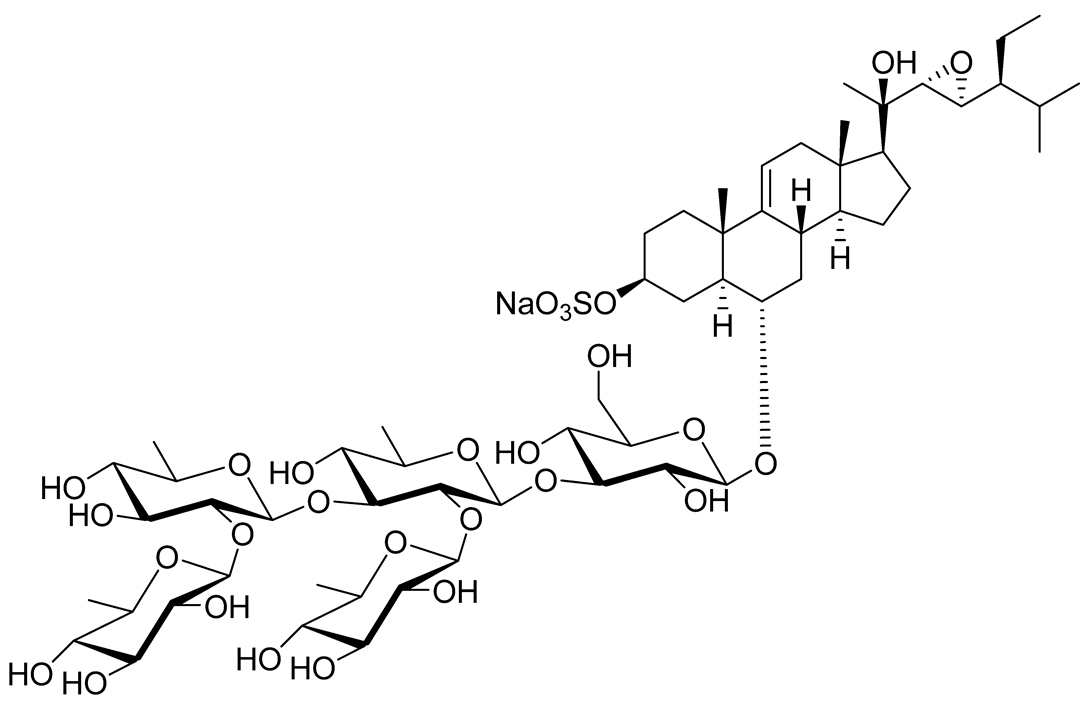 |
[93] | ||
| 18 | Archasteroside B | 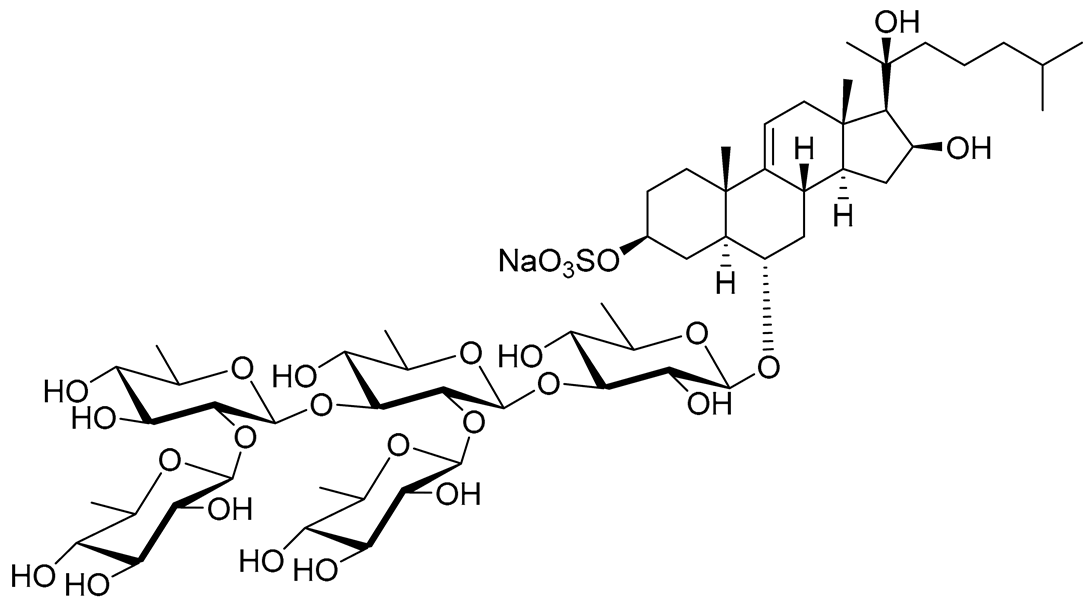 |
[93] | ||
| 19 | Halityloside A | 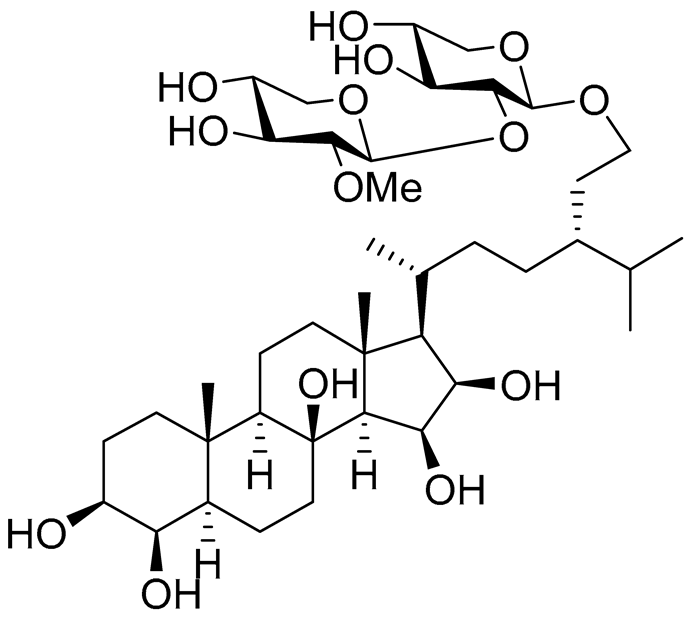 |
Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 48.59 ± 2.30 μM | [94] |
| 20 | Halityloside B | 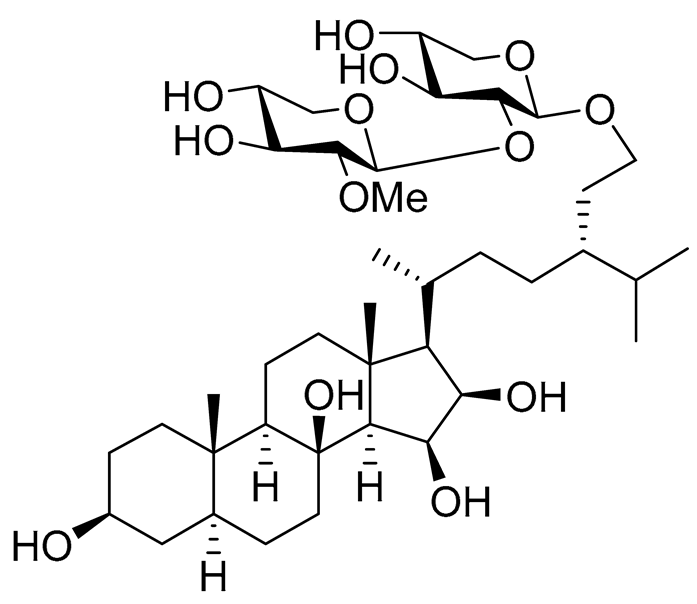 |
Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 39.68 ± 2.65 μM | [94] |
| 21 | Culcitoside C5 | 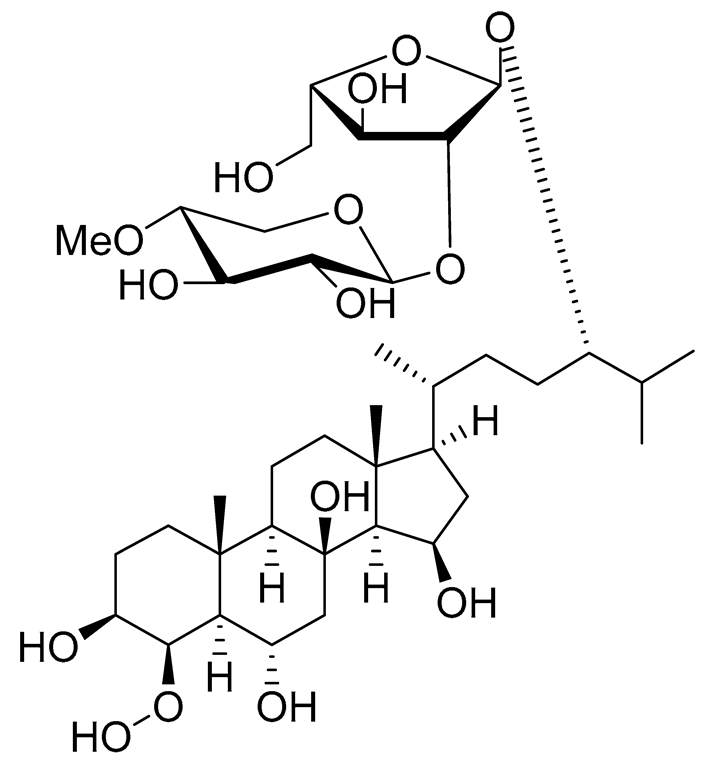 |
Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 57.08 ± 1.81 μM | [94] |
| 22 | Halityloside D | 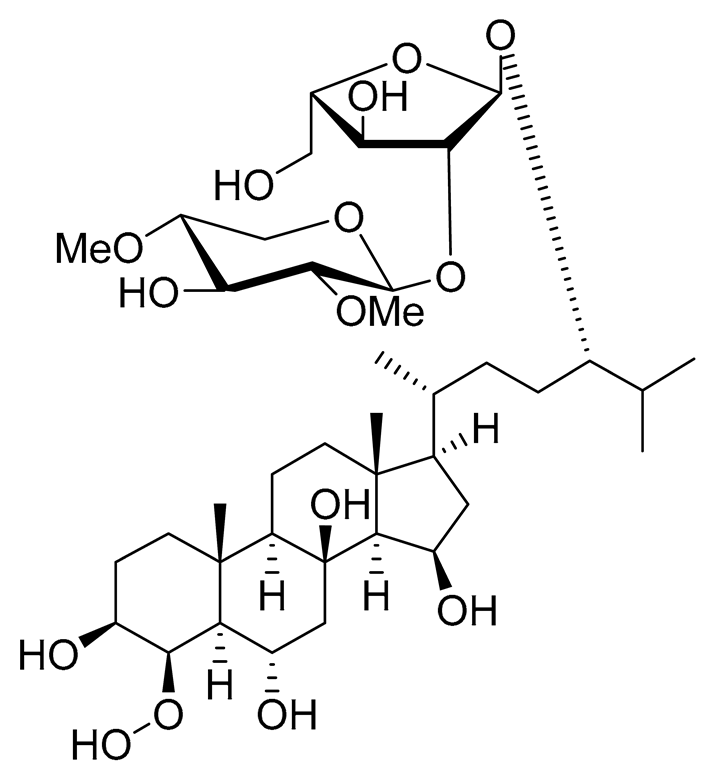 |
Starfish Culcita novaeguineae | Cytotoxic activity against prostate cancer cells LNCaP in SRB assay IC50 31.80 ± 1.59 μM | [94] |
| 23 | Spiculiferosides A |
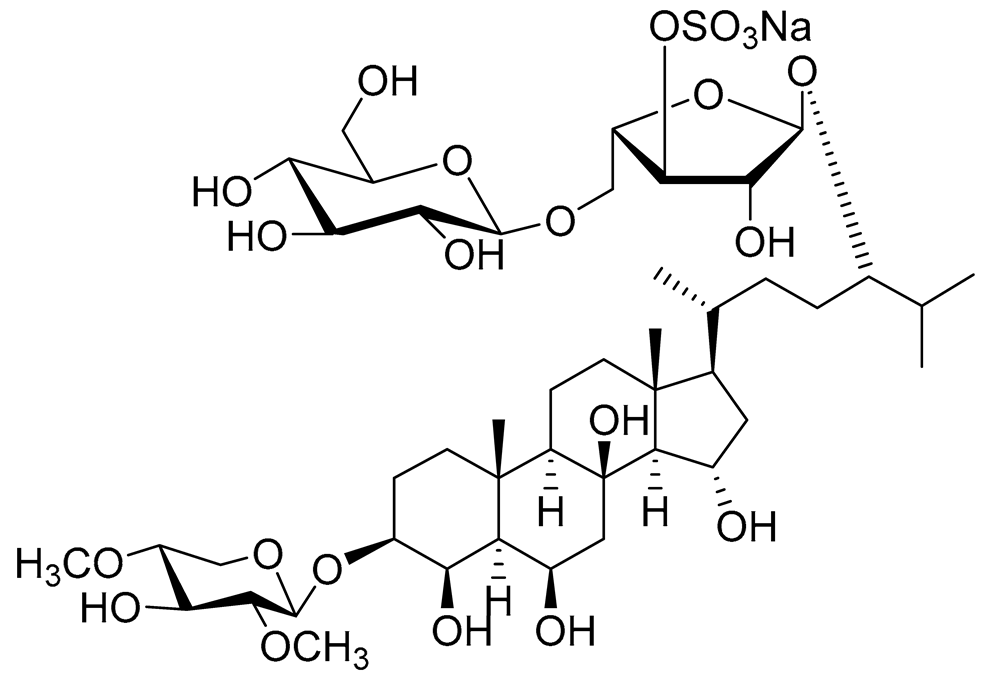 |
Starfish Henricia leviuscula spiculifera | Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 65% | [95] |
| 24 | Spiculiferosides B |
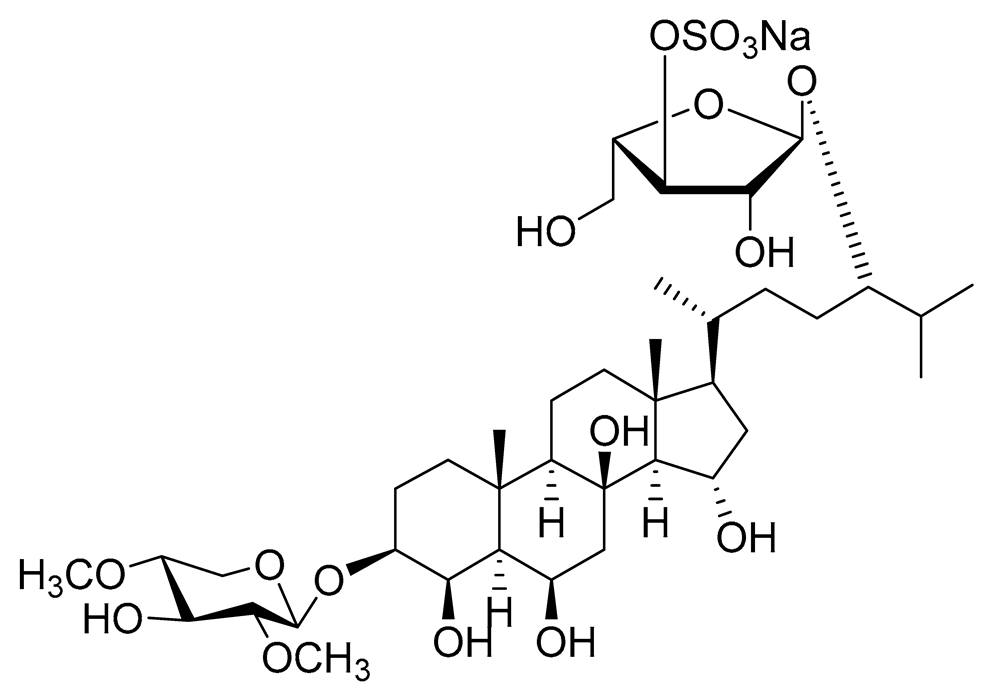 |
Starfish Henricia leviuscula spiculifera | Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 81% | [95] |
| 25 | Spiculiferosides C |
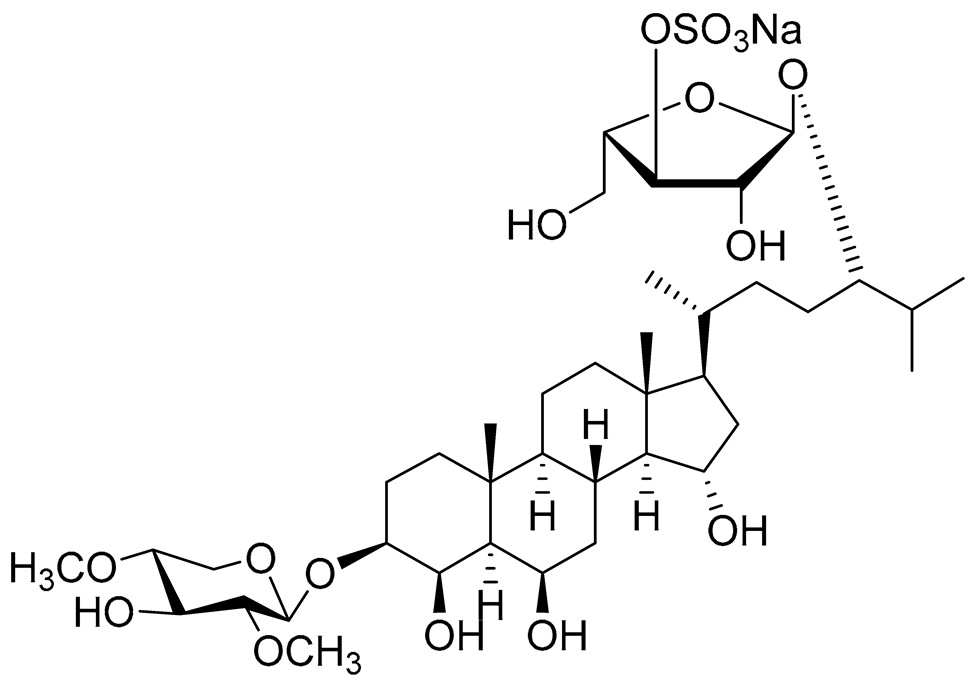 |
Starfish Henricia leviuscula spiculifera | Cytotoxic activity against colorectal carcinoma cells HCT 116 in MTS assay IC50 87.6 μM; Inhibition of colony formation colorectal carcinoma cells HCT 116 at concentration 40 μM was 87% |
[95] |
| 26 | (20R,22E)-24-norcholesta-5,22-diene-3β,21-diol 3,21-disulfate disodium salt | 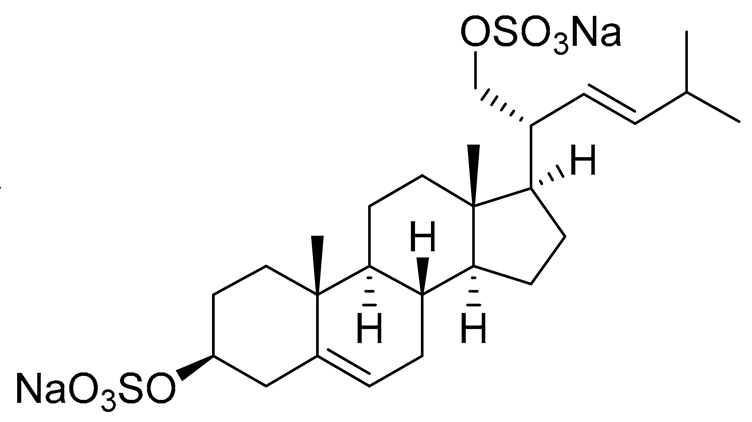 |
Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 76% | [112] |
| 27 | (20R,22E)-24-nor-5α-cholest-22-ene-3β,21-diol 3,21-disulfate disodium salt | 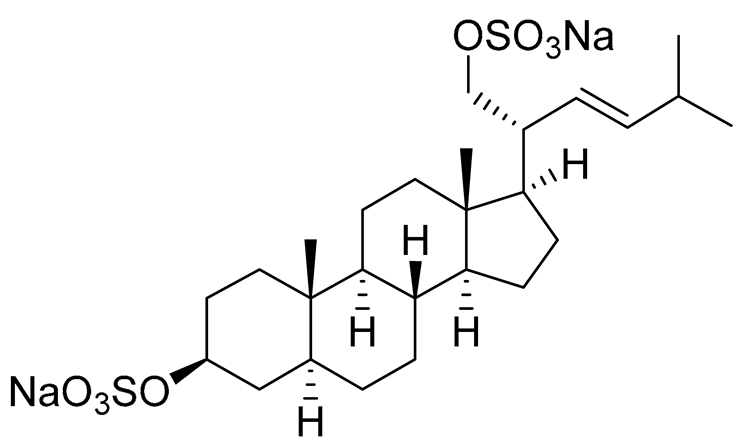 |
Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 86% | [112] |
| 28 | (20R)-7-oxo-24-methylcholesta-5,24(28)-diene-3β ,21-diyl disulfate disodium salt |
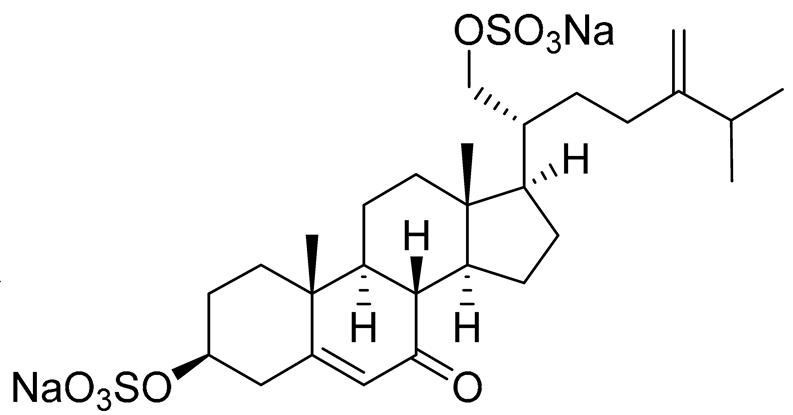 |
Starfish Pteraster marsippus | Cytotoxic activity of the mixture of 28 and 29 against human breast carcinoma cells ZR-75-1 in MTS assay IC50 90.4 μM | [96] |
| 29 | (20R)-7-oxo-24-methyl-5α-cholest-24(28)-ene-3 β,21-diyl disulfate disodium salt |
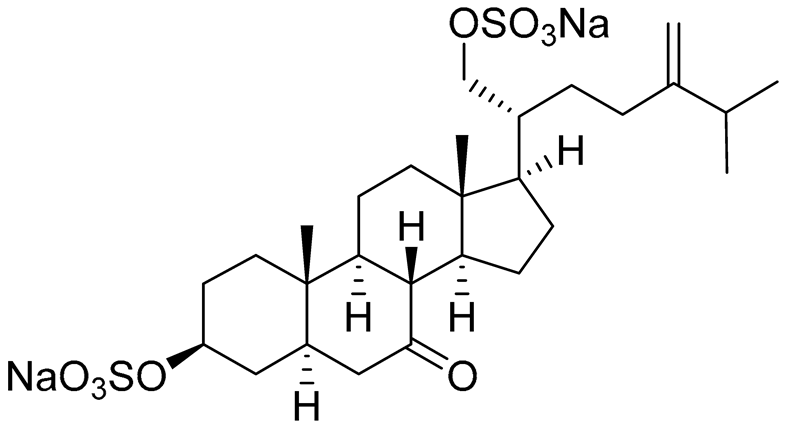 |
Starfish Pteraster marsippus | Cytotoxic activity of the mixture of 28 and 29 against human breast carcinoma cells ZR-75-1 in MTS assay IC50 90.4 μM | [96] |
| 30 | (20S,22R)-24-metylcholesta-5,24-diene-3β,22-diol 3,22-disulfate disodium salt | 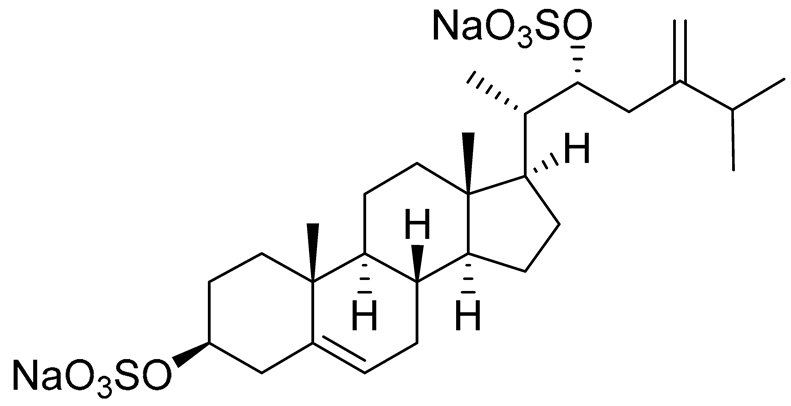 |
Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 71% | [112] |
| 31 | (20S,22R)-24-metyl-5α-cholest-24-ene-2β,3α,22-triol 3,22-disulfate disodium salt | 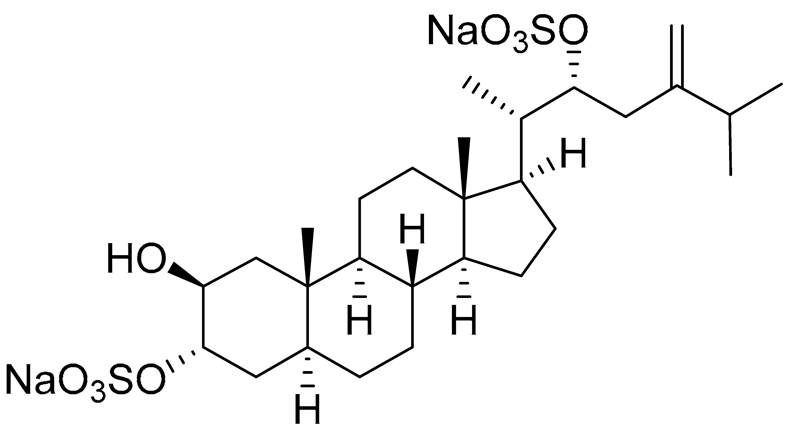 |
Starfish Pteraster marsippus | Inhibition of colony formation breast cancer cells T-47D at concentration 50 μM was 79% | [112] |
| 32 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 5′Z,11′Z-octadecadienoate | 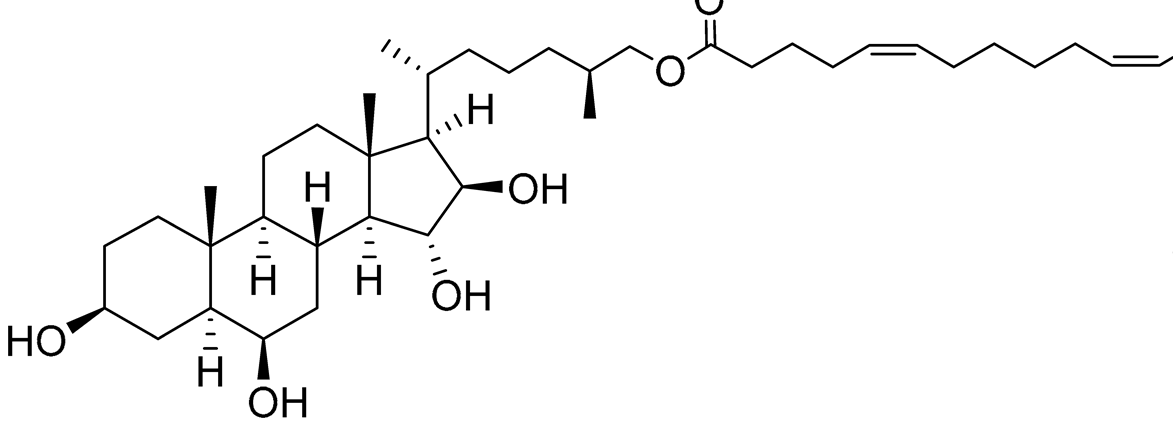 |
Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 36% | [97] |
| 33 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 11'Z-octadecenoate | 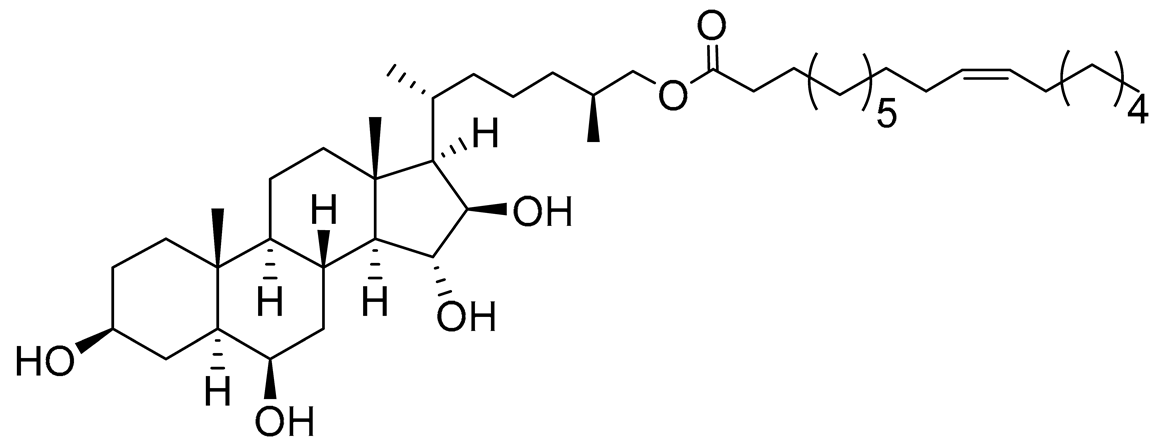 |
Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 73% | [97] |
| 34 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 5'Z,11'Z-eicosadienoate | 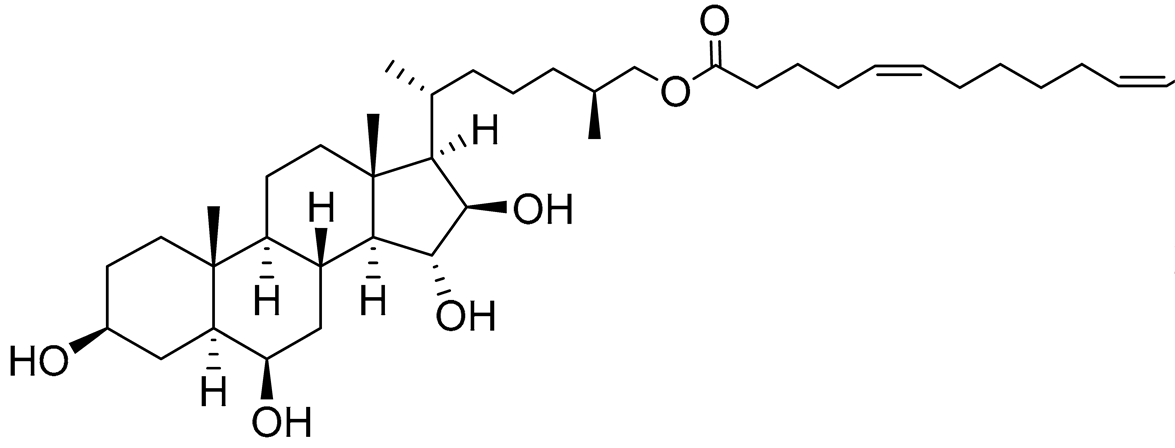 |
Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 30% | [97] |
| 35 | (25S)-5α-cholestane-3β,6β,15α,16β-tetraol-26-yl 7'Z-eicosenoate | 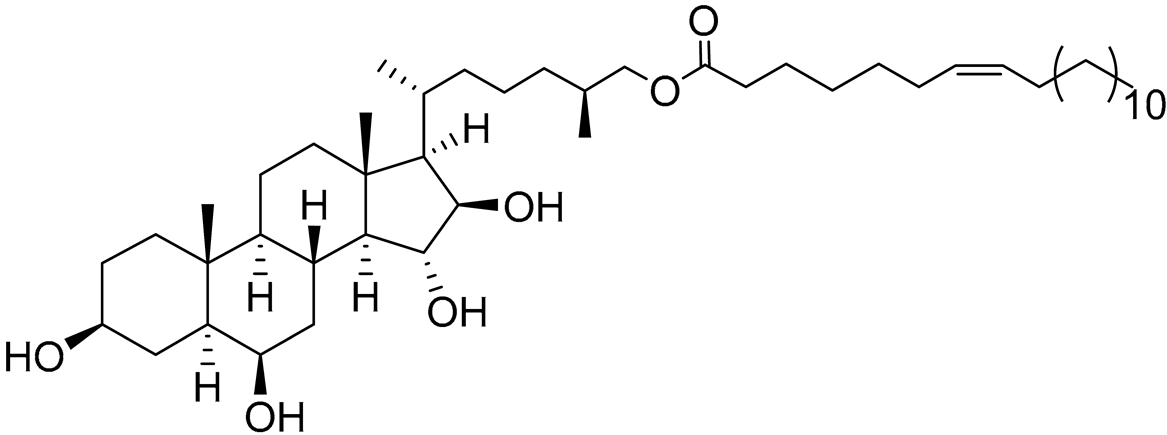 |
Starfish Ceramaster patagonicus | Inhibitory activity against migration of colorectal carcinoma cells HCT 116 was 24% | [97] |
| 36 | (23R)-methoxycholest-5,24-dien-3β-ol | 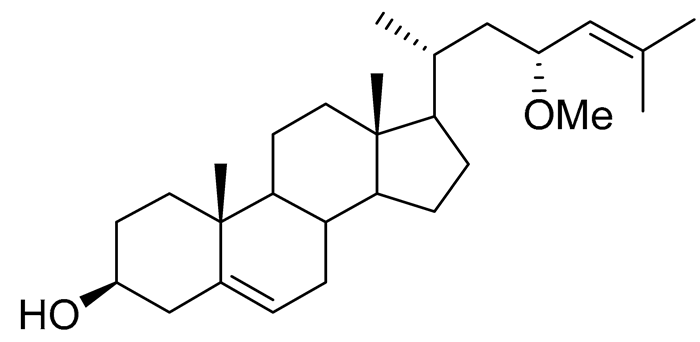 |
Colonial bryozoan Cryptosula pallasiana | Cytotoxic activity against hepatocellular carcinoma cells HepG2, gastric carcinoma SGC-7901 and leukemia cells HL-60 in MTT assay IC50 12.34 ± 0.12, 18.37 ± 0.17 and 17.64 ± 0.32 μM | [98] |
| 37 | Cerevisterol | 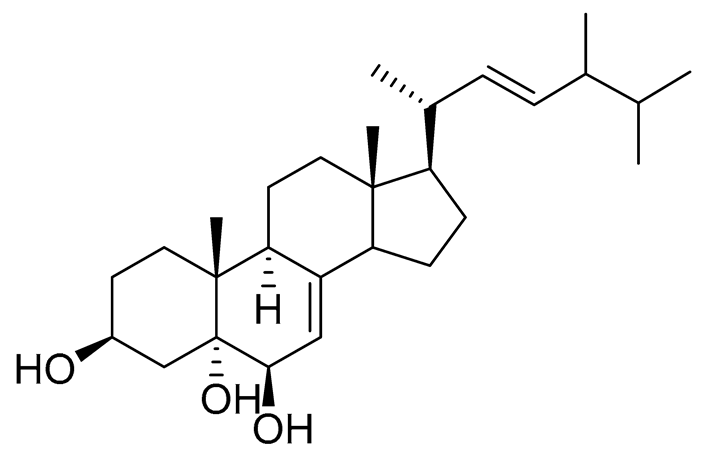 |
Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549 and breast cancer cells MCF-7 in MTT assay was not detected | [99] |
| 38 | Ergosterol peroxide | 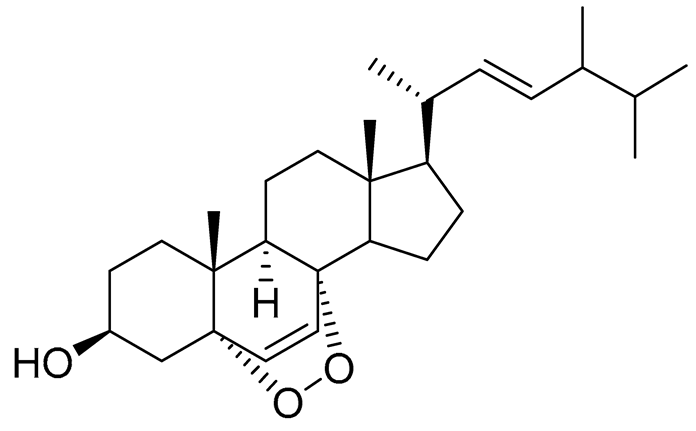 |
Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549 and breast cancer cells MCF-7 in MTT assay IC50 16.22, 22.48 and 27.11 μM | [99] |
| 39 | (3β,5α,22E)-ergosta-6,8(14),22-triene-3,5-diol | 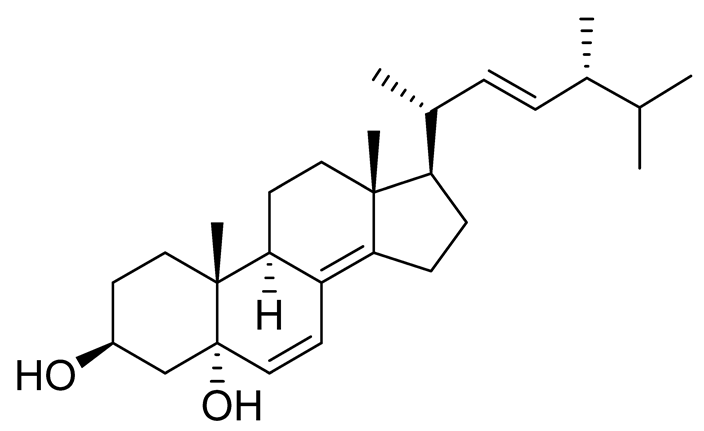 |
Marine fungus Penicillium levitum | Cytotoxic activity against hepatocellular carcinoma cells HepG2, lung carcinoma cells A549 and breast cancer cells MCF-7 in MTT assay IC50 2.89, 18.51 and 16.47 μM | [99] |
| 40 | (24E)-stigmasta-24(28)-en-3,6-dione | 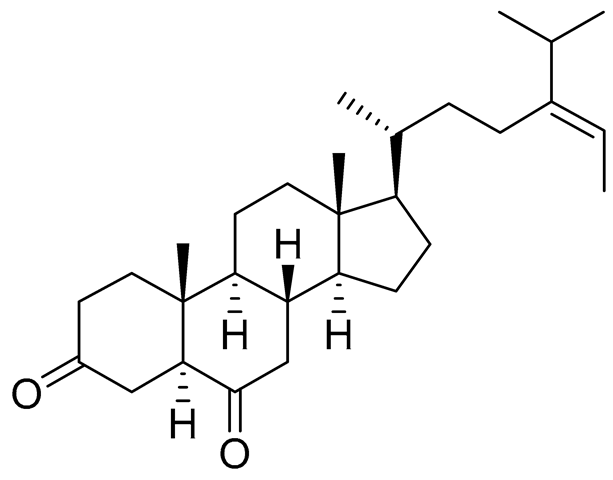 |
Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3 and prostate cancer cells LNCaP in MTT assay IC50 31.27 ± 1.50, 40.59 ± 3.10 and 19.80 ± 3.84 μM | [100] |
| 41 | Fucosterol | 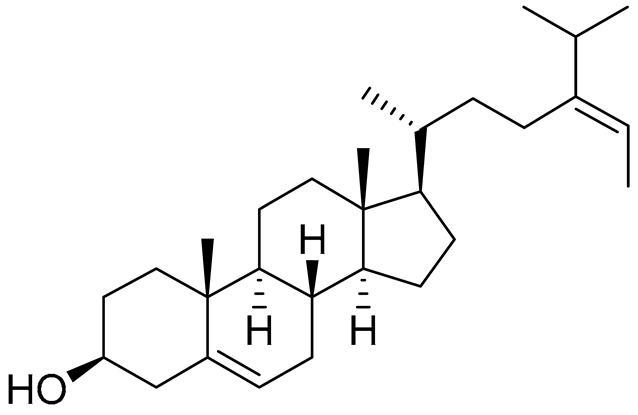 |
Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3 and prostate cancer cells LNCaP in MTT assay IC50 12.38 ± 2.47, 2.14 ± 0.33 and 1.38 ± 0.07 μM | [100] |
| 42 | Saringosterol | 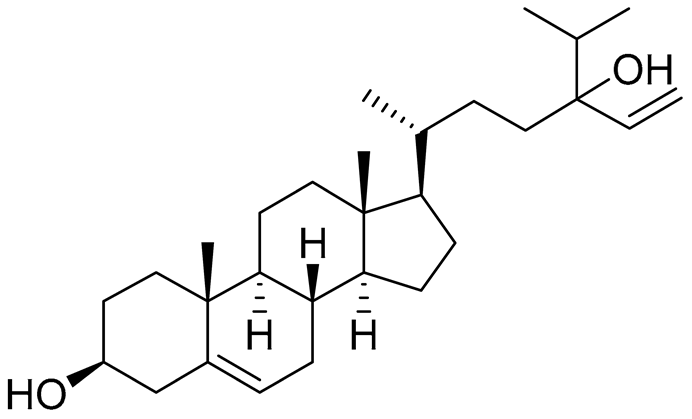 |
Green algae Tydemania expeditionis | Cytotoxic activity against prostate cancer cells DU-145, prostate cancer cells PC-3 and prostate cancer cells LNCaP in MTT assay IC50 >50, >50 and 41.60 ± 4.26 μM | [100] |
| 43 | Cholest-8-ene-3β,5α,6β,7α-tetraol | 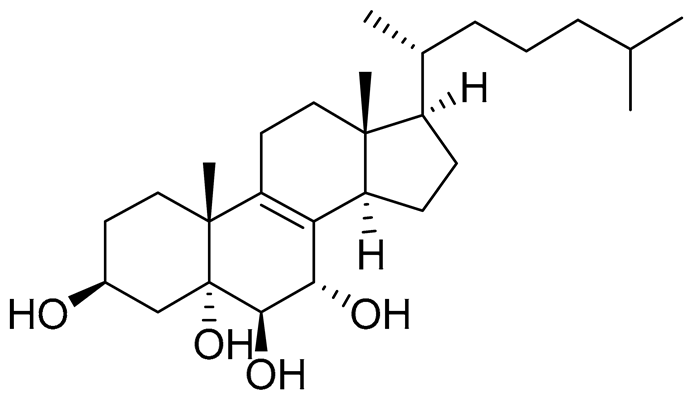 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 40.43 ± 1.45 μM | [101] |
| 44 | Cholest-8(14)-ene-3β,5α,6β,7α-tetraol | 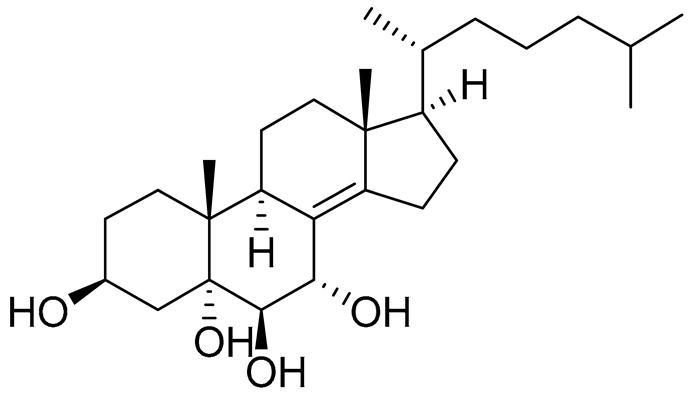 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 5.49 ± 0.22 μM | [101] |
| 45 | Cholest-7-ene-3β,5α,6β-triol | 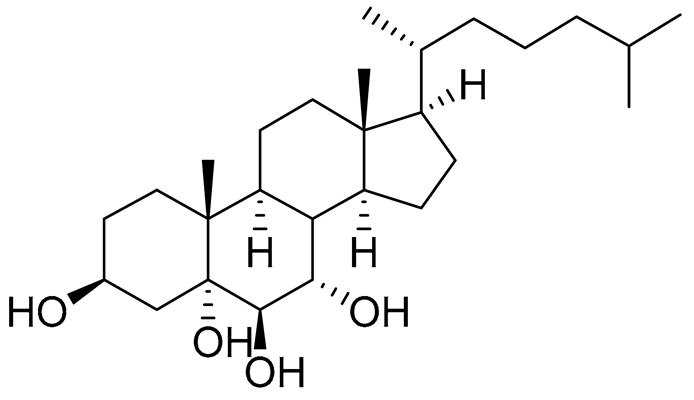 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 74.06 ± 3.46 μM | [101] |
| 46 | Cholest-7-ene-3β,5α,6α,9α-tetraol | 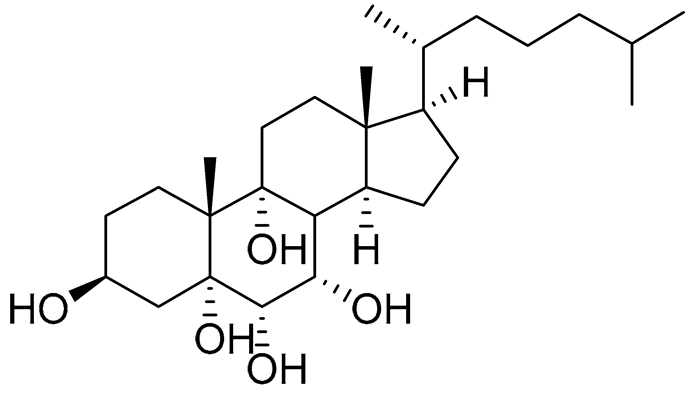 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 27.41 ± 0.50 μM | [101] |
| 47 | Cholest-7-ene-6-one-3β,5α,9α-triol | 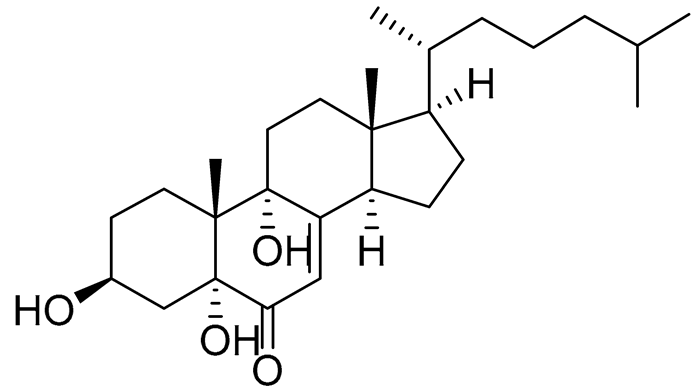 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 24.40 ± 0.46 μM | [101] |
| 48 | Cholest-5-ene-3β,7α-diol | 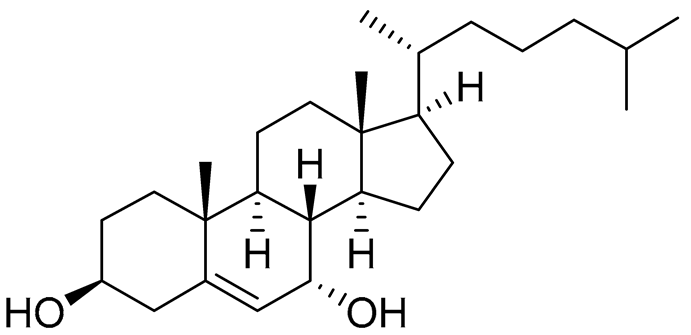 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 29.22 ± 0.17 μM | [101] |
| 49 | Cholest-5-ene-3β,7β-diol | 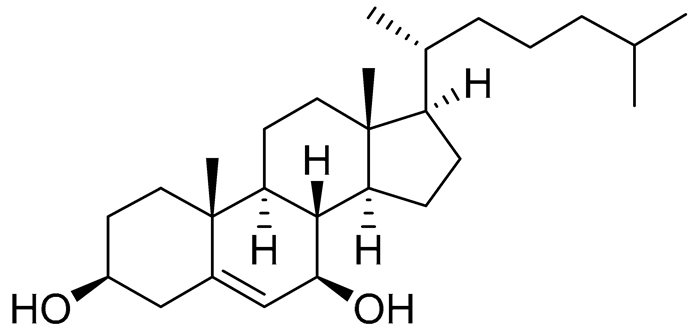 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 27.94 ± 0.63 μM | [101] |
| 50 | Cholest-5-ene-7β-methoxy-3β-ol | 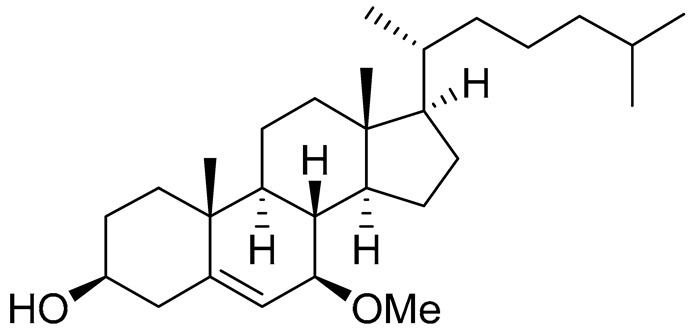 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 9.22 ± 0.67 μM | [101] |
| 51 | Campesterol | 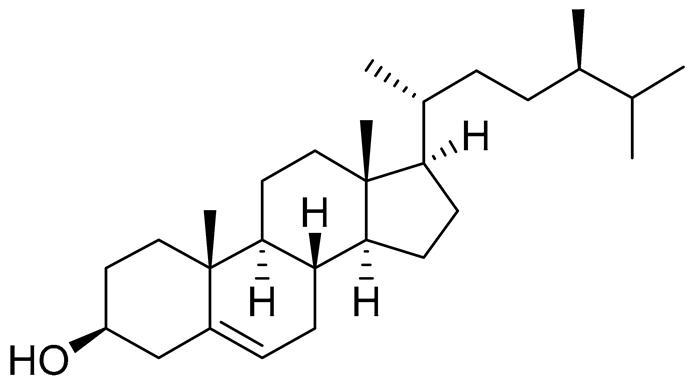 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 22.26 ± 0.59 μM | [101] |
| 52 | Cholest-5-ene-3β-sulfate sodium solt | 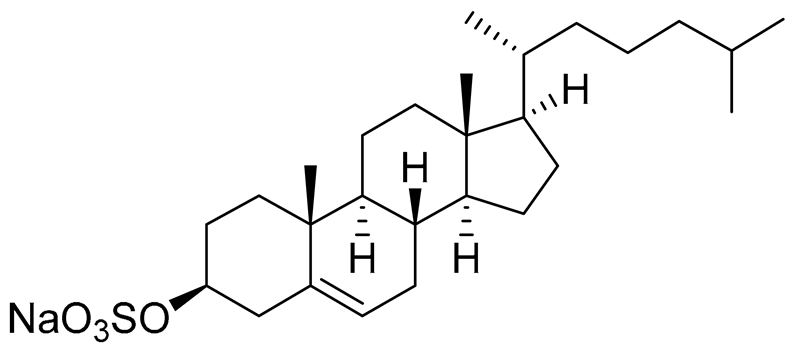 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 68.87 ± 6.08 μM | [101] |
