Submitted:
05 September 2025
Posted:
08 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Participant Recruitment and Study Sites
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Collection and Analysis
3. Results
3.1. General Demographics
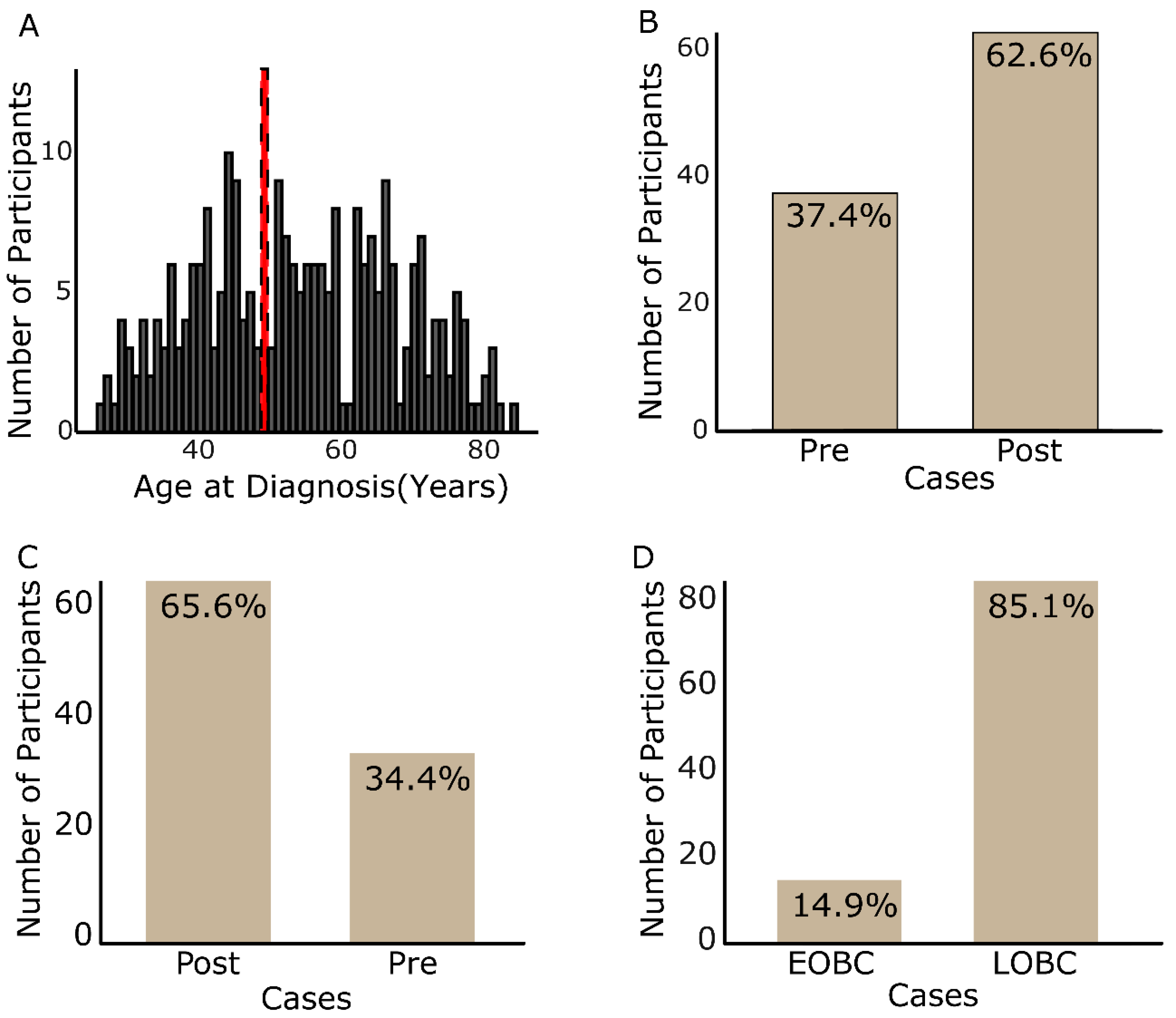
3.1.1. Participants’ Age Profiles and Case Group Incidence
3.1.2. Participants’ Reproductive Factors
3.2. Medical History and Clinical Presentations
3.3. Participants’ Lifestyle and Family History
3.4. Regression Analysis and Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2024, 74, 229–263. [Google Scholar] [CrossRef]
- GLOBOCAN. Cancer Today. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=cancer&group_populations=1&populations=288&sexes=2&types=0&age_end=9&cancers=20 (accessed on October 17, 2024).
- Black, E.; Richmond, R. Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward. Globalization and Health 2019, 15, 3–3. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Addai, B.W.; Adewole, I.; Ainsworth, V.; Alaro, J.; Alatise, O.I.; Ali, Z.; Anderson, B.O.; Anorlu, R.; Avery, S.; et al. Cancer in sub-Saharan Africa: a Lancet Oncology Commission. The Lancet Oncology 2022, 23, e251–e312. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN. Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bubbles?types=1&sexes=1_2&mode=cancer&group_populations=1&multiple_populations=1&multiple_cancers=1&cancers=20&populations=903_904_905_908_909_935&apc=cat_ca20v1.5_ca23v-1.5&group_cancers=1&bar_mode=stacked&years=2035 (accessed on October 17, 2024).
