1. Introduction
Fludioxonil is currently in review in the European Union (EU) under Regulation (EC) 1107/2009 as part of the Active Ingredient Renewal (AIR) program.
Fludioxonil is a broad-spectrum contact fungicide with residual activity, highly active against the many diseases caused by Ascomycetes, Basidiomycetes and Deuteromycetes. In Europe it can be used for foliar, seed treatment and post-harvest applications on a wide range of crops, including a considerable number of minor uses. Fludioxonil is a unique fungicide for seed and soil borne disease control and a key tool for farmers to control Fusarium, Microdochium nivale, Botrytis and many more diseases affecting the development of crops.
Fludioxonil is a phenylpyrrole based chemical derived from the natural product pyrrolnitrin. Fludioxonil interferes with the transport mechanisms of the fungal cell which inhibits spore germination, growth of germ tubes and mycelia before penetration of the plant tissues. When used as seed treatment, there is limited uptake into the seed and little translocation within the seedlings. Fludioxonil does not show any systemic properties and inhibits fungal development on the surface of the plant.
This mode of action is specific to phenylpyrroles (Fungicide Resistance Action Committee (FRAC) group 12) of which fludioxonil is the only commercially available example. Fludioxonil is necessary to control serious threat to plant health which cannot be contained by other available means including non-chemical methods. Fludioxonil is demonstrated to be essential according to the EFSA protocol for a huge number of crops where no or limited alternatives are available for the control that fludioxonil provides in the EU.
Understanding the environmental fate of agrochemicals is essential for risk assessment under EU Regulation (EC) No 1107/2009 and comparable frameworks. However, the evolution of regulatory conclusions over time can create challenges when it comes to the assessment of agrochemicals as part of the European AIR program. One such example relates to the data requirements for the identification of metabolites generated under aerobic soil conditions. When fludioxonil was submitted in the EU for the first time in 2003, it was concluded at an EFSA Expert Meeting that a metabolic fraction (labelled as MF2) generated in an aerobic soil metabolism study (as referenced in ANSES 2024, Section B8.1.1.1.1; Report K-CA B8.1.1.1/02 conducted by Abildt, U., 1991) [
3] consisted of at least two single compounds which did not meet the criteria for soil and groundwater assessment. However, at the time of active ingredient renewal, the position changed resulting in the need to generate additional data on MF2. Since no samples were available from the original study performed 30 years previously, a new study was designed and performed to attempt to replicate, as closely as possible, the Abildt study conducted in 1991 with the principal aim to generate metabolite fraction MF2 for further investigation as per the requirements under EU Regulation (EC) No 1107/2009. The study was designed to include two temperature and moisture regimes, to enhance the chance of generating the previously observed metabolite fraction. In addition, in line with Organisation for Economic Co-operation and Development (OECD) (2002a) Test Guideline 307 [
4], the study would determine the degradation rate of [pyrrole-4-
14C]-fludioxonil, characterise any metabolite formation, and assess mineralisation and non-extractable residue formation.
2. Materials and Methods
The study was conducted in accordance with Good Laboratory Practice and OECD (2002a) Test Guideline 307 along with modifications to ensure the same study conditions as the original aerobic soil study by Abildt.