| 53 | Cholest-6-ene-5α,8α-epidioxy-3β-ol | 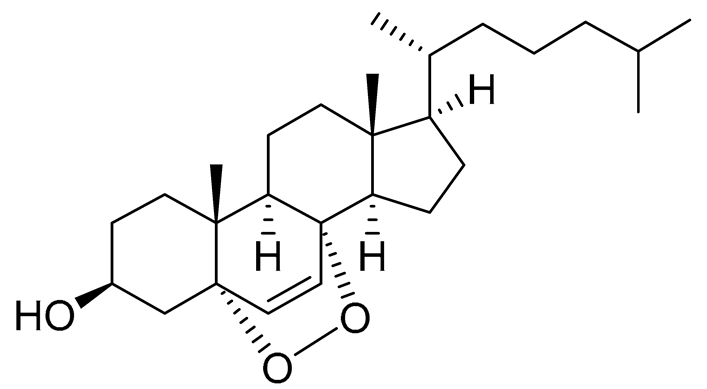 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay IC50 6.99 ± 0.28 μM | [101] |
| 54 | Cholest-5-ene-3β-ol | 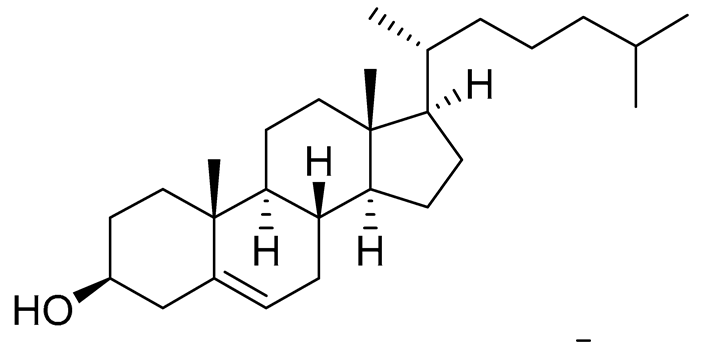 |
Sea urchin Diadema savignyi | Cytotoxic activity against prostate cancer cells PC-3 in MTT assay was not detected | [101] |
| 55 | Klyflaccisteroid A | 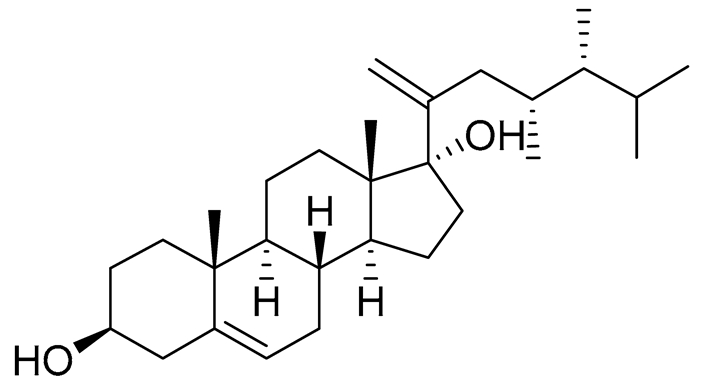 |
Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549 and murine leukemia cells P388 in Alamar Blue assay ED50 >20, 7.7 and >20 μg mL-1 | 10.1039/c4ra13977a |
| 56 | Klyflaccisteroid F | 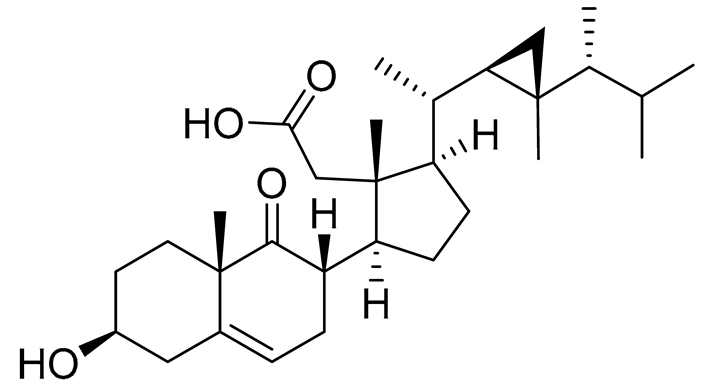 |
Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549 and murine leukemia cells P388 in Alamar Blue assay ED50 >20, 14.5 and 17.9 μg mL-1 | [102] |
| 57 | Klyflaccisteroid C | 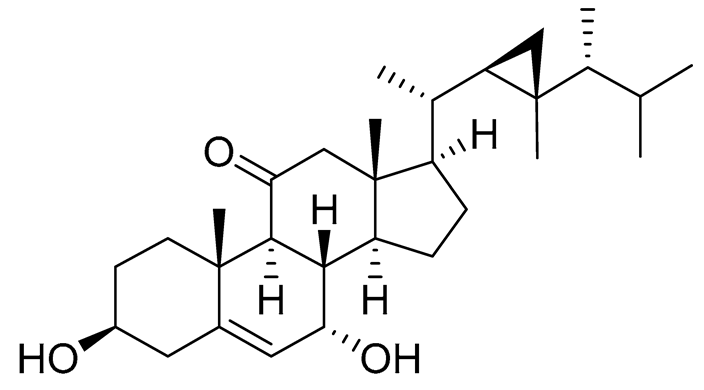 |
Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29, lung cancer cells A549 and murine leukemia cells P388 in Alamar Blue assay ED50 8.2, 6.1 and 10.8 μg mL-1 | [102] |
| 58 | Klyflaccisteroid E | 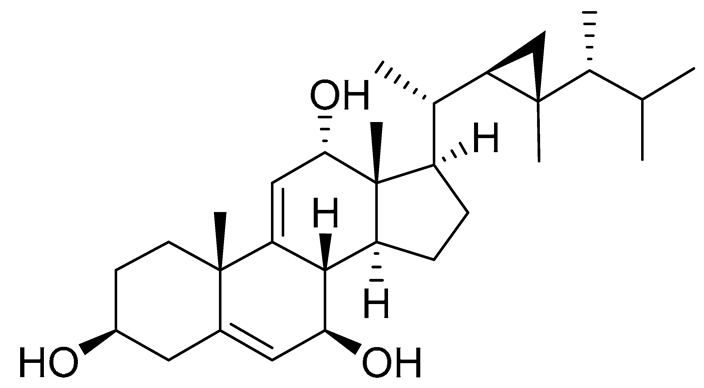 |
Soft coral Klyxum flaccidum | Cytotoxic activity against colon cancer cells HT-29 and murine leukemia cells P388 in Alamar Blue assay ED50 6.9 and 3.7 μg mL-1 | [102] |
| 59 | Ergosta-24(28)-ene-3β,5α,6β-triol-6-acetate | 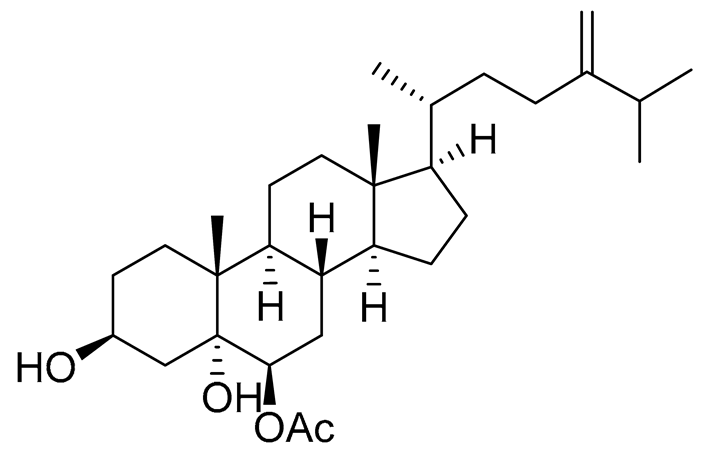 |
Soft coral Sinularia conferta | Cytotoxic activity against lung cancer cells A549, cervical adenocarcinoma cells HeLa and pancreatic epithelioid carcinoma cells PANC-1 in MTT assay IC 50 3.64 ± 0.18, 19.34 ± 0.42 and 1.78 ± 0.69 μM |
[113] |
| 60 | Dendronestadione | 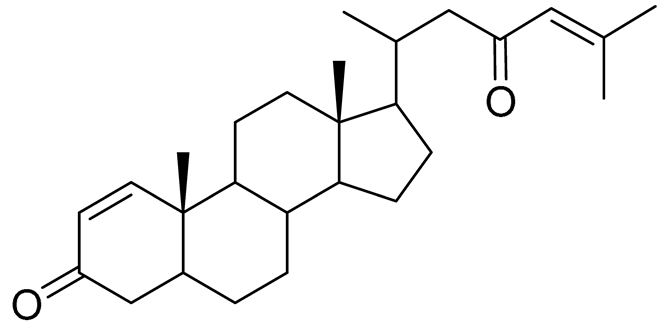 |
Soft coral Dendronephthya | Cytotoxic activity against hepatocellular carcinoma cells HepG2, colon cancer cells HT-29 and prostate cancer cells PC-3 in MTT assay IC50 19.1 ± 1.81, 32.4 ± 2.84 and 7.8 ± 0.80 μM |
[114] |
| 61 | (22E)-4α,24-dimethyl-5α-cholesta-22,24(28)-dien-3β,8β-diol | 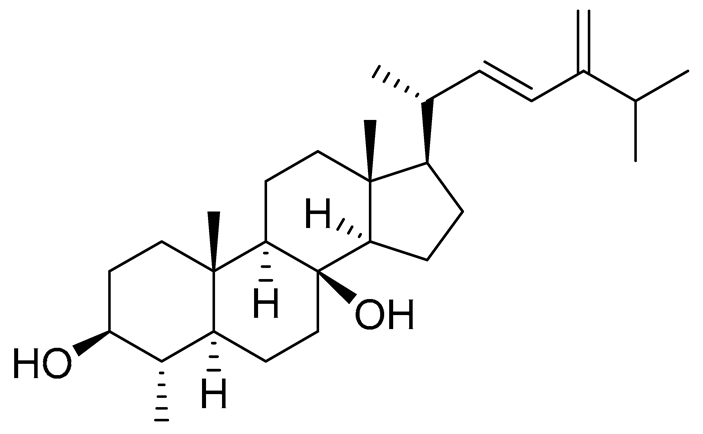 |
Soft coral Litophyton mollis | Cytotoxic activity against hepatocellular carcinoma cells HepG2, breast cancer cells MCF-7 and lung carcinoma cells NCI-H1299 in SRB assay IC50 > 50 μM in all cases | [104] |
| 62 | (22E,24R)-7β-acetoxy-24-methylcholesta-5,22-dien-3β,19-diol | 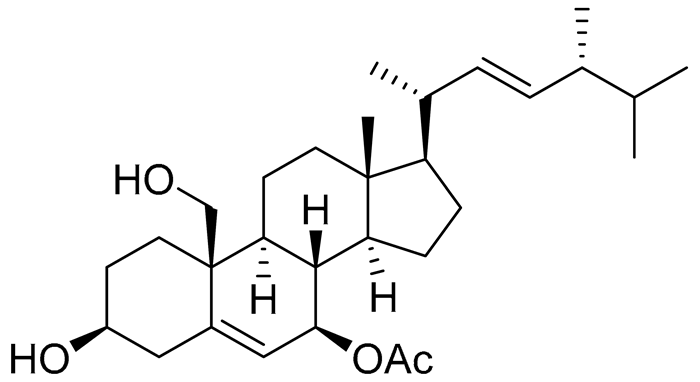 |
Soft coral Litophyton mollis | Cytotoxic activity against hepatocellular carcinoma cells HepG2, breast cancer cells MCF-7 and lung carcinoma cells NCI-H1299 in SRB assay IC50 32.5, 8.4 and 15.1 μM | [104] |
3. Potential Glucocorticoid Receptor Modulators from Marine Natural Products
| No | Name | Structure | Source | Anti-Inflammatory Effects | Reference |
| 56 | Klyflaccisteroid F | 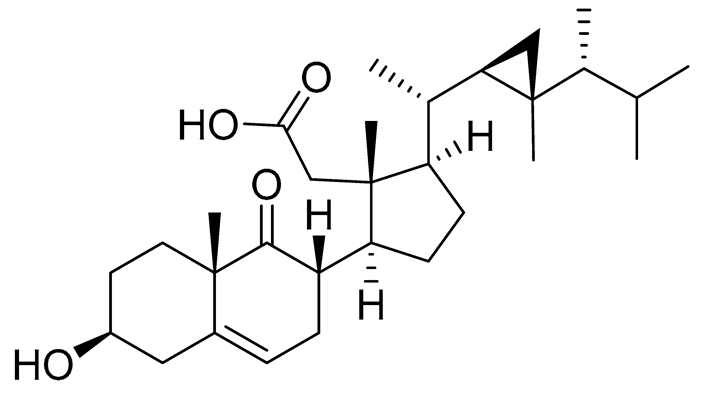 |
Soft coral Klyxum flaccidum | Activity in inhibiting the superoxide anion generation 88.26 ±35.86 % at 10 μM and activity in inhibiting against elastase release 104.22 ± 6.55 % at 10 μM in N-formyl-methionyl-leucyl-phenylalanine/ cytochalasin B (fMLP/CB)-induced neutrophils. |
[102] |
| 57 | Klyflaccisteroid C | 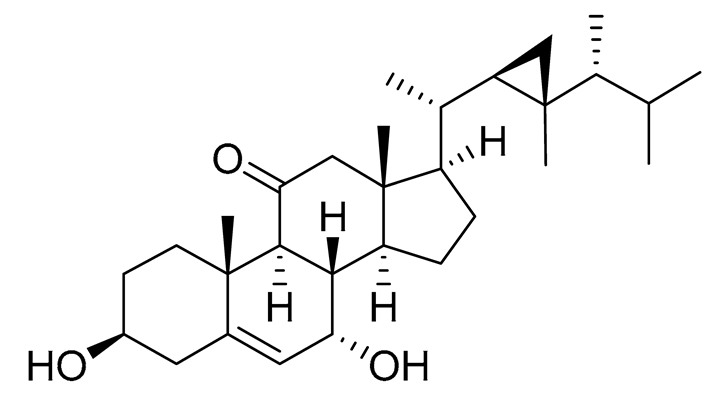 |
Soft coral Klyxum flaccidum | Activity in inhibiting the superoxide anion generation 76.24 ± 5.64 % at 10 μM and activity in inhibiting against elastase release 88.38 ± 1.19 % at 10 μM in N-formyl-methionyl-leucyl-phenylalanine/ cytochalasin B (fMLP/CB)-induced neutrophils. |
[102] |
| 63 | (22E,24S)-9a,15a-dihydroxyergosta-4,6,8(14),22- tetraen-3-one 15-palmitate |
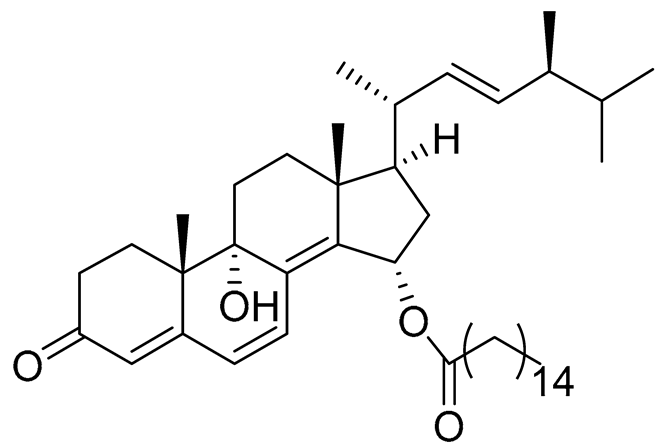 |
Marine fungus Penicillium oxalicum HL-44 | Inhibition of expression of pro-inflammatory cytokines TNF-α and INF-β1 on DMXAA-stimulated Raw264.7 cells by 68% and 94% at 20 μM | [138] |
| 64 | (22E, 24R)-ergosta-5,7,22-trien-3β-ol | 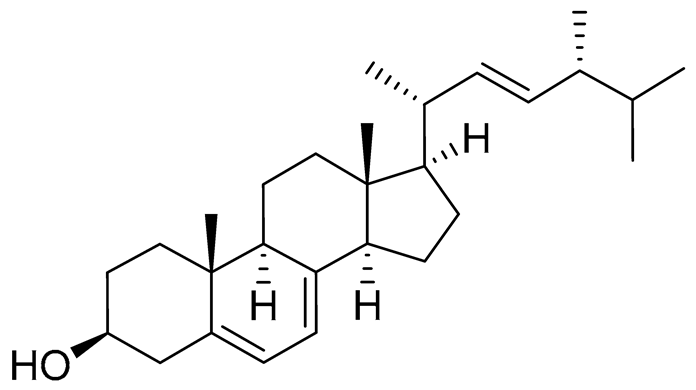 |
Mangrove fungus Amorosia sp. | Inhibition of expression of pro-inflammatory cytokines TNF-α, IL-6 and MCP-1 on LPS-activated RAW264.7 M1-type cells by 55%, 50% and 50% at 10 μM | [139] |
| 65 | Ergosterol | 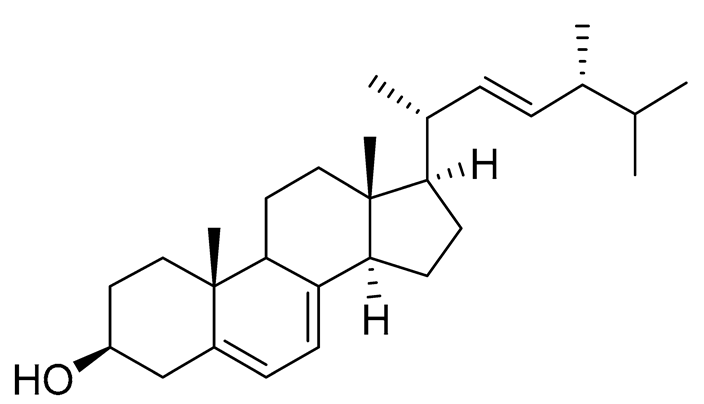 |
Marine fungus Samsoniella hepiali W7 | Inhibition of NO production in LPS-activated BV-2-microglia cells by 32.9 ± 1.6 % at 1 μM | [140] |
| 66 | Arctiol | 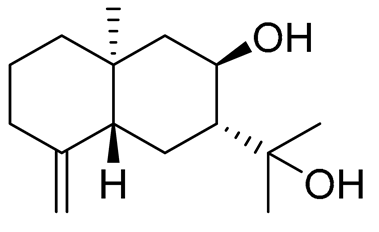 |
Marine fungus Eutypella sp. F0219 | Inhibition of NO production in LPS- treated BV-2-microglia cells by 71% at 20 μM | [141] |
| 67 | Persteroid | 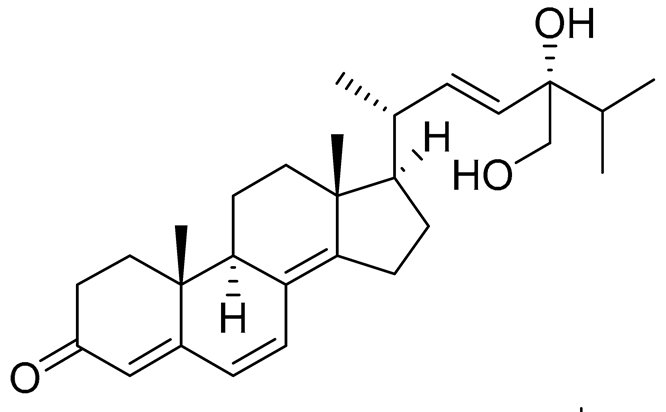 |
Marine fungus Penicillium sp. ZYX-Z-143 | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 25.81 ± 0.92 μM |
[142] |
| 37 | Cerevisterol | 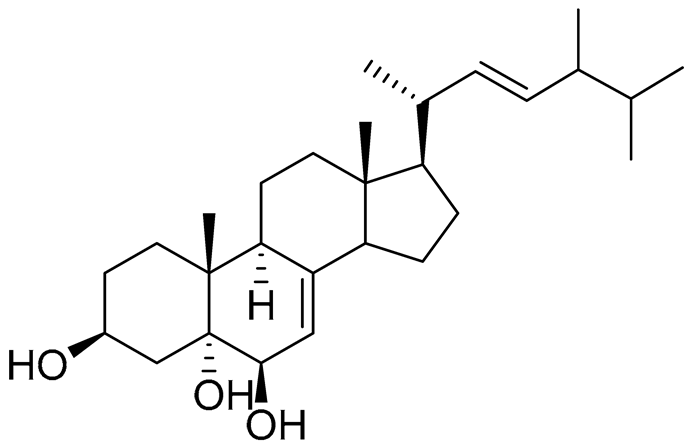 |
Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 25.45 μg/mL |
[99] |
| 38 | Ergosterol peroxide | 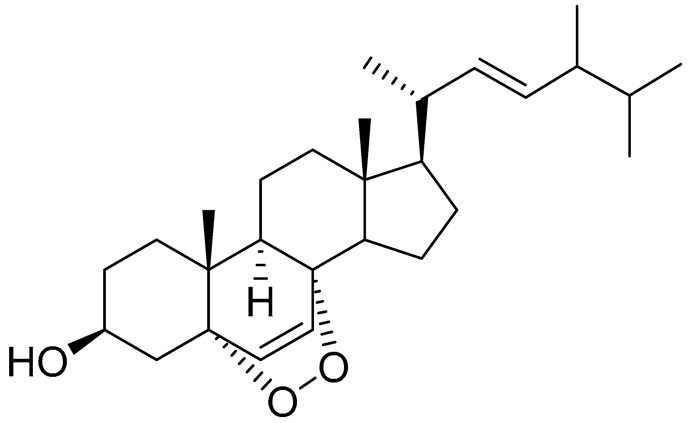 |
Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 2.85 μg/mL |
[99] |
| 39 | (3β,5α,22E)-ergosta-6,8(14),22-triene-3,5-diol | 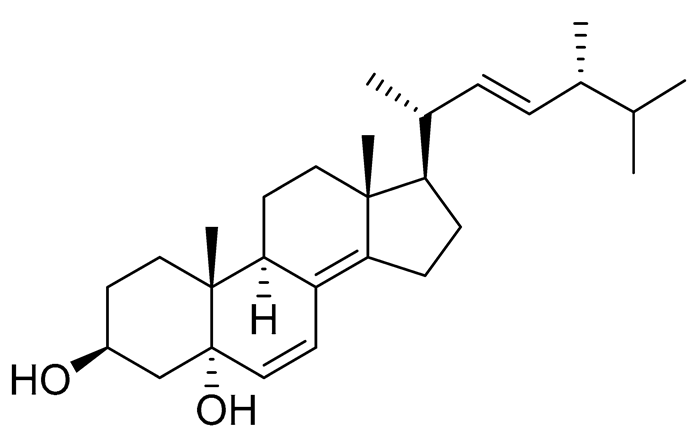 |
Marine fungus Penicillium levitum | NO half-maximal inhibitory concentration on LPS-stimulated RAW 264.7 cells IC50 2.79 μg/mL |
[99] |
| 68 | Splenocin A | 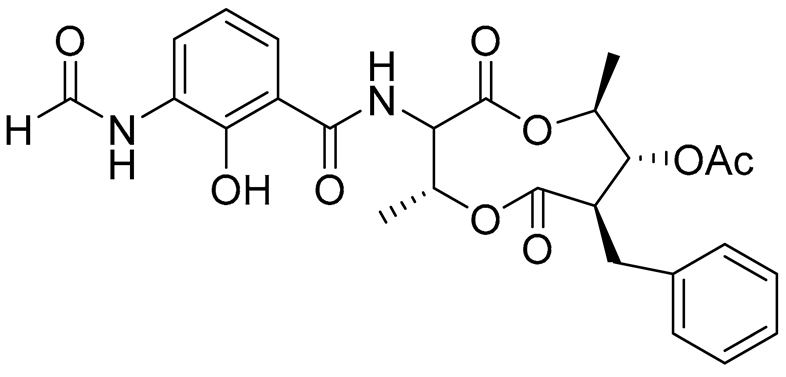 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 on TH2 cells (helper T lymphocytes) IC50 3.1 ± 1.2 nM | [143] |
| 69 | Splenocin B | 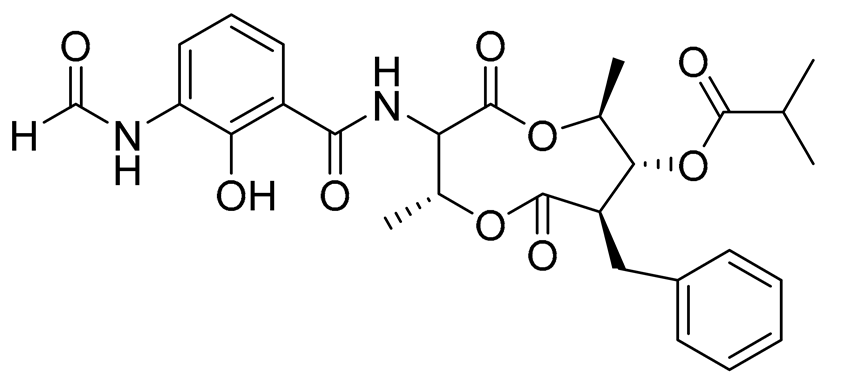 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 1.8 ± 0.2 and 1.6 ± 0.02 nM | [143] |
| 70 | Splenocin C | 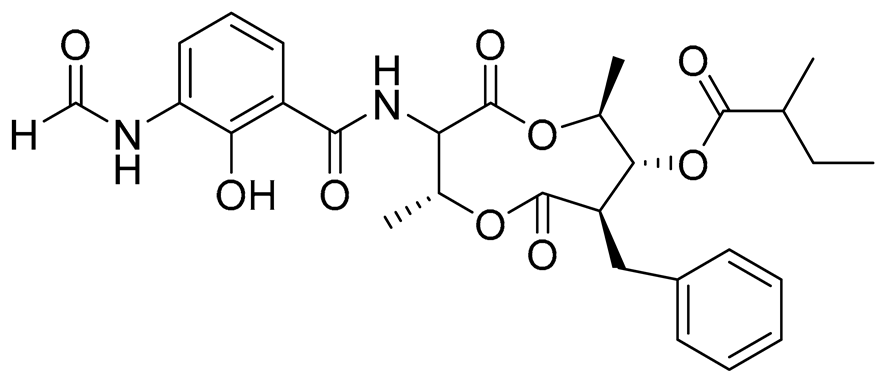 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 6.7 ± 0.2 and 7.3 ± 4.2 nM | [143] |
| 71 | Splenocin D | 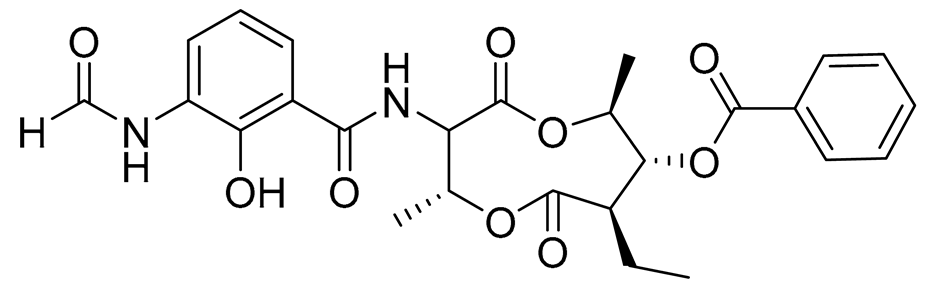 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 47.9 ± 2.9 and 43.7 ± 3.5 nM | [143] |
| 72 | Splenocin E | 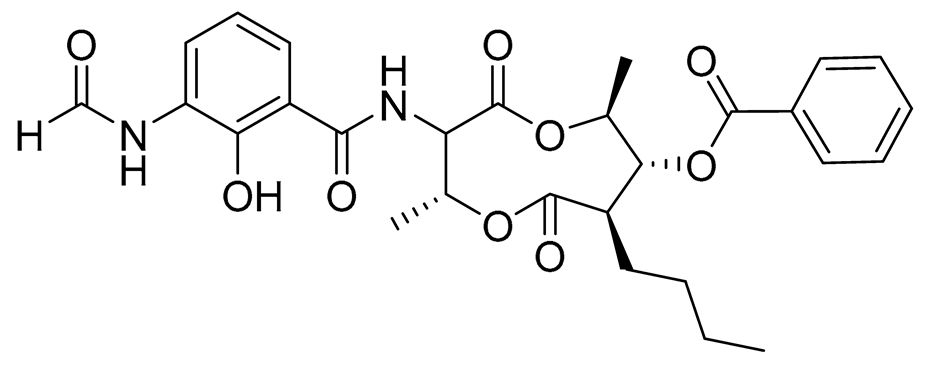 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 16.6 ± 1.8 and 15.9 ± 1.1 nM | [143] |
| 73 | Splenocin F | 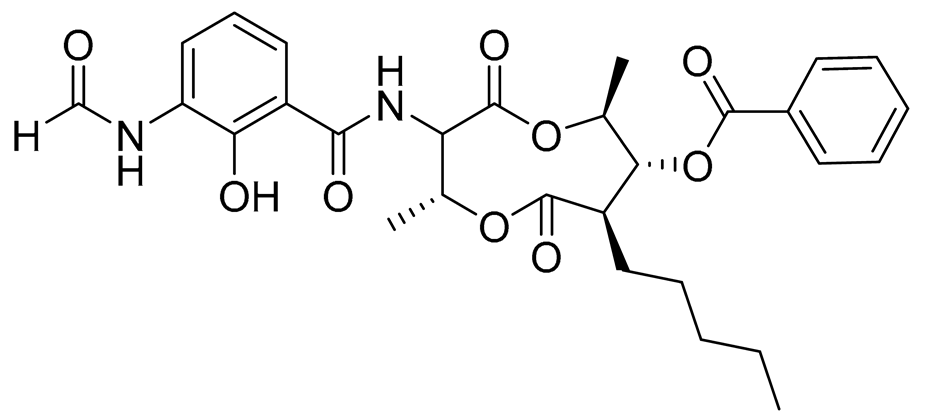 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 9.4 ± 2.8 and 6.8 ± 0.3 nM | [143] |
| 74 | Splenocin G | 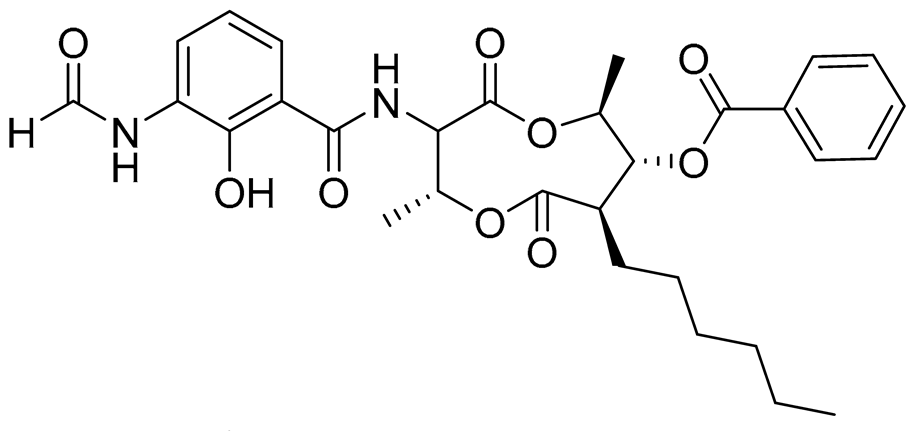 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 5 ± 0.4 and 5.2 ± 0.1 nM | [143] |
| 75 | Splenocin H | 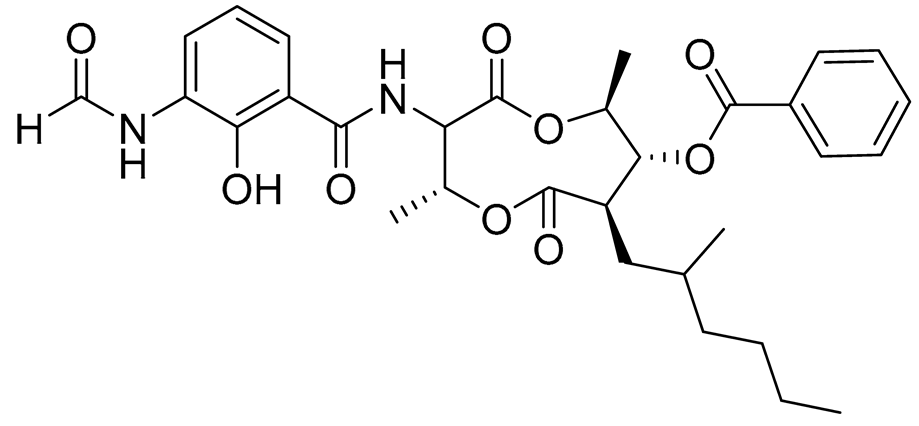 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 4.3 ± 0.5 and 5.1 ± 0.1 nM | [143] |
| 76 | Splenocin I | 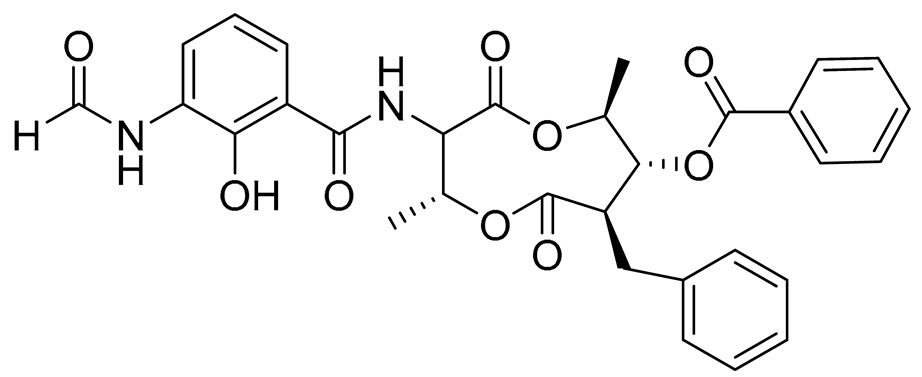 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 15.8 ± 1.0 and 15.2 ± 1.3 nM | [143] |
| 77 | Splenocin J | 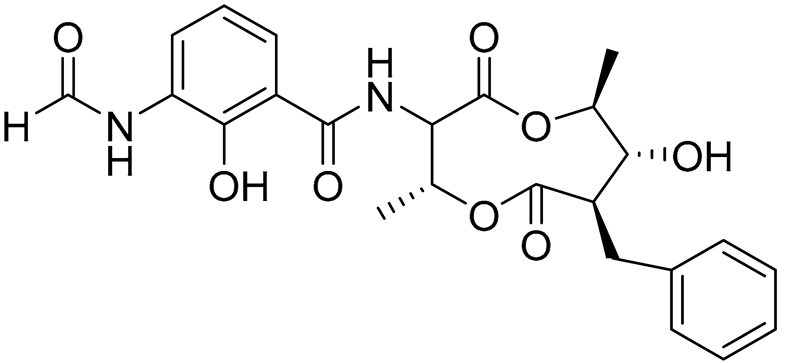 |
Marine bacterium Streptomyces sp. | Inhibition of expression of pro-inflammatory cytokines IL-5 and IL-13 on TH2 cells (helper T lymphocytes) IC50 1022.7 ± 52.3 and 826.3 ± 187.6 nM | [143] |
| 41 | Fucosterol | 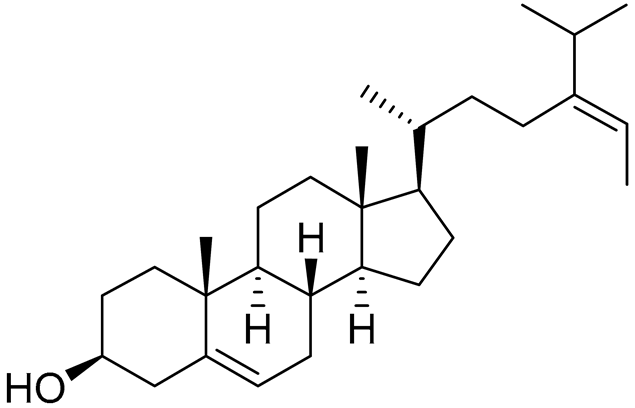 |
Green algae Tydemania expeditionis | Hydrophobic interaction with GR via Leu563, Phe623, Leu608, and Met604 (molecular docking analysis) |
