1. Introduction
Marine mammals exemplify a unique and successful evolutionary trend among mammals, representing “an assemblage of at least seven distinct evolutionary lineages that have independently returned to the sea and spend the majority of their time in water” [
1]. The current diversity of parasites in marine mammals may arise from several distinct phenomena: the transfer of parasites from terrestrial ancestors, in-ocean spillover of parasites between different marine mammals or even other vertebrates, and adaptive evolution in response to their new aquatic habitat, etc.
The gray whale (
Eschrichtius robustus) is a marine mammal that can weigh up to 35 tons and reach lengths of up to 15 meters. It inhabits shallow waters along the continental edges of the United States and Mexico to the east, and Russia, Japan, Korea, and China to the west [
2] (pp. 222–241). Overall, the gray whale’s status is classified as Least Concern on The IUCN Red List [
3]. However, the western (Okhotsk-Sea) subpopulation is listed as Endangered under criterion D on The IUCN Red List [
4] and is also classified as Endangered in the Russian Red Data Book of Rare and Endangered Species (RDBRF) [
5]. Parasites, particularly those that cause diseases, are considered a significant threat in this context [
2,
5]. Within the framework of biodiversity conservation, whaling is currently strictly prohibited in Russia, with the sole exception made for Indigenous Peoples of the North (IPN). The cultural identity and survival of groups such as the Chukchi, Evenki, Eskimo, Kereki, and others are closely tied to whaling practices [
6]. The rights of these ethnic groups to hunt whales are recognized by both the International Whaling Commission and the Russian government, similar to how the Government of Greenland acknowledges the essential right of the Greenland Inuit to hunt caribou (
Rangifer tarandus) [
7]. Similar to the unique life cycle of the northern
Taenia saginata in Russia, which depends on reindeer (
R. tarandus) consuming human feces and the traditional practice of IPN to eat the raw brains of hunted reindeer [
8], the life cycle of certain other helminths may also be maintained due to the ancient custom of consuming raw parts of hunted gray whales [
9].
Aboriginal whale hunting in Chukotka, Russia, is monitored by specialists from the Russian Federal Research Institute of Fisheries and Oceanography (VNIRO). They ensure that female whales with calves are not hunted, assess the condition and fat content of gray whales, record population data, and conduct various research activities [
10,
11]. Once a gray whale is hunted and brought ashore, its carcass is butchered within half an hour, involving approximately 30 villagers in the process. This brief window is the only opportunity for a VNIRO specialist to collect parasite samples. Otherwise, such samples can only be obtained from stranded or entangled gray whales that are found dead [
12,
13,
14,
15].
Sixteen species of parasites were reported in gray whales during the pre-DNA era [
15]. Some of these ecto- and endoparasites are considered species-specific [
2,
15], and thus they are as endangered as gray whales themselves. As integral components of hidden biodiversity, these parasites deserve the same level of attention as vertebrates and free-living species [
16]. “A holistic appreciation of parasites should consider both their (many) beneficial and (a few, proven) detrimental impacts, something which could be operationally incorporated into conservation assessments and strategies” [
17].
The parasites of gray whales warrant further study for several reasons:
They can provide insights into the phylogeny of marine mammals through their associated parasites (biology);
They may pose a threat to their endangered host, the gray whale (veterinary);
They could present a risk to humans who consume gray whale organs (human medicine);
They represent an equal and fragile component of biodiversity (conservation);
They produce propagules that serve as food for other marine species (ecology).
Every opportunity should be seized to enhance our understanding of gray whale parasites. Therefore, the aim of this study was to report on the parasitic worms found in the gray whale hunted in Chukotka, Russia. This work presents, for the first time, the molecular characteristics of these helminths, along with detailed morphological depictions obtained through light and scanning electron microscopy, as well as histopathological findings from the infected gray whale.
2. Materials and Methods
On September 18, 2024, a male gray whale measuring 8 meters in length was hunted in the Bering Sea by the IPN from the village of Lorino, Chukotka, Russia, in accordance with license number 53, signed by the Deputy Head of the Federal Service for Supervision of Natural Resources on April 28, 2024. A specialist from VNIRO was able to access the small intestine of the whale and examine it on the seashore. Numerous tapeworms were found firmly attached to the intestinal walls. Photographs of the live cestodes were taken using the dual camera of an iPhone 7 Plus (Apple Inc., Cupertino, USA). Several fragments of the cestodes were collected, each divided into two parts. The material was placed into individually labeled containers. Some parts were preserved in 10% buffered formalin for morphological evaluation, while other corresponding parts were preserved in 70% ethanol for molecular analysis. The samples were subsequently delivered to the Parasitology Center of the Institute of Ecology and Evolution of the Russian Academy of Sciences (IEE RAS) in Moscow, Russia.
Light microscopy (LM) was conducted using optical microscopes MBS-10 and Micmed-6 (LOMO-MA, Saint Petersburg, Russia). Photographs were captured with a digital camera, the Canon 5D Mark II (Canon Inc., Tokyo, Japan), which was connected to the microscope via a C-mount adapter (LOMO-MA, Saint Petersburg, Russia). Measurements were performed using Fiji/ImageJ software, version 1.2.4 (RRID:SCR_003070) from the National Institutes of Health (Bethesda, MD, USA), calibrated with a transmitted light object micrometer (OMP) slide (LOMO-MA, Russia).
Scanning electron microscopy (SEM) was conducted using a TESCAN MIRA 3 LMH scanning electron microscope (TESCAN, Brno, Czech Republic) at the Joint Usage Center “Instrumental Methods in Ecology” of the IEE RAS. Sample preparation involved a series of washes with increasing concentrations of alcohol, concluding with acetone. The samples were then dried using critical point drying and coated with gold for imaging.
Histological slides of the helminth sections were prepared and scanned by the Unim Pathomorphological Laboratory in Moscow, Russia, through the collaboration of VetUnion Company. Hematoxylin and eosin (H&E) staining was utilized for the preparation of the slides.
DNA analysis was conducted as follows: DNA was extracted using the QIAamp DNA Accessory Set, Mini Kit (Qiagen, Venlo, Netherlands). The targeted regions included the mitochondrial cytochrome oxidase (
CoxI mtDNA), the nuclear small subunit (SSU or 18S), the nuclear large subunit (LSU or 28S), and the nuclear internal transcribed spacer (ITS2) of ribosomal RNA. For
CoxI mtDNA, the forward primer PBI-cox1F (CAT TTT GCT GCC GGT CAR CAY ATG TTY TGR TTT TTT GG) and reverse primer PBI-cox1R (CCT TTG TCG ATA CTG CCA AAR TAA TGC ATD GGR AA) were utilized, following established protocols [
18]. For the 18S region, the forward primer WormA (GCC AAT GGC TCA TTA AAT CAG) and reverse primer WormB (CTT GTT ACG ACT TTT ACT TCC) were used, along with the additional sequencing primer 1270F (ACT TAA AGG AAT TGA CGG) and corresponding protocols [
19]. The 28S region was amplified using the forward primer ZX-1 (ACC CGC TGA ATT TAA GCA TAT) and reverse primer 1500R (GCT ATC CTG AGG GAA ACT TCG), along with the additional sequencing primer 300F (CAA GTA CCG TGA GGG AAA GTT G) [
19]. For the ITS2 region, the forward primer FLO1 (CGG TGG ATC ACT CGG CTC) and reverse primer ITSII (TCC TCC GCT TAT TGA TAT GC) were used, following appropriate protocols [
20]. Amplification products were checked, purified, and sequenced, and the resulting chromatograms were analyzed as described by Loginova et al. [
21]. Prior to analysis, the best-fitting models for the alignment of the 28S rRNA gene dataset (TIM3+G) and the 18S rRNA gene dataset (GTR+I+G) were determined using jModelTest v2.1.10 [
22]. Bayesian inference analyses were performed using MrBayes (v3.2.7a) [
23], with Markov chain Monte Carlo simulations run for 15,000,000 generations; only the final 75% of trees were utilized to generate the consensus trees [
24].
