Submitted:
15 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
Background: Leishmaniasis is a zoonotic vector-borne disease and a significant global public health concern worldwide and in Algeria. In This study we have investigated the potential role of ticks and fleas as carriers of Leishmania in endemic regions of Algeria. Methods: Adult ectoparasites were collected from reservoir dogs and cohabiting animals across three provinces: Tizi-Ouzou (northeast), M'Sila (southeast), and Tébessa (extreme east). A subset of 247 ectoparasites was randomly selected for Leishmania DNA screening using ITS1-PCR. Results: Morphological identification revealed two tick species, Rhipicephalus turanicus (378 specimens) and Rhipicephalus sanguineus s.l (127 specimens), and one flea species, Ctenocephalides felis (94 specimens). Dogs were the most heavily infested hosts (74.12%), followed by sheep (9.51%) and cats (9.34%). Leishmania DNA was detected in 36.43% (90/247) of the tested specimens, with higher positivity in ticks (41.32%) compared to fleas (17.64%). Infection rates varied by host species, with dogs harboring the majority of positive ectoparasites (62/90), primarily R. sanguineus s.l (19/30) and R. turanicus (40/115). Leishmania DNA was also detected in ectoparasites collected from cats and sheep, whereas goats and rabbits were free from Leishmania DNA. Conclusions: This investigation highlights the high detection rate of Leishmania DNA in ticks and fleas from animals in Algerian endemic regions, indicating exposure to infected hosts. Together with previous reports, these findings support the view that ticks and fleas may act as incidental hosts or mechanical carriers of the parasite. However, their role in parasite transmission remains unconfirmed and warrant further investigation, particularly through studies assessing vector competence. These results emphasize the need for additional research to clarify the contribution of these ectoparasites to Leishmania transmission and multi-host dynamics.
