Submitted:
26 November 2025
Posted:
27 November 2025
You are already at the latest version
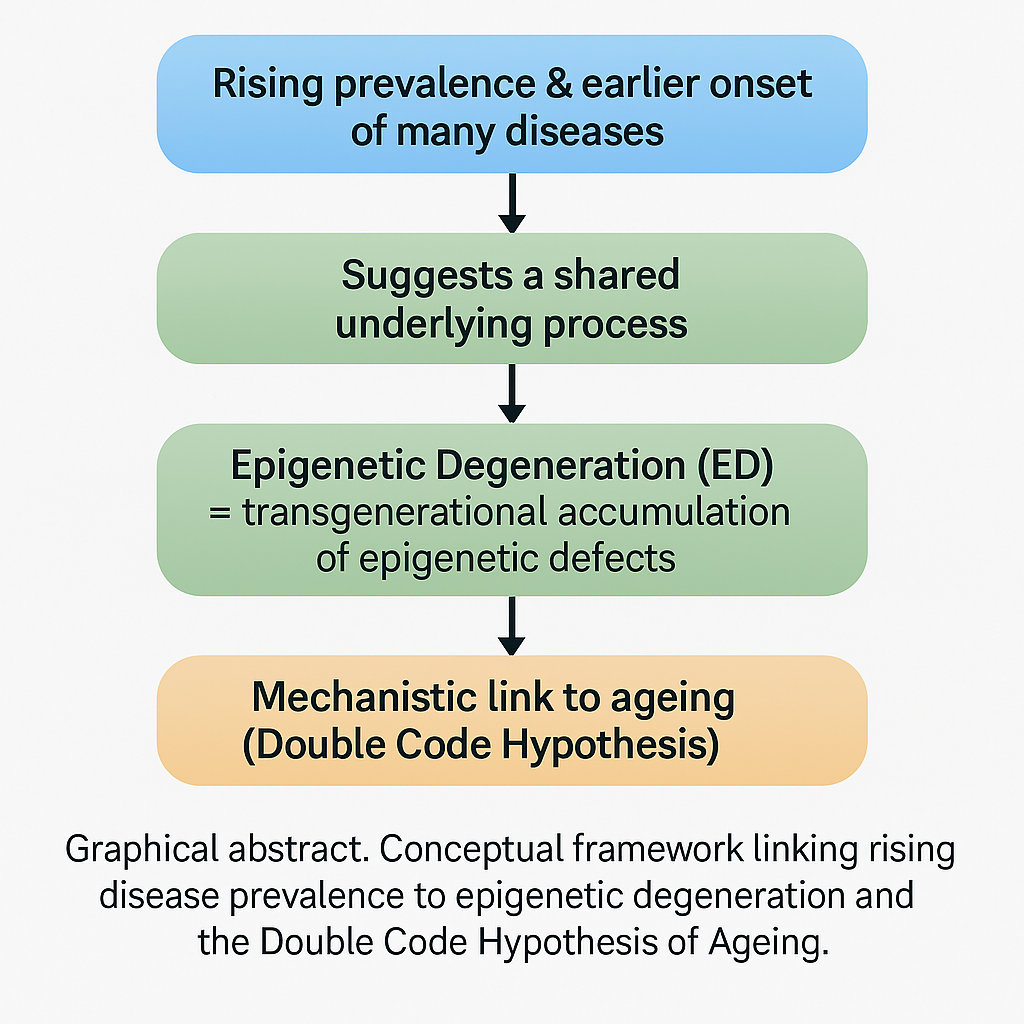
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Global Trend Lines Determination
2.2. Statistical Analysis
- Comparison of Temporal Prevalence Dynamics Across Mental and Physical Conditions (Figure 4)

- Geometric-Mean Aggregation
- Progressive Filtering Thresholds
- ANCOVA Analysis
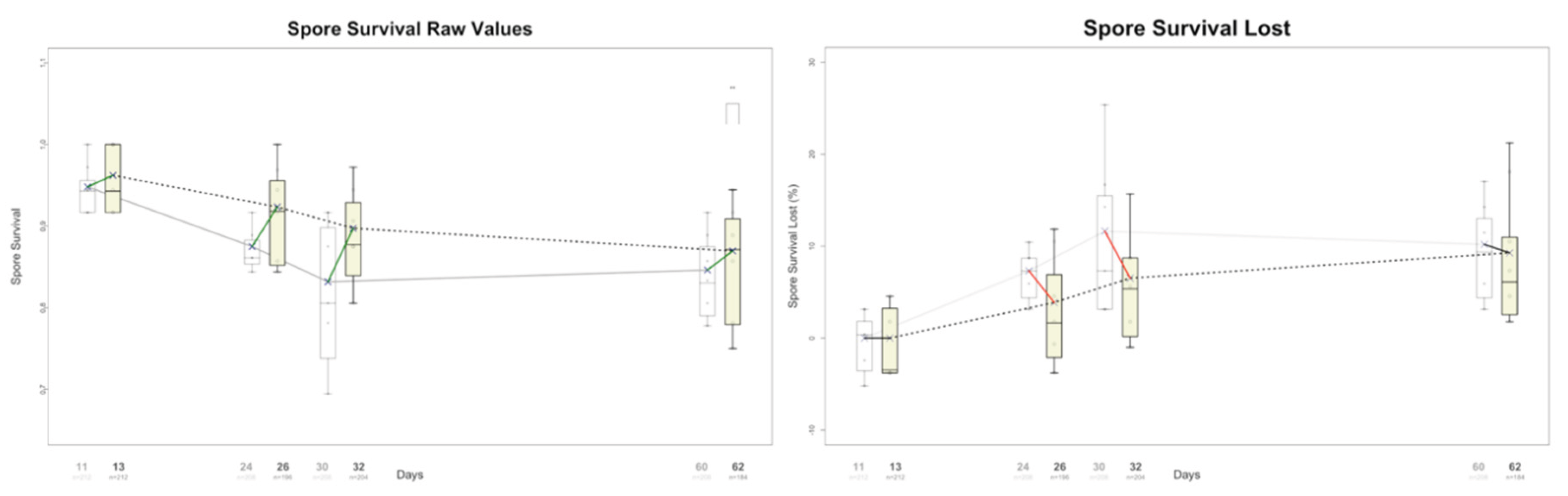
- Control Analysis Removing Deterministic Early Monotonic Stretches (Supplementary Figure S1)
3. Results
3.1. Evidence of Population-Based Epigenetic Degeneration in Human Populations
3.1.1. Examples of Epigenetic Degeneration in Human Populations: Somatic Diseases
3.1.2. Examples of Epigenetic Degeneration: Mental Health Issues
3.2. The Comorbidities Snowball and the Ladder Effect
3.2.1. The Comorbidities Snowball
3.2.2. The Ladder Effect
4. Discussion
4.1. Different Explanations, One Common Root
4.2. The Common Root’s Uncomfortable Implication
4.3. The Comorbidities Snowball Nature of Multimorbidity
4.4. The Principle of Pre-Determined Post-Processing
4.4.1. Multimorbidity: The Signature of Inheritance
4.4.2. Beyond the Principle of Labelled Lines
4.5. Evolutionary Consideration
4.6. Epidemiological Considerations
4.7. Social Considerations
Supplementary Materials
Author Contributions
Funding
Competing interests
References
- X. Marsellach, Ageing is not just ageing Zenodo (2025).
- X. Marsellach, The Principle of Continuous Biological Information Flow as the Fundamental Foundation for the Biological Sciences. Implications for Ageing Research Preprints (2021).
- X. Marsellach, A non-Lamarckian model for the inheritance of the epigenetically-coded phenotypic characteristics: a new paradigm for Genetics, Genomics and, above all, Ageing studies bioRxiv (2018).
- X. Marsellach, A non-genetic meiotic repair program inferred from spore survival values in fission yeast wild isolates: a clue for an epigenetic ratchet-like model of ageing bioRxiv (2017).
- A. Korolenko, M. K. Skinner, Generational stability of epigenetic transgenerational inheritance facilitates adaptation and evolution Epigenetics 19, 2380929 (2024).
- G. Witzany, Ed., Epigenetics in Biological Communication. (Springer Nature Switzerland, Cham, 2024).
- O. Karin, E. A. Miska, B. D. Simons, Epigenetic inheritance of gene silencing is maintained by a self-tuning mechanism based on resource competition Cell Systems 14, 24–40. e11 (2023).
