Submitted:
08 September 2025
Posted:
11 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
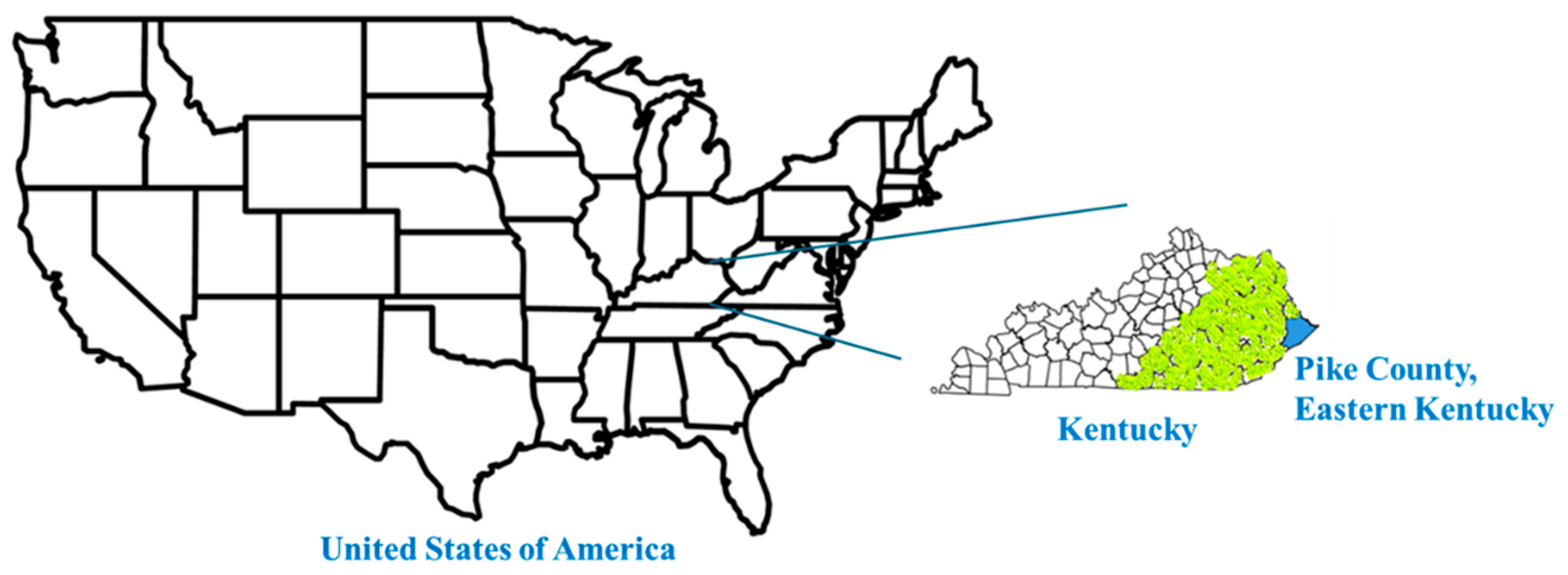
2. Study Design
- Can you remember or know of a local Eastern Kentucky plant used in your household for a medicinal purpose?
- Do you know the name of that plant (local/common name)?
- Do you know what part of that plant (fruit, leaf, root, etc.) is used for its benefit?
- Do you know the clinical or medical indication for which it is used?
- Do you know how the plant is prepared, including any special instructions, for using it for its medicinal purpose?
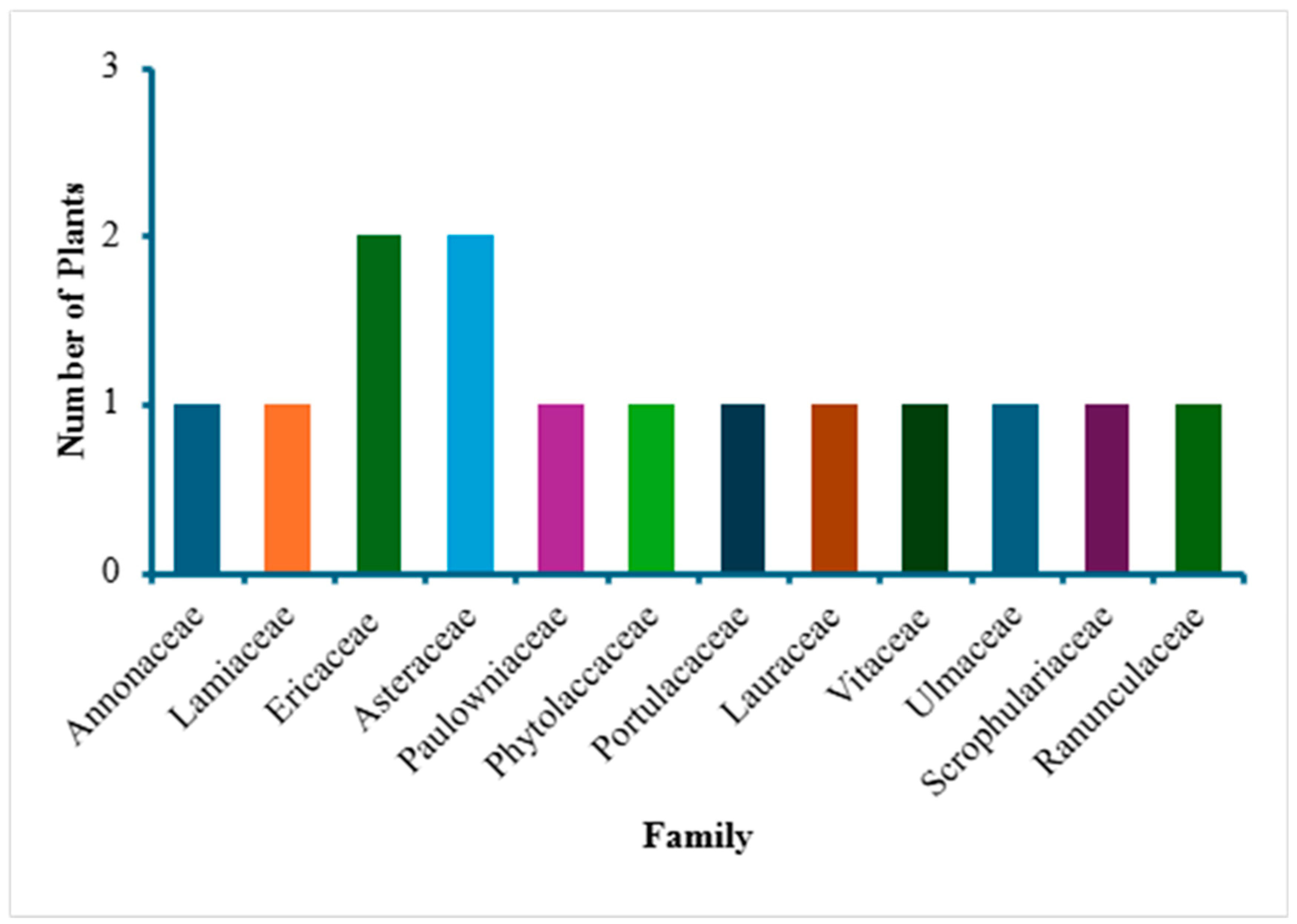
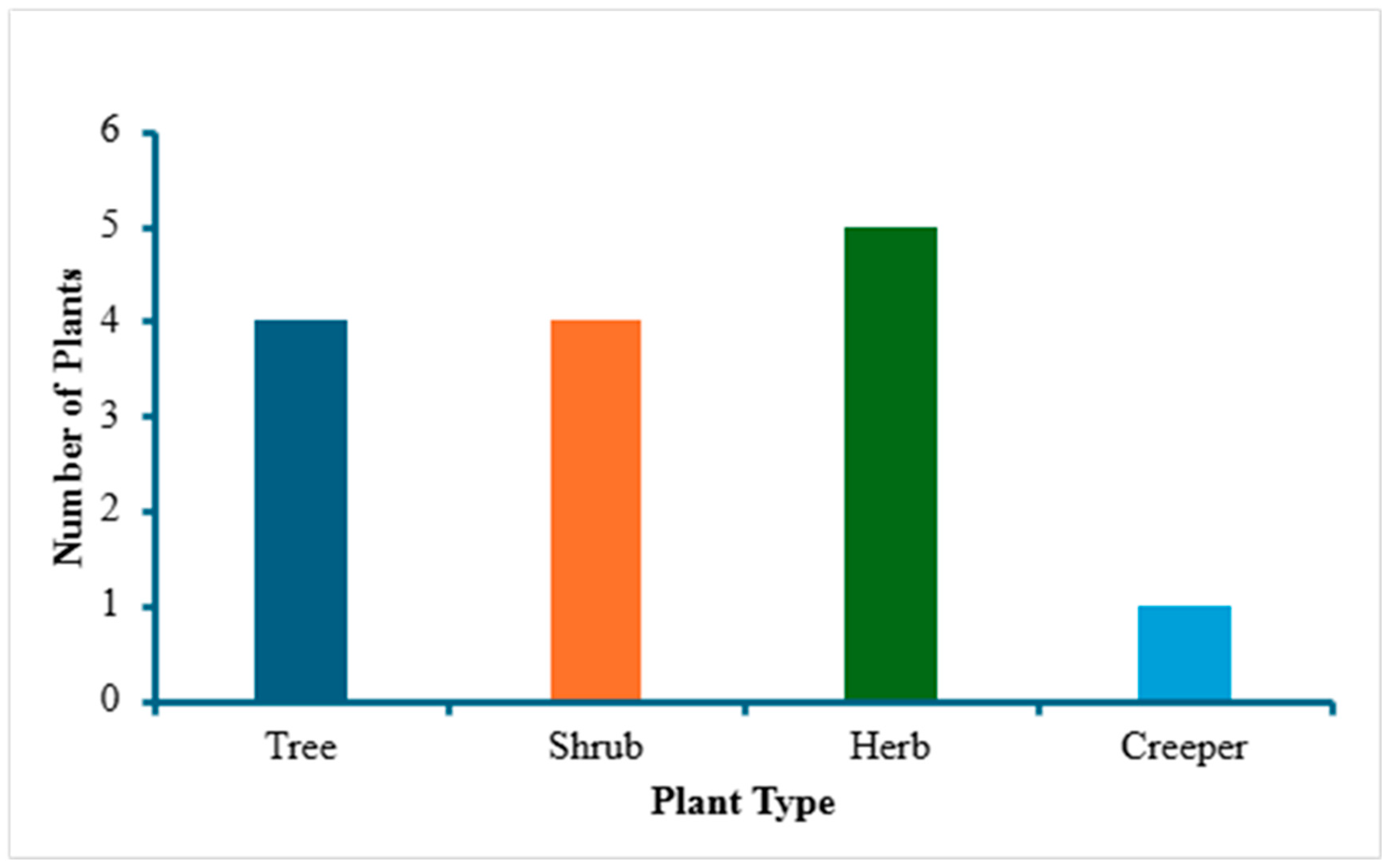
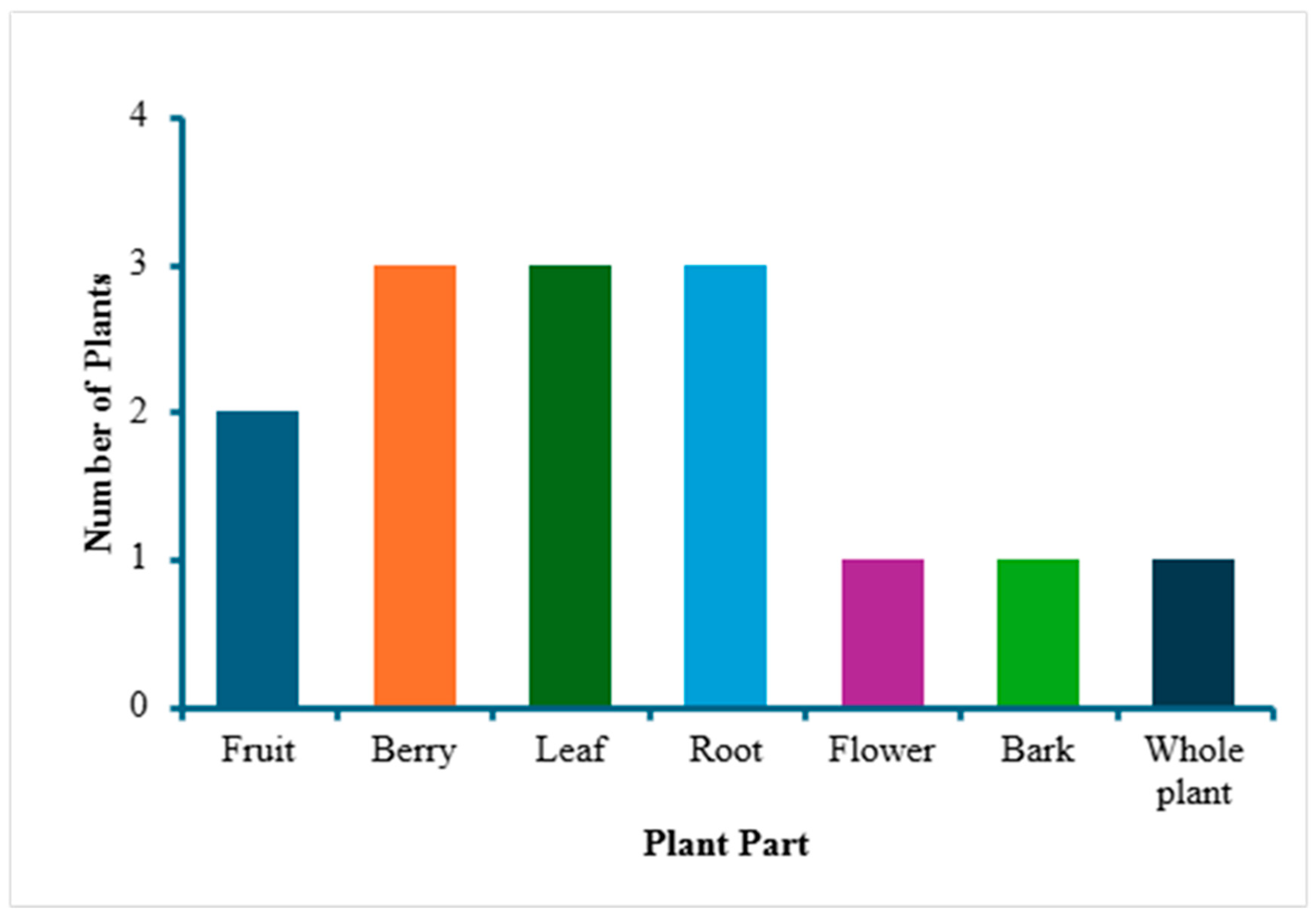