Keywords:
1. Introduction
2. Materials and Methods
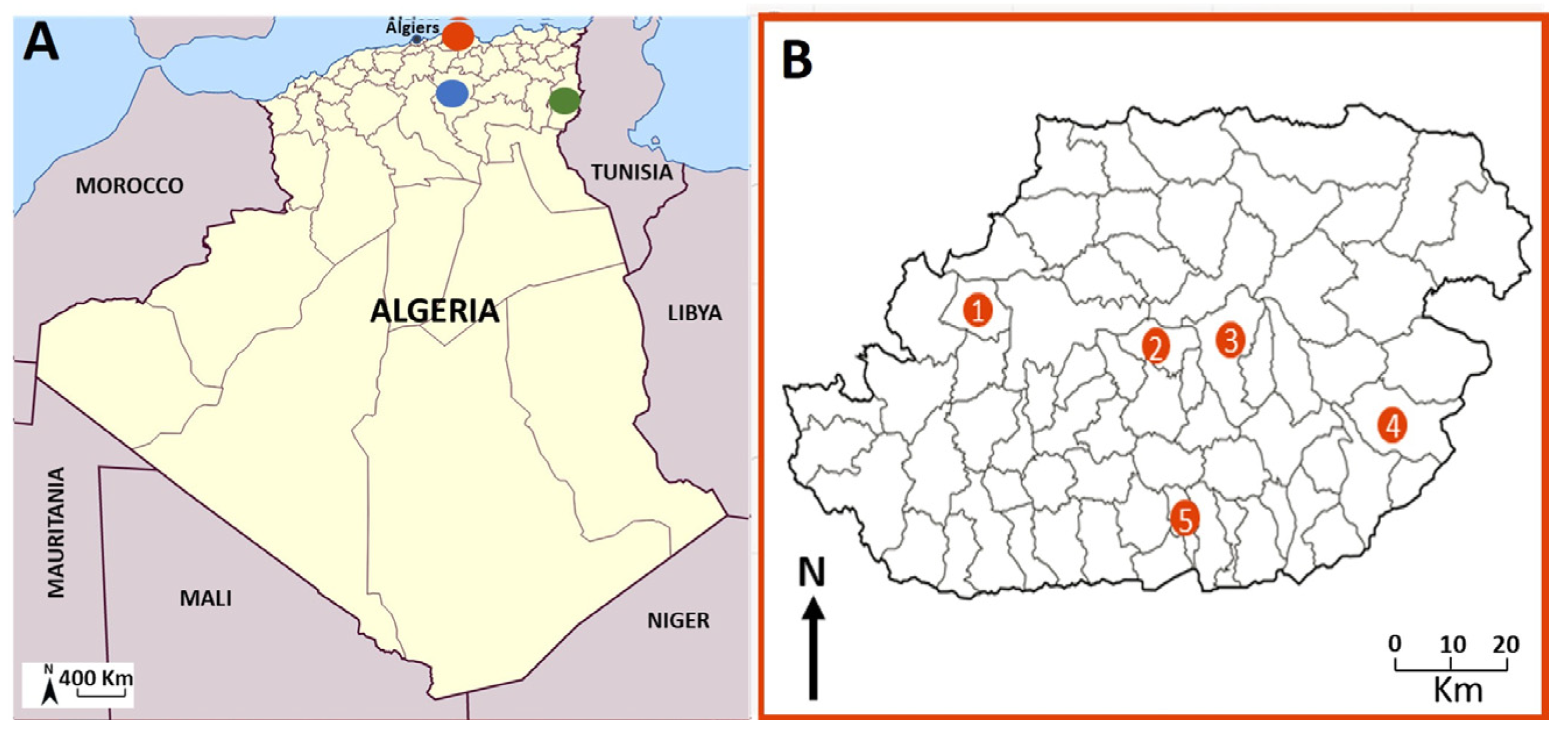
2.1. Study Area
2.2. Sampling Collection
2.3. Morphological Identification of Ticks and Fleas
2.3.1. Tick Identification
2.3.2. Flea Identification
2.4. Leishmania Molecular Detection
2.5. Statistical Analysis
2.5.1. Multivariable Analysis of Factors Associated with Leishmania DNA Detection in Ectoparasites
2.5.2. Logistic Regression Model
2.6. Ethical Considerations
3. Results
3.1. Morphological Identification of Ticks and Fleas
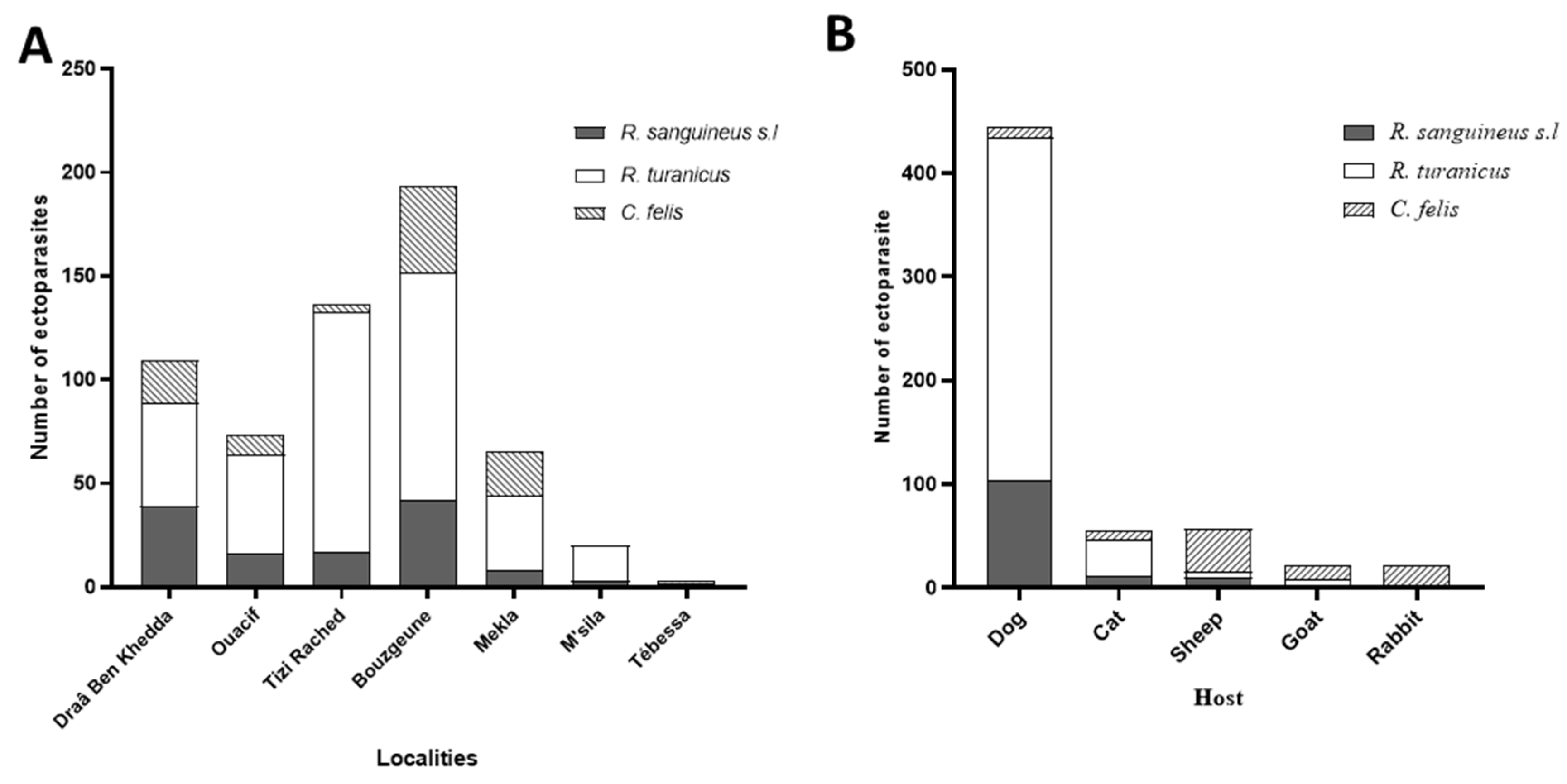
3.1.1. Geographic Distribution
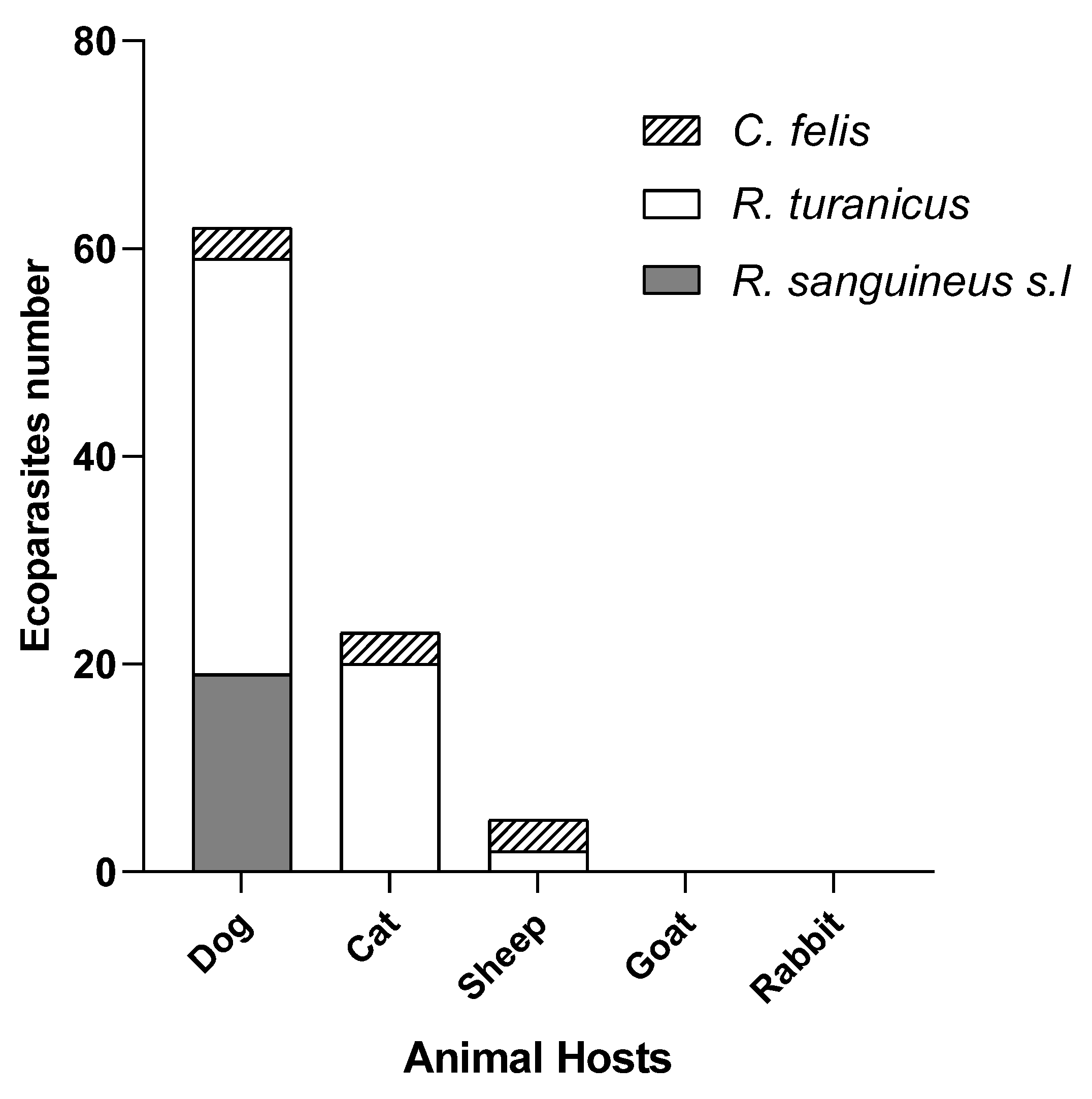
3.1.2. Host Distribution
3.1.3. Species Composition and Prevalence
3.2. Detection of Leishmania DNA in Ectoparasites
3.2.1. Leishmania DNA Prevalence by Ectoparasite Species and Host Animal
3.2.2. Geographic Variation in Leishmania DNA Detection Rate
3.2.3. Host Specific Leishmania DNA Detection Patterns
| Ectoparasites/hosts | R. sanguineus s.l | R. turanicus | C. felis | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| N° positive | (%) | N° positive | (%) | N° positive | (%) | N° positive | (%) | ||
| Tizi -Ouzou | Draâ Ben Khedda | 5♀ /7 | 42.85 | 6 (4♂, 2♀) /10 | 60.00 | 2♀/2 | 100.00 | 13/19 | 68.42 |
| Ouacif | 5 (3♂, 2♀) /8 | 62.5 | 8 (3♂, 5♀) /24 | 33.33 | - | - | 13/32 | 40.62 | |
| Tizi Rached | 5♂/8 | 62.5 | 30 (11♂, 19♀) /91 | 32.96 | 3♀/3 | 100.00 | 38/102 | 37.25 | |
| Bouzgeune | 0/13 | 0.00 | 0/12 | 0.00 | 4♀/35 | 11.42 | 4/60 | 6.66 | |
| Mekla | - | - | - | - | 0/11 | 0.00 | 0/11 | 0.00 | |
| Total | 15/36 | 41 | 44/137 | 32.11 | 9/51 | 17.64 | 68/224 | 30.35 | |
| M’sila | 3♂/3 | 100.00 | 17(6♂, 11♀) /17 | 100.00 | - | - | 20/20 | 100.00 | |
| Tébessa | 1♂/2 | 50.00 | 1♂/1 | 100.00 | - | - | 2/3 | 66.66 | |
| Total | 19/41 | 46.34 | 62/155 | 40.00 | 9/51 | 17.64 | 90/247 | 36.43 | |
| Ectoparasite/hosts | R. sanguineus | R. turanicus | C. felis | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N° positive | (%) | N° positive | (%) | N° positive | (%) | N° i positive | (%) | |