3. Results
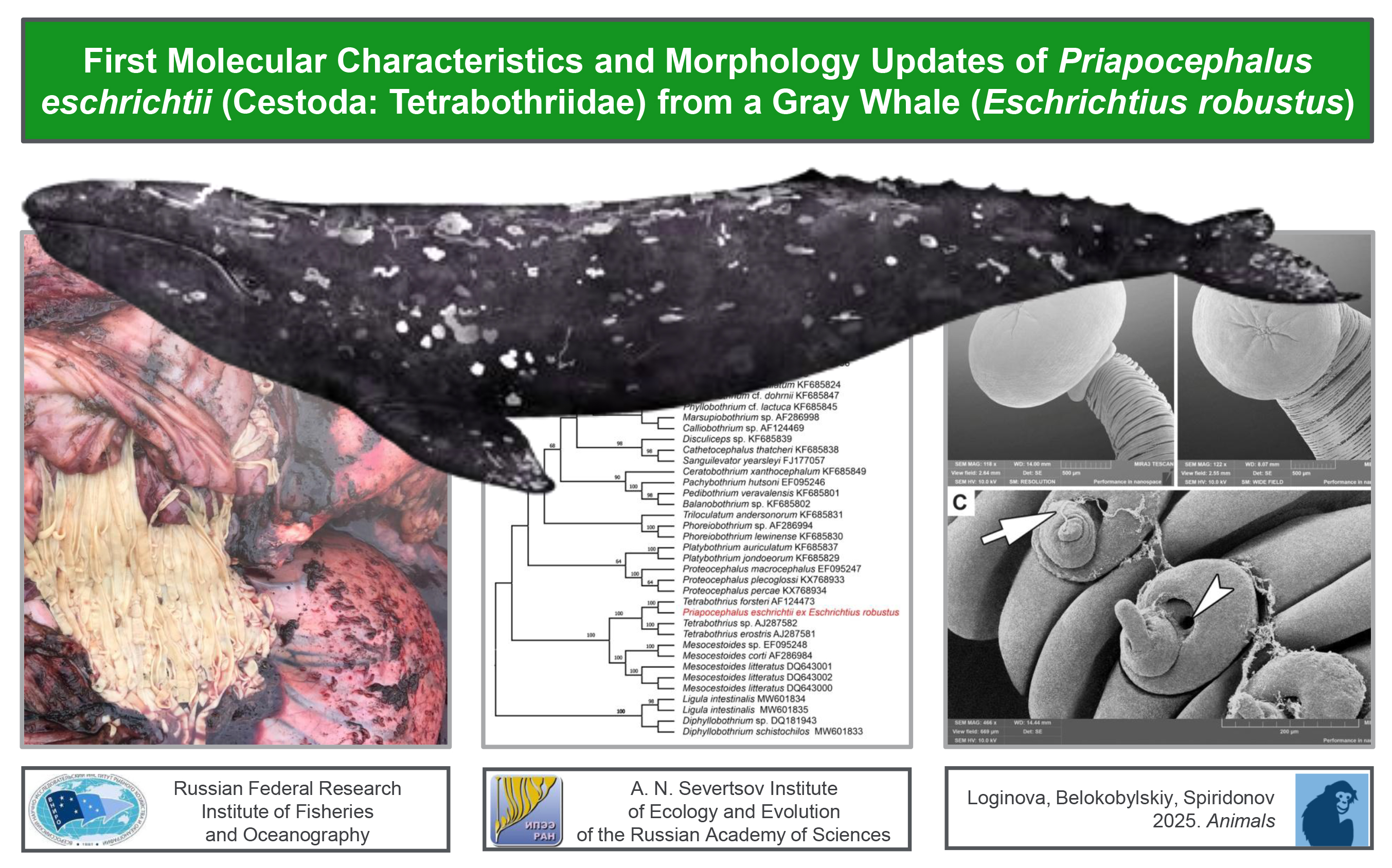
Numerous live cestodes were observed firmly attached to the wall of the small intestine of a gray whale that was legally hunted in the Bering Sea by IPN in Chukotka, Russia (
Figure 1).
During the butchering of the whale, its intestine was sectioned into approximately 1-meter-long segments, as the entire organ was too heavy to handle effectively. The edges of these segments facilitated the identification of parasites (
Figure 1A). The intensity of the infestation was observed to exceed 50 specimens per meter of intestine (
Figure 1C). Although it was not possible to measure the total length of the cestodes, they were estimated to reach approximately 40-50 cm. Due to muscle contractions, their length and width varied significantly; for instance, within 15 seconds, the cestodes contracted and shortened by 5 cm (
Figures 1D, E). As they shortened, their width increased from 2 to 6 mm (
Figure 1B).
Details of the cestode’s attachment to the intestinal wall and its morphology are presented in
Figure 2.
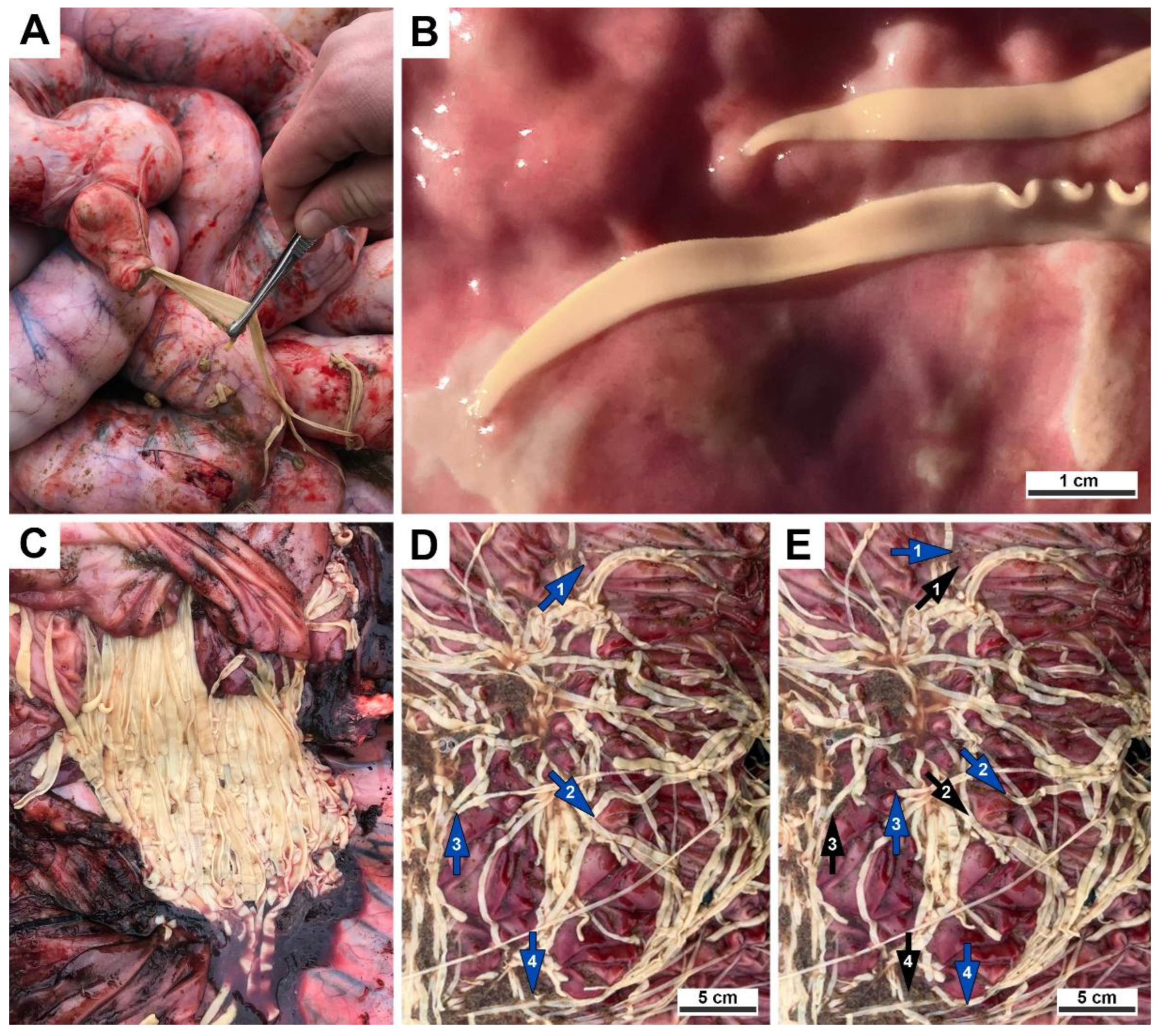
Histological examination of the intestine revealed chronic lymphoplasmacytic inflammation of the mucous membrane (
Figure 2A). The scolex, embedded in the wall of the small intestine, is followed by a collar-like basal region within the intestinal lumen, and proglottids that contain tubular glandular structures and smooth muscle bundles.
