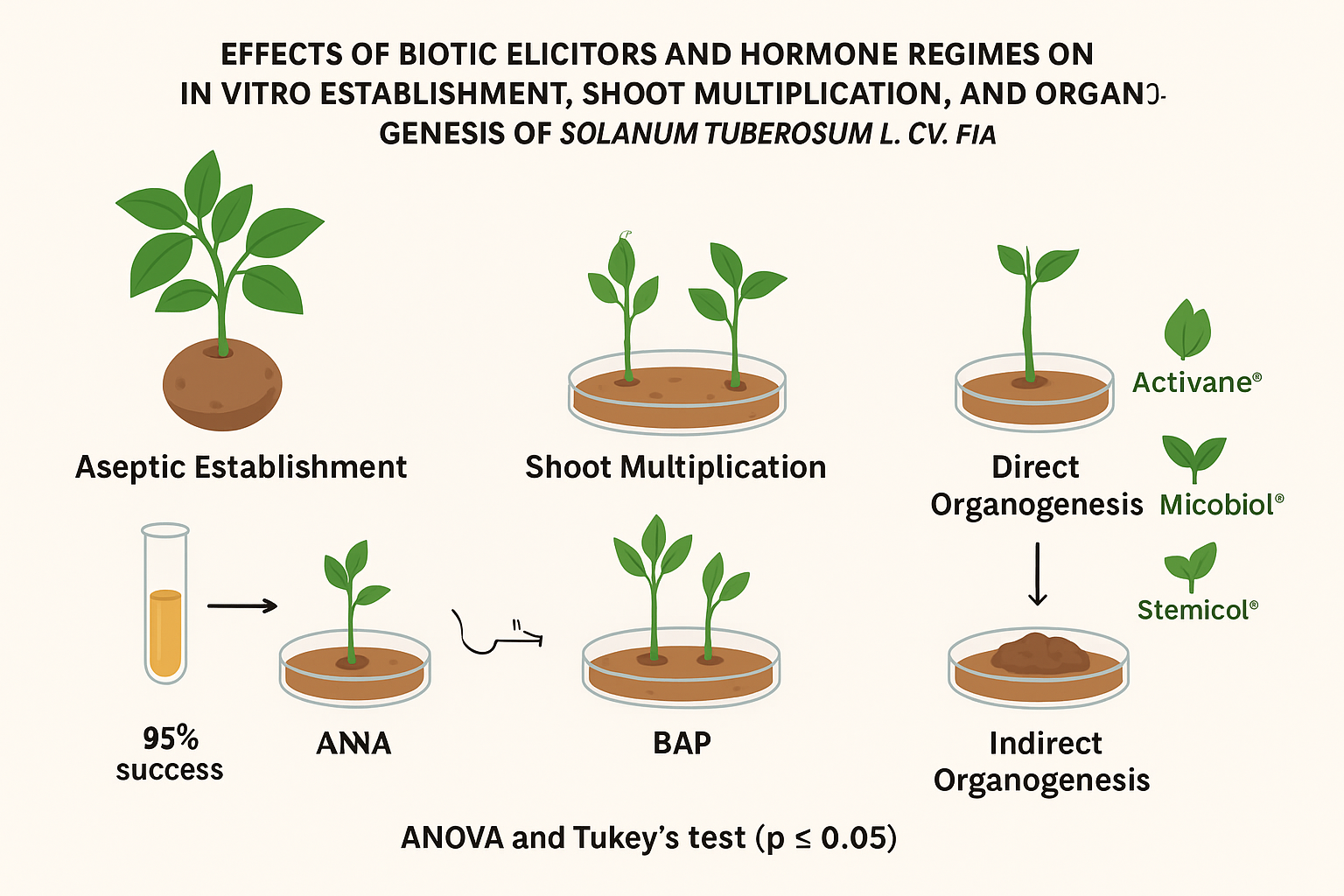
1. Introduction
Potato (
Solanum tuberosum L.) is one of the world’s most important food crops, with its origin traced back to the northern region of Lake Titicaca in the southern Andes of Peru [
1,
2,
3]. The earliest domestication events of potato occurred between 6,000 and 10,000 years ago, involving ancestral species such as
Solanum bukasovii and
S. multidissectum [
4,
5]. Following initial cultivation in the coastal regions of Chile, potatoes spread gradually across continents and climates, evolving into the diverse forms collectively known today as
Solanum tuberosum [
6,
7,
8,
9]. Peru remains a global center of potato genetic diversity, harboring over 3,000 native varieties that are still cultivated by smallholder farmers on more than 300,000 hectares, underscoring the crop’s cultural and economic significance [
10,
11,
48].
In recent decades, advances in plant biotechnology have revolutionized crop production, with plant tissue culture playing a central role in these innovations [
12,
13]. In vitro culture techniques have become preferred alternatives to conventional propagation methods, offering advantages such as rapid multiplication rates, pathogen-free plant production, and improved uniformity [
13,
14,
15]. Specifically, in vitro propagation of potato through axillary bud culture is widely adopted worldwide to produce both plantlets and microtubers, which are critical components of disease-free seed programs [
16,
17]. These propagules, developed in aseptic and controlled environments using artificial media, can be transitioned to field conditions for further multiplication as minitubers [
18]. Moreover, in vitro-derived plant material serves as a key resource for germplasm conservation and facilitates international germplasm exchange, supporting global efforts for crop improvement and biodiversity preservation [
19].
As the global population grows and climate change intensifies the threat of pests and diseases, sustainable and efficient crop production technologies are increasingly vital [
20]. Among explant sources for in vitro propagation, meristematic tissues are preferred due to their reduced microbial load and lower incidence of systemic pathogens, a consequence of limited pathogen migration to shoot apices [
21,
22]. Central to in vitro regeneration is the process of organogenesis, whereby differentiated plant cells are induced to dedifferentiate and redifferentiate to form new organs and complete plantlets [
23]. This process requires cells to be competent and responsive to developmental signals, typically mediated by phytohormones and other biochemical cues [
24]. Organogenesis can be categorized as direct or indirect; direct organogenesis involves shoot or root formation directly from explant tissues without an intervening callus phase, whereas indirect organogenesis proceeds via a callus intermediate, which later regenerates shoots [
25,
26].
