Submitted:
30 August 2025
Posted:
02 September 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Natural Biosynthesis Pathways
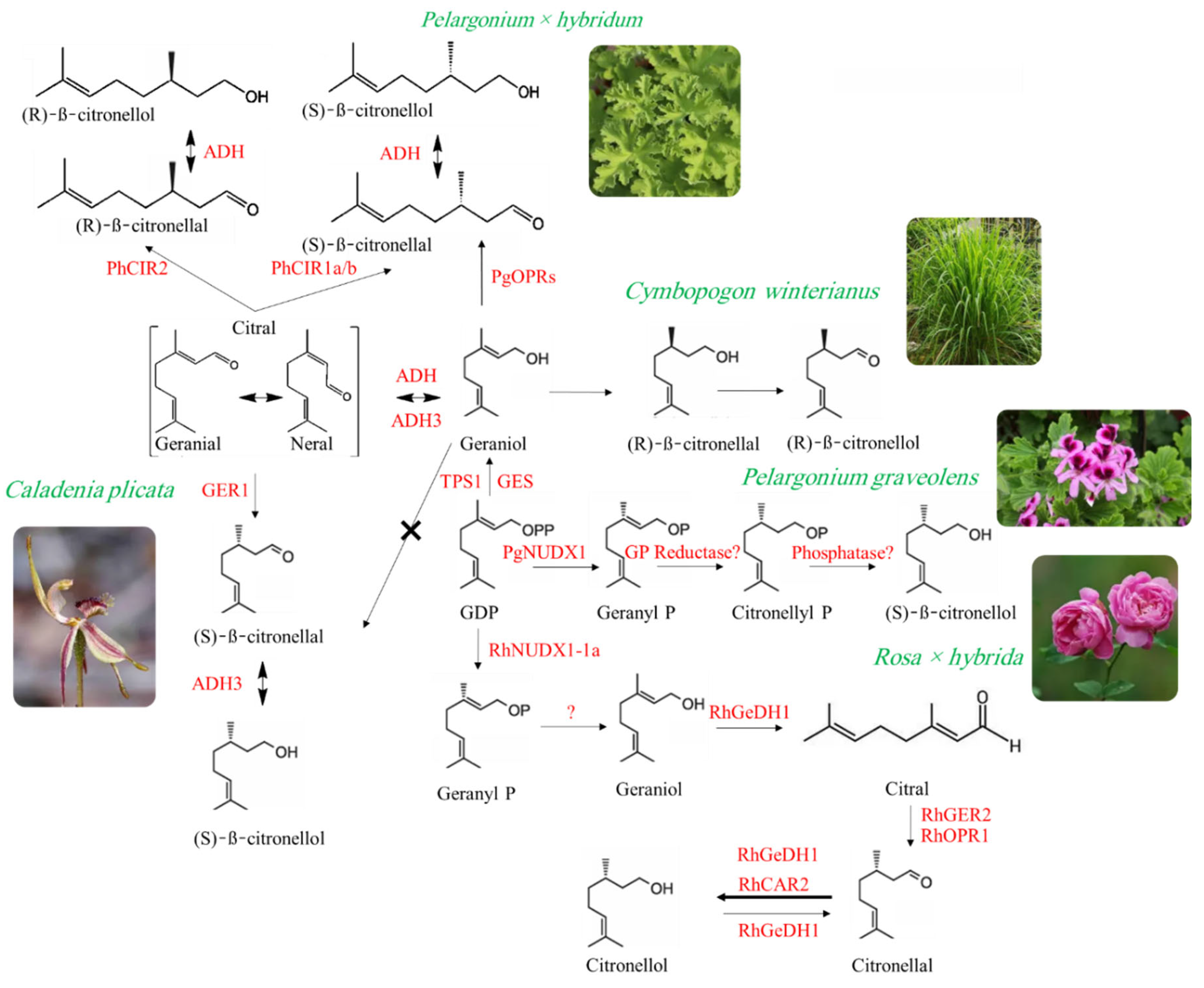
2.1. Citronellol Production in Plants: Key Enzymes and Metabolic Steps
2.2. Comparative Biosynthetic Routes: Cytosolic vs Plastidic Mechanisms
2.3. Role of Stereoisomerism in Biological Activity
3. Metabolic Engineering and Synthetic Biology
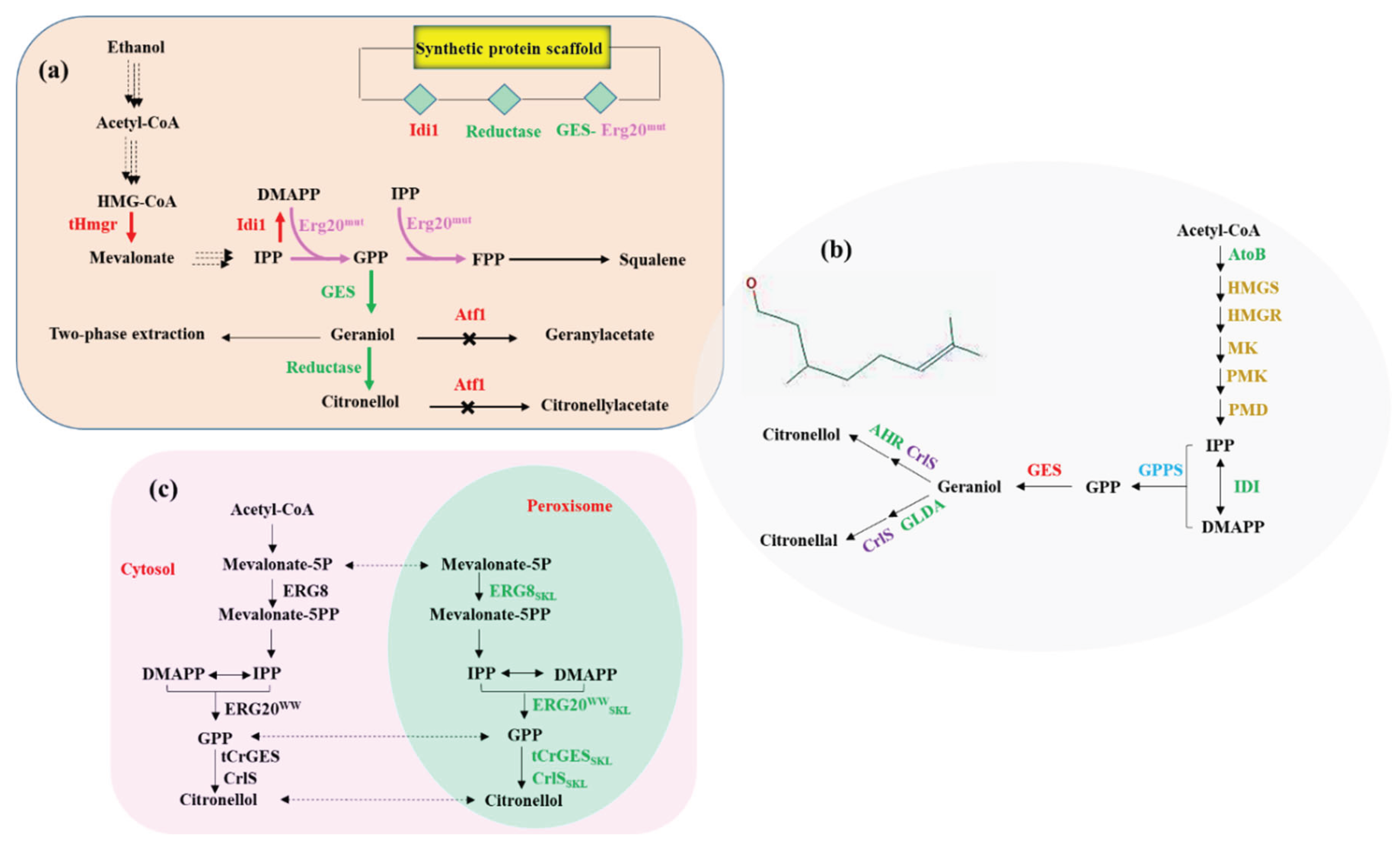
3.1. Construction of Biosynthetic Pathways in Microbes
3.2. Enzyme Cascade Design: Optimizing Flux Through GPP → Geraniol → Citronellol
4. Biotransformation and Catalysis
4.1. Whole-Cell Conversion Systems: Engineered Yeasts and Fungi
4.2. Enzyme-Specific Conversions: Reductases and Alcohol Dehydrogenases
4.3. Conversion of Citronellol to Rose Oxide and Other Fragrance Derivatives
5. Fermentation and Bioprocess Development
5.1. Gas-Phase Bioreactors
5.2. Two-Phase Partitioning Bioreactors (TPPBs)
5.3. Integration and Industrial Relevance
6. Analytical Tools and Characterization
6.1. GC-MS for Profiling and Quantification
6.2. Chiral HPLC for Enantiomer Separation
6.3. Molecular Docking and Computational Tools for Bioactivity Predictions
6.3.1. Computational Docking and Simulation Studies of Citronellal and Citronellol: From Neuroprotection to Vector Control
6.3.2. Scoring Functions and Theoretical Frameworks for Predicting Citronellal-Derived Reactions and Terpenoid Biosynthesis
6.3.3. Machine Learning Approaches for Predicting Bioactivity and Formulation Outcomes of Citronellol-Rich Essential Oils
6.3.4. Integrating Docking with ADME/Tox Profiling to Identify Citronellol-Related Compounds as Therapeutic Candidates
6.3.5. Artificial Intelligence and Systems-Level Approaches in Phytochemical and Biosynthetic Pathway Research
7. Applications and Functional Studies
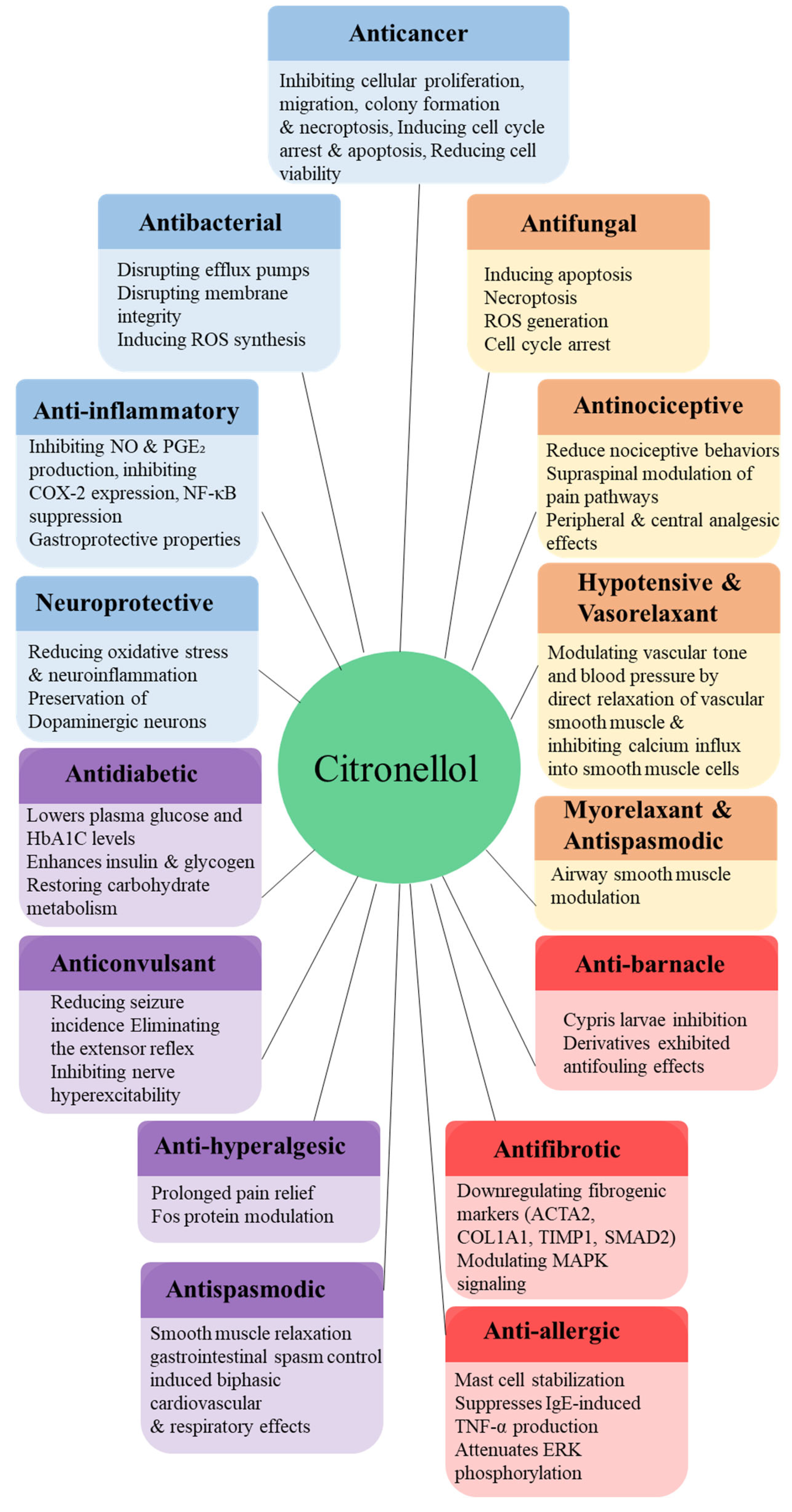
7.1. Biological Activities
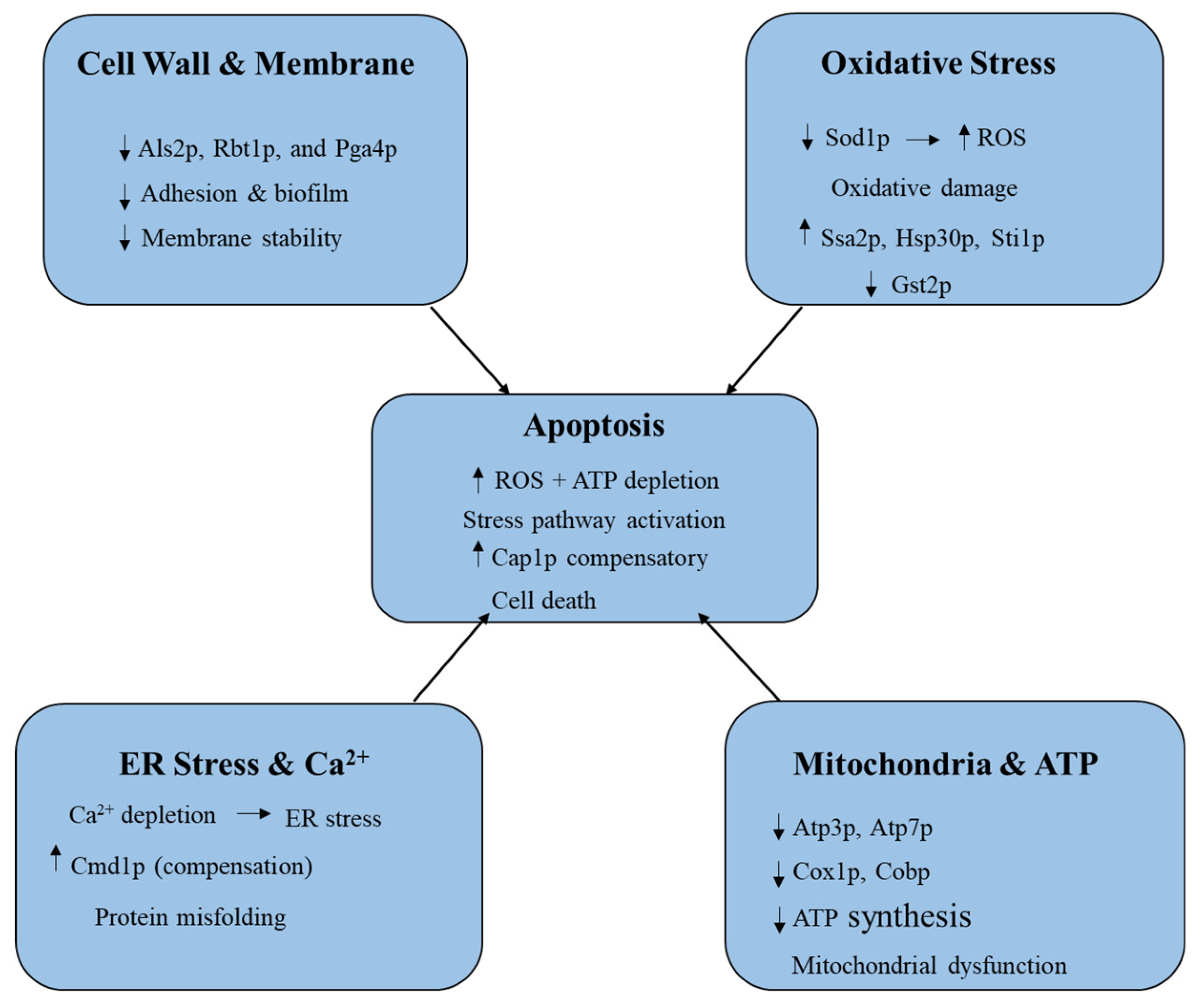
7.1.1. Antimicrobial Activity
7.1.2. Antifungal Activity
7.1.3. Anti-Inflammatory Potential
7.1.4. Neuroprotective Effects
7.1.5. Anticancer Effects
7.1.6. Antinociceptive Effect
7.1.7. Hypotensive and Vasorelaxant Effects
7.1.8. Myorelaxant and Antispasmodic Effects
7.1.9. Anti-Barnacle Activity
7.1.10. Anti-Allergic Activity
7.1.11. Antifibrotic Agent