[144] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, I.K.; Sawyers, C.L. The Emergence of Resistance to Targeted Cancer Therapeutics. Pharmacogenomics 2002, 3, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Khamisipour, G.; Jadidi-Niaragh, F.; Jahromi, A.S.; zandi, K.; Hojjat-Farsangi, M. Mechanisms of Tumor Cell Resistance to the Current Targeted-Therapy Agents. Tumor Biology 2016, 37, 10021–10039. [Google Scholar] [CrossRef]
- Gentile, C.; Martorana, A.; Lauria, A.; Bonsignore, R. Kinase Inhibitors in Multitargeted Cancer Therapy. Curr Med Chem 2017, 24. [Google Scholar] [CrossRef]
- Valerio, L.; Matrone, A. Multikinase and Highly Selective Kinase Inhibitors in the Neoadjuvant Treatment of Patients with Thyroid Cancer. Explor Target Antitumor Ther 2025, 6. [Google Scholar] [CrossRef]
- Buzatu, I.M.; Tataranu, L.G.; Duta, C.; Stoian, I.; Alexandru, O.; Dricu, A. A Review of FDA-Approved Multi-Target Angiogenesis Drugs for Brain Tumor Therapy. Int J Mol Sci 2025, 26, 2192. [Google Scholar] [CrossRef]
- Díaz Vico, T.; Martínez-Amores Martínez, B.; Mihic Góngora, L.; Jiménez-Fonseca, P.; Peinado Martín, P.; Grao Torrente, I.; García Muñoz-Nájar, A.; Durán-Poveda, M. Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing. Cancers (Basel) 2025, 17, 1800. [Google Scholar] [CrossRef]
- Flauto, F.; Damiano, V. The Efficacy and Safety of Multi-Kinase Inhibitors in Adrenocortical Carcinoma: A Systematic Review and Single-Arm Meta-Analysis. Cancers (Basel) 2025, 17, 2004. [Google Scholar] [CrossRef]
- Guo, C.; Gasparian, A.V.; Zhuang, Z.; Bosykh, D.A.; Komar, A.A.; Gudkov, A.V.; Gurova, K. V 9-Aminoacridine-Based Anticancer Drugs Target the PI3K/AKT/MTOR, NF-ΚB and P53 Pathways. Oncogene 2009, 28, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Prasad, S.; Webb, L.J.; Aggarwal, B.B. Cancer Drug Discovery by Repurposing: Teaching New Tricks to Old Dogs. Trends Pharmacol Sci 2013, 34, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Swamidass, S.J. Mining Small-Molecule Screens to Repurpose Drugs. Brief Bioinform 2011, 12, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy. Journal of Investigative Dermatology 2018, 138, 1935–1944. [Google Scholar] [CrossRef]
- Blatt, J.; Corey, S.J. Drug Repurposing in Pediatrics and Pediatric Hematology Oncology. Drug Discov Today 2013, 18, 4–10. [Google Scholar] [CrossRef]
- McCabe, B.; Liberante, F.; Mills, K.I. Repurposing Medicinal Compounds for Blood Cancer Treatment. Ann Hematol 2015, 94, 1267–1276. [Google Scholar] [CrossRef]
- Koval, A.; Ahmed, K.; Katanaev, V.L. Inhibition of Wnt Signalling and Breast Tumour Growth by the Multi-Purpose Drug Suramin through Suppression of Heterotrimeric G Proteins and Wnt Endocytosis. Biochemical Journal 2016, 473, 371–381. [Google Scholar] [CrossRef]
- Xi, Z.; Jie, W.; Long, Z.; Shasha, S. A Review of Thalidomide and Digestive System Related Diseases. Front Oncol 2025, 15. [Google Scholar] [CrossRef]
- de Miranda Drummond, P.L.; de Araújo Silva, C.; de Souza, J.E.; Magno, B.A.R.; Battaglia, G.M.; Candido, R.C.F.; Menezes de Pádua, C.A.; Pereira, B.G. Efficacy and Safety of Thalidomide for Oncology-Related Uses Approved in Brazil: An Overview of Systematic Reviews. Journal of Oncology Pharmacy Practice 2025. [CrossRef]
- Chen, Y.; Yu, D.; Zhu, D.; Muthusamy, S.; Deshpande, M.; Kiruthiga, N.; Theivendren, P.; Rajalakshmi, K.; Wu, S.; Zhu, C. Exploring Alkaloids and Flavonoids from Natural Sources: Emerging Natural Agents for Inhibiting Cervical Cancer Progression through Apoptosis Induction, Anti-Inflammatory Effects, and Oxidative Stress Reduction. Pathol Res Pract 2025, 272, 156092. [Google Scholar] [CrossRef]
- Al Khalily, I.; Megantara, S.; Aulifa, D. Targeting Molecular Pathways in Breast Cancer Using Plant-Derived Bioactive Compounds: A Comprehensive Review. J Exp Pharmacol 2025, Volume 17, 375–401. [Google Scholar] [CrossRef]
- Dodonova, S.A.; Zhidkova, E.M.; Kryukov, A.A.; Valiev, T.T.; Kirsanov, K.I.; Kulikov, E.P.; Budunova, I.V.; Yakubovskaya, M.G.; Lesovaya, E.A. Synephrine and Its Derivative Compound A: Common and Specific Biological Effects. Int J Mol Sci 2023, 24, 17537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yao, S.; Chen, L.; Dong, Q.; Luo, R.; Zheng, K.; Liu, J.; Liu, Y.; Chen, Y.; et al. Inflammatory Bowel Disease Induces Colorectal Cancer: Risk Factors, Triggering Mechanisms, and Treatment With Phyto-Derivatives. Phytotherapy Research 2025, 39, 3386–3418. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, N.; Sarıtaş, S.; Bechelany, M.; Karav, S. Polyphenols in Foods and Their Use in the Food Industry: Enhancing the Quality and Nutritional Value of Functional Foods. Int J Mol Sci 2025, 26, 5803. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Chingizova, E.A.; Pislyagin, E.A.; Yurchenko, E.A.; Klimovich, A.A.; Zelepuga, Elena. A.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S. Mechanisms of Action of Sea Cucumber Triterpene Glycosides Cucumarioside A0-1 and Djakonovioside A Against Human Triple-Negative Breast Cancer. Mar Drugs 2024, 22, 474. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Leshchenko, E.V.; Berdyshev, D.V.; Popov, R.S.; Chingizova, E.A.; Shlyk, N.P.; Gerasimenko, A.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Chausova, V.E.; et al. New Piperazine Derivatives Helvamides B–C from the Marine-Derived Fungus Penicillium Velutinum ZK-14 Uncovered by OSMAC (One Strain Many Compounds) Strategy. Nat Prod Bioprospect 2024, 14, 32. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Girich, E.V.; Yurchenko, E.A. Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Mar Drugs 2021, 19, 88. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Yurchenko, A.N.; Van Minh, C.; Aminin, D.L. Achievements in the Study of Marine Low-Molecular Weight Biologically Active Metabolites from the Vietnamese Territorial Waters as a Result of Expeditions Aboard the Research Vessel ‘Akademik Oparin’ (2004–2017). Chem Biodivers 2019, 16. [Google Scholar] [CrossRef]
- Hajdaś, G.; Koenig, H.; Pospieszny, T. Recent Advances in Steroid Discovery: Structural Diversity and Bioactivity of Marine and Terrestrial Steroids. Int J Mol Sci 2025, 26, 3203. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones. Nutr Metab (Lond) 2010, 7, 47. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, R. Revisiting the Role of Steroidal Therapeutics in the 21st Century: An Update on FDA Approved Steroidal Drugs (2000–2024). RSC Med Chem 2025, 16, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Nguyen, T.; Choi, H.A. Pharmacologic Characteristics of Corticosteroids. Journal of Neurocritical Care 2017, 10, 53–59. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am J Clin Nutr 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.J.; Yang, J.; Young, M.J.; Cole, T.J. Mechanisms of Ligand-Mediated Modulation of Mineralocorticoid Receptor Signaling. Mol Cell Endocrinol 2025, 600, 112504. [Google Scholar] [CrossRef]
- Martinez, G.J.; Appleton, M.; Kipp, Z.A.; Loria, A.S.; Min, B.; Hinds, T.D. Glucocorticoids, Their Uses, Sexual Dimorphisms, and Diseases: New Concepts, Mechanisms, and Discoveries. Physiol Rev 2024, 104, 473–532. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Lange, C.A.; Levin, E.R. Membrane-Initiated Estrogen, Androgen, and Progesterone Receptor Signaling in Health and Disease. Endocr Rev 2022, 43, 720–742. [Google Scholar] [CrossRef]