- Y. Takahashi, M. Morales Valencia, Y. Yu, Y. Ouchi, K. Takahashi, M. N. Shokhirev, K. Lande, A. E. Williams, C. Fresia, M. Kurita, T. Hishida, K. Shojima, F. Hatanaka, E. Nuñez-Delicado, C. R. Esteban, J. C. Izpisua Belmonte, Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice Cell 186, 715–731.e19 (2023).
- E. Jablonka, M. J. Lamb, Inheritance Systems and the Extended Evolutionary Synthesis (Cambridge University Press, Cambridge, 2020).
- E. Jablonka, M. J. Lamb, Evolution in four dimensions, revised edition: Genetic, epigenetic, behavioral, and symbolic variation in the history of life (MIT press, 2014).
- S. Gissis, S. B. Gissis, E. Jablonka, A. Zeligowski, Transformations of Lamarckism: from subtle fluids to molecular biology (MIT press, 2011).
- E. Jablonka, M. J. Lamb, Epigenetic inheritance and evolution: the Lamarckian dimension (Oxford University Press, 1995).
- C. Spadafora, Transgenerational epigenetic reprogramming of early embryos: a mechanistic model Environ Epigenet 6, dvaa009 (2020).
- M. K. Skinner, Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept that Facilitates Neo-Darwinian Evolution Genome Biol Evol 7, 1296–1302 (2015).
- A. Surachman, E. Hamlat, A. S. Zannas, S. Horvath, B. Laraia, E. Epel, Grandparents’ educational attainment is associated with grandchildren’s epigenetic-based age acceleration in the National Growth and Health Study Soc Sci Med 355, 117142 (2024).
- E. D. Watson, “Epigenetics of transgenerational inheritance of disease” in Epigenetics in Human Disease, Ed. (Elsevier, 2024), pp. 989–1030.
- J. Švorcová, Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals Genes (Basel) 14, 120 (2023).
- N. Deshe, Y. Eliezer, L. Hoch, E. Itskovits, E. Bokman, S. Ben-Ezra, A. Zaslaver, Inheritance of associative memories and acquired cellular changes in C. elegans Nat Commun 14, 4232 (2023).
- G. Sabarís, M. H. Fitz-James, G. Cavalli, Epigenetic inheritance in adaptive evolution Ann N Y Acad Sci 1524, 22–29 (2023).
- N. Liberman, M. H. Rothi, M. V. Gerashchenko, C. Zorbas, K. Boulias, F. G. MacWhinnie, A. K. Ying, A. Flood Taylor, J. Al Haddad, H. Shibuya, L. Roach, A. Dong, S. Dellacona, D. L. J. Lafontaine, V. N. Gladyshev, E. L. Greer, 18S rRNA methyltransferases DIMT1 and BUD23 drive intergenerational hormesis Mol Cell 83, 3268–3282.e7 (2023).