7.1.12. Antidiabetic Effects
7.1.13. Anticonvulsant Activity
7.1.14. Antispasmodic Effects
7.2. Use in Green Insect Repellents and Cosmetics
7.2.1. Citronella Candles and Sprays for Short-Term Mosquito Deterrence
7.2.2. Topical Lotions and Wristbands for Personal Protection
7.2.3. Essential Oil Diffusers for Ambient Repellent Effects
8. Challenges and Future Prospects
8.1. Limitations in Yield, Toxicity, and Pathway Bottlenecks
8.2. Strategies for Improving Microbial Tolerance and Efficiency
8.3. Emerging Technologies: AI-Guided Metabolic Design and Machine Learning in Fermentation Control
9. Conclusions
Data availability Statement
Conflicts of Interest
References
- Martinelli, L.; Bihanic, C.; Bony, A.; Gros, F.; Conart, C.; Fiorucci, S.; et al. Citronellol biosynthesis in pelargonium is a multistep pathway involving progesterone 5β-reductase and/or iridoid synthase-like enzymes. Plant Physiology. 2024, 194, 1006–1023. [Google Scholar] [CrossRef]
- Bergman, M.E.; Bhardwaj, M.; Phillips, M.A. Cytosolic geraniol and citronellol biosynthesis require a Nudix hydrolase in rose-scented geranium (Pelargonium graveolens). The Plant Journal. 2021, 107, 493–510. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Yan, H.; Bao, T.; Shan, X.; Caissard, J.-C.; et al. The complexity of volatile terpene biosynthesis in roses: Particular insights into β-citronellol production. Plant Physiology. 2024, 196, 1908–1922. [Google Scholar] [CrossRef]
- Santos, P.L.; Matos, J.P.S.; Picot, L.; Almeida, J.R.; Quintans, J.S.; Quintans-Junior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities-A systematic review. Food and Chemical Toxicology. 2019, 123, 459–469. [Google Scholar] [CrossRef]
- Iqbal, U.; Malik, A.; Sial, N.T.; Mehmood, M.H.; Nawaz, S.; Papadakis, M.; et al. β-Citronellol: a potential anti-inflammatory and gastro-protective agent-mechanistic insights into its modulatory effects on COX-II, 5-LOX, eNOS, and ICAM-1 pathways through in vitro, in vivo, in silico, and network pharmacology studies. Inflammopharmacology. 2024, 32, 3761–3784. [Google Scholar] [CrossRef]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nature communications. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Li, Y.; Pfeifer, B.A. Heterologous production of plant-derived isoprenoid products in microbes and the application of metabolic engineering and synthetic biology. Current opinion in plant biology. 2014, 19, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Vavitsas, K.; Fabris, M.; Vickers, C.E. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes. 2018, 9, 520. [Google Scholar] [CrossRef]