In recent years, elicitors have garnered considerable attention in plant tissue culture as biotic or abiotic compounds that can stimulate secondary metabolism and enhance plant defense mechanisms, even under sterile in vitro conditions [
27,
28]. These molecules offer promising alternatives or supplements to traditional plant growth regulators by potentially improving morphogenetic responses, growth rates, and overall in vitro culture efficiency [
29,
30,
31]. For example, oligosaccharide elicitors applied to potato cultures have been demonstrated to boost shoot regeneration and root development by enhancing antioxidant enzyme activity and modulating gene expression pathways involved in organogenesis [
32]. This integration of elicitors into tissue culture systems could thus represent a novel strategy to optimize micropropagation protocols, reduce reliance on synthetic hormones, and improve plant quality.
The overarching objective of this study was to evaluate the effects of three commercially available elicitors Activane®, Micobiol®, and Stemicol® on the morphogenetic responses and growth performance of Solanum tuberosum L. cv. Fianna under in vitro conditions. The specific aims included assessing callus induction, shoot and root development, and overall cellular multiplication efficiency. By systematically investigating these elicitors’ influence, this research sought to elucidate their potential to partially or fully replace conventional phytohormones while improving tissue culture outcomes. The findings contribute to the growing body of knowledge on elicitor-assisted micropropagation and offer practical insights for enhancing the productivity and quality of potato tissue culture systems.
2. Materials and Methods
2.1. Experimental Location
The study was performed at the Plant Tissue Culture Laboratory, Faculty of Agronomy, Autonomous University of Nuevo León, located on the Campus of Agricultural Sciences in Colonia Ex Hacienda El Canadá, Av. Francisco Villa s/n, General Escobedo, Nuevo León, Mexico. The experiments were conducted from January to October 2021 under controlled laboratory conditions.
2.2. Preparation and Pre-Disinfestation Protocol for Explant Collection from Potato Tubers (Solanum tuberosum L. cv. Fianna)
Potato tubers (
Solanum tuberosum L. cv. Fianna) were first washed with liquid soap and rinsed thoroughly with potable water to remove surface debris. To reduce microbial contamination, the tubers were then immersed in a bactericidal-fungicidal solution containing 1 g L⁻¹ each of benomyl and oxytetracycline for two hours. After this treatment, they were rinsed again with potable water and air-dried at room temperature for one week. Bud development was initiated by incubating the tubers in complete darkness at 25 ± 2 °C for four weeks. Following this period, to minimize etiolation and improve the quality of emerging buds for explant preparation, tubers were exposed to a controlled photoperiod of 16 hours light (54 µmol m⁻² s⁻¹) and 8 hours darkness for two additional weeks under the same temperature conditions. Shoots excised from the treated tubers were washed with liquid soap, followed by a rinse in 1% sodium hypochlorite (Cloralex
®) and potable water. To further lower the microbial load, the excised shoots were agitated for 40 minutes in a sterilant solution containing benomyl (2 g L⁻¹), amistar (2 g L⁻¹), oxytetracycline (2 g L⁻¹), sucrose (30 g L⁻¹), citric acid (500 mg L⁻¹), ascorbic acid (500 mg L⁻¹), and a commercial bactericide (2 mL L⁻¹). After a final rinse with purified water, the explants were transferred aseptically to a laminar flow hood for surface sterilization (
Figure 1).
2.3. Aseptic Establishment of Explants
Explants underwent surface sterilization in 6% sodium hypochlorite (Cloralex
®, 15% active ingredient) diluted to 15% v/v with 0.02% Tween-20 for 15 minutes, followed by triple rinsing with sterile distilled water. Explants were then cultured on Murashige and Skoog (MS) basal medium [
33] supplemented with vitamins, 100 mg L⁻¹ myo-inositol, 30 g L⁻¹ sucrose, and solidified with 4.3 g L⁻¹ Phytagel™. The pH was adjusted to 5.8 ± 0.02. Culture vessels containing 30 mL of medium were incubated under controlled conditions: 16-hour photoperiod at 54 µmol m⁻² s⁻¹ light intensity, 8 hours darkness, and 24 ± 2 °C temperature. Explants were observed over nine weeks to assess aseptic establishment percentage, shoot and root formation, and growth parameters (
Table 1). This method proved effective in achieving high asepsis rates (~95%) and vigorous morphogenic responses, consistent with similar studies [
34,
35].
2.4. Multiplication Stage
Shoots approximately 2 cm in height from the establishment phase were used as explants for multiplication. These were cultured on MS medium supplemented with vitamins, 30 g L⁻¹ sucrose, and solidified with 4.3 g L⁻¹ Phytagel™. The medium pH was adjusted to 5.7 ± 0.02. Four hormonal treatments were evaluated: control (no growth regulators), and three treatments with 0.2 mg L⁻¹ NAA combined with increasing concentrations of BAP at 0.5, 1.0, and 1.5 mg L⁻¹. Cultures were maintained under the same controlled conditions as before. After eight weeks, shoot number, length, root number, root length, and internode count were measured to determine the effects of hormonal combinations on shoot proliferation and rooting (
Table 2). Hormonal optimization revealed significant improvements in shoot and root growth, particularly at moderate BAP levels (T2 and T3), confirming prior observations on cytokinin-auxin balance in potato micropropagation [
29,
36].
2.5. Elicitor Treatments for Direct In Vitro Organogenesis
Shoots (~2 cm) from multiplication were used as explants in experiments testing the effects of biotic elicitors Activane
®, Micobiol
®, and Stemicol
® on direct organogenesis. The culture medium consisted of MS basal salts with vitamins, 100 mg L⁻¹ myo-inositol, 30 g L⁻¹ sucrose, and 4.3 g L⁻¹ Phytagel™, pH 5.8 ± 0.02. After sterilization, elicitors were added aseptically at three concentrations each: Activane
® (0.3, 0.5, 0.7 g L⁻¹), Micobiol
® (0.3, 0.5, 0.7 mL L⁻¹), and Stemicol
® (0.3, 0.5, 0.7 g L⁻¹), alongside a control without elicitor. Explants were cultured under the same photoperiod and temperature for three months. Morphogenic responses including shoot/root initiation time, shoot/root number, and length were recorded (
Figure 2). Results demonstrated that mid-range elicitor doses significantly enhance organogenesis and shoot proliferation, confirming the role of elicitors as growth regulator substitutes or enhancers [
29,
35].