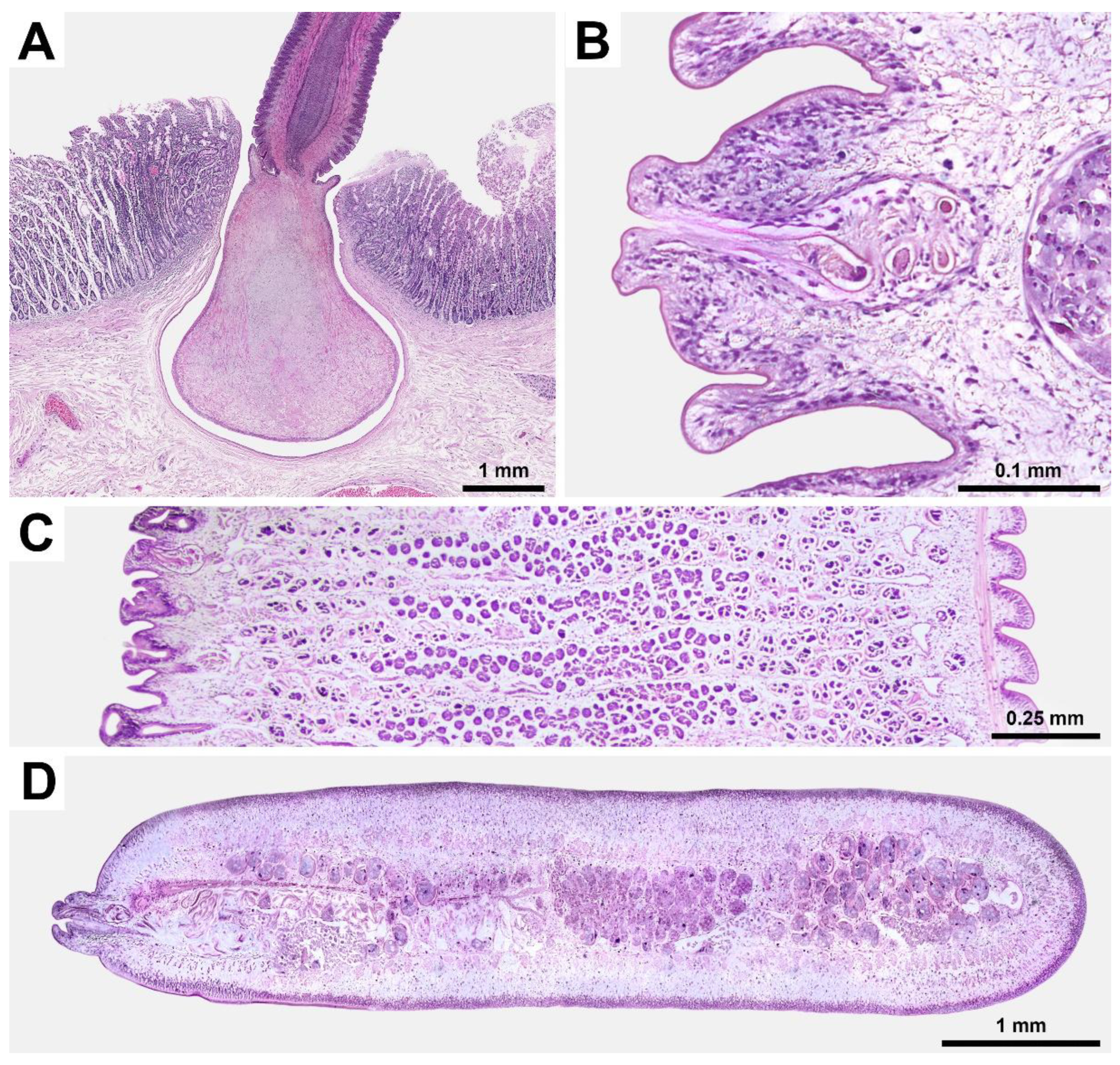
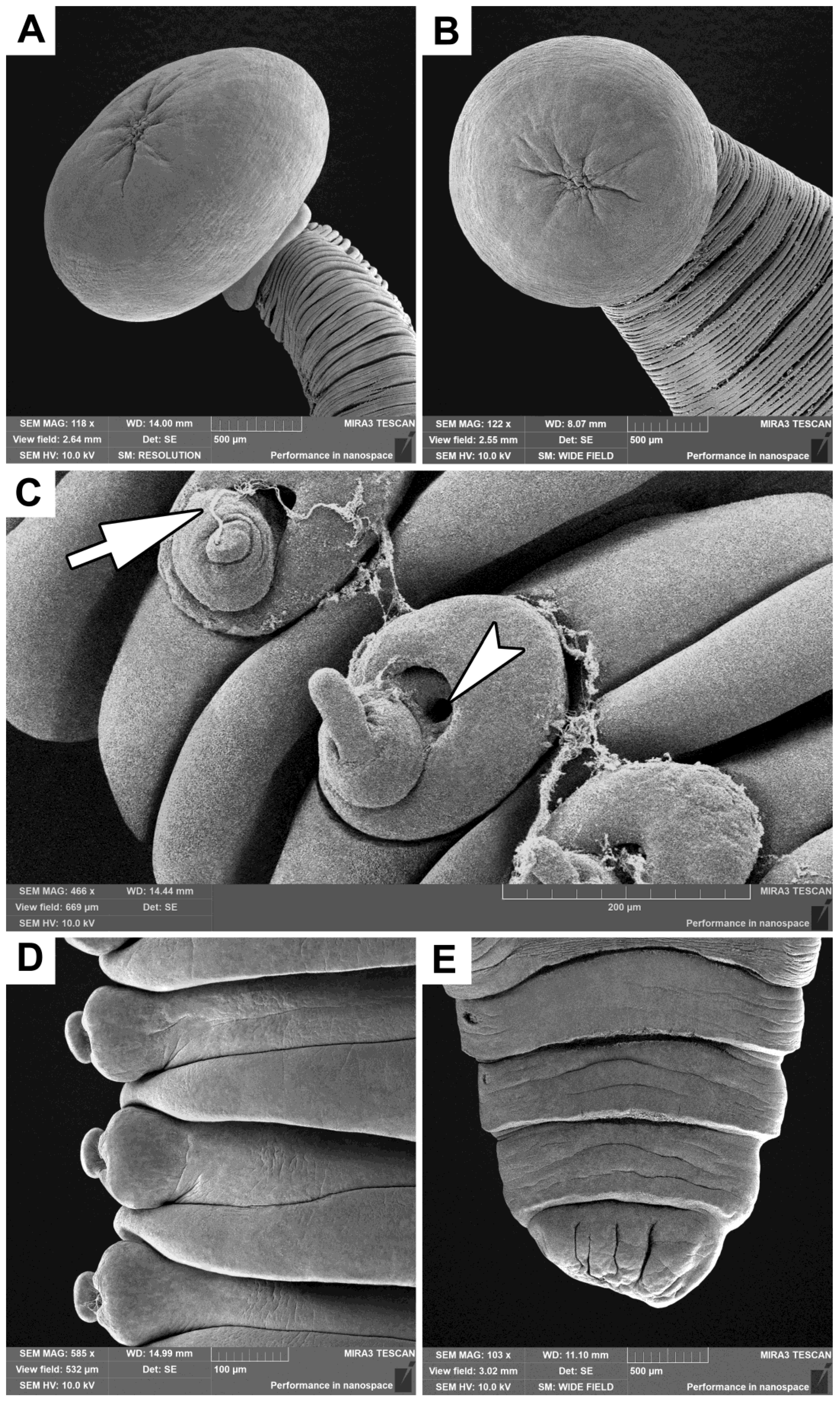
The morphology of the obtained cestodes, studied using LM and SEM, is presented in
Figure 3 and
Figure 4.**.