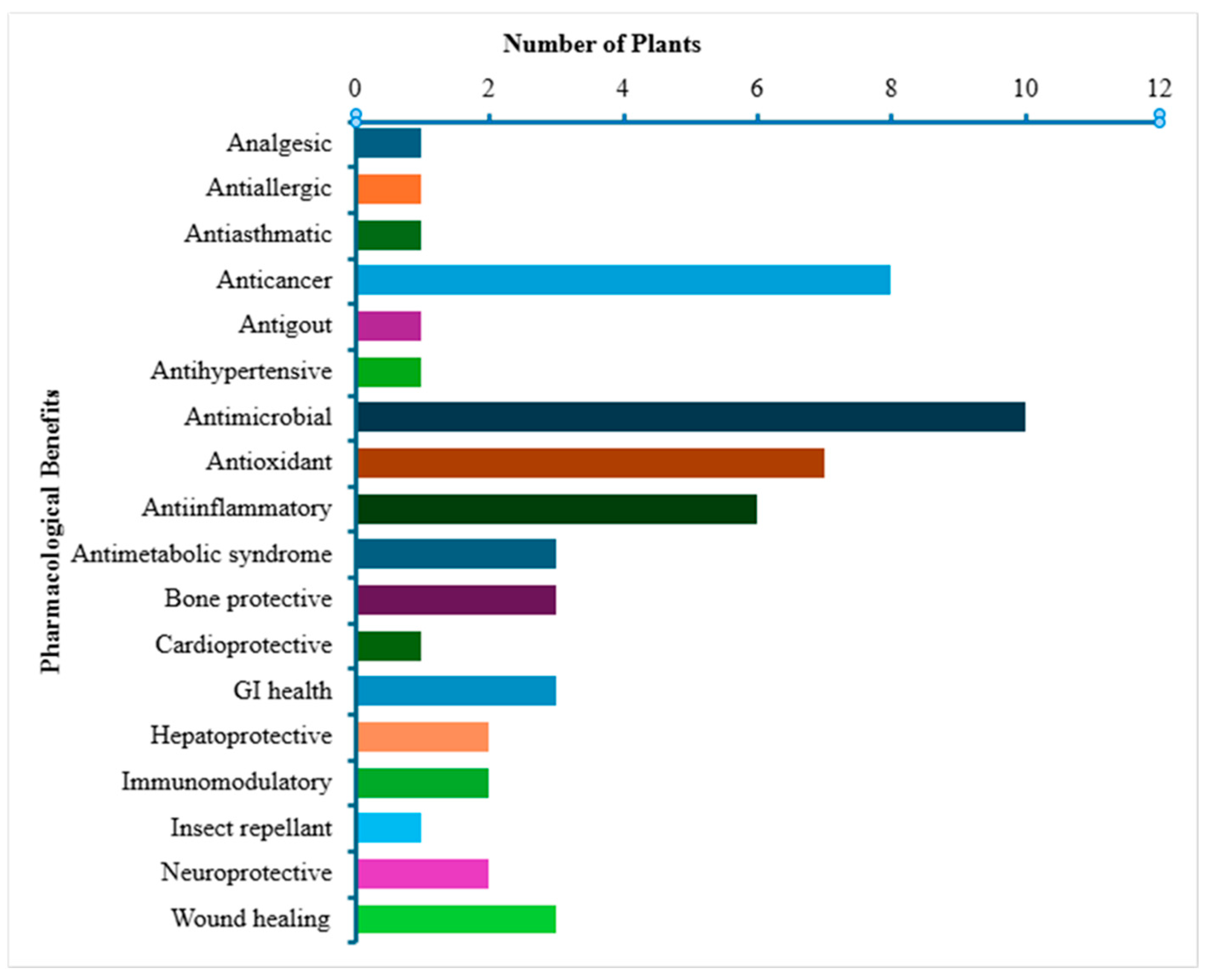
3. Results and Discussion
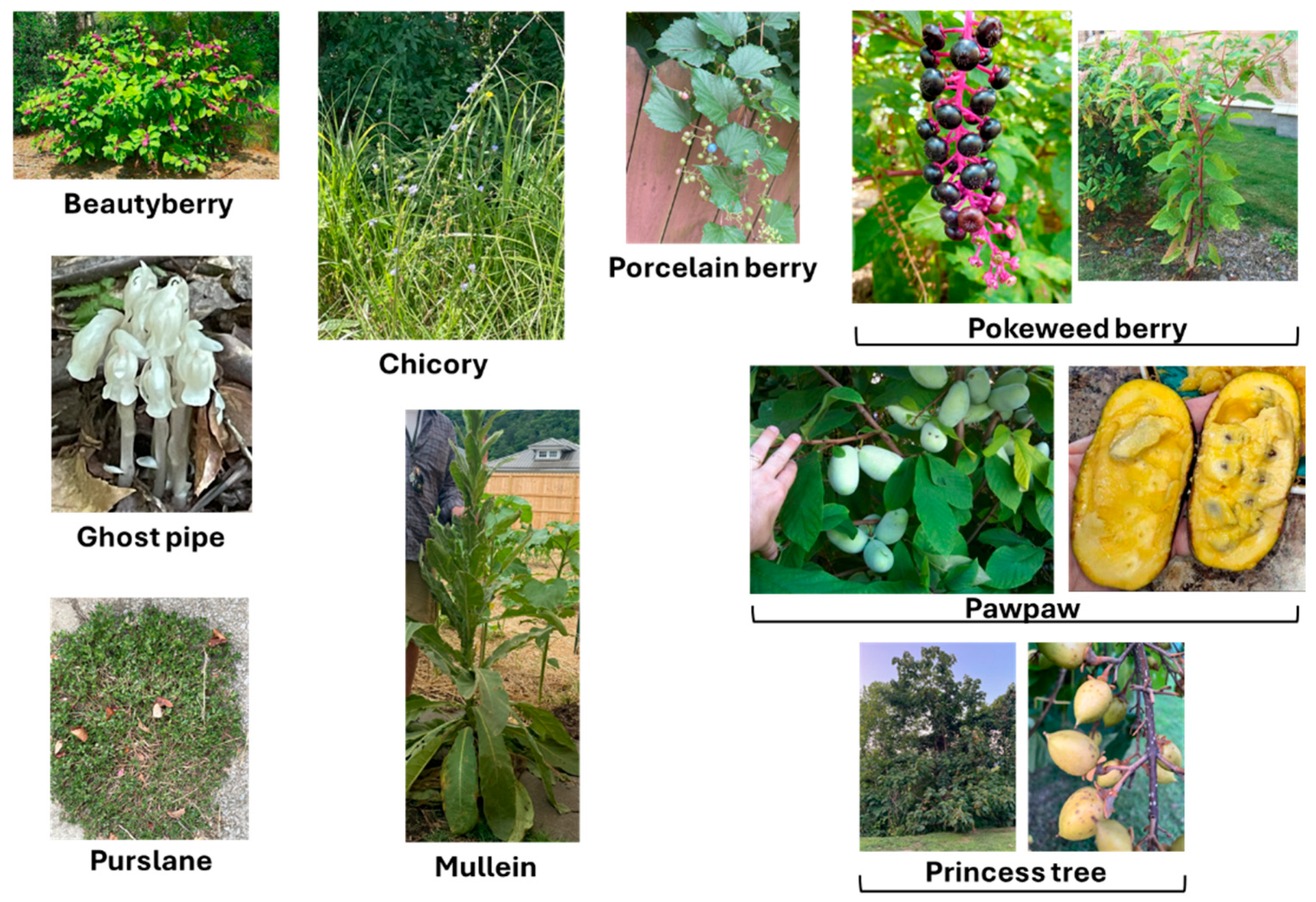
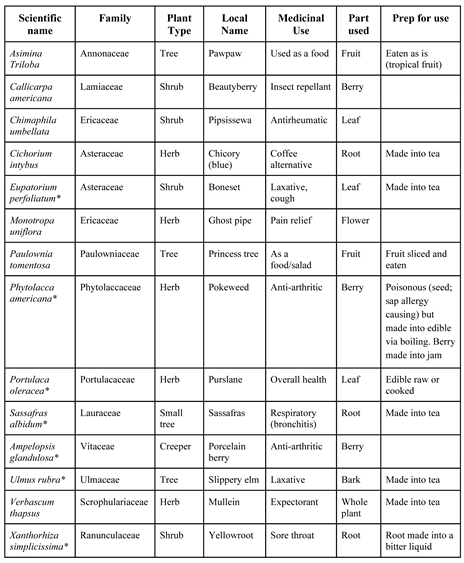
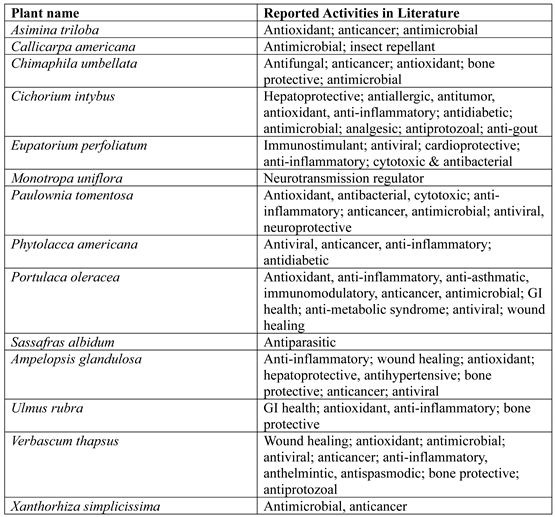
3.1. Asimina Triloba (L.) Dunal
3.2. Callicarpa americana L.
3.3. Chimaphila umbellata (L.) Barton
3.4. Cichorium intybus L.
3.5. Eupatorium Perfoliatum L.
3.6. Monotropa uniflora L.
3.7. Paulownia Tomentosa (Thunb.) Steud
3.8. Phytolacca americana L.
3.9. Portulaca Oleracea L.
3.10. Sassafras albidum (Nutt.) Nees
3.11. Ampelopsis glandulosa (Wall.) Momiy
3.12. Ulmus rubra Muhl
3.13. Verbascum thapsus Linnaeus
3.14. Xanthorhiza simplicissima Marshall
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghayur, M.N. Science across borders: 5th annual natural health product research conference - March 26-29, 2008, Toronto, Canada. Evid. Based Complement. Altern. Med. 2010, 7, 391-395. [CrossRef]
- Ghayur, M.N.; Janssen, L.J. A natural way to cardiovascular health. Nat. Rev. Cardiol. 2010, 7, 1-2. [CrossRef]
- Farnsworth, N.R.; Soejarto, D.D. Global importance of medicinal plants. In: The Conservation of Medicinal Plants; Akerele, O., Heywood, V., Synge, H., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 25-51.
- Light, P.D.; Gladstar, R.; Wood, M. Southern Folk Medicine: Healing Traditions from the Appalachian Fields and Forests; North Atlantic Books: Berkeley, CA, USA, 2018.
- Light, P.D. A history of southern and Appalachian folk medicine. J. Am. Herbalists Guild 2008, 8, 27-38.