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast cancer (Dove Medical Press) 2021, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Stoll, C.R.; Anandarajah, A.; Doering, M.; Colditz, G.A. Modifiable risk factors in women at high risk of breast cancer: a systematic review. Breast cancer research : BCR 2023, 25. [Google Scholar] [CrossRef]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncology 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Galukande, M.; Wabinga, H.; Mirembe, F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World journal of surgical oncology 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Gueye, M.; Gueye, S.; Gueye, M.; Diallo, M.; Gassama, O.; Biaye, B.; Lemine, A.; Niasse, A.; Mbodji, A.; Moreau, J. A hospital based case control study of female breast cancer risk factors in a Sub-Saharan African country. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2016, 5, 2328–2332. [Google Scholar] [CrossRef]
- Shulman, L.P. Genetic and Genomic Factors in Breast Cancer. In Management of the Patient at High Risk for Breast Cancer. In Management of the Patient at High Risk for Breast Cancer, Hansen, N.M., Ed.; Springer New York: New York, NY, 2013; pp. 29–47. [Google Scholar]
- Anothaisintawee, T.; Wiratkapun, C.; Leedsitthichai, P.; Kasamesup, V.; Wongwaisayawan, S.; Srinakarin, J.; Thakkinstian, A. Risk factors of breast cancer: a systematic review and meta-analysis. journals.sagepub.com 2013, 25, 368–387. [Google Scholar] [CrossRef]
- Hackney, L. Aetiology and Epidemiology of Breast Cancer. In Digital Mammography: A Holistic Approach, Mercer, C., Hogg, P., Kelly, J., Eds.; Springer International Publishing: Cham, 2022; pp. 51–69. [Google Scholar]
- Admoun, C.; Mayrovitz, H.N. The Etiology of Breast Cancer. In Breast Cancer. In Breast Cancer, Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, 2022; pp. 21–30. [Google Scholar]
- Hamajima, N.; Hirose, K.; Tajima, K.; Rohan, T.; Friedenreich, C.M.; Calle, E.E.; Gapstur, S.M.; Patel, A.V.; Coates, R.J.; Liff, J.M.; et al. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. The Lancet. Oncology 2012, 13, 1151–1151. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive Factors and Breast Cancer. Epidemiologic Reviews 1993, 15, 36–47. [Google Scholar] [CrossRef]
- Colditz, G.A.; Rosner, B. Cumulative Risk of Breast Cancer to Age 70 Years According to Risk Factor Status: Data from the Nurses' Health Study. American Journal of Epidemiology 2000, 152, 950–964. [Google Scholar] [CrossRef]
- Mullooly, M.; Khodr, Z.G.; Dallal, C.M.; Nyante, S.J.; Sherman, M.E.; Falk, R.; Liao, L.M.; Love, J.; Brinton, L.A.; Gierach, G.L. Epidemiologic Risk Factors for In Situ and Invasive Breast Cancers Among Postmenopausal Women in the National Institutes of Health-AARP Diet and Health Study. American journal of epidemiology 2017, 186, 1329–1340. [Google Scholar] [CrossRef]
- Catsburg, C.; Miller, A.B.; Rohan, T.E. Active cigarette smoking and risk of breast cancer. International journal of cancer 2015, 136, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miller, A.B.; Rohan, T.E. Cigarette smoking and breast cancer risk: update of a prospective cohort study. Breast cancer research and treatment 2006, 100, 293–299. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.A.; Goyal, A.; Terry, M.B. Alcohol Intake and Breast Cancer Risk: Weighing the Overall Evidence. Current breast cancer reports 2013, 5, 208–221. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. British journal of cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Van Den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Holmberg, L.; Howe, G.R.; Marshall, J.R.; et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 1998, 279, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Heath, C.W.; Miracle-McMahill, H.L.; Coates, R.J.; Liff, J.M.; Franceschi, S.; Talamini, R.; Chantarakul, N.; Koetsawang, S.; RachawatRachawat, D.; et al. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727. [Google Scholar] [CrossRef]
- Hang, Z.; Xun, L.; Jing, F.; Yang, W. Oral contraceptive use and risk of breast cancer: a meta-analysis of prospective cohort studies. The European journal of contraception & reproductive health care : the official journal of the European Society of Contraception 2012, 17, 402–414. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Suzuki, R.; Saji, S.; Toi, M.; Gnant, M.; Markopoulos, C. Impact of body mass index on breast cancer in accordance with the life-stage of women. Frontiers in Oncology 2012, 2, 123–123. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study - ClinicalKey. Lancet Global Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Organization, W.H. Menopause. Available online: https://www.who.int/news-room/fact-sheets/detail/menopause (accessed on December 5, 2024).
- Review, W.P. Age of Menopause by Country 2024. Available online: https://worldpopulationreview.com/country-rankings/age-of-menopause-by-country (accessed on October 18, 2024).
- Yisma, E.; Eshetu, N.; Ly, S.; Dessalegn, B. Prevalence and severity of menopause symptoms among perimenopausal and postmenopausal women aged 30-49 years in Gulele sub-city of Addis Ababa, Ethiopia. BMC women's health 2017, 17, 124–124. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, R.; Adami, H.-O.; Harirchi, I.; Akrami, R.; Zendehdel, K. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC Cancer 2014, 14, 343–343. [Google Scholar] [CrossRef]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiologic Reviews 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Trentham-Dietz, A.; Sprague, B.L.; Hampton, J.M.; Miglioretti, D.L.; Nelson, H.D.; Titus, L.J.; Egan, K.M.; Remington, P.L.; Newcomb, P.A. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case–control studies. Breast Cancer Research and Treatment 2014, 145, 165–175. [Google Scholar] [CrossRef]
- Sidibe, E.H. La ménopause en Afrique. Annales d'Endocrinologie 2005, 66, 105–107. [Google Scholar] [CrossRef]
- Galal, S. Population of Africa 2021, by age group. Available online: https://www.statista.com/statistics/1226211/population-of-africa-by-age-group/ (accessed on October 5, 2024).
