1. Introduction
Post-blast forensic investigations require precise chemical characterization of residues deposited on a wide range of substrates, often involving materials that are highly fragmented, chemically heterogeneous, and environmentally contaminated. The complexity of these samples presents significant analytical challenges, particularly when explosive residues are unevenly distributed or occur at trace levels. Understanding the chemical composition of such materials is critical for reconstructing detonation mechanisms, identifying explosive formulations, and supporting legal proceedings.
Among nitrate-based formulations, ammonium nitrate fuel oil (ANFO) remains one of the most frequently encountered explosives owing to its accessibility, low cost, and high detonation efficiency [
1,
2]. Following detonation, ANFO residues typically consist of nitrate salts and hydrocarbon components, which exhibit different binding affinities toward metallic, soil, and polymeric substrates. This heterogeneity complicates residue extraction and identification, especially in mixed or composite explosive events, where overlapping signals or partial combustion can obscure analytical signatures [
3].
Traditional single-technique approaches such as direct solvent rinsing or cotton swabbing often provide incomplete recovery from irregular or large fragments, resulting in reduced analytical sensitivity [
4]. Recent advances in forensic chemistry have emphasized the need for integrated analytical workflows that combine multiple extraction and instrumental techniques—such as sequential swabbing, multi-solvent extraction, syringe filtration, and orthogonal instrumental analysis—to enhance residue recovery and data reliability [
5,
6].