- Agnoletto, A.; Brisken, C. Hormone Signaling in Breast Development and Cancer. In; 2025; pp. 279–307.
- Quistini, A.; Chierigo, F.; Fallara, G.; Depalma, M.; Tozzi, M.; Maggi, M.; Jannello, L.M.I.; Pellegrino, F.; Mantica, G.; Terracciano, D.; et al. Androgen Receptor Signalling in Prostate Cancer: Mechanisms of Resistance to Endocrine Therapies. Res Rep Urol 2025, Volume 17, 211–223. [Google Scholar] [CrossRef]
- Chakrabarti, D.; Albertsen, P.; Adkins, A.; Kishan, A.; Murthy, V.; Parker, C.; Pathmanathan, A.; Reid, A.; Sartor, O.; Van As, N.; et al. The Contemporary Management of Prostate Cancer. CA Cancer J Clin 2025. [CrossRef]
- Mansour, R.; Abunasser, M.; Sharaf, B.; Abdel-Razeq, H. Update in the Clinical Utilization of Chemoprevention for Breast Cancer: A Narrative Review. Front Oncol 2025, 15. [Google Scholar] [CrossRef]
- Zulkipli, N.N.; Zakaria, R.; Wan Taib, W.R. Bibliometric Analysis of The Global Research Trends on The Application of Tamoxifen in The Treatment of Breast Cancer Over The Past 50 Years. Malaysian Journal of Medical Sciences 2025, 32, 35–55. [Google Scholar] [CrossRef]
- Hiltunen, J.; Helminen, L.; Paakinaho, V. Glucocorticoid Receptor Action in Prostate Cancer: The Role of Transcription Factor Crosstalk. Front Endocrinol (Lausanne) 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Posani, S.H.; Gillis, N.E.; Lange, C.A. Glucocorticoid Receptors Orchestrate a Convergence of Host and Cellular Stress Signals in Triple Negative Breast Cancer. J Steroid Biochem Mol Biol 2024, 243, 106575. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.B.; Conzen, S.D. Glucocorticoid Receptor-Mediated Oncogenic Activity Is Dependent on Breast Cancer Subtype. J Steroid Biochem Mol Biol 2024, 243, 106518. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Grigoreva, D.D.; Kirsanov, K.I.; Osipova, A.V.; Kulikov, E.P.; Mertsalov, S.A.; Belitsky, G.A.; Budunova, I.; Yakubovskaya, M.G.; et al. Nutritional Sensor REDD1 in Cancer and Inflammation: Friend or Foe? Int J Mol Sci 2022, 23, 9686. [Google Scholar] [CrossRef]
- Faggiano, A.; Mazzilli, R.; Natalicchio, A.; Adinolfi, V.; Argentiero, A.; Danesi, R.; D’Oronzo, S.; Fogli, S.; Gallo, M.; Giuffrida, D.; et al. Corticosteroids in Oncology: Use, Overuse, Indications, Contraindications. An Italian Association of Medical Oncology (AIOM)/ Italian Association of Medical Diabetologists (AMD)/ Italian Society of Endocrinology (SIE)/ Italian Society of Pharmacology (SIF) Multidisciplinary Consensus Position Paper. Crit Rev Oncol Hematol 2022, 180, 103826. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Yemelyanov, A.Yu.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. Antitumor Effect of Non-Steroid Glucocorticoid Receptor Ligand CpdA on Leukemia Cell Lines CEM and K562. Biochemistry (Moscow) 2011, 76, 1242–1252. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Kirsanov, K.; Popa, A.; Belitsky, G.; Yakubovskaya, M.; Gordon, L.I.; Rosen, S.T.; Budunova, I. Combination of a Selective Activator of the Glucocorticoid Receptor Compound A with a Proteasome Inhibitor as a Novel Strategy for Chemotherapy of Hematologic Malignancies. Cell Cycle 2013, 12, 133–144. [Google Scholar] [CrossRef]
- Lesovaya, E.; Yemelyanov, A.; Swart, A.C.; Swart, P.; Haegeman, G.; Budunova, I. Discovery of Compound A - a Selective Activator of the Glucocorticoid Receptor with Anti-Inflammatory and Anti-Cancer Activity. Oncotarget 2015, 6, 30730–30744. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Chudakova, D.; Baida, G.; Zhidkova, E.M.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. The Long Winding Road to the Safer Glucocorticoid Receptor (GR) Targeting Therapies. Oncotarget 2022, 13, 408–424. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Tilova, L.R.; Fetisov, T.I.; Kirsanov, K.I.; Kulikov, E.P.; Enikeev, A.D.; Budunova, I.V.; Badun, G.A.; Chernysheva, M.G.; Shirinian, V.Z.; et al. Synthesis and Anti-Cancer Activity of the Novel Selective Glucocorticoid Receptor Agonists of the Phenylethanolamine Series. Int J Mol Sci 2024, 25, 8904. [Google Scholar] [CrossRef]
- Zhidkova, E.M.; Oleynik, E.S.; Mikhina, E.A.; Stepanycheva, D.V.; Grigoreva, D.D.; Grebenkina, L.E.; Gordeev, K.V.; Savina, E.D.; Matveev, A.V.; Yakubovskaya, M.G.; et al. Synthesis and Anti-Cancer Activity In Vitro of Synephrine Derivatives. Biomolecules 2024, 15, 2. [Google Scholar] [CrossRef]
- Clarisse, D.; Van Moortel, L.; Van Leene, C.; Gevaert, K.; De Bosscher, K. Glucocorticoid Receptor Signaling: Intricacies and Therapeutic Opportunities. Trends Biochem Sci 2024, 49, 431–444. [Google Scholar] [CrossRef]
- Van Moortel, L.; Gevaert, K.; De Bosscher, K. Improved Glucocorticoid Receptor Ligands: Fantastic Beasts, but How to Find Them? Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef]
- Hsu, S.-J.; He, M.; Salomé-Abarca, L.F.; Choi, Y.H.; Wang, M. Uncovering Anti-Inflammatory Activity of Ginsenoside Rg1 in a Wound-Inured Zebrafish Model by GC-MS-Based Chemical Profiling. Planta Med 2025. [CrossRef] [PubMed]
- Jakob, F.; Hennen, S.; Gautrois, M.; Khalil, F.; Lockhart, A. Novel Selective Glucocorticoid Receptor Modulator GRM-01 Demonstrates Dissociation of Anti-Inflammatory Effects from Adverse Effects on Glucose and Bone Metabolism. Front Pharmacol 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Savinkova, A.V.; Mertsalov, S.A.; Kirsanov, K.I.; Belitsky, G.A.; Yakubovskaya, M.G.; Lesovaya, E.A. A Brief Overview of the Paradoxical Role of Glucocorticoids in Breast Cancer. Breast Cancer (Auckl) 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.M.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells 2020, 9, 1107. [Google Scholar] [CrossRef]
- Karra, A.G.; Tziortziou, M.; Kylindri, P.; Georgatza, D.; Gorgogietas, V.A.; Makiou, A.; Krokida, A.; Tsialtas, I.; Kalousi, F.D.; Papadopoulos, G.E.; et al. Boswellic Acids and Their Derivatives as Potent Regulators of Glucocorticoid Receptor Actions. Arch Biochem Biophys 2020, 695, 108656. [Google Scholar] [CrossRef]
- Morsy, M.A.; Patel, S.S.; El-Sheikh, A.A.K.; Savjani, J.K.; Nair, A.B.; Shah, J.N.; Venugopala, K.N. Computational and Biological Comparisons of Plant Steroids as Modulators of Inflammation through Interacting with Glucocorticoid Receptor. Mediators Inflamm 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Brown, M.N.; Fuhr, R.; Beier, J.; Su, H.-L.; Chen, Y.; Forsman, H.; Hamrén, U.W.; Jackson, H.; Aggarwal, A. Efficacy and Safety of AZD7594, an Inhaled Non-Steroidal Selective Glucocorticoid Receptor Modulator, in Patients with Asthma: A Phase 2a Randomized, Double Blind, Placebo-Controlled Crossover Trial. Respir Res 2019, 20, 37. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, T.; Zhang, F.; Lei, C.; Zhu, Y.; Luo, G.; Xiao, D. Bioequivalence Study of Fluticasone Propionate Nebuliser Suspensions in Healthy Chinese Subjects. Front Pharmacol 2025, 15. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Donaldson, K.; Strem, M.; Zann, V.; Leung, P.; Sweet, S.; Connor, A.; Combs, D.; Belanoff, J. Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin Pharmacol Drug Dev 2018, 7, 408–421. [Google Scholar] [CrossRef]