- Jiang, G.; Yao, M.; Wang, Y.; Xiao, W.; Yuan, Y. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae. Metabolic Engineering. 2021, 66, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ribeaucourt, D.; Hofler, G.T.; Yemloul, M.; Bissaro, B.; Lambert, F.; Berrin, J.-G.; et al. Tunable production of (R)-or (S)-citronellal from geraniol via a bienzymatic cascade using a copper radical alcohol oxidase and old yellow enzyme. ACS catalysis. 2022, 12, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Mermat, N.; Ferroukhi, O.; Peulon-Agasse, V.; Bayle, J.P.; Guermouche, M.H.; Cardinael, P. Original mesogenic citronellol-based stationary phase for both normal-and reversed-phase HPLC modes: properties and applications. Chromatographia. 2020, 83, 1495–1508. [Google Scholar] [CrossRef]
- Ye, M.; Ye, Y.; Du, Z.; Chen, G. Cell-surface engineering of yeasts for whole-cell biocatalysts. Bioprocess and biosystems engineering. 2021, 44, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Lecourt, M.; Chietera, G.; Jullien, F.; Blerot, B.; Antoniotti, S. From biosynthesis in plants to post-biosynthetic enzymatic conversion. Generation of odor-impact rose oxides from citronellol-rich essential oils. Botany Letters. 2023, 170, 15–27. [Google Scholar]
- Zhang, B.; Du, H.; Zheng, Y.; Sun, J.; Shen, Y.; Lin, J.; et al. Design and engineering of whole-cell biocatalyst for efficient synthesis of (R)-citronellal. Microbial Biotechnology. 2022, 15, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, A.S.; Milagre, C.D.; Hollmann, F. Alcohol dehydrogenases as catalysts in organic synthesis. Frontiers in Catalysis. 2022, 2, 900554. [Google Scholar] [CrossRef]
- Chadha, A.; Padhi, S.K.; Stella, S.; Venkataraman, S.; Saravanan, T. Microbial alcohol dehydrogenases: recent developments and applications in asymmetric synthesis. Organic & Biomolecular Chemistry. 2024, 22, 228–251. [Google Scholar]