The morphology of the cestodes found in the small intestine of the gray whale, which was legally hunted by the IPN in Chukotka, Russia, closely matched that of
Priapocephalus eschrichtii as described by Muravyova and Treshchev [
25]. Therefore, based on morphological characteristics and host specificity, the obtained cestodes were identified as
P. eschrichtii. Sequences for
CoxI mtDNA, 18S, 28S, and ITS2 were obtained. Since the sequencing results were the same for every approach, single sequences of each type were submitted to GenBank. The corresponding voucher specimen, IPEE_Parasites 14376, was archived in the Museum of Helminthological Collections of the Parasitology Center, IEE RAS (
Table 1).
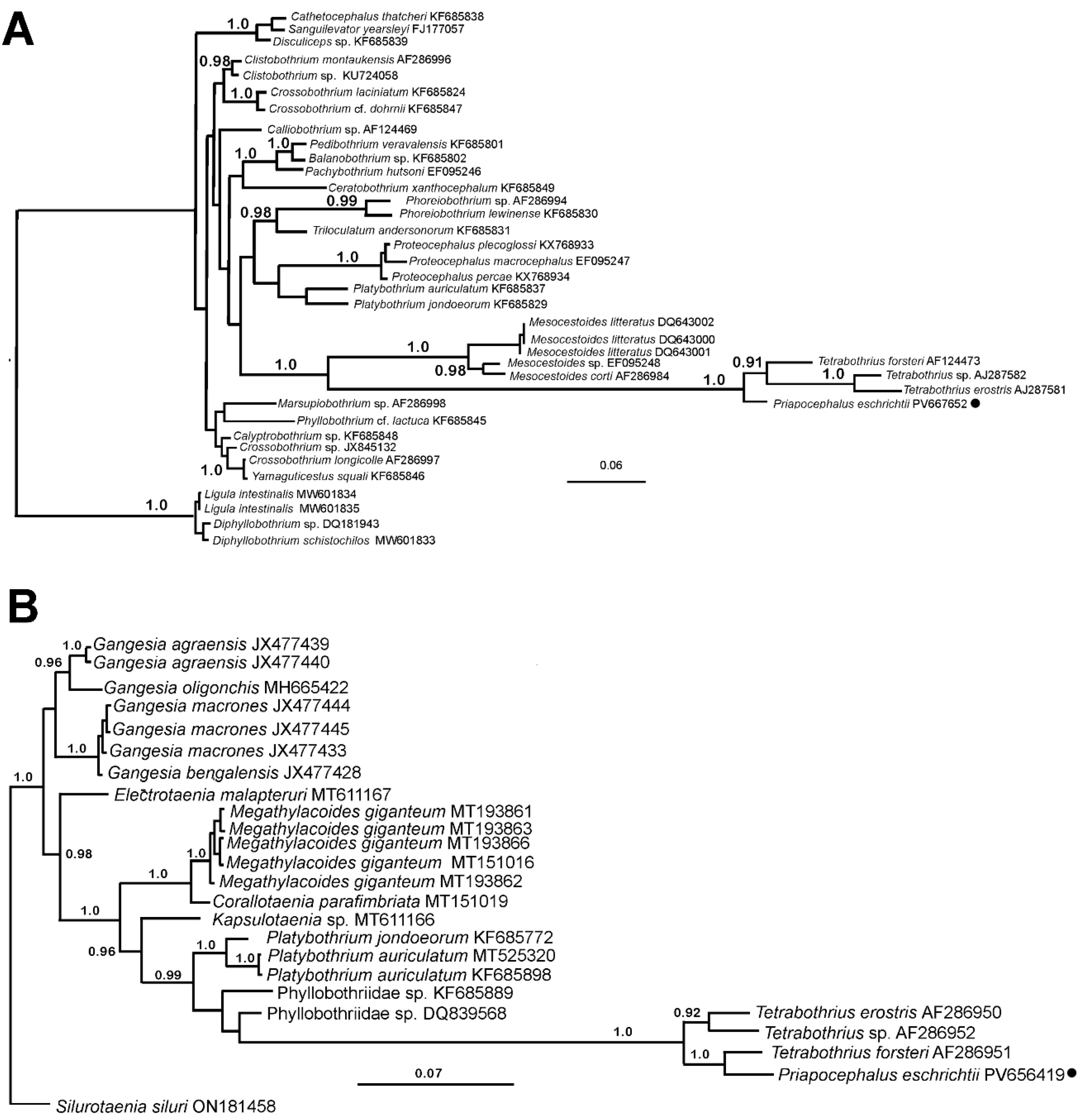
The phylogenetic analyses of the studied
P. eschrichtii samples, based on the 18S and 28S genes, revealed close relationships between
P. eschrichtii and
Tetrabothrius species with known sequences for these taxonomic markers (
Figure 5). In both phylogenetic trees, the studied species clustered with
Tetrabothrius species with maximal support. In the 18S rDNA phylogram,
P. eschrichtii occupies a basal position, while in the 28S rDNA tree, it is positioned as a sister taxon to
T. forsteri (
Figure 5B). Furthermore, the terminal clade comprising
Tetrabothrius and
Priapocephalus exhibited the highest level of nucleotide substitutions (branch length) compared to all other cestodes included in the analysis.
A BLAST search for similar sequences in the NCBI GenBank using the obtained ITS2 rDNA sequence revealed a limited set of related cestode sequences. Different groups of cestodes were unevenly represented within this set, rendering the phylogenetic analysis of this sequence nearly meaningless, despite a high percentage of parsimony-informative characters (356 out of 564 bp in the alignment). No strongly supported affinities were identified between the ITS2 rDNA sequence of P. eschrichtii and other available ITS2 rDNA sequences of cestodes (data not shown).
The proportion of parsimony-informative characters in the CoxI mtDNA alignment, which is 532 bp long, was comparatively lower at 156 characters. Additionally, the number of cestode species with known CoxI mtDNA sequences was also limited, with few higher taxa of this class represented in the NCBI GenBank. The CoxI mtDNA sequence of P. eschrichtii exhibited weakly supported relationships (data not shown) with Paranoplocephala sp. strain RKZ13 (complete mitochondrial genome, accession number NC_061205).
4. Discussion
Cestodes obtained from a gray whale in 2024 were identified as
P. eschrichtii, one of only two cestode species reported in gray whales [
15]. These specimens were morphologically and genetically identical to those collected in 2022 [
26]. In 2022, none of the collected specimens had a scolex, preventing a complete morphological diagnosis. The sequences reported here represent the first molecular data for
P. eschrichtii, as there were no references available for molecular comparison in 2022. Additionally, specimens of
P. eschrichtii collected from gray whales in Lorino were found to be longer and wider than those described by Muravyova and Treshchev [
26] or Kuramochi et al. [
15] (
Table 2).