- Akakpo, P.K.; Imbeah, E.G.; Edusei, L.; Naporo, S.; Ulzen-Appiah, K.; Clegg-Lamptey, J.N.; Dedey, F.; Nsaful, J.; Affram, N.; Wiafe, B.; et al. Clinicopathologic characteristics of early-onset breast cancer: a comparative analysis of cases from across Ghana. BMC Women's Health 2023, 23, 5–5. [Google Scholar] [CrossRef] [PubMed]
- Anim, J.T. Breast diseases: Review of surgical material in Korle Bu hospital 1977-1978. Ghana medical journal 1979, 18, 30–33, doi:http://197.255.68.203/handle/123456789/3953. [Google Scholar]
- Mensah, A.C.; Yarney, J.; Nokoe, S.K.; Opoku, S.; Clegg-Lamptey, J.N. Survival Outcomes of Breast Cancer in Ghana: An Analysis of Clinicopathological Features. OALib 2016, 03, 1–11. [Google Scholar] [CrossRef]
- Ghartey, N.F.J.; Anyanful, A.; Eliason, S.; Mohammed Adamu, S.; Debrah, S. Pattern of Breast Cancer Distribution in Ghana: A Survey to Enhance Early Detection, Diagnosis, and Treatment. International Journal of Breast Cancer 2016, 2016, 3645308–3645308. [Google Scholar] [CrossRef]
- O'Neil, A. Age structure in Ghana 2022. 2024.
- Kwawukume, E.Y.; Ghosh, T.S.; Wilson, J.B. Menopausal age of Ghanaian women. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 1993, 40, 151–155. [Google Scholar] [CrossRef]
- Setorglo, J.; Keddey, R.S.; Agbemafle, I.; Kumordzie, S.; Steiner-Asiedu, M. Determinants of Menopausal Symptoms among Ghanaian Women. Current Research Journal of Biological Sciences 2012, 4, 507–512. [Google Scholar]
- Edmund, D.M.; Naaeder, S.B.; Tettey, Y.; Gyasi, R.K. Breast cancer in Ghanaian women: what has changed? American journal of clinical pathology 2013, 140, 97–102. [Google Scholar] [CrossRef]
- Clegg-lamptey, J.N.A.; Hodasi, W.M. A study of breast cancer in korle bu teaching hospital: assessing the impact of health education. Ghana medical journal 2007, 41. [Google Scholar] [CrossRef]
- Bosompem, K.; Yorke, J.; Buckman, T.A.; Brenu, S.G.; Nyantakyi, M.; Aitpillah, F.S.K.; Kyei, I.; Adinku, M.O.; Yorke, D.A.; Obirikorang, C.; et al. Comparative analysis of breast cancer characteristics in young premenopausal and postmenopausal women in Ghana. Scientific Reports 2024, 14, 2704–2704. [Google Scholar] [CrossRef] [PubMed]
- Siddig, A.; Din, T.A.D.A.A.T.; Nafi, S.N.M.; Yahya, M.M.; Sulong, S.; Rahman, W.F.W.A. The Unique Biology behind the Early Onset of Breast Cancer. Genes 2021, 12, 372–372. [Google Scholar] [CrossRef]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Bleyer, A.; Johnson, R.H. Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years. JCO Oncology Practice 2021, 17, 305–313. [Google Scholar] [CrossRef]
- Chelmow, D.; Pearlman, M.D.; Young, A.; Bozzuto, L.; Dayaratna, S.; Jeudy, M.; Kremer, M.E.; Scott, D.M.; O’Hara, J.S. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstetrics and gynecology 2020, 135, 1457–1478. [Google Scholar] [CrossRef]
- Laven, J.S.E. Genetics of Early and Normal Menopause. Seminars in reproductive medicine 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Thasneem, K.; Kalarani, I.B.; Jayaprasad, P.; Mohammed, V.; Veerabathiran, R. Genes linked with early menopause and the pathogenesis of its associated diseases: a systematic review. Middle East Fertility Society Journal 2022, 27, 1–11. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Jackson, C.A.; Rowlands, J.V.; Mishra, G.D. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. International journal of epidemiology 2014, 43, 1542–1562. [Google Scholar] [CrossRef]
- Cortés, Y.I.; Marginean, V. Key factors in menopause health disparities and inequities: Beyond race and ethnicity. Current Opinion in Endocrine and Metabolic Research 2022, 26, 100389–100389. [Google Scholar] [CrossRef]
- Wise, L.A.; Krieger, N.; Zierler, S.; Harlow, B.L. Lifetime socioeconomic position in relation to onset of perimenopause. Journal of Epidemiology and Community Health 2002, 56, 860–860. [Google Scholar] [CrossRef] [PubMed]
- Mobley, D.L.; Dill, K.A. Binding of small-molecule ligands to proteins: "what you see" is not always "what you get". Structure (London, England : 1993) 2009, 17, 489–498. [Google Scholar] [CrossRef]
- Sighoko, D.; Kamaté, B.; Traore, C.; Mallé, B.; Coulibaly, B.; Karidiatou, A.; Bourgeois, D. Breast cancer in pre-menopausal women in West Africa: analysis of temporal trends and evaluation of risk factors associated with reproductive life. Elsevier 2013, 22, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Kleer, C.G.; Martin, I.; Awuah, B.; Nsiah-Asare, A.; Takyi, V.; Braman, M.; E. Quayson, S.; Zarbo, R.; Wicha, M.; et al. African Ancestry and Higher Prevalence of Triple-Negative Breast Cancer: Findings From an International Study. Cancer 2010, 116, 4926–4932. [Google Scholar] [CrossRef]
- Akarolo-Anthony, S.N.; Ogundiran, T.O.; Adebamowo, C.A. Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Research : BCR 2010, 12, S8–S8. [Google Scholar] [CrossRef]
- Ghana Statistical, S. Demographic and Health Survey 1993; Accra, 1994/12// 1994.