- Kuna, P.; Aurivillius, M.; Jorup, C.; Prothon, S.; Taib, Z.; Edsbäcker, S. Efficacy and Tolerability of an Inhaled Selective Glucocorticoid Receptor Modulator – AZD5423 – in Chronic Obstructive Pulmonary Disease Patients: Phase II Study Results. Basic Clin Pharmacol Toxicol 2017, 121, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Werkström, V.; Prothon, S.; Ekholm, E.; Jorup, C.; Edsbäcker, S. Safety, Pharmacokinetics and Pharmacodynamics of the Selective Glucocorticoid Receptor Modulator AZD5423 after Inhalation in Healthy Volunteers. Basic Clin Pharmacol Toxicol 2016, 119, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Conrado, D.J.; Krishnaswami, S.; Shoji, S.; Kolluri, S.; Hey-Hadavi, J.; McCabe, D.; Rojo, R.; Tammara, B.K. Predicting the Probability of Successful Efficacy of a Dissociated Agonist of the Glucocorticoid Receptor from Dose–Response Analysis. J Pharmacokinet Pharmacodyn 2016, 43, 325–341. [Google Scholar] [CrossRef]
- Bareille, P.; Hardes, K.; Donald, A.C. Efficacy and Safety of Once-Daily GW870086 a Novel Selective Glucocorticoid in Mild-Moderate Asthmatics: A Randomised, Two-Way Crossover, Controlled Clinical Trial. Journal of Asthma 2013, 50, 1077–1082. [Google Scholar] [CrossRef]
- Harcken, C.; Scholl, P.; Nabozny, G.; Thomson, D.; Bianchi, D. Clinical Profile of the Functionally Selective Glucocorticoid Receptor Agonist BI 653048 in Healthy Male Subjects. Expert Opin Investig Drugs 2019, 28, 489–496. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Chingizova, E.A.; Aminin, D.L.; Yurchenko, A.N. [Marine Fungi: In Search of New Antibacterial Drugs]. Molekuliarnaia biologiia 2025, 59, 43–59. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Kirichuk, N.N.; Chausova, V.E.; Popov, R.S.; Zhuravleva, O.I.; Chingizova, E.A.; Yurchenko, E.A.; Isaeva, M.P.; Yurchenko, A.N. Differences in Metabolite Profiles and Bioactivities of Intra-Strain Variants of Marine Fungus Penicillium Antarcticum KMM 4668. Metabolites 2025, 15, 77. [Google Scholar] [CrossRef]
- Chingizova, E.A.; Yurchenko, E.A.; Chingizov, A.R.; Klimovich, A.A.; Pislyagin, E.A.; Menchinskaya, E.S.; Kuzmich, A.S.; Trinh, P.T.H.; Ngoc, N.T.D.; Van, T.T.T.; et al. The Effects of Marine Fungal Asterripeptides A–C on In Vitro and In Vivo Staphylococcus Aureus Skin Infection. Pharmaceuticals 2024, 17, 1345. [Google Scholar] [CrossRef]
- Belousova, E.B.; Zhuravleva, O.I.; Yurchenko, E.A.; Oleynikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Chausova, V.E.; Khudyakova, Y.V.; Menshov, A.S.; Popov, R.S.; et al. New Anti-Hypoxic Metabolites from Co-Culture of Marine-Derived Fungi Aspergillus Carneus KMM 4638 and Amphichorda Sp. KMM 4639. Biomolecules 2023, 13, 741. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Oleinikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Pelageev, D.N.; Rasin, A.B.; Menshov, A.S.; Popov, R.S.; Kim, N.Yu.; Chingizova, E.A.; et al. New Antibacterial Chloro-Containing Polyketides from the Alga-Derived Fungus Asteromyces Cruciatus KMM 4696. Journal of Fungi 2022, 8, 454. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Chingizova, E.A.; Antonov, A.S.; Shlyk, N.P.; Borkunov, G.V.; Berdyshev, D.V.; Chausova, V.E.; Kirichuk, N.N.; Khudyakova, Y.V.; Chingizov, A.R.; et al. New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium Yezoense KMM 4679. Mar Drugs 2024, 22, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic Drimane-Type Sesquiterpenes from Co-Culture of the Marine-Derived Fungi Aspergillus Carneus KMM 4638 and Beauveria Felina (=Isaria Felina) KMM 4639. Mar Drugs 2022, 20, 584. [Google Scholar] [CrossRef] [PubMed]
- Krautforst, K.; Kulbacka, J.; Fornasier, M.; Mocci, R.; Porcheddu, A.; Pusceddu, A.; Moccia, D.; Murgia, S.; Bazylińska, U. Caulerpin Delivery via Pluronic-Free Cubosomes: Unlocking the Therapeutic Potential of a Pigment from an Invasive Marine Algae. Mol Pharm 2025, 22, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Atanu, M.S.H.; McDermid, K.J.; Wall, M.M.; Chang, B.L.; Wongwiwatthananukit, S.; Chang, L.C. Bioactive Constituents from the Edible Seaweed Halymenia Hawaiiana (Rhodophyta). Pharm Biol 2025, 63, 447–459. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Soltanmohammadi, F.; Kashkouie jahromi, M.; Javanmardi, F.; Roosta, A.; Zareian Jahromi, M.; Parsaei, A. Potential Anticancer Properties of Tetraselmis Suecica Extract against Oral and Colorectal Cancer Cells. Medical Oncology 2025, 42, 282. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-Derived Pharmaceuticals - Challenges and Opportunities. Biomol Ther (Seoul) 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Hussain, A.; Sohail, A.; Akash, M.S.H.; Iqbal, S.; Rehman, K.; Imran, M.; Khan, S.; Ayub, M.A.; Wang, D.; Ahmed, D.; et al. Marine Life as a Source of Anti-Prostate Cancer Agents: An Updated Overview (2003–2023). Naunyn Schmiedebergs Arch Pharmacol 2025, 398, 7971–8074. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Li, Q.; Su, M.; Guo, Y.; Jin, X. Recent Advances in Natural Products Derived from Marine Echinoderms and Endophytic Microbes: Chemical Insights and Therapeutic Potential. Mar Drugs 2025, 23, 33. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar Drugs 2024, 22, 433. [Google Scholar] [CrossRef]
- Islam, F.; Mitra, S.; Emran, T. Bin; Khan, Z.; Nath, N.; Das, R.; Sharma, R.; Awadh, A.A. Al; Park, M.N.; Kim, B. Natural Small Molecules in Gastrointestinal Tract and Associated Cancers: Molecular Insights and Targeted Therapies. Molecules 2022, 27, 5686. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat Prod Rep 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hafez, M.S.M.; Aziz Okbah, M.A. El; Ibrahim, H.A.H.; Hussein, A.A.E.R.; El Moneim, N.A.A.; Ata, A. First Report of Steroid Derivatives Isolated from Starfish Acanthaster Planci with Anti-Bacterial, Anti-Cancer and Anti-Diabetic Activities. Nat Prod Res 2022, 36, 5545–5552. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.M.; Phong, N.V.; Thao, N.P.; Binh, P.T.; Thao, D.T.; Thanh, N. Van; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Minh, C. Van Dendrodoristerol, a Cytotoxic C20 Steroid from the Vietnamese Nudibranch Mollusk Dendrodoris Fumata. J Asian Nat Prod Res 2020, 22, 193–200. [Google Scholar] [CrossRef]
- Quang, T.H.; Lee, D.; Han, S.J.; Kim, I.C.; Yim, J.H.; Kim, Y.; Oh, H. ChemInform Abstract: Steroids from the Cold Water Starfish Ctenodiscus Crispatus with Cytotoxic and Apoptotic Effects on Human Hepatocellular Carcinoma and Glioblastoma Cells. ChemInform 2015, 46. [Google Scholar] [CrossRef]
- Xiao, D.-J.; Peng, X.-D.; Deng, S.-Z.; Ma, W.-J.; Wu, H.-M. Structure Elucidation of (3E)-Cholest-4-En-3,6-Dione-3-Oxime in Marine Sponge Cinachyrella Australiensis from the South China Sea. Chin. J. Org. Chem. 2005, 25, 1606–1609. [Google Scholar]