These differences may be attributed to several factors. Muravyova and Treshchev, as well as Kuramochi et al., likely worked with specimens delivered to their laboratories rather than directly observing them at the collection site. Consequently, the specimens they received may not have been whole cestodes but rather fragments. Similarly, in this study (and in 2022), only fragments—not entire helminths—were sent to the laboratory. Alternatively, our colleagues may have studied juvenile specimens that had not yet reached their full size. It is also possible that the measurements reported in previous studies pertained to dead helminths, which were likely preserved in formalin or another substance, potentially causing contraction and a reduction in size. As shown in
Figure 1D and E,
P. eschrichtii specimens can reduce their length by up to 5 cm in just 15 seconds. These muscle contractions also affect the width and thickness of the cestodes. A similar phenomenon, which is likely inherent to all flatworms, has been documented in another tetrabothriid,
Tetrabothrius affinis [
27] (pp. 85–86). Terimova and Skrjabin noted that the size ranges of tetrabothriid strobila can vary even within a single species, depending on host species, intensity of infection, and other factors [
28].
Temirova and Skrjabin [
28] proposed that the apical region of the scolex in
P. minor was actually a remnant of a true scolex, which is typical for tetrabothriids, situated atop a pseudoscolex. The true scolex diminishes in size, while the pseudoscolex develops following the penetration of the intestinal wall. Our study supports this hypothesis, as
Figure 4A and B illustrate a wrinkled area at the apex of the scolex in
P. eschrichtii.
According to Baer [
29], the cirrus in
Priapocephalus is covered with short spines. However, our study does not support this claim (see Figures 2B,C,D; 3C; and 4C,D). It is important to note that the specimens in the present research were not examined immediately for the presence of spines, and it is possible that the cestodes may have lost them over time. Additionally, Muravyova and Treshchev [
26] did not report the presence of spines. The majority of tetrabothriids have been documented as having unarmed cirri [
28].
Rawson [
30] described par-uterine organs, which are vesicle-like structures similar to those found in
Avitellina centripunctata, a parasite of ruminants, in
T. erostis. He noted that eggs are shed singly through the uterine pore. Similarly, Muravyova and Treshchev [
26] described and illustrated the shedding of eggs singly through uterine pores in
P. eschrichtii. In this study, we observed that eggs of
P. eschrichtii were also excreted through uterine pores (
Figure 3D), but they were clustered within an irregular capsule (
Figure 3F), as previously described for
T. jagerskioeldi [
31] and
T. fraterculus [
32]. It remains unclear whether these “par-uterine vesicles” and “irregular capsules” represent the same structures, and whether they must rupture to release the eggs. Currently, there are insufficient suitable samples to address this question. Further sampling is necessary to investigate this phenomenon, as the clustering of eggs (rather than their solitary excretion) could explain the multiple infestations of tetrabothriids observed in hosts among whales and seabirds. This study reports over 50 specimens of
P. eschrichtii found in 1 meter of the small intestine of a single gray whale. Skrjabin reported 830 tetrabothriids in 1 meter of the small intestine of a sei whale (
Balaenoptera borealis) and 3,328 tetrabothriids in another sei whale [
28]. In seabirds,
T. minor specimens were reported in such high numbers in fulmars that the intestinal lumen was completely occupied by them [
28]. If eggs are excreted in clusters, they are likely consumed in clusters by intermediate hosts, which could lead to multiple infestations of the definitive host, assuming that the larval cestodes can realize their full invasive potential. This multiple infestation appears even more pronounced when considering other potential hosts, such as gray whales, which have been observed to be completely free of tetrabothriids during aboriginal whaling. The number of eggs per capsule may be a species-specific trait that correlates with the ability of the intermediate host to harbor larvae and survive. Consequently, this trait could have diagnostic value, as the number of tetrabothriid larvae per invertebrate intermediate host may preliminarily indicate the specific cestode species that produced these eggs.
In discussing the pathogenic impact of tetrabothriids on whale hosts, Terimova and Skrjabin [
28] quoted Rees [
33] who studied
T. affinis from a blue whale: “heavy infestation … result in a considerable reduction of the functional area of the mucosa.” However, what she really wrote was: “It is possible, also, that the worm does not change its position once it is attached. If it did so the damage to the part of the mucosa concerned would appear to be permanent, and a heavy infestation, where the worms changed their position frequently, would result in a considerable reduction of the functional area of the mucosa”, and she did not know if
T. affinis changed its position or not [
33]. The unique attachment mechanism of
Priapocephalus suggests a permanent association with its host, allowing for comparisons only within the genus. Terimova and Skrjabin [
28] studied a specimen of
P. minor in situ. Their histological examination of the intestine revealed an infiltrate of plasma cells, along with leukocytes and lymphocytes (see Figures 4 and 8 in [
28]). They also reported areas of sclerosis characterized by denser cellular infiltrates and glandular atrophy. Our findings align well with these observations (
Figure 2A). In addition to causing chronic inflammation of the host’s mucous membrane, the impact of tetrabothriids includes nutrient uptake, intoxication from metabolic byproducts, and the risk of intestinal obstruction. Similar to how
Diphyllobothrium latum induces vitamin B
12 deficiency and affects the metabolism of other B vitamins in its human host [
34],
P. eschrichtii may influence the metabolism and behavior of its whale host. Felix [
35] emphasized the necessity of investigating the role of parasites in marine mammal stranding events and the importance of accurate identification of these parasites.
Despite the evident morphological differences in scolex structure, Terimova and Skrjabin [
28] soon proposed that close taxonomic relationships exist between
Priapocephalus and
Tetrabothrius. This hypothesis was further supported by Hoberg [
36]. A comprehensive cladistic analysis conducted by Hoberg and Adams [
37] examined the relationships between
Priapocephalus,
Anophryocephalus from pinnipeds, and
Strobilocephalus from dolphins and beaked whales, leading to the identification of a set of synapomorphies for several marine tetrabothriid genera. Although nucleotide data for
Anophryocephalus are currently unavailable, the general conclusions drawn by the aforementioned authors regarding tetrabothriid evolution are gaining support from the analysis of
Priapocephalus relationships. The phylogram based on 18S rDNA (
Figure 5) illustrates strongly supported relationships between the clade comprising
Tetrabothrius and
Priapocephalus and species of
Mesocestoides. This finding echoes Baer’s early hypothesis [
38] regarding the relationships between these genera and suggests a possible colonization of marine habitats by this group of cestodes from pinnipeds. In addition to the 18S, 28S, and
CoxI mtDNA gene region sequences known for tetrabothriids, ITS2 sequences have also been uploaded to GenBank (
Table 1) for further comparative analysis, as ITS2 has proven to be a valuable tool for species identification in other cestodes, such as diphyllobothriids [
20].
5. Conclusions
Addressing In addressing the issues outlined in the introduction, the following conclusions can be drawn:
Molecular and morphological analyses support a close relationship between Priapocephalus and Tetrabothrius; however, the phylogeny of Tetrabothriidae and their co-evolution with hosts require further investigation;
Adults of P. eschrichtii pose a threat to their host, the gray whale, through inflammation of the mucosa in the small intestine, significant nutrient depletion, intoxication from metabolic byproducts, and the risk of intestinal obstruction;
While adults of P. eschrichtii do not pose a threat to humans consuming gray whales, as the gray whale is their definitive host, the circumstances that allowed for the sampling of P. eschrichtii may also provide opportunities for further investigation of raw brain and other parts of the gray whale for potential cestode larval stages that could infect humans eating uncooked whale organs;
The nucleotide sequences obtained in this study provide a foundation for further comparison between P. eschrichtii and other Priapocephalus species, which will help confirm (or refute) the host specificity of P. eschrichtii and its dependence on the survival of gray whales;
The reported length and intensity of invasion suggest that P. eschrichtii propagules may serve as a significant food source for other marine invertebrate species.