2.1. Test Substance
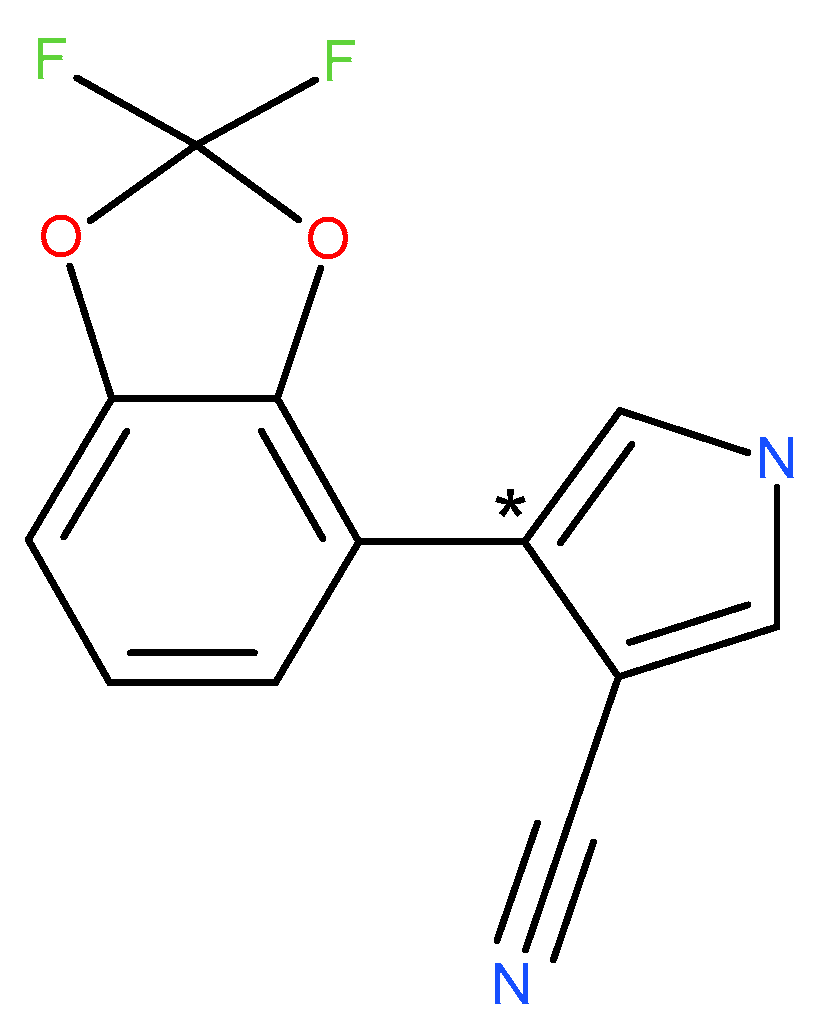
[Pyrrole-4-
14C]-fludioxonil (4-(2,2-difluoro-1,3-benzodioxol-4-yl)-1H-pyrrole-3-carbonitrile) (batch CFQ44124; 99.5% radiochemical purity, pale yellow solid) was supplied by Selcia Limited. The molecule was radiolabelled in the pyrrole ring (specific activity 5.06 MBq/mg). The radiochemical purity of the application solution was 100.0% before the first application and 99.7% after the last treatment. This demonstrated the stability of [pyrrole-4-
14C]-fludioxonil in the application solution during the application procedure. The structure of fludioxonil is given in
Figure 1.
2.2. Test System
Two agricultural soils were sourced from locations in Switzerland (Les Evouettes, grid reference 46°22 N, 6°55 E) and the United States (Conklin, grid reference 43º07 N 85º50 W). Les Evouettes (silt loam, Switzerland) was chosen as this was the soil used in the Abildt (1991) study where MF2 was generated at >5% for two consecutive sampling timepoints. However, the soil characteristics of the batch of Les Evouettes resampled for this work, were shown to be different from the original batch sampled for the Abildt (1991) study. Therefore, in order to provide the best chance of regenerating the metabolite observed, it was determined that a second soil should be used in the study that had more similar soil physico-chemical characteristics to the original Les Evouettes batch. A thorough search of our internal soil database was conducted and the soil identified as being the most similar to the soil used in the original study was Conklin (sandy clay loam, USA). The study was initiated within three months of sampling (91 days and 74 days for Les Evouettes and Conklin soils, respectively), in line with the requirements of OECD (2002a) test guideline 307. The physico-chemical properties of the two soils used in this study along with the original batch of Les Evouettes soil used in Abildt 1991are summarised in
Table 1.