- Krochmal, A. Medicinal Plants and Appalachia. Econ. Bot. 1968, 22, 332–337. [CrossRef]
- Pardieu, S. Biodiversity in Eastern Kentucky: Effects of Habitat Change, Surface Top Mining, and Current Reclamation Practices. Undergraduate Thesis, Louisville, KY, USA, Bellarmine University, 2023.
- Boggs, C.; Shiferawe, K.; Karsten, E.; Hamlet, J.; Altheide, S.T.; Marion, J.W. Evaluation of a Tetracycline-Resistant E. coli Enumeration Method for Correctly Classifying E. coli in Environmental Waters in Kentucky, USA. Pathogens 2023, 12, 1090. [PubMed]
- Ghayur, M.N. Natural products breaking through. Pharmacog. Mag. 2006, 2, 1-2.
- Alam-Siddiqui, F.; Ghayur, A.; Ul-Haq, Z.; Ghayur, M.N. Herbal Medicine for the Mind: Traditionally Used Medicinal Plants for Memory Loss from the Indian Subcontinent. Future Integr. Med. 2025, 4, 116-127. [CrossRef]
- Gautam, D.; Regmi, K.; Shrestha, S.; Karki, P. Medicinal Plants as A Natural Immunity Booster Against Covid 19: A Review. Kalika J. Multidiscip. Stud. 2024, 6, 145-167. [CrossRef]
- Begum, S.; Hassan, S.I.; Siddiqui, B.S.; Ifzal, R.; Perwaiz, S.; Kiran, T.; Shaheen, F.; Ghayur, M.N.; Gilani, A.H. Preparation, structure and spasmolytic activities of some derivatives of harmine series of alkaloids. Nat. Prod. Res. 2006, 20, 213-227. [CrossRef]
- Ghayur, M.N.; Janssen, L.J. Nephroprotective drugs from traditionally used Aboriginal medicinal plants. Kid. Int. 2010, 77, 471-472. [CrossRef]
- Krochmal, A.; Walters, R.S.; Doughty, R.M. A Guide to Medicinal Plants of Appalachia; Forest Service, U. S. Department of Agriculture: Upper Darby, PA, USA, 1969.
- Wharton, M.E.; Barbour, R.W. Trees & Shrubs of Kentucky; The University Press of Kentucky: Lexington, KY, USA, 1973.
- Jones, R.L. Plant life of Kentucky - An Illustrated Guide to the Vascular Flora; The University Press of Kentucky: Lexington, KY, USA, 2005.
- Clark, J.B. The Vascular Flora of Breaks Interstate Park, Pike County, Kentucky, and Dickenson County, Virginia. Master Thesis, Eastern Kentucky University, Richmond, KY, USA, 2012.
- Nam, J.S.; Jang, H.L.; Rhee, Y.H. Antioxidant Activities and Phenolic Compounds of Several Tissues of Pawpaw (Asimina triloba [L.] Dunal) Grown in Korea. J. Food Sci. 2017, 82, 1827-1833. [CrossRef]
- Nam, J.; Park, S.; Oh, H.; Jang, H.; Rhee, Y.H. Phenolic profiles, antioxidant and antimicrobial activities of pawpaw pulp (Asimina triloba [L.] Dunal) at different ripening stages. J. Food Sci. 2019, 84, 174–182. [CrossRef]
- Coothankandaswamy, V.; Liu, Y.; Mao, S.C.; Morgan, J.B.; Mahdi, F.; Jekabsons, M.B.; Nagle, D.G.; Zhou, Y.D. The alternative medicine pawpaw and its acetogenin constituents suppress tumor angiogenesis via the HIF-1/VEGF pathway. J. Nat. Prod. 2010, 73, 956-61. [CrossRef] [PubMed]
- He, Y.; Tong, J.; Li, Z.; Yao, L.; Chen, C.; Wan, L.; Ma, W.; Zheng, X.; Cho, N.; Huang, B. Anticancer and chemo-sensitizing effects of annonacin via p53-mediated DNA damage in ovarian cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167971. [CrossRef] [PubMed]
- Monsen, P.J.; Luzzio, F.A. Antiangiogenic Activity and Chemical Derivatization of the Neurotoxic Acetogenin Annonacin Isolated from Asimina triloba. J. Nat. Prod. 2018, 81, 1905-1909. [CrossRef]
- Nam, J.S.; Park, S.Y.; Lee, H.J.; Lee, S.O.; Jang, H.L.; Rhee, Y.H. Correlation Between Acetogenin Content and Antiproliferative Activity of Pawpaw (Asimina triloba [L.] Dunal) Fruit Pulp Grown in Korea. J. Food Sci. 2018, 83, 1430-1435. [CrossRef]
- Moerman, D.E. Native American Ethnobotany; Timber Press Inc.: Portland, OR, USA, 1998.
- Setzer, W.N. The Phytochemistry of Cherokee Aromatic Medicinal Plants. Medicines 2018, 5, 121. [CrossRef] [PubMed]
- Dettweiler, M.; Melander, R.J.; Porras, G.; Risener, C.; Marquez, L.; Samarakoon, T.; Melander, C.; Quave, C.L. A Clerodane diterpene from callicarpa americana resensitizes methicillin-resistant staphylococcus aureusto β-lactam antibiotics. ACS Infect. Dis. 2020, 6, 1667–1673. [CrossRef]
- Cantrell, C.L.; Klun, J.A.; Bryson, C.T.; Kobaisy, M.; Duke, S.O. Isolation and identification of mosquito bite deterrent terpenoids from leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) beautyberry. J. Agric. Food Chem. 2005, 53, 5948–5953. [CrossRef]
- Carroll, J.F.; Cantrell, C.L.; Klun, J.A.; Kramer, M. Repellency of two terpenoid compounds isolated from Callicarpa americana (Lamiaceae) against Ixodes scapularis and Amblyomma americanum ticks. Exp. Appl. Acarol. 2007, 41, 215–224. [CrossRef]
- Purja, S.; Kim, M.; Elghanam, Y.; Shim, H.J.; Kim, E. Efficacy and safety of vancomycin compared with those of alternative treatments for methicillin-resistant Staphylococcus aureus infections: An umbrella review. J. Evid. Based Med. 2024, 17, 729-739. [CrossRef]
- Bomfim, D.P.; da Rocha, M.A.D.; Sanudo, A.; Bagatin, E. A Prospective Randomized Trial Comparing Quality of Life in Adult Female Acne Treated with Azelaic Acid 15% Gel versus Oral Spironolactone. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2335-2343. [CrossRef]
- Valente Duarte de Sousa I.C. An update on the pharmacological management of acne vulgaris: the state of the art. Expert Opin. Pharmacother. 2024, 25, 2177-2190. [CrossRef]
- Zhu, C.; Wei, B.; Li, Y.; Wang, C. Antibiotic resistance rates in Cutibacterium acnes isolated from patients with acne vulgaris: a systematic review and meta-analysis. Front. Microbiol. 2025, 16, 1565111. [CrossRef]
- Pineau, R.M.; Hanson, S.E.; Lyles, J.T.; Quave, C.L. Growth Inhibitory Activity of Callicarpa americana Leaf Extracts Against Cutibacterium acnes. Front. Pharmacol. 2019, 10, 1206. [CrossRef] [PubMed]
- Hamilton, J.P.; Godden, G.T.; Lanier, E.; Bhat, W.W.; Kinser, T.J.; Vaillancourt, B.; Wang, H.; Wood, J.C.; Jiang, J.; Soltis, P.S.; et al. Generation of a chromosome-scale genome assembly of the insect-repellent terpenoid-producing Lamiaceae species, Callicarpa americana. GigaScience 2020, 9, giaa093. [CrossRef] [PubMed]
- Ali, U.; Khan, M.M.; Khan, N.; Haya, R.T.; Asghar, M.U.; Abbasi, B.H. Chimaphila umbellata; a biotechnological perspective on the coming-of-age prince’s pine. Phytochem. Rev. 2023, Jun 8, 1-16. [CrossRef] [PubMed]
- Blakey, Y.C. On the Use of the Chimaphila Umbellata in the Treatment of Fungus Articuli, or White Swelling. Med. Exam. (Phila.) 1846, 2, 585-587.