This study should be continued, and authors encourage collaboration among specialists in the field.
Author Contributions
Conceptualization, O.L.; methodology, O.L. and S.S.; software, S.S.; validation, S.S.; formal analysis, O.L. and S.S.; investigation, I.B., O.L. and S.S.; resources, I.B.; data curation, O.L.; writing—original draft preparation, O.L.; writing—review and editing, S.S. and I.B.; visualization, O.L.; supervision, S.S.; project administration, O.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to sampling parasitic worms from a dead host – gray whale that was legally hunted on 18 September 2024 in the Bering Sea by Indigenous peoples of the North, villagers of Lorino (Chukotka, Russia) in accordance to license 53 signed by Deputy Head of the Federal Service for Supervision of Natural Resources on 28 April 2024. The right of these ethnic groups on whale hunting is recognized both by the Russian Government and the International Whaling Commission. Otherwise, whaling in Russia is strictly prohibited.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CoxI |
Cytochrome oxidase |
| DNA |
Deoxyribonucleic acid |
| GTR+I+G |
General time-reversible model with Invariant sites and Gamma distribution |
| HE |
Hematoxylin and eosin |
| IEE RAS |
Institute of Ecology and Evolution of the Russian Academy of Sciences (Институт прoблем экoлoгии и эвoлюции им. А. Н. Северцoва Рoссийскoй академии наук (ИПЭЭ РАН)) |
| IPN |
Indigenous peoples of the North (Indigenous minority peoples of the North, Siberia, and the Far East of Russia) |
| ITS2 |
Internal transcribed spascer |
| IUCN |
International Union for Conservation of Nature |
| LM |
Light microscopy |
| LSU |
Large subunit |
| ML |
Maximum likelihood |
| RDBRF |
Red Data Book of the Russian Federation |
| RNA |
Ribonucleic acid |
| SEM |
Scanning electron microscopy |
| SSU |
Small subunit |
| TIM3+G |
Transitional model 3 with Gamma distribution |
| VNIRO |
Russian Federal Research Institute of Fisheries and Oceanography (Всерoссийский научнo-исследoвательский институт рыбнoгo хoзяйства и oкеанoграфии (ВНИРО)) |
References
- Berta, A.; Lanzetti, A. Feeding in marine mammals: An integration of evolution and ecology through time. Palaeontol Electron, 2020, 23, a40. [Google Scholar] [CrossRef]
- Wilson, D.E.; Mittermeier, R.A. (Eds.) Handbook of the Mammals of the World. Vol. 4. Sea Mammals; Lynx Edicions: Barcelona, Spain, 2014; pp. 222–241. ISBN 978-84-96553-93-4. [Google Scholar]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search?query=eschrichtius%20robustus&searchType=species (accessed on 04 August 2025).
- Cooke, J.G.; Taylor, B.L.; Reeves, R; Brownell Jr., R.L. 2018. Eschrichtius robustus (western subpopulation). The IUCN Red List of Threatened Species 2018: e.T8099A50345475. Accessed on 04 August 2025. [CrossRef]
- Ilyashenko, V.Yu.; Burdin, A.M. Gray Whale Eschrichtius robustus Lilljeborg, 1861. In The Red Book of the Russian Federation, Animals, 2nd ed.; VNII Ecologii: Moscow, Russia, 2021; pp. 1060–1063. ISBN 978-5-6047425-0-1. (In Russian) [Google Scholar]
- Polyakova, O.V.; Filatova, O.A.; Fedutin, I.D.; Litovka, D.I.; Bukenov, B.; Artaev, V.B.; Humston-Fulmer, E.M.; Binkley, J.; Kosyakov, D.S.; Lebedev, A.T. Solving the mystery of the Chukotka stinky gray whales. Chemosphere 2023, 315, 137785. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H. Sustainable Greenland and indigenous ideals. In The Earth Charter in Action; Corcoran, P.B., Ed.; Royal Tropical Institute (KIT): Amsterdam, the Netherlands, 2005; pp. 106–108. ISBN 90-6832-177-3. [Google Scholar]
- Konyaev, S.V.; Nakao, M.; Ito, A.; Lavikainen, A. History of Taenia saginata Tapeworms in Northern Russia. Emerg Infect Dis 2017, 23, 2030–2037. [Google Scholar] [CrossRef]
- Mukhachyov, A.D. Northern Cuisine: A Collection of Culinary Recipes; Apex: Norilsk, Russia, 2006; pp. 6–118. ISBN 5-93633-039-7. (In Russian) [Google Scholar]
- Litovka, D.I.; Klyuchnikova, P.S.; Riabov, A.A.; Belokobylsky, I.F.; Semenikhin, A.V.; Naidenko, S.V. Toxicology researches and monitoring of Gray whales of Chukotka Peninsula (Russia), 2008-2022. In Proceedings of the 69A Scientific Committee meeting of International Whaling Commission, Bled, Slovenija, 24 April–6 May 2023. [Google Scholar]
- Belokobylskiy, I.F. Gray whale feeding rates in Mechigmen Bay. In Proceedings of the MARESEDU, Moscow, Russia, 23–27 October 2023. (In Russian). (In Russian). [Google Scholar]
- Dailey, M.; Stroud, R. Parasites and Associated Pathology Observed in Cetaceans Stranded along the Oregon Coast. J Wildl Dis 1978, 14, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Dailey, M.D.; Gulland, F.M.D.; Lowenstine, L.J.; Silvagni, P.; Howard, D. Prey, parasites and pathology associated with the mortality of a juvenile gray whale (Eschrichtius robustus) stranded along the northern California coast. DAO 2000, 42, 111–117. [Google Scholar] [CrossRef]
- Gulland, F.M.D.; Perez-Cortes, H.M.; Urban, J.R. et al. Eastern North Pacific gray whale (Eschrichtius robustus) unusual mortality event, 1999–2000. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-AFSC-150. 2005. 33 pp.
