Submitted:
31 August 2025
Posted:
01 September 2025
You are already at the latest version
Abstract
Keywords:
Background
Methods.
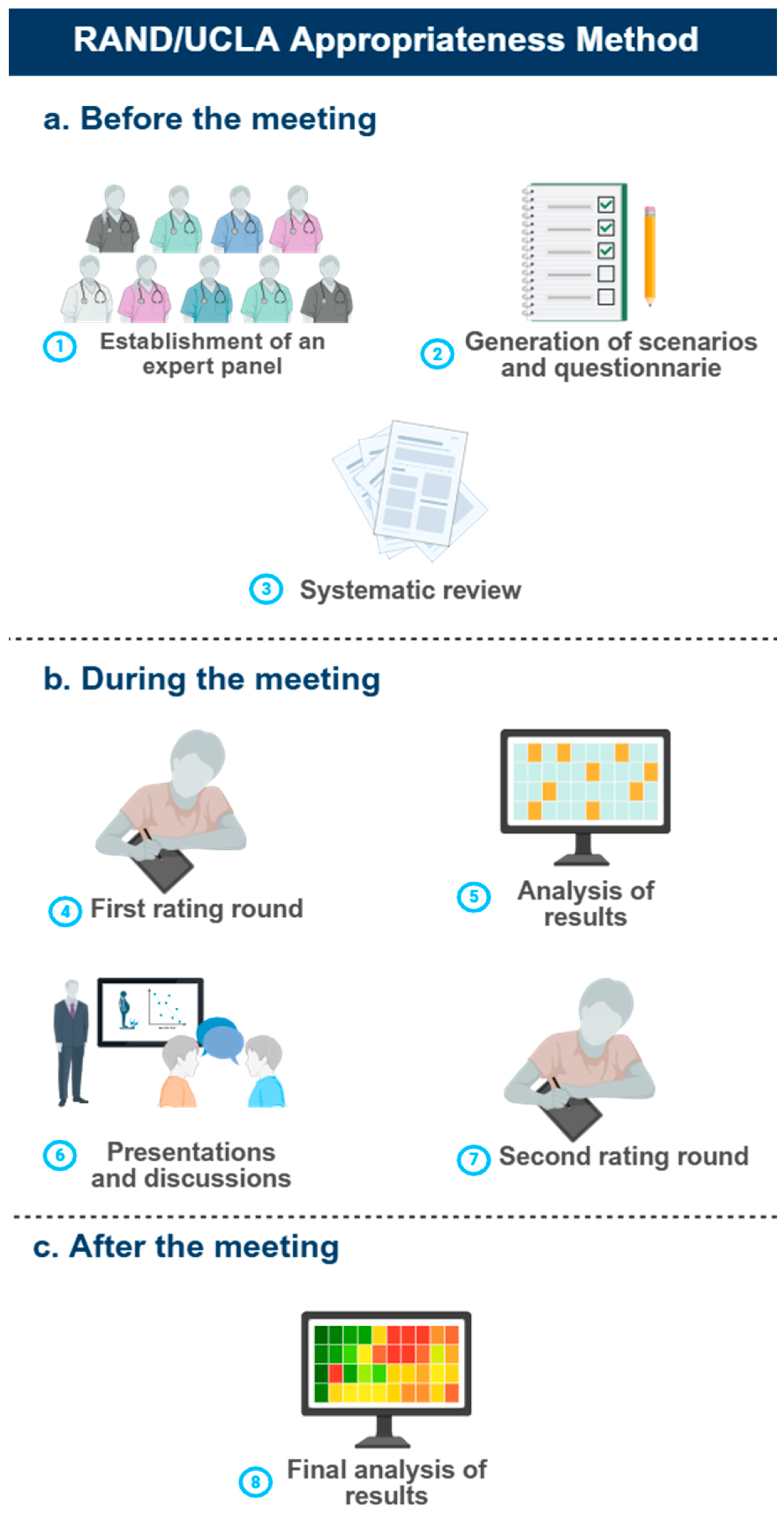
RAND/UCLA Appropriateness Method
Establishment of an Expert Panel
Generation of Scenarios
Systematic Review
Two-Round Consensus
Statistical Analysis
Ethical Compliance
Results
Summary of Participants and Answers
Appropriateness of the Intervention for Managing Obesity-Related Outcomes in Patients with Specific Comorbidities
| All patients with excess adiposity | Patients with excess adiposity and age 65 or more | Patients with excess adiposity receiving antidepressants | Patients with excess adiposity and anxiety disorder | Patients with excess adiposity and binge-eating disorder | Patients with excess adiposity and history of pancreatitis | Patients with excess adiposity and history of cholelithiasis | Patients with excess adiposity and Metabolic dysfunction–associated steatotic liver disease | Patients with excess adiposity and arrythmia | Patients with excess adiposity and heart failure | Patients with excess adiposity and hypertension | Patients with excess adiposity and high risk of cardiovascular disease | Patients with excess adiposity and chronic kidney disease | BMI | |
| Reducing excess adiposity | 9 | 9 | 9 | 8.5 | 8.5 | 8 | 8 | 9 | 9 | 9 | 9 | 8.5 | 8 | 25–29.9 kg/m² |
| 9 | 9 | 9 | 8.5 | 8.5 | 8 | 8 | 9 | 9 | 9 | 9 | 8.5 | 8 | 30–34.9 kg/m² | |
| 9 | 9 | 9 | 8.5 | 8 | 8 | 8 | 9 | 9 | 9 | 9 | 8.5 | 8 | 35–39.9 kg/m² | |
| 9 | 9 | 9 | 8.5 | 8 | 8 | 8 | 9 | 9 | 9 | 9 | 8.5 | 8 | >40 kg/m² | |
| Reducing waist circumference | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 9 | 9 | 9 | 9 | 9 | 25–29.9 kg/m² |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 9 | 9 | 9 | 9 | 9 | 30–34.9 kg/m² | |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 9 | 9 | 8.5 | 9 | 9 | 35–39.9 kg/m² | |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 9 | 9 | 9 | 9 | 9 | >40 kg/m² | |
| Reducing weight regain | 9 | 9 | 8.5 | 9 | 9 | 8 | 8 | 9 | 9 | 8 | 9 | 9 | 9 | 25–29.9 kg/m² |
| 9 | 9 | 8.5 | 9 | 9 | 8 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 30–34.9 kg/m² | |
| 9 | 9 | 8.5 | 9 | 9 | 8 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 35–39.9 kg/m² | |
| 9 | 9 | 8.5 | 9 | 9 | 8 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | >40 kg/m² | |
| Reducing low-grade chronic systemic inflammation and oxidative stress | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 25–29.9 kg/m² |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 30–34.9 kg/m² | |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 35–39.9 kg/m² | |