Instrumental methods remain indispensable in explosive residue analysis. Gas Chromatography–Mass Spectrometry (GC–MS) provides high sensitivity for organic explosive components, while Fourier Transform Infrared (FTIR) spectroscopy and Ion Chromatography (IC) are essential for detecting inorganic residues such as nitrates, chlorides, and perchlorates [
6,
7]. However, the analytical reliability of these methods depends strongly on the effectiveness of sampling, filtration, and pre-concentration steps applied to complex debris.
Accordingly, this study develops and validates an integrated forensic analytical framework for the characterization of complex and fragmented post-blast materials. The proposed workflow combines sequential swabbing, solvent extraction, syringe filtration, and spatial subsampling, coupled with GC–MS, Thin Layer Chromatography (TLC), and FTIR spectroscopy [
7,
8]. The approach enables simultaneous detection of both organic and inorganic residues, strengthening the evidentiary interpretation of heterogeneous post-blast exhibits in forensic casework.
2. Materials and Methods
2.1. Evidence Collection
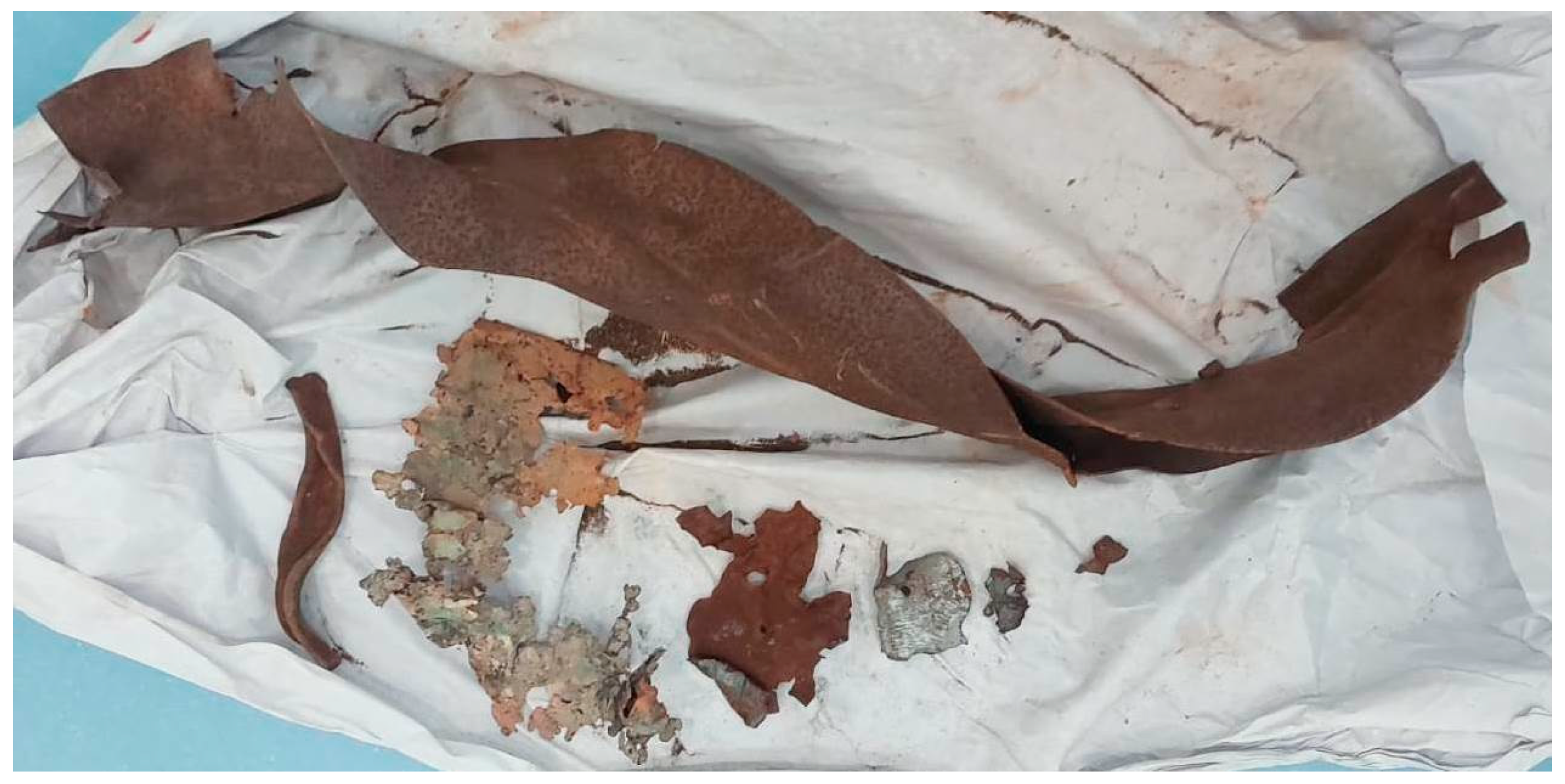
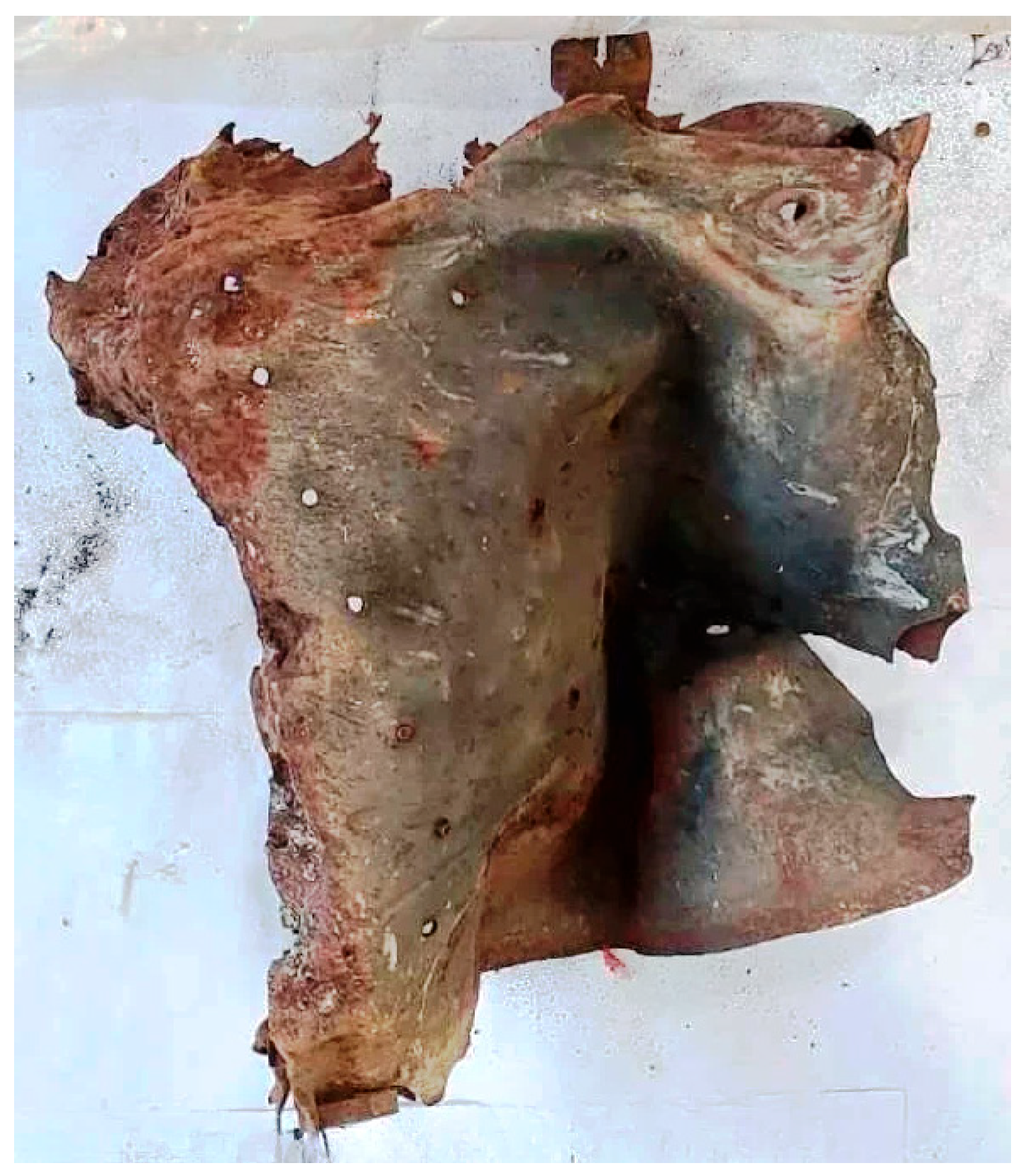
Post-blast materials were collected from a crime scene involving an accidental detonation of illegally stored explosive substances in a warehouse. The blast caused severe fragmentation of metallic and polymeric structures, dispersing debris over a wide area. Heterogeneous exhibits, including metallic spades, large and small metal fragments, nails, plastic containers, wires, and structural components, were collected alongside soil samples from both the blast epicenter and control areas (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). These materials were representative of the complex and composite matrices typically encountered in post-blast forensic investigations [
8].