2.3. Analytical Techniques
Quantification of radioactivity in liquid samples was performed by liquid scintillation counting (LSC) using either TriCarb 1900CA or TriCarb 2700 LSC counters (Packard Instruments). Post extraction solid samples were dried, and aliquots were analyzed for radioactive content by sample oxidation using an OX 500 sample oxidiser (Zinsser Analytic) to convert any radioactive components to [14C]-CO2, which was then trapped in a suitable trapping agent and analyzed by LSC.
HPLC was performed using a Water 2487/ Agilent 1260 DAD UV detector system. Radiodetection was via a Berthold LB 509 radiodetector.
The limit of quantification for LSC and HPLC was <1% of applied radioactivity (AR) as specified in OECD 307.
2.4. Estimation of Degradation Kinetics
The rate of degradation of [pyrrole-4-
14C]-fludioxonil was estimated using the Computer Assisted Kinetic Evaluation (CAKE) software version 3.3 [
5]. The rate of degradation was estimated by fitting single first-order kinetics (SFO) to the data.
Input data sets for modelling were derived from individual data of combined soil extracts for each time-point. All data points were unweighted. For optimal goodness of fit, the initial values were also allowed to be estimated by the model. Total mass balance results (sum of all extractable and non-extractable radioactivity) were used for parent as input data for time 0 samples, according to the Forum for the co-ordination of pesticide fate models and their use (FOCUS) [
6].
2.5. Aerobic Soil Metabolism Study Design
The rate of degradation of [pyrrole-4-14C]-fludioxonil was investigated in two different soils under aerobic conditions at two test conditions, identical to that used in the original study, for each soil; 20 °C at 30% of the field capacity (FC) and 10 °C at 60% FC. [Pyrrole-4-14C]-labelled fludioxonil was applied to each sieved soil sample at an application rate of 1,500 g fludioxonil/ha (soil concentration of 2 mg/kg dry soil assuming incorporation to a depth of 5 cm and a bulk density of 1.5 g/cm3).
The treated soils were then incubated for up to 365 days in continuous darkness at a constant temperature of 10.1 ± 0.5 °C and 21.0 ± 0.1 °C. For Les Evouettes soil, duplicate whole soil samples were selected for analysis at 0, 12, 30, 61, 91, 127, 272 and 365 days after treatment (DAT). For Conklin soil, duplicate samples were taken after 0, 14, 30, 63, 98, 118, 180, 272 and 365 DAT. The soil samples were extracted with a range of solvents, identical to that used in the original study, and analyzed by HPLC. Full details are provided in
Appendix A.1.
3. Results
3.1. Mass Balance
The overall mass balances in all soil samples were determined to be 98.4 ± 3.2% and 102.6 ± 2.0% AR in Les Evouettes soil incubated at 20 °C and 10 °C, respectively and 101.2 ± 2.7% and 102.9 ± 1.1% AR in Conklin soil incubated at 20 °C and 10 °C, respectively.
The values for mass balance of individual soil samples ranged from 90.1% to 106.9% AR throughout the study. Full details are provided in the Supporting Material, Tables S1-4.
3.2. Transformation of Parent
The same analytical work up procedure used in the original study was applied to the soil extracts generated in this study prior to profiling. It was observed that the analytical work-up procedure resulted in artefact formation as [pyrrole-4-14C]-fludioxonil was seen to degrade in the combined extracts during a concentration step. Further investigations were carried out to develop an optimised methodology to minimise degradation, and hence artefact formation, as much as possible. Concentration steps were repeated when considered necessary. Artefact formation was particularly evident when comparing profiles of earlier sampling intervals which showed a greater degree of degradation of fludioxonil than in later sampling intervals. The work-up procedure was changed to the modified procedure for later sampling intervals [127 DAT (Les Evouettes soil) and 118 DAT (Conklin soil) and all samples of 272 DAT or later]. Aliquots of the pooled extracts were concentrated using the optimised methodology under reduced pressure, removing only acetone. Even with the modified workup procedure, degradation and formation of artefacts could not be completely excluded as evidenced by components accounting for up to 5.5% AR (i.e., M3 corresponding to MF2 in the Abildt (1991) study) at 0 DAT in the Conklin soil. However, in all HPLC chromatograms, parent was the dominant radioactive fraction, which demonstrated that artefact formation had been limited as much as possible. Since the initial workup procedure was the same as that carried out in the original study, it cannot be excluded that the components observed in the original Abildt (1991) study may, at least to some extent, have been formed because of artefact formation.