- Galván, I.J.; Mir-Rashed, N.; Jessulat, M.; Atanya, M.; Golshani, A.; Durst, T.; Petit, P.; Amiguet, V.T.; Boekhout, T.; Summerbell, R.; et al. Antifungal and antioxidant activities of the phytomedicine pipsissewa, Chimaphila umbellata. Phytochem. 2008, 69, 738–746. [CrossRef]
- Shin, B.K.; Kim, J.; Kang, K.S.; Piao, H.S.; Park, J.H.; Hwang, G.S. A new naphthalene glycoside from Chimaphila umbellata inhibits the RANKL-stimulated osteoclast differentiation. Arch. Pharm. Res. 2015, 38, 2059-2065. [CrossRef]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L.G. Antimicrobial activity of natural products from the flora of Northern Ontario, Canada. Pharm. Biol. 2015, 53, 800-806. [CrossRef]
- Birsa, M.L.; Sarbu, L.G. Health Benefits of Key Constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [CrossRef]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties-A Review. Molecules 2021, 26, 1814. [CrossRef] [PubMed]
- Peña-Espinoza, M.; Romero-Uzqueda, Y.; Valente, A.H.; de Roode, M.; Simonsen, H.T.; Thamsborg, S.M.; Williams, A.R.; López-Muñoz, R. Anti-protozoal activity and metabolomic analyses of Cichorium intybus L. against Trypanosoma cruzi. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 43–53. [CrossRef]
- Pieroni, A.; Janiak, V.; Dürr, C.M.; Lüdeke, S.; Trachsel, E.; Heinrich, M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother. Res. 2002, 16, 467-473. [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B.; Jiang, Z.; Guo, F.; Yang, T. Cichorium intybus L. Extract Suppresses Experimental Gout by Inhibiting the NF-κB and NLRP3 Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4921. [CrossRef]
- Amatjan, M.; Li, N.; He, P.; Zhang, B.; Mai, X.; Jiang, Q.; Xie, H.; Shao, X. A Novel Approach Based on Gut Microbiota Analysis and Network Pharmacology to Explain the Mechanisms of Action of Cichorium intybus L. Formula in the Improvement of Hyperuricemic Nephropathy in Rats. Drug Des. Devel. Ther. 2023, 17, 107-128. [CrossRef]
- Kent, J.T. Eupatorium perfoliatum. The Homoeopathic Physician 1887, 7, 55-58. [PubMed]
- Lockwood, T.T. On the Use of Eupatorium perfoliatum, Thoroughwort Boneset. Buffalo Med. J. Monthly Rev. Med. Surg. Sci. 1847, 3, 197-198. [CrossRef]
- Derksen, A.; Kühn, J.; Hafezi, W.; Sendker, J.; Ehrhardt, C.; Ludwig, S.; Hensel, A. Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus. J. Ethnopharmacol. 2016, 188, 144-152. [CrossRef]
- Tomassone, D. Homeopathic Eupatorium perfoliatum in the treatment of COVID-19. Homeopathy 2023, 112, 70-72. [CrossRef]
- Roy, A.; Sarkar, A.; Roy, A.K.; Ghorai, T.; Nayak, D.; Kaushik, S.; Das, S. Ultradiluted Eupatorium perfoliatum Prevents and Alleviates SARS-CoV-2 Spike Protein-Induced Lung Pathogenesis by Regulating Inflammatory Response and Apoptosis. Diseases 2025, 13, 36. [CrossRef] [PubMed]
- Nayak, D.; Kaur, L.; Bhalerao, R.; Nahar, K.; Ram, H.; Sharma, P.; Gupta, A.; Singh, S.; Khurana, A.; Manchanda, R.K. Effectiveness of Eupatorium perfoliatum 30C in Preventing Dengue Fever-A Prospective, Community-Based, Open Label, Parallel Cohort Study in Delhi, India. Homeopathy 2025, 114, 163-172.
- Nayak, D.; Bhalla, R.; Bhalerao, R.; Kaur, L.; Gupta, A.; Dev, V.; Singh, S.; Khurana, A.; Manchanda, R.K. Effectiveness of Eupatorium perfoliatum 30C in Prevention of Dengue Fever and Acute Febrile Illness during 2017 Dengue Outbreak in Urban Slums of Delhi: A Prospective, Open-Label, Community-Based, Parallel Cohort Study. Complement. Med. Res. 2023, 30, 471-480. [CrossRef] [PubMed]
- Sinha, M.; Chakraborty, U.; Kool, A.; Chakravarti, M.; Das, S.; Ghosh, S.; Thakur, L.; Khuranna, A.; Nayak, D.; Basu, B.; et al. In-vitro antiviral action of Eupatorium perfoliatum against dengue virus infection: Modulation of mTOR signaling and autophagy. J. Ethnopharmacol. 2022, 282, 114627.
- Gao, Y.; Zhang, Y.; Fan Y. Eupafolin ameliorates lipopolysaccharide-induced cardiomyocyte autophagy via PI3K/AKT/mTOR signaling pathway. Iran J. Basic Med. Sci. 2019, 22, 1340-1346.
- Maas, M.; Deters, A.M.; Hensel, A. Anti-inflammatory activity of Eupatorium perfoliatum L. extracts, eupafolin, and dimeric guaianolide via iNOS inhibitory activity and modulation of inflammation-related cytokines and chemokines. J. Ethnopharmacol. 2011, 137, 371-381. [CrossRef]
- Habtemariam, S.; Macpherson, A.M. Cytotoxicity and antibacterial activity of ethanol extract from leaves of a herbal drug, boneset (Eupatorium perfoliatum). Phytother. Res. 2000, 14, 575-577. [CrossRef] [PubMed]
- Wang, Q.Y.; Chen, H.P.; Tao; H., Li, X.; Zhao, Q.; Liu JK. Penidaleodiolides A and B, cage-like polyketides with neurotransmission-regulating activity from the soil fungus penicillium daleae L3SO. Org. Lett. 2024, 26, 7632-7637.
- Anez, S.G.; Burkhart, E.P.; Kellogg, J.J. Ghost Pipe Then and Now: the Influence of Digital Media on the Medicinal Use of Monotropa uniflora in the United States. Econ. Bot. 2025. [CrossRef]
- Schneiderová, K.; Šmejkal, K. Phytochemical profile of Paulownia tomentosa (Thunb). Steud. Phytochem. Rev. 2015, 14, 799–833. [CrossRef]
- Ryu, H.W.; Park, Y.J.; Lee, S.U.; Lee, S.; Yuk, H.J.; Seo, K.H.; Kim, Y.U.; Hwang, B.Y.; Oh, S.R. Potential Anti-inflammatory Effects of the Fruits of Paulownia tomentosa. J. Nat. Prod. 2017, 80, 2659–2665. [CrossRef]
- Molčanová, L.; Treml, J.; Brezáni, V.; Maršík, P.; Kurhan, S.; Trávníček, Z.; Uhrin, P.; Šmejkal, K. C-geranylated flavonoids from Paulownia tomentosa Steud. fruit as potential anti-inflammatory agents. J. Ethnopharmacol. 2022, 296, 115509. [CrossRef]
- Lee, J.W.; Seo, K.H.; Ryu, H.W.; Yuk, H.J.; Park, H.A.; Lim, Y.; Ahn, K.S.; Oh, S.R. Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. J. Ethnopharmacol. 2018, 210, 23-30. [CrossRef]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vaněk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polanský, O.; et al. Anti-inflammatory Activity of Natural Geranylated Flavonoids: Cyclooxygenase and Lipoxygenase Inhibitory Properties and Proteomic Analysis. J. Nat. Prod. 2017, 80, 999-1006. [CrossRef]
- Hanáková, Z.; Hošek, J.; Babula, P.; Dall’Acqua, S.; Václavík, J.; Šmejkal, K. C-Geranylated Flavanones from Paulownia tomentosa Fruits as Potential Anti-inflammatory Compounds Acting via Inhibition of TNF-alpha Production. J. Nat. Prod. 2015, 78, 850-863. [CrossRef]
- Park, E.S.; Hwang, Y.S.; Ryu, H.W.; Yoon, H.R.; Kim, J.T.; Lim, J.S.; Cho, H.J.; Lee, H.G. Paulownin elicits anti-tumor effects by enhancing NK cell cytotoxicity through JNK pathway activation. Front. Pharmacol. 2024, 15, 1439079. [CrossRef]
- Kang, M.J.; Ryu, H.W.; Oh, E.S.; Song, Y.N.; Huh, Y.H.; Park, J.Y.; Oh, S.M.; Lee, S.Y.; Park, Y.J.; Kim, D.Y.; et al. Diplacone Isolated from Paulownia tomentosa Mature Fruit Induces Ferroptosis-Mediated Cell Death through Mitochondrial Ca(2+) Influx and Mitochondrial Permeability Transition. Int. J. Mol. Sci. 2023, 24, 7057. [CrossRef]
- Molčanová, L.; Kauerová, T.; Dall’Acqua, S.; Maršík, P.; Kollár, P.; Šmejkal, K. Antiproliferative and cytotoxic activities of C-Geranylated flavonoids from Paulownia tomentosa Steud. Fruit. Bioorg. Chem. 2021, 111, 104797. [CrossRef]
- Singh, M.P.; Park, K.H.; Khaket, T.P.; Kang, S.C. CJK-7, a Novel Flavonoid from Paulownia tomentosa Triggers Cell Death Cascades in HCT-116 Human Colon Carcinoma Cells via Redox Signaling. Anticancer Agents Med. Chem. 2018, 18, 428-437. [CrossRef]