| Dogs | 19 (12♂, 7♀) /30 | 63.33 | 40 (17♂, 23♀) /115 | 34.78 | 3♀/3 | 100 | (62/148) | 41.89 |
| Cats | 0/9 | 0.00 | 20 (8♂, 12♀) /34 | 58.82 | 3♀/10 | 30 | (23/53) | 43.39 |
| Sheeps | 0/2 | 0.00 | 2 (2♀) /6 | 33.33 | 3♀/14 | 21.42 | (5/22) | 22.72 |
| Goats | - | - | - | 0/13 | 0.00 | (0/13) | 0.00 | |
| Rabbits | - | - | - | 0/11 | 0.00 | (0/11) | 0.00 | |
| Total | 19/41 | 46.34% | 62/155 | 40.00% | 9/51 | 17.64% | 90/247 | 36.43% |
3.2.4. Influence of Arthropod Sex on Leishmania DNA Detection Rate
3.2.5. Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAAES | Algerian Association for Animal Experimentation Sciences |
| ANOVA | Analysis of variance |
| bp | base pair(s) |
| CanL | Canine leishmaniasis |
| CCL | Chronic cutaneous leishmaniasis |
| CI | Confidence interval |
| CL | Cutaneous leishmaniasis |
| CTAB | Cetyltrimethylammonium bromide |
| DNA | Deoxyribonucleic acid |
| GPS | Global Positioning System |
| ITS1 | Internal transcribed spacer 1 |
| KOH | Potassium hydroxide |
| (L.) | Leishmania (genus abbreviation) |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative polymerase chain reaction |
| RFLP | Restriction fragment length polymorphism |
| s.l. | sensu lato (in the broad sense) |
| s.s. | sensu stricto (in the strict sense) |
| spp. | multiple species within a genus |
| SCL | Sporadic cutaneous leishmaniasis |
| TE (buffer) | Tris–EDTA buffer |
| UV | Ultraviolet |
| VL | Visceral leishmaniasis |
| WHO | World Health Organization |
| ZCL | Zoonotic cutaneous leishmaniasis |
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [CrossRef]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, Mona.; Lin, Z.; Beshah, Abate.; Yajima, Aya. Global leishmaniasis surveillance/ Surveillance mondiale de la leishmaniose : 2019-2020, une période de reference pour la feuille de route a l'horizon 2030. 2019-2020, a baseline for the 2030 roadmap. World Health Organization, Weekly epidemiological record 2021. 96(35): pp. 401-420. ISNN0049-8114.