- Gabriel, C.A.; Domchek, S.M. Breast cancer in young women. Breast Cancer Research 2010, 12, 212–212. [Google Scholar] [CrossRef]
- Preston, D.L.; Mattsson, A.; Holmberg, E.; Shore, R.; Hildreth, N.G.; Boice, J.D. Radiation Effects on Breast Cancer Risk: A Pooled Analysis of Eight Cohorts. Radiation research 2002, 158, 220–235. [Google Scholar] [CrossRef]
- White, A.J.; Bradshaw, P.T.; Hamra, G.B. Air pollution and Breast Cancer: A Review. Current epidemiology reports 2018, 5, 92–100. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Davis Lynn, B.C.; Edusei, L.; Titiloye, N.; Adjei, E.; Clegg-Lamptey, J.N.; Yarney, J.; Wiafe-Addai, B.; Awuah, B.; Duggan, M.A.; et al. Reproductive factors and risk of breast cancer by tumor subtypes among Ghanaian women: A population-based case-control study. International journal of cancer 2020, 147, 1535–1547. [Google Scholar] [CrossRef]
- Factbook, T.W. Mother's mean age at first birth Available online:. Available online: https://www.cia.gov/the-world-factbook/field/mothers-mean-age-at-first-birth/ (accessed on December 5, 2024).
- Robertson, C.; Primic-Zakelj, M.; Boyle, P.; Hsieh, C.C. Effect of parity and age at delivery on breast cancer risk in Slovenian women aged 25-54 years. International Journal of Cancer 1997, 73, 1–9. [Google Scholar] [CrossRef]
- Nguyen, B.; Venet, D.; Lambertini, M.; Desmedt, C.; Salgado, R.; Horlings, H.M.; Rothé, F.; Sotiriou, C. Imprint of parity and age at first pregnancy on the genomic landscape of subsequent breast cancer. Breast Cancer Research 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.T.; Colditz, G.A.; Palmer, J.R.; Partridge, A.H.; Rosner, B.A.; Tamimi, R.M. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat 2013, 142, 165–175. [Google Scholar] [CrossRef]
- Hinkula, M.; Pukkala, E.; Kyyrönen, P.; Kauppila, A. Grand multiparity and the risk of breast cancer: population-based study in Finland. Cancer causes & control 2001, 12, 491–500. [Google Scholar] [CrossRef]
- Review, W.P. Age of Menarche by Country 2024. Available online: https://worldpopulationreview.com/country-rankings/age-of-menarche-by-country (accessed on October 18, 2024).
- Adadevoh, S.W.K.; Agble, T.K.; Hobbs, C.; Elkins, T.E. Menarcheal age in Ghanaian school girls. International Journal of Gynecology and Obstetrics 1989, 30, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ameade, E.P.K.; Garti, H.A. Age at Menarche and Factors that Influence It: A Study among Female University Students in Tamale, Northern Ghana. PloS one 2016, 11, e0155310–e0155310. [Google Scholar] [CrossRef] [PubMed]
- Reagan, P.B.; Salsberry, P.J.; Fang, M.Z.; Gardner, W.P.; Pajer, K. African-American/white differences in the age of menarche: Accounting for the difference. Social Science & Medicine 2012, 75, 1263–1270. [Google Scholar] [CrossRef]
- Garenne, M. Age at menarche in Nigerian demographic surveys. Journal of Biosocial Science 2021, 53, 745–757. [Google Scholar] [CrossRef]
- Estuardo, J.; Irastorza, L.; García Rodríguez, F.; Figueroa Preciado, G.; Marín, I.H.; Ayala, A.R. Early menarche as a risk factor of breast cancer. Ginecología y Obstetricia de México 2006, 74, 568–572. [Google Scholar]