[Pyrrole-4-14C]-fludioxonil decreased under all study conditions in both soils throughout the incubation period.
For the Les Evouettes soil, the level of [pyrrole-4-14C]-fludioxonil at application (0 DAT) was 87.9% AR (at 20 °C) and 100.2% AR (at 10 °C). At the end of incubation period, [pyrrole-4-14C]-fludioxonil was detected with a mean amount of 13.3% AR at 20 °C, whereas at 10 °C [pyrrole-4-14C]-fludioxonil was detected with a mean amount of 72.5% AR. The rapid decline observed at the 20 °C incubation was between 182 DAT (68.4%) and 272 DAT (21.7% AR), which was accompanied by a significant increase in trapped 14CO2.
For the Conklin soil, at 0 DAT, the test item recovered was 95.6% AR (20 °C) and 93.3% AR (10 °C) declining to 70.6% (20 °C) and 82.2% AR (10 °C) at 365 days after treatment.
Full details for both soils are provided in the Supporting Material, Tables S5-8.
3.3. Mineralization
Degradation of [pyrrole-4-14C]-fludioxonil with time was confirmed by formation of 14CO2 (up to a maximum of 42.7% AR) which corresponded to complete mineralisation. More mineralisation was observed at the higher temperatures for both soils.
For the Les Evouettes soil, the level of 14CO2 formation (%AR, max) at the end of the incubation period was 42.7% AR in the 20 °C incubations and 11.6% AR in the 10 °C incubations.
For the Conklin soil, the level of 14CO2 formation (%AR, max) at the end of the incubation period was 7.6% AR in the 20 °C incubations and 4.9% AR in the 10 °C incubations.
The mineralisation to 14CO2 was confirmed by precipitation with barium hydroxide.
Full details for both soils are provided in the Supporting Material, Tables S1-4.
3.4. Non-Extractable Residues
Alongside the increasing mineralisation to 14CO2, increased levels of unextracted residues over time were observed.
For the Les Evouettes soil, the level of unextracted residues at the end of the incubation period was 43.4% AR in the 20 °C incubations and 14.6% AR in the 10 °C incubations.
For the Conklin soil, the level of unextracted residues at the end of the incubation period was 18.2% AR in the 20 °C incubations and 12.2% AR in the 10 °C incubations.
Full details for both soils are provided in the Supporting Material, Tables S1-4.