- L. L. Schmitz, V. Duque, In utero exposure to the Great Depression is reflected in late-life epigenetic aging signatures Proc Natl Acad Sci U S A 119, e2208530119 (2022).
- A. Lempradl, Germ cell-mediated mechanisms of epigenetic inheritance Semin Cell Dev Biol 97, 116–122 (2020).
- P. Norouzitallab, K. Baruah, D. Vanrompay, P. Bossier, Can epigenetics translate environmental cues into phenotypes Sci Total Environ 647, 1281–1293 (2019).
- Y. Wang, H. Liu, Z. Sun, Lamarck rises from his grave: parental environment-induced epigenetic inheritance in model organisms and humans Biol Rev Camb Philos Soc 92, 2084–2111 (2017).
- X. Marsellach, The Double Code Hypothesis of Ageing Preprints (2025).
- H. Ledford, Why are so many young people getting cancer? What the data say Nature 627, 258–260 (2024).
- A. Licari, P. Magri, A. De Silvestri, A. Giannetti, C. Indolfi, F. Mori, G. L. Marseglia, D. Peroni, Epidemiology of Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis J Allergy Clin Immunol Pract 11, 2547–2556 (2023).
- J. W. van Straalen, S. de Roock, G. Giancane, E. Alexeeva, E. Koskova, P. Mesa-Del-Castillo Bermejo, F. Zulian, A. Civino, D. Montin, N. M. Wulffraat, N. Ruperto, J. F. Swart, P. R. I. N. T. O. Paediatric Rheumatology International Trials Organisation, Prevalence of familial autoimmune diseases in juvenile idiopathic arthritis: results from the international Pharmachild registry Pediatr Rheumatol Online J 20, 103 (2022).
- C. Moscheo, M. Licciardello, P. Samperi, M. La Spina, A. Di Cataldo, G. Russo, New Insights into Iron Deficiency Anemia in Children: A Practical Review Metabolites 12, 289 (2022).
- M. S. Lo, “Lupus in children” in Systemic Lupus Erythematosus, Ed. (Elsevier, 2021), pp. 527–533.
- Y. Sahin, Celiac disease in children: A review of the literature World J Clin Pediatr 10, 53–71 (2021).
- K. Wong, A. Dahlmann-Noor, Myopia and its progression in children in London, UK: a retrospective evaluation J Optom 13, 146–154 (2020).
- S. M. Langan, A. D. Irvine, S. Weidinger, Atopic dermatitis Lancet 396, 345–360 (2020).
- P. Song, Y. Zhang, J. Yu, M. Zha, Y. Zhu, K. Rahimi, I. Rudan, Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis JAMA Pediatr 173, 1154–1163 (2019).
- N. Lascar, J. Brown, H. Pattison, A. H. Barnett, C. J. Bailey, S. Bellary, Type 2 diabetes in adolescents and young adults The lancet Diabetes & endocrinology 6, 69–80 (2018).
- C. C. Caldwell, S. K. Saikaly, R. P. Dellavalle, J. A. Solomon, Prevalence of pediatric alopecia areata among 572,617 dermatology patients Journal of the American Academy of Dermatology 77, 980–981 (2017).
- I. N. Ackerman, J. L. Kemp, K. M. Crossley, A. G. Culvenor, R. S. Hinman, Hip and Knee Osteoarthritis Affects Younger People, Too J Orthop Sports Phys Ther 47, 67–79 (2017).
- K. Sahoo, B. Sahoo, A. K. Choudhury, N. Y. Sofi, R. Kumar, A. S. Bhadoria, Childhood obesity: causes and consequences J Family Med Prim Care 4, 187–192 (2015).
- I. Asher, N. Pearce, Global burden of asthma among children Int J Tuberc Lung Dis 18, 1269–1278 (2014).
- C. C. Patterson, G. G. Dahlquist, E. Gyürüs, A. Green, G. Soltész, EURODIAB Study Group, Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study Lancet 373, 2027–2033 (2009).
- A. M. Branum, S. L. Lukacs, Food allergy among children in the United States Pediatrics 124, 1549–1555 (2009).
- GBD 2021 Diseases and Injuries Collaborators, Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021 Lancet 403, 2133–2161 (2024).
- I. R. Hambleton, R. Caixeta, S. M. Jeyaseelan, S. Luciani, A. J. M. Hennis, The rising burden of non-communicable diseases in the Americas and the impact of population aging: a secondary analysis of available data Lancet Reg Health Am 21, 100483 (2023).
- P. Charalampous, V. Gorasso, D. Plass, S. M. Pires, E. von der Lippe, A. Mereke, J. Idavain, K. Kissimova-Skarbek, J. N. Morgado, C. H. Ngwa, I. Noguer, A. Padron-Monedero, M. J. Santi-Cano, R. Sarmiento, B. Devleesschauwer, J. A. Haagsma, COST Action CA18218 Participants, Burden of non-communicable disease studies in Europe: a systematic review of data sources and methodological choices Eur J Public Health 32, 289–296 (2022).
- P. J. Hotez, L. Peiperl, Noncommunicable Diseases: A Globalization of Disparity? PLoS Med 12, e1001859 (2015).
- I. M. Michalek, P. Koczkodaj, M. Michalek, F. L. Caetano Dos Santos, Unveiling the silent crisis: global burden of suicide-related deaths among children aged 10-14 years World J Pediatr (2024).
- T. Gupta, P. Gehlawat, “Assessment and Management of Emerging Personality Disorders in Adolescents” Eds. 2023), pp. 255–267.