2.2. Reagents and Preparation of Extracts
All reagents used were of analytical grade. Diethyl ether (Finar), acetone (Advent Chembio Pvt. Ltd.), sodium hydroxide, and pyridine (SRL) were employed as extraction solvents, while demineralized water (DM) obtained from Labogen Fine Chem Industry, Ludhiana, was used for aqueous and alkaline extractions. Whatman-42 filter paper and Allpure nylon syringe filters (0.22 μm) from Membrane Solutions were used to remove particulates from extracts. These reagents and materials were selected to enable comprehensive recovery of both organic (hydrocarbon, nitroaromatic) and inorganic (nitrate, chloride, sulfate) explosive residues [
9,
10].
Oversized and irregular fragments were gently cleaned of loose debris prior to extraction. Sequential swabbing and solvent extraction were then applied to each exhibit to ensure representative sampling of its entire surface area.
2.3. Extraction and Analytical Workflow
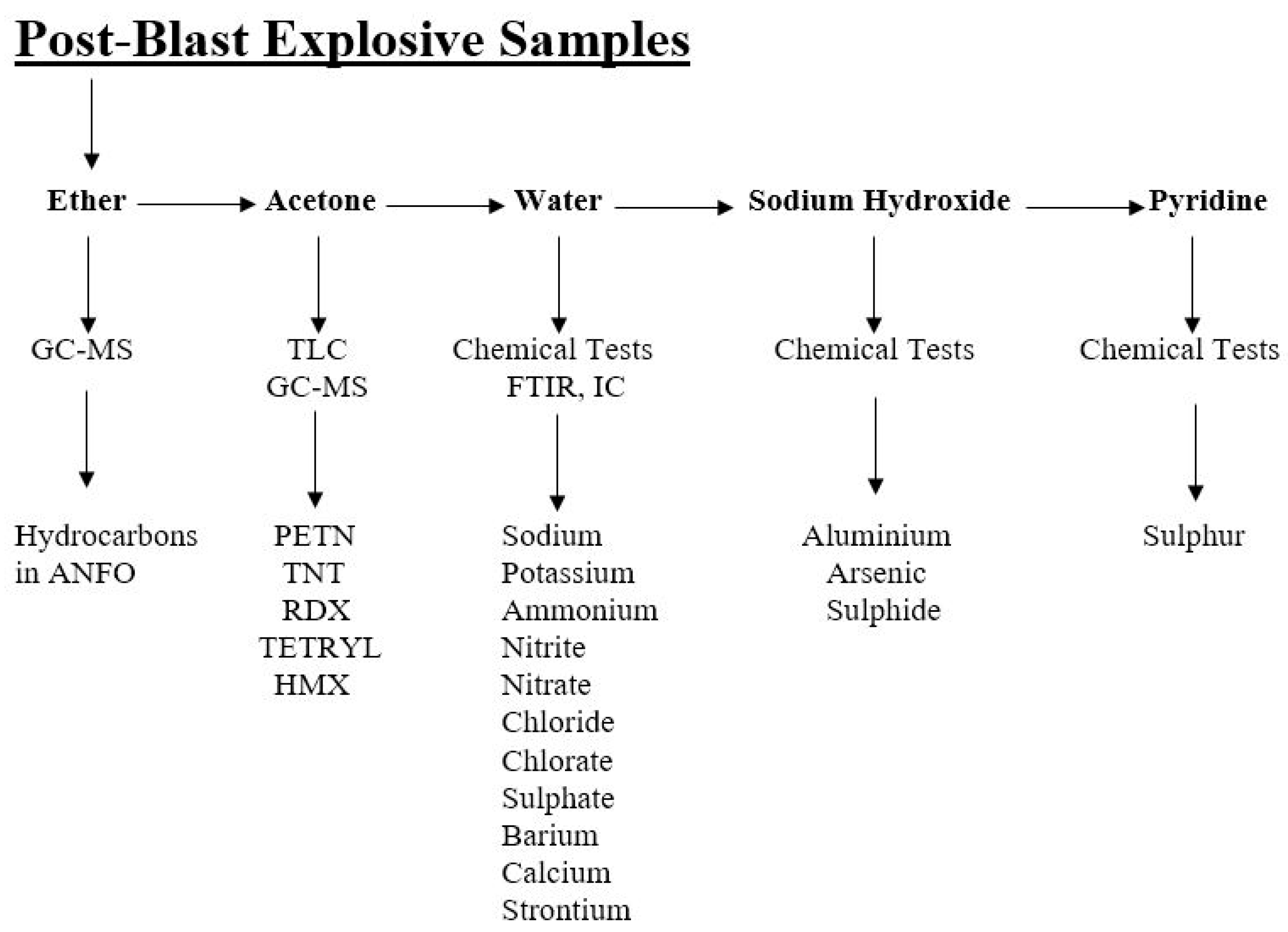
A multi-stage sequential swabbing protocol was applied to all exhibits to recover a wide range of explosive residues while minimizing sample loss. Each sample was swabbed successively with absorbent cotton saturated in ether, acetone, water, sodium hydroxide, and pyridine, thereby collecting organic, polar organic, and inorganic fractions in a systematic sequence (
Figure 7 and
Figure 8). Extracts were filtered through 0.22 μm syringe filters and concentrated to approximately 2–5 mL by evaporation at room temperature [
8,
9].