- Assimina, Z.; Charilaos, K.; Fotoula, B. Leishmaniasis: An overlooked public health concern. Health Science Journal 2008, 2(4), pp. 196-205. ISSN: 1108-7366.
- Lemma, W.; , Bizuneh, A.; Tekie, H.; Belay, H.; Wondimu, Hirut.; Kassahun, A.; Shiferaw, W.; Balkew, M.; Abassi, I.; Baneth, G.; Hailu, A. Preliminary study on investigation of zoonotic visceral leishmaniasis in endemic foci of Ethiopia by detecting Leishmania infections in rodents. Asian Pacific Journal of Tropical Medicine 2017, 10(4): pp. 418-422. [CrossRef]
- Dedet, J.P.; Addadi, K.; Belazzoug, S. Les phlébotomes (Diptera, Psychodidae) d’Algérie. Cahiers-ORSTOM. Entomologie Médicale et Parasitologie 1984, 22, 99–127.
- Burza, S., Croft S.L.; Boelaert, M. Leishmaniasis–authors' reply. The lancet 2019, 393(10174): p. 872-873. [CrossRef]
- Eddaikra, N.; Ait-Oudhia, K.; Kherrachi, I.; Oury, B.; Moulti-Mati, F.; Benikhlef, R.; Harrat, Z.; Sereno, D. Antimony susceptibility of Leishmania isolates collected over a 30-year period in Algeria. PLoS Negl. Trop. Dis 2018, 12(3), e0006310. [CrossRef]
- Benikhlef, R.; Aoun, K.; Boudrissa, A.; Ben Abid, Me.; Cherif, K.; Aissi, W.; Benrekta, S.; Boubidi, S.C.; Späth, G.F.; Bouratbine, A.; Denis, S.; Harrat, Z. Cutaneous leishmaniasis in Algeria; highlight on the focus of M’Sila. Microorganisms 2021. 9(5): p. 962. [CrossRef]
- Eddaikra, N.; Benikhlef, R.; Sereno, D. A Century of Epidemiological Advances in Cutaneous and Visceral Leishmaniasis in Algeria. J Parasitol Res 2025: p. 2102270. [CrossRef]
- Boubou, N. Etude géodemographique et climatique de la problématique de l’eau en algerie. geodemographic’s and climate’s study of water’s problems in algeria. Le Journal de l'Eau et de l'Environnement 2015, 14(26): p. 48-61.
- Randa, G.; Samir, Z.; Hamid, B. Association between climatic changes and leishmaniasis incidence in Biskra district, Algeria. J. Entomol. Zool. Stud 2017, 5, 43–49.