- Xu, S.; Kang, A.; Tian, Y.; Li, X.; Qin, S.; Yang, R.; Guo, Y. Plant Flavonoids with Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). ACS Infect. Dis. 2024, 10, 3086-3097. [CrossRef]
- Bouqellah, N.A.; Hussein, E.T.; Abdel Razik, A.B.; Ahmed, M.F.; Faraag, A.H.I. Development of transgenic Paulownia trees expressing antimicrobial thionin genes for enhanced resistance to fungal infections using chitosan nanoparticles. Microb. Pathog. 2024, 191, 106659. [CrossRef]
- Škovranová, G.; Molčanová, L.; Jug, B.; Jug, D.; Klančnik, A.; Smole-Možina, S.; Treml, J.; Tušek Žnidarič, M.; Sychrová, A. Perspectives on antimicrobial properties of Paulownia tomentosa Steud. fruit products in the control of Staphylococcus aureus infections. J. Ethnopharmacol. 2024, 321, 117461. [CrossRef]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela-M, R.E.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated flavonoids from Paulownia tomentosa fruits with antimicrobial potential and synergistic activity with antibiotics. Pharm. Biol. 2016, 54, 1398-1407. [CrossRef]
- Navrátilová, A.; Schneiderová, K.; Veselá, D.; Hanáková, Z.; Fontana, A.; Dall’Acqua, S.; Cvačka, J.; Innocenti, G.; Novotná, J.; Urbanová, M.; et al. Minor C-geranylated flavanones from Paulownia tomentosa fruits with MRSA antibacterial activity. Phytochem. 2013, 89, 104-113. [CrossRef]
- Smejkal, K.; Chudík, S.; Kloucek, P.; Marek, R.; Cvacka, J.; Urbanová, M.; Julínek, O.; Kokoska, L.; Slapetová, T.; Holubová, P.; et al. Antibacterial C-geranylflavonoids from Paulownia tomentosa Fruits. J. Nat. Prod. 2008, 71, 706-709. [CrossRef]
- Magurano, F.; Micucci, M.; Nuzzo, D.; Baggieri, M.; Picone, P.; Gioacchini, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; Mari, M.; et al. A potential host and virus targeting tool against COVID-19: Chemical characterization, antiviral, cytoprotective, antioxidant, respiratory smooth muscle relaxant effects of Paulownia tomentosa Steud. Biomed. Pharmacother. 2023, 158, 114083. [CrossRef]
- Ji, P.; Chen; C.; Hu, Y.; Zhan, Z.; Pan, W.; Li, R.; Li, E; Ge, H.M.; Yang, G. Antiviral activity of Paulownia tomentosa against enterovirus 71 of hand, foot, and mouth disease. Biol. Pharm. Bull. 2015, 38, 1-6. [CrossRef]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Yuk, H.J.; Kim, D.W.; Kim, H.J.; Lee, W.S.; Park, K.H. Cholinestrase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg. Med. Chem. 2012, 20, 2595-2602. [CrossRef]
- Kim, S.K.; Cho, S.B.; Moon, H.I. Neuroprotective effects of a sesquiterpene lactone and flavanones from Paulownia tomentosa Steud. against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Phytother. Res. 2010, 24, 1898-1900. [CrossRef]
- Domashevskiy, A.V.; Goss, D.J. Pokeweed antiviral protein, a ribosome inactivating protein: activity, inhibition and prospects. Toxins (Basel) 2015, 7, 274-298. [CrossRef] [PubMed]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Ni, B.; Guo, C.X.; Mao, X. Inhibition of Japanese encephalitis virus infection in vitro and in vivo by pokeweed antiviral protein. Virus Res. 2013, 171, 89–96. [CrossRef]
- Attitalla, I.H. Anti-colon activity in ethanolic extract of Phytolacca americana. Pak. J. Biol. Sci. 2011, 14, 914–915. [CrossRef]
- George Thompson, A.M.; Iancu, C.V.; Nguyen, T.T.; Kim, D.; Choe, J.Y. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci. Rep. 2015, 5, 12804. [CrossRef]
- Hassan, Y.; Ogg, S.; Ge, H. Expression of novel fusion antiviral proteins ricin a chain-pokeweed antiviral proteins (RTA-PAPs) in Escherichia coli and their inhibition of protein synthesis and of hepatitis B virus in vitro. BMC biotech. 2018, 18, 47. [CrossRef]
- Mattera, M.; Pilla, N.; Aguzzi, A.; Gabrielli, P.; DiLena, G.; Durazzo, A.; Lucarini, M. Portulaca oleracea L.: literature quantitative research analysis. Nat. Prod. Res. 2024, 12, 1-10. [CrossRef] [PubMed]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 925631.
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Antioxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [CrossRef] [PubMed]
- Zhang, Z.; Qiao, D.; Zhang, Y.; Chen, Q.; Chen, Y.; Tang, Y.; Que, R.; Chen, Y.; Zheng, L.; Dai, Y.; et al. Portulaca oleracea L. Extract Ameliorates Intestinal Inflammation by Regulating Endoplasmic Reticulum Stress and Autophagy. Mol. Nutr. Food Res. 2022, 66, e2100791. [CrossRef]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y. Q.; et al. Edible exosome-like nanoparticles from Portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J. Nanobiotech. 2023, 21, 309. [CrossRef]
- Ebrahimian, Z.; Razavi, B.M.; Mousavi Shaegh, S.A.; Hosseinzadeh, H. Effects of Portulaca oleracea L. (purslane) on the metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2022, 25, 1275–1285. [PubMed]
- Malek, F.; Boskabady, M.H.; Borushaki, M.T.; Tohidi, M. Bronchodilatory effect of Portulaca oleracea in airways of asthmatic patients. J. Ethnopharmacol. 2004, 93, 57-62. [CrossRef]
- Askari, V.R.; Rezaee, S.A.; Abnous, K.; Iranshahi, M.; Boskabady, M.H. The influence of hydro-ethanolic extract of Portulaca oleracea L. on Th(1)/Th(2) balance in isolated human lymphocytes. J. Ethnopharmacol. 2016, 194: 1112-1121.
- Kaveh, M.; Eidi, A.; Nemati, A.; Boskabady, MH. The Extract of Portulaca oleracea and Its Constituent, Alpha Linolenic Acid Affects Serum Oxidant Levels and Inflammatory Cells in Sensitized Rats. Iran. J. Allergy Asthma Immunol. 2017, 16, 256-270.
- Kaveh, M.; Eidi, A.; Nemati, A.; Boskabady, M.H. Modulation of lung inflammation and immune markers in asthmatic rats treated by Portulaca oleracea. Avicenna J. Phytomed. 2017, 7, 409-416.
- Shakeri, F.; Ghorani, V.; Saadat, S.; Gholamnezhad, Z.; Boskabady, MH. The Stimulatory Effects of Medicinal Plants on β2-adrenoceptors of Tracheal Smooth Muscle. Iran. J. Allergy Asthma Immunol. 2019, 18, 12-26. [CrossRef] [PubMed]
- Khazdair, M.R.; Anaeigoudari, A.; Kianmehr, M. Anti-Asthmatic Effects of Portulaca Oleraceaand its Constituents, a Review. J. Pharmacopunct. 2019, 22, 122–130. [CrossRef]
- Weng, Q.; Yuan, K.; Zhang, H.; Xiong, J.; Wang, C.; Xu, G. Determination of dopamine and norepinephrine in Portulaca oleracea L. by micellar electrokinetic capillary chromatography with amperometric detection. Se Pu. 2005, 23, 18-21. [PubMed]
- Zhou, X.; Li, Y.; Li, T.; Cao, J.; Guan, Z.; Xu, T.; Jia, G.; Ma, G.; Zhao, R. Portulaca oleracea L. Polysaccharide Inhibits Porcine Rotavirus In Vitro. Animals 2023, 13, 2306. [CrossRef]
- Wei, H.; Chen, Z.; Lai, W.; Wang, W.; Bian, X.; Zhang, L.; Li, X. Aqueous extracts of Portulaca oleracea L. alleviate atopic dermatitis by restoring skin barrier function. Front. Pharmacol. 2025, 16, 1591394. [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Li, W.; Hu, Q.; Huang, H.; Xu, X.; Du, B.; Li, P. Probiotic-fermented Portulaca oleracea L. alleviated DNFB-induced atopic dermatitis by inhibiting the NF-kappaB signaling pathway. J. Ethnopharmacol. 2023, 313, 116613. [CrossRef]
- Lv, W.J.; Huang, J.Y.; Li, S.P.; Gong, X.P.; Sun, J.B.; Mao, W.; Guo, SN. Portulaca oleracea L. extracts alleviate 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Front. Nutr. 2022, 9, 986943. [CrossRef]
- Wang. L.; Zhang, Y.; Geng, S.; Ma, L.; Wang, Y.; Han, D.; Fan, G.; Zhang, W.; Lv, Y.; Ma, J. A Chinese drug-compatibility-based approach to purslane hydrogels for acute eczema therapy. Front. Pharmacol. 2025, 16, 1504120.