- Okonofua, F.E.; Lawal, A.; Bamgbose, J.K. Features of menopause and menopausal age in Nigerian women. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 1990, 31, 341–345. [Google Scholar] [CrossRef]
- Reynolds, P. Smoking and breast cancer. Journal of mammary gland biology and neoplasia 2013, 18, 15–23. [Google Scholar] [CrossRef]
- González-Jiménez, E.; García, P.A.; Aguilar, M.J.; Padilla, C.A.; Álvarez, J. Breastfeeding and the prevention of breast cancer: a retrospective review of clinical histories. Journal of clinical nursing 2014, 23, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Biessy, C.; Joffe, M.; Cubasch, H.; Norris, S.; Vorster, H.H.; Taljaard-Krugell, C.; Gunter, M.J.; Rinaldi, S. Reproductive factors and risk of breast cancer in black South African women. Cancer Causes Control 2021, 32, 415–415. [Google Scholar] [CrossRef]
- Butt, S.; Borgquist, S.; Anagnostaki, L.; Landberg, G.; Manjer, J. Breastfeeding in relation to risk of different breast cancer characteristics. BMC Research Notes 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Stordal, B. Breastfeeding reduces the risk of breast cancer: A call for action in high-income countries with low rates of breastfeeding. Cancer Medicine 2022, 12, 4616–4616. [Google Scholar] [CrossRef]
- Bothou, A.; Zervoudis, S.; Iliadou, M.; Pappou, P.; Iatrakis, G.; Tsatsaris, G.; Peitsidis, P.; Gerende, A.; Chalkidou, A.; Anthoulaki, X.; et al. Breastfeeding and Breast Cancer Risk: Our Experience and Mini-review of the Literature. Materia Socio-Medica 2022, 34, 28–28. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Bothou, A.; Eirini, O.; Daglas, M.; Iliadou, M.; Antoniou, E.; Palaska, E. Breastfeeding and its Association with Breast Cancer: a Systematic Review of the Literature. Mædica 2024, 19, 106–106. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulou, A.; Chatzinikolaou, S.; Kyriopoulos, I.; Bletsa, G.; Kaparelou, M.; Liontos, M.; Dimopoulos, M.A.; Zagouri, F. The Mutational Landscape of Early-Onset Breast Cancer: A Next-Generation Sequencing Analysis. Frontiers in Oncology 2022, 11, 797505–797505. [Google Scholar] [CrossRef]
- Ssentongo, P.; Oh, J.S.; Amponsah-Manu, F.; Wong, W.; Candela, X.; Acharya, Y.; Ssentongo, A.E.; Dodge, D.G. Breast Cancer Survival in Eastern Region of Ghana. Frontiers in Public Health 2022, 10, 880789–880789. [Google Scholar] [CrossRef]
- Limaiem, F.; Ahmad, F. Mucinous Breast Carcinoma. In StatPearls; StatPearls Publishing: 2023.
- Hercules, S.M.; Alnajar, M.; Chen, C.; Mladjenovic, S.M.; Shipeolu, B.A.; Perkovic, O.; Pond, G.R.; Mbuagbaw, L.; Blenman, K.R.; Daniel, J.M. Triple-negative breast cancer prevalence in Africa: a systematic review and meta-analysis. BMJ Open 2022, 12, e055735–e055735. [Google Scholar] [CrossRef]
- Espina, C.; McKenzie, F.; dos-Santos-Silva, I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Annals of Epidemiology 2017, 27, 659–659. [Google Scholar] [CrossRef]