- Bounoua, L.; Kahime, K.; Houti, L.; Blakey, T.; Ebi, K.L.; Zhang, P.; Imhoff, M.L.; Thome, K.J.; Dudek, C.; Sahabi, S.A. Linking Climate to Incidence of Zoonotic Cutaneous Leishmaniasis (L. major) in Pre-Saharan North Africa. Int. J. Environ. Res. Public Health 2013, 10(8), 3172–3191. [CrossRef]
- Wenyon, C.M. The transmission of leishmania infections: A review. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1932, 25(5): p. 319-348.
- Bayon, H. Demonstration of specimens relating to the transmission of artificial cultures of Leishmania infantum to mice and rats. The British Medical Journal, 1912: p. 1197-1199.
- Adler, S. and Theodor, O. The experimental transmission of cutaneous leishmaniasis to man from Phlebotomus papatasii. Annals of Tropical Medicine & Parasitology, 1925, 19(3): p. 365-371.
- Sergent, E.; Sergent, E.; Parrot, L.; Donatien, A.; Beguet, M. Sergent, E. Transmission du clou de Biskra par le phlébotome (Phlebotomus papatasi Scop.). CR Acad Sci, 1921, 173: p. 1030-1032.
- Wenyon, C.M. Experiments on the Behaviour of Leishmania and allied Flagellates in Bugs and Fleas, with some Remarks on Previous Work. 1912.
- Dantas-Torres, F. Ticks as vectors of Leishmania parasites. Trends in parasitology, 2011, 27(4): p. 155-159. [CrossRef]
- Rioux, J.A; Lanotte, G.; Croset, H.; Houin, R.; Guy, Y.; Debet, J.P. Ecology of leishmaniasis in the south of France. 3. Comparison of susceptibility of Phlebotomus ariasi and Rhipicephalus turanicus to infection by Leishmania donovani. 1972.
- Blanc, G.; Caminopetros, J. Transmission of Mediterranean EA by a Tick. 1930.
- Gherbi, R.; Bounechada, M.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Dantas-Torres, F.; Otranto, D. Phlebotomine sand flies and Leishmania species in a focus of cutaneous leishmaniasis in Algeria. PLoS Negl Trop Dis, 2020, 18;14(2):e0008024. [CrossRef]
- Harrat, Z.; Pratlong, F.; Belazzoug, S.; Dereure, J.; Deniau, M.; Rioux, J.A.; Belkaid, M.; Dedet, J.P. Leishmania infantum and L. major in Algeria. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1996, 90(6): p. 625-629. [CrossRef]
- Ferreira, M. G.; Fattori, K. R.; Souza, F.; Lima, V. M. Potential role for dog fleas in the cycle of Leishmania spp. Vet parasitol, 2009, 165(1-2): p. 150-154. [CrossRef]
- Malamos, B. Experiments with Leishmania. IV. Experiments in the Transmission of Kala-azar by Ticks, R. sanguineus. 1938.
- McKenzie, K.K. A study of the transmission of canine leishmaniasis by the tick, Rhipicephalus sanguineus (latreille), and an ultrastructural comparison of the promastigotes (arthropod, biological). 1984: Oklahoma State University.
- Dantas- Torres, F.; Lorusso, V.; Testini, G.; de Paiva-Cavalcanti, M.; Figueredo, L. A.; Stanneck, D.; Mencke, N.; Brandão-Filho, S. P.; Alves, L. C.; Otranto, D. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol res, 2010, 106: p. 857-860. [CrossRef]
- Coutinho, M. T.; Bueno, L. L.; Sterzik, A.; Fujiwara, R. T.; Botelho, J. R.; De Maria, M.; Genaro, O.; Linardi, P. M. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Vet parasitol, 2005, 128(1-2): p. 149-155. [CrossRef]
- Coutinho, M.T.; Linardi, P. M. Can fleas from dogs infected with canine visceral leishmaniasis transfer the infection to other mammals? Vet Parasitol, 2007, 147(3-4): p. 320-325. [CrossRef]
- Azarm, A.; Dalimi, A.; Mohebali, M.; Mohammadiha, A.; Pirestani, M.; Zarei, Z.; Zahraei-Ramazani, A. Molecular Identification of Leishmania infantum kDNA in Naturally Infected Dogs and Their Fleas in an Endemic Focus of Canine Visceral Leishmaniasis in Iran. J Arthropod Borne Dis, 2022, 16(3): p. 243. [CrossRef]
- Kernif, T.; Medrouh, B.; Eddaikra, N.; Oury, B. ;Holzmuller, P.; Sereno, D. Ticks as vectors of Trypanosomatidae with medical or veterinary interest: Insights and implications from a comprehensive systematic review and meta-analysis. Heliyon, 2024. 10(24). [CrossRef]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors, 2010, 3: p. 1-11. [CrossRef]
- Paz, G. F.; Ribeiro, M. F.; Michalsky, E. M.; da Rocha Lima, A. C.; França-Silva, J. C.; Barata, R. A.; Fortes-Dias, C. L.; Dias, E. S. Evaluation of the vectorial capacity of Rhipicephalus sanguineus (Acari: Ixodidae) in the transmission of canine visceral leishmaniasis. Parasitol Res, 2010, 106: p. 523-528. [CrossRef]
- Solano-Gallego, L.; Rossi, L.; Scroccaro, A. M.; Montarsi, F.; Caldin, M.; Furlanello, T.; Trotta, M. Detection of Leishmania infantum DNA mainly in Rhipicephalus sanguineus male ticks removed from dogs living in endemic areas of canine leishmaniosis. Parasites & vectors, 2012, 5: p. 98. [CrossRef]
- Trotta, M.; Nicetto, M.; Fogliazza, A.; Montarsi, F.; Caldin, M.; Furlanello, T.; Solano-Gallego, L. Detection of Leishmania infantum, Babesia canis, and rickettsiae in ticks removed from dogs living in Italy. Ticks Tick Borne Dis, 2012. 3(5-6): p. 294-7. [CrossRef]
- Petersen, C.A.; Barr, S. C. Canine leishmaniasis in North America: emerging or newly recognized? Vet Clin North Am Small Anim Pract, 2009. 39(6): p. 1065-74, vi. [CrossRef]
- Benikhlef, R.; Harrat, Z.; Aoun, K. Epidemiological profile of cutaneous leishmaniasis due to Leishmania tropica in Algeria and Tunisia. J Biol Méd, 2019, 8(31): p. 215-223.