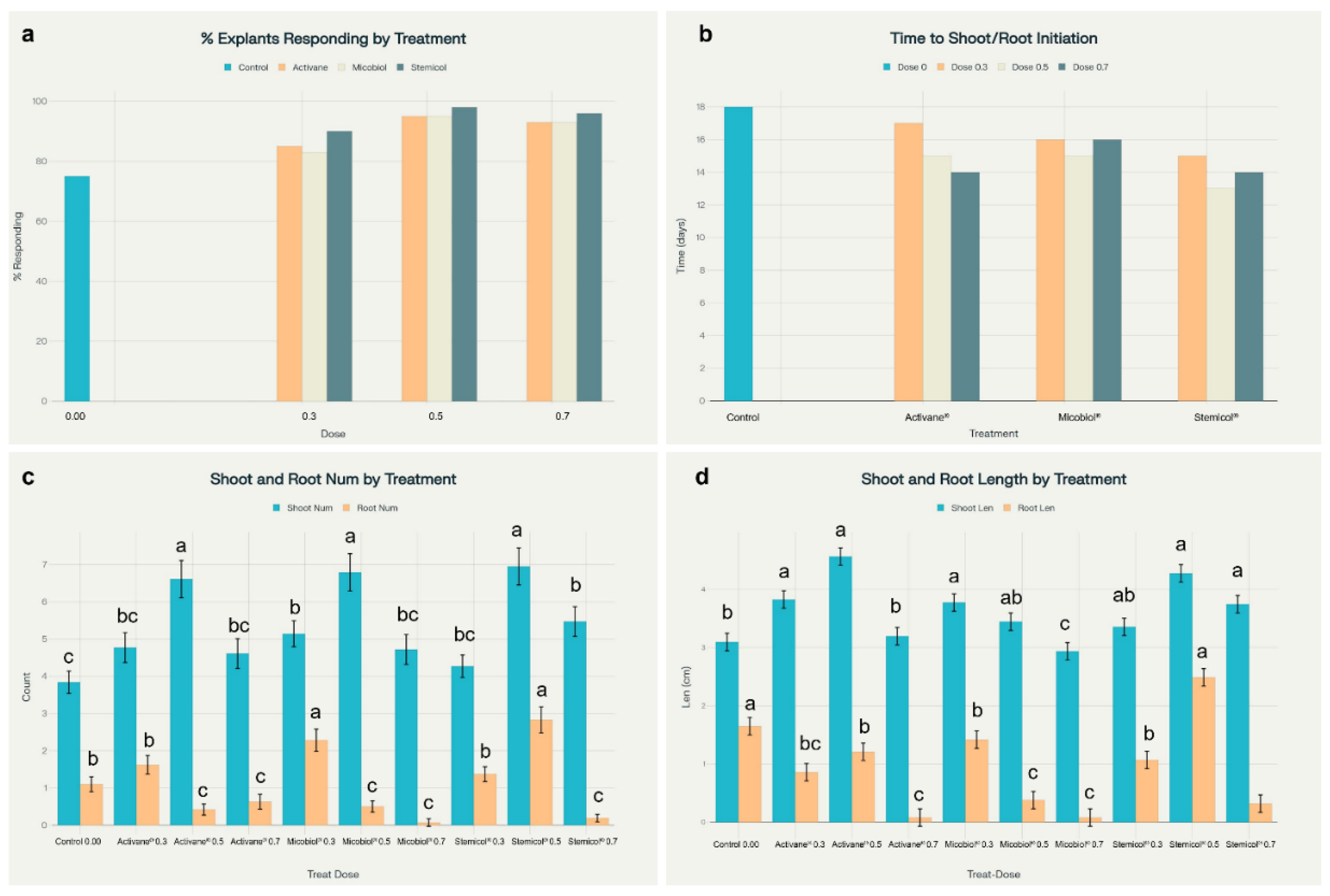
Figure 2.
Elicitor treatments enhanced in vitro regeneration of Solanum tuberosum cv. Fianna. (a) Explant response was highest with Stemicol® 0.5 g L⁻¹ (98%) compared to the control (75%). (b) Time to shoot/root initiation was shortest under Stemicol® 0.5 (13 days). (c) The greatest shoot and root numbers were observed in Stemicol® 0.5 (6.95 shoots, 2.83 roots) and Micobiol® 0.3 (2.28 roots). (d) Shoot and root lengths were maximized with Activane® 0.5 (4.57 cm) and Stemicol® 0.5 (2.49 cm), respectively. Statistical differences are indicated by different letters (Tukey’s HSD, p < 0.05).
Figure 2.
Elicitor treatments enhanced in vitro regeneration of Solanum tuberosum cv. Fianna. (a) Explant response was highest with Stemicol® 0.5 g L⁻¹ (98%) compared to the control (75%). (b) Time to shoot/root initiation was shortest under Stemicol® 0.5 (13 days). (c) The greatest shoot and root numbers were observed in Stemicol® 0.5 (6.95 shoots, 2.83 roots) and Micobiol® 0.3 (2.28 roots). (d) Shoot and root lengths were maximized with Activane® 0.5 (4.57 cm) and Stemicol® 0.5 (2.49 cm), respectively. Statistical differences are indicated by different letters (Tukey’s HSD, p < 0.05).
2.6. Elicitor Treatments for Indirect Organogenesis
For indirect organogenesis, shoots (~2 cm) were cultured on MS medium supplemented with 3.0 mg L⁻¹ 2,4-D and 0.5 mg L⁻¹ kinetin, along with the same basal medium components as above. Elicitors Activane
®, Micobiol
®, and Stemicol
® were added at the same concentrations as in the direct organogenesis experiments. Cultures were incubated in complete darkness at 24 ± 2 °C for three months. Parameters measured included callus induction percentage, fresh weight, diameter, texture, and color (
Figure 4). Elicitor treatments, especially Stemicol
® and Micobiol
® at mid-to-high concentrations, significantly improved callus quality and mass, corroborating earlier reports on elicitor-induced callogenesis and secondary metabolite production [
37,
38].