Filtered extracts were analyzed as follows:
Ether extracts → GC–MS for hydrocarbons and fuel oil detection (ANFO identification).
Acetone extracts → TLC and GC–MS for organic high explosives (e.g., TNT, RDX, PETN).
Water and NaOH extracts → Chemical tests and FTIR for inorganic ions (nitrate, chloride, sulfate).
Pyridine extracts → Identification of elemental sulfur and related residues.
This integrated workflow facilitated cross-validation across analytical techniques, significantly improving the reliability of residue identification in mixed and complex post-blast matrices [
6,
8,
9].
2.4. Thin Layer Chromatography
Silica gel 60G F254 Plates with a thickness of 200 micrometer and size of 20 x 20 cm were used for Thin Layer Chromatography (TLC) analysis. Chloroform, Acetone, Toluene and Cyclo hexane used for the test.[
8,
9,
10,
11]
Pre-coated TLC plates were activated by placing them in an air oven at 110 °C for 30 minutes. One hundred milliliters of solvent [chloroform : acetone (1:1) and toluene : cyclohexane (7:3)] was taken in two different developing chambers (for 20 × 20 cm TLC plates), covered with a lid, and allowed to saturate for at least 30 min. The concentrated acetone extract of each sample was spotted on a pre-coated TLC plate along with reference standards of high explosives, leaving 2 cm from one edge at the bottom of the TLC plate and maintaining a minimum distance of 1.5 cm between two spots. The TLC plate was placed vertically in the developing chamber and allowed to develop until the solvent front rose to 10 cm from the spots by capillary action. After completion, the plate was removed and left at room temperature for the eluent to evaporate.[
8,
9,
10,
11]
The TLC plate was developed by spraying with 5% diphenylamine (DPA) in 95% ethanol, and the colour produced was noted. The plate was then placed under UV light (254 nm) to observe fluorescence and subsequently sprayed with concentrated sulphuric acid, and the resulting colours were recorded. The colours were compared with the Rf values for identification.[
8,
9,
10,
11]
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