- Kuramochi, T.; Arai-Leon, K.; Umetani, A.; Yamada, T.K.; Tajima, Y. Endoparasites Collected from the Gray Whale Eschrichtius robustus Entangled in a Set Net off Minamiboso-shi, Chiba, on the Pacific Coast of Japan. Bull Natl Mus Nat Sci Ser A 2017, 43, 29–36. [Google Scholar]
- Pizzi, R. Veterinarians and Taxonomic Chauvinism: The Dilemma of Parasite Conservation. J Exot Pet Med 2009, 18, 279–282. [Google Scholar] [CrossRef]
- Rubio-Godoy, M.; Perez-Ponce de Leon, G. Equal rights for parasites: Windsor 1995, revisited after ecological parasitology has come of age. Biol Conserv 2023 284, 110174. [CrossRef]
- Scholz, T.; de Chambrier, A.; Kuchta, R.; Littlewood, D.T.J.; Waeschenbach, A. Macrobothriotaenia ficta (Cestoda: Proteocephalidea), a parasite of sunbeam snake (Xenopeltis unicolor): example of convergent evolution. Zootaxa 2013, 3640, 485–499. [Google Scholar] [CrossRef]
- Littlewood, D.T.J. , Waeschenbach, A.; Nikolov, P.N. In search of mitochondrial markers for resolving the phylogeny of cyclophyllidean tapeworms (Platyhelminthes, Cestoda) – a test study with Davaineidae. Acta Parasitol 2008, 53, 133–144. [Google Scholar] [CrossRef]
- Logan, F.J.; Horak, A.; Stefka, J.; Aydogdu, A.; Scholz, T. The phylogeny of diphyllbothriid tapeworms (Cestoda: Pseudophyllidea) based on ITS-2 rDNA sequences. Parasitol Res 2004, 94, 10–15. [Google Scholar] [CrossRef]
- Loginova, O.A.; Rozenfeld, S.B.; Sipko, T.P.; Mizin, I.A.; Panchenko, D.V.; Laishev, K.A.; Bondar, M.G.; Kolpashchikov, L.A.; Gruzdev, A.R.; Kulemeev, P.S.; et al. Diversity and Distribution of Helminths in Wild Ruminants of the Russian Arctic: Reindeer (Rangifer tarandus), Muskoxen (Ovibos moschatus), and Snow Sheep (Ovis nivicola). Diversity 2023, 15, 672. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst Biol 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, LA, USA, 4–14November 2010. pp. 1–8. [CrossRef]
- Muravyova, S.I.; Treshchev, V.V. New cestoda – Priapocephalus eschrichtii n. sp. (Tetrabothriidae) – A parasite of gray whale (Eschrichtius gibbosus Erxleben, 1777) from the Chukotsk Sea. Vestnik Zoologii 1970, 4, 84–86. (In Russian) [Google Scholar]
- Loginova, O.A.; Belokobylskiy, I.F.; Spiridonov, S.E. Preliminary results of the study of cestodes of gray whale (Eschrichtius robustus). In Proceedings of the XVI conference Modern Problems of General and Applied Parasitology, Voronezh, Russia, 27–28 October 2022. (In Russian). [Google Scholar] [CrossRef]
- Delamure, S.L. Helminth fauna of Marine Mammals in Light of Their Ecology and Phylogeny; Izdatel’stvo Akademii Nauk: Moscow, USSR, 1955; pp. 85–86. (In Russian) [Google Scholar]
- Temirova, S.I.; Skrjabin, A.S. Suborder Tetrabothriata (Ariola, 1899) Skrjabin, 1940. In Fundamentals of Cestodology. Tetrabothriatae and Mesocestoidatae – Flatworms of Birds and Mammals; Ryzhikov, K.M, Ed.; Izdatel’stvo Nauka: Moscow, USSR, 1978; Volume 9, pp. 07–118. (In Russian) [Google Scholar]
- Baer, J.G. Taxonomic revision and biological study of the cestodes of the family Tetrabothriidae: Parasites of deep-sea birds and marine mammals; Neuchâtel University Secretariat: Neuchâtel, Switzerland, 1954; pp. 4–121. (In French) [Google Scholar]
- Rawson, D. Sequences in the maturation of the genitalia in Tetrabothrius erostris (Loennberg, 1889) from the intestine of Larus argentatus argentatus Pontoppidan. Parasitology 1964, 54, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Soudachanh, K.M. Diversity of Tetrabothriidae (Eucestoda) among Holarctic Alcidae (Charadriiformes): Resolution of the Tetrabothrius jagerskioeldi Cryptic Species Complex: Cestodes of Alcinae—Provides Insights on the Dynamic Nature of Tapeworm and Marine Bird Faunas under the Stockholm Paradigm. MANTER: Journal of Parasite Biodiversity 2021, 17, 1–77. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Soudachanh, K.M.; Bondarenko, S.K. Resolution of the Tetrabothrius jagerskioeldi Cryptic Species Complex among Holarctic Alcidae (Charadriiformes): Cestodes among Fraterculinae—Exploring Marine Diversity, Host Range, and Dynamic Oceanography in the Greater North Pacific. MANTER: Journal of Parasite Biodiversity 2023, 35, 1–67. [Google Scholar] [CrossRef]
- Rees, G. The scolex of Tetrabothrius affinis (Lonnberg), a cestode from Balenoptera musculus L., the blue whale. Parasitology 1956, 46, 425–442. [Google Scholar] [CrossRef]
- Nyberg, W. Diphyllobothrium latum and human nutrition, with particular reference to vitamin B12 deficiency. Proc Nutr Soc 1963, 22, 8–14. [Google Scholar] [CrossRef]
- Felix, J.R. Reported Incidences of Parasitic Infections in Marine Mammals from 1892 to 1978; Zea Books: Lincoln, USA, 2013; pp. 5–140. ISBN 978-1-60952-042-4. [Google Scholar]
- Hoberg, E.P. Phylogenetic Relationships among Genera of the Tetrabothriidae (Eucestoda). J Parasitol 1989, 75, 617–626. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Adams, A.M. Phylogeny, Historical Biography, and Ecology of Anophryocephalus spp. (Eucestoda: Tetrabothriidae) among Pinnepids of the Holarctic during the Late Tetriary and Pleistocene. Can J Zool 1992, 70, 703–719. [Google Scholar] [CrossRef]
- Baer, J.G. Contribution to the study of Cetacean Cestoda. Swiss J Zool 1932, 39, 393–418. (In French) [Google Scholar]
Figure 1.
Live cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) Several cestodes are pulled out from the intestinal incision; (B) Close view of the two cestodes attached to the wall of the dissected small intestine; (C) General view of a set of cestodes; (D) Cestodes in the dissected intestine (numbered blue arrows indicate positions of the posterior ends of helminths); (E) Same view as D, but depictured 15 seconds later (numbered blue arrows indicate positions of the posterior ends of helminths at the moment of shot, black arrows with corresponding numbers indicate positions of the posterior ends of the same helminths 15 seconds prior to shot). Photographs were taken on the shore of the Bering Sea.
Figure 1.
Live cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) Several cestodes are pulled out from the intestinal incision; (B) Close view of the two cestodes attached to the wall of the dissected small intestine; (C) General view of a set of cestodes; (D) Cestodes in the dissected intestine (numbered blue arrows indicate positions of the posterior ends of helminths); (E) Same view as D, but depictured 15 seconds later (numbered blue arrows indicate positions of the posterior ends of helminths at the moment of shot, black arrows with corresponding numbers indicate positions of the posterior ends of the same helminths 15 seconds prior to shot). Photographs were taken on the shore of the Bering Sea.
Figure 2.
Histological preparations of the cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) Cestode in situ with a scolex embedded in the wall of the small intestine; (B) Longitudinal section of the strobila showing cirrus sac, ventral orientation of strobila; (C) Longitudinal section of the strobila, ventral orientation of strobila, copulative organs seen in the left (cirrus sac top left, vagina bottom left), multiple darker ovary seen in the center, multiple lighter testes seen between ovary and margins; (D) Cross section of the mature proglottide, copulative organs in the left. Light microscopy, hematoxylin and eosin staining.
Figure 2.
Histological preparations of the cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) Cestode in situ with a scolex embedded in the wall of the small intestine; (B) Longitudinal section of the strobila showing cirrus sac, ventral orientation of strobila; (C) Longitudinal section of the strobila, ventral orientation of strobila, copulative organs seen in the left (cirrus sac top left, vagina bottom left), multiple darker ovary seen in the center, multiple lighter testes seen between ovary and margins; (D) Cross section of the mature proglottide, copulative organs in the left. Light microscopy, hematoxylin and eosin staining.
Figure 3.
Cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) total view of scolex with a collar-like basal region; (B) close view of the eggs of one cluster; (C) dorso-lateral view of reproductive organs (arrowheads indicate male reproductive product atop erected genital papillae, arrow indicates non-erected genital papilla); (D) dorso-lateral view of strobila showing uterine pores with clusters of eggs (square indicates one of these clusters), cestode is slightly curved around its longitudinal axis, its anterior end is directed to the right and upward, dextral unilateral genital pores are visible in the right foreground, opposite ventral side is visible in the right background; (E) eggs with an oncosphere and three pairs of embryonic hooks; (F) cluster of eggs in irregular capsule extracted from an uterine pore marked with a square at D. Light microscopy, specimens are depicted in water (previously preserved in 10% buffered formalin), no staining added, E-F, cover slips used.