- Ohene-Yeboah, M.; Adjei, E. Breast cancer in Kumasi, Ghana. Ghana Med J 2012, 46, 8–13. [Google Scholar]
- Jiagge, E.M.; Oppong, J.K.; Awuah, B.; Newman, L.A.; Wicha, M.; Merajver, S. Characteristics of breast cancer in Ghana and prevalence of aggressive disease and high mortality. Journal of Clinical Oncology 2014, 32, 596–596. [Google Scholar] [CrossRef]
- Jedy-Agba, E.; McCormack, V.; Adebamowo, C.; dos-Santos-Silva, I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. The Lancet Global Health 2016, 4, e923–e935. [Google Scholar] [CrossRef]
- Ahearn, T.U.; Choudhury, P.P.; Derkach, A.; Wiafe-Addai, B.; Awuah, B.; Yarney, J.; Edusei, L.; Titiloye, N.; Adjei, E.; Vanderpuye, V.; et al. Breast Cancer Risk in Women from Ghana Carrying Rare Germline Pathogenic Mutations. Cancer Epidemiology Biomarkers and Prevention 2022, 31, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
| Menopausal Description | Onset Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Overall N = 2621 |
Post N = 1721 |
Pre N = 901 |
p-value2 | EOBC N = 391 |
LOBC N = 2231 |
p-value3 | |
| Age at Diagnosis | 262 | 54 (27, 85) | 63 (48, 85) | 41 (27, 47) | 35 (27, 39) | 57 (40, 85) | |||
| Menopause age | 120 | 48 (27, 60) | 49 (32, 60) | 42 (27, 45) | <0.001 | 27 (27, 27) | 48 (32, 60) | 0.087 | |
| Menarche age | 192 | 15 (10, 25) | 15 (10, 25) | 15 (11, 19) | 0.005 | 14 (11, 17) | 15 (10, 25) | <0.001 | |
| Primiparity age | 233 | 24 (15, 54) | 23 (15, 54) | 27 (15, 41) | <0.001 | 27 (16, 35) | 24 (15, 54) | 0.024 | |
| Parity | 259 | 3 (0, 9) | 3 (0, 9) | 2 (0, 7) | <0.001 | 1 (0, 4) | 3 (0, 9) | <0.001 | |
| Gravidity | 232 | 4 (0, 10) | 4 (0, 10) | 3 (0, 10) | <0.001 | 2 (0, 5) | 4 (0, 10) | <0.001 | |
| Tribe | 246 | 0.10 | 0.070 | ||||||
| Akan | 110 (45%) | 74 (45%) | 36 (44%) | 16 (50%) | 94 (44%) | ||||
| Ewe | 53 (22%) | 29 (18%) | 24 (29%) | 11 (34%) | 42 (20%) | ||||
| Ga-Adangbe | 60 (24%) | 46 (28%) | 14 (17%) | 3 (9.4%) | 57 (27%) | ||||
| Others | 23 (9%) | 15 (9.1%) | 8 (9.8%) | 2 (6.3%) | 21 (9.8%) | ||||
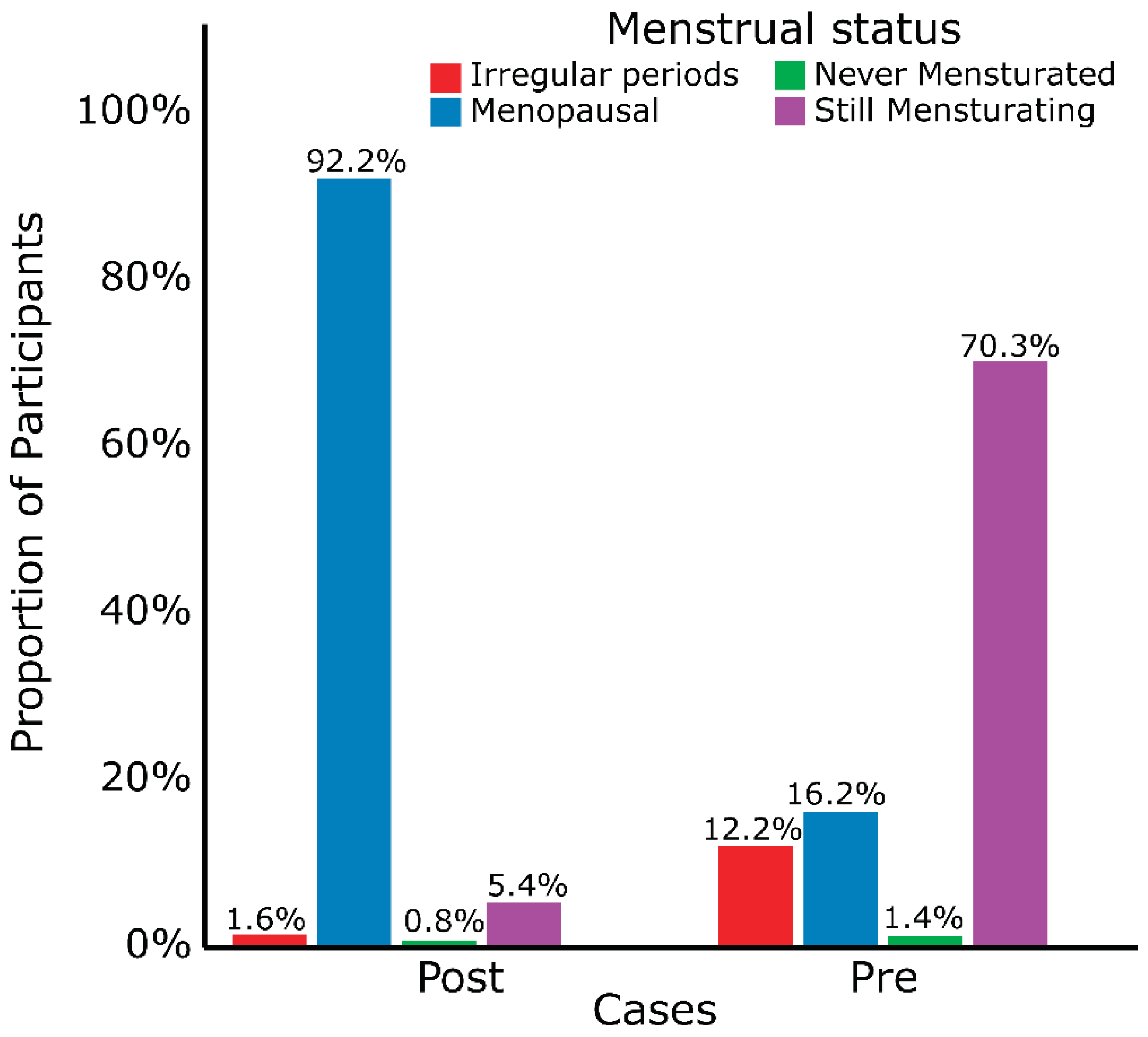
| Menstrual status | 262 | <0.001 | <0.001 | ||||||
| Postmenopausal | 171 (65%) | 155 (90%) | 16 (18%) | 2 (5.1%) | 169 (76%) | ||||
| Premenopausal | 67 (26%) | 7 (4%) | 60 (67%) | 29 (74%) | 38 (17%) | ||||
| others | 24 (9%) | 10 (6%) | 14 (16%) | 8 (21%) | 16 (7.2%) | ||||
| Occupation | 262 | <0.001 | <0.001 | ||||||
| Others | 67 (26%) | 31 (18%) | 36 (40%) | 18 (46%) | 49 (22%) | ||||
| Student/Unemployed/Retired | 72 (27%) | 67 (39%) | 5 (5.6%) | 3 (8%) | 69 (31%) | ||||
| Trader/Businesswoman | 123 (47%) | 74 (43%) | 49 (54%) | 18 (46%) | 105 (47%) | ||||
| Residence | 252 | 0.2 | 0.7 | ||||||
| Peri-Urban | 133 (53%) | 94 (57%) | 39 (45%) | 19 (51%) | 114 (53%) | ||||
| Rural | 7 (3%) | 5 (3%) | 2 (2%) | 0 (0%) | 7 (3.3%) | ||||
| Urban | 112 (44%) | 66 (40%) | 46 (53%) | 18 (49%) | 94 (44%) | ||||
| Detection | 201 | 0.3 | 0.2 | ||||||
| ME | 64 (32%) | 39 (29%) | 25 (37%) | 6 (22%) | 58 (33%) | ||||
| SE | 137 (68%) | 94 (71%) | 43 (63%) | 21 (78%) | 116 (67%) | ||||
| Mammogram | 253 | 0.009 | <0.001 | ||||||