- Rudzka, A.; Zdun, B.; Antos, N.; Montero, L.M.; Reiter, T.; Kroutil, W.; et al. Biocatalytic characterization of an alcohol dehydrogenase variant deduced from Lactobacillus kefir in asymmetric hydrogen transfer. Communications Chemistry. 2023, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chemical reviews. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Malinowski, J.J. Two-phase partitioning bioreactors in fermentation technology. Biotechnology advances. 2001, 19, 525–538. [Google Scholar] [CrossRef]
- Doig, S.; Boam, A.; Leak, D.; Livingston, A.; Stuckey, D. Optimisation of the kinetics of the stereoselective reduction of geraniol to citronellol in a two liquid phase system. Biocatalysis and Biotransformation. 1998, 16, 27–44. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Xiang, H.; Zhu, H.; Lu, X.; Feng, B. Two-phase fermentation systems for microbial production of plant-derived terpenes. Molecules. 2024, 29, 1127. [Google Scholar] [CrossRef]
- Subash, P.; Kumar, K.S.; Rao, K.S.; Khute, S. Advanced Chromatography Analytical Methods for the Isolation and Identification of Natural Drug Molecules. 2025.
- Chen, L.; Dean, B.; Liang, X. A technical overview of supercritical fluid chromatography-mass spectrometry (SFC-MS) and its recent applications in pharmaceutical research and development. Drug Discovery Today: Technologies. 2021, 40, 69–75. [Google Scholar] [CrossRef]
- Vamshi, G.; DSNBKP; Sampath, A.; Dammalli, M.; Kumar, P.; BS G; et al. Possible cerebroprotective effect of citronellal: molecular docking, MD simulation and in vivo investigations. Journal of Biomolecular Structure and Dynamics. 2024, 42, 1208–1219. [Google Scholar]
- Santos, P.L.; Brito, R.G.; Oliveira, M.A.; Quintans, J.S.; Guimaraes, A.G.; Santos, M.R.; et al. Docking, characterization and investigation of β-cyclodextrin complexed with citronellal, a monoterpene present in the essential oil of Cymbopogon species, as an anti-hyperalgesic agent in chronic muscle pain model. Phytomedicine. 2016, 23, 948–957. [Google Scholar] [CrossRef]
- Okoli, B.J.; Ladan, Z.; Mtunzi, F.; Hosea, Y.C. Vitex negundo L. Essential oil: odorant binding protein efficiency using molecular docking approach and studies of the mosquito repellent. Insects. 2021, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Patsilinakos, A. Machine learning applications to essential oils and natural extracts. 2020.