Figure 3.
Cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) total view of scolex with a collar-like basal region; (B) close view of the eggs of one cluster; (C) dorso-lateral view of reproductive organs (arrowheads indicate male reproductive product atop erected genital papillae, arrow indicates non-erected genital papilla); (D) dorso-lateral view of strobila showing uterine pores with clusters of eggs (square indicates one of these clusters), cestode is slightly curved around its longitudinal axis, its anterior end is directed to the right and upward, dextral unilateral genital pores are visible in the right foreground, opposite ventral side is visible in the right background; (E) eggs with an oncosphere and three pairs of embryonic hooks; (F) cluster of eggs in irregular capsule extracted from an uterine pore marked with a square at D. Light microscopy, specimens are depicted in water (previously preserved in 10% buffered formalin), no staining added, E-F, cover slips used.
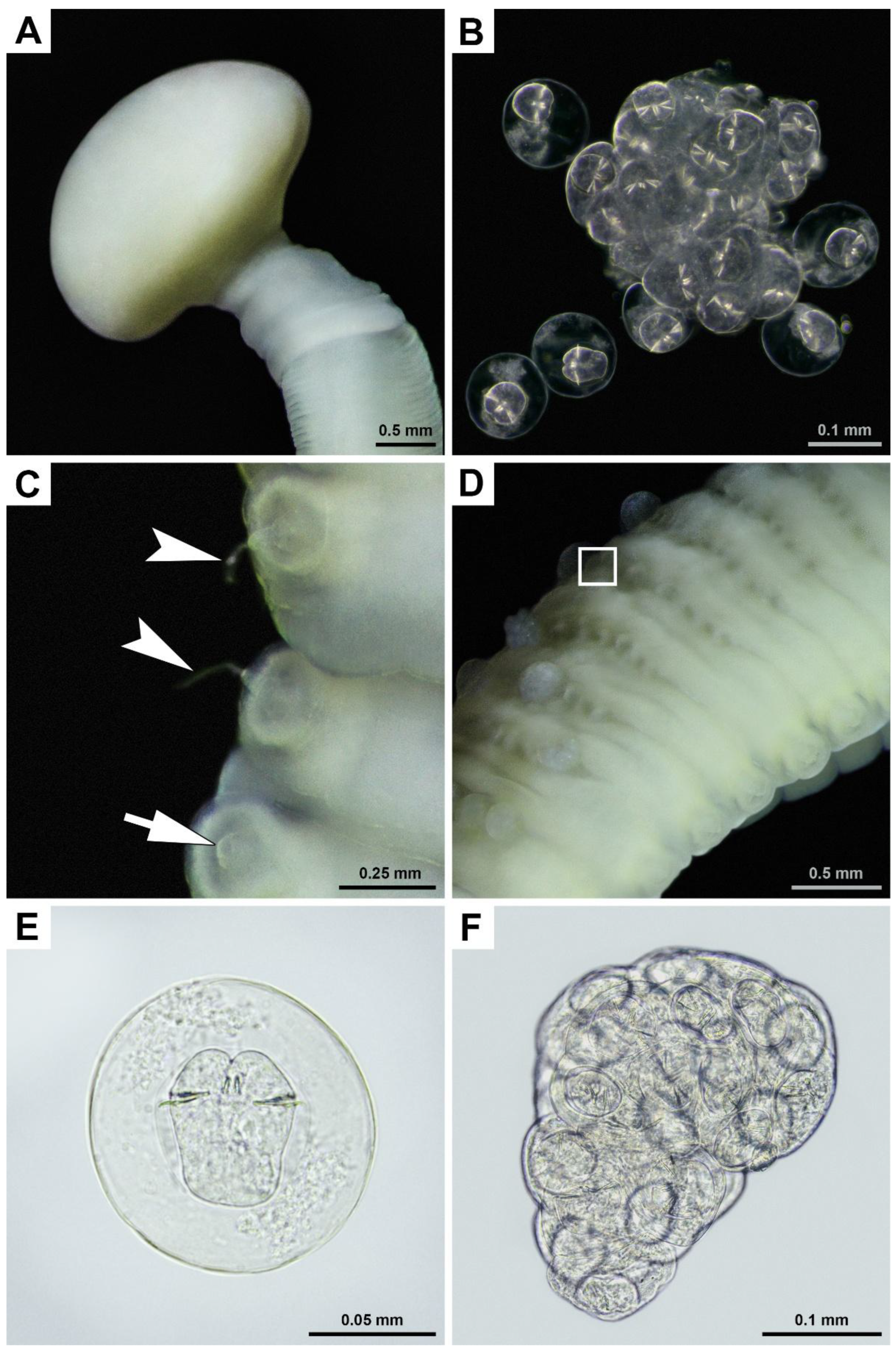
Figure 4.
Scanning electron images of cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) lateral view of scolex with a collar-like basal region; (B) apical view of the same scolex and ventral view of the beginning of strobila; (C) ventro-lateral view of reproductive organs (arrow indicates male reproductive product atop erected genital papillae, arrowhead indicates vagina opening); (D) ventral view of strobila showing unilateral genital pores in the left; (E) ventral view of posterior end of cestode, the two penultimate proglottids show genital pores in the left.
Figure 4.
Scanning electron images of cestodes found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) lateral view of scolex with a collar-like basal region; (B) apical view of the same scolex and ventral view of the beginning of strobila; (C) ventro-lateral view of reproductive organs (arrow indicates male reproductive product atop erected genital papillae, arrowhead indicates vagina opening); (D) ventral view of strobila showing unilateral genital pores in the left; (E) ventral view of posterior end of cestode, the two penultimate proglottids show genital pores in the left.
Figure 5.
Phylogenetic trees of the two genes for species identification of P. eschrichtii found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) 18S (SSU) gene region analyzed, Maximum Likelihood (ML) method was used according to GTR+G+I model with 500 bootstrap replicates; (B) 28S (LSU) gene region analyzed, ML TIM3+G. Significant bootstrap support values (≥ 50%) are shown next to the branches. The studied isolates are indicated with black circles.
Figure 5.
Phylogenetic trees of the two genes for species identification of P. eschrichtii found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia: (A) 18S (SSU) gene region analyzed, Maximum Likelihood (ML) method was used according to GTR+G+I model with 500 bootstrap replicates; (B) 28S (LSU) gene region analyzed, ML TIM3+G. Significant bootstrap support values (≥ 50%) are shown next to the branches. The studied isolates are indicated with black circles.
Table 1.
GenBank accession numbers of nucleotide sequences corresponding to the voucher specimen, IPEE_Parasites 14376, of P. eschrichtii found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia.
Table 1.
GenBank accession numbers of nucleotide sequences corresponding to the voucher specimen, IPEE_Parasites 14376, of P. eschrichtii found in the small intestine of a gray whale legally hunted by IPN in Chukotka, Russia.
| Gene (region) |
Base pair number |
Accession number |
|
CoxI mtDNA |
522 |
PV656058 |
| 18S (SSU) |
2085 |
PV667652 |
| 28S (LSU) |
1486 |
PV656419 |
| ITS2 |
548 |
PV658287 |
Table 2.
Comparative measurements of specimens of P. eschrichtii found in the small intestine of a gray whale legally hunted in 2024 by IPN in Chukotka, Russia and literature data. All measurements are given in millimeters.
Table 2.
Comparative measurements of specimens of P. eschrichtii found in the small intestine of a gray whale legally hunted in 2024 by IPN in Chukotka, Russia and literature data. All measurements are given in millimeters.
| Source |
Maximum length |
Maximum width |
| Muravyova and Treshchev, 1970 [26] |
93 |
2.3 |
| Kuramochi et al., 2017 [15] |
60 |
2.9 |
| Loginova et al., 2022 [26] |
≤100 |
4 |
| Present study1
|
400-500 |
6 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).