| 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | >40 kg/m² |
Appropriateness of the Intervention as an Adjunct to Conventional Pharmacotherapy
| Cardiovascular | Musculoskeletal | Reproductive | Metabolic | Daily-life limitations | Hepatic | Respiratory | BMI | |
| GLP-1 RA | 8 | 8 | 8.5 | 8 | 8 | 8.5 | 8 | 25–29.9 kg/m² |
| 8 | 8 | 8.5 | 8 | 8 | 8.5 | 8 | 30–34.9 kg/m² | |
| 8 | 8 | 8.5 | 8 | 8 | 8.5 | 8 | 35–39.9 kg/m² | |
| 8 | 8 | 8.5 | 8 | 8 | 8.5 | 8 | >40 kg/m² | |
| Naltrexone/Bupropion | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 25–29.9 kg/m² |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 30–34.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 35–39.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | >40 kg/m² | |
| Orlistat | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 25–29.9 kg/m² |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 30–34.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 35–39.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | >40 kg/m² | |
| Phentermine | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 25–29.9 kg/m² |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 30–34.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | 35–39.9 kg/m² | |
| 8 | 8 | 8 | 8 | 8 | 8 | 8 | >40 kg/m² |
Appropriateness of the Intervention in a Set of Special Clinical Situations
| BMI | ||
| Prevention of progression to clinical obesity | 8 | 25–29.9 kg/m² |
| 8 | 30–34.9 kg/m² | |
| 8 | 35–39.9 kg/m² | |
| 8 | >40 kg/m² | |
| GLP-1 RA dose reduction | 9 | 25–29.9 kg/m² |
| 9 | 30–34.9 kg/m² | |
| 9 | 35–39.9 kg/m² | |
| 9 | >40 kg/m² | |
| Maintenance of low-dose GLP-1 RA receptor agonist therapy | 9 | 25–29.9 kg/m² |
| 9 | 30–34.9 kg/m² | |
| 9 | 35–39.9 kg/m² | |
| 9 | >40 kg/m² | |
| Prior to initiating pharmacological treatment or bariatric surgery | 8.5 | 25–29.9 kg/m² |
| 8.5 | 30–34.9 kg/m² | |
| 8.5 | 35–39.9 kg/m² | |
| 8.5 | >40 kg/m² | |
| Prevention of weight regain | 9 | 25–29.9 kg/m² |
| 9 | 30–34.9 kg/m² | |
| 9 | 35–39.9 kg/m² | |
| 9 | >40 kg/m² | |
| Transition to a medication-free period | 9 | 25–29.9 kg/m² |
| 9 | 30–34.9 kg/m² | |
| 9 | 35–39.9 kg/m² | |
| 9 | >40 kg/m² |
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonist |
| GIP | Glucose-dependent insulinotropic polypeptide |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| PICO | Population, Intervention, Comparison, Outcome |
| PICOS | Population, Intervention, Comparison, Outcome, Study design |