3.5. Metabolite Profile
Initial HPLC chromatographic conditions used in the study (referred to as HPLC Method 13, see Supporting
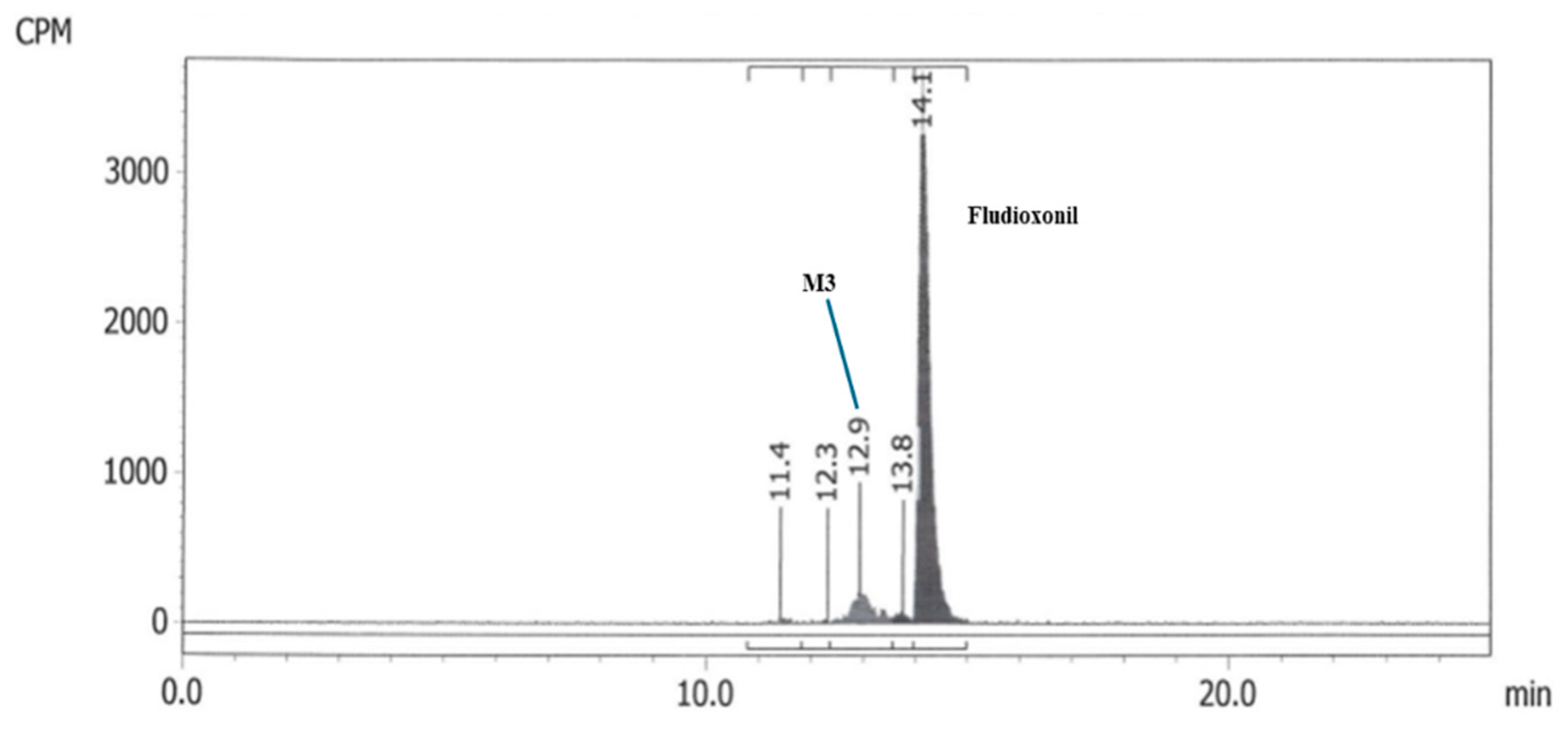
Materials Table S13) were the same as those used in the Abildt (1991) study. Based on relative retention times compared to parent, it was concluded that M3 (see
Figure 3) corresponded to unknown metabolite fraction MF2 (see
Figure 2) from the original aerobic metabolism study by Abildt (1991). Only this M3 fraction was present at levels between 5 and 10% AR at two consecutive timepoints in the analysed soil extracts, both in the 60% FC/10 °C Les Evouettes soil incubation (61 and 91 DAT) samples and the 60% FC/10 °C Conklin soil incubation (30 and 63 DAT) samples.