- Sharma, A.D.; Kaur, I.; Chauhan, A. In vitro anti-mucormycosis and anti-aspergillosis potential of essential oils and molecular docking of principal components from Cymbopogon khasianus and Cymbopogon citratus. Chemistry Africa. 2023, 6, 2835–2848. [Google Scholar] [CrossRef]
- Reinhardt, J.K.; Craft, D.; Weng, J.-K. Toward an integrated omics approach for plant biosynthetic pathway discovery in the age of AI. Trends in Biochemical Sciences. 2025. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.; Jeong, D.; Lee, J.-C. Synthesis of citronellol-derived antibacterial polymers and effect of thioether, sulfoxide, sulfone, and ether functional groups on their bactericidal activity. Macromolecules. 2023, 56, 3406–3420. [Google Scholar] [CrossRef]
- Lopez, G.P.; Roque, L.B.; Igal, K.; Espinosa, E.G.; Bellotti, N. Citronellol-functionalized natural silica: a biogenic approach for antifungal and antibacterial material applications. Frontiers in Chemistry. 2025, 13, 1535787. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.L.S.; Campina, F.F.; do Socorro Costa, M.; da Cruz, R.P.; de Freitas, T.S.; Dos Santos, A.T.L.; et al. Antibacterial and modulatory activities of β-cyclodextrin complexed with (+)-β-citronellol against multidrug-resistant strains. Microbial Pathogenesis. 2021, 156, 104928. [Google Scholar] [CrossRef]
- Pereira, F.d.O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.d.L.; Oliveira WAd Lima, E.d.O. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharmaceutical biology. 2015, 53, 228–234. [Google Scholar] [CrossRef]
- Su, Y.-W.; Chao, S.-H.; Lee, M.-H.; Ou, T.-Y.; Tsai, Y.-C. Inhibitory effects of citronellol and geraniol on nitric oxide and prostaglandin E2 production in macrophages. Planta medica. 2010, 76, 1666–1671. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Parekh, K.A.; Ojha, S.K.; Beiram, R. Effect of citronellol on oxidative stress, neuroinflammation and autophagy pathways in an in vivo model of Parkinson's disease. Heliyon 2022, 8(. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer effect of Citrus hystrix DC. leaf extract and its bioactive constituents citronellol and, citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceuticals. 2020, 13, 476. [Google Scholar]
- Demirel, S. Geraniol and β-citronellol participate in the vasorelaxant effects of Rosa damascena Miller essential oil on the rat thoracic aorta. Fitoterapia. 2022, 161, 105243. [Google Scholar] [CrossRef]
- Ribeiro-Filho, H.V.; de Souza Silva, C.M.; de Siqueira, R.J.; Lahlou, S.; dos Santos, A.A.; Magalhães, P.J.C. Biphasic cardiovascular and respiratory effects induced by β-citronellol. European journal of pharmacology. 2016, 775, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Krobthong, S.; Yingchutrakul, Y.; Thongsri, Y.; et al. Proteomic analysis reveals proteins involved in the mode of action of β-Citronellol identified from Citrus hystrix DC. Leaf against Candida albicans. Frontiers in microbiology. 2022, 13, 894637. [Google Scholar]
- Yue, Y.; Li, C.; Zhang, T.; Park, S. Neuroprotective Effects of SELFormer-Selected β-Citronellol and β-Caryophyllene in Vagotomized Ischemic Stroke Model Through Direct Brain Protection and Gut Microbiota Modulation. BioFactors. 2025, 51, e70031. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Dos Santos, P.L.; Quintans, J.S.; de Lucca Júnior, W.; Araújo, A.A.; Saravanan, S.; et al. Citronellol, a natural acyclic monoterpene, attenuates mechanical hyperalgesia response in mice: Evidence of the spinal cord lamina I inhibition. Chemico-Biological Interactions. 2015, 239, 111–117. [Google Scholar] [CrossRef]
- Bastos, J.F.; Moreira, Í.J.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; et al. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic & Clinical Pharmacology & Toxicology. 2010, 106, 331–337. [Google Scholar]
- Vasconcelos, T.; Ribeiro-Filho, H.; Lucetti, L.; Magalhães, P. β-Citronellol, an alcoholic monoterpene with inhibitory properties on the contractility of rat trachea. Brazilian Journal of Medical and Biological Research. 2015, 49, e4800. [Google Scholar] [CrossRef]
- Tanikawa, A.; Fujihara, T.; Nakajima, N.; Maeda, Y.; Nogata, Y.; Yoshimura, E.; et al. Anti-Barnacle Activities of Isothiocyanates Derived from β-Citronellol and Their Structure–Activity Relationships. Chemistry & Biodiversity. 2023, 20, e202200953. [Google Scholar]
- Kobayashi, Y.; Sato, H.; Yorita, M.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; et al. Inhibitory effects of geranium essential oil and its major component, citronellol, on degranulation and cytokine production by mast cells. Bioscience, Biotechnology, and Biochemistry. 2016, 80, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Potup, P.; Thongsri, Y.; Daowtak, K.; et al. Investigating the antifibrotic effects of β-citronellol on a TGF-β1-stimulated LX-2 hepatic stellate cell model. Biomolecules. 2024, 14, 800. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Gonçalves, J.C.R.; Quintans-Júnior, L.; Cruz, J.S.; Araújo, D.A.M.; de, A.l.m.e.i.d.a.RN. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neuroscience letters. 2006, 401, 231–235. [Google Scholar] [CrossRef]
- Sadraei, H.; Asghari, G.; Emami, S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Research in Pharmaceutical Sciences. 2013, 8, 17. [Google Scholar]
- Iovinella, I.; Caputo, B.; Cobre, P.; Manica, M.; Mandoli, A.; Dani, F.R. Advances in mosquito repellents: effectiveness of citronellal derivatives in laboratory and field trials. Pest Management Science. 2022, 78, 5106–5112. [Google Scholar] [CrossRef]
- Songkro, S.; Hayook, N.; Jaisawang, J.; Maneenuan, D.; Chuchome, T.; Kaewnopparat, N. Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2012, 72, 339–355. [Google Scholar] [CrossRef]
- Gilpin, S.; Hui, X.; Maibach, H. In vitro human skin penetration of geraniol and citronellol. DERM. 2010, 21, 41–48. [Google Scholar] [CrossRef]
- Cagliero C, Bechis G, Marengo A, Sgorbini B, Bicchi C, Rubiolo P, editors. Essential oil components as GC-compatible hydrophobic deep eutectic solvents to extract regulated compounds from water-based fragrances. 53rd INTERNATIONAL SYMPOSIUM ON ESSENTIAL OILS Book of Abstract; 2023.
- Wang, X.; Zhang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Wang, Z.; et al. Advances in microbial production of geraniol: From metabolic engineering to potential industrial applications. Critical Reviews in Biotechnology. 2025, 45, 727–742. [Google Scholar] [CrossRef]
- Singh, K.; Kaloni, D.; Sehgal, K.; Pan, S.; Sarethy, I.P. Essential oils: An update on their biosynthesis and genetic strategies to overcome the production challenges. Plant-derived bioactives: production, properties and therapeutic applications. 2020:33-60.