| RAND/UCLA | RAND/University of California, Los Angeles (Appropriateness Method) |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor-alpha |
| POMC | Pro-opiomelanocortin |
| S. cerevisiae | Saccharomyces cerevisiae |
References
- Rubino F, Cummings DE, Eckel RH, et al.: Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. Published Online First: 9 January 2025. [CrossRef]
- Gaskin CJ, Cooper K, Stephens LD, Peeters A, Salmon J, Porter J: Clinical practice guidelines for the management of overweight and obesity published internationally: A scoping review. Obesity Reviews. 2024, 25. [CrossRef]
- Ng M, Gakidou E, Lo J, et al.: Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021. The Lancet. 2025, 405:813–38. [CrossRef]
- Chávez-Manzanera EA, Vera-Zertuche JM, Kaufer-Horwitz M, et al.: Mexican Clinical Practice Guidelines for Adult Overweight and Obesity Management. Curr Obes Rep. 2024. [CrossRef]
- Shi Q, Wang Y, Hao Q, et al.: Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. The Lancet. 2024, 403:e21–31. [CrossRef]
- van Baak MA, Mariman ECM: Physiology of Weight Regain after Weight Loss: Latest Insights. Curr Obes Rep. 2025, 14. [CrossRef]
- Alali M, Alqubaisy M, Aljaafari MN, et al.: Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules. 2021, 26. [CrossRef]
- Puri V, Nagpal M, Singh I, et al.: A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients. 2022, 14. [CrossRef]
- Mancebo-Molina R, Castañe-Sitjas FX, Cuñé-Castellana J, et al.: LIGANDO DE GRASAS OBTEN IDO A PARTIR DE LA BIOMASA DEL PROCEDIMIENTO DE ELABORACIÓN DE LA CERVEZA. (2014). , 1–52.
- Jung EY, Lee JW, Hong YH, Chang UJ, Suh HJ: Low dose yeast hydrolysate in treatment of obesity and weight loss. Prev Nutr Food Sci. 2017, 22:45–9. [CrossRef]
- Valero-Pérez M, Bermejo LM, López-Plaza B, García MA, Palma-Milla S, Gómez-Candela C: Regular consumption of LIPIGO® promotes the reduction of body weight and improves the rebound effect of obese people undergo a comprehensive weight loss program. Nutrients. 2020, 12:1–14. [CrossRef]
- Santas J, Lázaro E, Cuñé J: Effect of a polysaccharide-rich hydrolysate from Saccharomyces cerevisiae (LipiGo®) in body weight loss: randomised, double-blind, placebo-controlled clinical trial in overweight and obese adults. J Sci Food Agric. 2017, 97:4250–7. [CrossRef]
- Batsis JA, Apolzan JW, Bagley PJ, et al.: A Systematic Review of Dietary Supplements and Alternative Therapies for Weight Loss. Obesity. 2021, 29:1102–13. [CrossRef]
- Fitch Kathryn: The Rand/UCLA appropriateness method user’s manual. Rand; 2001.