2.7. Experimental Design and Statistical Analysis
This study employed a completely randomized design (CRD) across all experimental stages to ensure statistical robustness and minimize bias. During the in vitro establishment stage, 70 Magenta boxes were used, each containing 30 mL of MS medium and four explants, to assess aseptic establishment and initial morphogenesis. For the multiplication phase, four hormone treatments were evaluated, with each treatment replicated six times using four explants per box. This facilitated a reliable analysis of shoot and root development, including internode formation, under varying auxin and cytokinin combinations. In the regeneration stage, three biotic elicitors Activane®, Micobiol®, and Stemicol® were tested at three concentrations each, alongside a control, to examine their impact on direct and indirect organogenesis. Each treatment had six replicates, each with four explants. This factorial design enabled the identification of optimal elicitor concentrations for shoot induction and callus development. Quantitative variables such as shoot/root number and length, internode count, explant response percentage, and callus biomass were subjected to analysis of variance (ANOVA), and treatment means were compared using Tukey’s HSD test at a 5% significance level. All statistical analyses were performed using SPSS version 20. This rigorous design and analysis framework ensured reliable, reproducible results, advancing insights into elicitor-driven micropropagation strategies in potato biotechnology.
3. Results and Discussion
3.1. Aseptic Establishment and Morphogenic Response of Solanum tuberosum cv. Fianna
The aseptic establishment and subsequent morphogenic development of
Solanum tuberosum cv. Fianna under in vitro conditions demonstrated high viability and reproducibility (Tab 1). Over a nine-week incubation period, the explants showed consistent growth responses in terms of shoot and root formation, with an average asepsis rate of 94.77%, confirming the effectiveness of the disinfection protocol employed. The initial asepsis reached 100% in week 1, with slight declines in subsequent weeks (minimum 91.18% in week 3), suggesting robust explant tolerance to the 15% NaOCl and Tween-20 sterilization method. These results are in line with findings by [
34,
35], where similar surface sterilants achieved asepsis rates above 90% in potato explants. Comparable aseptic efficiency was also observed in the micropropagation studies by [
39] on Ethiopian cultivars.
Shoot formation initiated by week 2 (0.7 shoots/explant) and peaked in week 8 with 2.65 shoots per explant, averaging 1.6 shoots/explant overall. The shoot length followed a parallel trajectory, reaching 3.0 cm by week 9 (average 1.58 cm). These results reflect the effectiveness of hormone regulation and MS-based media in inducing shoot morphogenesis, consistent with previous reports by [
35,
40], who optimized shoot proliferation in TIS (Temporary Immersion Systems) and static cultures using cytokinins like BAP and GA₃. The linear growth trend aligns with patterns seen in [
41], who demonstrated the positive effects of growth regulators on callus induction and subsequent regeneration from potato explants under in vitro conditions.
Rooting was not observed until week 4, likely due to the early prioritization of shoot organogenesis. Once initiated, root formation increased progressively to 2.0 roots/explant by week 9 (average 0.8). Root length mirrored this trend, with a maximum of 2.53 cm and an average of 1.15 cm. Similar delayed but successful rooting responses have been documented by [
34,
39], where auxin supplementation (NAA or IBA) post-shoot development facilitated stable root induction. Furthermore, [
40] emphasized the importance of precise hormone balance in optimizing both shoot and root development in temporary immersion systems. Their findings support the observed morphological characteristics in this study, affirming that culture conditions and hormone regimes critically influence shoot-root coordination.
3.2. In Vitro Morphogenic Response of Potato cv. Fianna to BAP and ANA
The combination of BAP (6 Benzylaminopurine) and ANA (1 Naphthaleneacetic acid) significantly influenced shoot and root development in
Solanum tuberosum cv. Fianna explants under in vitro conditions (
Table 2). Treatment T2 (0.2 mg L⁻¹ ANA + 0.5 mg L⁻¹ BAP) resulted in the highest shoot number (6.95 a), shoot length (6.98 cm a), and root number (6.10 a), followed by T3 (0.2 mg L⁻¹ ANA + 1.0 mg L⁻¹ BAP), which also performed well across variables except internode number. In contrast, T4, with the highest BAP concentration (1.5 mg L⁻¹), significantly reduced shoot number (4.86 c) and internode count (4.58 c), indicating a threshold above which cytokinin becomes inhibitory rather than stimulatory.
These results align with [
36], who emphasized the importance of maintaining a balanced hormonal status in in vitro potato cultures. Excessive cytokinins may suppress apical dominance or trigger stress responses that reduce morphogenic efficiency. Our findings support previous observations by [
29,
34], where moderate hormonal inputs enhanced meristematic activity and cell division. Moreover, the improved root and shoot elongation in T2 and T3 reflect synergistic auxin cytokinin interactions known to govern organogenesis.
Root length also followed this trend, with T2 to T4 outperforming the control, though T4 achieved the longest roots (5.31 cm a). These results agree with [
40], who demonstrated that optimized hormonal and environmental parameters in temporary immersion systems (TISs) significantly improved root and shoot morphology in potato explants. Like their findings, our results affirm the effectiveness of specific hormone regimes in enhancing not only shoot multiplication but also underground organ development essential for acclimatization and tuberization. Additionally, internodal development peaked in T1 (control), suggesting that high shoot proliferation in other treatments may reduce spacing between nodes due to compact growth, a tradeoff observed in other micropropagation studies [
40,
42].
3.3. Direct In Vitro Organogenesis in Potato cv. Fianna Improved by Biotic Elicitor Applications
The application of elicitors Activane
®, Micobiol
®, and Stemicol
® at various concentrations significantly enhanced the morphogenic response of
Solanum tuberosum cv. Fianna under in vitro conditions. The percentage of explants responding increased notably across all treatments, ranging from 83% to 98%, compared to only 75% in control. Notably, the shortest time to shoot and root initiation (13 days) was observed in the Stemicol
® 0.5 g L⁻¹ treatment, indicating its effectiveness in promoting early morphogenesis. These results are visualized in
Figure 2 and
Figure 3, which illustrates treatment-specific differences in callus mass and morphology.