- Shubina, L.K.; Makarieva, T.N.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; Dmitrenok, P.S.; von Amsberg, G.; Stonik, V.A. Gracilosulfates A–G, Monosulfated Polyoxygenated Steroids from the Marine Sponge Haliclona Gracilis. Mar Drugs 2020, 18, 454. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Abd-Elrazek, A.E.E.; Hassanean, H.A.; Youssef, D.T.A.; van Soest, R. New Compounds from the Red Sea Marine Sponge Echinoclathria Gibbosa. Phytochem Lett 2014, 9, 51–58. [Google Scholar] [CrossRef]
- Holland, I.P.; McCluskey, A.; Sakoff, J.A.; Gilbert, J.; Chau, N.; Robinson, P.J.; Motti, C.A.; Wright, A.D.; van Altena, I.A. Steroids from an Australian Sponge Psammoclema Sp. J Nat Prod 2009, 72, 102–106. J Nat Prod 2009, 72, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Dyshlovoy, S.A.; Long, P.Q.; Stonik, V.A. Two New Asterosaponins, Archasterosides A and B, from the Vietnamese Starfish Archaster Typicus and Their Anticancer Properties. Bioorg Med Chem Lett 2010, 20, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Ngoan, B.T.; Hanh, T.T.H.; Vien, L.T.; Diep, C.N.; Thao, N.P.; Thao, D.T.; Thanh, N. Van; Cuong, N.X.; Nam, N.H.; Thung, D.C.; et al. Asterosaponins and Glycosylated Polyhydroxysteroids from the Starfish Culcita Novaeguineae and Their Cytotoxic Activities. J Asian Nat Prod Res 2015, 17, 1010–1017. [Google Scholar] [CrossRef]
- Kicha, A.A.; Tolkanov, D.K.; Malyarenko, T.V.; Malyarenko, O.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V.; Dmitrenok, P.S. Sulfated Polyhydroxysteroid Glycosides from the Sea of Okhotsk Starfish Henricia Leviuscula Spiculifera and Potential Mechanisms for Their Observed Anti-Cancer Activity against Several Types of Human Cancer Cells. Mar Drugs 2024, 22, 294. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Malyarenko, O.S.; Ermakova, S.P.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V. Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster Marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures. Mar Drugs 2022, 20, 164. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Malyarenko, O.S.; Zakharenko, V.M.; Kotlyarov, I.P.; Kalinovsky, A.I.; Popov, R.S.; Svetashev, V.I.; Ivanchina, N.V. New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster Patagonicus and Their Anticancer Activity. Mar Drugs 2020, 18, 260. [Google Scholar] [CrossRef]
- Tian, X.-R.; Gao, Y.-Q.; Tian, X.-L.; Li, J.; Tang, H.-F.; Li, Y.-S.; Lin, H.-W.; Ma, Z.-Q. New Cytotoxic Secondary Metabolites from Marine Bryozoan Cryptosula Pallasiana. Mar Drugs 2017, 15, 120. [Google Scholar] [CrossRef]
- Hoang, C.K.; Le, C.H.; Nguyen, D.T.; Tran, H.T.N.; Luu, C.V.; Le, H.M.; Tran, H.T.H. Steroid Components of Marine-Derived Fungal Strain Penicillium Levitum N33.2 and Their Biological Activities. Mycobiology 2023, 51, 246–255. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Tian, H.-Y.; Li, J.; Jin, L.; Luo, C.; Ye, W.-C.; Jiang, R.-W. Steroids with Inhibitory Activity against the Prostate Cancer Cells and Chemical Diversity of Marine Alga Tydemania Expeditionis. Fitoterapia 2012, 83, 973–978. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.J.; Kang, J. Il; Kang, H.K.; Cuong, N.X.; Nam, N.H.; Kiem, P. Van; Minh, C. Van; Kim, Y.H. Steroidal Constituents from the Edible Sea Urchin Diadema Savignyi Michelin Induce Apoptosis in Human Cancer Cells. J Med Food 2015, 18, 45–53. [Google Scholar] [CrossRef]
- Tsai, C.-R.; Huang, C.-Y.; Chen, B.-W.; Tsai, Y.-Y.; Shih, S.-P.; Hwang, T.-L.; Dai, C.-F.; Wang, S.-Y.; Sheu, J.-H. New Bioactive Steroids from the Soft Coral Klyxum Flaccidum. RSC Adv 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.M.; Asfour, H.Z.; Ayyad, S.-E.N.; Khedr, A. ; Badria, Farid. A.; Al-lihaibi, S.S. Cytotoxic Effects of Three New Metabolites from Red Sea Marine Sponge, Petrosia Sp. Environ Toxicol Pharmacol 2014, 37, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.H.; Abdel-Tawab, A.M.; A. E.; Hamed, E.S.; El-Ablack, F.Z.; N. Ayyad, S.-E. Cytotoxic Evaluation of New Polyhydroxylated Steroids from the Red Sea Soft Coral Litophyton Mollis (Macfadyen, 1936). Nat Prod Res 2024, 38, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Wang, H.-N.; Sun, L.-X.; Sun, J.; Jin, S.-H.; Dai, F.-X.; Sai, C.-M.; Zhang, Z. Bioactive Steroids from Marine-Derived Fungi: A Review (2015–2023). J Asian Nat Prod Res 2025, 1–27. [Google Scholar] [CrossRef]
- Khaledi, M.; Moradipoodeh, B.; Moradi, R.; Baghbadorani, M.A.; Mahdavinia, M. Antiproliferative and Proapoptotic Activities of Sea Cucumber H. Leucospilota Extract on Breast Carcinoma Cell Line (SK-BR-3). Mol Biol Rep 2022, 49, 1191–1200. [Google Scholar] [CrossRef]
- Heidary Jamebozorgi, F.; Yousefzadi, M.; Firuzi, O.; Nazemi, M.; Zare, S.; Chandran, J.N.; Schneider, B.; Baldwin, I.T.; Jassbi, A.R. Cytotoxic Furanosesquiterpenoids and Steroids from Ircinia Mutans Sponges. Pharm Biol 2021, 59, 573–581. [Google Scholar] [CrossRef]
- Hadisaputri, Y.E.; Andika, R.; Sopyan, I.; Zuhrotun, A.; Maharani, R.; Rachmat, R.; Abdulah, R. Caspase Cascade Activation During Apoptotic Cell Death of Human Lung Carcinoma Cells A549 Induced by Marine Sponge Callyspongia Aerizusa. Drug Des Devel Ther 2021, Volume 15, 1357–1368. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Rodríguez-Pérez, C.; Herranz-López, M.; Martín-García, B.; Gómez-Caravaca, A.-M.; Arráez-Román, D.; Segura-Carretero, A.; Barrajón-Catalán, E.; Micol, V. Marine Invertebrate Extracts Induce Colon Cancer Cell Death via ROS-Mediated DNA Oxidative Damage and Mitochondrial Impairment. Biomolecules 2019, 9, 771. [Google Scholar] [CrossRef]
- Thi Duy Ngoc, N.; Yurchenko, E.A.; Thi Hoai Trinh, P.; Menchinskaya, E.S.; Thi Dieu, T.V.; Savagina, A.D.; Minin, A.; Thinh, P.D.; Khanh, H.H.N.; Thi Thanh Van, T.; et al. Secondary Metabolites of Vietnamese Marine Fungus Penicillium Chermesinum 2104NT-1.3 and Their Cardioprotective Activity. Reg Stud Mar Sci 2025, 81, 104003. [Google Scholar] [CrossRef]
- Huong, P.T.M.; Phong, N.V.; Thao, N.P.; Binh, P.T.; Thao, D.T.; Thanh, N. Van; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Minh, C. Van Dendrodoristerol, a Cytotoxic C20 Steroid from the Vietnamese Nudibranch Mollusk Dendrodoris Fumata. J Asian Nat Prod Res 2020, 22, 193–200. [Google Scholar] [CrossRef]
- Kicha, A.A.; Malyarenko, T.V.; Kuzmich, A.S.; Malyarenko, O.S.; Kalinovsky, A.I.; Popov, R.S.; Tolkanov, D.K.; Ivanchina, N.V. Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster Marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells. Mar Drugs 2024, 22, 43. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Thanh, N. Van; Chi, N.T.P.; Dang, N.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P. Van; Minh, C. Van Cytotoxic Steroids from the Vietnamese Soft Coral <I>Sinularia Conferta</I> Chem Pharm Bull (Tokyo) 2017, 65, 300–305. [CrossRef]
- Ghandourah, M.A. Cytotoxic Ketosteroids from the Red Sea Soft Coral Dendronephthya Sp. Open Chem 2023, 21. Open Chem 2023, 21. [Google Scholar] [CrossRef]
- Adcock, I.M.; Mumby, S. Glucocorticoids. In; 2016; pp. 171–196.