- Heydarirad, G.; Rastegar, S.; Haji-Abdolvahab, H.; Fuzimoto, A.; Hunter, J.; Zare, R.; Pasalar, M. Efficacy and safety of purslane (Portulaca oleracea) for mild to moderate chronic hand eczema; A randomized, double-blind, placebo-controlled clinical trial. Explore (NY) 2024, 20, 401-410. [CrossRef]
- Qu, L.; Wang, F.; Ma, X. The extract from Portulaca oleracea L. rehabilitates skin photoaging via adjusting miR-138-5p/Sirt1-mediated inflammation and oxidative stress. Heliyon 2023, 9, e21955. [CrossRef]
- Wei, J.; Quan, Q.; Wang, P.; Wang, Y.; Huo, T.; An, Q. Portulaca oleracea extract relieves skin barrier damage induced by increased photosensitivity after GA peeling. Cutan. Ocul. Toxicol. 2022, 41, 257-263. [CrossRef] [PubMed]
- Budiawanm, A.; Purwanto, A.; Puradewa, L.; Cahyani, E.D.; Purwaningsih, C.E. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023, 13, 9871-9877. [CrossRef]
- Alves Barros, A.S.; Oliveira Carvalho, H.; Dos Santos, I.V.F.; Taglialegna, T.; Dos Santos Sampaio, T.I.; Duarte, J.L.; Fernandes, C.P.; Tavares Carvalho, J.C. Study of the non-clinical healing activities of the extract and gel of Portulaca pilosa L. in skin wounds in Wistar rats: A preliminary study. Biomed. Pharmacother. 2017, 96, 182-190. [CrossRef]
- Rashed, A.N.; Afifi, F.U.; Disi, A.M. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131-136. [CrossRef]
- Perlmutter, J.; Cogan, R.; Wiseman, M.C. Treatment of Atopic Dermatitis, Dermatophytes, and Syphilis by Indigenous Peoples Prior to 1850. J. Cut. Med. Surg. 2022, 26, 198–200. [CrossRef]
- “Oil of Sassafras for Neuralgia.” The Dental Register 1886, 40, 256.
- Pulivarthi, D.; Steinberg, K.M.; Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial Activity of Compounds Isolated from Sassafras albidum. Nat. Prod. Commun. 2015, 10, 1229-1230. [CrossRef] [PubMed]
- Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial Potential of Tropical Rainforest Plant Extracts. Medicines (Basel, Switzerland) 2014, 1, 32–55. [CrossRef]
- Hu, L.; Wu, F.; He, J.; Zhong, L.; Song, Y.; Shao, H. Cytotoxicity of safrole in HepaRG cells: studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 2019, 49, 1504-1515. [CrossRef]
- Sadati, S.N.; Ardekani, M.R.; Ebadi, N.; Yakhchali, M.; Dana, A.R.; Masoomi, F.; Khanavi, M.; Ramezany, F. Review of Scientific Evidence of Medicinal Convoy Plants in Traditional Persian Medicine. Pharmacog. Rev. 2016, 10, 33–38.
- Wu, M.J.; Yen, J.H.; Wang, L.; Weng, C.Y. Antioxidant activity of Porcelainberry (Ampelopsis brevipedunculata (Maxim.) Trautv.). Am. J. Chin. Med. 2004, 32, 681-693. [CrossRef]
- Choi, Y.A.; Yu, J.H.; Jung, H.D.; Lee, S.; Park, P.H.; Lee, H.S.; Kwon, T.K.; Shin, T.Y.; Lee, S.W.; Rho, M.C.; et al. Inhibitory effect of ethanol extract of Ampelopsis brevipedunculata rhizomes on atopic dermatitis-like skin inflammation. J. Ethnopharmacol. 2019, 238, 111850. [CrossRef]
- Park, J.Y.; Kim, M.J.; Choi, Y.A.; Lee, S.W.; Lee, S.; Jang, Y.H.; Kim, S.H. Ethanol Extract of Ampelopsis brevipedunculata Rhizomes Suppresses IgE-Mediated Mast Cell Activation and Anaphylaxis. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 5083956. [CrossRef] [PubMed]
- Le, M.Q.; Kim, M.S.; Song, Y.S.; Noh; W.N.; Chun, S.C.; Yoon, D.Y. Water-extracted Ampelopsis brevipedunculata downregulates IL-1beta,CCL5, and COX-2 expression via inhibition of PKC-mediated JNK/NF-kappaB signaling pathways in human monocytic cells. J. Pharmacol. Sci. 2014, 126, 359-369.
- Jang, H.J.; Lee, S.J.; Lim, H.J.; Jung, K.; Lee, S.; Park, C.S.; Lee, S.W; Rho, MC. Inhibitory Effects of Compounds and Extracts from Ampelopsis brevipedunculata on IL-6-Induced STAT3 Activation. Biomed Res. Int. 2018, 2018, 3684845. [CrossRef] [PubMed]
- Bak, S.G.; Lim, H.J.; Won, Y.S.; Park, E.J.; Kim, Y.H.; Lee, S.W.; Oh, J.H.; Kim, J.E.; Lee, M.J.; Lee, S.; et al. Effect of Ampelopsis brevipedunculata (Maxim.) Trautv extract on a model of atopic dermatitis in HaCaT cells and mice. Food Sci. Nutr. 2023, 11, 6616-6625. [CrossRef]
- Chang, C.I.; Chien, W.C.; Huang, K.X.; Hsu, J.L. Anti-Inflammatory Effects of Vitisinol A and Four Other Oligostilbenes from Ampelopsis brevipedunculata var. Hancei. Molecules 2017, 22, 1195. [CrossRef]
- Huang, T.Y.; Lin, J.Y.; Su, W.T. Coaxial nanofibers encapsulated with Ampelopsis brevipedunculata extract and green synthesized AgNPs for wound repair. Colloids Surf. B Biointerfaces 2024, 235, 113771. [CrossRef]
- Huang, T.Y.; Wang, Y.W.; Liao, H.X.; Su, W.T. Sprayable hydroxypropyl chitin/collagen extract of Ampelopsis brevipedunculata hydrogel accelerates wound healing. J. Wound Care 2024, 33, S10-S23. [CrossRef]
- Yum, M.J.; Koppula, S.; Kim, J.S.; Shin, G.M.; Chae, Y.J.; Yoon, T.; Chun, C.S.; Lee, J.D.; Song, M. Protective effects of Ampelopsis brevipedunculata against in vitro hepatic stellate cells system and thioacetamide-induced liver fibrosis rat model. Pharm. Biol. 2017, 55, 1577-1585. [CrossRef]
- Yabe, N.; Matsui, H. Ampelopsis brevipedunculata (Vitaceae) extract inhibits a progression of carbon tetrachloride-induced hepatic injury in the mice. Phytomedicine 2000, 7, 493-498. [CrossRef] [PubMed]
- Yang, L.L.; Yen, K.Y.; Kiso, Y.; Hikino, H. Antihepatotoxic actions of Formosan plant drugs. J. Ethnopharmacol. 1987, 19, 103-110. [CrossRef]
- Yabe, N.; Tanaka, K.; Matsui, H. An ethanol-extract of Ampelopsis brevipedunculata (Vitaceae) berries decreases ferrous iron-stimulated hepatocyte injury in culture. J. Ethnopharmacol. 1998, 59, 147-159. [CrossRef] [PubMed]
- Yabe, N.; Matsui, H. Ampelopsis brevipedunculata (Vitaceae) extract stimulates collagen synthesis through superoxide generation in the serum-free cultures of rat dermal fibroblasts and Ito cells. J. Ethnopharmacol. 1997, 56, 67-76. [CrossRef]
- Yabe, N.; Matsui H. Effects of Ampelopsis brevipedunculata (Vitaceae) extract on hepatic M cell culture: function in collagen biosynthesis. J. Ethnopharmacol. 1997, 56, 31-44. [CrossRef] [PubMed]
- Su, P.S.; Doerksen, R.J.; Chen, S.H.; Sung, W.C.; Juan, C.C.; Rawendra, R.D.; Chen, C.R.; Li, J.W.; Aisha-Huang, T.C.; et al. Screening and profiling stilbene-type natural products with angiotensin-converting enzyme inhibitory activity from Ampelopsis brevipedunculata var. hancei (Planch.) Rehder. J. Pharm. Biomed. Anal. 2015, 108, 70-77. [CrossRef]
- Kim, J.Y.; Park, S.H.; Oh, H.M.; Kwak, S.C.; Baek, J.M.; Lee, M.S.; Rho, M.C.; Oh, J. Ampelopsis brevipedunculata extract prevents bone loss by inhibiting osteoclastogenesis in vitro and in vivo. Molecules 2014, 19, 18465-18478. [CrossRef]
- Lee, H.; Lin, J.Y. Antimutagenic activity of extracts from anticancer drugs in Chinese medicine. Mutat. Res. 1988, 204, 229-234. [CrossRef] [PubMed]
- Sun, X.; Guan, Y.X.; Luo, X.L.; Yu, Y.A.; Gao, H.Z. Observation of the efficacy of Ampelopsis brevipedunculata Trautv. in the treatment of herpes zoster. J. Tradit. Chin. Med. 1986, 6, 17-18. [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury - Slippery Elm. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599741/ (accessed on 04 Sep 2025).