- Förster-Fromme, K.; Jendrossek, D. Catabolism of citronellol and related acyclic terpenoids in pseudomonads. Applied microbiology and biotechnology. 2010, 87, 859–869. [Google Scholar] [CrossRef]
- Devi, K.; Mishra, S.K.; Sahu, J.; Panda, D.; Modi, M.K.; Sen, P. Genome wide transcriptome profiling reveals differential gene expression in secondary metabolite pathway of Cymbopogon winterianus. Scientific reports. 2016, 6, 21026. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, H.; Yijing, W.; Wan, X.; Ming, C.; Huitang, P.; et al. Functional characterization of LcTPS1 and LcTPS14 explains the biosynthesis of citronellol, citronellal and linalool in Lagerstroemia caudata. Industrial Crops and Products. 2024, 209, 118033. [Google Scholar]
- Zhao, Q.; Zhang, M.; Gu, L.; Yang, Z.; Li, Y.; Luo, J.; et al. Transcriptome and volatile compounds analyses of floral development provide insight into floral scent formation in Paeonia lactiflora ‘Wu Hua Long Yu’. Frontiers in Plant Science. 2024, 15, 1303156. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Chávez, Á.; Ferrer, A.; Phillips, M.A. Distinct metabolic pathways drive monoterpenoid biosynthesis in a natural population of Pelargonium graveolens. Journal of Experimental Botany. 2020, 71, 258–271. [Google Scholar] [CrossRef]
- Iijima, M.; Kenmoku, H.; Takahashi, H.; Lee, J.-B.; Toyota, M.; Asakawa, Y.; et al. Characterization of 12-oxophytodienoic acid reductases from rose-scented geranium (Pelargonium graveolens). Natural Product Communications. 2016, 11, 1934578X1601101201. [Google Scholar] [CrossRef]
- Xu, H.; Bohman, B.; Wong, D.C.; Rodriguez-Delgado, C.; Scaffidi, A.; Flematti, G.R.; et al. Complex sexual deception in an orchid is achieved by co-opting two independent biosynthetic pathways for pollinator attraction. Current Biology. 2017, 27, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annual review of plant biology. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Blerot, B.; Martinelli, L.; Prunier, C.; Saint-Marcoux, D.; Legrand, S.; Bony, A.; et al. Functional analysis of four terpene synthases in rose-scented Pelargonium cultivars (Pelargonium× hybridum) and evolution of scent in the Pelargonium genus. Frontiers in Plant Science. 2018, 9, 1435. [Google Scholar] [CrossRef]
- Bohrer, A.-S.; Kopriva, S.; Takahashi, H. Plastid-cytosol partitioning and integration of metabolic pathways for APS/PAPS biosynthesis in Arabidopsis thaliana. Frontiers in Plant Science. 2015, 5, 751. [Google Scholar] [CrossRef]
- Morcelli, S.R.; Bull, É.S.; Terra, W.S.; Moreira, R.O.; Borges, F.V.; Kanashiro, M.M.; et al. Synthesis, characterization and antitumoral activity of new cobalt (II) complexes: Effect of the ligand isomerism on the biological activity of the complexes. Journal of Inorganic Biochemistry. 2016, 161, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Aseri, M.L.; Padmanabhan, D. A review of drug isomerism and its significance. International journal of applied and basic medical research. 2013, 3, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Elsharif, S.A.; Buettner, A. Influence of the chemical structure on the odor characters of β-citronellol and its oxygenated derivatives. Food Chemistry. 2017, 232, 704–711. [Google Scholar] [CrossRef]
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Júnior, F.; et al. (R)-(+)-β-Citronellol and (S)-(−)-β-Citronellol in Combination with Amphotericin B against Candida spp. International journal of molecular sciences. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Li, L.; Jiang, H.; Xu, T.; Wang, X. Engineering Bienzymatic Cascade for Efficient Biosynthesis of Citronellal and Citronellol. Biotechnology and Bioengineering. 2025. [Google Scholar] [CrossRef]
- Lu, Z.; Evans, S.; McDonnell, L.; Bandari, N.C.; Weng, Y.; Jin, W.; et al. Exploiting the Geranylgeranyl-Pyrophosphate-Sensing N-Terminal Domain of HMG-CoA Reductase 2 to Regulate Farnesyl Pyrophosphate Synthase (Erg20p) for Improved Monoterpene Production in Saccharomyces cerevisiae. Yeast. 2025. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, H.; Shan, M.; Wu, F.; Jiang, G.; Yao, M.; et al. Systematic Engineering To Enhance Citronellol Production in Yeast. Journal of Agricultural and Food Chemistry. 2025. [Google Scholar] [CrossRef]
- Ferrer-Carbonell, C.; Villa, R.; Viskaal, I.; Opperman, D.J.; Paul, C.E. Enzymatic Synthesis of Enantiopure (R)-Citronellal from Geraniol via a Short-Chain Dehydrogenase and Ene Reductase. Advanced Synthesis & Catalysis 2025, 2500060. [Google Scholar]
- Maróstica Jr, M.R.; Pastore, G.M. Biotransformation of citronellol in rose-oxide using cassava wastewater as a medium. Food Science and Technology. 2006, 26, 690–696. [Google Scholar] [CrossRef]
- Pimentel, M.; Molina, G.; Bertucci, T.; Pastore, G. Biotransformation of citronellol in rose oxide by Pseudomonas spp. Chemical Engineering Transactions. Chemical Engineering Transactions. 2012, 27, 295–300. [Google Scholar]
- Pinto, A.; Contente, M.L.; Tamborini, L. Advances on whole-cell biocatalysis in flow. Current Opinion in Green and Sustainable Chemistry. 2020, 25, 100343. [Google Scholar] [CrossRef]
- Ma, G.; Zhu, X.; Zhang, D.; Li, H.; Lin, J.; Wei, D. Design of a Self-Sufficient Whole-Cell Cascade for the Production of (R)-Citronellal from Geraniol. Journal of Agricultural and Food Chemistry. 2024, 72, 26305–26315. [Google Scholar] [CrossRef]
- Oda, S.; Sugai, T.; Ohta, H. Syntheses of Optically Active Citronellol, Citronellal, and Citronellic Acid by Microbial Oxidation and Double Coupling System in an Interface Bioreactor. Bulletin of the Chemical Society of Japan. 2000, 73, 2819–2823. [Google Scholar] [CrossRef]
- Demyttenaere, J.C.; Vanoverschelde, J.; De Kimpe, N. Biotransformation of (R)-(+)-and (S)-(−)-citronellol by Aspergillus sp. and Penicillium sp., and the use of solid-phase microextraction for screening. Journal of Chromatography A. 2004, 1027, 137–146. [Google Scholar] [CrossRef]
- Bhat, S.V.; Mestry, S.J.; Gupta, M.O. Biotechnological transformation of citronellene and citronellol by fungus Rhizopus oryzae (ATCC 9363) leading to interesting conversions through oxidations and rearrangements. 2022.