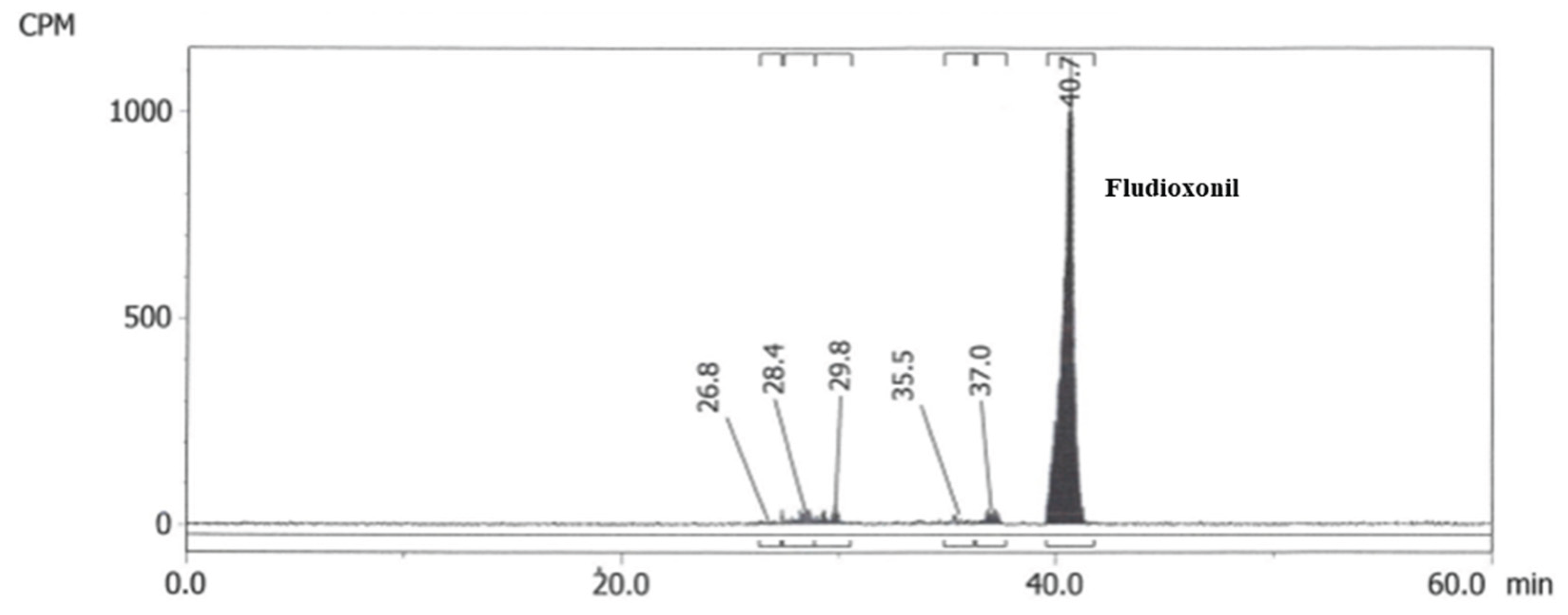
Figure 2.
Chromatogram from the original Abildt (1991) aerobic soil metabolism study showing the presence of metabolite fraction MF2 (Les Evouettes, 362 days after application at 30% field capacity and 20 °C).
Figure 2.
Chromatogram from the original Abildt (1991) aerobic soil metabolism study showing the presence of metabolite fraction MF2 (Les Evouettes, 362 days after application at 30% field capacity and 20 °C).
Figure 3.
Chromatogram from the new aerobic soil metabolism study (Les Evouettes, 91 days after application at 60% field capacity and 10 °C) showing the presence of a component, M3, at a relative retention time consistent with that of MF2.
Figure 3.
Chromatogram from the new aerobic soil metabolism study (Les Evouettes, 91 days after application at 60% field capacity and 10 °C) showing the presence of a component, M3, at a relative retention time consistent with that of MF2.
Using an improved chromatographic system (HPLC Method 12, see Supporting
Materials Table S14), it was shown that metabolite fraction M3 consisted of at least two components, which individually never exceeded 5% AR at consecutive timepoints (an example chromatogram is shown in
Figure 4). In conclusion, a total of fourteen metabolic fractions (after separation of M3 into subfractions) were detected in the soil extracts, of which none exceeded 5% AR over two consecutive timepoints.