Shoot proliferation was significantly improved across all elicitor treatments compared to the control. The highest shoot numbers were observed in explants treated with Stemicol
® at 0.5 g L⁻¹ (6.95 a), Micobiol
® at 0.5 mL L⁻¹ (6.79 a), and Activane
® at 0.5 g L⁻¹ (6.61 a), all showing a significant advantage over the control (3.84 c). Shoot elongation followed a similar trend, with the longest shoots recorded in Activane
® 0.5 (4.57 cm a) and Stemicol
® 0.5 (4.28 cm a), suggesting that moderate concentrations of these elicitors enhance shoot architecture. These findings agree with [
29,
34] and [
35], who demonstrated that intermediate levels of elicitors stimulate endogenous cytokinin-like responses, resulting in enhanced cell division and meristem activity. Likewise, [
34] reported improved shoot elongation with elicitor applications, attributing it to increased nutrient uptake and hormonal regulation. [
43] also highlighted the positive impact of certain antimicrobial compounds such as β-lactam antibiotics in promoting shoot organogenesis in potato cultivars, reinforcing the potential dual role of elicitor-type treatments in both microbial suppression and morphogenic stimulation. The limited shoot growth in the control treatment corroborates the observations of [
42], who noted restricted shoot proliferation in the absence of external growth regulators.
Root development was also significantly influenced by elicitor treatments. The highest root number (2.83 a) and root length (2.49 cm a) were achieved with Stemicol
® at 0.5 g L⁻¹, followed by Micobiol
® at 0.3 mL L⁻¹. In contrast, higher concentrations (0.7 levels) across all elicitors showed diminished root formation, suggesting a threshold beyond which elicitor efficacy declines or becomes inhibitory. These observations align with studies by [
36,
40], who highlighted the importance of maintaining optimal hormonal balance to promote coordinated shoot and root development. Furthermore, [
29] emphasized the role of elicitor-induced hormonal crosstalk in enhancing root organogenesis by modulating auxin signaling pathways. The results indicate that mid-level doses of Micobiol
® and Stemicol® are most effective for promoting balanced morphogenesis in
Solanum tuberosum cv. Fianna under in vitro conditions, supporting their application in commercial micropropagation systems.
Figure 3.
Direct in vitro organogenesis in Solanum tuberosum cv. Fianna improved by biotic elicitor applications. Representative shoot development after four weeks of culture under different elicitor treatments: Control, Activane® 0.5 g L⁻¹, Micobiol® 0.5 mL L⁻¹, and Stemicol® 0.5 g L⁻¹. Enhanced shoot proliferation and elongation are clearly observed in elicitor-treated explants compared to the control, indicating the positive effect of biotic elicitors on direct organogenesis.
Figure 3.
Direct in vitro organogenesis in Solanum tuberosum cv. Fianna improved by biotic elicitor applications. Representative shoot development after four weeks of culture under different elicitor treatments: Control, Activane® 0.5 g L⁻¹, Micobiol® 0.5 mL L⁻¹, and Stemicol® 0.5 g L⁻¹. Enhanced shoot proliferation and elongation are clearly observed in elicitor-treated explants compared to the control, indicating the positive effect of biotic elicitors on direct organogenesis.
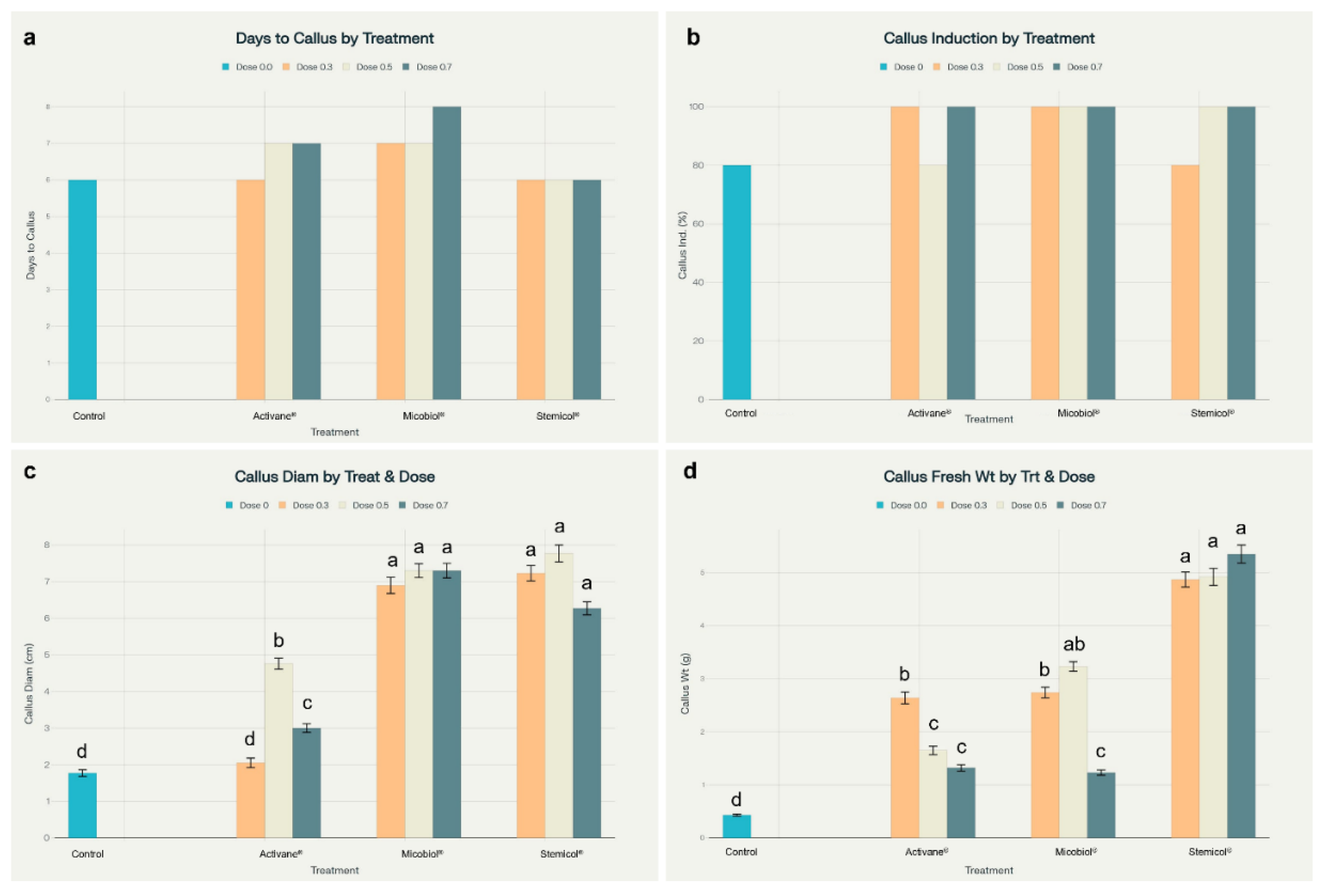
3.4. Enhanced Indirect Organogenesis in Potato cv. Fianna through Elicitor Treatments
The use of elicitors significantly influenced indirect organogenesis in
Solanum tuberosum L. cv. Fianna. Notably, Stemicol
® and Micobiol
® treatments produced superior callogenic responses, as reflected in parameters such as callus initiation time, fresh weight, diameter, and induction percentage. For example, Stemicol
® at 0.7 g L⁻¹ generated the highest callus fresh weight (5.35 ± 0.17 g) and diameter (6.27 ± 0.18 cm), with 100 percent callus induction. These results are also visualized in
Figure 4 and
Figure 5, which illustrates treatment-specific differences in callus mass and morphology.