- Belkacemi, S.; Ouazzi, L. Etude rétrospective des cas des leishmanioses cutanée et viscérale entre 2007 et 2015 dans la région de Tizi-Ouzou et étude de six cas de la leishmaniose cutanée diagnostiqués au CHU de Belloua 2016, Doctoral dissertation, Université Mouloud Mammeri.
- Mouloua, A.; Boubidi, S. C.; Bouiba, L.; Mezai, G.; Madiou, M.; Harrat, Z. Environmental impact on the distribution of leishmaniasis in the focus of Tizi-Ouzou (Algeria). Rev Méd Vét, 2017, 168(10/12), 252-261, ref. 252017.
- Moulinier, C. Parasitologie et mycologie médicales: eléments de morphologie et de biologie. Arthropodes. Champignons ou mycètes: hors texte couleur]. Troisième partie. Quatrième partie. Éditions Médicales Internationales. 2002, 2743004886.
- Walker, A.R. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports Edinburgh. 2003, 74.
- Duchemin, J.B. Biogéographie des puces de Madagascar. Doctoral dissertation, Paris 12 2003. p. 253 p.
- Beaucournu, J.C.; Launay, H. Les puces (siphonaptera) de France et du Bassin méditerranéen occidental. Paris: Fédération Française des Sociétés de Sciences Naturelles 1990, Google Scholar.
- Estrada-Peña, A. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species. University of Zaragoza, 2004.
- Šlapeta, J., Chandra, S.; Halliday, B. The tropical lineage of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). International Journal for Parasitology 2021, 51 (6), 431-436. [CrossRef]
- Lodhi, M.A., Ye, GN., Weeden, N.F.; Reisch, B. I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 1994, 12(1), p. 6-13. [CrossRef]
- Schonian,G.; Nasereddin, A.; Dinse, N.; Schweynoch, C.; Schallig, H.D.; Presber, W.; Jaffe, C.L. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis 2003, 47, 349–358. [CrossRef]
- Kebbi, R.; Nait-Mouloud, M.; Hassissen, L.; Ayad, A. Seasonal activity of ticks infesting domestic dogs in Bejaia province, Northern Algeria. Onderstepoort J Vet Res 2019, 17;86(1):e1-e6. [CrossRef]
- Djouaher, T.; Chahed, S.; Beneldjouzi, A.; Eddaikra, N.; Brahmi K. Diversity of hard tick (Acari: Ixodidae) infesting small ruminants in some breeding farms in Tizi-Ouzou area (Northern Algeria). Bul Soc Roy Sc de Liège, 2023, 92(1). [CrossRef]
- Benikhlef, R.; Harrat, Z.; Toudjine, M.; Djerbouh, A.; Bendali-Braham, S.; Belkaid, M. Detection of Leishmania infantum MON-24 in the dog. ]. Med Trop 2004, 64(4): p. 381-3.
- Benikhlef, R.; Aoun, K.; Bedoui, K.; Harrat, Z.; Bouratbine, A. First identifications of Leishmania infantum MON-80 in dogs in Algeria and Tunisia. Rev Méd Vét 2009, 160(10): p. 460-462.
- Sidhoum, N.R.; Boucheikhchoukh, M.; Mechouk, N.; Deak, G. An overview of fleas (Siphonaptera) in wild and domestic mammals from Algeria with new data from the central north and south of the country. Acta Trop 2023, 247, p. 107004. [CrossRef]
- Bitam, I.; Parola, P.; De La Cruz, K.D.; Matsumoto, K.; Baziz, B.; Rolain, J. M.; Belkaid, M.; Raoult, D. First molecular detection of Rickettsia felis in fleas from Algeria. Am J Trop Med Hyg 2006, 74(4):p. 532-535. PMID: 16606979.
- Mans, B.J. Paradigms in tick evolution. Trends Parasitol 2023, 39(6): p. 475-486. [CrossRef]
- Durden, L.A.; Hinkle, N. C. Chapter 10 - Fleas (Siphonaptera). Medical and Veterinary Entomology (Third Edition), G.R. Mullen and L.A. Durden, Editors. Academic Press 2019,. p. 145-169. [CrossRef]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int J Infect Dis 2010, 14(8):e667-76. [CrossRef]
- Boucheikhchoukh, M.; Mechouk, N.; Benakhla, A.; Raoult, D.; Parola, P. Molecular evidence of bacteria in Melophagus ovinus sheep keds and Hippobosca equina forest flies collected from sheep and horses in northeastern Algeria. Comp Immunol Microbiol Infect Dis 2019, 65:103-109. [CrossRef]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol 2014, 30(4):205-14. [CrossRef]
- Bedouhene, A.; Kelanemer, R.; Medrouh, B.; Kernif, T.; Saidi, F.; Tail, G.; Ziam, H. Seasonal Dynamics and Predilection Sites of Ticks (Acari: Ixodidae) Feeding on Cows in the Western Parts of the Djurdjura, Algeria. Frontiers in Tropical Diseases 2022, 3, 856179.