This finding infers that unknown fraction MF2 from the Abildt (1991) study also consisted of multiple components with no individual component exceeding 5% AR at consecutive timepoints (MF2 was present at 5.9% AR and 7.7% AR at consecutive sampling timepoints of 30 DAT and 61 DAT in the Abildt (1991) study).
No other unassigned metabolite, present in chromatographed fractions, exceeded 5% AR at consecutive timepoints.
Full details for both soils are provided in the Supporting Material,
Tables S5-S12.
3.6. Rate of Degradation of Fludioxonil
The rate of degradation in the soils for [pyrrole-4-
14C]-fludioxonil was calculated using SFO kinetics using the data from total extracts. The DegT
50 values (SFO) were determined to be 191 and 740 days in soil Les Evouettes at 20 °C and 10 °C, respectively and 896 and 1360 days in soil Conklin at 20 °C and 10 °C, respectively. Full details are provided in Supporting Material,
Table S15.
4. Discussion
The rate of degradation of [pyrrole-4-14C]-fludioxonil was investigated under two different conditions: 20 °C incubation with soil moisture of 30% (FC) and 10 °C incubation with a soil moisture of 60% (FC). Two different soils were selected for use in the study. The first soil, Les Evouettes (a silt loam soil), was selected as it was the soil used in the original study. However, the physico-chemical soil properties of this batch of soil were not a good match to the batch used in the original aerobic soil metabolism study. Therefore, a second soil, Conklin (a sandy loam soil), with more similar properties was selected to improve the chances of generating the same metabolite profile observed in the original soil study.
There was evidence of degradation and artefact formation during the work up of soil extracts. To minimize this as much as possible, selected soil extracts were subjected to an optimized work-up methodology. The improved work-up procedures significantly reduced artefact formation, but could not entirely eliminate it, as evidenced in the 0 DAT analysis of Conklin soil. Consequently, this could have resulted in an over-estimation of the level of degradates formed as a result of metabolism in both this and the original aerobic soil metabolism study.
In the study, the metabolic fraction labelled as M3, corresponded to the MF2 fraction observed in the original Abildt study (based on M3 having the same relative retention time as MF2 when using the original HPLC analytical method from the Abildt study). With improved resolving chromatography, M3 was shown to consist of at least two components, of which neither exceeded 5% AR at two consecutive timepoints.
5. Conclusions
Our work builds upon established soil metabolism methodologies while addressing the unique challenge of replicating historical study conditions when original samples are no longer available.
This study successfully replicated the original Abildt (1991) study from over 30 years ago in forming one unknown fraction that was > 5% AR at two consecutive timepoints within an aerobic soil incubation system. This unknown metabolite fraction, labelled as M3, was shown by relative retention time to correspond to unknown metabolite fraction MF2 from the original aerobic metabolism study.
Further to this, there was also evidence of degradation and artefact formation during the work up of soil extracts. Since the work up procedure was the same as that carried out in the original Abildt (1991) study, it cannot be excluded that the components observed in the original Abildt study, including unknown metabolite fraction MF2 may, at least to some extent, have been formed because of artefact formation.
Further investigations using an HPLC method with improved chromatographic resolution, demonstrated that M3 consisted of at least two subfractions, of which neither exceeded 5% of the applied active ingredient at two consecutive timepoints. This finding infers that unknown fraction MF2 from the Abildt (1991) study can also be characterised as consisting of multiple components with no individual component exceeding 5% of the applied active ingredient at consecutive timepoints. Hence, unknown aerobic soil fraction MF2 does not meet the criteria for identification and soil/groundwater assessment. This study provides a methodological framework for addressing similar challenges with legacy studies.
Author Contributions
Conceptualization, J.D.B., S.N.E.; methodology, J.D.B. and S.N.E.; investigation, J.D.B. and S.N.E.; data curation, J.D.B. and S.N.E.; writing—original draft preparation, J.D.B. and S.N.E; writing—review and editing, J.D.B., S.N.E. All authors have read and agreed to the published version of the manuscript.
Funding
The studies were conducted at 3rd party contract research organisations and funded by Syngenta Ltd.