Figure 4.
Elicitor treatments improved callus development in Solanum tuberosum cv. Fianna. (a) Days to callus formation were consistent across treatments. (b) Callus induction reached 100% in most treated groups. (c) Callus diameter was significantly larger in Micobiol® and Stemicol® treatments. (d) Callus fresh weight peaked under Stemicol®, significantly exceeding the control. Different letters indicate significant differences (Tukey’s HSD, p < 0.05).
Figure 4.
Elicitor treatments improved callus development in Solanum tuberosum cv. Fianna. (a) Days to callus formation were consistent across treatments. (b) Callus induction reached 100% in most treated groups. (c) Callus diameter was significantly larger in Micobiol® and Stemicol® treatments. (d) Callus fresh weight peaked under Stemicol®, significantly exceeding the control. Different letters indicate significant differences (Tukey’s HSD, p < 0.05).
These findings align with [
37], who emphasized the genotype-dependent response of potato varieties to in vitro culture conditions and highlighted the importance of optimizing culture media to enhance morphogenic potential. Similarly, [
44] reported efficient callus induction and shoot regeneration in potato explants under long-day conditions with suitable hormonal treatments, supporting our results that the combination of elicitors and optimized media can accelerate organogenesis. The sensitivity of indirect organogenesis to hormonal and abiotic factors has been previously documented. [
45] demonstrated that plant growth regulators and salt stress significantly affect callus induction and shoot regeneration in potato, which is consistent with our observation that higher doses of Activane® may induce stress responses, as indicated by delayed callus color change and looser callus texture. In terms of callus quality, Micobiol
® treatments at 0.5 and 0.7 mL L⁻¹ yielded friable calli, a trait favorable for subsequent shoot regeneration. [
46] reported a similar correlation between friable callus morphology and high-frequency shoot regeneration in
Sansevieria trifasciata, highlighting the critical role of callus structure in regeneration efficiency.
Figure 5.
Enhanced indirect organogenesis in Solanum tuberosum cv. Fianna through elicitor treatments. Representative callus formation from explants cultured under different elicitor treatments: Control, Activane® 0.5 g L⁻¹, Micobiol® 0.5 mL L⁻¹, and Stemicol® 0.5 g L⁻¹. Elicitor treatments, particularly Micobiol® and Stemicol®, resulted in more vigorous, compact, and friable calli with greater biomass compared to the control. These visual differences reflect the stimulatory effect of elicitors on callogenesis and the potential for improved regeneration efficiency.
Figure 5.
Enhanced indirect organogenesis in Solanum tuberosum cv. Fianna through elicitor treatments. Representative callus formation from explants cultured under different elicitor treatments: Control, Activane® 0.5 g L⁻¹, Micobiol® 0.5 mL L⁻¹, and Stemicol® 0.5 g L⁻¹. Elicitor treatments, particularly Micobiol® and Stemicol®, resulted in more vigorous, compact, and friable calli with greater biomass compared to the control. These visual differences reflect the stimulatory effect of elicitors on callogenesis and the potential for improved regeneration efficiency.
Further, elicitors have also been shown to enhance secondary metabolite production and biomass accumulation in plants, as reviewed by [
38,
47] in
Stevia rebaudiana. These studies suggest elicitor treatments not only promote morphogenesis but also enhance metabolic activity, which may explain the improved callus vigor observed in our treatments. The relatively rapid callus initiation time of 6 to 8 days observed here parallels results from Kirillov et al. (2022), who demonstrated fast callogenesis through precise hormone balancing, and complements previous observations by [
44] regarding efficient propagation under optimized culture conditions.
4. Conclusions
This study demonstrates the effectiveness of biotic elicitors Activane®, Micobiol®, and Stemicol® in enhancing in vitro morphogenesis of Solanum tuberosum L. cv. Fianna across different stages of micropropagation. The sterilization and culture protocols adopted ensured high asepsis and viability, enabling reproducible shoot and root formation during the establishment phase. Optimal hormonal combinations, particularly 0.2 mg L⁻¹ ANA with 0.5–1.0 mg L⁻¹ BAP, significantly boosted shoot and root proliferation during the multiplication stage. Notably, mid-range concentrations of Micobiol® and Stemicol® (0.5 mL/g L⁻¹) were most effective in promoting direct organogenesis, reducing initiation time and enhancing shoot-root architecture. Indirect organogenesis was also improved, with Stemicol® at 0.7 g L⁻¹ producing robust, compact calli with the highest fresh weight and diameter. These findings highlight the dual role of elicitors in stimulating morphogenic responses and potentially enhancing metabolite production, offering a promising approach to replacing or supplementing conventional growth regulators. The integration of elicitor treatments into micropropagation protocols can increase efficiency, reduce costs, and contribute to sustainable in vitro propagation systems for commercial potato production and germplasm conservation.