- Ohashi, Y.; Huang, S.; Maeda, I. Biosyntheses of geranic acid and citronellic acid from monoterpene alcohols by Saccharomyces cerevisiae. Bioscience, Biotechnology, and Biochemistry. 2021, 85, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Singh, M.; Singh, U.; Jatav, S.; Sangwan, R.S.; Mishra, B.B. Iodosylbenzene (PhIO) mediated synthesis of rose oxide from β-citronellol and its application for in situ rose oxide enrichment led valorization of citronella essential oil. Journal of Cleaner Production. 2018, 172, 1765–1771. [Google Scholar] [CrossRef]
- Arifin, A.A.; Don, M.M.; Uzir, M.H. Baker's yeast mediated biotransformation of geraniol into citronellol using a continuous-closed-gas-loop bioreactor (CCGLB) system. Biochemical engineering journal. 2011, 56, 219–224. [Google Scholar] [CrossRef]
- Muñoz, R.; Daugulis, A.J.; Hernández, M.; Quijano, G. Recent advances in two-phase partitioning bioreactors for the treatment of volatile organic compounds. Biotechnology Advances. 2012, 30, 1707–1720. [Google Scholar] [CrossRef]
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS-and GC/MS-based metabolomics: State of the field and future prospects. TrAC Trends in Analytical Chemistry. 2016, 78, 23–35. [Google Scholar] [CrossRef]
- Chen, B.-G.; Chang, C.D.; Wang, C.-T.; Chen, Y.-J.; Chang, W.-T.; Wang, S.-M.; et al. A novel approach to evaluate the extent and the effect of cross-contribution to the intensity of ions designating the analyte and the internal standard in quantitative GC-MS analysis. Journal of the American Society for Mass Spectrometry. 2011, 19, 598–608. [Google Scholar] [CrossRef]
- Kaur, J.; Goswami, D.; Saraf, M. Response surface methodology: a comparative optimization of antifungal metabolite production by Trichoderma viride and Trichoderma harzianum using solid-state fermentation. Biomass Conversion and Biorefinery 2025, 1–24. [Google Scholar] [CrossRef]
- Stilo F, Franchina FA, Cordero C, Focant J-F, editors. Comprehensive two-dimensional gas chromatography coupled to mass spectrometry (GC× GC-TOF MS): discrimination of Italian extra virgin olive oils (EVOOs) from different regions and exploration of high resolution (HR) mass spectrometry information. 12th Multidimensional Chromatography Workshop-Guidebook; 2021.
- Scriba, G.K. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends in Analytical Chemistry. 2019, 120, 115639. [Google Scholar]
- Ikai, T.; Okamoto, Y. Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chemical Reviews. 2009, 109, 6077–6101. [Google Scholar] [CrossRef]
- Ilisz, I.; Berkecz, R.; Péter, A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. Journal of pharmaceutical and biomedical analysis. 2008, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, D.; Wu, Y.; Lin, Z.; Wang, L.; Wang, L.; et al. Analysis of monoterpenol Isomers in hops and beers: comparison of methods with a chiral column and a nonchiral column. Journal of the American Society of Brewing Chemists. 2017, 75, 149–155. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Sun, L.; Liang, A.; Duan, J.; Zhang, Y.; et al. Preparation of novel chiral stationary phases based on chiral metal-organic cages enable extensive HPLC enantioseparation. Analytica Chimica Acta. 2025, 1337, 343541. [Google Scholar] [CrossRef] [PubMed]
- Mookdasanit, J.; Tamura, H. Comparative enantioseparation of monoterpenes by HPLC on three kinds of chiral stationary phases with an on-line optical rotatory dispersion under reverse phase mode. Food Science and Technology Research. 2002, 8, 367–372. [Google Scholar] [CrossRef]
- de Brito, G.A.; Rocha de Oliveira, P.F.; de Andrade Silva, C.M.; de Araújo Neto, M.F.; Leite, F.H.A.; Mesquita, P.R.R.; et al. Identification of bioactive compounds against Aedes aegypti (Diptera: Culicidae) by bioassays and in silico assays. Chemistry & Biodiversity. 2021, 18, e2100242. [Google Scholar]
- Dakpa, G.; Chiang, Y.-T.; Lin, L.-Y.; Tsao, N.-W.; Wang, C.-H.; Pérez-Sánchez, H.; et al. Essential oil-derived compounds target core fatigue-related genes: A network pharmacology and molecular Docking approach. PLoS One. 2025, 20, e0314125. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.; Song, Q. Lemongrass essential oil and its major component citronellol: evaluation of larvicidal activity and acetylcholinesterase inhibition against Anopheles sinensis. Parasitology Research. 2024, 123, 315. [Google Scholar] [CrossRef]
- de Souza, B.o.z.z.i. A. Theoretical investigation of the Pictet-Spengler reaction between dopamine and (S)-citronellal catalyzed by the enzyme (S)-norcoclaurine synthase. 2021.
- Dendera, W. Generation of a virtual library of terpenes using graph theory, and its application in exploration of the mechanisms of terpene biosynthesis: RHODES UNIVERSITY, SOUTH AFRICA; 2018.