- Zeroual, F.; Bitam, I.; Ouchene, N.; Leulmi, H.; Aouadi, A.; Benakhla, A. Identification and seasonal dynamics of ticks on wild boar (Sus scrofa) in the extreme north-east of Algeria. Bull Soc Zool Fr 2014, 139, 245-253.
- Morel, P.C. ; Vassiliades, G. Les Rhipicephalus du groupe sanguineus: espèces africaines (Acariens: Ixodoidea). Revue d’élevage et de médecine vétérinaire des pays tropicaux, 1962, 15(4): p. 343-386.
- Meddour, A. Clés d’identification des Ixodina (Acarina) d’Algérie. Sciences & Technologie. C, Biotechnologies 2006: p. 32-42.
- Benchikh-Elfegoun, M.; Benakhla, A.; Bentounsi, B.; Bouattour, A.; Piarroux, R. Identification and seasonal kinetics of parasitic ticks in cattle in the region of Taher (Jijel) Algeria. Ann. Vet. Med 2007, 151, 209-214.
- Bouchama, B., Dik, B., Benia, F., Mouffok, C. Dynamique saisonnière des tiques (Acari: Ixodidae) parasites des bovins dans la région semi-aride de la wilaya de Sétif Algérie. Bull. Soc. Zool. Fr 2020, 145(2), 71-81.
- Mechouk, N.; Mihalca, A.D.; Deak, G.; Bouslama, Z. Synopsis of the ticks of Algeria with new hosts and localities records. Parasit Vectors 2022, 27;15(1):302. [CrossRef]
- Bitam, I.; Parola, P.; Matsumoto, K.; Rolain, J.M.; Baziz, B.; Boubidi, S.C.; Harrat, Z.; Belkaid, M.; Raoult, D. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann N Y Acad Sci 2006, 1078:368-72. [CrossRef]
- Colombo, F.A.; Odorizzi, R.M.; Laurenti, M.D.; Galati, E.A.; Canavez, F.; Pereira-Chioccola, V.L. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res 2011, 109(2):267-74. [CrossRef]
- Pereira, A.; Parreira, R.; Cristóvão, J.M.; Vitale, F.; Bastien, P.; Campino. L.; Maia, C. Leishmania infantum strains from cats are similar in biological properties to canine and human strains. Vet Parasitol 2021, 298:109531. [CrossRef]
- Costa-Val, A.P.D.; Coura, F.M.; Barbieri, J.M.; Diniz, L.; Sampaio, A.; Reis, J.K.P.D.; Bueno, B.L.; Gontijo, C.M.F. Serological study of feline leishmaniasis and molecular detection of Leishmania infantum and Leishmania braziliensis in cats (Felis catus). Rev Bras Parasitol Vet 2020, 8;29(2):e003520. PMID: 32520088. [CrossRef]
- Fernandez-Gallego, A.; Feo Bernabe, L.; Dalmau, A.; Esteban-Saltiveri, D.; Font, A.; Leiva, M.; Ortuñez-Navarro, A.; Peña, M.T.; Tabar, M.D.; Real-Sampietro, L.; Saló, F.; Lloret, A.; Bardagí, M. Feline leishmaniosis: diagnosis, treatment and outcome in 16 cats. J Feline Med Surg 2020, 22(10):993-1007. [CrossRef]
- Baneth, G.; Nachum-Biala, Y.; Zuberi, A.; Zipori-Barki, N.; Orshan, L.; Kleinerman, G.; Shmueli-Goldin, A.; Bellaiche, M.; Leszkowicz-Mazuz, M.; Salant, H.; Yasur-Landau, D. Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasit Vectors 2020, 20;13(1):115. [CrossRef]
- Qi, Y.; Zhang, J.; André, M.R.; Qin, T. Editorial: New insights in the microbe-vector interaction. Front Microbiol 2024, 16;15:p. 1364989. [CrossRef]

| Samples sites and GPS | Hosts | Number of ticks or fleas per animal host | Ticks or fleas species | Sex of ticks / fleas | Leishmania DNA detection (n) | |||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Negative | Positive | |||||
| Draâ Ben Khedda | 36° 44′ 06″ N, 3° 57′ 20″ E | Dog | 5 | R. sanguineus s.l | 5 | 0 | 0 | 5♀ |
| 4 | R. turanicus | 0 | 4 | 0 | 4♂ | |||