- C. F. Sun, H. Xie, V. Metsutnan, J. H. Draeger, Y. Lin, M. S. Hankey, A. S. Kablinger, The mean age of gender dysphoria diagnosis is decreasing Gen Psychiatr 36, e100972 (2023).
- K. A. Shaw, D. McArthur, M. M. Hughes, A. V. Bakian, L.-C. Lee, S. Pettygrove, M. J. Maenner, Progress and disparities in early identification of autism spectrum disorder: autism and developmental disabilities monitoring network, 2002-2016 Journal of the American Academy of Child & Adolescent Psychiatry 61, 905–914 (2022).
- S. Steinsbekk, B. Ranum, L. Wichstrøm, Prevalence and course of anxiety disorders and symptoms from preschool to adolescence: a 6-wave community study Journal of Child Psychology and Psychiatry 63, 527–534 (2022).
- S. Hendriks, K. Peetoom, C. Bakker, R. Koopmans, W. van der Flier, J. Papma, F. Verhey, D. E. S. G. Young-Onset, M. de Vugt, S. Köhler, Global incidence of young-onset dementia: A systematic review and meta-analysis Alzheimers Dement (2022).
- J. Tkacz, B. L. Brady, Increasing rate of diagnosed childhood mental illness in the United States: Incidence, prevalence and costs Public Health Pract (Oxf) 2, 100204 (2021).
- M. K. Singh, R. M. Post, D. J. Miklowitz, B. Birmaher, E. Youngstrom, B. Goldstein, C. Soutullo, D. Axelson, K. D. Chang, M. P. DelBello, A commentary on youth onset bipolar disorder Bipolar Disord 23, 834–837 (2021).
- J. M. Twenge, A. B. Cooper, T. E. Joiner, M. E. Duffy, S. G. Binau, Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017 J Abnorm Psychol 128, 185–199 (2019).
- C. Hollis, Schizophrenia in children and adolescents BJPsych Advances 21, 333–341 (2015).
- D. E. Nicholls, R. Lynn, R. M. Viner, Childhood eating disorders: British national surveillance study Br J Psychiatry 198, 295–301 (2011).
- PLOS Medicine Editors, Multimorbidity: Addressing the next global pandemic PLoS Med 20, e1004229 (2023).
- S. R. Chowdhury, D. Chandra Das, T. C. Sunna, J. Beyene, A. Hossain, Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis EClinicalMedicine 57, 101860 (2023).
- C. J. M. Whitty, C. MacEwen, A. Goddard, D. Alderson, M. Marshall, C. Calderwood, F. Atherton, M. McBride, J. Atherton, H. Stokes-Lampard, W. Reid, S. Powis, C. Marx, Rising to the challenge of multimorbidity BMJ 368, l6964 (2020).
- D. M. Carlson, B. C. Yarns, Managing medical and psychiatric multimorbidity in older patients Ther Adv Psychopharmacol 13, 20451253231195274 (2023).
- A. Wędrychowicz, M. Minasyan, A. Pietraszek, J. Centkowski, M. Stręk, J. Różańska, K. Chełmecka, B. Zdzierak, M. Wilk, P. Czekańska, P. Pacut, Z. Grzenda-Adamek, J. Małek, M. Ciechanowska, M. Stelmach, J. Nazim, J. B Starzyk, Increased prevalence of celiac disease and its clinical picture among patients with diabetes mellitus type 1 - observations from a single pediatric center in Central Europe Pediatr Endocrinol Diabetes Metab 27, 1–6 (2021).
- L. P. Boulet, Obesity and atopy Clin Exp Allergy 45, 75–86 (2015).
- F. Cristofori, C. Fontana, A. Magistà, T. Capriati, F. Indrio, S. Castellaneta, L. Cavallo, R. Francavilla, Increased prevalence of celiac disease among pediatric patients with irritable bowel syndrome: a 6-year prospective cohort study JAMA Pediatr 168, 555–560 (2014).
- N. R. Lee, B. K. Kim, N. Y. Yoon, S. Y. Lee, S. Y. Ahn, W. S. Lee, Differences in Comorbidity Profiles between Early-Onset and Late-Onset Alopecia Areata Patients: A Retrospective Study of 871 Korean Patients Ann Dermatol 26, 722–726 (2014).
- J. L. Friedlander, W. J. Sheehan, S. N. Baxi, L. S. Kopel, J. M. Gaffin, A. Ozonoff, C. Fu, D. R. Gold, W. Phipatanakul, Food Allergy and Increased Asthma Morbidity in a School-Based Inner-City Asthma Study The Journal of Allergy and Clinical Immunology: In Practice 1, 479–484 (2013).
- R. Kramer, C. M. Aarnio-Peterson, L. A. Conard, K. R. Lenz, A. Matthews, Eating disorder symptoms among transgender and gender diverse youth Clin Child Psychol Psychiatry 29, 30–44 (2024).
- S. Chidambaram, M. C. Theni, K. Kaliappan, L. Earnesteen, J. Jeganathan, M. C. Madurai, S. M. C. Government, M. C. Coimbatore, The Prevalence of Depressive Symptoms in Schizophrenia: A Cross-Sectional Study Path of Science 9, 4001–4006 (2023).
- A. M. Rahnejat, M. Babaie, M. Ebrahimi, R. Sepahvand, A. Taghva, H. S. Barzoki, M. Ghasemzadeh, The prevalence of cluster B personality disorder in young male homosexuals (2022).
- E. Hisle-Gorman, C. A. Landis, A. Susi, N. A. Schvey, G. H. Gorman, C. M. Nylund, D. A. Klein, Gender Dysphoria in Children with Autism Spectrum Disorder LGBT Health 6, 95–100 (2019).