- Patsilinakos, A.; Artini, M.; Papa, R.; Sabatino, M.; Božović, M.; Garzoli, S.; et al. Machine learning analyses on data including essential oil chemical composition and in vitro experimental antibiofilm activities against Staphylococcus species. Molecules. 2019, 24, 890. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I. Essential oil from Cymbopogon citratus exhibits “anti-aspergillosis” potential: in-silico molecular docking and in vitro studies. Bulletin of the National Research Centre. 2022, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Salaria, D.; Rolta, R.; Sharma, N.; Patel, C.N.; Ghosh, A.; Dev, K.; et al. In vitro and in silico antioxidant and anti-inflammatory potential of essential oil of Cymbopogon citratus (DC.) Stapf. of North-Western Himalaya. Journal of Biomolecular Structure and Dynamics. 2022, 40, 14131–14145. [Google Scholar] [CrossRef]
- Cardeal dos Santos, A.N.; Oliveira PEGd da Cruz Freire, J.E.; Araújo dos Santos, S.; Júnior, J.E.R.H.; Andrade CRd et, a.l. Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies. International Journal of Molecular Sciences. 2025, 26, 7671. [Google Scholar] [CrossRef]
- Liao, L.; Xie, M.; Zheng, X.; Zhou, Z.; Deng, Z.; Gao, J. Molecular insights fast-tracked: AI in biosynthetic pathway research. Natural Product Reports. 2025. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Shringi, H.; Efferth, T.; Ramamoorthy, S. Artificial intelligence driven approaches in phytochemical research: trends and prospects. Phytochemistry Reviews 2025, 1–16. [Google Scholar] [CrossRef]
- da Silva, A.J.A.; de Sousa Silveira, Z.; Macêdo, N.S.; dos Santos Barbosa, C.R.; da Silva Sousa, Â.E.; da Silva, T.F.; etal., *!!! REPLACE !!!*. Evaluation of the antibacterial and inhibitory activity of the NorA efflux pump in Staphylococcus aureus by citronellol. Biologia 2025, 1–15. [Google Scholar] [CrossRef]
- Wei, T.; Regeard, C.; Barroca-Aubry, N.; Roger, P.; Aymes-Chodur, C. Chemoenzymatic oxidation of citronellol and geraniol: Synthesis and antibacterial activity assessment. Colloids and Surfaces B: Biointerfaces. 2025, 253, 114723. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Lotfipoor, F.; Delazar, A.; Razavi, S.M.; Asnaashari, S.; Kasebi, N.; et al. Chemical composition, and antibacterial and free-radical-scavenging activities of the essential oils of a citronellol producing new chemotype of Thymus pubescens Boiss. & Kotschy ex Celak. Records of Natural Products. 2011, 5, 184. [Google Scholar]
- Zhang, J.; Liu, H.; Yao, J.; Ma, C.; Yang, W.; Lei, Z.; et al. Plant-derived citronellol can significantly disrupt cell wall integrity maintenance of Colletotrichum camelliae. Pesticide Biochemistry and Physiology. 2024, 204, 106087. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Sosinska, G.J.; De Groot, P.W.; Brul, S. Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS yeast research. 2009, 9, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Cleary, I.; MacGregor, N.; Saville, S.; Thomas, D. Investigating the function of Ddr48p in Candida albicans. Eukaryotic Cell. 2012, 11, 718–724. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryotic cell. 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Widiyarti G, Handayani S, Hanafi M, editors. Synthesis and cytotoxic activity of citronellol esters. AIP Conference Proceedings; 2018: AIP Publishing LLC.
- Mamur, S. Investigation of Cytotoxic Effect of Monoterpenes Beta-Citronellol and (-)-Menthone in Human Breast Cancer (MCF-7) Cell Line. Adnan Menderes Üniversitesi Sağlık Bilimleri Fakültesi Dergisi. 2019, 3, 111–119. [Google Scholar]
- Yu, W.-N.; Lai, Y.-J.; Ma, J.-W.; Ho, C.-T.; Hung, S.-W.; Chen, Y.-H.; et al. Citronellol induces necroptosis of human lung cancer cells via TNF-α pathway and reactive oxygen species accumulation. in vivo. 2019, 33, 1193–1201. [Google Scholar] [CrossRef]
- Brito, R.G.; Santos, P.L.; Prado, D.S.; Santana, M.T.; Araújo, A.A.; Bonjardim, L.R.; et al. Citronellol reduces orofacial nociceptive behaviour in mice–evidence of involvement of retrosplenial cortex and periaqueductal grey areas. Basic & clinical pharmacology & toxicology. 2013, 112, 215–221. [Google Scholar]
- Brito, R.G.; Guimarães, A.G.; Quintans, J.S.; Santos, M.R.; De Sousa, D.P.; Badaue-Passos Jr, D.; et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. Journal of natural medicines. 2012, 66, 637–644. [Google Scholar] [CrossRef]
- Srinivasan, S.; Muruganathan, U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chemico-biological interactions. 2016, 250, 38–46. [Google Scholar] [CrossRef]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; et al. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. Journal of Vector Ecology. 2009, 34, 2–8. [Google Scholar] [CrossRef]
- Revay, E.E.; Junnila, A.; Xue, R.-D.; Kline, D.L.; Bernier, U.R.; Kravchenko, V.D.; et al. Evaluation of commercial products for personal protection against mosquitoes. Acta tropica. 2013, 125, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Awaluddin, N.; Awaluddin, S.W.; Bachri, N.; Mointi, S.S. The Formulation of Reed Diffuser is A Combination of Cinnamon (Cinnamomomum Verum) and Citronella (Cymbopogon Nardus) Essential Oil as An Anti-Stress Aromatheraphy. Jurnal Penelitian Pendidikan IPA. 2023, 9, 1960–1967. [Google Scholar] [CrossRef]
- Ratnesakera, D.; Nayanthara, K. Efficacy of cinnamon and citronella oil vapours in the control of Callosobruchus chinensis L. in bulk stored green gram. Journal of Food and Agriculture 2013, 3. [Google Scholar] [CrossRef]
- Soares-Castro, P.; Soares, F.; Santos, P.M. Current advances in the bacterial toolbox for the biotechnological production of monoterpene-based aroma compounds. Molecules. 2020, 26, 91. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Zavala, A.; Diaz-Perez, C.; Cervantes, C.; Diaz-Perez, A.; Campos-Garcia, J. The atu and liu clusters are involved in the catabolic pathways for acyclic monoterpenes and leucine in Pseudomonas aeruginosa. Applied and environmental microbiology. 2006, 72, 2070–2079. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, G.B.; Kim, Y.; Lee, S.Y. Applications of artificial intelligence to enzyme and pathway design for metabolic engineering. Current Opinion in Biotechnology. 2022, 73, 101–107. [Google Scholar] [CrossRef]
- Vindman, C.; Trump, B.; Cummings, C.; Smith, M.; Titus, A.J.; Oye, K.; et al. The convergence of AI and synthetic biology: the looming deluge. arXiv preprint 2024, arXiv:240418973. [Google Scholar]
- Bolmanis, E.; Galvanauskas, V.; Grigs, O.; Vanags, J.; Kazaks, A. Leveraging Historical Process Data for Recombinant P. pastoris Fermentation Hybrid Deep Modeling and Model Predictive Control Development. Fermentation. 2025, 11, 411. [Google Scholar]
- Hjort, C.L.; Heum, H.E.B. Fermentation Prediction Through Machine Learning and Its Potential Use in Production Planning and Control: NTNU; 2023.
- Yee, C.S.; Zahia-Azizan, N.A.; Abd Rahim, M.H.; Mohd Zaini, N.A.; Raja-Razali, R.B.; Ushidee-Radzi, M.A.; et al. Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods. Fermentation. 2025, 11, 323. [Google Scholar] [CrossRef]
- Cai, S.; Mao, Z.; Wang, Z.; Yin, M.; Karniadakis, G.E. Physics-informed neural networks (PINNs) for fluid mechanics: A review. Acta Mechanica Sinica. 2021, 37, 1727–1738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