| Dog | 24 | R. sanguineus s.l | 14 | 10 | _/ | _/ | ||
| 40 | R. turanicus | 24 | 16 | /_ | _/ | |||
| Dog | 6 | C. felis | 4 | 2 | _/ | _/ | ||
| Sheep | 2 | R. sanguineus s.l | 1 | 1 | 2 | 0 | ||
| 6 | R. turanicus | 6 | 0 | 4 | 2♀ | |||
| Sheep | 8 | R. sanguineus s.l | 6 | 2 | /_ | /_ | ||
| Sheep | 2 | C. felis | 2 | 0 | 0 | 2♀ | ||
| Sheep | 12 | C. felis | 9 | 3 | _/ | _/ | ||
| Ouacif | 36° 31′ 25″ N, 4° 12′ 20″ E | Dog | 8 | R. sanguineus s.l | 3 | 5 | _/ | _/ |
| 24 | R. turanicus | 12 | 12 | /_ | _/ | |||
| Dog | 8 | R. sanguineus s.l | 3 | 5 | 3 | 5 (2♀, 3♂) | ||
| 24 | R. turanicus | 12 | 12 | 16 | 8 (5♀, 3♂) | |||
| Rabbit | 9 | C. felis | 8 | 1 | _/ | _/ | ||
| Tizi Rached | 36° 33′ 45″ N, 2° 32′ 00″ E | Dog | 5 | R. sanguineus s.l | 0 | 5 | 0 | 5♂ |
| 59 | R. turanicus | 37 | 22 | 49 | 10 (7♀, 3♂) | |||
| Dog | 9 | R. sanguineus s.l | 1 | 8 | /_ | /_ | ||
| 25 | R. turanicus | 8 | 17 | _/ | /_ | |||
| Cat | 3 | R. sanguineus s.l | 0 | 3 | 3 | 0 | ||
| 32 | R. turanicus | 16 | 16 | 12 | 20 (12♀, 8♂) | |||
| Dog | 3 | C. felis | 3 | 0 | 0 | 3♀ | ||
| Bouzgeune | 36° 37′ 00″ N, 4° 28′ 47″ E | Dog | 7 | R. sanguineus s.l | 6 | 1 | 7 | 0 |
| 10 | R. turanicus | 3 | 7 | 10 | 0 | |||
| Dog | 24 | R. sanguineus s.l | 11 | 13 | /_ | _/ | ||
| 92 | R. turanicus | 63 | 29 | _/ | _/ | |||
| Cat | 6 | R. sanguineus s.l | 6 | 0 | 6 | 0 | ||
| 2 | R. turanicus | 1 | 1 | 2 | 0 | |||
| Cat | 3 | R. sanguineus s.l | 3 | 0 | _/ | _/ | ||
| Goat | 2 | R. sanguineus s.l | 2 | 0 | _/ | /_ | ||
| 6 | R. turanicus | 6 | 0 | _/ | /_ | |||
| Goat | 13 | C. felis | 9 | 4 | 13 | 0 | ||
| Sheep | 12 | C. felis | 11 | 1 | 11 | 1♀ | ||
| Sheep | 6 | C. felis | 4 | 2 | _/ | _/ | ||
| Cat | 10 | C. felis | 10 | 0 | 7 | 3♀ | ||
| Mekla | 36° 41′ 16″ N, 4° 16′ 05″ E | Dog | 8 | R. sanguineus s.l | 0 | 8 | / | _/ |
| 36 | R. turanicus | 17 | 19 | /_ | _/ | |||
| Rabbit | 11 | C. felis | 11 | 0 | 11 | 0 | ||
| Rabbit | 1 | C. felis | 1 | 0 | _/ | _/ | ||
| Sheep | 9 | C. felis | 8 | 1 | _/ | _/ | ||
| M'sila | 35° 42′ 07″ N, 4° 32′ 48″ E | Dog | 3 | R. sanguineus s.l | 0 | 3 | 0 | 3♂ |
| 17 | R. turanicus | 11 | 6 | 0 | 17 (11♀, 6♂) | |||
| Messloula | 35° 24′ 19″ N, 8° 06′ 59″ E | Dog | 2 | R. sanguineus s.l | 1 | 1 | 1 | 1♂ |
| 1 | R. turanicus | 1 | 0 | 0 | 1♂ | |||
| Total ectoparasites | 599 | 359 | 240 | / | / | |||
| 505 ticks / 94 fleas | 279 ticks /80 fleas | 226 ticks / 14 fleas | / | / | ||||
| Total ectoparasites tested for Leishmania DNA | 247 | 155♀ | 92♂ | 157 (101♀, 56♂) | 90 (53♀, 37♂) | |||
| Ectoparasites | Positive | Negative | P valus>0.05 | ||
|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ||
| R. sanguineus s.l | 7 | 12 | 15 | 7 | 0.06 |
| R. turanicus | 37 | 25 | 49 | 44 | 0.32 |
| C. felis | 9 | 0 | 37 | 5 | 0.999 |
| Total | 53 | 37 | 101 | 56 | 247 |
| Predictor | Coefficient | Std. Error | z-score | p-value | 95% CI (Lower) | 95% CI (Upper) |
|---|---|---|---|---|---|---|
| Intercept | -0.213 | 0.509 | -0.42 | 0.675 | -1.210 | 0.784 |
| R. sanguineus s.l | +0.147 | 0.593 | 0.25 | 0.804 | -1.015 | 1.310 |
| R. turanicus | -0.122 | 0.533 | -0.23 | 0.819 | -1.166 | 0.922 |
| Dog | -0.050 | 0.336 | -0.15 | 0.882 | -0.708 | 0.608 |
| Sheep | -0.992 | 0.627 | -1.58 | 0.113 | -2.221 | 0.236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