- Masuda E, Ozsvath K, Vossler J, et al.: The 2020 appropriate use criteria for chronic lower extremity venous disease of the American Venous Forum, the Society for Vascular Surgery, the American Vein and Lymphatic Society, and the Society of Interventional Radiology. J Vasc Surg Venous Lymphat Disord. 2020, 8:505-525.e4. [CrossRef]
- Woo K, Ulloa J, Allon M, et al.: Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg. 2017, 65:1089-1103.e1. [CrossRef]
- Saust LT, Siersma VD, Bjerrum L, Hansen MP: Development of quality indicators for the diagnosis and treatment of urinary tract infections in general practice: a RAND appropriateness method. BMJ Open Qual. 2023, 12:e002156. [CrossRef]
- Broder MS, Gibbs SN, Yermilov I: An Adaptation of the RAND/UCLA Modified Delphi Panel Method in the Time of COVID-19. J Healthc Leadersh. 2022, Volume 14:63–70. [CrossRef]
- Carson-Stevens A, Campbell S, Bell BG, et al.: Identifying ‘avoidable harm’ in family practice: a RAND/UCLA Appropriateness Method consensus study. BMC Fam Pract. 2019, 20:134. [CrossRef]
- Saavedra-Fuentes N, Carmona-Montesinos E, Castañeda-Hernández G, et al.: Appropriateness of Ketoanalogues of Amino Acids, Calcium Citrate, and Inulin Supplementation for CKD Management: A RAND/UCLA Consensus. Nutrients. 2024, 16:2930. [CrossRef]
- Grunvald E, Shah R, Hernaez R, et al.: AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. 2022, 163:1198–225. [CrossRef]
- Congressional Budget Office: How Would Authorizing Medicare to Cover Anti-Obesity Medications Affect the Federal Budget? Washington, D.C.: Congressional Budget Office. (2024). Accessed: April 9, 2025. https://www.cbo.gov/publication/60816.
- Hong KB, Jung EY, Kim JH, Chang UJ, Suh HJ: Yeast hydrolysate as a functional anti-obesity ingredient: appetite suppressive effects of yeast hydrolysate in food deprived mice. Progress in Nutrition. 2015, 17:262–4.
- Jung EY, Cho MK, Hong YH, Kim JH, Park Y, Chang UJ, Suh HJ: Yeast hydrolysate can reduce body weight and abdominal fat accumulation in obese adults. Nutrition. 2014, 30:25–32. [CrossRef]
- Mosikanon K, Arthan D, Kettawan A, Tungtrongchitr R, Prangthip P: Yeast β–Glucan Modulates Inflammation and Waist Circumference in Overweight and Obese Subjects. J Diet Suppl. 2017, 14:173–85. [CrossRef]
- Bertuccioli A, Cardinali M, Biagi M, Moricoli S, Morganti I, Zonzini GB, Rigillo G: Nutraceuticals and herbal food supplements for weight loss: Is there a prebiotic role in the mechanism of action? Microorganisms. 2021, 9. [CrossRef]
- Bays H: Phentermine, topiramate and their combination for the treatment of adiposopathy ('sick fat) and metabolic disease. Expert Rev Cardiovasc Ther. 2010, 8:1777–801. [CrossRef]
- Grilo CM, Lydecker JA, Morgan PT, Gueorguieva R: Naltrexone + Bupropion Combination for the Treatment of Binge-eating Disorder with Obesity: A Randomized, Controlled Pilot Study. Clin Ther. 2021, 43:112-122.e1. [CrossRef]
| Parameters | Inclusion Criteria |
| Population | Overweight or obese patients |
| Intervention | Bioactive peptide-rich Saccharomyces cerevisiae hydrolysates + lifestyle modifications (medical nutrition therapy, structured exercise, sleep hygiene, and stress management) |
| Comparison | Placebo |
| Outcomes | Changes in body weight, fat composition/mass, BMI, and abdominal perimeter |
| Study Design | Blinded, randomized, placebo-controlled clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