- Ried, K.; Travica, N.; Dorairaj, R.; Sali, A. Herbal formula improves upper and lower gastrointestinal symptoms and gut health in Australian adults with digestive disorders. Nutr. Res. 2020, 76, 37–51. [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Uchitel, S.; Denniston, K.; Chopra, D.; Mills, P.J.; Peterson, S.N. Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. J. Altern. Complement. Med. 2018, 24, 656-665. [CrossRef]
- Jeong, C.; Lee, C.H.; Seo, J.; Park, J.H.Y.; Lee, K.W. Catechin and flavonoid glycosides from the Ulmus genus: Exploring their nutritional pharmacology and therapeutic potential in osteoporosis and inflammatory conditions. Fitoterapia 2024, 178, 106188. [CrossRef]
- Aleman, R.S.; Page, R.; Cedillos, R.; Montero-Fernández, I.; Fuentes, J.A.M.; Olson, D.W.; Aryana, K. Influences of Yogurt with Functional Ingredients from Various Sources That Help Treat Leaky Gut on Intestinal Barrier Dysfunction in Caco-2 Cells. Pharm. 2023, 16, 1511. [CrossRef]
- Taleb, S.; Saeedi, M. The effect of the Verbascum Thapsus on episiotomy wound healing in nulliparous women: a randomized controlled trial. BMC Complement. Med. Ther. 2021, 21, 166. [CrossRef]
- Shahbaz, F.; Akhter, N.; Shahid, M.; Riaz, M.; Anjum, F.; Hussain, F. Ultrasound Assisted Extraction and Characterization of Bioactives From Verbascum thapsus Roots to Evaluate Their Antioxidant and Medicinal Potential. Dose Response 2022, 20, 15593258221097665. [CrossRef]
- Zhang, N.; Baran, A.; Valioglu, F.; Teng, L.; Atalar, M.N.; Keskin, C.; Wang, X.X.; Hatipoğlu, A.; Baran, M.F.; Abdelsalam, A.H.; et al. Antioxidant, AChE inhibitory, and anticancer effects of Verbascum thapsus extract. Cell Mol. Biol. 2023, 69, 211-216. [CrossRef]
- Beghelli, D.; Zallocco, L.; Angeloni, C.; Bistoni, O.; Ronci, M.; Cavallucci, C.; Mazzoni, M.R.; Nuccitelli, A.; Catalano, C.; Hrelia, S; et al. Dietary Supplementation with Boswellia serrata, Verbascum thapsus, and Curcuma longa in Show Jumping Horses: Effects on Serum Proteome, Antioxidant Status, and Anti-Inflammatory Gene Expression. Life (Basel) 2023, 13, 750. [CrossRef]
- Kavousi, H.R.; Karimi, M.R.; Neghab, M.G. Assessment the copper-induced changes in antioxidant defense mechanisms and copper phytoremediation potential of common mullein (Verbascum thapsus L.). Environ. Sci. Pollut. Res. Int. 2021, 28, 18070-18080. [CrossRef]
- Mahdavi, S.; Amiradalat, M.; Babashpour, M.; Sheikhlooei, H.; Miransari, M. The Antioxidant, Anticarcinogenic and Antimicrobial Properties of Verbascum thapsus L. Med. Chem. 2020, 16, 991-995.
- Ferrucci, V.; Miceli, M.; Pagliuca, C.; Bianco, O.; Castaldo, L.; Izzo, L.; Cozzolino, M.; Zannella, C.; Oglio, F.; Polcaro, A.; et al. Modulation of innate immunity related genes resulting in prophylactic antimicrobial and antiviral properties. J. Transl. Med. 2024, 22, s574. [CrossRef]
- Escobar, F.M.; Sabini, M.C.; Zanon, S.M.; Tonn, C.E.; Sabini, L.I. Antiviral effect and mode of action of methanolic extract of Verbascum thapsus L. on pseudorabies virus (strain RC/79). Nat. Prod. Res. 2012, 26, 1621-1625. [CrossRef] [PubMed]
- Rajbhandari, M.; Mentel, R.; Jha, P.K.; Chaudhary, R.P.; Bhattarai, S.; Gewali, M.B.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral activity of some plants used in Nepalese traditional medicine. Evid. Based Complement. Alternat. Med. 2009, 6, 517-522. [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [CrossRef]
- Zhao, Y.L.; Wang, S.F.; Li, Y.; He, Q.X.; Liu, K.C.; Yang, Y.P.; Li, X.L. Isolation of chemical constituents from the aerial parts of Verbascum thapsus and their antiangiogenic and antiproliferative activities. Arch. Pharm. Res. 2011, 34, 703-707. [CrossRef]
- Ali, N.; Ali-Shah, S.W.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I.; Ali, W. Anthelmintic and relaxant activities of Verbascum Thapsus Mullein. BMC Complement. Altern. Med. 2012, 12, 29. [CrossRef] [PubMed]
- Calabrese, G.; Zappalà, A.; Dolcimascolo, A.; Acquaviva, R.; Parenti, R.; Malfa, G.A. Phytochemical Analysis and Anti-Inflammatory and Anti-Osteoarthritic Bioactive Potential of Verbascum thapsus L. (Scrophulariaceae) Leaf Extract Evaluated in Two In Vitro Models of Inflammation and Osteoarthritis. Molecules 2021, 26, 5392.
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398-1404. [CrossRef] [PubMed]
- Fakhrieh-Kashan, Z.; Arbabi, M.; Delavari, M.; Mohebali, M.; Hooshyar, H. Induction of Apoptosis by Alcoholic Extract of Combination Verbascum thapsus and Ginger officinale on Iranian Isolate of Trichomonas vaginalis. Iran. J. Parasitol. 2018, 13, 72–78.
- Mehriardestani, M.; Aliahmadi, A.; Toliat, T.; Rahimi, R. Medicinal plants and their isolated compounds showing anti-Trichomonas vaginalis- activity. Biomed. Pharmacother. 2017, 88, 885-893. [CrossRef] [PubMed]
- Kashan, Z.F.; Arbabi, M.; Delavari, M.; Hooshyar, H.; Taghizadeh, M.; Joneydy, Z. Effect of Verbascum thapsus ethanol extract on induction of apoptosis in Trichomonas vaginalis in vitro. Infect. Disord. Drug Targets 2015, 15, 125-130. [CrossRef] [PubMed]
- Baratto, L.C.; Päßler U. Plants of the USA: recordings on native North American useful species by Alexander von Humboldt. J. Ethnobiol. Ethnomed. 2024, 20, 87. [CrossRef] [PubMed]
- Cicero, A.F.; Baggioni, A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016, 928, 27-45.
- Okunade, A.L.; Hufford, C.D.; Richardson, M.D.; Peterson, J.R.; Clark, A.M. Antimicrobial properties of alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 1994, 83, 404–406. [CrossRef]
- Wu, Y.C.; Yamagishi, T.; Lee, K.H. Cytotoxic isoquinoline alkaloids from Xanthorhiza simplicissima. Gaoxiong Yi Xue Ke Xue Za Zhi. 1989, 5, 409-411.
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