The Ether, Acetone and dried water extracts were examined using a Thermo Fisher Scientific Nicolet iS20 FTIR spectrometer instrument, which equipped with an IR source, an attenuated total reflectance (ATR) accessory, a DTGS detector and KBr beam splitter from Thermo Fisher Scientific. The instrument was operated at resolution of 4.000 between wavenumber 4000 cm-1 to 400 cm-1. The analysis was performed by scanning the background and sample using the Thermo Scientific OMNIC software. The sample was scanned 64 times to obtain a characteristic spectrum. The spectrum was searched using correlation search type in the libraries of the instrument to identify the sample.[
8,
9,
10,
11]
2.6. Methodological Challenges
The analysis of complex and fragmented post-blast materials presents challenges due to non-uniform residue deposition, variable surface composition, and extensive environmental contamination. Swabbing remains the most effective recovery method for oversized and irregular fragments, but factors such as cotton quality, solvent compatibility, and extraction efficiency affect reproducibility [
3,
5]. Integration of syringe filtration and sequential solvent extraction substantially reduced matrix interference and enhanced GC–MS signal clarity [
6,
9]. These findings underscore the importance of multi-modal analytical integration for accurate and reproducible forensic characterization of post-blast materials.
3. Observations
3.1. TLC and GC-MS Examinations
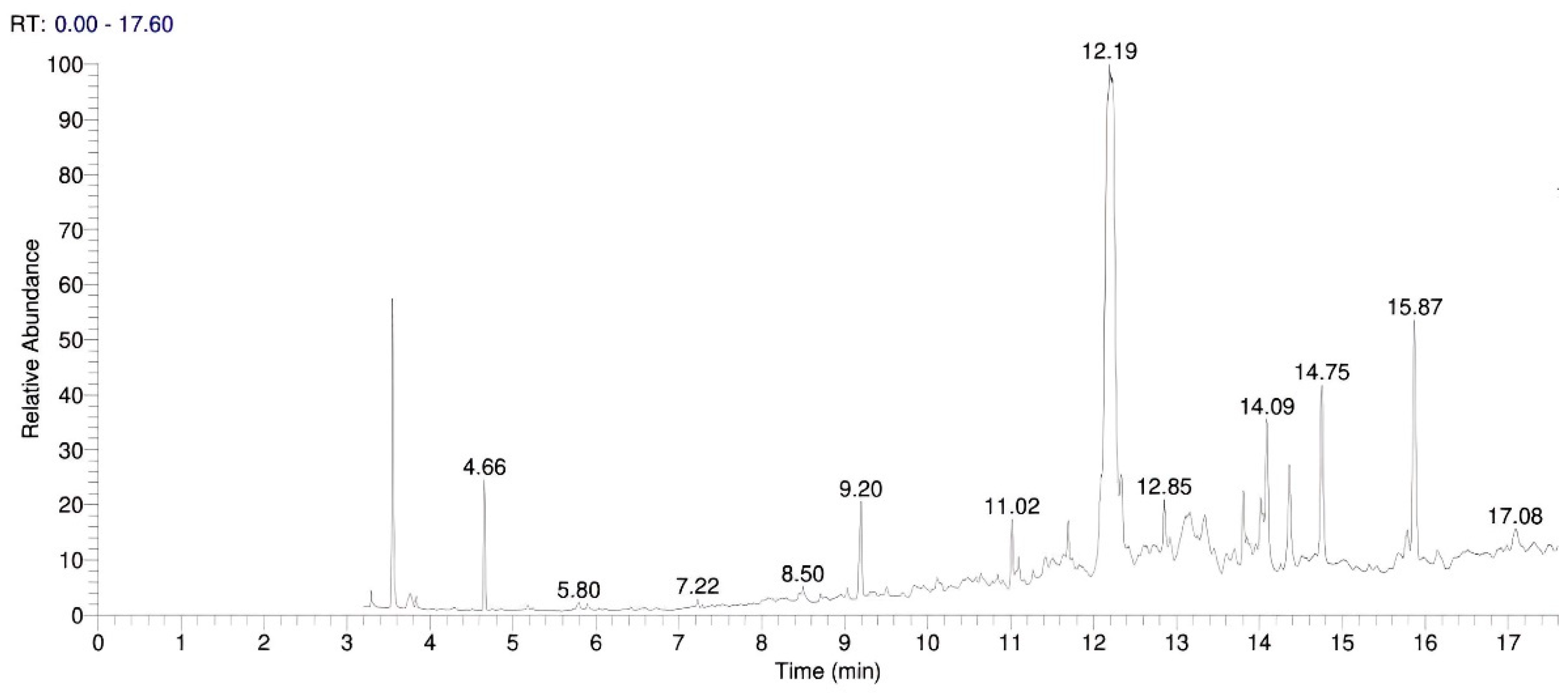
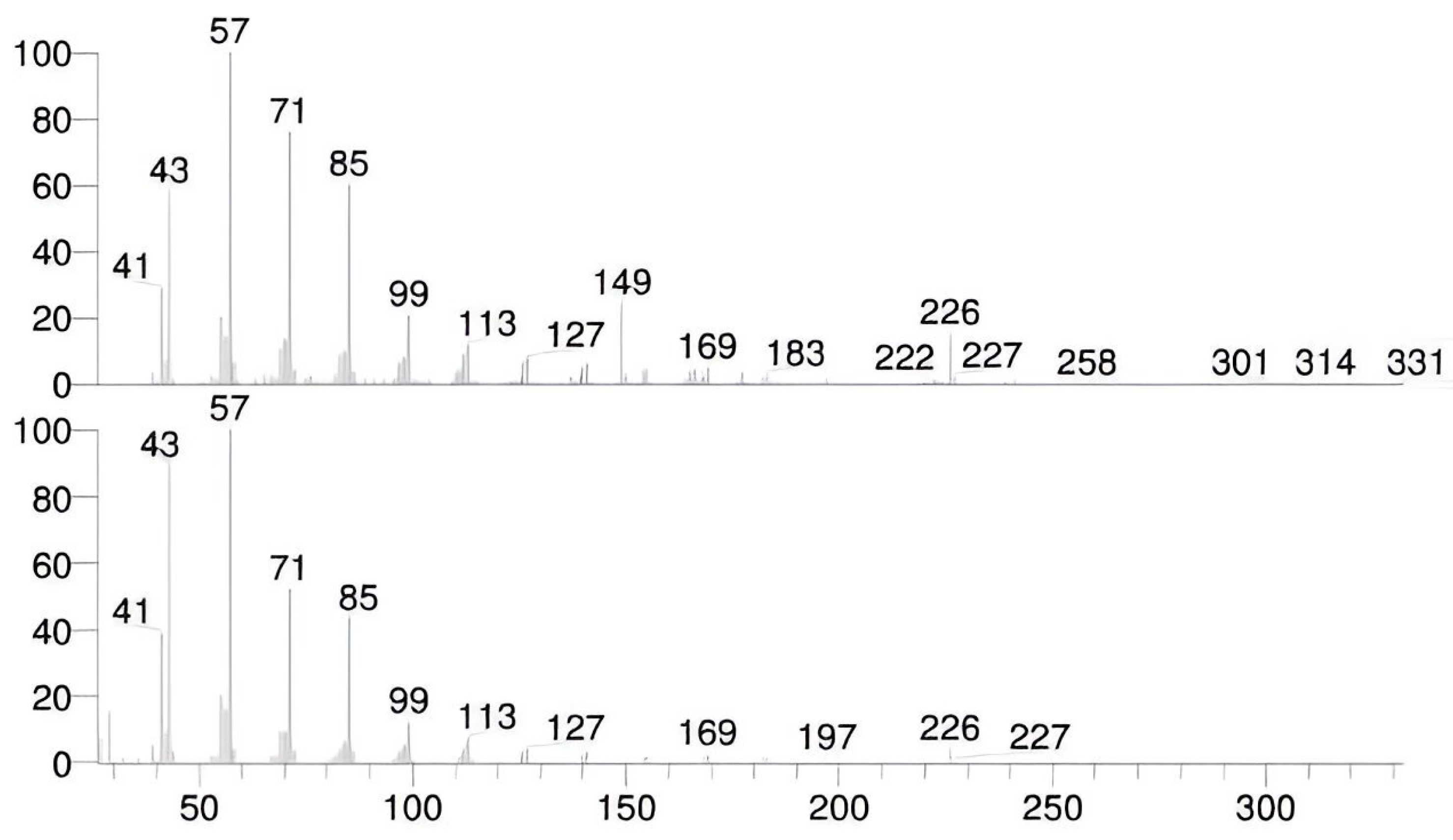
High-boiling fractions of petroleum hydrocarbons identified in the GC-MS analysis of the Ether extracts of the exhibit are shown in the
Figure 1. The compound name was identified by the GC-MS library as Hexadecane at the RT 14.02 having SI value of 792 and RSI value is 929. The GC-MS spectrum has a peak area of 136911064.09 and peak height of 37343910.88, as shown in
Figure 9 of the Total Ion Chromatogram (TIC). [
12,
13]
The mass spectra of the forensic case sample obtained by analysis using the GC-MS method, along with the reference library of mass spectra, are shown in
Figure 10. The x axis presents abundance and y axis presents m/z values. [
12,
13]
No High Explosives were identified through Chemical Examination, TLC and GC-MS of the Acetone Extract.