- Tan, C.K.; Wahli, W. A Trilogy of Glucocorticoid Receptor Actions. Proceedings of the National Academy of Sciences 2016, 113, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Specificity and Sensitivity of Glucocorticoid Signaling in Health and Disease. Best Pract Res Clin Endocrinol Metab 2015, 29, 545–556. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. Journal of Allergy and Clinical Immunology 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Pofi, R.; Caratti, G.; Ray, D.W.; Tomlinson, J.W. Treating the Side Effects of Exogenous Glucocorticoids; Can We Separate the Good From the Bad ? Endocr Rev 2023, 44, 975–1011. [Google Scholar] [CrossRef]
- De Bosscher, K.; Beck, I.M.; Ratman, D.; Berghe, W. Vanden; Libert, C. Activation of the Glucocorticoid Receptor in Acute Inflammation: The SEDIGRAM Concept. Trends Pharmacol Sci 2016, 37, 4–16. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective Glucocorticoid Receptor Modulation: New Directions with Non-Steroidal Scaffolds. Pharmacol Ther 2015, 152, 28–41. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for Modulation of the Nuclear Receptor Superfamily. Nat Rev Drug Discov 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Hudson, W.H.; Vera, I.M.S. de; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.G.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic Glucocorticoid Receptor-Binding Sites Pervade Genomic NF-ΚB Response Elements. Nat Commun 2018, 9, 1337. [Google Scholar] [CrossRef]
- Hudson, W.H.; Kossmann, B.R.; de Vera, I.M.S.; Chuo, S.-W.; Weikum, E.R.; Eick, G.N.; Thornton, J.W.; Ivanov, I.N.; Kojetin, D.J.; Ortlund, E.A. Distal Substitutions Drive Divergent DNA Specificity among Paralogous Transcription Factors through Subdivision of Conformational Space. Proceedings of the National Academy of Sciences 2016, 113, 326–331. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Wuescher, L.; Marino, J.S.; Hill, J.W.; Eng, C.; Hinds, T.D. Glucocorticoid Receptor β Stimulates Akt1 Growth Pathway by Attenuation of PTEN. Journal of Biological Chemistry 2014, 289, 17885–17894. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ben Hamida, O.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dexamethasone, a Synthetic Glucocorticoid, Induces the Activity of Androgen Receptor in Human Dermal Papilla Cells. Skin Pharmacol Physiol 2022, 35, 299–304. [Google Scholar] [CrossRef]
- Burnstein, K.L.; Maiorino, C.A.; Dai, J.L.; Cameron, D.J. Androgen and Glucocorticoid Regulation of Androgen Receptor CDNA Expression. Mol Cell Endocrinol 1995, 115, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Ogara, M.F.; Sanz, R.T.; Vicent, G.P. Choosing the Right Partner in Hormone-Dependent Gene Regulation: Glucocorticoid and Progesterone Receptors Crosstalk in Breast Cancer Cells. Front Endocrinol (Lausanne) 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Nordeen, S.K. Overlapping but Distinct Gene Regulation Profiles by Glucocorticoids and Progestins in Human Breast Cancer Cells. Molecular Endocrinology 2002, 16, 1204–1214. [Google Scholar] [CrossRef]
- Hundertmark, S.; Buhler, H.; Rudolf, M.; Weitzel, H.; Ragosch, V. Inhibition of 11 Beta-Hydroxysteroid Dehydrogenase Activity Enhances the Antiproliferative Effect of Glucocorticosteroids on MCF-7 and ZR-75-1 Breast Cancer Cells. Journal of Endocrinology 1997, 155, 171–180. [Google Scholar] [CrossRef]
- Meyer, M.-E.; Gronemeyer, H.; Turcotte, B.; Bocquel, M.-T.; Tasset, D.; Chambon, P. Steroid Hormone Receptors Compete for Factors That Mediate Their Enhancer Function. Cell 1989, 57, 433–442. [Google Scholar] [CrossRef]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the Glucocorticoid Receptor Is Associated with Poor Prognosis in Estrogen Receptor-Negative Breast Cancer. Cancer Res 2011, 71, 6360–6370. [Google Scholar] [CrossRef]
- West, D.C.; Pan, D.; Tonsing-Carter, E.Y.; Hernandez, K.M.; Pierce, C.F.; Styke, S.C.; Bowie, K.R.; Garcia, T.I.; Kocherginsky, M.; Conzen, S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Molecular Cancer Research 2016, 14, 707–719. [Google Scholar] [CrossRef]
- Karmakar, S.; Jin, Y.; Nagaich, A.K. Interaction of Glucocorticoid Receptor (GR) with Estrogen Receptor (ER) α and Activator Protein 1 (AP1) in Dexamethasone-Mediated Interference of ERα Activity. Journal of Biological Chemistry 2013, 288, 24020–24034. [Google Scholar] [CrossRef]
- Miranda, T.B.; Voss, T.C.; Sung, M.-H.; Baek, S.; John, S.; Hawkins, M.; Grøntved, L.; Schiltz, R.L.; Hager, G.L. Reprogramming the Chromatin Landscape: Interplay of the Estrogen and Glucocorticoid Receptors at the Genomic Level. Cancer Res 2013, 73, 5130–5139. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.C.; Schiltz, R.L.; Sung, M.-H.; Yen, P.M.; Stamatoyannopoulos, J.A.; Biddie, S.C.; Johnson, T.A.; Miranda, T.B.; John, S.; Hager, G.L. Dynamic Exchange at Regulatory Elements during Chromatin Remodeling Underlies Assisted Loading Mechanism. Cell 2011, 146, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Volden, P.A.; Conzen, S.D. The Influence of Glucocorticoid Signaling on Tumor Progression. Brain Behav Immun 2013, 30, S26–S31. [Google Scholar] [CrossRef]
- Pang, C.; Chen, Y.-H.; Bian, H.-H.; Zhang, J.-P.; Su, L.; Han, H.; Zhang, W. Anti-Inflammatory Ergosteroid Derivatives from the Coral-Associated Fungi Penicillium Oxalicum HL-44. Molecules 2023, 28, 7784. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Ye, Y.; Xu, Z.; Zhao, Q.; Luo, X.; Liu, Y.; Guo, P. Anti-Inflammatory Compounds from the Mangrove Endophytic Fungus Amorosia Sp. SCSIO 41026. SCSIO 41026. Front Microbiol 2022, 13. [Google Scholar] [CrossRef]
- Zou, Z.-B.; Wu, T.-Z.; Yang, L.-H.; He, X.-W.; Liu, W.-Y.; Zhang, K.; Xie, C.-L.; Xie, M.-M.; Zhang, Y.; Yang, X.-W.; et al. Hepialiamides A–C: Aminated Fusaric Acid Derivatives and Related Metabolites with Anti-Inflammatory Activity from the Deep-Sea-Derived Fungus Samsoniella Hepiali W7. Mar Drugs 2023, 21, 596. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Su, R.; Chen, M.-T.; Li, J.-Y.; Chen, H.-Y.; Yang, L.; Liu, F.-F.; Liu, J.; Xu, C.-J.; Li, W.-S.; et al. Ent-Eudesmane Sesquiterpenoids with Anti-Neuroinflammatory Activity from the Marine-Derived Fungus Eutypella Sp. F0219. Phytochemistry 2024, 223, 114121. [Google Scholar] [CrossRef]
- Dai, L.-T.; Yang, L.; Wang, Z.-P.; Guo, J.-C.; Ma, Q.-Y.; Xie, Q.-Y.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Persteroid, a New Steroid from the Marine-Derived Fungus Penicillium Sp. ZYX-Z-143. Nat Prod Res 2024, 1–8. [CrossRef]
- Strangman, W.K.; Kwon, H.C.; Broide, D.; Jensen, P.R.; Fenical, W. Potent Inhibitors of Pro-Inflammatory Cytokine Production Produced by a Marine-Derived Bacterium. J Med Chem 2009, 52, 2317–2327. [Google Scholar] [CrossRef]
- Hannan, Md.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering Molecular Mechanism of the Neuropharmacological Action of Fucosterol through Integrated System Pharmacology and In Silico Analysis. Mar Drugs 2019, 17, 639. [Google Scholar] [CrossRef]