Author Contributions
Conceptualization, M.C.O.Z. A.K.C. A.I.L. and M.J.F.; Methodology, O.S. and R.E.A.; Formal Analysis, P.O.-A. and Y.C.; Investigation, M.J.F. A.K.C. and M.C.O.Z.; Writing Original Draft, M.J.F. and A.K.C.; Review and Editing, M.C.O.Z. A.K.C. A.I.L. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the National Council for the Humanities, Sciences, and Technologies (CONAHCYT) as graduate student scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors express their gratitude for the support provided by the National Council for the Humanities, Sciences, and Technologies (CONAHCYT), the Faculty of Agronomy of the Autonomous University of Nuevo Leon (FAUANL), and Lida de México SA DE CV.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kennedy, G.; Raneri, J.E.; Stoian, D.; Attwood, S.; Burgos, G.; Ceballos, H.; Ekesa, B.; Johnson, V.; Low, J.W.; Talsma, E.F. Roots, Tubers and Bananas: Contributions to Food Security. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 231–256. [Google Scholar] [CrossRef]
- Vincent, C.; Alyokhin, A.; Giordanengo, P. Potatoes and their Pests—Setting the Stage. In Insect Pests of Potato; Alyokhin, A., Vincent, C., Giordanengo, P., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 3–8. [Google Scholar] [CrossRef]
- Spooner, D.M.; McLean, K.; Ramsay, G.; Waugh, R.; Bryan, G.J. A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proc. Natl. Acad. Sci. USA 2005, 102, 14694–14699. [Google Scholar] [CrossRef] [PubMed]
- Sukhotu, T.; Hosaka, K. Origin and evolution of Andigena potatoes revealed by chloroplast and nuclear DNA markers. Genome 2006, 49, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, L. Potato origin and production. In Advances in Potato Chemistry and Technology, 2nd ed.; Academic Press: San Diego, CA, USA, 2016; pp. 1–32. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Crisovan, E.; Hamilton, J.P.; Kim, J.; Laimbeer, P.; Leisner, C.P.; Manrique-Carpintero, N.C.; Newton, L.; Pham, G.M.; Vaillancourt, B.; et al. Genome Diversity of Tuber-Bearing Solanum Uncovers Complex Evolutionary History and Targets of Domestication in the Cultivated Potato. Proc. Natl. Acad. Sci. USA 2017, 114, E9999–E10008. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Jia, Y.; Zhang, J.; He, X.; Wang, X.; Yu, J.; Bai, Y.; Li, S.; Yin, D.; Li, F.; Du, Y. Genome Evolution and Diversity of Wild and Cultivated Potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- Adekanmbi, T.; Wang, X.; Basheer, S.; Liu, S.; Yang, A.; Cheng, H. Climate change impacts on global potato yields: a review. Environ. Res. Clim. 2024, 3, 012001. [Google Scholar] [CrossRef]
- Jennings, S.A.; Koehler, A.-K.; Nicklin, K.J.; Deva, C.; Sait, S.M.; Challinor, A.J. Global potato yields increase under climate change with adaptation and CO2 fertilisation. Front. Sustain. Food Syst. 2020, 4, 519324. [Google Scholar] [CrossRef]
- Fajardo-Escoffié, J.L. Size, Color, and Freshness: Standards and Heritage of Native Potatoes in Peru. Food Foodways 2022, 30, 165–184. [Google Scholar] [CrossRef]
- Spanoghe, M.; Marique, T.; Nirsha, A.; Esnault, F.; Lanterbecq, D. Genetic Diversity Trends in the Cultivated Potato: A Spatiotemporal Overview. Biology 2022, 11, 604. [Google Scholar] [CrossRef]
- Tabei, Y.; Muranaka, T. Preface to the Special Issue “Technology in Tissue Culture toward Horizon of Plant Biotechnology”. Plant Biotechnol. 2020, 37, 117–120. [Google Scholar] [CrossRef]
- Abdelhafez, A.; Mahmoud, M.; Elhosieny, B.; Mohamed, M.; Abdallah, M. Production of Potato Tubers and Seedlings Using Plant Tissue Culture and Aeroponic for Potato Cultivation. Int. J. Holist. Res. 2024, 1–5. [Google Scholar] [CrossRef]
- Dalleh, M.; Borjac, J.; Younes, G.; Choueiri, E.; Chehade, A.; Elbitar, A. In Vitro Propagation and Microtuberization of Potato (Solanum tuberosum L.) Spunta Variety in Lebanon. Adv. Hortic. Sci. 2023, 37, 243–253. [Google Scholar] [CrossRef]
- Abu Zeid, I.M.; Soliman, H.I.A.; Metwali, E.M.R. In vitro evaluation of some high yield potato (Solanum tuberosum L.) cultivars under imposition of salinity at the cellular and organ levels. Saudi J. Biol. Sci. 2022, 29, 2541–2551. [Google Scholar] [CrossRef]
- Boubaker, H.; Saadaoui, W.; Dasgan, H.Y.; Tarchoun, N.; Gruda, N.S. Enhancing Seed Potato Production from In Vitro Plantlets and Microtubers through Biofertilizer Application: Investigating Effects on Plant Growth, Tuber Yield, Size, and Quality. Agronomy 2023, 13, 2541. [Google Scholar] [CrossRef]
- Sembiring, R.; Hayati, M.; Kesumawati, E. Formation of potato microtubers (Solanum tuberosum L.) by using BAP and coconut water in the in vitro culture. In Proceedings of the 1st International Conference on Agriculture and Bioindustry 2019; IOP Conf. Ser.: Earth Environ. Sci. IOP Publishing: Bristol, UK, 2020; Volume 425, p. 012072. [Google Scholar] [CrossRef]
- Mamiya, K.; Tanabe, K.; Onishi, N. Production of Potato (Solanum tuberosum L.) Microtubers Using Plastic Culture Bags. Plant Biotechnol. 2020, 37, 233–238. [Google Scholar] [CrossRef]
- Gopal, J.; Minocha, J.L.; Dhaliwal, H.S. Microtuberization in Potato (Solanum tuberosum L.). Plant Cell Rep. 1998, 17, 794–798. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The Persistent Threat of Emerging Plant Disease Pandemics to Global Food Security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef] [PubMed]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Khatun, Z.; Eaton, T.E.-J.; Hossen, M.I.; Haque, M.K.; Soren, E.B. Generation of Virus-Free Potato Plantlets through Meristem Culture and Their Field Evaluation. Am. J. Plant Sci. 2020, 11, 1827–1846. [Google Scholar] [CrossRef]