- R. George, M. A. Stokes, Sexual Orientation in Autism Spectrum Disorder Autism Research 11, 133–141 (2018).
- J. Matsuo, Y. Kamio, H. Takahashi, M. Ota, T. Teraishi, H. Hori, A. Nagashima, R. Takei, T. Higuchi, N. Motohashi, H. Kunugi, Autistic-like traits in adult patients with mood disorders and schizophrenia PLoS One 10, e0122711 (2015).
- D. F. Connor, L. A. Doerfler, ADHD with comorbid oppositional defiant disorder or conduct disorder: discrete or nondistinct disruptive behavior disorders J Atten Disord 12, 126–134 (2008).
- R. S. McIntyre, J. K. Soczynska, A. Bottas, K. Bordbar, J. Z. Konarski, S. H. Kennedy, Anxiety disorders and bipolar disorder: a review Bipolar Disorders 8, 665–676 (2006).
- A. R. Mayer, D. S. Kosson, Handedness and Psychopathy Cognitive and Behavioral Neurology 13, (2000).
- T. D. Wade, C. M. Bulik, M. Neale, K. S. Kendler, Anorexia nervosa and major depression: shared genetic and environmental risk factors Am J Psychiatry 157, 469–471 (2000).
- J. M. Bailey, Homosexuality and mental illness Archives of general psychiatry 56, 883–884 (1999).
- P. Satz, M. F. Green, Atypical handedness in schizophrenia: some methodological and theoretical issues Schizophr Bull 25, 63–78 (1999).
- G. Langs, F. Quehenberger, K. Fabisch, G. Klug, H. Fabisch, H. G. Zapotoczky, Prevalence, patterns and role of personality disorders in panic disorder patients with and without comorbid (lifetime) major depression Acta Psychiatrica Scandinavica 98, 116–123 (1998).
- M. Adesuyan, Y. H. Jani, D. Alsugeir, R. Howard, I. C. K. Wong, L. Wei, R. Brauer, Trends in the incidence of dementia in people with hypertension in the UK 2000 to 2021 Alzheimers Dement (Amst) 15, e12466 (2023).
- D. Ciciulla, V. X. Soriano, V. McWilliam, J. J. Koplin, R. L. Peters, Systematic Review of the Incidence and/or Prevalence of Eating Disorders in Individuals With Food Allergies J Allergy Clin Immunol Pract 11, 2196–2207.e13 (2023).
- N. Launders, L. Kirsh, D. P. J. Osborn, J. F. Hayes, The temporal relationship between severe mental illness diagnosis and chronic physical comorbidity: a UK primary care cohort study of disease burden over 10 years Lancet Psychiatry 9, 725–735 (2022).
- A. Annamalai, U. Kosir, C. Tek, Prevalence of obesity and diabetes in patients with schizophrenia World J Diabetes 8, 390–396 (2017).
- A. Lopez-de-Andrés, M. I. Jiménez-Trujillo, V. Hernández-Barrera, J. M. de Miguel-Yanes, M. Méndez-Bailón, N. Perez-Farinos, C. de Burgos Lunar, J. Cárdenas-Valladolid, M. Á. Salinero-Fort, R. Jiménez-García, P. Carrasco-Garrido, Trends in the prevalence of depression in hospitalized patients with type 2 diabetes in Spain: analysis of hospital discharge data from 2001 to 2011 PLoS One 10, e0117346 (2015).
- A. Raevuori, J. Haukka, O. Vaarala, J. M. Suvisaari, M. Gissler, M. Grainger, M. S. Linna, J. T. Suokas, The increased risk for autoimmune diseases in patients with eating disorders PLoS One 9, e104845 (2014).
- L. B. Ceretta, G. Z. Réus, H. M. Abelaira, L. K. Jornada, M. T. Schwalm, N. J. Hoepers, C. D. Tomazzi, K. G. Gulbis, R. A. Ceretta, J. Quevedo, Increased prevalence of mood disorders and suicidal ideation in type 2 diabetic patients Acta Diabetol 49 Suppl 1, S227–34 (2012).
- A. Darby, P. Hay, J. Mond, F. Quirk, P. Buttner, L. Kennedy, The rising prevalence of comorbid obesity and eating disorder behaviors from 1995 to 2005 Int J Eat Disord 42, 104–108 (2009).
- J. Janson, T. Laedtke, J. E. Parisi, P. O’Brien, R. C. Petersen, P. C. Butler, Increased risk of type 2 diabetes in Alzheimer disease Diabetes 53, 474–481 (2004).
- R. J. Anderson, K. E. Freedland, R. E. Clouse, P. J. Lustman, The prevalence of comorbid depression in adults with diabetes: a meta-analysis Diabetes Care 24, 1069–1078 (2001).
- D. L. Morris, S. M. Montgomery, M. L. Galloway, R. E. Pounder, A. J. Wakefield, Inflammatory bowel disease and laterality: is left handedness a risk Gut 49, 199–202 (2001).
- L. Wu, Y. He, B. Jiang, D. Sun, J. Wang, M. Liu, S. Yang, Y. Wang, Trends in prevalence, awareness, treatment and control of hypertension during 2001-2010 in an urban elderly population of China PloS one 10, e0132814 (2015).
- M. Ten Have, R. Verheul, A. Kaasenbrood, S. van Dorsselaer, M. Tuithof, M. Kleinjan, R. de Graaf, Prevalence rates of borderline personality disorder symptoms: a study based on the Netherlands Mental Health Survey and Incidence Study-2 BMC Psychiatry 16, 249 (2016).
- H. Haller, H. Cramer, R. Lauche, F. Gass, G. J. Dobos, The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review BMC Psychiatry 14, 128 (2014).