Data Availability Statement
Restrictions apply to the datasets. Relevant data from the studies described in this paper have been included in the article and supplementary materials. Access to the study reports are restricted due to data confidentiality.
Conflicts of Interest
The authors are employees of Syngenta Ltd. The authors have no other known conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIR |
Active ingredient renewal |
| AR |
Applied radioactivity |
| CAKE |
Computer Assisted Kinetic Evaluation |
| DAT |
Days after treatment |
| EFSA |
European Food Safety Authority |
| EU |
European Union |
| FC |
Field capacity |
| FOCUS |
Forum for the co-ordination of pesticide fate models and their use |
| FRAC |
Fungicide Resistance Action committee |
| HPLC |
High-performance liquid chromatography |
| LSC |
Liquid scintillation counting |
| OECD |
Organisation for Economic Co-operation and Development |
| SFO |
Single first-order |
Appendix A
Appendix A.1: Detailed Method Description
The rate of degradation of [pyrrole-4-14C]-fludioxonil was investigated in two different soils under aerobic conditions at two different test conditions for each soil; 20 °C at 30% of the field capacity (FC) and 10 °C at 60% FC. Soil was sieved to ≤ 2mm and 100 g (dry weight equivalent) was weighed into 1 L glass metabolism flasks. [Pyrrole-4-14C]-labeled fludioxonil was applied to each soil sample at an application rate of 1,500 g a.i./ha (soil concentration of 2 mg/kg dry soil assuming incorporation to a depth of 5 cm and a bulk density of 1.5 g/cm3).
The treated soils were then incubated for up to 365 days in continuous darkness at a constant temperature of 10.1 ± 0.5 °C and 21.0 ± 0.1 °C. Moistened air was passed over the soil surface throughout to flush the exhaust gases through a solution of 2M NaOH to trap evolved [14C]-CO2 following complete mineralization of the test compound (radioassayed by liquid scintillation counting (LSC)).
For Les Evouettes soil, duplicate whole soil samples were selected for analysis at 0, 12, 30, 61, 91, 127, 272 and 365 days after treatment (DAT). For Conklin soil, duplicate samples were taken after 0, 14, 30, 63, 98, 118, 180, 272 and 365 DAT. The soil samples were extracted sequentially, as per the original study, by shaking at room temperature with 2 x acetonitrile:water (8:2, v/v), 3 x acetone and then performing a Soxhlet extraction with acetone (8 h). Extracts were separated by centrifugation at 3900 rpm for 10 min. All extracts were combined, radioassayed by LSC and analyzed by HPLC.
The mass balance for each sample was calculated by summation of the recovered radioactivity in the soil extract, soil post-extraction residue (by sample oxidation/LSC) and trapped in the 2M NaOH traps.
References
- European Parliament and Council. Regulation (EC) No 1107/2009 of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Official Journal of the European Union 2009, L309, 1–50. [Google Scholar]
- EFSA (2019) PRAPeR Expert Meeting 07 (13–15 November 2006) Fludioxonil. Available online upon request via the Connect.EFSA platform; accessed 12 September 2025.
- ANSES- French Agency for Food, Environmental and Occupational Health & Safety (2024) Fludioxonil Renewal Assessment Report Volume 3–B.8 (AS). (Background documents). Available online: https://open.efsa.europa.eu/questions/EFSA-Q-2016-00481?search=fludioxonil (accessed on 12 September 2025).
- OECD Guideline for the Testing of Chemicals. Test Guideline No. 307 Aerobic and Anaerobic Transformation in Soil. Adopted 24 April 2002.
- Computer Assisted Kinetic Evaluation (CAKE) Version 3.3 (Release), Capgemini Plc, Abingdon, Oxfordshire, UK. Available online: https://cake-kinetics.org/ (accessed on 12 September 2025).
- FOCUS. Generic guidance for estimating persistence and degradation kinetics from environmental studies on pesticides in EU registration, December 2014.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).