- Dagla, H.R. Organogenesis in Plant Tissue Culture. Resonance 2012, 17, 8–18. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of Plant Tissue Culture. In Plant Cell Culture Protocols, 2nd ed.; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 9–27. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New Insights into Tissue Culture Plant-Regeneration Mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xiong, Y.; Guo, B.; Yan, H.; Jian, S.; Ren, H.; Zhang, X.; Li, Y.; Zeng, S.; Wu, K.; Zheng, F.; Teixeira da Silva, J.A.; Xiong, Y.; Ma, G. Shoot organogenesis and somatic embryogenesis from leaf and root explants of Scaevola sericea. Sci. Rep. 2020, 10, 11343. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Dogramaci, M.; Sarkar, D.; Finger, F.L.; Shetty, K.; Fugate, K.K. Natural Elicitors Enhanced Suberin Polyphenolic Accumulation in Wounded Potato Tuber Tissues. Front. Plant Sci. 2024, 15, 1384602. [Google Scholar] [CrossRef]
- Cham, A.K.; Ojeda Zacarías, M.C.; Lozoya Saldaña, H.; Vázquez Alvarado, R.E.; Olivares Sáenz, E.; Alvarado Gómez, O.G. Effects of Elicitation on In Vitro Regeneration of Two Tomato (Solanum lycopersicum L.) Cultivars in Tissue Culture. J. Exp. Biol. Agric. Sci. 2024, 12, 106–123. [Google Scholar] [CrossRef]
- Chintha, P.; Sarkar, D.; Ramakrishna, R.; Dogramaci, M.; Lulai, E.C.; Shetty, K. Biological elicitors to enhance wound healing responses in cut potato tubers. Sci. Hortic. 2023, 319, 112152. [Google Scholar] [CrossRef]
- Sokolova, D.O.; Królicka, A.; Czajkowski, R. Elicitation of potato plants to increase their resistance against Soft Rot Pectobacteriaceae bacteria. Eur. J. Plant Pathol. 2025, 171, 67–80. [Google Scholar] [CrossRef]
- Umemoto, N.; Yasumoto, S.; Yamazaki, M.; Asano, K.; Akai, K.; Lee, H.J.; Akiyama, R.; Mizutani, M.; Nagira, Y.; Saito, K.; Muranaka, T. Integrated Gene-Free Potato Genome Editing Using Transient Transcription Activator-like Effector Nucleases and Regeneration-Promoting Gene Expression by Agrobacterium Infection. Plant Biotechnol. 2023, 40, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Forest, M.J.; Ojeda Zacarías, M.C.; Lozoya Saldaña, H.; Olivares Sáenz, E.; Cham, A.K. The impact of elicitation on potato (Solanum tuberosum L.) production, enzymatic and antioxidant activity in Nuevo León, Mexico. J. Exp. Biol. Agric. Sci. 2023, 11, 572–580. [Google Scholar] [CrossRef]
- Ariste, A.; Ojeda Zacarías, M. del C.; Lozoya Saldaña, H.; Olivares Sáenz, E.; García Zambrano, E.A.; Ibarra López, A.; Cham, A.K. Optimized in vitro micropropagation and microtuber production in potato (Solanum tuberosum L.) through apical buds using hormone regulation and tissue culture techniques. J. Exp. Biol. Agric. Sci. 2025, 13, 86–96. [Google Scholar] [CrossRef]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Savelieva, E.M.; Arkhipov, D.V.; Deigraf, S.V.; Romanov, G.A. Global view on the cytokinin regulatory system in potato. Front. Plant Sci. 2020, 11, 613624. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.M.; Oros, P.; Cătană, C.; Antofie, M.M.; Sand, C.S. In vitro cultivation of purple-fleshed potato varieties: Insights into their growth and development. Horticulturae 2023, 9, 425. [Google Scholar] [CrossRef]
- Miladinova-Georgieva, K.; Geneva, M.; Stancheva, I.; Petrova, M.; Sichanova, M.; Kirova, E. Effects of different elicitors on micropropagation, biomass, and secondary metabolite production of Stevia rebaudiana Bertoni – A review. Plants 2022, 12, 153. [Google Scholar] [CrossRef]
- Hajare, S.T.; Chauhan, N.M.; Kassa, G. Effect of growth regulators on in vitro micropropagation of potato (Solanum tuberosum L.) Gudiene and Belete varieties from Ethiopia. Sci. World J. 2021, 2021, 5928769. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Sapakhova, Z.; Kanat, R.; Akhmetzhanova, D.; Abilda, Z.; Toishimanov, M.; Raissova, N.; Otynshiyev, M.; Zhambakin, K.; Shamekova, M. The impact of the growth regulators and cultivation conditions of temporary immersion systems (TISs) on the morphological characteristics of potato explants and microtubers. Agronomy 2024, 14, 1782. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Ahmad, R.; Ahmed, B.; Khan, T.U.; Yasin, M. Effect of growth regulators on callus induction and plant regeneration in potato (Solanum tuberosum L.) explants. Sarhad J. Agric. 2023, 39, 140–146. [Google Scholar] [CrossRef]
- Sota, V.; Bekheet, S.; Kongjika, E. Effect of growth regulators on micropropagation and in vitro tuberization of Solanum tuberosum L. cv. Vermosh. South West. J. Hortic. Biol. Environ. 2020, 11, 67–81. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Enhancement of shoot organogenesis of potato cultivars ‘Kufri Pukhraj’ and ‘Kufri Chipsona 1’ by β-lactam antibiotics. Potato Res. 2022, 65, 137–151. [Google Scholar] [CrossRef]
- Kumlay, A.M.; Ercisli, S. Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnol. Biotechnol. Equip. 2015, 29, 1075–1084. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Jerry, A.N.; Khalil, A.I. Effect of plant growth regulators and sodium chloride on indirect organogenesis of three cultivars of potato plant (Solanum tuberosum L.) via in vitro culture. AAB Bioflux 2017, 9, 103–110. [Google Scholar]
- García-Hernández, E.; Loera-Quezada, M.M.; Morán-Velázquez, D.C.; et al. Indirect organogenesis for high-frequency shoot regeneration of two cultivars of Sansevieria trifasciata Prain differing in fiber production. Sci. Rep. 2022, 12, 8507. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, B.; Fareed, A.; Ahmad, N.; et al. Elicitors directed in vitro growth and production of stevioside and other secondary metabolites in Stevia rebaudiana (Bertoni) Bertoni. Sci. Rep. 2024, 14, 14714. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Jia, Y.; Zhang, J.; He, X.; Wang, X.; Yu, J.; Bai, Y.; Li, S.; Yin, D.; Li, F.; Du, Y. Genome Evolution and Diversity of Wild and Cultivated Potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).