- R. Isomaa, A. L. Isomaa, M. Marttunen, R. Kaltiala-Heino, K. Björkqvist, The prevalence, incidence and development of eating disorders in Finnish adolescents: a two-step 3-year follow-up study Eur Eat Disord Rev 17, 199–207 (2009).
- S. A. Swanson, S. J. Crow, D. Le Grange, J. Swendsen, K. R. Merikangas, Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement Arch Gen Psychiatry 68, 714–723 (2011).
- W. H. Organization, Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (2011).
- American Diabetes Association, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020 Diabetes Care 43, S14–S31 (2020).
- S. Fernandes, Prediction of a rise in antisocial personality disorder through cross-generational analysis Prediction of an Increase in Personality Disorder (2019).
- J. Coid, S. Ullrich, Antisocial personality disorder is on a continuum with psychopathy Compr Psychiatry 51, 426–433 (2010).
- L. K. Twells, I. Janssen, J. L. Kuk, “Epidemiology of Adult Obesity” Eds. (Obesity Canada, Edmonton, Canada, 2020),.
- H. Devlin, More than 800 million people around the world have diabetes, study finds The Guardian (2024).
- M. Stobbe, CDC finds nearly 1 in 3 US youth have prediabetes, but experts question scant data Associated Press (2024).
- S. Marsh, Half a billion young people will be obese or overweight by 2030, report finds The Guardian (2025).
- S. McManus, One in four young people in England have mental health condition, NHS survey finds The Guardian (2025).
- M. Stobbe, American kids have become increasingly unhealthy over nearly two decades, new study finds Associated Press (2024).
- S. Simmons-Duffin, Autism rates keep rising. What RFK Jr. and the CDC plan to do next NPR (2025).
- J. Mason, Trump administration cut autism-related research by 26% so far in 2025 Reuters (2025).
- B. Editorial, What’s going on at HHS? RFK Jr.’s staffing choices raise alarm The Washington Post (2025).
- R. J. Rocque, C. Beaudoin, R. Ndjaboue, L. Cameron, L. Poirier-Bergeron, R.-A. Poulin-Rheault, C. Fallon, A. C. Tricco, H. O. Witteman, Health effects of climate change: an overview of systematic reviews BMJ Open 11, e046333 (2021).
- D. P. Strachan, Hay fever, hygiene, and household size BMJ 299, 1259–1260 (1989).
- J.-F. Bach, The effect of infections on susceptibility to autoimmune and allergic diseases N Engl J Med 347, 911–920 (2002).
- P. Elwood, J. Galante, J. Pickering, S. Palmer, A. Bayer, Y. Ben-Shlomo, M. Longley, J. Gallacher, Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study PLoS One 8, e81877 (2013).
- T. Hrncir, Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options Microorganisms 10, 578 (2022).
- A. Portela, M. Esteller, Epigenetic modifications and human disease Nat Biotechnol 28, 1057–1068 (2010).
- P. E. Griffiths, P. Bourrat, Integrating evolutionary, developmental and physiological mismatch Evol Med Public Health 11, 277–286 (2023).
- M. D. Anway, A. S. Cupp, M. Uzumcu, M. K. Skinner, Epigenetic transgenerational actions of endocrine disruptors and male fertility Science 308, 1466–1469 (2005).
- M. Ben Maamar, Y. Wang, E. E. Nilsson, D. Beck, W. Yan, M. K. Skinner, Transgenerational sperm DMRs escape DNA methylation erasure during embryonic development and epigenetic inheritance Environ Epigenet 9, dvad003 (2023).
- P. W. S. Hill, H. G. Leitch, C. E. Requena, Z. Sun, R. Amouroux, M. Roman-Trufero, M. Borkowska, J. Terragni, R. Vaisvila, S. Linnett, H. Bagci, G. Dharmalingham, V. Haberle, B. Lenhard, Y. Zheng, S. Pradhan, P. Hajkova, Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte Nature 555, 392–396 (2018).
- J. A. Hackett, M. A. Surani, DNA methylation dynamics during the mammalian life cycle Philos Trans R Soc Lond B Biol Sci 368, 20110328 (2013).
- M. Cowley, R. J. Oakey, Resetting for the next generation Mol Cell 48, 819–821 (2012).
- H. Kobayashi, T. Sakurai, M. Imai, N. Takahashi, A. Fukuda, O. Yayoi, S. Sato, K. Nakabayashi, K. Hata, Y. Sotomaru, Y. Suzuki, T. Kono, Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks PLoS Genet 8, e1002440 (2012).
- Z. D. Smith, M. M. Chan, T. S. Mikkelsen, H. Gu, A. Gnirke, A. Regev, A. Meissner, A unique regulatory phase of DNA methylation in the early mammalian embryo Nature 484, 339–344 (2012).
- R. Hirasawa, H. Chiba, M. Kaneda, S. Tajima, E. Li, R. Jaenisch, H. Sasaki, Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development Genes Dev 22, 1607–1616 (2008).
- E. Li, Chromatin modification and epigenetic reprogramming in mammalian development Nat Rev Genet 3, 662–673 (2002).
- P. D. Gluckman, M. A. Hanson, Living with the past: evolution, development, and patterns of disease Science 305, 1733–1736 (2004).
- B. T. Heijmans, E. W. Tobi, A. D. Stein, H. Putter, G. J. Blauw, E. S. Susser, P. E. Slagboom, L. H. Lumey, Persistent epigenetic differences associated with prenatal exposure to famine in humans Proc Natl Acad Sci U S A 105, 17046–17049 (2008).