| No | 35 (14%) | 16 (10%) | 19 (22%) | 12 (32%) | 23 (11%) | ||||
| Yes | 218 (86%) | 149 (90%) | 69 (78%) | 26 (68%) | 192 (89%) | ||||
| Menopausal Description | Onset Description | |||||||
|---|---|---|---|---|---|---|---|---|
| Reproductive Factors | N | Overall N = 2621 |
Post N = 1721 |
Pre N = 901 |
p-value2 | EOBC N = 391 |
LOBC N = 2231 |
p-value2 |
| Primiparity | 233 | 0.002 | 0.10 | |||||
| 24 | 14 (6%) | 12 (8%) | 2 (2.7%) | 0 (0%) | 14 (6.7%) | |||
| >24 | 109 (47%) | 62 (39%) | 47 (64%) | 16 (67%) | 93 (44%) | |||
| <24 | 110 (47%) | 85 (53%) | 25 (34%) | 8 (33%) | 102 (49%) | |||
| Menarche | 192 | 0.012 | 0.002 | |||||
| 15 | 48 (25%) | 27 (23%) | 21 (28%) | 10 (33%) | 38 (23%) | |||
| >15 | 66 (34%) | 50 (42%) | 16 (22%) | 2 (6.7%) | 64 (40%) | |||
| <15 | 78 (41%) | 41 (35%) | 37 (50%) | 18 (60%) | 60 (37%) | |||
| Menopausal age | 120 | <0.001 | 0.5 | |||||
| 48 | 11 (9%) | 11 (10%) | 0 (0%) | 0 (0%) | 11 (9.2%) | |||
| >48 | 55 (46%) | 55 (50%) | 0 (0%) | 0 (0%) | 55 (46%) | |||
| <48 | 54 (45%) | 44 (40%) | 10 (100%) | 1 (100%) | 53 (45%) | |||
| Gravidity | 262 | 0.018 | <0.001 | |||||
| 0 | 44 (17%) | 24 (14%) | 20 (22%) | 15 (38%) | 29 (13%) | |||
| 4 | 58 (22%) | 40 (23%) | 18 (20%) | 5 (13%) | 53 (24%) | |||
| >4 | 77 (29%) | 60 (35%) | 17 (19%) | 4 (10%) | 73 (33%) | |||
| <4 | 83 (32%) | 48 (28%) | 35 (39%) | 15 (38%) | 68 (30%) | |||
| Parity | 262 | <0.001 | <0.001 | |||||
| 0 | 23 (9%) | 6 (4%) | 17 (19%) | 14 (36%) | 9 (4%) | |||
| 3 | 73 (28%) | 49 (28%) | 24 (27%) | 7 (18%) | 66 (30%) | |||
| >3 | 82 (31%) | 64 (37%) | 18 (20%) | 3 (7.7%) | 79 (35%) | |||
| <3 | 84 (32%) | 53 (31%) | 31 (34%) | 15 (38%) | 69 (31%) | |||
| Breastfeed | 242 | 0.5 | 0.14 | |||||
| No | 12 (5%) | 7 (4%) | 5 (6%) | 3 (11%) | 9 (4.2%) | |||
| Yes | 230 (95%) | 157 (96%) | 73 (94%) | 24 (89%) | 206 (96%) | |||
| Breastfeeding duration | 222 | 0.11 | 0.6 | |||||
| 6-12 | 63 (28%) | 48 (31%) | 15 (22%) | 5 (24%) | 58 (29%) | |||
| >12 | 149 (67%) | 97 (63%) | 52 (76%) | 16 (76%) | 133 (66%) | |||
| <6 | 10 (5%) | 9 (6%) | 1 (2%) | 0 (0%) | 10 (5%) | |||
| Menopausal Description | Onset Description | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N | Overall N = 2621 |
Post N = 1721 |
Pre N = 901 |
p-value2 | EOBC N = 391 |
LOBC N = 2231 |
p-value2 |
| Tumours | 212 | 0.5 | >0.9 | |||||
| Carcinoma NST | 2 (1%) | 1 (1%) | 1 (1.3%) | 0 (0%) | 2 (1%) | |||
| Ductal Carcinoma in situ | 28 (13%) | 19 (14%) | 9 (12%) | 6 (16%) | 22 (13%) | |||
| Inflammatory Carcinoma | 2 (1%) | 2 (2%) | 0 (0%) | 0 (0%) | 2 (1%) | |||
| Invasive Carcinoma NST | 119 (56%) | 67 (51%) | 52 (64%) | 20 (54%) | 99 (57%) | |||
| Invasive Ductal Carcinoma | 38 (18%) | 24 (18%) | 14 (18%) | 8 (22%) | 30 (17%) | |||
| Invasive Lobular Carcinoma | 10 (5%) | 9 (7%) | 1 (1.3%) | 1 (3%) | 9 (5%) | |||
| Other Malignant | 13 (6%) | 9 (7%) | 4 (5.2%) | 2 (5%) | 11 (6%) | |||
| Tumour Stage | 47 | 0.5 | 0.8 | |||||
| I | 9 (19%) | 4 (15%) | 5 (25%) | 3 (27%) | 6 (17%) | |||
| II | 29 (62%) | 19 (70%) | 10 (50%) | 6 (55%) | 23 (64%) | |||