3.2. Chemical Examinations
The Low Explosives identified in the Water, Alkali and Pyridine extracts of the smaller-sized and oversized exhibits are listed
Table 1. Except for the control soil, all the exhibits yielded positive results for low explosives. [
12,
13]
3.3. FTIR Analysis
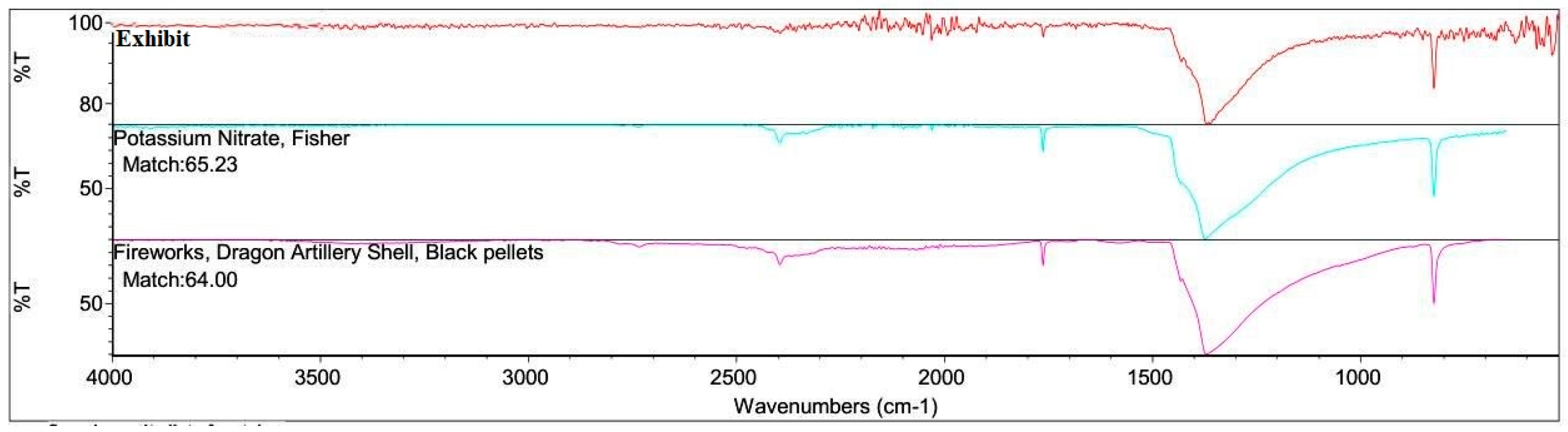
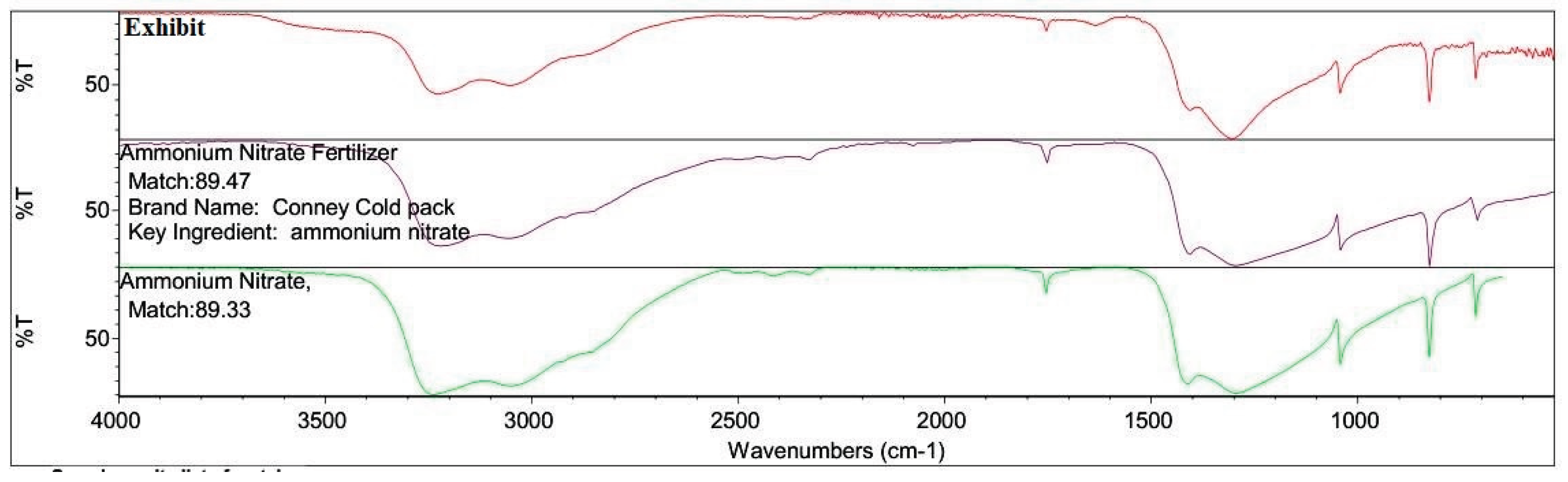
High Explosives were not identified in the FTIR analysis of the Acetone Extract. However, Potassium Nitrate and Ammonium Nitrate (Low Explosives) were identified in the Water Extract. The FTIR spectra are shown in
Figure 11 and
Figure 12.