- R. Yehuda, N. P. Daskalakis, A. Lehrner, F. Desarnaud, H. N. Bader, I. Makotkine, J. D. Flory, L. M. Bierer, M. J. Meaney, Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring American Journal of Psychiatry 171, 872–880 (2014).
- S. Accordini, L. Calciano, A. Johannessen, L. Portas, B. Benediktsdóttir, R. J. Bertelsen, L. Bråbäck, A.-E. Carsin, S. C. Dharmage, J. Dratva, B. Forsberg, F. Gomez Real, J. Heinrich, J. W. Holloway, M. Holm, C. Janson, R. Jögi, B. Leynaert, A. Malinovschi, A. Marcon, J. Martínez-Moratalla Rovira, C. Raherison, J. L. Sánchez-Ramos, V. Schlünssen, R. Bono, A. G. Corsico, P. Demoly, S. Dorado Arenas, D. Nowak, I. Pin, J. Weyler, D. Jarvis, C. Svanes, Ageing Lungs in European Cohorts (ALEC) Study, A three-generation study on the association of tobacco smoking with asthma Int J Epidemiol 47, 1106–1117 (2018).
- S. Tonegawa, Somatic generation of antibody diversity Nature 302, 575–581 (1983).
- G. Matarese, The link between obesity and autoimmunity Science 379, 1298–1300 (2023).
- H.-J. Lin, C.-C. Wei, C.-Y. Chang, T.-H. Chen, Y.-A. Hsu, Y.-C. Hsieh, H.-J. Chen, L. Wan, Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation EBioMedicine 10, 269–281 (2016).
- D. Moulin, J. Sellam, F. Berenbaum, J. Guicheux, M.-A. Boutet, The role of the immune system in osteoarthritis: mechanisms, challenges and future directions Nature Reviews Rheumatology 21, 221–236 (2025).
- W. Cheng, Z. Du, B. Lu, Chronic low-grade inflammation associated with higher risk and earlier onset of cardiometabolic multimorbidity in middle-aged and older adults: a population-based cohort study Sci Rep 14, 22635 (2024).
- I. Ioakeim-Skoufa, F. González-Rubio, M. Aza-Pascual-Salcedo, C. Laguna-Berna, B. Poblador-Plou, J. Vicente-Romero, H. Coelho, A. Santos-Mejías, A. Prados-Torres, A. Moreno-Juste, A. Gimeno-Miguel, Multimorbidity patterns and trajectories in young and middle-aged adults: a large-scale population-based cohort study Front Public Health 12, 1349723 (2024).
- D. Furman, J. Campisi, E. Verdin, P. Carrera-Bastos, S. Targ, C. Franceschi, L. Ferrucci, D. W. Gilroy, A. Fasano, G. W. Miller, A. H. Miller, A. Mantovani, C. M. Weyand, N. Barzilai, J. J. Goronzy, T. A. Rando, R. B. Effros, A. Lucia, N. Kleinstreuer, G. M. Slavich, Chronic inflammation in the etiology of disease across the life span Nature Medicine 25, 1822–1832 (2019).
- R. Cordova, V. Viallon, E. Fontvieille, L. Peruchet-Noray, A. Jansana, K.-H. Wagner, C. Kyrø, A. Tjønneland, V. Katzke, R. Bajracharya, M. B. Schulze, G. Masala, S. Sieri, S. Panico, F. Ricceri, R. Tumino, J. M. A. Boer, W. M. M. Verschuren, Y. T. van der Schouw, P. Jakszyn, D. Redondo-Sánchez, P. Amiano, J. M. Huerta, M. Guevara, Y. Borné, E. Sonestedt, K. K. Tsilidis, C. Millett, A. K. Heath, E. K. Aglago, D. Aune, M. J. Gunter, P. Ferrari, I. Huybrechts, H. Freisling, Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases:multinational cohort study The Lancet Regional Health – Europe 35, (2023).
- C. K. Ward-Caviness, J. Moyer, A. Weaver, R. Devlin, D. Diaz-Sanchez, Associations between PFAS occurrence and multimorbidity as observed in an electronic health record cohort Environ Epidemiol 6, e217 (2022).
- K. Mendoza, S. A. Smith-Warner, S. L. Rossato, N. Khandpur, J. E. Manson, L. Qi, E. B. Rimm, K. J. Mukamal, W. C. Willett, M. Wang, F. B. Hu, J. Mattei, Q. Sun, Ultra-processed foods and cardiovascular disease: analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies Lancet Reg Health Am 37, 100859 (2024).
- M. A. Beier, S. Setoguchi, T. Gerhard, J. Roy, D. Koffman, D. Mendhe, J. Madej, B. L. Strom, M. J. Blaser, D. B. Horton, Early childhood antibiotics and chronic pediatric conditions: a retrospective cohort study J Infect Dis (2025).
- C. Hoskinson, C. Petersen, S. E. Turvey, How the early life microbiome shapes immune programming in childhood asthma and allergies Mucosal Immunol 18, 26–35 (2025).
- W. Huang, Z. Zhang, M. Colucci, L. Deng, M. Yang, X. Huang, X. Zhou, Y. Jin, E. Lazzarini, C. Balbi, O. Juanola, A. Valdata, S. Bressan, Y. Zhan, F. Qi, Q. Wei, L. Yang, X. Zou, S. Qiu, The mixed effect of Endocrine-Disrupting chemicals on biological age Acceleration: Unveiling the mechanism and potential intervention target Environ Int 184, 108447 (2024).
- C. Symeonides, E. Aromataris, Y. Mulders, J. Dizon, C. Stern, T. H. Barker, A. Whitehorn, D. Pollock, T. Marin, S. Dunlop, An Umbrella Review of Meta-Analyses Evaluating Associations between Human Health and Exposure to Major Classes of Plastic-Associated Chemicals Ann Glob Health 90, 52 (2024).