- Hagiwara, H.; Wakita, K.; Inada, Y.; Hirose, S. Fucosterol Decreases Angiotensin Converting Enzyme Levels with Reduction of Glucocorticoid Receptors in Endothelial Cells. Biochem Biophys Res Commun 1986, 139, 348–352. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Khmel, O.O.; Nesterenko, L.E.; Aminin, D.L. The Kelch/Nrf2 Antioxidant System as a Target for Some Marine Fungal Metabolites. Oxygen 2023, 3, 374–385. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Popov, R.S.; Yurchenko, E.A.; Stonik, V.A. Oxysterols from a Marine Sponge Inflatella Sp. and Their Action in 6-Hydroxydopamine-Induced Cell Model of Parkinson’s Disease. Mar Drugs 2018, 16, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Afiyatullov, S.Sh.; Vishchuk, O.S.; Denisenko, V.A.; Slinkina, N.N.; Smetanina, O.F. Decumbenone C, a New Cytotoxic Decaline Derivative from the Marine Fungus Aspergillus Sulphureus KMM 4640. Arch Pharm Res 2012, 35, 1757–1762. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Zhuravleva, O.I.; Khmel, O.O.; Oleynikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Chausova, V.E.; Kalinovsky, A.I.; Berdyshev, D.V.; Kim, N.Y.; et al. New Cyclopiane Diterpenes and Polyketide Derivatives from Marine Sediment-Derived Fungus Penicillium Antarcticum KMM 4670 and Their Biological Activities. Mar Drugs 2023, 21, 584. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.Sh.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Yu. Secondary Metabolites from a Marine-Derived Fungus Aspergillus Carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef]
- Afiyatullov, S.Sh.; Zhuravleva, O.I.; Antonov, A.S.; Leshchenko, E.V.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Pislyagin, E.A.; Kim, N.Yu.; Berdyshev, D.V.; et al. Piltunines A–F from the Marine-Derived Fungus Penicillium Piltunense KMM 4668. Mar Drugs 2019, 17, 647. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Kalinovsky, A.I.; Pushilin, M.A.; Glazunov, V.P.; Khudyakova, Y.V.; Kirichuk, N.N.; Ermakova, S.P.; Dyshlovoy, S.A.; Yurchenko, E.A.; et al. Oxirapentyns F–K from the Marine-Sediment-Derived Fungus Isaria Felina KMM 4639. J Nat Prod 2014, 77, 1321–1328. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Chingizova, E.A.; Oleinikova, G.K.; Starnovskaya, S.S.; Antonov, A.S.; Kirichuk, N.N.; Menshov, A.S.; Popov, R.S.; Kim, N.Yu.; Berdyshev, D.V.; et al. Anthraquinone Derivatives and Other Aromatic Compounds from Marine Fungus Asteromyces Cruciatus KMM 4696 and Their Effects against Staphylococcus Aureus. Mar Drugs 2023, 21, 431. [Google Scholar] [CrossRef]
- Dalinova, A.; Smirnov, S.; Dubovik, V.; Senderskiy, I.; Stepanycheva, E.; Kovalenko, N.; Berestetskiy, A. Production and Toxicological Characterization of Secondary Metabolites of Pyrenophora Tritici-Repentis. Journal of Plant Diseases and Protection 2025, 132, 149. [Google Scholar] [CrossRef]
- Afiyatullov, Sh.Sh.; Leshchenko, E.V.; Sobolevskaya, M.P.; Antonov, A.S.; Denisenko, V.A.; Popov, R.S.; Khudyakova, Yu. V.; Kirichuk, N.N.; Kuz’mich, A.S.; Pislyagin, E.A.; et al. New Thomimarine E from Marine Isolate of the Fungus Penicillium Thomii. Chem Nat Compd 2017, 53, 290–294. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kirichuk, N.N.; Khudyakova, Yu. V.; Yurchenko, E.A.; Afiyatullov, Sh.Sh. Metabolites of the Marine Fungus Penicillium Citrinum Associated with a Brown Alga Padina Sp. Chem Nat Compd 2016, 52, 111–112. Chem Nat Compd 2016, 52, 111–112. [Google Scholar] [CrossRef]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Trinh, P.T.H.; Ngoc, N.T.D.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective Metabolites from Vietnamese Marine Derived Fungi of Aspergillus and Penicillium Genera. Mar Drugs 2020, 18, 608. [Google Scholar] [CrossRef]
- Yurchenko, A.; Trinh, P.; Girich (Ivanets), E.; Smetanina, O.; Rasin, A.; Popov, R.; Dyshlovoy, S.; von Amsberg, G.; Menchinskaya, E.; Thanh Van, T.; et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus Flocculosus. Mar Drugs 2019, 17, 579. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Kalinovsky, A.I.; Pushilin, M.A.; Slinkina, N.N.; Yurchenko, E.A.; Afiyatullov, Sh.Sh. 4-Methoxy-3-Methylgoniothalamin from Marine-Derived Fungi of the Genus Penicillium. Russian Chemical Bulletin 2011, 60, 760–763. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Phan, T.T.H.; Ngo, N.T.D.; Zhuravleva, O.I.; Rasin, A.B.; Dyshlovoy, S.A.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. Auroglaucin-Related Neuroprotective Compounds from Vietnamese Marine Sediment-Derived Fungus Aspergillus Niveoglaucus. Nat Prod Res 2020, 34, 2589–2594. [Google Scholar] [CrossRef]
- Antonov, A.S.; Leshchenko, E.V.; Zhuravleva, O.I.; Dyshlovoy, S.A.; von Amsberg, G.; Popov, R.S.; Denisenko, V.A.; Kirichuk, N.N.; Afiyatullov, S.Sh. Naphto-Γ-Pyrones from the Marine-Derived Fungus Aspergillus Foetidus. Nat Prod Res 2021, 35, 131–134. [Google Scholar] [CrossRef]
- Afiyatullov, Sh.Sh.; Leshchenko, E.V.; Sobolevskaya, M.P.; Gerasimenko, A.V.; Khudyakova, Yu. V.; Kirichuk, N.N.; Mikhailov, V.V. New 3-[2′(R)-Hydroxybutyl]-7-Hydroxyphthalide from Marine Isolate of the Fungus Penicillium Claviforme. Chem Nat Compd 2015, 51, 111–115. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, Sh.Sh. Metabolites of the Marine Fungus Aspergillus Candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem Nat Compd 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Berdyshev, D.V.; Yurchenko, E.A.; Antonov, A.S.; Borkunov, G.V.; Kirichuk, N.N.; Chausova, V.E.; Kalinovskiy, A.I.; Popov, R.S.; Khudyakova, Y.V.; et al. Bioactive Polyketides from the Natural Complex of the Sea Urchin-Associated Fungi Penicillium Sajarovii KMM 4718 and Aspergillus Protuberus KMM 4747. Int J Mol Sci 2023, 24, 16568. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Min′ko, E.M.; Ivanets, E.V.; Afiyatullov, Sh.Sh. New Diorcinol J Produced by Co-Cultivation of Marine Fungi Aspergillus Sulphureus and Isaria Felina. Chem Nat Compd 2016, 52, 227–230. [Google Scholar] [CrossRef]
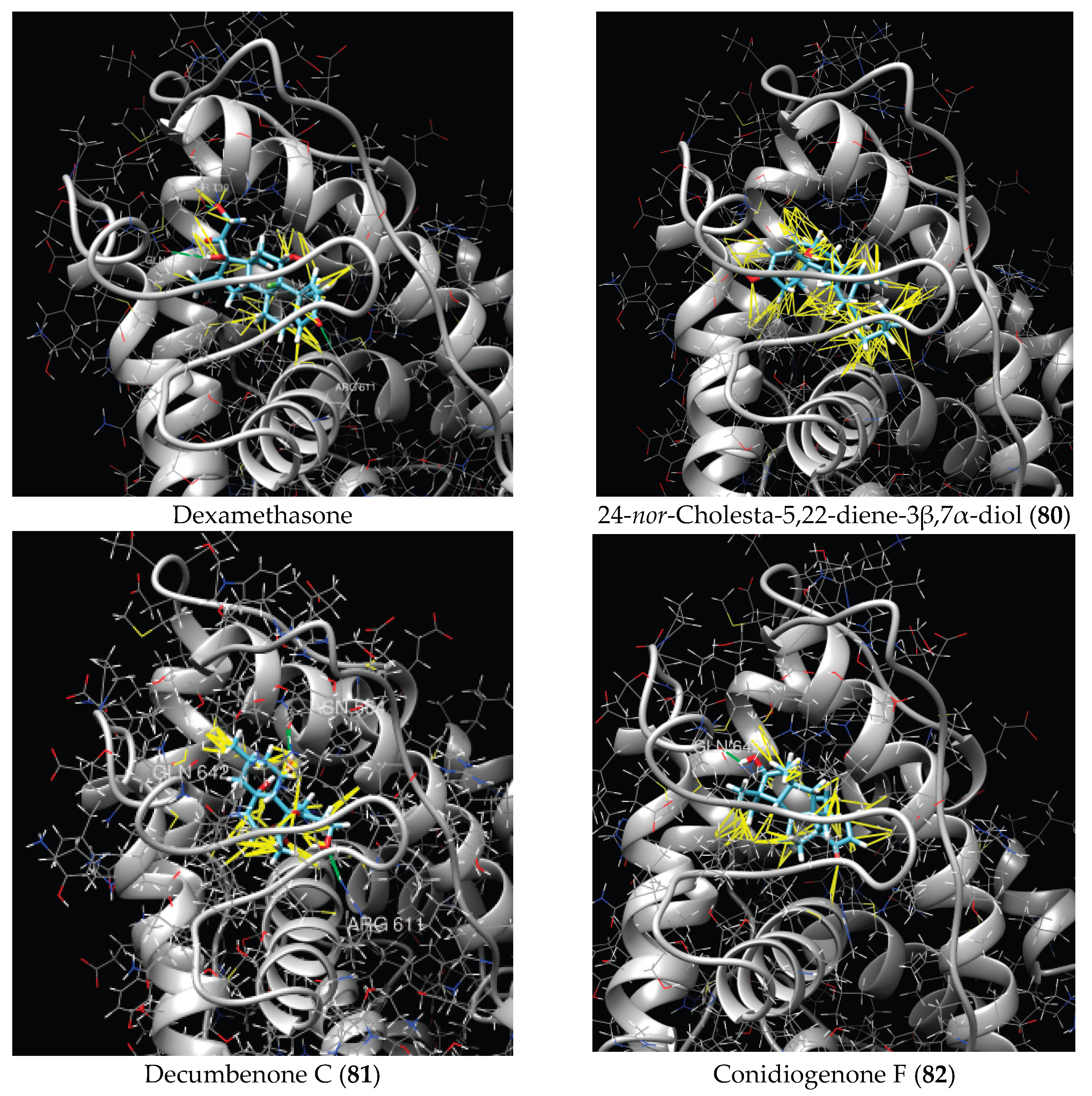
| No | Compound | Chemical Structure | ΔG, kcal/mol | FF Score, kcal/mol | H-Binding | Hydrophobic Interactions |
| 78 | 3β,15β-Dihydroxy-(22E, 24R)-ergosta-5,8(14),22-trien-7-one | 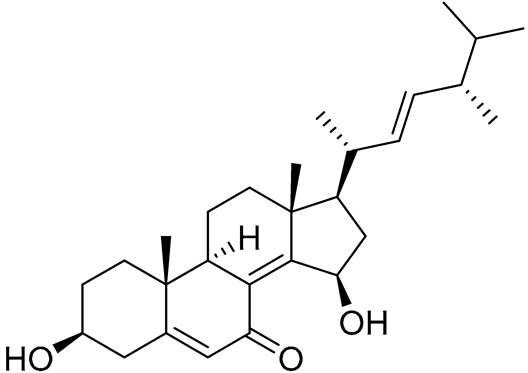 |
-7.23 | -1226.71 | - | Val538, ILe539, Lys576, Ala573, Leu544, Trp577 |
| 79 | 24-Methylcholesta-5,24(28)-diene-3β,4α-diol | 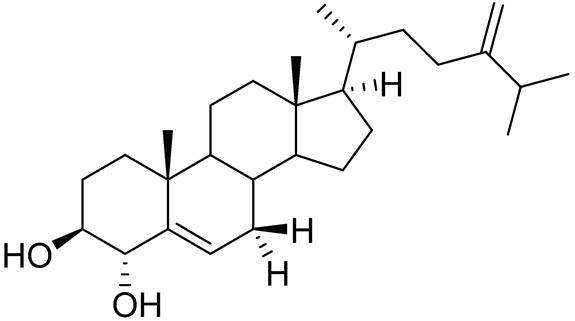 |
-7.40 | -1204.67 | H29 … Arg611 2.607 |
Val543, Trp610, Tyr660, |
| 80 | 24-nor-Cholesta-5,22-diene-3β,7α-diol | 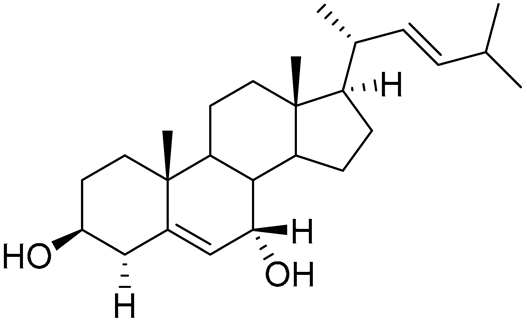 |
-6.37 | -1165.36 | - | Met604, Leu566, Leu732, Asn630, Leu563, Tyr735, Phe623, Leu608, Cys736 |
| 81 | Decumbenone C | 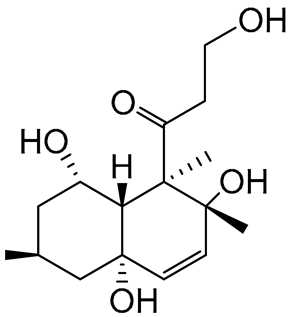 |
-8.23 | -1298.75 |
Arg611 … O 2.666 H24 … Asn564 1.682 |
Gly567, Trp600, Met604, Met601, Leu732, Met646, Phe623, Leu563, Met560 |
| 82 | Conidiogenone F | 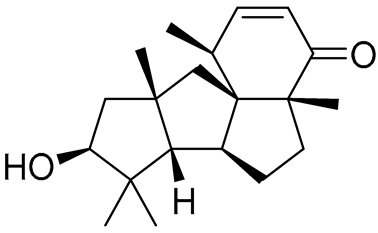 |
-8.46 | -1229.36 | - | Gly567, Met604, Met601, Leu732, Trp600, Cys736, Tyr735, Met560, Leu563, Met646, Phe623 |
| -7.91 | -1211.54 | H29 … Gln642 2.103 | Met560, Leu563, Leu753, Gly567, Met604, Met646, Leu732, Cys736 | |||
| Dexamethasone | 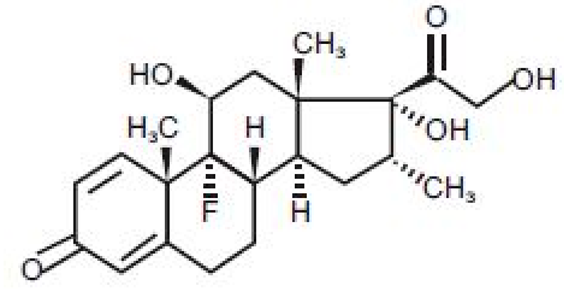 |
-10.19 | -1206.31 | Arg611 … O 2.146 H27 … Gln642 2.351 H26 … Thr739 2.115 |
Met560, Leu566, Gly567, Trp600, Met601, Met604, Phe623, Met646, Tyr735, Cys736, Thr739, Ile747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