The presence of high-boiling fractions of petroleum hydrocarbons and Ammonium Nitrate confirms the presence of Ammonium Nitrate Fuel Oil (ANFO) in the Oversized Exhibits. [
12,
13]
4. Results and Discussions
The forensic investigation of oversized and fragmented exhibits from the mixed ANFO–nitrate detonation provided consistent evidence of explosive residues across several analytical platforms.
4.1. Overview of Analytical Workflow
The integrated analytical framework applied in this study successfully addressed the challenges associated with complex and fragmented post-blast materials, enabling reliable detection of both organic and inorganic explosive residues across multiple analytical platforms. Sequential solvent extraction, syringe filtration, and multi-technique cross-verification provided improved recovery efficiency and analytical reproducibility, essential for heterogeneous forensic samples.
4.2. Organic Residue Detection (GC–MS and TLC)
Gas Chromatography–Mass Spectrometry (GC–MS) analysis of ether extracts revealed the presence of high-boiling petroleum hydrocarbons, characterized by distinct chromatographic peaks consistent with diesel fractions used in ammonium nitrate fuel oil (ANFO) mixtures. The hexadecane peak at a retention time (RT) of 14.02 min (similarity index = 792, reverse similarity index = 929) exhibited strong spectral correlation with the NIST mass spectral library, confirming the presence of hydrocarbon residues (
Figure 9 and
Figure 10).
These findings align with previous reports demonstrating the stability of long-chain alkanes in post-blast residues even under oxidative and environmental stress conditions [
1,
2]. The identification of these hydrocarbon signatures substantiates the hypothesis that the explosive formulation employed in the incident was ANFO-based.
No high explosives (such as TNT, RDX, PETN, or HMX) were detected in the acetone extracts via TLC or GC–MS. TLC chromatograms displayed no characteristic Rf values for nitroaromatic or nitramine explosives, suggesting that the device relied primarily on ANFO rather than composite or high-explosive formulations.
4.3. Inorganic Residue Detection (Chemical Tests and FTIR)
Chemical spot tests and instrumental analyses of aqueous and alkaline extracts confirmed the presence of several key ions associated with nitrate-based low explosives (
Table 1). Positive tests for nitrate, nitrite, ammonium, potassium, chloride, and sulfate were observed, whereas chlorate, perchlorate, and metallic additives (e.g., aluminum, magnesium) were absent. These results indicate the predominance of oxidizing salts typical of ANFO-type formulations and exclude the presence of common oxidizers found in high-explosive mixtures [
3,
7].
FTIR spectra of the water extracts exhibited strong absorption bands at 1384 cm⁻¹ (ν₃ NO₃⁻ stretching) and 824 cm⁻¹ (ν₂ NO₃⁻ bending), consistent with ammonium nitrate and potassium nitrate (
Figure 11 and
Figure 12). The IR spectra matched library references with high correlation coefficients (>95%), confirming the inorganic component of the explosive residues.
The combination of chemical spot testing and FTIR thus provided complementary confirmation of nitrate-based explosive residues, reinforcing the GC–MS results and strengthening evidentiary reliability through multi-method corroboration [
6,
7].
4.4. Workflow Efficiency and Analytical Reliability
Sequential swabbing and solvent extraction, when combined with syringe filtration, demonstrated enhanced residue recovery and reduced matrix interference compared with conventional direct solvent rinsing. The syringe filtration step effectively removed particulate contaminants that otherwise suppressed chromatographic ionization signals, leading to cleaner GC–MS spectra and improved detection limits [
4,
5].
This finding underscores the importance of integrating pre-analytical steps—such as filtration and solvent sequencing—into forensic explosive residue protocols. The improved clarity of chromatographic data and reproducibility of chemical test results across multiple exhibits validate the integrated analytical approach as a significant advancement for post-blast investigations involving complex debris matrices.
4.5. Forensic Interpretation
The concurrent detection of hydrocarbons (diesel range organics) and nitrate-based oxidizers provides definitive evidence for the presence of ammonium nitrate fuel oil (ANFO) in the post-blast debris. The absence of high explosives further suggests that the device was a bulk ANFO charge, rather than a composite or military-grade explosive system.
From a forensic standpoint, these results establish a direct link between the chemical signatures on the recovered fragments and the explosive formulation used in the detonation. The analytical convergence across GC–MS, TLC, FTIR, and classical spot tests reinforces the credibility of the evidentiary interpretation. This multi-technique confirmation is essential for judicial admissibility, as it minimizes false positives and analytical uncertainty [
1,
3,
6].