- N. E. Nnadi, D. A. Carter, Climate change and the emergence of fungal pathogens PLoS Pathog 17, e1009503 (2021).
- J. Sánchez-Valle, A. Valencia, Molecular bases of comorbidities: present and future perspectives Trends Genet 39, 773–786 (2023).
- S. A. Reyes-Diaz, B. A. Priego-Parra, H. R. Ordaz-Alvarez, E. L. Núñez-Jiménez, C. L. Dorantes-Nava, F. Higuera-de la Tijera, M. Amieva-Balmori, C. Velez, J. M. Remes-Troche, Unequal Burdens: Irritable Bowel Syndrome in Sexual and Gender Minority Communities vs Cisgender Heterosexual Individuals Clin Transl Gastroenterol (2025).
- T. Stein, S. Collins, J. St Louis, The prevalence of hypermobile Ehlers-Danlos syndrome at a gender-affirming primary care clinic SAGE Open Med 13, 20503121251315021 (2025).
- E. Tabor, D. Kneale, P. Patalay, Sexuality and respiratory outcomes in the UK: disparities, development and mediators in multiple longitudinal studies Public Health 247, 105886 (2025).
- O. Deraz, B. Caceres, C. G. Streed, L. B. Beach, X. Jouven, M. Touvier, M. Goldberg, M. Zins, J.-P. Empana, Sexual Minority Status Disparities in Life’s Essential 8 and Life’s Simple 7 Cardiovascular Health Scores: A French Nationwide Population-Based Study J Am Heart Assoc 12, e028429 (2023).
- D. Wegener, J. McFaline-Figueroa, R. Goldstein, Migraine Prevalence in Gender Identity Minorities (P14-12.004) Neurology 100, 4340 (2023).
- A. B. Simon, K. Norland, M. Blackburn, S. Zhao, X. Wang, R. A. Harris, Evidence of increased cardiovascular disease risk in left-handed individuals Front Cardiovasc Med 10, 1326686 (2023).
- A. Kallitsounaki, D. M. Williams, Autism Spectrum Disorder and Gender Dysphoria/Incongruence. A systematic Literature Review and Meta-Analysis J Autism Dev Disord 53, 3103–3117 (2023).
- T. G. Goetz, N. Adams, The transgender and gender diverse and attention deficit hyperactivity disorder nexus: A systematic review Journal of Gay & Lesbian Mental Health 1–18 (2022).
- J. Sherman, C. Dyar, J. McDaniel, N. T. Funderburg, K. M. Rose, M. Gorr, E. Morgan, Sexual minorities are at elevated risk of cardiovascular disease from a younger age than heterosexuals J Behav Med 45, 571–579 (2022).
- M. G. Maggio, P. Calatozzo, A. Cerasa, G. Pioggia, A. Quartarone, R. S. Calabrò, Sex and Sexuality in Autism Spectrum Disorders: A Scoping Review on a Neglected but Fundamental Issue Brain Sci 12, 1427 (2022).
- E. Nastou, S. Ocklenburg, M. Hoogman, M. Papadatou-Pastou, Handedness in ADHD: Meta-Analyses Neuropsychol Rev 32, 877–892 (2022).
- V. Warrier, D. M. Greenberg, E. Weir, C. Buckingham, P. Smith, M. C. Lai, C. Allison, S. Baron-Cohen, Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals Nat Commun 11, 3959 (2020).
- J. M. Nagata, K. T. Ganson, J. Tabler, A. J. Blashill, S. B. Murray, Disparities Across Sexual Orientation in Migraine Among US Adults JAMA Neurol 78, 117–118 (2020).
- C. C. Stewart, S. J. Swanson, D. S. Sabsevitz, M. E. Rozman, J. K. Janecek, J. R. Binder, Predictors of language lateralization in temporal lobe epilepsy Neuropsychologia 60, 93–102 (2014).
- N. S. Morfit, N. Y. Weekes, Handedness and immune function Brain and Cognition 46, 209–213 (2001).
- N. Geschwind, P. Behan, Left-handedness: association with immune disease, migraine, and developmental learning disorder Proceedings of the National Academy of Sciences 79, 5097–5100 (1982).
- R. Blanchard, Birth order and sibling sex ratio in homosexual versus heterosexual males and females Annu Rev Sex Res 8, 27–67 (1997).
- R. Blanchard, A. F. Bogaert, Homosexuality in men and number of older brothers Am J Psychiatry 153, 27–31 (1996).
- A. Ganna, K. J. H. Verweij, M. G. Nivard, R. Maier, R. Wedow, A. S. Busch, A. Abdellaoui, S. Guo, J. F. Sathirapongsasuti, R. T. 23andMe, P. Lichtenstein, S. Lundström, N. Långström, A. Auton, K. M. Harris, G. W. Beecham, E. R. Martin, A. R. Sanders, J. R. B. Perry, B. M. Neale, B. P. Zietsch, Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior Science 365, (2019).
- Y. Wang, J. Wang, Modelling and prediction of global non-communicable diseases BMC Public Health 20, 822 (2020).
- D. L. Hartl, A. G. Clark, Principles of Population Genetics (Sinauer Associates, Sunderland, MA, 2007).
- E. Mayr, “Change of genetic environment and evolution Evolution as a Process” 1st, J. Huxley, A. C. Hardy, E. B. Ford, Eds. (Allen & Unwin, London, 1954), pp. 157–180.
- M. Goldman, RFK Jr.’s vaccine pullback stokes fears of lost medical breakthroughs Axios (2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