| III | 7 (15%) | 3 (11%) | 4 (20%) | 2 (18%) | 5 (14%) | |||
| IV | 2 (4%) | 1 (3.7%) | 1 (5.0%) | 0 (0%) | 2 (5.6%) | |||
| Tumour Grade | 149 | 0.4 | 0.7 | |||||
| 1 | 31 (21%) | 22 (24%) | 9 (16%) | 5 (19%) | 26 (21%) | |||
| 2 | 76 (51%) | 46 (51%) | 30 (52%) | 12 (46%) | 64 (52%) | |||
| 3 | 42 (28%) | 23 (25%) | 19 (33%) | 9 (35%) | 33 (27%) | |||
| Molecular Classification | 152 | 0.7 | 0.9 | |||||
| HER2-enriched | 4 (3%) | 3 (3%) | 1 (1.8%) | 1 (3.8%) | 3 (2.4%) | |||
| HR- | 29 (19%) | 15 (16%) | 14 (25%) | 6 (23%) | 23 (18%) | |||
| HR-_HER2_equivocal | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 1 (0.8%) | |||
| HR+ | 85 (56%) | 56 (58%) | 29 (52%) | 13 (50%) | 72 (57%) | |||
| HR+_HER2_equivocal | 8 (5%) | 6 (6%) | 2 (3.6%) | 1 (3.8%) | 7 (5.6%) | |||
| TNBC | 25 (16%) | 15 (16%) | 10 (18%) | 5 (19%) | 20 (16%) | |||
| Symptoms | 262 | 0.5 | 0.14 | |||||
| Lump in breast | 159 (60.7%) | 101 (59%) | 58 (64%) | 29 (74%) | 130 (58%) | |||
| Others | 62 (23.7%) | 41 (24%) | 21 (23%) | 7 (18%) | 55 (25%) | |||
| Painful breast | 41 (15.6%) | 30 (17%) | 11 (12%) | 3 (7.7%) | 38 (17%) | |||
| Menopausal Description | Onset Description | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Overall N = 2621 |
Post N = 1721 |
Pre N = 901 |
p-value2 | EOBC N = 391 |
LOBC N = 2231 |
p-value2 |
| Contraceptives | 244 | 0.3 | 0.005 | |||||
| No | 174 (71%) | 116 (73%) | 58 (67%) | 18 (51%) | 156 (75%) | |||
| 70 (29%) | 42 (27%) | 28 (33%) | 17 (49%) | 53 (25%) | ||||
| Alcohol | 242 | 0.8 | 0.9 | |||||
| Daily | 3 (1%) | 3 (2%) | 0 (0%) | 0 (0%) | 3 (1%) | |||
| Monthly | 9 (4%) | 6 (4%) | 3 (3%) | 1 (3%) | 8 (3%) | |||
| Not at all | 177 (73%) | 113 (73%) | 64 (73%) | 30 (81%) | 147 (72%) | |||
| Weekly | 2 (1%) | 1 (1%) | 1 (1%) | 0 (0%) | 2 (1%) | |||
| Yearly | 51 (21%) | 31 (20%) | 20 (23%) | 6 (16%) | 45 (22%) | |||
| Smoking | 251 | 0.5 | >0.9 | |||||
| No | 249 (99%) | 160 (99%) | 89 (100%) | 38 (100%) | 211 (99%) | |||
| Yes | 2 (0.8%) | 2 (1%) | 0 (0%) | 0 (0%) | 2 (0.9%) | |||
| Bleaching cream | 262 | 0.070 | 0.3 | |||||
| No | 228 (87%) | 145 (84%) | 83 (92%) | 36 (92%) | 192 (86%) | |||
| Yes | 34 (13%) | 27 (16%) | 7 (7.8%) | 3 (7.7%) | 31 (14%) | |||
| Family History | 262 | 0.7 | 0.5 | |||||
| No | 227 (87%) | 150 (87%) | 77 (86%) | 35 (90%) | 192 (86%) | |||
| Yes | 35 (13%) | 22 (13%) | 13 (14%) | 4 (10%) | 31 (14%) | |||
| Knowledge | 242 | 0.10 | 0.8 | |||||
| Yes | 190 (79%) | 119 (75%) | 71 (85%) | 27 (77%) | 163 (79%) | |||
| No | 52 (21%) | 39 (25%) | 13 (15%) | 8 (23%) | 44 (21%) | |||
| Premenopausal | EOBC | |||
|---|---|---|---|---|
| Predictor | ORa (95%CI) | p-value | ORa (95% CI) | p-value |
| Occupation | ||||
| Employed/Self-Employed | __ | __ | __ | __ |
| Unemployed | 0.118 (0.045–0.310) |
<0.001 | 0.183 (0.051–0.656) |
0.009 |
| Primiparity | ||||
| <24 | __ | __ | __ | __ |
| ≥24 | 2.108 (1.151–3.859) |
0.016 | __ | __ |
| Parity | ||||
| Parous | __ | __ | __ | __ |
| Nulliparous | __ | __ | 13.491 (5.059–35.980) |
<0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