4.6. Summary of Key Outcomes
The integrated workflow combining sequential swabbing, solvent extraction, and syringe filtration provided superior recovery efficiency for complex and fragmented post-blast materials.
GC–MS confirmed the presence of diesel-range hydrocarbons, while FTIR and chemical testing verified ammonium nitrate and potassium nitrate, confirming ANFO-type residues.
The absence of high explosives supports the conclusion that the detonation resulted from a bulk ANFO charge rather than a composite mixture.
The study demonstrates that analytical integration significantly enhances sensitivity, reproducibility, and interpretive robustness in forensic explosive investigations.
4.7. Implications for Forensic Practice
This work demonstrates the value of adopting integrated analytical methodologies in forensic explosive residue analysis. By aligning sampling, extraction, and instrumental techniques into a cohesive workflow, analysts can improve both detection reliability and interpretive accuracy, even in the most complex post-blast scenarios. The methods described here contribute to a standardized framework that can be adapted for routine casework and future research within forensic chemistry laboratories.
5. Conclusions
This study demonstrates the effectiveness of an integrated forensic protocol for investigating oversized and fragmented exhibits from mixed ANFO–nitrate detonations in a field setting. By combining sequential swabbing, solvent extraction, syringe filtration, and complementary analytical techniques (GC–MS, TLC, chemical spot tests, and FTIR), the reliable detection of both organic (fuel oil hydrocarbons) and inorganic (nitrate-based) residues was achieved.
The results highlight three key conclusions:
The residue recovery efficiency was maximized by adapting the workflows to oversized exhibits, with syringe filtration proving particularly valuable.
Analytical corroboration across GC–MS, TLC, chemical tests, and FTIR reinforced evidentiary strength and reduced uncertainty.
Forensic reconstruction confirmed the exclusive use of ANFO, providing insights into the nature of the explosive device and its deployment strategy.
These findings emphasize that forensic protocols must be tailored to the scale and heterogeneity of post-blast exhibits to ensure accurate residue recovery and it’s interpretation. The methodological advances described herein contribute to strengthening forensic investigations of large-scale detonation events and support judicial processes by providing scientifically validated evidences.
Author Contributions
Conceptualization, D.K.; methodology, D.K.; validation, D.K.; formal analysis, D.K.; investigation, D.K.; writing—original draft preparation, D.K.; writing—review and editing, D.K.; visualization, D.K.; and supervision, D.K. The author has read and agreed to the published version of this manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The author declares that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the author upon reasonable request.
Acknowledgments
I would like to express my gratitude to the Director, Central Forensic Science Laboratory, Pune, for continuous support and for providing the necessary infrastructure for this study.
Conflicts of Interest
The author declares no conflicts of interest
References
- Braga, J. W. B.; Logrado, L. P. L. Evaluation of interferents in sampling materials for analysis of post-explosion residues (explosive emulsion/ANFO) using gas chromatography–mass spectrometry (GC–MS). J. Forensic Sci. 2024, 70(1), 1–9.
- De Vooght-Johnson, R. Contamination concerns for GC–MS analysis of explosive residues. Anal. Sci. 2024, Wiley Analytical Science.
- Smith, J.; Brown, K. Post-blast explosive residue: A review of formation and dispersion theories and experimental research. RSC Adv. 2014, 4(41), 12345–12360. [CrossRef]
- Doe, A.; Lee, P. Sampling of explosive residues: The use of a gelatine-based medium for the recovery of ammonium nitrate. Forensic Sci. Int. 2020, 310, 110234. [CrossRef]
- Green, D.; White, S. Recent advances in ambient mass spectrometry of trace explosives. J. Mass Spectrom. 2018, 53(9), 873–889. [CrossRef]
- Johnson, T. The application of mass spectrometry to explosive casework: Opportunities and challenges. In Applications of Mass Spectrometry for the Provision of Forensic Intelligence; Royal Society of Chemistry: London, UK, 2017; pp. 201–223.
- Yinon, J. Detection of explosives by Fourier transform infrared spectrometry. J. Forensic Sci. 1995, 40(5), 865–870. [CrossRef]
- Kumar, D. Explosive device reconstruction through chemical and trace evidence analysis: A homicide case investigation. Preprint (Version 1), Research Square, 17 August 2025. [CrossRef]
- Central Forensic Science Laboratory Pune. Working Procedure Manual; Doc. No.: CFSL/PUNE/WPM/EXPL/11, Issue No. 01; Directorate of Forensic Science Services, Ministry of Home Affairs: Pune, India, 2022.
- Kumar, D.; Prajakta, U. K. Optimizing forensic detection of explosive substances: Extended column analysis of TNT. Int. J. Res. Appl. Sci. Eng. Technol. 2025. [CrossRef]
- Kumar, D.; Prajakta, U. K. Thermal decomposition approach for PETN detection in improvised explosive devices. Int. J. Innov. Res. Technol. 2025, 11(12). [CrossRef]
- KumarD. Analytical Techniques for Forensic Investigation of Oversized and Fragmented Exhibits in Mixed Explosive Detonations. ChemRxiv. 2025; This content is a preprint and has not been peer-reviewed. [CrossRef]
- Kumar, D. Analytical Techniques for Forensic Investigation of Oversized and Fragmented Exhibits in Mixed Explosive Detonations. Preprints 2025, 2025090885. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).